Abstract
Background: Insulin initiation and/or titration for type 2 diabetes (T2DM) is often delayed as it is a resource-intensive process, often requiring frequent exchange of information between a patient and their diabetes healthcare professional, such as a credentialed diabetes educator (CDE) for insulin dose adjustment (IDA). Existing models of IDA are unlikely to meet the increasing service demand unless efficiencies are increased. Mobile health (mHealth), a subset of Ehealth, has been shown to improve glycaemic control through enhanced self-management and feedback leading to improved patient satisfaction and could simultaneously reduce costs. Considering the potential benefits of mHealth, we have developed an innovative mHealth-based care model to support patients and clinicians in diabetes specialist community outreach and telehealth clinics, that is, REthinking Model of Outpatient Diabetes care utilizing EheaLth – Insulin Dose Adjustment (REMODEL-IDA). This model primarily aims to improve the glycaemic management of patients with T2DM on insulin, with the secondary aims of improving healthcare service delivery efficiency and the patients’ experience. Methods/Design: A two-arm pilot randomized controlled trial (RCT) will be conducted for 3 months with 44 participants, randomized at a 1:1 ratio to receive either the mHealth-based model of care (intervention) or routine care (control), in diabetes specialist community outreach and telehealth clinics. The intervention arm will exchange information related to blood glucose levels via the Mobile Diabetes Management System developed for outpatients with T2DM. They will receive advice on insulin titration from the CDE via the mobile-app and receive automated text-message prompts for better self-management based on their blood glucose levels and frequency of blood glucose testing. The routine care arm will be followed up via telephone calls by the CDE as per usual practice. The primary outcome is change in glycated haemoglobin, a marker of glycaemic management, at 3 months. Patient and healthcare provider satisfaction, and time required to perform IDA by healthcare providers in both arms will be collected. This pilot study will guide the conduct of a large-scale pragmatic RCT in regional Australia.
Keywords: diabetes, digital health, ehealth, insulin dose adjustment, insulin titration, mHealth, telehealth, telemedicine
Introduction
Type 2 diabetes (T2DM) is a progressive disease that involves stepwise addition of multiple glucose- lowering agents over time, with insulin being the most potent among them, to achieve adequate individual glycaemic targets.1,2 The benefits of intensive glycaemic management in the prevention of diabetes-related complications are well established.3–5 The current T2DM-management position statement from the Australian Diabetes Society recommends early commencement of insulin if glycaemic targets are not achieved, especially if the glycated haemoglobin (HbA1c) is above 9% (75 mmol/mol).1 Insulin initiation and/or titration is a resource-intensive process requiring patient education and skill development.6 This process requires a diabetes health professional, often a credentialed diabetes educator (CDE), to commence a programme for insulin dose adjustment (IDA). It requires synchronous contact between the patient and health professional, relay of blood glucose levels (BGLs), interpretation of variables influencing glucose levels and calculating new insulin doses for the patient to implement. The IDA service benefits both the patient, in achieving better clinical outcomes by appropriate insulin initiation/intensification, and the referring doctor, in improving efficiency by upgrading the role of nurse educators to adjust insulin doses according to local protocols.7,8
The current IDA process has some limitations: (a) inefficiencies of time management when trying to ensure synchronous communication by phone between the health professional and patient; (b) lack of readily available patient BGLs data; (c) the possibility of transcription errors using conventional approaches of data recording via the telephone. These factors can lead to a suboptimal patient and health professional experience and hinder patient self-management.
With the incidence and prevalence of diabetes increasing9 in a background of regional workforce shortages, continuing the current model of IDA services10 is unsustainable. Insulin initiation is also often delayed,11,12 particularly in primary care, and clinician-related therapeutic inertia can be an important factor that contributes to this delay13,14 A process that can simplify initiation and titration of insulin may assist this clinician-related therapeutic inertia.13,15
There is emerging evidence that mobile health (mHealth), a subset of Ehealth, can assist in the management of chronic disease such as diabetes.16–18 mHealth can provide a platform for better integrated care with data availability in a systematic and organized manner,19 it can be cost-saving compared with traditional care,20,21 it can provide automated customized instant feedback,20 and improve patient satisfaction.22 These benefits of mHealth could assist in overcoming some of the limitations of the current model of IDA by enhancing self-management, organized care and easier patient access and feedback to healthcare professionals. Considering these benefits, we have designed an innovative mHealth model of IDA, that is, REthinking Model of Outpatient Diabetes care utilising EheaLth – Insulin Dose Adjustment (REMODEL-IDA), utilizing the Mobile Diabetes Management System (MDMS). The MDMS system is described further in the ‘Methods’ section but in summary comprises an app for smartphones, and a web-based clinical portal. The mobile app enables patients to use a Bluetooth-enabled glucose meter to upload their BGL readings automatically to the clinical portal. The mobile app also provides an insulin diary that allows patients to manually enter the dose and time of their insulin injections. These data are subsequently transmitted and uploaded to the clinical portal via the internet. The primary aim of this study is to examine the effects of REMODEL-IDA on glycaemic control at 3 months in patients with T2DM. The secondary aims are to examine IDA service delivery efficiency and patient satisfaction. We hypothesize that the REMODEL-IDA model will improve glycaemic control through improved patient self-management and feedback.
Methods
Setting
The sites for recruitment will be the Princess Alexandra Hospital (PAH) diabetes specialist outreach community clinics and PAH diabetes telehealth clinic. These clinics accept referrals from general practitioners (GPs) for management of patients with diabetes where the ongoing management is beyond the capacity of the patient’s usual GP. The diabetes specialist outreach community clinics are a model of integrated primary–secondary care where patients are seen in a community-based general practice by an advanced-skill GP and a CDE, both supervised by a visiting endocrinologist from the PAH. An advanced-skill GP is an experienced doctor who has undertaken further training in diabetes management.23,24 The diabetes telehealth clinic is led by an endocrinologist and is usually supported by a GP or CDE at the recipient end.25–27 The diabetes telehealth clinic offers teleconsultations to most hospital and health services across regional and remote Western Queensland. Both clinics review patients at regular (4 weeks to 6 months) scheduled outpatient consultations: the interval between these consultations is determined by the doctor based on the patient’s glycaemic management and comorbidities. Where clinically appropriate, a proportion of patients are referred to the IDA service either for insulin initiation and/or insulin titration to achieve optimal glycaemic targets. Both clinics serve a disadvantaged population. The specialist community outreach clinics serve a population with a high proportion of low socio-economic status and the telehealth clinic serves a population with otherwise limited access to specialist services.
Current model of IDA service
The indications and limitations of IDA have been described earlier. Patients not meeting glycaemic targets who need to initiate insulin or adjust current insulin regimes are referred by the treating endocrinologist to the IDA service. The IDA service is performed by a CDE under the supervision of an endocrinologist. The CDE provides initial face-to-face education with the patient. Then over 4–6 weeks via once or twice weekly phone calls, the CDE obtains a combination of fasting, pre-meal and postprandial BGL data and provides advice to the patient on adjustment of insulin doses, if required, and general diabetes management. Most patients achieve individual target glucose levels usually within 4–6 weeks and are discharged from the service after assessment by the CDE. For the remaining individuals, a decision is made to either continue with IDA or advance their clinic appointment to identify other variables that may be influencing their glycaemic management.
Description of MDMS
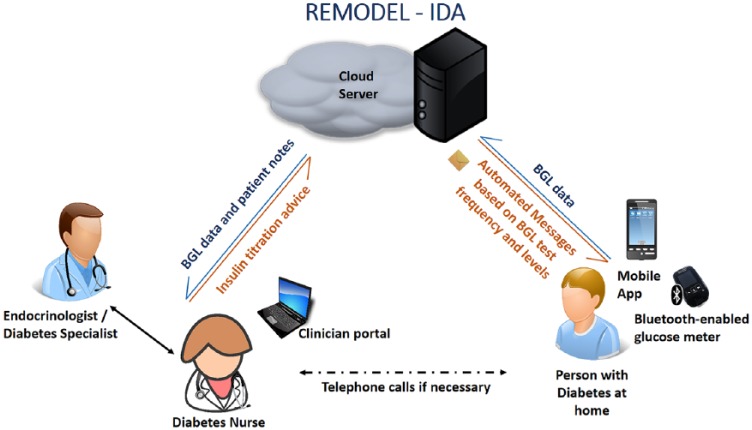
In partnership with the Australian E-Health Research Centre, the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the Department of Diabetes and Endocrinology at PAH, the Centre for Online Health, a sub-centre of the Centre for Health Services research, The University of Queensland, developed MDMS to support the transformation of the provision of specialist diabetes care. This system is modelled on a highly successful CSIRO platform that supports in-home cardiac rehabilitation leading to improvements in adherence and clinical outcomes.28 The system has been modified to enable real-time monitoring of BGLs in patients with diabetes, and to support patient care. The system comprises an app for iOS- (Apple) and android-based smartphones, and a web-based clinical portal. The mobile app enables patients to use a Bluetooth-enabled glucose meter (Accu-Chek® Guide, Roche Diagnostics GmbH, Basel, Switzerland) to upload their BGL readings automatically to the clinical portal. The mobile app screen enables a review of BGL and their trends. The mobile app also provides an insulin diary that allows patients to manually enter the dose and time of their insulin injections, along with a free text comment for each dose (e.g. ‘before dinner’). These data are subsequently transmitted and uploaded to the clinical portal via the internet. The clinical portal presents the uploaded data in graphical and tabular formats for the CDEs and endocrinologists to monitor and manage a patient’s condition. Integrated alerts, which can be customized by clinicians, highlight out-of-range measures. Through the portal, the clinicians can review patients’ BGL data and insulin dosages and send messages to patients’ mobile phones. A summary of their diabetes care is also displayed based on the clinical information entered. Patients receive optional automated individual text-messages based on the frequency of BGL testing and BGL values. The text-messages are triggered when patients have severely low or high BGL values (<2.5 and ⩾ 25mmol/L). All patients also receive automated text-messages based on BGL values and the frequency of BGL self-monitoring twice a week. Positive feedback messages are sent if these parameters are within clinically recommended targets. If parameters are outside of targets, the messages serve as prompts to check BGLs as recommended, to consider reasons for out-of-range BGL measurements and to seek medical advice if required. The messages also contain links to the national Diabetes Australia website for additional information regarding self-management of diabetes.
We obtained patient and clinician feedback on the MDMS through a proof-of-concept trial with patients with diabetes who had stable glycaemic management.29 This facilitated enhancements to the MDMS. The MDMS will be utilized in the intervention REMODEL-IDA (see the section on the intervention group below).
Study design
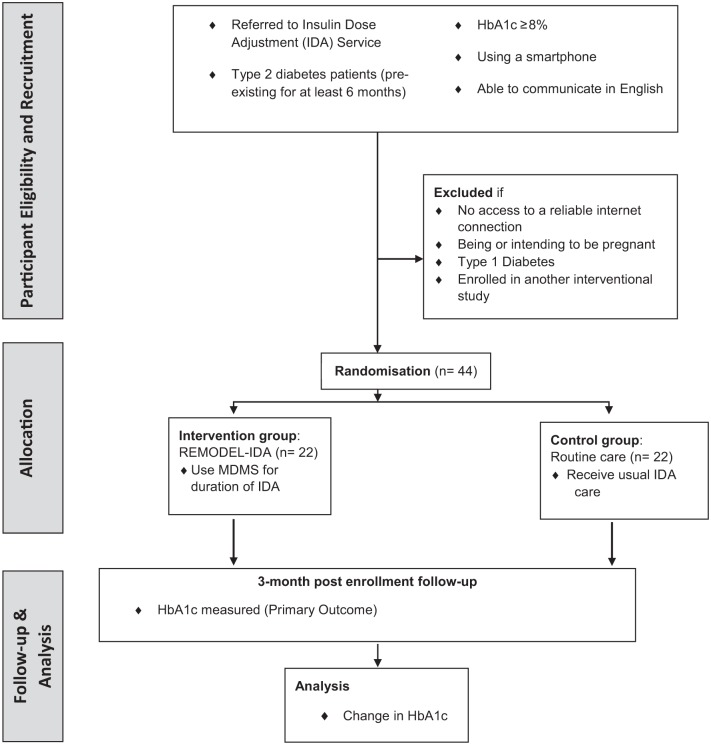
A two-group pilot randomized controlled trial (RCT) will be conducted for 3 months as shown in Figure 1. Patients referred by the endocrinologist to the IDA service will be randomized to the intervention (REMODEL-IDA) utilizing the MDMS or control (routine care). The setting will be the sites as described above. The IDAs for the PAH diabetes specialist outreach clinics will be performed by the CDE at each site. For the PAH diabetes telehealth service, the insulin dose adjustment will be managed by the CDE at PAH except for patients of the South West Queensland Health Service where the adjustment will be conducted by the local CDE.
Figure 1.
Study design.
HbA1c, glycated haemoglobin; MDMS, mobile diabetes management system; REMODEL-IDA, REthinking MOdel of Diabetes care utilizing EheaLth – Insulin Dose Adjustment.
Participants
A total of 44 patients with T2DM requiring IDA services will be recruited at the participating sites. The inclusion criteria are: (a) people with T2DM pre-existing for at least 6 months; (b) age 16 years or older; (c) HbA1c 8% (64 mmol/mol) or greater (performed in the last 4 weeks before enrolment); (d) able to use a smartphone daily; (e) able to read and write in English.
Exclusion criteria will be: (a) having no access to a reliable internet connection (reliable internet connection is defined as access to 4G, 3G or Wi-Fi connection); (b) being or intending to be pregnant; (c) having type 1 diabetes; (d) enrolled in another interventional study.
Outcomes
The primary outcome measure will be change in HbA1c. Secondary outcome measures will be: (a) serum fructosamine (short-term marker of glycaemic management over the preceding 2 weeks); (b) number of persons continuing to engage and completing IDA; (c) total time spent in IDA; (d) clinician time spent in IDA; (e) hypoglycaemic events in the preceding 2 weeks at baseline, 4 weeks and 3 months recorded and/or self-reported by the patient; (f) percentage of patients achieving HbA1c 7.5% (58 mmol/mol) or less; (g) patient satisfaction and acceptability at 4 weeks; (h) healthcare provider satisfaction from the CDE; (i) number of diabetes-related visits to the CDE, GP and/or hospital visits.
Sample size and power calculation
We will recruit 44 patients with 22 patients in each arm, allowing for 10% attrition. This sample size for the pilot study was chosen based on previous studies.30,31
Ethics
Human Research Ethics Committee approval was obtained through the Metro South Health Human Research Ethics Committee (Ref. HREC/18/QPAH/42) and registered with Australia New Zealand Clinical Trials Registry. Trial registry ACTRN12618000412235.
Recruitment and randomization procedure
The principal researcher will explain the study protocol to the clinical staff at the participating sites. A project officer will liaise with staff to identify potential participants for the study from those referred to the IDA service. The project officer will then screen patients for eligibility (see eligibility criteria in section on participants) and recruit patients who meet the eligibility criteria after obtaining informed consent. Ethics-approved Patient Information and Consent Forms will be used. When the patients consent to participate, they will be randomized using the Research Electronic Data Capture (REDCap) software. They will be stratified according to their residential address as regional and nonregional. Regional is defined as all areas classified under RA2 (Inner Regional), RA3 (Outer Regional), RA4 (Remote) and RA5 (Very Remote) as per the Australian Standard Geographical Standard Remoteness Areas 2011.32,33 Nonregional is defined as RA1 (Major Cities). Based on a conservative recruitment rate of two patients per week, it will take 22 weeks to complete recruitment and a further 13 weeks for data collection.
Control group
Patients referred for IDA and randomized to the control group will receive routine care provided by the clinical staff as mentioned in the section on the current model of IDA service above. The control group will be provided with standard glucose meters if their current glucose meter is more than 2 years old and followed up as per the current model of care
Intervention group
The intervention group (see Figure 2) will use the MDMS as described earlier for IDAs. All other aspects of care including face-to-face diabetes education will be similar to the control group and is as stated in the section on the current model of IDA service above.
Figure 2.
New model of diabetes care for insulin dose adjustment using MDMS (mobile diabetes management system).
BGL, blood glucose level.
For the intervention group, the researcher will install and demonstrate the mobile app on participants’ individual smartphones, and provide and demonstrate the use of the Accu-Chek® Guide, which pairs via Bluetooth to the mobile app and sends blood glucose recordings automatically to a web-portal daily. The participants will be followed up for the duration of their enrolment into the IDA service, which can last up to 4–6 weeks. The CDE will provide advice twice weekly to the patient on insulin titration through messages sent via the mobile app. The participants who complete the IDA due to meeting blood glucose concentration targets before 3 months will be discharged from the MDMS but contacted at 3 months by the project officer to obtain an HbA1c. The study will be terminated for participants who continue to be in the IDA at 3 months and an HbA1c obtained. As stated above most participants will be discharged usually around 4–6 weeks, but a minority could continue with the IDA at the discretion of the CDE.
Data collection
Study data (see Table 1) will be collected and managed using REDCap electronic data-capture tools hosted at the University of Queensland. REDCap is a secure, web-based application designed to support data capture for research studies, providing: (a) an intuitive interface for validated data entry; (b) audit trails for tracking data manipulation and export procedures; (c) automated export procedures for seamless data downloads to common statistical packages; (d) procedures for importing data from external sources.34
Table 1.
Schedule of enrolment, interventions and assessments.
| MDMS for IDA | Study period – 3 months | |||
|---|---|---|---|---|
| Consent | Allocation | Post-allocation | Close-out | |
| TIMEPOINT | 0 | 4 weeks | 3 months | |
| ENROLMENT: | ||||
| Eligibility screen | X | |||
| Informed consent | X | |||
| Allocation | X | |||
| INTERVENTION: MDMS |

|
|||
| ASSESSMENTS: | ||||
| Primary outcome | ||||
| Change in HbA1c | X | X | ||
| Secondary outcomes | ||||
| Serum fructosamine, patient satisfaction survey, | X | X | ||
| Patient acceptability of MDMS survey | X | |||
| Self-reported hypoglycaemic events survey | X | X | X | |
| Healthcare provider (CDE) satisfaction survey | X | |||
| Completion rate of IDA | X | |||
| Clinician time, diabetes-related visits to CDE/GP/hospital |

|
|||
CDE, credentialed diabetes educator; GP, general practitioner; HbA1c, glycated haemoglobin; IDA, insulin dose adjustment; MDMS, mobile diabetes management system.
Analysis
Statistical analysis
All analyses will be based on ‘intention to treat’ and per protocol (Protocol Version 3 26/10/2018). The primary analysis will be intention to treat. A secondary analysis will be based on per protocol. Differences between groups at baseline and the relevant endpoints will be compared using either the Student’s t test or Mann–Whitney U-test as appropriate. A p value of less than 0.05 will be considered statistically significant. A subgroup analysis of the participants based on their location, regional versus urban, will be conducted.
Conclusion
This paper describes the protocol of a pilot RCT using MDMS for IDA in T2DM for specialist community outreach and diabetes telehealth clinics across regional Queensland, Australia. This protocol builds on the previous proof-of-concept study utilizing MDMS, which showed promising levels of adherence and usability.29 This study will assess a model for IDA (REMODEL-IDA) that combines mHealth interventions such as automated, personalized text-messages, provision of visual tracking of BGL patterns and feedback regarding insulin titration via the mobile app (MDMS). Given the potential benefits of mHealth in assisting patient self-management, this should lead to improved clinical outcomes as measured by HbA1c. Other possible benefits of this new model of care include improved healthcare service delivery efficiency and patient and health professional satisfaction. This pilot study will inform recruitment yield, retention and acceptability from patients with T2DM and clinicians to guide the conduct of a large pragmatic RCT assessing the benefits of REMODEL-IDA for IDA in relevant patients across regional and remote Queensland, Australia. This has the potential to be integrated into the routine diabetes care of patients with T2DM in the community and regional Australia.
Supplemental Material
Supplemental material, REMODEL-IDA_PICF_Patient_Information_and_Consent_FormV2_11022018 for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism
Supplemental Material
Supplemental material, spirit_check_list_ for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism
Supplemental Material
Supplemental material, Trial_Registration_Data_Set_1 for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Authors’ note: Conceived and designed the experiments: AM, AR, LG, FF, DB and DD. Analyzed the data: N/A. Wrote the first draft of the manuscript: AM. Contributed to the writing of the manuscript: AM, AR, LG, FF, DB and DD. Agree with manuscript results and conclusions: N/A. Jointly developed the structure and arguments for the paper: AM. Made critical revisions and approved final version: AM, AR, LG, FF, DB, DD and MK. All authors reviewed and approved of the final manuscript. Farhad Fatehi is now affiliated with Tehran University of Medical Sciences, School of Advanced Technologies in Medicine, Tehran, Iran.
Funding: This project is funded by a grant from the Telehealth Seed Funding, Queensland Health and Metro South Health, Queensland Health. AM is a recipient of a Diabetes Queensland Scholarship. AM and DB are financially supported by the Centre of Research Excellence in Telehealth funded by the National Health and Medical Research Council (NHMRC; grant ID: APP1061183). FF is financially supported by the Queensland Government through an Advance Queensland Research Fellowship. We acknowledge Roche Australia for the provision of glucose meters.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this paper.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Anish Menon  https://orcid.org/0000-0002-8176-0292
https://orcid.org/0000-0002-8176-0292
Contributor Information
Anish Menon, The University of Queensland, Centre for Health Services Research, and the Department of Diabetes and Endocrinology, Princess Alexandra Hospital, Woollongabba, Australia Ground Floor Building 33, Princess Alexandra Hospital Campus, Brisbane, Australia 4102.
Leonard Gray, The University of Queensland, Centre for Health Services Research, Brisbane, Australia.
Farhad Fatehi, The University of Queensland, Centre for Health Services Research, Brisbane, Australia, and CSIRO Australian eHealth Research Centre, Brisbane, Australia.
Dominique Bird, The University of Queensland, Centre for Health Services Research, Brisbane, Australia.
Darsy Darssan, The University of Queensland, School of Public Health, Brisbane, Australia.
Mohan Karunanithi, CSIRO Australian eHealth Research Centre, Brisbane, Australia.
Anthony Russell, Department of Diabetes and Endocrinology, Princess Alexandra Hospital, Woolloongabba, Australia, and The University of Queensland, Faculty of Medicine, Brisbane, Australia.
References
- 1. Gunton JE, Cheung NW, Davies TM, et al. ; Australian Diabetes Society. A new blood glucose management algorithm for type 2 diabetes: a position statement of the Australian Diabetes Society. Med J Aust 2016; 201: 650–653. [DOI] [PubMed] [Google Scholar]
- 2. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32(Suppl. 2): S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Control G, Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52: 2288–2298. [DOI] [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 6. Lau AN, Tang T, Halapy H, et al. Initiating insulin in patients with type 2 diabetes. CMAJ 2012; 184: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Association of Diabetes Educators. Diabetes educators play a critical role in successful insulin management. Chicago, IL: American Association of Diabetes Educators, 2017. [Google Scholar]
- 8. Furler J, O’Neal D, Speight J, et al. Supporting insulin initiation in type 2 diabetes in primary care: results of the Stepping Up pragmatic cluster randomised controlled clinical trial. BMJ 2017; 356: j783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Global report on diabetes. World Health Organization, 2016. http://www.who.int/iris/handle/10665/204871 [Google Scholar]
- 10. Murray RB, Wilson A. Work-readiness and workforce numbers: the challenges. Med J Aust 2017; 206: 433–434. [DOI] [PubMed] [Google Scholar]
- 11. Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control – do specialists differ from primary care physicians? Diabetes Care 2005; 28: 600–606. [DOI] [PubMed] [Google Scholar]
- 12. Shah BR, Hux JE, Laupacis A, et al. Diabetic patients with prior specialist care have better glycaemic control than those with prior primary care. J Eval Clin Pract 2005; 11: 568–575. [DOI] [PubMed] [Google Scholar]
- 13. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes 2017; 11: 3–12. [DOI] [PubMed] [Google Scholar]
- 14. Funnell MM. Overcoming barriers to the initiation of insulin therapy. Clin Diabetes 2007; 25: 36–38. [Google Scholar]
- 15. Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab 2018; 20: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonoto BC, de Araujo VE, Godoi IP, et al. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. JMIR Mhealth Uhealth 2017; 5: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Xue H, Huang Y, et al. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv Nutr 2017; 8: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kitsiou S, Pare G, Jaana M, et al. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS One 2017; 12: e0173160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nundy S, Dick JJ, Goddu AP, et al. Using mobile health to support the chronic care model: developing an institutional initiative. Int J Telemed Appl 2012; 2012: 871925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fatehi F, Gray LC, Russell AW. Mobile health (mHealth) for diabetes care: opportunities and challenges. Diabetes Technol Ther 2017; 19: 1–3. [DOI] [PubMed] [Google Scholar]
- 21. Hayes DF, Markus HS, Leslie RD, et al. Personalized medicine: risk prediction, targeted therapies and mobile health technology. BMC Med 2014; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mora P, Buskirk A, Lyden M, et al. Use of a novel, remotely connected diabetes management system is associated with increased treatment satisfaction, reduced diabetes distress, and improved glycemic control in individuals with insulin-treated diabetes: first results from the Personal Diabetes Management Study. Diabetes Technol Ther 2017; 19: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russell AW, Baxter KA, Askew DA, et al. Model of care for the management of complex Type 2 diabetes managed in the community by primary care physicians with specialist support: an open controlled trial. Diabet Med 2013; 30: 1112–1121. [DOI] [PubMed] [Google Scholar]
- 24. Russell AW, Donald M, Borg SJ, et al. Clinical outcomes of an integrated primary-secondary model of care for individuals with complex type 2 diabetes: a non-inferiority randomised controlled trial. Diabetologia 2019; 62: 41–52. [DOI] [PubMed] [Google Scholar]
- 25. Menon A, Gray LC, Fatehi F, et al. A comparison of characteristics of patients seen in a tertiary hospital diabetes telehealth service versus specialist face-to-face outpatients. J Telemed Telecare 2017; 23: 842–849. [DOI] [PubMed] [Google Scholar]
- 26. Fatehi F, Gray LC, Russell AW. Telemedicine for clinical management of diabetes – a process analysis of video consultations. J Telemed Telecare 2013; 19: 379–382. [DOI] [PubMed] [Google Scholar]
- 27. Fatehi F, Martin-Khan M, Smith AC, et al. Patient satisfaction with video teleconsultation in a virtual diabetes outreach clinic. Diabetes Technol Ther 2015; 17: 43–48. [DOI] [PubMed] [Google Scholar]
- 28. Varnfield M, Karunanithi M, Lee CK, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014; 100: 1770–1779. [DOI] [PubMed] [Google Scholar]
- 29. Ding H, Fatehi F, Russell AW, et al. User experience of an innovative mobile health program to assist in insulin dose adjustment: outcomes of a proof-of-concept trial. Telemed J E Health 2018; 24: 536–543. [DOI] [PubMed] [Google Scholar]
- 30. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther 2016; 18: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasmussen OW, Lauszus FF, Loekke M. Telemedicine compared with standard care in type 2 diabetes mellitus: a randomized trial in an outpatient clinic. J Telemed Telecare 2016; 22: 363–368. [DOI] [PubMed] [Google Scholar]
- 32. Commonwealth Department of Health, Standard. Medicare remoteness classification (ASGS-RA) N, 2015. [Google Scholar]
- 33. Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): Volume 5 Remoteness Structure. Canberra: Australian Bureau of Statistics, 2011. [Google Scholar]
- 34. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, REMODEL-IDA_PICF_Patient_Information_and_Consent_FormV2_11022018 for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism
Supplemental material, spirit_check_list_ for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism
Supplemental material, Trial_Registration_Data_Set_1 for Mobile-based insulin dose adjustment for type 2 diabetes in community and rural populations: study protocol for a pilot randomized controlled trial by Anish Menon, Leonard Gray, Farhad Fatehi, Dominique Bird, Darsy Darssan, Mohan Karunanithi and Anthony Russell in Therapeutic Advances in Endocrinology and Metabolism