Abstract
Hepatitis A virus (HAV) and hepatitis E virus (HEV) cause acute, self-limiting hepatic infections that are usually spread by the fecal–oral route in humans. Naturally occurring and experimental infections are possible in a variety of nonhuman primates and, in the case of HEV, a number of other species. Many advances in understanding the pathogenesis of these viruses have come from studies in experimental animals. In general, animals infected with these viruses recapitulate the histologic lesions seen in infected humans, but typically with less severe clinical and histopathological manifestations. This review describes the histopathologic changes associated with HAV and HEV infection in humans and experimental animals.
Of the five hepatotropic human viruses classically associated with hepatitis in humans, hepatitis A virus (HAV) and hepatitis E virus (HEV) are distinguished by being predominantly transmitted by the fecal–oral route and by rarely causing chronic liver injury associated with persistent infection. Progressive liver disease associated with persistent infection has never been documented with HAV, and only recently have long-lasting infections with HEV been recognized in immunocompromised persons (Kamar et al. 2011). Thus, both viruses cause primarily acute, self-limiting liver injury. Fulminant infections associated with massive hepatocellular necrosis can occur with either virus, and prolonged cholestasis is a well-recognized complication of HAV infection (Taylor et al. 2006; Jung et al. 2010; Agrawal et al. 2012), but typically there is little need for invasive diagnostic measures including biopsy of the liver. Thus, primary human material is rarely available for histopathologic examination. On the other hand, infections with either virus, both naturally acquired and experimental, are possible in multiple species of nonhuman primates (see Li and Wakita 2018) and, to a lesser extent, smaller mammals. Most of what we know of the pathogenesis of HAV and HEV comes from studies in experimental animals. In general, animals infected with these viruses recapitulate the histologic lesions seen in infected humans, albeit typically with less severe clinical and histopathological manifestations.
HEPATITIS A VIRUS
HAV infection results in hepatic inflammation that is most often self-limiting. Clinically, however, there is a broad range of possible outcomes following infection (see Shin and Jeong 2018). Liver disease is generally considered to be relatively mild and often asymptomatic in very young children (Benenson et al. 1980). Liver injury is more significant in adults in whom jaundice is more likely. Fulminant hepatitis occurs in ∼0.5% of infected persons, and the case fatality rate is ∼0.2% (Jung et al. 2010). HAV antigen has been detected in organs other than the liver, but no associated inflammation has been reported.
HAV is highly conserved antigenically, and only a single serotype exists. However, seven HAV genotypes have been identified: four genotypes (I, II, III, and VII) are of human origin and the other three (IV, V, VI) are of nonhuman primate origin (see Smith and Simmonds 2018). The primate viruses are similar to human HAV, although they are genetically heterogeneous (Lemon et al. 1987). Naturally occurring HAV infection occurs in nonhuman primates, including the great apes (chimpanzee), Old World macaques (cynomolgus and stump-tailed macaques), baboons, and the African vervet monkey, as well as New World species (owl monkeys, marmosets, and tamarins) (Dienstag et al. 1976; Coursaget et al. 1981; Lemon et al. 1982; Burke and Heisey 1984; Balayan 1992; Bennett et al. 2016). Experimental infections have been produced by either oral or intravenous inoculation in all of these species, except baboons. Clinically evident disease is usually not apparent in these animals, although fulminant hepatitis A has been reported in a chimpanzee (Abe and Shikata 1982). In addition to nonhuman primates, naturally occurring infections with closely related hepatoviruses have recently been discovered in bats, rodents, seals, shrews, and hedgehogs (Drexler et al. 2015; Siebert et al. 2017; see Sander et al. 2018). However, detailed descriptions of hepatic histopathology are lacking in these other mammals. Some evidence suggests that guinea pigs are permissive for infection with HAV, but they do not develop disease (Hornei et al. 2001). In contrast, genetically modified mice with specific deficits in type 1 interferon signaling support robust replication of the virus and some types of genetic knockouts develop severe hepatic inflammation (Hirai-Yuki et al. 2016).
Histopathology of Acute Hepatitis A in Humans
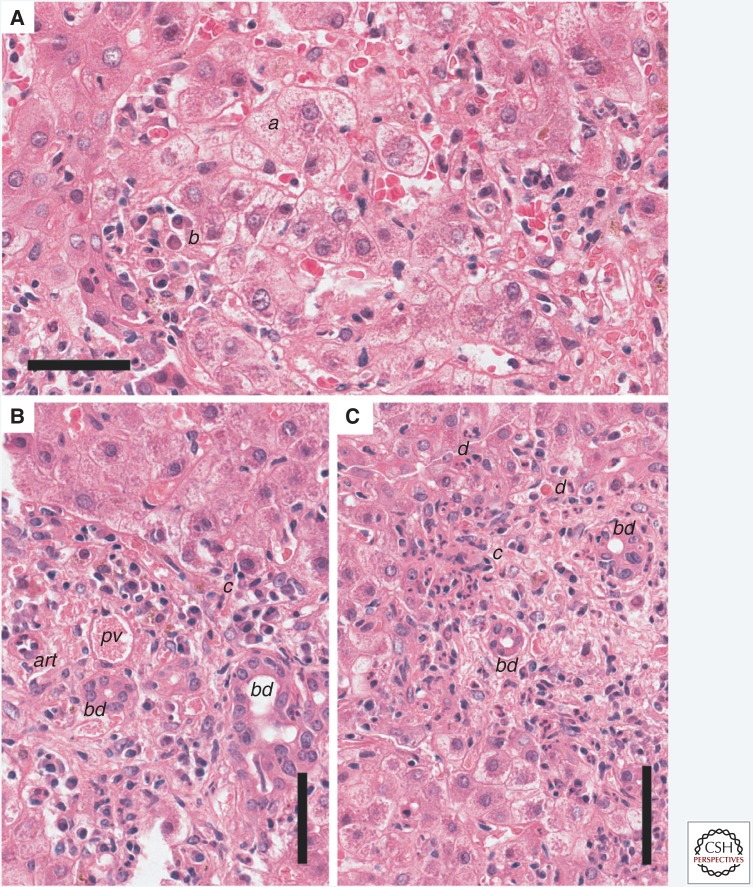
Acute type A viral hepatitis has a typical histologic appearance in humans (Teixeira et al. 1982). HAV appears to be noncytopathic in vivo, as it generally is in cell culture. The earliest stages of infection are attended by little histologic response, although variation in the staining quality of hepatocytes can be seen. Once host immune responses are initiated, the affected liver is characterized by a combination of inflammatory cell infiltration with macrophages and endothelial cell activation, and injury to or necrosis of hepatocytes. Typical histologic features include hepatocellular ballooning: a swelling of viable hepatocytes up to twice the normal diameter, with clearing of the cytoplasm and variation in the size and shape of both nuclei and cytoplasm (Fig. 1A). These changes are accentuated in the centrilobular region of the liver parenchyma. Hepatocyte swelling, apoptotic death, and necrosis of hepatocytes leads to a disordered arrangement of the hepatic plates referred to as lobular disarray (Fig. 1A).
Figure 1.
Section of liver from a human patient with acute hepatitis A stained with hematoxylin and eosin (H&E). (A) The normal arrangement of the hepatic plates is disordered by hepatocyte swelling and parenchymal inflammatory infiltrates causing lobular disarray. Affected hepatocytes (a) are swollen and the cytoplasm has a lacy appearance. Clusters of plasma cells (b) are present within the parenchyma. (B) A portal tract inflammatory infiltrate consisting of lymphocytes and macrophages extends through the limiting plate and is associated with injured hepatocytes (c), resulting in piecemeal necrosis. (C) Piecemeal necrosis (c) associated with adjacent remnants of necrotic or apoptotic hepatocytes. Scale bars, 50 µm. bd, bile ducts; pv, portal vein; art, hepatic artery branch; d, apoptotic hepatocyte.
Hepatic inflammation generally peaks coincident with maximal serum alanine aminotransferase (ALT) elevation. The inflammatory cell population consists of lymphocytes primarily. However, plasma cells can be abundant in the inflamed HAV-infected liver, leading to some histologic overlap with autoimmune hepatitis. This distinguishes hepatitis A from other types of acute viral hepatitis in which plasma cells are less common in the inflammatory infiltrate. There is also a tendency for greater inflammatory involvement of the periportal region than with other viruses. Portal inflammation may extend beyond the margins of the portal tract into the adjacent parenchyma causing hepatocyte injury, a process termed piecemeal necrosis (Fig. 1B,C). This typically involves <25% of the circumference of the portal tract, but the presence of piecemeal necrosis can suggest a diagnosis of other forms of hepatitis (Abe et al. 1982; Kryger and Christoffersen 1983).
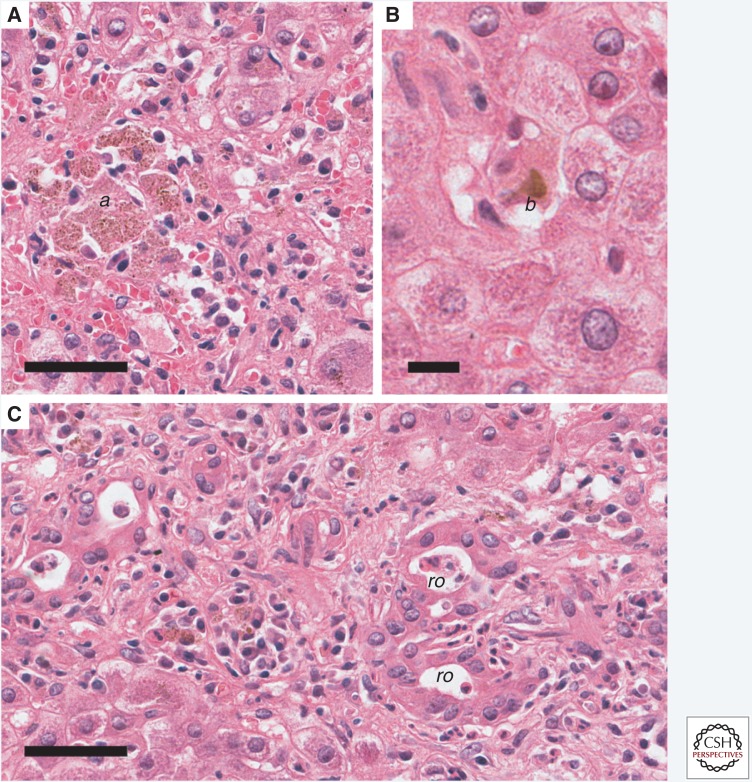
Individual affected hepatocytes may be deeply eosinophilic in hematoxylin and eosin (H&E)-stained sections, often with condensed, deeply basophilic nuclear remnants. The morphologic appearance of such dying cells, which are referred to as acidophilic bodies or Councilman bodies in the older literature (after the American pathologist, William Councilman), is suggestive of an apoptotic death. Such cells are typically found within the parenchyma adjacent to lymphocytes. Fragments of apoptotic hepatocytes can be extruded into the sinusoids and phagocytosed by Kupffer cells, liver resident macrophages. Edema can enlarge the outline of the portal tracts, and reticulin stains can reveal expansion of the portal tract connective tissue with focal areas of hepatocyte loss. The Kupffer cells are typically reactive, characterized by hyperplasia and hypertrophy. The cytoplasm is expanded and often contains glycogen or other polysaccharides staining with Periodic Acid Schiff (PAS) stain and/or hemosiderin (Fig. 2A).
Figure 2.
Acute hepatitis A in a human. Staining with hematoxylin and eosin (H&E). (A) Aggregates of pigmented macrophages (a) within the parenchyma are a common feature of hepatitis A virus (HAV) infection. Scale bar, 50 µm. (B) Cholestasis (b) is often evident within canaliculi of the centrilobular region. Scale bar, 12.5 µm. (C) Rosette formation (ro) caused by hepatocytes arranged around a dilated canaliculus may occur when cholestasis is prominent. Scale bar, 50 µm.
In some cases, the major lesion within the liver is perivenular injury. Cholestasis also occurs. It is marked histologically by the formation of bile plugs (Fig. 2B), typically most evident in the centrilobular canaliculi, and can vary from minimal to marked. Cholestasis may be disproportionate to the degree of hepatocellular injury, and occasionally persists for weeks following peak enzyme elevation (Gordon et al. 1984). Fibrosis is not a feature of the pathology caused by the acute nature of the infection.
The relative proportion of each of these features may vary with the magnitude of the inoculum size, age of the infected individual, as well as the duration of infection and individual variation in the host inflammatory response. Genetic variation in the virulence of different HAV strains has been suggested, but it is not well documented (Fujiwara et al. 2002). In a minority of patients, regions of necrosis can bridge portal tracts or form connections to central regions. Necrosis that affects entire lobules is termed “massive necrosis” with “submassive necrosis,” describing necrosis affecting only portions of the lobule. Hepatocellular necrosis is often accompanied by a prominent periportal proliferation of bipotential precursor cells capable of forming biliary ducts or hepatocytes (Fig. 2C). These small basophilic cells form cords or small ductules in a process called a ductular reaction. With sufficient loss of viable parenchyma, fulminant hepatic failure and death may ensue, although this is quite uncommon.
During recovery, thickened hepatic plates two cells across and multinucleate hepatocytes may be present. Kupffer cell activation often persists. Hepatocellular mitoses become more common.
Histopathology of HAV Infection in the Chimpanzee
Chimpanzees (Pan troglodytes) were the first nonhuman primates shown to develop hepatitis following inoculation with HAV (Deinhardt et al. 1962; see Li and Wakita 2018). Histologic lesions tend to recapitulate those seen in infected humans, although there is a tendency for the chimpanzees to be less severely affected than adult humans (Dienstag et al. 1975; Maynard 1975; Maynard et al. 1975; Popper et al. 1980). Age-related effects, however, may be involved as infected human infants tend to have milder symptoms than adults, and many of the chimpanzees that have been studied are relatively young. Despite the tendency to have milder disease than humans, fulminant hepatitis has been described in an HAV-infected chimpanzee (Abe and Shikata 1982; Theamboonlers et al. 2012).
The most thorough characterization of the histopathology of the liver in HAV-infected chimpanzees was reported by Popper et al. in 1980 (Popper et al. 1980). The initial alteration, coincident with elevations of serum aminotransferase activities, was observed 1 to 5 weeks following oral or percutaneous inoculation of the virus and consisted of focal necrosis (Fig. 3B). Hepatocellular alterations developed mainly in the periportal region. Periportal infiltrates consisted primarily of lymphocytes and macrophages containing PAS-positive, diastase-resistant material. Necrotic hepatocytes were intermixed with the periportal inflammatory infiltrate (Fig. 3B). Lymphocytes and macrophages were aggregated in the portal tracts along with considerable numbers of plasma cells.
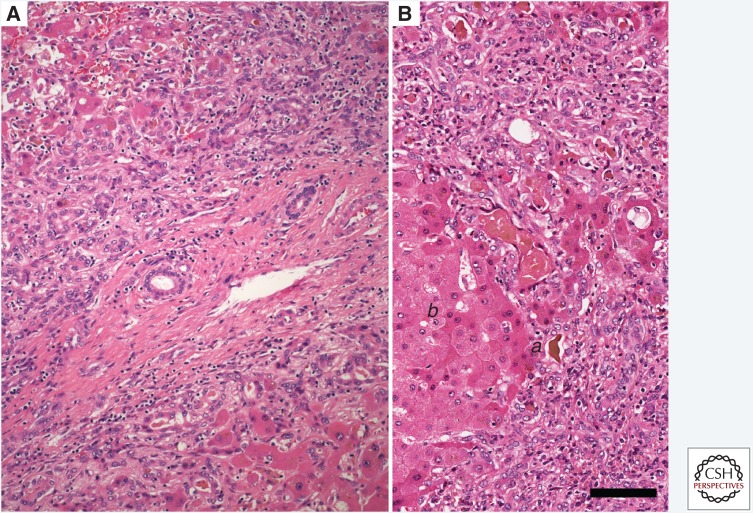
Figure 3.
Sequential liver biopsies taken from an acutely infected adult chimpanzee (Pan troglodytes) inoculated intravenously with HM175 virus. Staining with hematoxylin and eosin (H&E). (A) Pre-infection; alanine aminotransferase (ALT) = 25 IU/L. There is modest periportal inflammation (a) with hemosiderin-laden macrophages. (B) 3 weeks postinoculation; ALT = 319 IU/L. Periportal inflammatory infiltrates are significantly increased and extend into the parenchyma, representing early piecemeal necrosis (b) with a prominent apoptotic/necrotic hepatocyte bordered by inflammatory cells. The hepatic plates are moderately disorganized. (C) 4 weeks postinoculation; ALT = 392 IU/L (peak value). There is more prominent inflammation extending into the parenchyma with diffuse moderate-to-severe hepatocellular swelling and a binucleate hepatocyte. There is marked lobular disarray with disorganized hepatic cords. (D) 10 weeks postinoculation; ALT = 35. Inflammatory infiltrates are reduced and the normal architecture of the hepatic plates has been restored. (Images from Lanford et al. 2011; reproduced, with permission, from The National Academy of Sciences © 2011.)
At a later stage in the infection, there was piecemeal necrosis, characterized by disruption of the limiting plate of the portal tract (Fig. 3B), adjacent hepatocellular necrosis, and a proliferation of bile ductules. In some cases, bridging portal-to-portal necrosis was present. Portal tracts were conspicuously expanded, and broad bands of inflammatory infiltrates appeared to bridge adjacent portal tracts. At the time of maximal inflammation (Fig. 3C), foci of necrotic/apoptotic cells were evident throughout much of the parenchyma, although the centrilobular zone was usually unaffected. Apoptotic bodies were present within the sinusoids and between intact hepatocytes. Cellular infiltration was particularly dense around bile ducts, sometimes involving the duct wall, although the epithelium remained intact. The frequency of hepatocellular mitoses was increased, but the number of multinuclear giant cells remained relatively constant. Also, scattered mononuclear inflammatory cells were noted in the wall of large tributaries of the hepatic veins.
Over the ensuing 6 to 8 weeks, the intralobular inflammation and apoptosis was seen to recede (Fig. 3D). The portal inflammation became less conspicuous, but hemosiderin-containing macrophages remained numerous. Serum enzyme levels returned to normal before the resolution of the histologic lesions.
HAV Infection in Cynomolgus Macaques
Cynomolgus macaques (Macaca fasicularis) have been found to be naturally infected with HAV (Burke and Heisey 1984). While closely related, the virus recovered from cynomolgus macques appears to be specific to these primates as it differs genetically from human HAV and is classified in a distinct genotype (Nainan et al. 1991; see Smith and Simmonds 2018). Cynomolgus macaques are also susceptible to infection with human HAV (Shevtsova et al. 1988; Amado et al. 2010), and the histopathology of the liver following intravenous inoculation with a human HAV isolate (HAF-203) has been described (Amado et al. 2010). Initially, swelling of hepatocytes and microvesicular steatosis were evident by day 7 postinoculation. The highest level of fecal shedding of HAV occurred 15 days after inoculation and was accompanied by the first evidence of hepatic inflammation and serum ALT elevations. This sequence is very similar to what has been observed in experimentally infected chimpanzees (Lanford et al. 2011). Scattered apoptotic hepatocytes were present at day 17 postinfection, as were typical ballooned hepatocytes and reactive Kupffer cells. The portal tracts were maximally infiltrated by lymphocytes and other mononuclear cells, causing expansion of the portal tracts at around 20–45 days postinfection when ALT levels were most elevated. Multinucleate hepatocytes were evident as soon as 7 days postinfection and again in biopsies at 30 days postinfection. Infected animals were not clinically ill at any point following infection. No significant hepatic lesions were observed in a single control animal.
As mentioned above, autochthonously transmitted nonhuman primate viruses are genetically distinct from human isolates, and this may affect their pathogenicity (Emerson et al. 1991; Tsarev et al. 1991).
Old World primates, other than cynomolgus macaques, may not be good models of human HAV infection as they generally develop little or no evidence of liver injury when infected with human strains of HAV (see Li and Wakita 2018). Rhesus macaques and stump-tailed macaques (Macaca arctoides) developed hepatitis when infected with nonhuman primate-derived virus, but not human-derived HAV (Andzhaparidze et al. 1987). In another study, stump-tailed macaques developed enzyme elevations following infection with a human-derived virus, but no histology was described (Mao et al. 1981). Similarly, an HAV isolate from African green monkeys (AGM-27) was shown to cause disease in New World tamarins (Saguinas mystax) and some Old World primates (African green monkey [Chlorocebus sp.] and cynomolgus macaques [M. fasicularis]), but not in chimpanzees (Emerson et al. 1991).
New World Primates: Owl Monkeys
Owl monkeys (Aotus trivirgatus) were recognized to be susceptible to infection with HAV following the discovery of extensive transmission of virus (PA-21 strain) among newly captured animals held within a primate colony in Panama (Lemon et al. 1982). Subsequent studies showed that infection can be achieved experimentally by either oral or intravenous inoculation of virus. The course of infection and the severity of hepatitis varies between studies and individual animals (Keenan et al. 1984; Asher et al. 1995). A study of the natural history of experimental infection via the intravenous route showed viral shedding in feces within 4 days, with fecal shedding peaking just before biochemical evidence of liver inflammation (Lemon 1994).
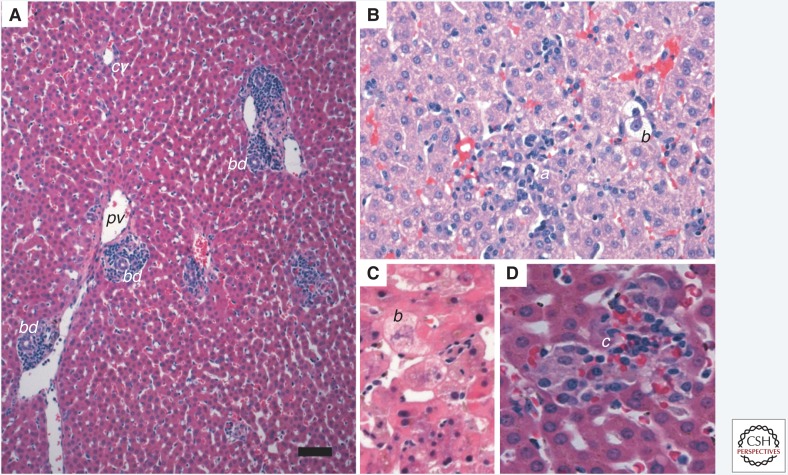
In a hallmark study of orally infected owl monkeys by Asher et al. (1995), histologic abnormalities were described in liver tissue from animals necropsied 4 or 24 hours after infection. The changes described included increased eosinophilic cytoplasmic staining in centrilobular hepatocytes, pyknotic nuclei, and individualization of hepatocytes surrounding the terminal hepatic venules. An important caveat, however, is that these animals were not biopsied before infection for comparison. At 3 days, diffuse swelling of hepatocytes was present throughout the hepatic lobules with clusters of polymorphonuclear leucocytes in sinusoids. Neutrophils often bordered ballooned hepatocytes. Only a few neutrophils were present in sinusoidal spaces at 5 days and only a few hepatocytes had increased cytoplasmic eosinophilia. At 7 days, in addition to occasional neutrophils in sinusoids, diffuse fatty changes were seen in hepatocytes. Two weeks postinoculation, changes in the liver were similar to those found in an animal necropsied 3 days after inoculation: hepatocytes again showed diffuse ballooning with clear cytoplasm and numerous clusters of neutrophils in the lobules. Neutrophils were the only inflammatory cells seen at that time; they were not numerous and were distributed randomly throughout the lobules although the centrilobular regions were spared. The accumulation of iron in Kupffer cells and portal macrophages as well as in macrophages of spleens and lymph nodes was first noted. Portal inflammation and hepatocellular necrosis (Fig. 4A) were observed in these orally infected owl monkeys 3 weeks into the infection, when infectious virus was recovered in cell cultures inoculated with serum, fecal extracts, and throat washings (Asher et al. 1995). At that time, hepatocytes had diffuse accumulations of microvesicular fat in the cytoplasm and enlarged pale nuclei. Occasional apoptotic hepatocytes, not accompanied by inflammatory cells, were also present in the lobules. Portal inflammation was mild and consisted mostly of lymphocytes and enlarged macrophages that were filled with hemosiderin. By 4 weeks after infection, the portal tracts were expanded by an influx of inflammatory cells (Fig. 4B), most of which were lymphocytes, and macrophages surrounding bile ducts. Subsequently, inflammation involved all portal areas and extended across the limiting plates into the lobules. Piecemeal necrosis, characterized by damage to the limiting plate and apoptotic hepatocytes (acidophilic bodies), was evident. By 8 weeks after inoculation, inflammatory infiltrates comprised of small lymphocytes, plasma cells, and macrophages were still extensive but confined to portal areas without signs of hepatocellular necrosis. Over the course of the infection, the amount of iron in Kupffer cells increased, and was accompanied by hypertrophy and proliferation of these cells (Asher et al. 1995).
Figure 4.
Acute hepatitis A in a New World owl monkey (Aotus trivirgatus). Staining with hematoxylin and eosin (H&E). (A) Portal tracts are infiltrated with lymphocytes and macrophages primarily. There is no piecemeal necrosis and no derangement of the lobular architecture. Scale bar, 100 µm. bd, bile duct; pv, portal vein; cv, central vein. (B) Inflammation within the parenchyma is characterized by scattered lymphocyte aggregates (a) and accompanied by modest lobular disarray. Ballooning degeneration (b) of hepatocytes is also evident. (C) Ballooning degeneration (b) of hepatocytes generally occurs early in hepatitis A virus infection in owl monkeys. (D) Kupffer cell aggregates (c) may form in the later stages of hepatitis A infection, with or without iron, as seen here.
In a second study by Keenan et al. (1984), liver biopsies were obtained between 16 and 24 days postintravenous inoculation of two different strains of HAV: PA-21 (gtIII), which was recovered from owl monkeys in Panama (Lemon et al. 1982), and HM175 virus (genotype [gt]I), recovered from an infected human in Australia (see Lemon and Walker 2018). Histopathologic changes were variable but, generally similar to those in the orally infected animals in the Asher study, were present in both. However, there was no evidence of inflammation in the region of the terminal hepatic venules. Hepatic lipidosis was not evident and there were fewer neutrophils seen. By 28–35 days postinoculation, liver enzymes had returned close to baseline and most lesions were diminished in severity, although some mild residual inflammation persisted in portal tracts in several animals. Cholestasis was not evident histologically (nor in the Asher study above), and there was no difference in the clinical course or hepatic histopathology of animals infected with HAV from these two genotypes.
Viral antigen was detected by staining with monoclonal anti-HAV antibodies in epithelial cells of intestinal crypts and in the lamina propria of the ileum in owl monkeys following oral challenge, but inflammation was not observed (Asher et al. 1995). These microscopic findings represent the best evidence available that HAV is capable of infecting cells within the gastrointestinal tract. Careful efforts were made to confirm the specificity of the staining. Virus was also isolated in cell culture from jejunum, ileum, and colonic tissue, but could also be isolated from intestinal contents. However, similar studies performed in tamarins (Saguinus mystax; see Li and Wakita 2018) failed to find any evidence of HAV antigen within intestinal tissues (Mathiesen et al. 1980).
New World Primates: Marmosets and Tamarins
Marmosets (Callithrix sp.) were among the first species to be confirmed to support transmission of HAV and were extensively used in early studies of hepatitis A pathogenesis (Deinhardt et al. 1967; Holmes et al. 1969). At least three species of marmosets are susceptible to infection, including S. mystax, Saguinas labiatus, and other species in this genus, as well as Callithrix jacchus (Deinhardt et al. 1975; Ebert et al. 1978; Baptista et al. 1993). Following either oral or intravenous inoculation, the activities of liver enzymes (isocitrate dehydrogenase [ICD]) become increased in the serum, histologic evidence of hepatic injury is evident and, with time, anti-HAV antibodies develop. Hepatic inflammation can occur as soon as 11 days following infection, and typically peaks at ∼4–6 weeks following infection (Ebert et al. 1978). The timing and extent of enzyme increases and hepatic inflammation vary with the inoculum, and especially its infectious titer. In early studies, fecal shedding of virus was reported to persist in the face of enzyme elevation (Bradley et al. 1977; Ebert et al. 1978). This differs from what has been observed with chimpanzees and owl monkeys, in which fecal shedding typically is diminished with the onset of hepatic inflammation. However, these early studies predate the availability of quantitative polymerase chain reaction (PCR)-based methods for assessing viral shedding.
HAV-infected marmosets show histologic changes that closely resemble those seen in infected human patients (Deinhardt et al. 1975). Initial histologic changes occur before enzyme elevations are evident and include an increase in the number and reactivity of sinusoidal lining cells, as well as scattered foci of individual hepatocyte necrosis and inflammatory infiltrates of the portal tracts. When enzyme elevations are at their peak, hepatocytes become heterogeneous with regard to staining qualities of the cytoplasm and nuclei. Most often, altered hepatocytes have a densely eosinophilic cytoplasm or patches of dense eosinophilia. Dense areas do not stain with PAS, indicating that there is a low glycogen content in these areas, but the PAS stain does accentuate the differences between hepatocytes caused by differences in glycogen content. Ballooned hepatocytes are present, characterized by expansion of the hepatocyte surface area in sections with diaphanous cytoplasm and clear, irregular vacuoles typical of glycogen. There are numerous infiltrates of lymphocytes and macrophages in the parenchyma, with scattered foci of hepatocellular necrosis and isolated acidophilic (apoptotic) hepatocytes. Regions of hepatocellular necrosis and inflammation can be large. There may be inflammation of the connective tissue of the central veins, with thickening of the vein wall and activation of endothelial cells. Kupffer cells become more prominent and often contain PAS-positive, diastase-resistant non-glycogenic material. Aggregates of these lining cells can form in the sinuosids.
Most strikingly, portal tract inflammation is prominent and diffuse, with inflammatory infiltrates consisting of lymphocytes, plasma cells, macrophages, and smaller numbers of neutrophils. Damage to the hepatocytes forming the limiting plate (piecemeal necrosis) can be evident (Deinhardt et al. 1975; Baptista et al. 1993). Plasma cells can be prominent, which is a characteristic feature of inflammation within the HAV-infected human liver. Proliferation of small-caliber bile ductules also occurs. Histologic lesions may persist in the liver despite the return of enzymes to the normal range (Deinhardt et al. 1967). In the cotton-topped marmoset (C. jacchus), there may be inflammation without significant enzyme elevations (Baptista et al. 1993).
Tamarins (Saguinus sp.), closely related to marmosets are also susceptible to infection with HAV (Karayiannis et al. 1986). Elevated serum liver enzyme activities and hepatic inflammation similar to that seen in marmosets have been described following experimental infection (Karayiannis et al. 1990).
Rodent Models: Guinea Pigs
Infectious HAV challenge of guinea pigs does not result in clinical evidence of infection or elevation of liver-related enzyme activities in serum, although HAV was detected in serum and feces by reverse transcription PCR (RT-PCR) or cell culture isolation for several weeks following inoculation of virus adapted to growth in guinea pig cell culture (Hornei et al. 2001). Hepatocyte swelling and rounding with cytoplasmic pallor was described. A proportion of hepatocytes had a deeply acidophilic cytoplasm and irregular outlines with evidence of nuclear pyknosis. Inflammatory cells, mainly lymphocytes, plasma cells, and macrophages in varying proportions were most abundant in regions with hepatocellular damage. Inflammatory cells, primarily lymphocytes, were observed surrounding individual hepatocytes, and multinucleate hepatocytes and activated Kupffer cells (common features of HAV infection in other species) were present. In this study, however, a control animal kept in the same room as the infected animals also developed confluent necrosis of the liver, raising some concern about the underlying cause of liver injury in animals challenged with HAV. The findings suggest that guinea pigs might be able to support limited HAV replication, but hepatic histopathology is not reflective of that seen in infected humans or nonhuman primates.
Genetically Modified Mice
Whereas normal mice are not susceptible to infection with HAV, a model of human hepatitis A has been described recently in knockout mice lacking expression of the type 1 interferon receptor (Ifnar1−/− mice) (Hirai-Yuki et al. 2016, 2018). Similar to primates, HAV replicates robustly in a highly hepatotropic fashion in these genetic knockouts, leading to viremia, fecal shedding of virus via the biliary tract, serum ALT elevations, and impressive inflammatory changes in the liver (Fig. 5). The histopathology of infected mice is characterized by multiple foci of apoptotic hepatocytes surrounded by small aggregates of lymphocytes and macrophages (Fig. 5A). In Ifnar1−/− mice challenged intravenously with high titer virus, the normal liver architecture may be completely destroyed, with large, confluent areas of inflammation. F4/80+CD11B+ macrophages and CD3−NK1.1+ natural killer (NK) cells are sharply increased in number, and both CD4+ and CD8+ CD3+ T lymphocytes are found in infiltrates. Remarkably, however, there is little to no inflammation of portal tracts distinguishing the inflammatory picture observed in these mice from that in experimentally infected nonhuman primates. This is likely reflective of the immunodeficient state of these animals. Appreciable amounts of viral RNA are present in the spleens of these mice during the peak stages of the infection, but there are no changes in the histology of the spleen.
Figure 5.
Hepatitis A in genetically deficient Ifnar1−/− mice that lack expression of the type I interferon (IFN)a/b receptor. Mice were inoculated intravenously with mouse-passaged HM175 virus (Hirai-Yuki et al. 2016). (A) Hematoxylin and eosin (H&E)-stained liver 28 days after inoculation of a 9-week-old female mouse. Serum alanine aminotransferase (ALT) was 57 IU/L, down from 474 IU/L on day 7 postinoculation (upper limits of normal = 30.2 IU/L). Scale bar, 50 µm. Inflammatory infiltrates comprised of foci of lymphocytes and macrophages are scattered throughout the parenchyma, in most cases surrounding remnants of apoptotic hepatocytes (a). Portal inflammation is not evident. (B) Liver collected 41 days after intravenous inoculation of a female Ifnar1−/−Ifngr1−/− double-knockout mouse with third mouse-passage virus; ALT was 201 IU/L. An inflammatory focus is associated with numerous apoptotic hepatocytes (a). Scale bar, 20 µm. (C) Liver from a male Ifnar1−/− mouse collected at necropsy 161 days after inoculation with fifth mouse-passage virus at 9 weeks of age; ALT was 100 IU/L after peaking at 414 IU/L on day 7. Extensive parenchymal infiltrates surround numerous apoptotic hepatocytes (b). Scale bar, 100 µm.
Other murine genetic knockouts are also susceptible to HAV infection, including mice lacking expression of mitochondrial antiviral signaling protein (Mavs−/− mice) and interferon regulatory factors (Irf3−/−Irf7−/− double-knockout mice). However, these knockouts do not develop any evidence of hepatic inflammation when infected with HAV, despite the virus replicating as well if not better than in Ifnar1−/− mice (Hirai-Yuki et al. 2016, 2018). These differences, coupled with evidence for IRF3 activation in the livers of infected Ifnar1−/− mice, suggest that hepatic inflammation arises from intrinsic hepatocellular apoptosis caused by MAVS- and IRF3-dependent (but interferon-independent) induction of proapoptotic interferon-stimulated genes (ISGs). Mice with specific defects in adaptive immunity (NSG and Rag1−/− mice) were nonpermissive for HAV infection, and prior depletion of T cells, NK/NK T cells, or macrophages had no impact on the development of inflammation in Ifnar1−/− mice (Hirai-Yuki et al. 2016).
HEPATITIS E VIRUS
There are four HEV genotypes that have distinct regional distributions and different host species range (see Smith and Simmonds 2018). gtI and gtII have caused large epidemics of hepatitis in developing countries, whereas gtIII and gtIV infect both a variety of animals as well as humans and cause sporadic outbreaks of hepatitis. Unlike HAV, HEV infections in humans often have a zoonotic origin. The virus is transmitted via the fecal–oral route through consumption of contaminated water or by ingestion of undercooked meats, especially pork, but also wild boar and venison (Matsuda et al. 2003; Malcolm et al. 2007). Infection is generally acute and self-limiting, but unlike HAV can persist in persons who are immunocompromised (Kamar et al. 2011). The clinical course of acute infection can vary from the common mild form of the disease to a fatal, fulminant hepatitis, which is particularly common in pregnant women (Rein et al. 2012). There is an increasing number of animal species known to be naturally infected with HEV. These include domestic and wild pigs, deer, rabbits, rats, chickens, moose, mongoose, ferrets, bats, and trout. Serologic evidence of infection extends to rhesus and cynomolgus macaques, goats, sheep, and cattle (Kabrane-Lazizi et al. 1999; Yugo et al. 2014). Cross-species susceptibility and zoonotic transmission have been shown (Yugo et al. 2014).
Histopathology of HEV Infection in Humans
There is less information available concerning the histopathology of HEV infection in humans compared with other types of viral hepatitis. The classical pattern of liver injury in acute hepatitis E includes typical types of lesions found in other forms of acute hepatitis (Peron et al. 2007b). These include ballooning of hepatocytes, scattered apoptotic (acidophilic) bodies, and infiltration of the portal tracts with lymphocytes. Neutrophils are relatively common in the parenchyma and the portal tracts, a feature not seen in other types of viral hepatitis, but lymphocytes are the predominant infiltrating cell type. There is also a cholestatic response to HEV infection. Cholestasis can be mild or moderate with plugged canaliculi, and lead to dilated ductules or a pseudoglandular arrangement of hepatocytes, particularly in the periportal regions. Infections marked by strong cholestatic responses may have less hepatocellular injury than more typical cases, and, in some regards, the cholestatic form of hepatitis E can resemble HAV infection. HEV-associated destructive cholangitis also has been described (Wendum et al. 2005). In more severe cases, there can be widespread hepatocellular necrosis and collapse of the parenchyma with a prominent ductular reaction (Fig. 6A). Surviving hepatocytes may be distended with foamy cytoplasm and pigmented macrophages are scattered throughout the parenchyma. Portal tracts are surrounded by a prominent ductular reaction. Inflammation of vessel wall and Kupffer cell hyperplasia can also be present. Reports from Western Europe indicate that autochthonous HEV infection may be associated with substantial portal inflammation, piecemeal necrosis, cholangiolitis, parenchymal injury, and even fulminant hepatic failure, and that histopathologic changes may be more severe than in patients with infections acquired in more endemic regions (Malcolm et al. 2007; Peron et al. 2007a).
Figure 6.
Hepatitis E in a hepatitis E virus (HEV)-infected patient. (A) Hematoxylin and eosin (H&E) stain. The periportal parenchyma contains a marked ductular reaction characterized by a proliferation of small-caliber ducts lined with pale basophilic epithelial cells extending from the margin of the portal tract. Prominent extracellular matrix separates the ductules. Abutting hepatocytes are in disarray and prominent bile plugs are evident in distended canaliculi and ductules. A mild lymphocytic infiltrate is dispersed through the portal tract and adjacent parenchyma. (B) H&E stain. Higher-magnification image from the same patient. Prominent cholestasis is evident in distended canaliculi with formation of small bile lake at the interface of a prominent ductular reaction on the right and a focus of hepatocytes (a) that are lightly vacuolated and lack normal sinusoidal arrangement. Scale bar, 100 µm.
HEV Infection in Animals
Generally speaking, animals infected with HEV have less hepatic inflammation and liver injury and more modest ALT elevations than infected humans (Halbur et al. 2001; Meng 2010). However, this may reflect a bias against the identification of mild infections in humans because of a lack of readily available diagnostic tests. A wide range of nonhuman primate species are susceptible to infection with various genotypes of HEV, including great apes (chimpanzees), Old World monkeys (cynomolgus, rhesus, and stump-tailed macaques, patas monkeys (Erythrocebus patas), and African green monkeys, as well as New World primates such as owl monkeys, squirrel monkeys (Saimiri sciureus), and tamarins (Fig. 7) (Wang and Wang 2016). Of these many species, cynomolgus macaques have been used most successfully in research.
Figure 7.
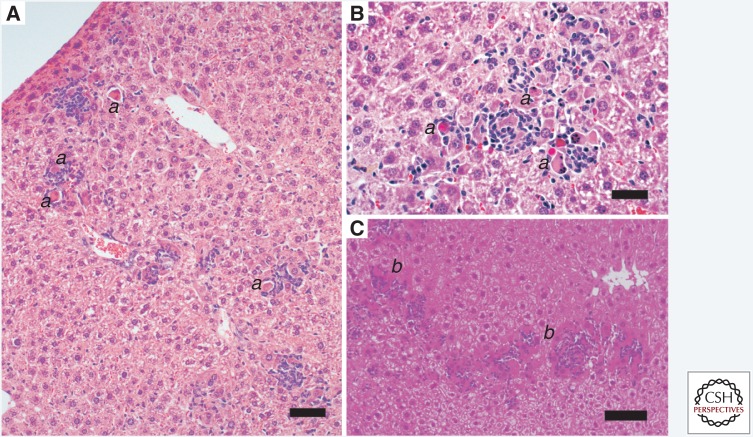
Hepatitis E in a rhesus macaque. (A) The hepatic architecture is well preserved. Focal lymphocytic infiltrates (a) are scattered throughout the parenchyma with mild lymphocytic infiltration (b) of the portal region. Scale bar, 100 µm. pv, portal vein; cv, central vein; ha, hepatic artery branch. (B) Focal lymphocytic parenchymal inflammatory infiltrate surrounding several necrotic hepatocytes.
Susceptible animal species can be infected by either oral or intravenous inoculation of virus, with the latter route considered most efficient (Gupta et al. 1990; Tsarev et al. 1994). The course of the infection parallels that in infected humans, with a 3- to 8-week incubation preceding peak elevation of liver enzyme activity in serum (Uchida et al. 1990; Ticehurst et al. 1992). Fecal viral shedding and viremia tend to peak simultaneously during the incubation and early phase of disease. Liver inflammation develops along with liver enzyme elevations. The infection is self-limited, and resolution occurs in all cases. Persistent infection, as described in immunocompromised patients, has not been observed in nonhuman primates.
Nonhuman Primates: HEV Infection in Chimpanzees
Chimpanzees (P. troglodytes) are variably susceptible to infection with HEV, with host factors likely affecting the severity of the disease. Serum aminotransaminase elevations occur following intravenous inoculation of the virus, but they are generally mild, rarely exceeding 100 IU/L (Yu et al. 2010) or 400 IU/L (Popper et al. 1980). Chimpanzees appear to have less ALT elevation following HEV infection than similarly infected cynomolgus and rhesus macaques (Purcell et al. 2013). Histologic features have been described by several investigators (Pfeifer et al. 1980; Popper et al. 1980; Feinstone et al. 1981; Purcell and Emerson 2001; Yu et al. 2010). Following an incubation period of 2–12 weeks, there may be swelling of hepatocytes with variation in their staining and alterations of the cytoplasm including clumping and lipid vacuolization (Popper et al. 1980). Sinusoidal lining cells may be diffusely reactive. Periportal lymphocytic infiltrates may develop, without piecemeal necrosis, as well as focal necrosis within the hepatic parenchyma (Yu et al. 2010). Lymphoid follicles are evident in some infected chimpanzees, although eosinophils are present within portal infiltrates in others (Popper et al. 1980). Occasionally, lymphocytes are seen to surround bile ducts or ductules with the biliary epithelium displaying mild variation in size and ratio of nucleus to cytoplasmic surface area. Lymphocytic infiltrates may also develop within the parenchyma along with an increase in macrophages and scattered PAS-positive Kupffer cells. Focal necrosis may develop and moderate numbers of apoptotic (acidophilic bodies) may be scattered within the parenchyma. During the course of the infection, there may be a transient alteration in the size of the nucleus in hepatocytes and the appearance of multinucleated hepatocytes (Popper et al. 1980). Cholestasis has not been reported. During recovery, there may be persistent evidence of activated sinusoidal lining cells, characterized by increased size and more prominent cytoplasm, particularly in the centrilobular regions, and irregular dilation of the sinusoids.
HEV Infection in Old World Monkeys: Cynomolgus Macaques
Naturally occurring HEV infection has been reported in cynomolgus and rhesus macaques (M. fasicularis), although the histology of these infections is not well characterized (Arankalle et al. 1994; Hirano et al. 2003; Yugo et al. 2014). Experimental infection of cynomolgus macaques with HEV produced no outward clinical signs (Bradley et al. 1987; Ticehurst et al. 1992). However, serum ALT elevations began ∼25 days following inoculation, coincident with evidence of hepatic inflammation. Increases in serum ALT activity are generally no more than threefold increased over baseline, although in one study the ALT level reached 600 IU/L in an individual animal. The severity of hepatic injury following HEV inoculation may vary with the titer of the inoculum as well as the responses of the individual animal, as might be expected in an outbred species (Soe et al. 1989; Tsarev et al. 1993; Purcell et al. 2013).
The characteristic histologic response in infected cynomolgus monkeys is marked by mild lymphocytic portal inflammatory infiltrates. The infiltrates have been described as less extensive than those seen in infected owl monkeys (Ticehurst et al. 1992). Ballooning hepatocytes, scattered apoptotic hepatocytes associated with lymphocytes and macrophages, and parenchymal lymphocytes are typical (Krawczynski et al. 2011). One severely affected animal was found to have lymphocytic infiltrates and edema of the portal tracts, focal hepatic necrosis, and both micro- and macrovesicular lipid in hepatocytes (Tsarev et al. 1993). Neutrophils were found in the portal tracts around portal veins as well as within the parenchyma. There was neither fibrosis nor other residual lesions.
Rhesus Macaque
Rhesus macaques (Macaca mulatta), like chimpanzees and cynomolgus macaques, are susceptible to infection with all four genotypes of HEV (Wang and Wang 2016). Histologic changes are similar to those seen in cynomolgus macaques (Tsarev et al. 1995; Krawczynski et al. 2011).
HEV Infection in New World Monkeys: Owl Monkeys
Owl monkeys (A. trivirgatus) infected intravenously with HEV derived from an outbreak in Mexico did not have overt signs of disease (Ticehurst et al. 1992). Serum ALT levels peaked 22–26 days following inoculation but were only modestly elevated (three to four times baseline) and there was only a minimal increase in serum bilirubin. Histologic changes in the liver were similarly limited. By day 23, lymphocytic infiltrates were evident in the portal tracts of three of five animals. One animal developed piecemeal necrosis with multiple apoptotic hepatocytes (acidophilic bodies), whereas another developed a parenchymal inflammatory infiltrate with an influx of plasma cells. However, two of five animals did not develop histologic evidence of inflammation. Swollen hepatocytes were apparent in one animal 69 days following inoculation (Ticehurst et al. 1992).
HEV Infection in Marmosets
Infected marmosets (Callithrix sp.) show relatively modest hepatic injury with minor elevations in serum ALT activity (Feinstone et al. 1981; Inoue et al. 1986). Histologic changes in experimentally infected animals consist mainly of lymphocytic infiltration of the portal tracts along with the presence of pigment-laden macrophages in both portal tracts and parenchyma, multifocal individual hepatocyte necrosis, occasional lymphocytic sinusoidal infiltration, and Kupffer cell activation (Inoue et al. 1986; Watanabe et al. 1987). Lymphocytes are frequently adjacent to injured hepatocytes. Cholestasis has not been observed. Hepatocyte ballooning and clumped cytoplasm was noted in one study, along with a general lack of parenchymal inflammation. Later in the course of infection, Kupffer cell proliferation was prominent as were apoptotic hepatocytes (acidophilic bodies), anisocytosis, and variations in the shape of the nucleus in hepatocytes (Feinstone et al. 1981; Bradley et al. 1987).
HEV Infection in Nonprimate Species: Rabbits
Rabbits can be naturally infected with a gtIII strain of HEV and have been experimentally infected with gtIV virus (Cossaboom et al. 2011, 2012). Natural infection is widespread in some regions, and virus recovered from rabbits is capable of infecting pigs as well as nonhuman primates. Histologic changes in the liver of infected adult rabbits are not well characterized, but inflammation appears to coincide with serum ALT elevation. Isolated foci of lymphocytic and histiocytic infiltrates were seen in one of several infected rabbits and foci of necrosis in another animal (Ma et al. 2010).
Pigs
There is widespread infection of domestic pigs with gtIII and gtIV viruses. Infected animals do not develop overt clinical signs, and generally have only minimal-to-moderate evidence of inflammation (Meng et al. 1997). Hepatic inflammation was more severe in pigs infected with HEV derived from a human source than with HEV recovered from pigs, suggesting some differences between strains (Halbur et al. 2001). Natural infection is associated with mild lymphocytic and plasmacytic portal and parenchymal infiltrates. Lymphocytes may be distributed along the sinusoids. Swollen hepatocytes and mild focal necrosis may occasionally occur. Pigs infected with a human strain, US-2, had similar, but more prominent lesions. Inflammation was maximal 20 days following intravenous inoculation of the virus and persisted for up to 55 days, with lesions diminishing toward the end of the infection (Halbur et al. 2001). Infected pigs can be a source of infection in humans consuming undercooked pork sausage or liver (Peron et al. 2007a,b).
Chickens
Avian HEV infection is widespread in the U.S. poultry population (Huang et al. 2002). Experimental infection of specific-pathogen-free chickens with avian HEV produces a mild hepatitis. Lymphocytic and heterophilic perivasculitis and vaculitits have been reported (Billam et al. 2005, 2009).
Rodents
Rats have been recognized to be naturally infected with HEV infection since 2009 (Johne et al. 2010). There are no overt clinical signs associated with the infection (Purcell et al. 2011; Li et al. 2013), and liver enzyme activity is not increased in serum (Purcell et al. 2011). There are only limited descriptions of histologic changes accompanying infection in laboratory rats. Mild hepatitis characterized by light lymphocytic portal inflammation and aggregates of lymphocytes within the parenchyma were noted. Scattered Kupffer cell aggregates were also present. We have tried, without success, to infect Ifnar1−/− knockout mice by intravenous inoculation of HEV.
CONCLUDING REMARKS
Animal models, particularly nonhuman primates, have significantly contributed to current understanding of the pathogenesis of HAV and HEV. Nonhuman primates are susceptible to infection with both HAV and HEV recovered from infected humans, and the inflammatory responses in the liver parallel those seen in infected humans. Experimental animal models have been exceptionally valuable in developing an understanding of the impact of these viruses on the histologic appearance of the liver, as liver tissue is rarely available for study from humans experiencing typical, uncomplicated hepatitis A or hepatitis E.
The pattern and timing of hepatic inflammation following infection with HAV in susceptible nonhuman primate species closely resembles that seen in infected humans. However, the degree of liver injury, reflected in enzyme elevations and hepatic inflammation, has generally been reported to be milder in nonhuman primates than in infected humans. There may be histologic evidence of cholestasis in infected nonhuman primates, but icterus and other overt clinical signs of disease such as lethargy and inappetence are not observed, despite being common in infected humans. To some extent, this may reflect the age of experimentally infected animals, as HAV infection is often mild and not recognized clinically in young children (Benenson et al. 1980). Nonetheless, in a carefully executed vaccine trial involving healthy children aged 2–16 years, each of the 25 placebo recipients who became infected was symptomatic and had elevated serum ALT activity (mean 1383 U/L with a range of 240–2850) and half were icteric (Werzberger et al. 1992). Similar overt evidence of liver injury has rarely been observed in experimentally infected nonhuman primates, although fulminant disease was reported in one chimpanzee (Theamboonlers et al. 2012). HEV infection similarly appears to result in lesser degrees of liver injury in experimentally infected animals than in humans. However, it seems likely that only the most severe HEV infections are recognized clinically in humans, and there is still much to be learned about the complete spectrum of human HEV infection.
Despite these potential differences in severity of the inflammatory response, the nature of the host response and clearance of infection seem to be quite similar, especially for hepatitis A. Distinctive features of HAV infection, which has a tendency toward more robust inflammation in the portal and periportal regions of the liver, at times progressing to piecemeal necrosis, is recapitulated in primate models, particularly in chimpanzees. The presence of large numbers of plasma cells is also a feature common to both animal models of HAV infection and infection in humans. Prominent cholestasis, on the other hand, is not commonly observed in animal models of HAV infection. Similarly, of the two main types of histopathologic response to HEV infection (inflammatory and cholestatic), inflammation is reproduced in animal models, albeit perhaps with less severity than in humans. Cholestatic responses to HEV infection have not been observed in animal models, but additional studies will be needed to accurately compare human and animal responses to HEV infection at a fine level of detail.
Footnotes
Editors: Stanley M. Lemon and Christopher Walker
Additional Perspectives on Enteric Hepatitis Viruses available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abe K, Shikata T. 1982. Fulminant type A viral hepatitis in a chimpanzee. Acta Pathol Jpn 32: 143–148. [DOI] [PubMed] [Google Scholar]
- Abe H, Beninger PR, Ikejiri N, Setoyama H, Sata M, Tanikawa K. 1982. Light microscopic findings of liver biopsy specimens from patients with hepatitis type A and comparison with type B. Gastroenterology 82: 938–947. [PubMed] [Google Scholar]
- Agrawal V, Goel A, Rawat A, Naik S, Aggarwal R. 2012. Histological and immunohistochemical features in fatal acute fulminant hepatitis E. Indian J Pathol Microbiol 55: 22–27. [DOI] [PubMed] [Google Scholar]
- Amado LA, Marchevsky RS, De Paula VS, Hooper C, Freire MdS, Gaspar AMC, Pinto MA. 2010. Experimental hepatitis A virus (HAV) infection in cynomolgus monkeys (Macaca fascicularis): Evidence of active extrahepatic site of HAV replication. Int J Exp Pathol 91: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andzhaparidze AG, Kazachkov Iu A, Balaian MS, Kusov I, Poleshchuk VF. 1987. Hepatitis A in Macaca fascicularis and M. arctoides infected by the Java monkey-55 strain of hepatitis A virus. Vopr Virusol 32: 440–448. [PubMed] [Google Scholar]
- Arankalle VA, Goverdhan MK, Banerjee K. 1994. Antibodies against hepatitis E virus in Old World monkeys. J Viral Hepat 1: 125–129. [DOI] [PubMed] [Google Scholar]
- Asher LV, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J Med Virol 47: 260–268. [DOI] [PubMed] [Google Scholar]
- Balayan MS. 1992. Natural hosts of hepatitis A virus. Vaccine 10: S27–S31. [DOI] [PubMed] [Google Scholar]
- Baptista ML, Marchevsky RS, Oliveira AV, Yoshida CF, Schatzmayr HG. 1993. Histopathological and immunohistochemical studies of hepatitis A virus infection in marmoset Callithrix jacchus. Exp Toxicol Pathol 45: 7–13. [DOI] [PubMed] [Google Scholar]
- Benenson MW, Takafuji ET, Bancroft WH, Lemon SM, Callahan MC, Leach DA. 1980. A military community outbreak of hepatitis type A related to transmission in a child care facility. Am J Epidemiol 112: 471–481. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Sibley SD, Lauck M, Weny G, Hyeroba D, Tumukunde A, Friedrich TC, O’Connor DH, Johnson CA, Rothman JM, et al. 2016. Naturally circulating hepatitis A virus in olive baboons, Uganda. Emerg Infect Dis 22: 1308–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billam P, Huang FF, Sun ZF, Pierson FW, Duncan RB, Elvinger F, Guenette DK, Toth TE, Meng XJ. 2005. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J Virol 79: 3429–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billam P, LeRoith T, Pudupakam RS, Pierson FW, Duncan RB, Meng XJ. 2009. Comparative pathogenesis in specific-pathogen-free chickens of two strains of avian hepatitis E virus recovered from a chicken with hepatitis–splenomegaly syndrome and from a clinically healthy chicken. Vet Microbiol 139: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DW, Gravelle CR, Cook EH, Fields RM, Maynard JE. 1977. Cyclic excretion of hepatitis A virus in experimentally infected chimpanzees: Biophysical characterization of the associated HAV particles. J Med Virol 1: 133–138. [DOI] [PubMed] [Google Scholar]
- Bradley DW, Krawczynski K, Cook EH Jr, McCaustland KA, Humphrey CD, Spelbring JE, Myint H, Maynard JE. 1987. Enterically transmitted non-A, non-B hepatitis: Serial passage of disease in cynomolgus macaques and tamarins and recovery of disease-associated 27- to 34-nm viruslike particles. Proc Natl Acad Sci 84: 6277–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DS, Heisey GB. 1984. Wild Malaysian cynomolgus monkeys are exposed to hepatitis A virus. Am J Trop Med Hyg 33: 940–944. [DOI] [PubMed] [Google Scholar]
- Cossaboom CM, Cordoba L, Dryman BA, Meng XJ. 2011. Hepatitis E virus in rabbits, Virginia, USA. Emerg Infect Dis 17: 2047–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossaboom CM, Cordoba L, Sanford BJ, Pineyro P, Kenney SP, Dryman BA, Wang Y, Meng XJ. 2012. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J Gen Virol 93: 1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursaget P, Levesque B, Gretillat E, Eyraud M, Ferrara L, Germain M. 1981. Hepatitis A virus in primates outside captivity. Lancet 2: 929–929. [Google Scholar]
- Deinhardt F, Courtois G, Dherte P, Osterrieth P, Ninane G, Henle G, Henle W. 1962. Studies of liver function tests in chimpanzees after inoculation with human infectious hapatitis virus. Am J Hyg 75: 311–321. [DOI] [PubMed] [Google Scholar]
- Deinhardt F, Holmes AW, Capps RB, Popper H. 1967. Studies on the transmission of human viral hepatitis to marmoset monkeys. I: Transmission of disease, serial passages, and description of liver lesions. J Exp Med 125: 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt F, Peterson D, Cross G, Wolfe L, Holmes AW. 1975. Hepatitis in marmosets. Am J Med Sci 270: 73–80. [DOI] [PubMed] [Google Scholar]
- Dienstag JL, Feinstone SM, Purcell RH, Hoofnagle JH, Barker LF, London WT, Popper H, Peterson JM, Kapikian AZ. 1975. Experimental infection of chimpanzees with hepatitis A virus. J Infect Dis 132: 532–545. [DOI] [PubMed] [Google Scholar]
- Dienstag JL, Davenport FM, McCollum RW, Hennessy AV, Klatskin G, Purcell RH. 1976. Nonhuman primate-associated viral hepatitis type A. Serologic evidence of hepatitis A virus infection. JAMA 236: 462–464. [PubMed] [Google Scholar]
- Drexler JF, Corman VM, Lukashev AN, van den Brand JM, Gmyl AP, Brunink S, Rasche A, Seggewibeta N, Feng H, Leijten LM, et al. 2015. Evolutionary origins of hepatitis A virus in small mammals. Proc Natl Acad Sci 112: 15190–15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert JW, Maynard JE, Bradley DW, Lorenz D, Krushak DH. 1978. Experimental infection of marmosets with hepatitis A virus. Primates Med 10: 295–299. [PubMed] [Google Scholar]
- Emerson SU, Tsarev SA, Purcell RH. 1991. Biological and molecular comparisons of human (HM-175) and simian (AGM-27) hepatitis A viruses. J Hepatol 13: S144–S145. [DOI] [PubMed] [Google Scholar]
- Feinstone SM, Alter HJ, Dienes HP, Shimizu Y, Popper H, Blackmore D, Sly D, London WT, Purcell RH. 1981. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis 144: 588–598. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Yokosuka O, Ehata T, Saisho H, Saotome N, Suzuki K, Okita K, Kiyosawa K, Omata M. 2002. Association between severity of type A hepatitis and nucleotide variations in the 5′ non-translated region of hepatitis A virus RNA: Strains from fulminant hepatitis have fewer nucleotide substitutions. Gut 51: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SC, Reddy KR, Schiff L, Schiff ER. 1984. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Int Med 101: 635–637. [DOI] [PubMed] [Google Scholar]
- Gupta H, Tandon BN, Sriramachari S, Joshi YK, Iyenger B. 1990. Animal transmission of enteric non-A, non-B hepatitis infection to Macaca mulatta by faeco-oral route. Indian J Med Res 91: 87–90. [PubMed] [Google Scholar]
- Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol 39: 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai-Yuki A, Hensley L, McGivern DR, Gonzalez-Lopez O, Das A, Feng H, Sun L, Wilson JE, Hu F, Feng Z, et al. 2016. MAVS-dependent host species range and pathogenicity of human hepatitis A virus. Science 353: 1541–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hirai-Yuki A, Whitmire JK, Joyce M, Tyrrell DL, Lemon SM. 2018. Murine models of hepatitis A virus (HAV) infection. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Ding X, Tran HT, Li TC, Takeda N, Sata T, Nakamura S, Abe K. 2003. Prevalence of antibody against hepatitis E virus in various species of non-human primates: Evidence of widespread infection in Japanese monkeys (Macaca fuscata). Jpn J Infect Dis 56: 8–11. [PubMed] [Google Scholar]
- Holmes AW, Wolfe L, Rosenblate H, Deinhardt F. 1969. Hepatitis in marmosets: Induction of disease with coded specimens from a human volunteer study. Science 165: 816–817. [DOI] [PubMed] [Google Scholar]
- Hornei B, Kammerer R, Moubayed P, Frings W, Gauss-Muller V, Dotzauer A. 2001. Experimental hepatitis A virus infection in guinea pigs. J Med Virol 64: 402–409. [DOI] [PubMed] [Google Scholar]
- Huang FF, Haqshenas G, Shivaprasad HL, Guenette DK, Woolcock PR, Larsen CT, Pierson FW, Elvinger F, Toth TE, Meng XJ. 2002. Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. J Clin Microbiol 40: 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue O, Nagataki S, Itakura H, Iida F, Shimizu Y, Yano M, Shikata T, Tandon BN. 1986. Liver morphology in marmosets infected with epidemic non-A, non-B hepatitis in India. Liver 6: 178–183. [DOI] [PubMed] [Google Scholar]
- Johne R, Heckel G, Plenge-Bonig A, Kindler E, Maresch C, Reetz J, Schielke A, Ulrich RG. 2010. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg Infect Dis 16: 1452–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YM, Park SJ, Kim JS, Jang JH, Lee SH, Kim JW, Park YM, Hwang SG, Rim KS, Kang SK, et al. 2010. Atypical manifestations of hepatitis A infection: A prospective, multicenter study in Korea. J Med Virol 82: 1318–1326. [DOI] [PubMed] [Google Scholar]
- Kabrane-Lazizi Y, Fine JB, Elm J, Glass GE, Higa H, Diwan A, Gibbs CJ Jr, Meng XJ, Emerson SU, Purcell RH. 1999. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg 61: 331–335. [DOI] [PubMed] [Google Scholar]
- Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, et al. 2011. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 140: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Karayiannis P, Jowett T, Enticott M, Moore D, Pignatelli M, Brenes F, Scheuer PJ, Thomas HC. 1986. Hepatitis A virus replication in tamarins and host immune response in relation to pathogenesis of liver cell damage. J Med Virol 18: 261–276. [DOI] [PubMed] [Google Scholar]
- Karayiannis P, Chitranukroh R, Fry M, Petrovic LM, Moore D, Scheuer PJ, Thomas HC. 1990. Protracted alanine aminotransferase levels in tamarins infected with hepatitis A virus. J Med Virol 30: 151–158. [DOI] [PubMed] [Google Scholar]
- Keenan CM, Lemon SM, LeDuc JW, McNamee GA, Binn LN. 1984. Pathology of hepatitis A infection in the owl monkey (Aotus trivirgatus). Am J Pathol 115: 1–8. [PMC free article] [PubMed] [Google Scholar]
- Krawczynski K, Meng XJ, Rybczynska J. 2011. Pathogenetic elements of hepatitis E and animal models of HEV infection. Virus Res 161: 78–83. [DOI] [PubMed] [Google Scholar]
- Kryger P, Christoffersen P. 1983. Liver histopathology of the hepatitis A virus infection: A comparison with hepatitis type B and non-A, non-B. J Clin Pathol 36: 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford RE, Feng Z, Chavez D, Guerra B, Brasky KM, Zhou Y, Yamane D, Perelson AS, Walker CM, Lemon SM. 2011. Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc Natl Acad Sci 108: 11223–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SM. 1994. The natural history of hepatitis A: The potential for transmission by transfusion of blood or blood products. Vox San 67: 19–23; discussion 24–16. [PubMed] [Google Scholar]
- *.Lemon SM, Walker CM. 2018. Hepatitis A virus and hepatitis E virus: Emerging and re-emerging enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SM, LeDuc JW, Binn LN, Escajadillo A, Ishak KG. 1982. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J Med Virol 10: 25–36. [DOI] [PubMed] [Google Scholar]
- Lemon SM, Chao SF, Jansen RW, Binn LN, LeDuc JW. 1987. Genomic heterogeneity among human and nonhuman strains of hepatitis A virus. J Virol 61: 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Li T-C, Wakita T. 2018. Small animal models of hepatitis E virus infection. Cold Spring Harb Perspect Med 10.1101/cshperspect.a032581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TC, Yoshizaki S, Ami Y, Suzaki Y, Yasuda SP, Yoshimatsu K, Arikawa J, Takeda N, Wakita T. 2013. Susceptibility of laboratory rats against genotypes 1, 3, 4, and rat hepatitis E viruses. Vet Microbiol 163: 54–61. [DOI] [PubMed] [Google Scholar]
- Ma H, Zheng L, Liu Y, Zhao C, Harrison TJ, Ma Y, Sun S, Zhang J, Wang Y. 2010. Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS ONE 5: e9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm P, Dalton H, Hussaini HS, Mathew J. 2007. The histology of acute autochthonous hepatitis E virus infection. Histopathology 51: 190–194. [DOI] [PubMed] [Google Scholar]
- Mao JS, Guo XY, Huang HY, Yu PH, Huang BZ, Ding ZC, Chen NL, Yu JH, Xie RY. 1981. Studies on the transmission of human hepatitis A virus to stump-tailed monkey. Sci Sin 24: 1590–1596. [PubMed] [Google Scholar]
- Mathiesen LR, Moller AM, Purcell RH, London WT, Feinstone SM. 1980. Hepatitis A virus in the liver and intestine of marmosets after oral inoculation. Infect Immun 28: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Okada K, Takahashi K, Mishiro S. 2003. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis 188: 944. [DOI] [PubMed] [Google Scholar]
- Maynard JE. 1975. Hepatitis A: Perspectives and recent advances. Am J Pathol 81: 683–694. [PMC free article] [PubMed] [Google Scholar]
- Maynard JE, Bradley DW, Gravelle CR, Ebert JW, Krushak DH. 1975. Preliminary studies of hepatitis A in chimpanzees. J Infect Dis 131: 194–197. [DOI] [PubMed] [Google Scholar]
- Meng XJ. 2010. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet Microbiol 140: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci 94: 9860–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nainan OV, Margolis HS, Robertson BH, Balayan M, Brinton MA. 1991. Sequence analysis of a new hepatitis A virus naturally infecting cynomolgus macaques (Macaca fascicularis). J Gen Virol 72: 1685–1689. [DOI] [PubMed] [Google Scholar]
- Peron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. 2007a. Fulminant liver failure from acute autochthonous hepatitis E in France: Description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat 14: 298–303. [DOI] [PubMed] [Google Scholar]
- Peron JM, Danjoux M, Kamar N, Missoury R, Poirson H, Vinel JP, Mansuy JM, Bureau C, Izopet J, Brousset P, et al. 2007b. Liver histology in patients with sporadic acute hepatitis E: A study of 11 patients from South-West France. Virchows Arch 450: 405–410. [DOI] [PubMed] [Google Scholar]
- Pfeifer U, Thomssen R, Legler K, Bottcher U, Gerlich W, Weinmann E, Klinge O. 1980. Experimental non-A, non-B hepatitis: Four types of cytoplasmic alteration in hepatocytes of infected chimpanzees. Virchows Arch B Cell Pathol Incl Mol Pathol 33: 233–243. [DOI] [PubMed] [Google Scholar]
- Popper H, Dienstag JL, Feinstone SM, Alter HJ, Purcell RH. 1980. The pathology of viral hepatitis in chimpanzees. Virchows Arch A Pathol Anat Histol 387: 91–106. [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. 2001. Animal models of hepatitis A and E. ILAR J 42: 161–177. [DOI] [PubMed] [Google Scholar]
- Purcell RH, Engle RE, Rood MP, Kabrane-Lazizi Y, Nguyen HT, Govindarajan S, St Claire M, Emerson SU. 2011. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis 17: 2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Engle RE, Govindarajan S, Herbert R, St Claire M, Elkins WR, Cook A, Shaver C, Beauregard M, Swerczek J, et al. 2013. Pathobiology of hepatitis E: Lessons learned from primate models. Emerg Microbe Infect 2: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. 2012. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology (Baltimore, Md) 55: 988–997. [DOI] [PubMed] [Google Scholar]
- *.Sander A-L, Corman VM, Lukashev AN, Drexler JF. 2018. Evolutionary origins of enteric hepatitis viruses. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova ZV, Lapin BA, Doroshenko NV, Krilova RI, Korzaja LI, Lomovskaya IB, Dzhelieva ZN, Zairov GK, Stakhanova VM, Belova EG, et al. 1988. Spontaneous and experimental hepatitis A in Old World monkeys. J Med Primatol 17: 177–194. [PubMed] [Google Scholar]
- *.Shin E-C, Jeong S-K. 2018. Natural history, clinical manifestations, and pathogenesis of hepatitis A. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert U, Rademaker M, Ulrich SA, Wohlsein P, Ronnenberg K, Prenger-Berninghoff E. 2017. Bacterial microbiota in harbor seals (Phoca vitulina) from the North Sea of Schleswig-Holstein, Germany, around the time of morbillivirus and influenza epidemics. J Wildl Dis 53: 201–214. [DOI] [PubMed] [Google Scholar]
- *.Smith DB, Simmonds P. 2018. Classification and genomic diversity of enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe S, Uchida T, Suzuki K, Komatsu K, Azumi J, Okuda Y, Iida F, Shikata T, Rikihisa T, Mizuno K, et al. 1989. Enterically transmitted non-A, non-B hepatitis in cynomolgus monkeys: Morphology and probable mechanism of hepatocellular necrosis. Liver 9: 135–145. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ. 2006. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology 44: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MR Jr, Weller IV, Murray A, Bamber M, Thomas HC, Sherlock S, Scheuer PJ. 1982. The pathology of hepatitis A in man. Liver 2: 53–60. [DOI] [PubMed] [Google Scholar]
- Theamboonlers A, Abe K, Thongmee C, Poovorawan Y. 2012. Complete coding sequence and molecular analysis of hepatitis A virus from a chimpanzee with fulminant hepatitis. J Med Primatol 41: 11–17. [DOI] [PubMed] [Google Scholar]
- Ticehurst J, Rhodes LL Jr, Krawczynski K, Asher LV, Engler WF, Mensing TL, Caudill JD, Sjogren MH, Hoke CH Jr, LeDuc JW, et al. 1992. Infection of owl monkeys (Aotus trivirgatus) and cynomolgus monkeys (Macaca fascicularis) with hepatitis E virus from Mexico. J Infect Dis 165: 835–845. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Emerson SU, Balayan MS, Ticehurst J, Purcell RH. 1991. Simian hepatitis A virus (HAV) strain AGM-27: Comparison of genome structure and growth in cell culture with other HAV strains. J Gen Virol 72: 1677–1683. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Emerson SU, Tsareva TS, Yarbough PO, Lewis M, Govindarajan S, Reyes GR, Shapiro M, Purcell RH. 1993. Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J Infect Dis 167: 1302–1306. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, Yarbough PO, Legters LJ, Moskal T, Purcell RH. 1994. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J Med Virol 43: 135–142. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, Rippy MK, Zack P, Shapiro M, Purcell RH. 1995. Experimental hepatitis E in pregnant rhesus monkeys: Failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J Infect Dis 172: 31–37. [DOI] [PubMed] [Google Scholar]
- Uchida T, Win KM, Suzuki K, Komatsu K, Iida F, Shikata T, Rikihisa T, Mizuno K, Soe S, Myint H, et al. 1990. Serial transmission of a putative causative virus of enterically transmitted non-A, non-B hepatitis to Macaca fascicularis and Macaca mulatta. Jpn J Exp Med 60: 13–21. [PubMed] [Google Scholar]
- Wang L, Wang L. 2016. Animal models for hepatitis E virus. Adv Exp Med Biol 948: 161–173. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Katagiri J, Kojima H, Kamimura T, Ichida F, Ashida M, Hamada C, Shibayama T. 1987. Studies on transmission of human non-A, non-B hepatitis to marmosets. J Med Virol 22: 143–156. [DOI] [PubMed] [Google Scholar]
- Wendum D, Nachury M, Yver M, Lemann M, Flejou JF, Janin A, Bertheau P. 2005. Acute hepatitis E: A cause of lymphocytic destructive cholangitis. Hum Pathol 36: 436–438. [DOI] [PubMed] [Google Scholar]
- Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G, et al. 1992. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med 327: 453–457. [DOI] [PubMed] [Google Scholar]
- Yu C, Boon D, McDonald SL, Myers TG, Tomioka K, Nguyen H, Engle RE, Govindarajan S, Emerson SU, Purcell RH. 2010. Pathogenesis of hepatitis E virus and hepatitis C virus in chimpanzees: Similarities and differences. J Virol 84: 11264–11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugo DM, Cossaboom CM, Meng XJ. 2014. Naturally occurring animal models of human hepatitis E virus infection. ILAR J 55: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]