Abstract
Hypertension affects more than 40% of adults worldwide and is associated with stroke, myocardial infarction, heart failure, and other cardiovascular diseases. It has also been shown to cause severe functional and structural damage to the brain, leading to cognitive impairment and dementia. Furthermore, it is believed that these cognitive impairments affect the mental ability to maintain productivity at work, ultimately causing social and economic problems. Because hypertension is a chronic condition that requires clinical treatment, strategies with fewer side effects and less-invasive procedures are needed. Physical exercise (PE) has proven to be an efficient and complementary tool for hypertension management, and its peripheral benefits have been widely supported by related studies. However, few studies have specifically examined the potential positive effects of PE on the brain in hypertensive individuals. This narrative review discusses the pathophysiological mechanisms that hypertension promotes in the brain, and suggests PE as an important tool to prevent and reduce cognitive damage caused by hypertension. We also provide PE recommendations for hypertensive individuals, as well as suggestions for promoting PE as a method for increasing cognitive abilities in the brain, particularly for hypertensive individuals.
Keywords: blood pressure, cerebral blood flow, clinical practice, cognition, physical exercise, rehabilitation
Introduction
Hypertension affects more than 40% of adults worldwide1 and is strongly associated with coronary artery disease, stroke, and heart failure.2 The prevalence of hypertension in older adults is high (ie, ~70%-80%),3 and increasing hypertension diagnoses in children and adolescents have been observed in recent years.4 Currently, the prevalence of high blood pressure (BP) is higher than 10% in American school-aged children,4 which is concerning given that hypertension can damage brain structures and functions, leading to impaired cognitive functions.5,6 Moreover, hypertension can elicit endothelial dysfunction, decreasing cerebral perfusion, and degenerating white matter.6 Taken together, this scenario is associated with an increased chance of individuals with hypertension developing vascular dementia, especially those who have been exposed to this condition for years.7 Furthermore, it has been observed that cerebrovascular damage (eg, microvascular rarefaction, ischemia, and impaired neurovascular coupling)6 and cerebral atrophy of the frontal lobe and hippocampus play a critical role in decreasing cognitive functions.8 Studies in animal models have shown that hypertension can decrease brain-derived neurotrophic factor (BDNF) levels and neurogenesis in the hippocampus, which can lead to impairment in memory acquisition.9 In humans, hypertension elicits alterations in the prefrontal cortex (PFC) volume and function, as well as its underlying areas,10,11 with consequent impairment of executive functions such as inhibitory control, attention, working memory, and judgment ability.10,12 Furthermore, brain damage caused by hypertension affects a person’s mental ability to work and decreases their productivity, leading to social and economic problems.10
Pharmacologic therapy for hypertension management has undoubtedly advanced in recent decades.13 However, patients with hypertension taking antihypertensive medications are exposed to side effects such as exacerbated electrolyte dysfunction, renal damage, exacerbated hypotensive effects, cerebral hypoperfusion, and syncope.13,14 Therefore, given that hypertension is a chronic disease that needs long-term treatment, therapeutic approaches with reduced side effects are needed for this population. Physical exercise (PE) has been recommended by several professional committees and organizations such as the American College of Sports Medicine, American Heart Association, Canadian Hypertension Education Program, and the European Society of Hypertension/European Society of Cardiology, as a cornerstone of non-pharmacologic therapy for hypertension.15 Specifically, the antihypertensive effects of PE such as reduced resting16 and ambulatory17 BP, as well as improvements in cardiorespiratory fitness,18 have been accepted as effective practices for cardiovascular health. However, the effects of PE on the cerebrovascular system and cognitive functions in individuals with hypertension are unclear. Almost all hypertension clinical practice guidelines provide recommendations regarding PE. They describe the benefits of PE in reducing BP levels and cardiovascular risk factors, as well as in increasing fitness level, improving body composition and quality of life, and decreasing mortality risk.3,19,20 However, there is little information about the effects of PE on the cerebrovascular system and cognitive functions in individuals with hypertension. This is a concerning issue given that hypertension is strongly associated with brain and cognitive dysfunctions.
Here we present the current evidence about the associations between BP and brain damage, as well as the benefits of PE on the cerebrovascular system and cognitive functions. Also, we argue that PE recommendations for individuals with hypertension should not only focus on the benefits on the cardiovascular system, but also on the cerebrovascular and cognitive functions.
Mechanisms Underlying Brain Differences Between Normotensive and Hypertensive Individuals
Individuals with hypertension are more susceptible to presenting brain structural and functional alterations and worse cognition than individuals without hypertension.6 In 1995, a prospective observational study involving 3735 older adults showed an association between higher systolic BP levels in midlife and lower cognitive function in later life.21 However, there are some inconsistencies in the literature pertaining to very old people. Some studies demonstrated an inverse relation between levels of BP and cognitive decline,22,23 whereas others showed a direct association.24 Recently, a review demonstrated physiological and neuroimaging damage to the brain caused by hypertension during the lifespan and reinforced the association between higher BP and lower cognitive function.12 Regarding damage caused by hypertension to the brain, we may highlight greater amyloid-β (Aβ) levels, cerebral small vessel disease, microbleeding, and lacunar infarction.12 In addition, higher systolic BP has been associated with smaller brain volume,25,26 increased white matter hyperintensities (WMHs),27 PFC damage, and hippocampal dysfunctions.8 This aggressive scenario and vascular cognitive impairment might progress to vascular dementia, a situation where an individual has cerebral function severely impaired, compromising daily activities.5 In sum, a hypertensive individual’s brain is characterized by lower volume with microinfarction in areas linked to cognitive function.
To elucidate the genesis of this issue and its consequences, it is important to understand the vascular impairment commonly found in hypertension and its implications for the brain. The renin-angiotensin system and sympathetic activation are described in individuals with high BP levels and this is linked to increased collagen production on the surrounding vessels.28 Collagen is a strong determining factor for passive diameter of arterial vessels and is cross-linked with itself, tightening the vessel wall, leading to inward remodeling and reducing luminal diameter.29,30 These structural changes are known as vessel remodeling, which refers to the adaptation to support higher pressure levels. This process has been observed in hypertensive individuals to reduce the mechanical stress on the arterial wall and protect microvessels from pulsatile stress.31 Vessel remodeling over the years can be harmful, as it leads to increased vascular resistance and vessel wall stiffening.32 As a long-term consequence of hypertension, collagen accumulation and elastin fragmentation elicit higher arterial stiffness, which is strongly associated with brain damage, stroke, and cognitive decline in individuals with hypertension.32
Due to increased arterial stiffness, there is an increase in arterial pulse wave velocity and pulsatile pressure, which may cause rarefaction of downstream capillaries, reduced cerebral blood flow (CBF), and decreased cerebrovascular reactivity.6 In addition to these deleterious changes, the arteriosclerosis process (loss of smooth muscle cells, deposits of fibro-hyaline material, reduced vessel lumen, and thickening of the vessel wall)6 contributes to reduce cerebral perfusion. Moreover, the presence of atherosclerotic lesions impairs the linear CBF and is a focus for thrombus building and embolization, which could lead to multiple ischemic lesions.32 These alterations might alter the blood vessel ability to use vasoactive substances and, together with the above-mentioned microvascular structural alterations, might compromise CBF and brain perfusion.6
Furthermore, evidence suggests that long-term high BP might disrupt the blood-brain barrier (BBB) by both angiotensin II action28 and hypoxia which damage cells and induce protein plasma extravasation and vascular and perivascular inflammation.6 A recent review33 suggests that the low-grade chronic inflammation found in hypertension pathology stimulates interactions between proinflammatory mediators and the BBB on the luminal side, modulating its permeability. Moreover, this modulation is associated to inflammation on the central nervous system, activating glial components, and thereby altering autonomic signaling and increasing sympathetic outflow.34 In addition, hypertension activates proteases, resulting in plasma extravasation, perivascular inflammation, and microbleeding.6 The endothelial damage also leads to higher vascular stress and decreased production of nitric oxide (NO).35 It should be noted that NO participates in BDNF production, linking endothelial function and cognition.33 BDNF has been related to improved synaptic plasticity, neurogenesis, and cognition.36 A study involving spontaneously hypertensive rats showed decreased endothelial nitric oxide synthase (eNOS), NO production by cerebral microvessels, and BDNF levels.33 All of these factors lead to microvascular rarefaction, lower collateral circulation within the brain, and less BDNF quantity. Considered together, these structural and molecular changes (see Figure 1) seem to impair anatomical and functional structures and disrupt the cortical and subcortical connections, implying a wide range of cognitive dysfunctions such as impaired memory, inhibitory control, decision-making, and processing speed. These negative changes lead to a reduced ability to perform daily functions, decreased self-care levels and quality of life, and also increased risk of morbidity and mortality.37
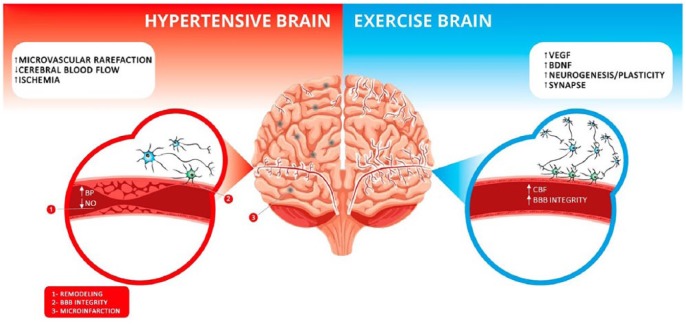
Figure 1.
The cerebral changes promoted by hypertension and exercise. Left (in red): it is shown that the brain of a hypertensive individual has increased microvascular rarefaction, decreased cerebral blood flow, and increased ischemic regions, illustrated by the gray points on the left side of the brain. In addition, hypertension causes vessel remodeling and microinfarctions, disrupts the brain-blood barrier integrity, and decreases nitric oxide availability. Right (in blue): the benefits that exercise induces are shown. It increases the levels of vascular endothelial growth factor (VEGF) which leads to angiogenesis, enhances brain-derived natriuretic factor (BDNF), a molecule related to neuronal survival and synapse formation, and promotes neurogenesis. In addition, physical exercise increases cerebral blood flow and improves blood-brain barrier integrity.
BBB, blood-brain barrier; BP, blood pressure; CBF, cerebral blood flow; NO, nitric oxide.
Hypertension and Cognitive Function
Excluding age, hypertension is the most important risk factor for cerebrovascular dysfunctions, triggering cognitive impairment and possibly dementia.6 These functional and structural alterations are the basis of hypertension-related cognitive decline.6,38 Brain susceptibility to multiple ischemic injuries, especially in vulnerable white matter areas, is critical for establishing vascular dementia.6 As a consequence, hypertension has been linked to worse cognitive performance.38
In a study with 14 337 adults aged between 45 and 64 years, The Atherosclerosis Risk in Communities (ARIC) showed that the hypertensive subjects had lower scores on processing speed and word fluency tests.39 Corroborating these data, the third National Health and Nutrition Examination Survey (NHANHES III) analyzed 6377 middle-aged individuals with high BP and found that these subjects had poorer overall cognition as measured by the Mini-Mental State Exam.40 Furthermore, a longitudinal study investigated the cognitive functioning of individuals aged ~25 years over a period of 25 years and found that high levels of BP were associated with worse performance on a wide range of cognitive tasks in midlife, including verbal memory, processing speed, and executive function.41 It has also been observed that higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) in subjects’ midlife periods were associated with worse performance on overall cognition, attention, and memory scores over a period of ~20 years.42 Overall, there is consistent evidence that higher BP in midlife is associated with impaired cognitive functions in midlife and later life,6 as well as executive function, whereas processing speed seems to be the most affected cognitive domain, although memory can also be affected.6
It is true that elevated BP is linked to vascular dementia, but it can also be responsible for the development of Alzheimer disease.6 Cerebral atherosclerosis and arterial stenosis processes lead to hypoperfusion. Lower CBF reduces Aβ clearance, whereas increases in Aβ production43 and Aβ accumulation represent a molecular basis to developing Alzheimer disease. Therefore, it is imperative to make patients with hypertension aware of these problems and how they can manage them, thereby increasing their quality of life.
To date, there is no specific recommendation regarding the exercise prescription considering the FITT (Frequency, Intensity, Type, and Time) principle to maximize cognitive functions. Despite this, the recent Physical Activity Guidelines for Americans44 supports, in an evidence-based manner, the benefits of exercise on cognitive functions in children, adolescents, adults, and older adults. Therefore, the types and amount of exercise recommended by this recent guideline should be strongly encouraged for all individuals to maintain or improve cognition. It should also be noted that the exercise recommendations vary for the different age groups. For example, children and adolescents should perform 60 minutes or more of moderate- to vigorous-intensity exercise daily. Adults should perform 150 to 300 minutes per week of moderate-intensity or 75 to 150 minutes per week of vigorous-intensity aerobic exercise, or an equivalent combination of moderate- and vigorous-intensity aerobic exercise. Also, they should perform muscle-strengthening activities at least 2 days per week. Older adults should add multicomponent activities, which involve more than 1 type of activity (ie, aerobic, muscle strengthening, and balance), such as dancing and sports. We highlight that the previous guidelines for exercise prescription for hypertension15 are in accordance with this new Physical Activity Guidelines for Americans.44
Peripheral Benefits of PE for Hypertension Management
It is well known that hypertension has 2 main treatment pillars: the non-pharmacologic and pharmacologic approaches. Depending on a patient’s hypertension classification (eg, stage I, II, or III), it may be possible to start only with lifestyle changes, advancing to drug therapy if or when necessary.45 However, even when medications are necessary, lifestyle intervention should be kept to maximize BP management.19 Different classes of antihypertensive medications can control BP efficiently. In addition, non-pharmacologic alternatives, such as PE, optimize the treatment when the pathology is already established, as well as prevent its development or hypertension-related complications.46 From a clinical perspective, the best strategies for hypertension treatment should combine lifestyle changes and pharmacologic intervention as result of the high burden of adherence to daily medication and the strong commitment required to maintain lifestyle changes.2 Thus, we cannot rule out that the BP-lowering effect commonly observed following an exercise program may be mediated by antihypertensive medication(s). For instance, previous research has compared aerobic exercise (dancing exercise for 12 weeks, 3 days/week, intensity of 50%-70% of heart rate [HR] reserve) plus antihypertensive drugs (co-amilozide and amlodipine) with antihypertensive drugs alone for lowering BP. As a result, it was found that, in a population of uncontrolled BP patients, PE for 12 weeks plus medications significantly lowered BP and reduced the use of antihypertensive medication.47 However, it should be noted that the BP-lowering effect of PE in hypertensive individuals can occur when they are under no antihypertensive medication(s). For example, Molmen-Hansen et al48 observed a BP-lowering effect on 24-hour ambulatory BP following 12 weeks of high-intensity interval (HIIT: 12 and 8 mm Hg for SBP and DBP, respectively) and moderate-intensity continuous training (MICT; 4.5 and 3.5 mm Hg for SBP and DBP, respectively) in stage 1 or 2 middle-aged hypertensive individuals under no antihypertensive medications. The medications of these patients were terminated 1 month before inclusion in the study.
The above-mentioned effect might be explained by the exercise ability to promote acute and chronic BP-lowering effects. The acute BP-lowering effect of exercise is a well-documented phenomenon called post-exercise hypotension (PEH).49 PEH is a reduction of post-exercise BP levels compared with pre-exercise values, which may be sustained for several hours in a laboratory setting49 and free-living conditions.50 The chronic BP-lowering effect is characterized by reduced resting16 and/or ambulatory17 BP after a period of regular exercise training. It should be noted that the chronic antihypertensive effect of exercise training is a result of physiological adjustments to repeated exercise training sessions.51 Interestingly, recent studies have reported that the magnitude of PEH (ie, acute BP-lowering effect) is positively correlated with the magnitude reduction in resting BP after an exercise training period (ie, chronic BP-lowering effect).52,53
Regarding the acute BP-lowering effect of exercise, both aerobic exercise and dynamic resistance exercises elicit PEH in individuals with hypertension.54,55 However, it is not known which exercise design (intensity, type, and time) can maximize PEH in individuals with hypertension. It should be noted that the hemodynamic determinants of PEH following aerobic exercise seem to be different between individuals with normal BP and hypertension. Brito et al56 showed that the main hemodynamic determinant of PEH is reduced systemic vascular resistance; however, the PEH in older adults overweight and hypertensive individuals is more associated with a decrease in cardiac output post exercise, especially due to a reduction in stroke volume. It is suggested that increased arterial stiffness, peripheral vascular resistance, and endothelial dysfunction associated with aging and hypertension may weaken post-exercise reduction of systemic vascular resistance, favoring reduced stroke volume and cardiac output in older adults.56 The impact of resistance exercise on hemodynamic determinants of PEH is less known.55
Chronic antihypertensive effects of exercise training on both resting and ambulatory BP have been demonstrated in the last 2 decades by several randomized controlled trials (RCTs).16,17,57 A meta-analysis conducted by Cornelissen and Smart16 showed that aerobic exercise training (moderate to high intensity, <210 minutes/week) reduces 8.3 and 5.2 mm Hg of resting SBP and DBP, respectively, in individuals with hypertension. Sosner et al17 showed that aerobic exercise training, regardless intensity, frequency, and duration, reduces ~4 and ~3 mm Hg of 24-hour ambulatory SBP and DBP, respectively, and this reduction is greater in individuals with resting BP higher than 130/85 mm Hg. More recently, MacDonald et al57 demonstrated that dynamic resistance training (moderate intensity - 65%-75% of 1 repetition max, ~3 days/week) reduces ~6 and ~5 mm Hg of resting SBP and DBP, respectively, in individuals with hypertension. Altogether, there is robust evidence to support that both aerobic and dynamic resistance exercise training elicit a chronic BP-lowering effect in individuals with hypertension, and that the magnitude of this effect is higher than 5 mm Hg for resting SBP and DBP. From a clinical perspective, a decrease of 5 mm Hg in SBP reduces the mortality due to stroke by 14%, mortality due to coronary heart disease by 9%, and all-cause mortality by 7%,58 and a decrease of 10 mm Hg reduces the risk of stroke by 27%, coronary heart disease by 17%, heart failure by 28%, and all-cause mortality by 13%.55
In addition to the specific effects on BP levels, when aerobic exercise training is accompanied by a substantial reduction in SBP (ie, >7.6 mm Hg) and/or prolonged duration (ie, >12 weeks), it decreases arterial stiffness in individuals with pre-hypertension and hypertension.59 It should be noted that, despite there being no differences in the magnitude of the BP-lowering effect between the MICT and HIIT,18 the latter improves the vascular function and cardiorespiratory fitness18 to a greater extent than MICT. Therefore, HIIT can be considered an alternative approach to traditional MICT for individuals with pre-hypertension and hypertension.18 As an important supplement to aerobic exercise, dynamic resistance exercise training elicits BP-lowering effects,57 as well as neuromuscular benefits such as increases in strength, power, and muscle mass60 that support its prescription for individuals with hypertension.
In this sense, Table 1 provides the current recommendations from 5 professional organizations.3,19,20,61 The table was based on the work of Pescatello et al15; however, we have added the Brazilian19 and Australian62 Guidelines for Hypertension and updated certain guidelines, such as the Canadian and European. The recommendations are based on the FITT principle.63
Table 1.
Professional recommendations regarding exercise for individuals with hypertension.
| Professional Associations and Committees | |||||
|---|---|---|---|---|---|
| FITT principle | Brazilian Society of Cardiology | Canadian Hypertension Education Program | European Society of Cardiology/European Society of Hypertension | American College of Cardiology and American Heart Association | National Heart Foundation of Australia |
| Frequency | 3-5 days/week | 4-7 days/week | 5-7 days/week | – | – |
| Intensity | Moderate intensity (50%-70% of HR reserve) | Moderate intensity | Moderate intensity | 65%-75% of HR reserve | Moderate/vigorous intensity |
| Time | 30-50 minutes/session | 30-60 minutes/session | 30 minutes/session | 90-150 minutes/week | 150-300 minutes (moderate intensity) or 75-150 minutes (vigorous intensity) |
| Type | Aerobic exercise | Aerobic exercise | Aerobic exercise | Aerobic exercise | Aerobic exercise |
| Primary evidence rating | Class IIa—Grade Ba | Grade Db | Class I—Grade Ac | Class I—Grade Ad | – |
| Complementary training | Resistance Training (2-3 days/week; 8-10 exercise for major muscle groups; 10-15 repetitions for each exercise; passive resting between 90 and 120 seconds) | Resistance training only for patients at stage I | Resistance training (2-3/week) | Resistance training (90-150 minutes/week; 50%-80% of HR reserve; 6 exercises; 3 sets/exercise; 10 repetitions/exercise) | Muscle stretching exercise for at least 2 days/week |
| Evidence rating | Class IIa—Grade Ba | Grade Db | – | – | – |
FITT, Frequency, Intensity, Time, and Type; HR, heart rate.
According to the Brazilian Society of Cardiology, Class IIa refers to “Evidence in favor. Most of studies approve it.” and Grade B refers to “Data obtained from less robust meta-analysis or obtained from only 1 randomized-controlled trial study or from non-observational studies.”
According to the Canadian Hypertension Education Program, Grade D is based on expert opinion alone.
According to the European Society of Cardiology and the European Society of Hypertension, Class I refers to “Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective” and Grade A refers to “Data derived from multiple randomized-controlled trial or meta-analysis.”
According to the American College of Cardiology and the American Heart Association, Class I refers to strong evidence and posits that the benefits are way greater than the risks. Grade A, according to both organizations, refers to “High quality evidence from more than 1 randomized-controlled trial; meta-analysis of high quality randomized-controlled trial; one or more randomized-controlled trial corroborated by high-quality registry studies.”
Molecular Brain Changes Promoted by PE
Some possible brain molecular changes can explain the benefits of PE. The human brain has the capacity to promote neurogenesis and prevent age-related cognitive decline. This process can upregulate neuronal cell proliferation on the hippocampus and improve the neuronal plasticity ability.64 It has also been observed that regular chronic exercise practice increases CBF in the dentate gyrus (hippocampal region).65 In addition, evidence suggests that exercise promotes increased levels of different neurotrophins in the human brain such as BDNF. BDNF has its gene and protein expression raised in the hippocampus after exercise, which mediates neuronal survival, plasticity, and synapse reinforcement.66,67 PFC and amygdala are also associated with increased BDNF levels after exercise.68 These areas are involved in executive functions, cognition, and emotional processing.69,70 Moreover, insulin-like growth factor 1 (IGF-1) is another neurotrophin raised by PE71 that is related to improved brain functions and is involved in memory and cerebral plasticity.71 In addition, IGF-1 modulates BDNF activity after exercise through enhancing its expression and signaling via its receptor, which culminates in higher BDNF levels in the brain.72 Interestingly, both IGF-1 and exercise-released catecholamines contribute to upregulating BDNF RNAm production.68 Furthermore, it is already known that PE by itself induces higher peripheral levels of IGF-1 and vascular endothelial growth factor (VEGF), both of which induce angiogenesis and neurogenesis on the brain.73 VEGF has mitotic activity in vascular endothelial cells causing their proliferation and migration, raising the cerebral vasculature, thus enabling more delivered oxygen and nutrients to the brain74 (see Figure 1).
Concomitantly, exercise leads to higher CBF. It has been observed that PE training with cognitive training is associated with higher CBF, higher metabolic activity in the hippocampus, and better memory compared with the control group that did not exercise.75 Also, exercise is associated to increased length, complexity, and density of some types of neuron dendrites76 and greater integrity of the BBB.77 Taking these processes together, it is suggested that PE promotes several molecular and structural adaptations that can improve cognitive functioning.
Expanding the Message: the Benefits of PE on the Brain and Cognition and Its Implications for Hypertension Management
PE has been shown to be a useful tool to improve cognition and mental health.78 Aerobic exercise has been related to increased attention, executive function, and memory.79 When aerobic exercise is combined with resistance training, it seems to promote even more positive effects on attention and working memory.19,80 Notably, not only older adults can benefit from PE. One study using a sample of 241 subjects aged between 15 and 71 years observed that PE can be beneficial for cognitive functions in both younger and older individuals.81 In children, cross-sectional data suggest that those with higher fitness levels have greater bilateral hippocampal volume. The authors demonstrated that the hippocampal volume mediated the relationship between cardiorespiratory fitness and cognitive outcomes.82 The same hippocampal changes can be viewed in older individuals who have enhanced fitness levels.83
Until this point, we have presented evidence herein that exercise can enhance cognitive functions in a healthy sample regardless of age. However, those who already have cognitive impairment can also use PE as a therapeutic tool for reducing cognitive damage already established, as well as delaying the advance of the impairment. Studies have demonstrated that exercise can promote greater speed processing and attention in patients with cognitive mild impairment.84 Patients with more serious dementia such as Alzheimer disease also showed cognitive improvements with exercise, as well as decreased caregiver distress.85 Thus, PE is shown not only to be important in prevention, but also to reverse some already established impairments.
Although the literature has a wide range of studies regarding the peripheral effects of exercise on hypertensive individuals, few works have been published about the central effects of exercise in this population. The amount of studies showing the importance of PE on cognition in hypertensive subjects is small. However, 1 study with 1094 hypertensive middle-aged adults showed that the treatment of BP in midlife may prevent cognitive decline in later life. Moreover, the authors showed that lower cardiovascular fitness and enhanced exercise BP and HR responses are associated with smaller brain volume 19 years later. The researchers argue that regular exercise is necessary to keep the BP at normal levels and to maintain brain health.86 Furthermore, a recent study using an animal model demonstrated that spontaneously hypertensive rats that performed 8 weeks of exercise training had decreased BBB leakage on brainstem areas when compared with rats that were kept sedentary for 8 weeks, suggesting another potential benefit of exercise on the brain of hypertensive individuals.77 Increased BBB leakage is shown to be associated with damage in white matter,87 which in turn may lead to cognitive impairment,88 stressing the importance of BBB integrity. Importantly, brainstem is related to cognition through a network involving the amygdala and the PFC and this link is bidirectional. For instance, the PFC has inhibitory functions over the amygdala, which has an excitatory function over the rostral ventricular lateral medulla and inhibitory function over the nucleus ambiguus.89 Thus, an impaired brainstem not only impacts peripheral functions, but can also impact important cerebral areas for cognitive functions, such as the PFC,87 which in turns may lead to cognitive impairment.88 Hence, BBB integrity is important to those patients. Another research demonstrated that spontaneously hypertensive rats present lower levels of hippocampal BDNF and that daily treadmill exercise (30 minutes for 1 week) was capable of increasing hippocampal BDNF levels.33 Following this line, a study had proposed that BDNF changes linked to PE in hypertensive rats are associated to changes in cerebral hemodynamics. The authors highlight that NO synthase inhibition and genetic hypertension blunt the BDNF increase by exercise.90 In addition, a research conducted in spontaneously hypertensive rats showed that the animals had increased levels of calcium on the brain after 1 hour of wheel running. The authors argued that the elevated levels of this ion, which plays a key role in dopamine production, may inhibit sympathetic activity via the D2 receptors in the brain.91 The production of dopamine begins in the ventral tegmental area, a midbrain that has projections to the striatum and frontal cortex. Importantly, the frontal cortex plays a key role in cognitive functions including learning, executive functions, and emotions.92,93 For instance, a positron emission tomography (PET) study revealed that subjects increase dopamine production on the striatum while performing executive function tests (set shifting and planning).94 Thus, greater levels of this catecholamine can be extremely beneficial to the brain of hypertensive patients. To our knowledge, there are no other studies approaching the relationship between PE, brain, and hypertension. However, in linking the previous studies to the general population and the hypertension physiopathology discussed throughout this study, we could speculate that PE could be positive for managing cognition and preventing brain impairment in hypertensive individuals.
Applicability in Daily Life
PE has been shown to be an efficient and complementary tool for treating and managing hypertension.15 Although the peripheral benefits of PE have been widely supported in the related literature,15 there are few studies examining the potential positive effects of PE on the brain of hypertensive individuals.
Despite the wide range of positive effects of PE, adherence to exercise programs is low in hypertensive individuals, achieving only 32% of this population in the United States.95 For instance, adherence to hypertension treatments and lifestyle changes has been investigated, including medicine usage, exercise, smoking, and diet. It was found that exercise had the lowest rates of adherence when compared with the other treatments.96 Cohen-Mansfield and Sommerstein97 have also shown that adherence to PE programs is a complex and multifactorial phenomenon that includes several subjective factors, such as self-efficacy perception and social support. The United States Preventive Services Task Force had recently highlighted that patients with risk factors for cardiovascular diseases should be encouraged to attend PE programs to improve health.98 Furthermore, a recent study including 4158 individuals showed that providing advice regarding the benefits of PE to patients with cardiovascular diseases is an effective method to keep them engaged in regular exercise programs.98 Thus, we emphasize that patients with hypertension must receive strong encouragement from (not limited to) medical doctors and other health professionals to increase the likelihood of adherence to exercise programs and guarantee the possible benefits in the brain and cognition described herein. Because it has been shown to be an effective way to increase patient adherence, we believe that PE prescription by a medical doctor should not only focus on the peripheral benefits, but also on the brain and cognitive improvements.
Of course, outreach to patients through medical and health professionals is just one example of how to increase awareness of the benefits that PE may provide to patients with hypertension. Other examples could include public service announcements and joint outreach campaigns funded by aforementioned heart and health committees.
Perspectives
Despite some studies having shown that PE can improve cerebrovascular function and structure, RCTs that focus on individuals with hypertension are needed. Specifically, future RCTs should investigate the effects of different types of PE (ie, aerobic and resistance exercise) on cerebrovascular structure and function, and its implications for cognitive performance in this population. It is important to know if moderate-intensity aerobic training, resistance training, or high-intensity exercise, for example, can have different impacts on the cognitive functions of these patients. The use of different methods to assess brain function and structure (eg, functional magnetic resonance imaging [fMRI], electroencephalography, and near-infrared spectroscopy) could enable more comprehensive understanding regarding the impact of PE on hypertensive subjects’ brains. Finally, it is extremely necessary to find strategies that engage these individuals in a regular exercise program. An effective strategy should start at the physician’s office, where the patient will learn about the connection between hypertension and possible brain injuries, and how PE may help mitigate those risks.
Conclusions
Here we present evidence showing that individuals with hypertension are more susceptible to deleterious changes in brain structure and function which may have negative implications for cognitive function, making this population more vulnerable to vascular dementia and Alzheimer disease. Although hypertension clinical practice guidelines provide recommendations on PE for individuals with high BP, its key statements do not include the probable benefits of PE on brain health. To date, limited information from RCTs is available about the effects of PE on cerebrovascular function and structure, as well as cognitive performance in individuals with hypertension. Therefore, RCTs are needed to investigate the effects of different types of PE (eg, aerobic and resistance exercise) on cerebrovascular structure and function and its implications for cognitive performance in individuals with hypertension. However, given the well-known benefits of PE on the brain, on cognitive performance, and its protective role against dementia,7 a rule of thumb for clinical practice is that the positive impact of PE on brain health must be publicized for patients with hypertension.
The aim of this study was to provide evidence that PE not only enhances peripheral parameters on hypertensive individuals, but can also improve brain functions through molecular and structural alterations. Exercise is widely shown to have BP-lowering effect, and it is recommended for prevention and treatment of hypertension. Also, the main point to be highlighted is that exercise is crucial for the brain’s health through different pathways. We have discussed the PE effects in increasing hippocampal volume and improving memory task performance, as well as in modulating cerebral hemodynamics and enhancing IGF-1,71 VEGF,73 and BDNF production,90 which are related directly or indirectly to cognitive functions.33 Moreover, we showed that exercise mitigates sympathetic hyperactivation,99 which may have positive implications on the CBF and cognition.89,100
Despite a wide range of benefits, the adherence to exercise is a problem among hypertensive patients. We believe that if patients become aware of how hypertension may impair memory, attention, and inhibitory control on their daily life, they would be more likely to engage in PE programs. In addition, we strongly suggest that medical doctors emphasize the importance of regular exercise for hypertension management to increase patient’s adherence to an exercise program. Moreover, due to the increasing aging rates worldwide,101 the message for patients with hypertension should focus on 3 pillars: the heart, the brain, and the cognitive benefits. Hypertensive patients should perform at least 150 minutes per week of moderate-intensity or 75 minutes per week of vigorous-intensity aerobic exercise, and muscle-strengthening activities at least 2 days per week, in the absence of specific contraindications. The simple message “sit less, walk more and exercise” could be implemented in clinical practice as an initial approach to encourage a more active lifestyle for hypertensive patients, especially those highly sedentary and unfit.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: MR and DC have written the first draft of the manuscript. EF and EC made critical revisions, suggestions and approval of the final version.
ORCID iD: Maria LM Rêgo  https://orcid.org/0000-0003-4218-8999
https://orcid.org/0000-0003-4218-8999
References
- 1. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557–576. [DOI] [PubMed] [Google Scholar]
- 2. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e136–e139. [DOI] [PubMed] [Google Scholar]
- 4. Meyers KE, Sethna CB. Thinking under pressure. J Pediatr. 2017;180:7–10. [DOI] [PubMed] [Google Scholar]
- 5. Muela HCS, Costa-Hong VA, Yassuda MS, et al. Hypertension severity is associated with impaired cognitive performance. J Am Heart Assoc. 2017;6:e004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68(6):e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trigiani LJ, Hamel E. An endothelial link between the benefits of physical exercise in dementia. J Cereb Blood Flow Metab. 2017;37:2649–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amenta F, Di Tullio MA, Tomassoni D. Arterial hypertension and brain damage—evidence from animal models (review). Clin Exp Hypertens. 2003;25:359–380. [DOI] [PubMed] [Google Scholar]
- 9. Shih Y-H, Tsai S-F, Huang S-H, et al. Hypertension impairs hippocampus-related adult neurogenesis, CA1 neuron dendritic arborization and long-term memory. Neuroscience. 2016;322:346–357. [DOI] [PubMed] [Google Scholar]
- 10. Grant H, Bhambhani Y, Singhal A. Hemodynamic changes in the prefrontal cortex during working memory in essential hypertension. J Am Soc Hypertens. 2015;9:628–639. [DOI] [PubMed] [Google Scholar]
- 11. Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. [DOI] [PubMed] [Google Scholar]
- 12. Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gudsoorkar PS, Tobe SW. Changing concepts in hypertension management. J Hum Hypertens. 2017;31:763–767. [DOI] [PubMed] [Google Scholar]
- 14. Testa G, Ceccofiglio A, Mussi C, et al. Hypotensive drugs and Syncope due to orthostatic hypotension in older adults with dementia (Syncope and dementia study). J Am Geriatr Soc. 2018;66(8):1532–1537. [DOI] [PubMed] [Google Scholar]
- 15. Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for hypertension: a prescription update integrating existing recommendations with emerging research. Curr Hypertens Rep. 2015;17:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sosner P, Guiraud T, Gremeaux V, Arvisais D, Herpin D, Bosquet L. The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta-analysis of selected moderators. Scand J Med Sci Sports. 2017;27:327–341. [DOI] [PubMed] [Google Scholar]
- 18. Costa EC, Hay JL, Kehler DS, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. 2018:2127–2142. [DOI] [PubMed] [Google Scholar]
- 19. Malachias M, Souza W, Plavnik F, et al. Capítulo 6—tratamento não medicamentoso. Arquivos Brasileiros de Cardiologia. 2016;107:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 21. Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667–1672. [DOI] [PubMed] [Google Scholar]
- 24. Rosano C, Abebe KZ, Aizenstein HJ, et al. Longitudinal systolic blood pressure characteristics and integrity of white matter tracts in a cohort of very old black and white adults. Am J Hypertens. 2015;28:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage. 2006;31:754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008;26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 27. Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. [DOI] [PubMed] [Google Scholar]
- 28. Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology. 2009;24:45–57. [DOI] [PubMed] [Google Scholar]
- 29. Bakker Buus CL, Spaan JA, Perree J, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. [DOI] [PubMed] [Google Scholar]
- 30. Bakker Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. [DOI] [PubMed] [Google Scholar]
- 31. Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. [DOI] [PubMed] [Google Scholar]
- 32. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monnier A, Prigent-Tessier A, Quirie A, et al. Brain-derived neurotrophic factor of the cerebral microvasculature: a forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol. 2017;219:790–802. [DOI] [PubMed] [Google Scholar]
- 34. Setiadi A, Korim WS, Elsaafien K, Yao ST. The role of the blood-brain barrier in hypertension. Exp Physiol. 2018;103:337–342. [DOI] [PubMed] [Google Scholar]
- 35. Bruno RM, Masi S, Taddei M, Taddei S, Virdis A. Essential hypertension and functional microvascular ageing. High Blood Press Cardiovasc Prev. 2018;25:35–40. [DOI] [PubMed] [Google Scholar]
- 36. Biojone C, Casarotto PC, Joca SR, Castren E. Interplay between nitric oxide and brain-derived neurotrophic factor in neuronal plasticity. CNS Neurol Disord Drug Targets. 2015;14:979–987. [DOI] [PubMed] [Google Scholar]
- 37. Rego MLM, Cabral DAR, Fontes EB. Cognitive deficit in heart failure and the benefits of aerobic physical activity. Arq Bras Cardiol. 2018;110:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gąsecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Gerontology. 1998;44:95–105. [DOI] [PubMed] [Google Scholar]
- 40. Obisesan TO, Obisesan OA, Martins S, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:353–364. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Liu S, Matsumoto Y, et al. Angiotensin type 1a receptor deficiency decreases amyloid β-protein generation and ameliorates brain amyloid pathology. Sci Rep. 2015;5:12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jordan J, Kurschat C, Reuter H. Arterial hypertension. Dtsch Arztebl Int. 2018;115:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moraes-Silva IC, Mostarda CT, Silva-Filho AC, Irigoyen MC. Hypertension and exercise training: evidence from clinical studies. Adv Exp Med Biol. 2017;1000:65–84. [DOI] [PubMed] [Google Scholar]
- 47. Maruf FA, Akinpelu AO, Salako BL, Akinyemi JO. Effects of aerobic dance training on blood pressure in individuals with uncontrolled hypertension on two antihypertensive drugs: a randomized clinical trial. J Am Soc Hypertens. 2016;10:336–345. [DOI] [PubMed] [Google Scholar]
- 48. Molmen-Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–160. [DOI] [PubMed] [Google Scholar]
- 49. Costa EC, Dantas TCB, de Farias Junior LF, et al. Inter- and intra-individual analysis of post-exercise hypotension following a single bout of high-intensity interval exercise and continuous exercise: a pilot study. Int J Sports Med. 2016;37:1038–1043. [DOI] [PubMed] [Google Scholar]
- 50. Dantas TCB, Farias Junior LF, Frazao DT, et al. A single session of low-volume high-intensity interval exercise reduces ambulatory blood pressure in normotensive men. J Strength Cond Res. 2017;31:2263–2269. [DOI] [PubMed] [Google Scholar]
- 51. da Nobrega AC. The subacute effects of exercise: concept, characteristics, and clinical implications. Exerc Sport Sci Rev. 2005;33:84–87. [DOI] [PubMed] [Google Scholar]
- 52. Hecksteden A, Grutters T, Meyer T. Association between postexercise hypotension and long-term training-induced blood pressure reduction: a pilot study. Clin J Sport Med. 2013;23:58–63. [DOI] [PubMed] [Google Scholar]
- 53. Kiviniemi AM, Hautala AJ, Karjalainen JJ, et al. Acute post-exercise change in blood pressure and exercise training response in patients with coronary artery disease. Front Physiol. 2014;5:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carpio-Rivera E, Moncada-Jimenez J, Salazar-Rojas W, Solera-Herrera A. Acute effects of exercise on blood pressure: a meta-analytic investigation. Arq Bras Cardiol. 2016;106:422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Casonatto J, Goessler KF, Cornelissen VA, Cardoso JR, Polito MD. The blood pressure-lowering effect of a single bout of resistance exercise: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2016;23:1700–1714. [DOI] [PubMed] [Google Scholar]
- 56. Brito LC, Queiroz ACC, Forjaz CLM. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz J Med Biol Res. 2014;47:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. MacDonald HV, Johnson BT, Huedo-Medina TB, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5:e003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 59. Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2014;173:361–368. [DOI] [PubMed] [Google Scholar]
- 60. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. [DOI] [PubMed] [Google Scholar]
- 61. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–525. [DOI] [PubMed] [Google Scholar]
- 62. Gabb GM, Mangoni AA, Anderson CS, et al. Guideline for the diagnosis and management of hypertension in adults—2016. Med J Aust. 2016;205:85–89. [DOI] [PubMed] [Google Scholar]
- 63. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000, https://search.library.wisc.edu/catalog/999931853902121. [Google Scholar]
- 64. Baek S-S. Role of exercise on the brain. J Exerc Rehabil. 2016;12:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. [DOI] [PubMed] [Google Scholar]
- 67. Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dinoff A, Herrmann N, Swardfager W, Lanctot KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. 2017;46:1635–1646. [DOI] [PubMed] [Google Scholar]
- 69. Miller EK. The prefrontal cortex and cognitive control. https://www.ncbi.nlm.nih.gov/pubmed/11252769. Accessed July 3, 2018. [DOI] [PubMed]
- 70. Morawetz C, Bode S, Baudewig J, Heekeren HR. Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Soc Cogn Affect Neurosci. 2017;12:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. [DOI] [PubMed] [Google Scholar]
- 72. Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. [DOI] [PubMed] [Google Scholar]
- 73. Fabel K, Fabel K, Tam B, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. [DOI] [PubMed] [Google Scholar]
- 74. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- 75. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. [DOI] [PubMed] [Google Scholar]
- 77. Buttler L, Jordao MT, Fragas MG, Ruggeri A, Ceroni A, Michelini LC. Maintenance of blood-brain barrier integrity in hypertension: a novel benefit of exercise training for autonomic control. Front Physiol. 2017;8:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. [DOI] [PubMed] [Google Scholar]
- 79. Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59:191–198. [DOI] [PubMed] [Google Scholar]
- 80. Boutcher YN, Boutcher SH. Exercise intensity and hypertension: what’s new. J Hum Hypertens. 2017;31:157–164. [DOI] [PubMed] [Google Scholar]
- 81. Hillman CH, Motl RW, Pontifex MB, et al. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychol. 2006;25:678–687. [DOI] [PubMed] [Google Scholar]
- 82. Chaddock L, Erickson KI, Prakash RS, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical activity and Alzheimer’s disease: a systematic review. J Gerontol A Biol Sci Med Sci. 2017;72:733–739. [DOI] [PubMed] [Google Scholar]
- 86. Spartano NL, Himali JJ, Beiser AS, et al. Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later. Neurology. 2016;86:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang CE, Wong SM, Uiterwijk R, et al. Blood-brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging [published online ahead of print March 23, 2018]. Brain Imaging Behav. doi: 10.1007/s11682-018-9855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Filley CM, Fields RD. White matter and cognition: making the connection. J Neurophysiol. 2016;116:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. [DOI] [PubMed] [Google Scholar]
- 90. Banoujaafar H, Van Hoecke J, Mossiat CM, Marie C. Brain BDNF levels elevation induced by physical training is reduced after unilateral common carotid artery occlusion in rats. J Cereb Blood Flow Metab. 2014;34:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Akiyama K, Sutoo D. Rectifying effect of exercise on hypertension in spontaneously hypertensive rats via a calcium-dependent dopamine synthesizing system in the brain. Brain Res. 1999;823:154–160. [DOI] [PubMed] [Google Scholar]
- 92. Ko JH, Strafella AP. Dopaminergic neurotransmission in the human brain: new lessons from perturbation and imaging. Neuroscientist. 2012;18:149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Drozak J, Bryła J. Dopamine: not just a neurotransmitter. Postepy Hig Med Dosw. 2005;59:405–420. [PubMed] [Google Scholar]
- 94. Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. Neuroimage. 2006;33:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mu L, Cohen AJ, Mukamal KJ. Prevalence and predictors of resistance and aerobic exercise among hypertensive adults in the United States. J Hum Hypertens. 2015;29:394–395. [DOI] [PubMed] [Google Scholar]
- 96. Uzun S, Kara B, Yokusoglu M, Arslan F, Yilmaz MB, Karaeren H. The assessment of adherence of hypertensive individuals to treatment and lifestyle change recommendations. Anadolu Kardiyol Derg. 2009;9:102–109. [PubMed] [Google Scholar]
- 97. Cohen-Mansfield J, Sommerstein M. Motivating inactive seniors to participate in physical activity: a pilot RCT. Am J Health Behav. 2019;43:195–206. [DOI] [PubMed] [Google Scholar]
- 98. Sreedhara M, Silfee VJ, Rosal MC, Waring ME, Lemon SC. Does provider advice to increase physical activity differ by activity level among US adults with cardiovascular disease risk factors? Fam Pract. 2018;35(4):420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–122. [DOI] [PubMed] [Google Scholar]
- 100. Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. [DOI] [PubMed] [Google Scholar]
- 101. OMS. Relatório mundial de envelhecimento e saúde. https://www.portaldoenvelhecimento.com.br/relatorio-mundial-de-envelhecimento-e-saude/. Up-dated 2015.