Abstract
There are many similarities in the epidemiology and transmission of hepatitis A virus (HAV) and hepatitis E virus (HEV) genotype (gt)3 infections in the United States. Both viruses are enterically transmitted, although specific routes of transmission are more clearly established for HAV than for HEV: HAV is restricted to humans and primarily spread through the fecal–oral route, while HEV is zoonotic with poorly understood modes of transmission in the United States. New cases of HAV infection have decreased dramatically in the United States since infant vaccination was recommended in 1996. In recent years, however, outbreaks have occurred among an increasingly susceptible adult population. Although HEV is the most common cause of acute viral hepatitis in developing countries, it is rarely diagnosed in the United States.
The incidence of hepatitis A virus (HAV) infection has been declining in the United States since infant vaccination was initially recommended in 1996 (see cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm). Infections in the United States are typically seen in travelers returning from endemic countries, but outbreaks have increasingly been linked to foods imported from endemic countries (Klevens et al. 2010; Collier et al. 2014). Community-wide outbreaks continue to occur in high-risk populations, such as men who have sex with men (MSM), the homeless, and persons who use illicit drugs, and appear to be more severe in older populations and populations with underlying medical conditions (Villano et al. 1997; Bialek et al. 2011; Collier et al. 2014; Centers for Disease Control and Prevention [CDC], unpubl.). Despite being a vaccine-preventable disease, the changing epidemiology of HAV has led to increased susceptibility in older persons who are more likely to suffer from more severe disease, highlighting the significance of HAV as an important pathogen in terms of public health and its burden on the medical system.
Hepatitis E virus (HEV), also an enterically transmitted RNA virus, is a significant cause of acute viral hepatitis worldwide. In developing countries, there are an estimated 20 million incident HEV infections annually, 3.3 million of which are symptomatic, resulting in an estimated 26,700 deaths in 2015 (Rein et al. 2012; Global Burden of Disease 2015 Mortality and Causes of Death Collaborators 2016). HEV infection has two distinct epidemiological profiles: in developing countries HEV genotype (gt)1 (and occasionally gt2 and gt4) presents as sporadic disease and as large outbreaks primarily associated with fecally contaminated drinking water, although in developed countries HEV gt3 presents as sporadic disease (Teshale et al. 2010). These isolated clinical cases were traditionally associated with travel to endemic areas; however, in the past two decades, locally acquired (autochthonous) HEV infection has been increasingly recognized in developed countries, including the United States.
EPIDEMIOLOGY OF HAV IN THE UNITED STATES
The success of childhood vaccination has made HAV infection rarer in the United States than in the past (Fig. 1) (Murphy et al. 2016). Despite this dramatic success, and the herd immunity it generates throughout the U.S. population, challenges persist as susceptible persons continue to be exposed to contaminated foods as well as other unidentified risks (Murphy et al. 2016).
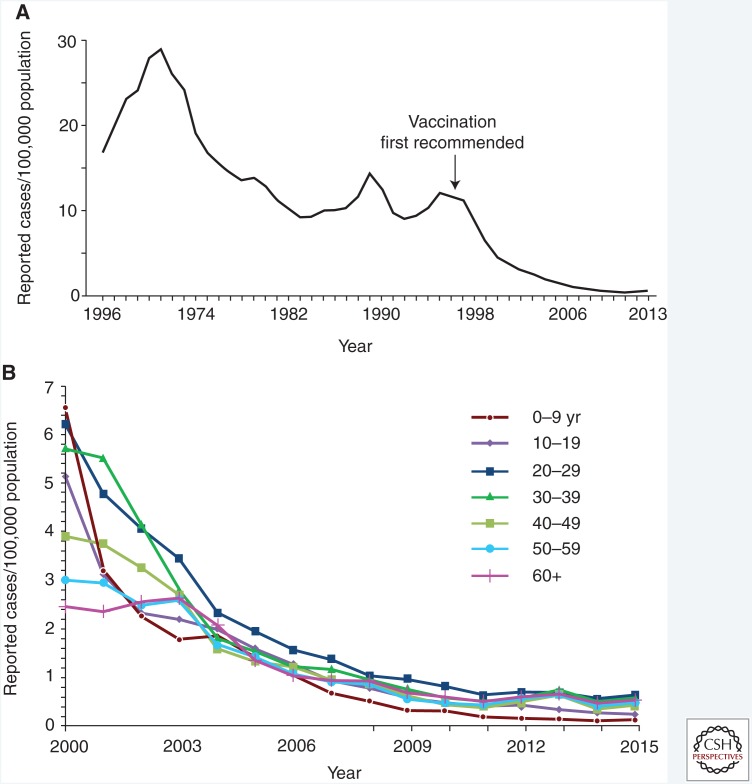
Figure 1.
Incidence of hepatitis A in the United States. (A) Cases of acute hepatitis A per 100,000 population reported to the National Notifiable Diseases Surveillance System, United States, 1966–2013. (Panel from Murphy et al. 2016; reproduced courtesy of the Centers for Disease Control and Prevention © 2016.) (B) Incidence of hepatitis A virus by age group—United States, 2000–2015. (Source: CDC, National Notifiable Diseases Surveillance System, 2015.)
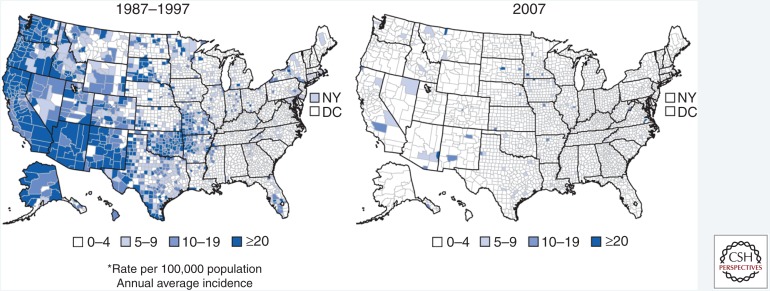
Patterns of HAV disease occur in three distinct ways according to endemicity of disease (Shapiro and Margolis 1993). In developing countries with poor sanitary infrastructure, high infection rates occur among exposed children who develop immunity without ever being symptomatic, and outbreaks are infrequent (Shapiro and Margolis 1993). Countries with intermediate levels of disease experience increased numbers of susceptible adults and occasional, large outbreaks (Shapiro and Margolis 1993). Countries with adequate sanitation infrastructure have low rates of infection and infrequent outbreaks as long as the disease is not introduced into the population from an external source (Shapiro and Margolis 1993; Klevens et al. 2015). In the United States, during the transition from intermediate-to-low HAV endemicity, temporal increases in HAV infection and community outbreaks arose every 10–15 years (Fig. 1A) (Murphy et al. 2016). Geographic variability of HAV disease was also more extreme in the United States prior to the availability of vaccine (Daniels et al. 2009). In 1995, the incidence of HAV infection in the United States ranged from 1.1 per 100,000 per year in Kentucky and New Hampshire to 86.7 per 100,000 in Oregon (Daniels et al. 2009). As vaccination coverage increased, incidence rates declined and temporal and geographic variability disappeared (Fig. 2) (Denniston et al. 2015). In 1996, 31,032 cases were reported to the CDC National Notifiable Diseases Surveillance System (NNDSS); after correcting for underreporting, it is estimated that around 80,000 cases occurred (Bell et al. 1998). In 2015, the most recent year for which data are available, 1398 cases were reported to NNDSS; after correcting for underreporting, it is estimated that ∼2800 cases occurred (see cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm).
Figure 2.
Annual average incidence of reported cases of acute hepatitis A in the United States by county, pre-vaccine (1987–1997) and post-vaccine (2007) introduction. (From Murphy et al. 2016; reproduced courtesy of the Centers for Disease Control and Prevention 2016.)
Previous studies showed that persons who cannot identify a source of exposure may acquire HAV infection from an asymptomatic or unrecognized case (Bell et al. 1998). In the pre-vaccination era, asymptomatic children, in particular, were thought to be the source of infection among cases with unknown risk factors (Bell et al. 1998). Outbreaks among children and staff at day care centers have been recognized since the 1970s (Shapiro and Hadler 1991). During an investigation of a community-wide outbreak, an asymptomatic child ≤6 years old was found in the households of 30% of cases (Bell et al. 1998). The epidemiologic paradigm of asymptomatic infected children being a frequent source of infection changed with the introduction of childhood hepatitis A vaccination.
During 1983–1995, HAV infection occurred primarily in persons 15 to 29 years of age, largely the result of local sustained outbreaks (Bell et al. 1998). Risk factor information was unknown in 52% of cases but the most frequently reported risk factors were history of intravenous drug use, and sexual or household contact with an HAV-infected case; travel was identified in only 4% of cases (Bell et al. 1998). In 2005–2007, however, among HAV cases with identified risk factor information, international travel was the most frequently described potential source of infection (Klevens et al. 2010). As the U.S. hepatitis A vaccination strategy has recommended vaccination for travelers since 1996, this suggests that there is a need for improved vaccine recommendation awareness among both travelers and clinical providers.
HAV does not produce long-lasting infection, and humans are the only natural reservoir, so reduction in disease incidence should be sustained by maintaining a high level of population immunity through ongoing routine vaccination of infants and young children (Bell et al. 1998). Recent increases in HAV infection are primarily affecting the adult population, particularly those who suffer from medical comorbidities (Collier et al. 2014, 2015; Klevens et al. 2015).
Data from the National Health and Nutrition Examination Survey (NHANES) show that the prevalence of antibodies against HAV (anti-HAV) among adults is low, likely because of the success of childhood immunization (Klevens et al. 2015). During 2007–2013, the prevalence of anti-HAV was below 20% among adults aged 30 to 49 years, which indicated a lack of immunity in this age group. This is thought to be because of the fact that 30- to 49-year-olds did not benefit from expansion of childhood hepatitis A vaccination recommendations in 1996 and later, and that there was also lack of HAV exposure when these individuals were children (Denniston et al. 2015; Klevens et al. 2015). A shift toward decreased HAV immunity among U.S.-born adults with increased use of hepatitis A vaccination and higher anti-HAV prevalence among children suggests that U.S. children are no longer a major source of HAV infection for susceptible adults (Klevens et al. 2015).
Despite vaccination efforts, large outbreaks have occurred because susceptible adults are increasingly being exposed to contaminated food imported from HAV-endemic countries (Collier et al. 2014; Ly and Klevens 2015). In 2014, 21,461,300 metric tons of fruits and vegetables were imported into the United States compared with 12,858,800 metric tons of fruits and vegetables imported in 2000 (see ers.usda.gov/data-products/us-food-imports/us-food-imports).
HAV infections among older individuals continue to pose public health and clinical challenges because HAV causes more severe disease among older patients with liver disease and comorbid medical conditions (Collier et al. 2015). The increase over time in the proportion of cases who are hospitalized reflects the simultaneous increase over time in HAV infection among the susceptible older adult population, resulting in more severe disease (Ly and Klevens 2015). Since 1996, it has been recommended that persons with chronic liver disease, including those with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, receive hepatitis A vaccination (Bell et al. 1996). Despite this recommendation, the 2011 Multiple Cause of Death (death certificate) data showed that 39% of deaths related to HAV infection had “other hepatitis” and 28% had “cirrhosis, fibrosis, and other liver diseases,” indicating vaccination was likely not received (Ly and Klevens 2015). Examination of hepatitis A immunity and vaccination among chronic HCV patients in a large U.S. cohort during 2006–2008 revealed that 56% showed immunity or had documented vaccination against hepatitis A (Henkle et al. 2015). In 2015, only 9% of adults with chronic liver disease had received hepatitis A vaccination nationwide (Williams et al. 2017). Although overall adult vaccination coverage remains low for all vaccines, clinicians should be aware of the hepatitis A vaccination recommendations and the changing epidemiology of hepatitis A infection in the United States, and consider vaccinating adults in recommended risk groups (Collier et al. 2015).
Molecular epidemiology is increasingly being used to understand transmission networks of persons with similar risk exposures. Molecular epidemiology alone usually has limited public health utility; however, when combined with reliable and accurate epidemiologic data, it has the potential to generate actionable public health information (Nainan et al. 2005; Klevens et al. 2010). More than 80% of surveillance specimens tested by the Division of Viral Hepatitis Laboratory at CDC are genotype IA, with ∼16% being genotype IB (Y Khudyakov, unpubl.). Both genotype IA and IB are the most common circulating genotypes in the world. Genotype IA is seen in North and South America and genotype IB in the Middle East and North Africa (Vaughan et al. 2014).
TRANSMISSION OF HAV WITHIN THE UNITED STATES
In the United States, transmission of HAV is typically person-to-person through the fecal–oral route and occurs among high-risk groups and populations likely to develop severe disease, including travelers to endemic countries, MSM, persons who use illicit drugs, and persons with chronic liver disease. Foodborne, waterborne, and blood transfusion– and organ transplantation–associated outbreaks are rare but continue to occur (Collier et al. 2014; Foster et al. 2017).
Among cases of HAV infection with risk factor data reported in the United States, ∼46% are attributable to international travel to endemic countries (Klevens et al. 2010). The risk of developing hepatitis A infection among susceptible travelers ranges from 3 to 20 per 1000 travelers (Khuroo 2003; Fiore et al. 2006). The proportion of cases attributable to international travel includes cases who did not travel but reported exposure to a traveler (Khuroo 2003). Vaccination has been recommended for international travelers since 1996, but awareness and uptake of the vaccine prior to travel has been lacking, with up to 65% of U.S. international travelers reportedly unaware that vaccination prior to travel was available or recommended, and vaccine coverage among travelers ∼16% in 2015 (Liu et al. 2015; Williams et al. 2017).
Outbreaks of HAV have been well documented among MSM in the United States (Cotter et al. 2003). Although it is possible that the virus could be transmitted sexually because of the short course of viremia, transmission is more likely the result of close personal contact and specific sexual practices, notably oral–anal or digital–anal contact (Bialek et al. 2011). Hepatitis A vaccine was recommended for MSM by the Advisory Committee on Immunization Practices (ACIP) in 1996, but vaccine uptake remains low (Williams et al. 2017).
HAV infection is also found at high frequency among persons who use illicit drugs (Villano et al. 1997). There is no evidence that injection of drugs contributes substantially to the high prevalence of HAV infection in this population, however, and it is theorized that transmission is not bloodborne but occurs by direct person-to-person contact related to crowding and poor hygiene (Villano et al. 1997).
In 2017, hundreds of reportedly homeless individuals were involved in multiple large hepatitis A outbreaks in the United States (CDC, unpubl.). Despite previously described outbreaks in this population, homeless individuals have not been considered a high-risk group under current ACIP hepatitis A vaccine recommendations (Bell et al. 1996; Tjon et al. 2005; Hennessey et al. 2009). Anti-HAV prevalence among homeless populations in the United States is unknown; few seroprevalence studies have been conducted and NHANES does not include this population. It is difficult to determine whether challenges in maintaining hygiene make homelessness an independent risk factor for HAV infection as homeless individuals may also have other HAV risk factors (Hennessey et al. 2009).
Community-wide epidemics, commonly seen in the pre-vaccine era, no longer occur as frequently as in the past, but large, common source foodborne outbreaks have occurred with increasing frequency in the United States in recent years. Hepatitis A is the only vaccine-preventable foodborne disease in the United States. Describing the epidemiology of foodborne HAV in the United States is challenging because of underreporting related to misdiagnosis, the long incubation period (which leads to difficulty in recalling food exposures), focal viral contamination, and cases being geographically and temporally widespread (Amon et al. 2005). HAV transmission related to food could be the result of contamination at the point of service, usually by an infected food handler, or contamination during growing, harvesting, processing, or distribution (Fiore 2004). A single food handler who works while infectious can transmit HAV to hundreds of persons, but as food handlers are not at high risk of hepatitis A infection owing to their occupation, universal hepatitis A vaccine for food handlers is not recommended at this time (Fiore 2004; Fiore et al. 2006; Sharapov et al. 2016). Mirroring the decline in hepatitis A incidence observed nationally, the number of HAV-infected food-handler cases has declined substantially since the implementation of childhood hepatitis A vaccination (Sharapov et al. 2016).
Food items contaminated before distribution are difficult to identify; once identified, it is often impossible to determine at what point in the cultivation, harvesting, or processing the contamination occurred (Fiore 2004). Recently, HAV-contaminated frozen strawberries, pomegranates, and scallops have been implicated in multiple outbreaks involving a large number of cases and multiple states (Collier et al. 2014; M Foster, unpubl.; M Viray, unpubl.). HAV outbreaks related to shellfish have the added contributing factors of inappropriate harvesting near known sources of sewage, inappropriate discharge of sewage near shellfish beds, and use of contaminated water to immerse harvested live shellfish. Shellfish concentrate hepatitis A virus through natural filtration processes (Enriquez et al. 1992). Foodborne HAV outbreaks can be prevented by reducing bare-hand contact with foods that are not subsequently cooked prior to consumption, excluding food handlers from duties that involve contact with food while infectious, providing sanitary facilities for field workers, and using treated water for rinsing produce or to make ice for packing (Fiore 2004).
Current water-treatment processes used in the United States make waterborne outbreaks of hepatitis A virus unlikely. Although waterborne HAV outbreaks have not been reported in the last 20 years, they have been reported previously in relation to wells, springs, and swimming pools (Bowen and McCarthy 1983; Bergeisen et al. 1985; Mahoney et al. 1992). Fecal contamination of these various water sources was typically from a nearby septic system. Prevention of waterborne HAV outbreaks is best accomplished by using and swimming in water that has been properly treated and/or chlorinated.
PARENTERAL TRANSMISSION
Parenteral transmission of HAV through contaminated blood products or needles is also rare, despite patients being viremic up to 30 days before symptom onset (Bower et al. 2000). Transmission of HAV after blood transfusion has been established previously, and prolonged infection and transmission of HAV in a solid organ transplant recipient was recently reported (Hughes et al. 2014; Foster et al. 2017). No screening tests for HAV infection are required for blood, organ, or tissue donation in the United States, although hepatitis A vaccination of transplantation candidates is recommended (Fiore et al. 2006; Hughes et al. 2014).
Nosocomial transmission of HAV is uncommon because hygienic practices are generally adhered to when patients are symptomatic enough to be hospitalized (Azimi et al. 1986). The use of contact precautions is recommended for health-care workers caring for patients with HAV who require diapers or are incontinent (Siegel et al. 2007). Health-care workers do not have increased prevalence of HAV infection; because of this and the rarity of nosocomial outbreaks, the hepatitis A vaccine is currently not a mandatory vaccination for health-care workers in the United States (Fiore et al. 2006; Guturu et al. 2012).
EPIDEMIOLOGY OF HEV IN THE UNITED STATES
HEV belongs to the genus Orthohepevirus within the Hepeviridae family. Genotypes capable of infecting humans are clustered within a single species, Orthohepevirus A, and include gt1, gt2, gt3, gt4, and gt7 (Smith et al. 2016). gt1 and gt2 infect only humans, whereas gt3 and gt4 have been isolated from a variety of mammalian species and gt7 strains have been isolated from camels (Woo et al. 2014). To date, autochthonous HEV infections reported in the United States have been caused by gt3 only (Drobeniuc et al. 2013).
The true incidence, prevalence, and risk of HEV infection in the United States are unknown. Clinical cases of HEV infection are rarely reported, and few cases of autochthonous acute hepatitis E have been documented in the United States (Drobeniuc et al. 2013). HEV is not a nationally notifiable disease in the United States; consequently, systematic surveillance is lacking (see cdc.gov/nndss/conditions/notifiable/2017). HEV seroprevalence data has been difficult to compare because of the inconsistent performance of research and commercially available HEV antibody serological assays (Mast et al. 1998; Bendall et al. 2010; Drobeniuc et al. 2010). The lack of a highly sensitive and specific Food and Drug Administration (FDA)-licensed assay is very likely to be a major barrier to diagnosis of HEV infection in the United States.
Other causes of liver injury and disease may take precedence over the diagnosis of HEV infection. Misdiagnosis of HEV infection as drug-induced liver injury further complicates accurate detection of HEV in the United States. Serum samples from 318 patients with suspected drug-induced liver injury participating in the Drug-Induced Liver Injury Network study were analyzed for the presence of HEV antibodies (Davern et al. 2011). The prevalence of antibodies to HEV immunoglobulin G and M (anti-HEV IgG and IgM) was 16% and 3%, respectively (Davern et al. 2011). Clinical reassessment of the anti-HEV IgM-positive patients led to the conclusion that HEV infection accounts for a small proportion of suspected drug-induced acute liver injury in the United States (Davern et al. 2011).
Using data from NHANES III (conducted among the noninstitutionalized U.S. population aged ≥6 years between 1988 and 1994), researchers estimated the force of incident HEV infection in the United States to be seven infections per 1000 susceptible persons per year (Table 1) (Faramawi et al. 2011). Recently, for the first time in the United States, a comparison was undertaken of the prevalence of anti-HEV IgG over time using the same serological assay in a population-based sample. Analyzing data from NHANES III (1988–1994) and the 2009–2010 NHANES survey; Teshale et al. (2015) determined that the prevalence of HEV antibody among those aged ≥6 years declined from 10.2% (95% confidence interval [CI], 9.1%–11.4%) during 1988–1994 to 6.0% (95% CI, 5.2%–6.8%) during 2009–2010. In both NHANES surveys, age was significantly associated with anti-HEV IgG positivity, with higher prevalence among older persons (Teshale et al. 2015).
Table 1.
Comparison of HAV and HEV characteristics in the United States
| Additional considerations | HAV | HEV |
|---|---|---|
| Viral taxonomy (family, genus) | Picornaviridae, Hepatovirus | Hepeviridae, Orthohepevirus |
| Primary route of transmission | Fecal–oral | Zoonotic (foodborne) |
| Zoonotic reservoir | No | Pigs, boars, and deer |
| Chronic infection | No | Yes, among immunocompromised persons |
| Severity | Generally mild, self-limited acute illness Increased severity with increased age at infection and in persons with preexisting liver disease Acute liver failure possible, but rare |
Most infections are clinically silent When symptoms occur, illness is generally mild and self-limited Increased severity in pregnant women and persons with preexisting liver disease Acute liver failure possible, but rare |
HAV, hepatitis A virus; HEV, hepatitis E virus.
Analyses of routine blood donations have provided an additional perspective on HEV seroprevalence in the United States. Xu et al. (2013) analyzed blood donations from the National Institutes of Health obtained in 2006 and 2012: the overall seroprevalence of HEV IgG was 18.8% among the 1939 donations, and a statistically significant (p < 0.01) decrease in seroprevalence was noted between the 2006 samples (21.8%) and the 2012 samples (16.0%). Only 0.4% of the total samples were HEV IgM reactive, and HEV RNA was not detected in any of the blood donations (Xu et al. 2013). Stramer et al. evaluated 18,829 routine blood donation samples obtained through the American Red Cross National Testing Laboratories in 2013. Only two donations were confirmed to be positive for HEV RNA, generating a viral RNA detection frequency of one in 9500 among U.S. blood donors (Stramer et al. 2016). In a subanalysis, 4499 samples underwent antibody testing; HEV IgG and IgM seroprevalence were 7.3% and 0.58% overall, respectively (Stramer et al. 2016).
The epidemiology of HEV among immunocompromised patients (e.g., organ transplant recipients, human immunodeficiency virus [HIV]-infected patients, patients with hematologic disorders, or chemotherapy recipients) differs from that among immunocompetent individuals. HEV primarily causes acute, self-limited infections. However, chronic HEV infection involving gt3 was reported in 2008 among solid organ transplant recipients in France who were receiving immunosuppressive drugs (Kamar et al. 2008). In a retrospective analysis of data from primarily European transplant centers, 65.9% of the 85 solid organ transplant recipients with recent HEV infection developed chronic HEV infection (Kamar et al. 2011). In the United States, Terrault et al. (2014) evaluated liver transplant recipients from nine geographically diverse regions between 1998 and 2009; HEV infection was found in 38% of patients prior to liver transplant, but incident infections after liver transplant were rare, with only 1.9% identified during follow-up (median 114 days), all of which were HEV RNA-negative. This suggests that HEV is not a significant cause of unexplained hepatitis among liver transplant recipients in the United States.
Several studies have shown that the prevalence of antibodies against HEV is somewhat higher among individuals who work with swine than among members of the general population. A cross-sectional survey comparing U.S. swine veterinarians to normal blood donors showed that 26.4% and 18.3%, respectively, were positive for anti-HEV IgG (Meng et al. 2002). Prevalence of anti-HEV IgG increased with age among both the swine veterinarians and normal blood donors (Meng et al. 2002). Another cross-sectional serosurvey compared HEV antibody prevalence between North Carolina swine workers and non-swine workers; 10.9% and 2.4% were anti-HEV-positive, respectively, despite a complete lack of history suggesting past clinical manifestations of hepatitis E in any of the anti-HEV-positive study participants (Withers et al. 2002). The serosurvey also examined swine and mice from farms in the same region as the swine workers: the overall antibody prevalence in swine was 34.5%, but varied from 10.0% to 91.7%, although no anti-HEV was detected in mice (Withers et al. 2002). In a survey of wild rat species from across the United States, ∼8% of Rattus spp. rats were positive for HEV by polymerase chain reaction (PCR) (Lack et al. 2012). HEV-positive rats were detected across the United States in both urban and rural areas, and genetic sequencing showed widespread infection with zoonotic HEVgt3 (Lack et al. 2012).
TRANSMISSION OF HEV IN THE UNITED STATES
HEV can be transmitted zoonotically from direct animal contact, fecal–orally from contaminated water or food, parenterally from blood components and solid organ transplants, and vertically from mother to child. Direct evidence of sexual transmission has not been shown. Additionally, person-to-person transmission of HEV has not been documented in the United States, nor have instances of transmission via vertical, parenteral, or waterborne routes.
Historically, in the United States, HEV infection was considered rare and documented only in association with travelers returning from HEV-endemic areas (Centers for Disease Control and Prevention 1993). Travel-related HEV infections in the United States have been identified because of gt1 (Asia and Africa) and gt4 (Asia) (Teshale et al. 2010; Drobeniuc et al. 2013). During the past two decades, however, multiple autochthonous HEV infections have been reported within the United States (Tsang et al. 2000; Amon et al. 2006; Curry et al. 2009; Tohme et al. 2011; Drobeniuc et al. 2013; Im et al. 2013; Te et al. 2013; Aderinto-Adike et al. 2014; Grewal et al. 2014; Sue et al. 2014; Terrault et al. 2014). Despite extensive epidemiological investigation, a clear source of HEV infection is often not apparent in many of the sporadic autochthonous cases reported in the United States (Tsang et al. 2000; Amon et al. 2006; Curry et al. 2009; Tohme et al. 2011).
ZOONOTIC TRANSMISSION
HEV gt3 and gt4 are zoonotic; pigs (domestic and wild) are a recognized reservoir and deer and rabbits serve as potential reservoirs for zoonotic HEV infection (Meng 2013). Swine veterinarians from eight states in the United States had 1.51 times the odds of being positive for HEV antibodies as normal blood donors who were matched on age and geography (Meng et al. 2002). In a cross-sectional serosurvey conducted in North Carolina, the second largest swine producing region in the United States, swine workers had a 4.5-fold higher HEV antibody prevalence than non-swine workers (Withers et al. 2002). In an effort to determine the presence of HEV RNA in commercial pig livers, researchers in Virginia tested 127 pig livers sold in grocery stores in the United States and found that 11% were positive for HEV RNA (Feagins et al. 2007). Furthermore, the research by Feagins et al. showed that inoculation with the contaminating virus could cause infection in specific pathogen-free pigs, raising public health concerns regarding the potential for foodborne HEV infection from virus surviving ex vivo in retail settings (Feagins et al. 2007).
In addition to zoonotic transmission through direct contact with animal reservoirs, HEV gt3 and gt4 are also transmissible through consumption of meat from animals that are viremic at the time of slaughter (Teo 2010; Meng 2013; Ankcorn and Tedder 2017). Although viral sequencing has shown direct linkages between HEV infection and consumption of raw or undercooked pork, boar, or venison in industrial countries outside the United States, no such compelling laboratory evidence has been reported in the United States (Tei et al. 2003; Li et al. 2005; Deest et al. 2007; Matsubayashi et al. 2008; Colson et al. 2010). Additionally, analysis of NHANES data did not show a significant association between HEV seropositivity and pork consumption (Teshale et al. 2015). To date, the potential for autochthonous foodborne HEV infection in the United States has only been posited in two case reports (Aderinto-Adike et al. 2014; Grewal et al. 2014). Grewal et al. (2014) reported on a 62-year-old immunocompetent woman with chronic hepatitis E who denied receiving blood products, recounted eating pork, oysters, and mussels, and noted several trips to Mexico in the years preceding illness onset. Although a specific source of infection was not identified, the investigators reported that “it is tempting to speculate that this patient may have acquired HEV infection by consuming pork or shellfish” (Grewal et al. 2014). Aderinto-Adike and colleagues (2014) presented two cases of locally acquired acute hepatitis E, one in a 56-year-old female from Louisiana, and the other in a 76-year-old female from Texas, whose only noted potential sources of infection were deer, and pig liver and deer meat, respectively.
Although no cases of hepatitis E have been directly linked to waterborne transmission or environmental contamination in the United States to date, there is growing concern that contaminated produce or shellfish could represent an emerging source of foodborne HEV transmission. HEV-infected humans and animals excrete large quantities of HEV in feces, and infectious virus has been isolated from swine manure slurry storage facilities in the Midwestern United States (Kasorndorkbua et al. 2005). Agricultural application or runoff of HEV-containing manure or feces could indirectly contaminate the surrounding environment or irrigation water, leading to downstream contamination of produce or shellfish and the possibility of foodborne transmission, similar to other enterically transmitted viruses, including hepatitis A virus (Shieh et al. 2007; Meng 2013; Ankcorn and Tedder 2017).
PARENTERAL TRANSMISSION
Transfusion transmission has been described in HEV-endemic and industrialized countries outside of the United States (Khuroo et al. 2004; Boxall et al. 2006; Matsubayashi et al. 2008; Hewitt et al. 2014). The overall risk of a viremic blood donation causing a subsequent infection in a recipient is estimated to be 42%; transfusion transmission has been documented from red blood cells, platelet products, fresh frozen plasma, and pooled granulocytes (Hewitt et al. 2014). In a recent evaluation of HEV in blood donors and recipients in the United States, no evidence of HEV transmission was identified among 362 prospectively followed blood recipients despite an HEV IgG seroprevalence of 18.8% among the donor population (Xu et al. 2013). However, researchers estimated that HEV could have been transmitted by transfusion in up to 0.8% of recipients (Xu et al. 2013). This suggests that transfusion-transmitted HEV infections may be occurring in the United States, but that larger studies will be necessary to determine the frequency with which this occurs.
Although HEV can be transmitted by organ transplantation, this has not been documented in the United States. The first reported case of HEV transmission from solid organ transplantation occurred in Germany after an orthotopic liver transplant recipient developed chronic HEV infection and cirrhosis following transplantation of an occultly infected donor liver (Schlosser et al. 2012). More recently, two additional cases of HEV gt3 transmission from solid organ transplantation were reported in France, the first such cases described involving transmission via infectious renal grafts (Pourbaix et al. 2017).
Although a variety of HEV transmission routes have been confirmed worldwide (e.g., fecal–oral, vertical, zoonotic, person-to-person, and parenteral), direct evidence to support most of these transmission routes is lacking in the United States. Additionally, zoonotic exposure and transmission cannot account for the vast majority of HEV IgG-positive persons nationwide; no clear associations with food consumption have been identified (Teshale et al. 2015). Further research efforts are required to determine the routes of transmission that account for the high HEV seroprevalence in the United States.
CONCLUDING REMARKS
HAV infections remain an important cause of morbidity and mortality in the United States, particularly among adults in key risk groups. Although hepatitis A rates have declined drastically since the introduction of effective vaccines, declining anti-HAV prevalence estimates among adults suggest that a substantial proportion of the U.S. adult population remains susceptible to hepatitis A at ages when risk of morbidity and mortality from HAV infection is highest. Although vaccinations have increased herd immunity, changes in epidemiology leading to a more susceptible adult population make it difficult to protect unvaccinated individuals who are exposed to the virus.
HEV infection (gt3) in the United States behaves markedly differently than in developing countries (mainly gt1). The contribution of HEV to the landscape of acute and chronic hepatitis in the United States remains enigmatic. Relevant risk factors of infection, pathogenicity, incidence, prevalence, and modes of transmission of HEV in the United States require further elucidation. The development of FDA-approved assays for HEV, as well as increased epidemiological studies, will be necessary to resolve the questions that persist regarding the epidemiology and transmission of HEV in the United States.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Editors: Stanley M. Lemon and Christopher Walker
Additional Perspectives on Enteric Hepatitis Viruses available at www.perspectivesinmedicine.org
REFERENCES
- Aderinto-Adike A, Schwartz MR, Monsour HP Jr. 2014. Acute hepatitis E in the US today occurs in diverse patient populations: Case reports. Gastroenterology Res 7: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon JJ, Devasia R, Xia G, Nainan OV, Hall S, Lawson B, Wolthuis JS, Macdonald PD, Shepard CW, Williams IT, et al. 2005. Molecular epidemiology of foodborne hepatitis A outbreaks in the United States, 2003. J Infect Dis 192:1323–1330. [DOI] [PubMed] [Google Scholar]
- Amon JJ, Drobeniuc J, Bower WA, Magaña JC, Escobedo MA, Williams IT, Bell BP, Armstrong GL. 2006. Locally acquired hepatitis E virus infection, El Paso, Texas. J Med Virol 78: 741–746. [DOI] [PubMed] [Google Scholar]
- Ankcorn MJ, Tedder RS. 2017. Hepatitis E: The current state of play. Transfus Med 27: 84–95. [DOI] [PubMed] [Google Scholar]
- Azimi PH, Roberto RR, Guralnik J, Livermore T, Hoag S, Hagens S, Lugo N. 1986. Transfusion-acquired hepatitis A in a premature infant with secondary nosocomial spread in an intensive care nursery. Am J Dis Child 140: 23–27. [DOI] [PubMed] [Google Scholar]
- Bell BP, Margolis HS, Shapiro CN. 1996. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 45(RR15): 1–30. [PubMed] [Google Scholar]
- Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS. 1998. The diverse patterns of hepatitis A epidemiology in the United States—Implications for vaccination strategies. J Infect Dis 178: 1579–1584. [DOI] [PubMed] [Google Scholar]
- Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. 2010. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol 82: 799–805. [DOI] [PubMed] [Google Scholar]
- Bergeisen GH, Hinds MW, Skaggs JW. 1985. A waterborne outbreak of hepatitis A in Meade County, Kentucky. Am J Public Health 75: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek SR, Barry V, Bell BP, Valleroy LA, Behel S, Mackellar DA, Secura G, Thiede H, McFarland W, Ford WL, et al. 2011. Seroprevalence and correlates of hepatitis A among HIV-negative American men who have sex with men. Sex Health 8: 343–348. [DOI] [PubMed] [Google Scholar]
- Bowen GS, McCarthy MA. 1983. Hepatitis A associated with a hardware store water fountain and a contaminated well in Lancaster County, Pennsylvania, 1980. Am J Epidemiol 117: 695–705. [DOI] [PubMed] [Google Scholar]
- Bower WA, Nainan OV, Han X, Margolis HS. 2000. Duration of viremia in hepatitis A virus infection. J Infect Dis 182: 12–17. [DOI] [PubMed] [Google Scholar]
- Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. 2006. Transfusion-transmitted hepatitis E in a “nonhyperendemic” country. Transfus Med 16: 79–83. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1993. Hepatitis E among US travelers, 1989–1992. JAMA 269: 845–846. [PubMed] [Google Scholar]
- Collier MG, Khudyakov YE, Selvage D, Adams-Cameron M, Epson E, Cronquist A, Jervis RH, Lamba K, Kimura AC, Sowadsky R, et al. 2014. Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: An epidemiological case study. Lancet Infect Dis 14: 976–981. [DOI] [PubMed] [Google Scholar]
- Collier MG, Tong X, Xu F. 2015. Hepatitis A hospitalizations in the United States, 2002–2011. Hepatology 61: 481–485. [DOI] [PubMed] [Google Scholar]
- Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. 2010. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 202: 825–834. [DOI] [PubMed] [Google Scholar]
- Cotter SM, Sansom S, Long T, Koch E, Kellerman S, Smith F, Averhoff F, Bell BP. 2003. Outbreak of hepatitis A among men who have sex with men: Implications for hepatitis A vaccination strategies. J Infect Dis 187: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Curry JA, Adams N, Crum-Cianflone NF. 2009. Acute hepatitis E virus (HEV) infection in an HIV-infected person in the US. Ann Intern Med 150: 226–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D, Grytdal S, Wasley A. 2009. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ 58: 1–27. [PubMed] [Google Scholar]
- Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH. 2011. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 141: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deest G, Zehner L, Nicand E, Gaudy-Graffin C, Goudeau A, Bacq Y. 2007. Autochthonous hepatitis E in France and consumption of raw pig meat. Gastroenterol Clin Biol 31: 1095–1097. [DOI] [PubMed] [Google Scholar]
- Denniston MM, Monina Klevens R, Jiles RB, Murphy TV. 2015. Self-reported hepatitis A vaccination as a predictor of hepatitis A virus antibody protection in U.S. adults: National Health and Nutrition Examination Survey 2007–2012. Vaccine 33: 3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobeniuc J, Greene-Montfort T, Le NT, Mixson-Hayden TR, Ganova-Raeva L, Dong C, Novak RT, Sharapov UM, Tohme RA, Teshale E, et al. 2013. Laboratory-based surveillance for hepatitis E virus infection, United States, 2005–2012. Emerg Infect Dis 19: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobeniuc J, Meng J, Reuter G, Greene-Montfort T, Khudyakova N, Dimitrova Z, Kamili S, Teo CG. 2010. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: Pangenotypic evaluation of performances. Clin Infect Dis 51: e24–e27. [DOI] [PubMed] [Google Scholar]
- Enriquez R, Frösner GG, Hochstein-Mintzel V, Riedemann S, Reinhardt G. 1992. Accumulation and persistence of hepatitis A virus in mussels. J Med Virol 37: 174–179. [DOI] [PubMed] [Google Scholar]
- Faramawi MF, Johnson E, Chen S, Pannala PR. 2011. The incidence of hepatitis E virus infection in the general population of the USA. Epidemiol Infect 139: 1145–1150. [DOI] [PubMed] [Google Scholar]
- Feagins A, Opriessnig T, Guenette D, Halbur P, Meng XJ. 2007. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol 88: 912–917. [DOI] [PubMed] [Google Scholar]
- Fiore AE. 2004. Hepatitis A transmitted by food. Clin Infect Dis 38: 705–715. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Wasley A, Bell BP. 2006. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 55: 1–23. [PubMed] [Google Scholar]
- Foster MA, Weil LM, Jin S, Johnson T, Hayden-Mixson TR, Khudyakov Y, Annambhotla PD, Basavaraju SV, Kamili S, Ritter JM, et al. 2017. Transmission of hepatitis A virus through combined liver-small intestine-pancreas transplantation. Emerg Infect Dis 23: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease 2015 Mortality and Causes of Death Collaborators. 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal P, Kamili S, Motamed D. 2014. Chronic hepatitis E in an immunocompetent patient: A case report. Hepatology 59: 347–348. [DOI] [PubMed] [Google Scholar]
- Guturu P, Cicalese L, Duchini A. 2012. Hepatitis A vaccination in healthcare personnel. Ann Hepatol 11: 326–329. [PubMed] [Google Scholar]
- Henkle E, Lu M, Rupp LB, Boscarino JA, Vijayadeva V, Schmidt MA, Gordon SC; Chronic Hepatitis Cohort Study (CHeCS) Investigators. 2015. Hepatitis A and B immunity and vaccination in chronic hepatitis B and C patients in a large United States cohort. Clin Infect Dis 60: 514–522. [DOI] [PubMed] [Google Scholar]
- Hennessey KA, Bangsberg DR, Weinbaum C, Hahn JA. 2009. Hepatitis A seroprevalence and risk factors among homeless adults in San Francisco: Should homelessness be included in the risk-based strategy for vaccination? Public Health Rep 124: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J. 2014. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet 384: 1766–1773. [DOI] [PubMed] [Google Scholar]
- Hughes JA, Fontaine MJ, Gonzalez CL, Layon AG, Goodnough LT, Galel SA. 2014. Case report of a transfusion-associated hepatitis A infection. Transfusion 54: 2202–2206. [DOI] [PubMed] [Google Scholar]
- Im GY, Sehgal V, Ward SC. 2013. A case of undulating fevers and elevated liver tests after pancreas–kidney transplantation. Semin Liver Dis 33: 89–94. [DOI] [PubMed] [Google Scholar]
- Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, et al. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Eng J Med 358: 811–817. [DOI] [PubMed] [Google Scholar]
- Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E. 2011. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 140: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Kasorndorkbua C, Opriessnig T, Huang F, Guenette D, Thomas P, Meng XJ, Halbur P. 2005. Infectious swine hepatitis E virus is present in pig manure storage facilities on United States farms, but evidence of water contamination is lacking. App Environ Microbiol 71: 7831–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuroo MS. 2003. Viral hepatitis in international travellers: Risks and prevention. Int J Antimicrob Agents 21: 143–152. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Kamili S, Yattoo GN. 2004. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol 19: 778–784. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Miller JT, Iqbal K, Thomas A, Rizzo EM, Hanson H, Sweet K, Phan Q, Cronquist A, Khudyakov Y, et al. 2010. The evolving epidemiology of hepatitis A in the United States: Incidence and molecular epidemiology from population-based surveillance, 2005–2007. Arch Intern Med 170: 1811–1818. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Denniston MM, Jiles-Chapman RB, Murphy TV. 2015. Decreasing immunity to hepatitis A virus infection among US adults: Findings from the National Health and Nutrition Examination Survey (NHANES), 1999–2012. Vaccine 33: 6192–6198. [DOI] [PubMed] [Google Scholar]
- Lack JB, Volk K, Van Den Bussche RA. 2012. Hepatitis E virus genotype 3 in wild rats, United States. Emerg Infect Dis 18: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, Kurata Y, Ishida M, Sakamoto S, Takeda N. 2005. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis 11: 1958–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Sharapov U, Klevens M. 2015. Patient awareness of need for hepatitis A vaccination (prophylaxis) before international travel. J Travel Med 22: 174–178. [DOI] [PubMed] [Google Scholar]
- Ly KN, Klevens RM. 2015. Trends in disease and complications of hepatitis A virus infection in the United States, 1999–2011: A new concern for adults. J Infect Dis 212: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney FJ, Farley TA, Kelso KY, Wilson SA, Horan JM, McFarland LM. 1992. An outbreak of hepatitis A associated with swimming in a public pool. J Infect Dis 165: 613–618. [DOI] [PubMed] [Google Scholar]
- Mast EE, Alter MJ, Holland PV, Purcell RH. 1998. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatology 27: 857–861. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K. 2008. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 48: 1368–1375. [DOI] [PubMed] [Google Scholar]
- Meng XJ. 2013. Zoonotic and foodborne transmission of hepatitis E virus. Semin Liver Dis 33: 41–49. [DOI] [PubMed] [Google Scholar]
- Meng X, Wiseman B, Elvinger F, Guenette D, Toth T, Engle R, Emerson S, Purcell R. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol 40: 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TV, Denniston MM, Hill HA, McDonald M, Klevens MR, Elam-Evans LD, Nelson NP, Iskander J, Ward JD. 2016. Progress toward eliminating hepatitis A disease in the United States. MMWR Suppl 65: 29–41. [DOI] [PubMed] [Google Scholar]
- Nainan OV, Armstrong GL, Han XH, Williams I, Bell BP, Margolis HS. 2005. Hepatitis A molecular epidemiology in the United States, 1996–1997: Sources of infection and implications of vaccination policy. J Infect Dis 191: 957–963. [DOI] [PubMed] [Google Scholar]
- Pourbaix A, Ouali N, Soussan P, Roque Afonso AM, Péraldi MN, Rondeau E, Peltier J. 2017. Evidence of hepatitis E virus transmission by renal graft. Transpl Infect Dis 10.1111/tid.12624. [DOI] [PubMed] [Google Scholar]
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. 2012. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 55: 988–997. [DOI] [PubMed] [Google Scholar]
- Schlosser B, Stein A, Neuhaus R, Pahl S, Ramez B, Krüger DH, Berg T, Hofmann J. 2012. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol 56: 500–502. [DOI] [PubMed] [Google Scholar]
- Shapiro CN, Hadler SC. 1991. Hepatitis A and hepatitis B virus infections in day-care settings. Pediatr Ann 20: 435–441. [DOI] [PubMed] [Google Scholar]
- Shapiro CN, Margolis HS. 1993. Worldwide epidemiology of hepatitis A virus infection. J Hepatol 18: S11–S14. [DOI] [PubMed] [Google Scholar]
- Sharapov UM, Kentenyants K, Groeger J, Roberts H, Holmberg SD, Collier MG. 2016. Hepatitis A infections among food handlers in the United States, 1993–2011. Public Health Rep 131: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y, Khudyakov Y, Xia G, Ganova-Raeva L, Khambaty F, Woods J, Veazey J, Motes M, Glatzer M, Bialek S. 2007. Molecular confirmation of oysters as the vector for hepatitis A in a 2005 multistate outbreak. J Food Prot 70: 145–150. [DOI] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Jackson M, Chiarello L; Health Care Infection Control Practices Advisory Committee. 2007. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control 35: S65–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Simmonds P, Izopet J, Oliveira-Filho EF, Ulrich RG, Johne R, Koenig M, Jameel S, Harrison TJ, Meng XJ, et al. 2016. Proposed reference sequences for hepatitis E virus subtypes. J Gen Virol 97: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer SL, Moritz ED, Foster GA, Ong E, Linnen JM, Hogema BM, Mak M, Chia CP, Dodd RY. 2016. Hepatitis E virus: Seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion 56: 481–488. [DOI] [PubMed] [Google Scholar]
- Sue PK, Pisanic N, Heaney CD, Nelson K, Schwarz KB, Valsamakis A, Forman M, Jackson AM, Ticehurst JR, Montgomery RA. 2014. Characteristics of post transplantation hepatitis E virus infection among solid organ transplant recipients at a North American transplant center. Hepatology 60: 535A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te H, Drobeniuc J, Kamili S, Dong C, Hart J, Sharapov U. 2013. Hepatitis E virus infection in a liver transplant recipient in the United States: A case report. Transplant Proc 45: 810–813. [DOI] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362: 371–373. [DOI] [PubMed] [Google Scholar]
- Teo C. 2010. Much meat, much malady: Changing perceptions of the epidemiology of hepatitis E. Clin Microbiol and Infect 16: 24–32. [DOI] [PubMed] [Google Scholar]
- Terrault N, Engle RE, Dodge JL, Freise C, Sherker AH, Farci P, Purcell RH. 2014. Hepatitis E virus (HEV) infection is rare cause of hepatitis among US liver transplant recipients. Hepatology 60: 534A–535A. [Google Scholar]
- Teshale EH, Hu DJ, Holmberg SD. 2010. The two faces of hepatitis E virus. Clin Infect Dis 51: 328–334. [DOI] [PubMed] [Google Scholar]
- Teshale EH, Denniston MM, Drobeniuc J, Kamili S, Teo CG, Holmberg SD. 2015. Decline in hepatitis E virus antibody prevalence in the United States from 1988–1994 to 2009–2010. J Infect Dis 211: 366–373. [DOI] [PubMed] [Google Scholar]
- Tjon GM, Götz H, Koek AG, de Zwart O, Mertens PL, Coutinho RA, Bruisten SM. 2005. An outbreak of hepatitis A among homeless drug users in Rotterdam, The Netherlands. J Med Virol 77: 360–366. [DOI] [PubMed] [Google Scholar]
- Tohme RA, Drobeniuc J, Sanchez R, Heseltine G, Alsip B, Kamili S, Hu DJ, Guerra F, Teshale EH. 2011. Acute hepatitis associated with autochthonous hepatitis E virus infection—San Antonio, Texas, 2009. Clin Infect Dis 53: 793–796. [DOI] [PubMed] [Google Scholar]
- Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. 2000. Acute hepatitis E infection acquired in California. Clin Infect Dis 30: 618–619. [DOI] [PubMed] [Google Scholar]
- Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, Khudyakov YE. 2014. Hepatitis A virus: Host interactions, molecular epidemiology and evolution. Infect Genet Evol 21: 227–243. [DOI] [PubMed] [Google Scholar]
- Villano SA, Nelson KE, Vlahov D, Purcell RH, Saah AJ, Thomas DL. 1997. Hepatitis A among homosexual men and injection drug users: More evidence for vaccination. Clin Infect Dis 25: 726–728. [DOI] [PubMed] [Google Scholar]
- Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. 2017. Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Surveill Summ 66: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, Boak MB, Vaughn DW. 2002. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg 66: 384–388. [DOI] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, et al. 2014. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis 20: 1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang RY, Schechterly CA, Ge S, Shih JW, Xia NS, Luban NL, Alter HJ. 2013. An assessment of hepatitis E virus (HEV) in US blood donors and recipients: No detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion 53: 2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]