Abstract
Setting
A survey of the prevalence of drug-resistant tuberculosis (DR-TB) in new and previously treated patients (PTPs) was performed in Burkina Faso from 2016 to 2017.
Design
In this cross-sectional survey, a structured questionnaire was administered to eligible smear-positive patients in all 86 diagnostic and treatment centers of the country to collect their socio-demographic characteristics and medical histories. Their sputa were tested using the Mycobacterium tuberculosis/rifampicin (MTB/RIF) Xpert assay. Those which were found to be positive for TB and rifampicin-resistant were also tested with GenoType MTBDRplus2.0 and MTBDRsl2.0. Univariate and multivariate logistic regressions were performed to determine risk factors associated with rifampicin resistance.
Results
Of the 1140 smear-positive patients enrolled, 995 new and 145 PTPs were positive for MTB complex by Xpert. Of these, 2.0% (20/995, 95% confidence interval (CI): 1.1–2.9) of the new cases and 14.5% (95% CI: 14.2–20.2) of the PTPs were resistant to rifampicin; 83% of them has multidrug-resistant tuberculosis (MDR-TB). None were pre-extensively drug-resistant TB (pre-XDR-TB) or XDR-TB. Only the previous treatment was significantly associated with rifampicin resistance, p < 0.0001.
Conclusion
Similar to global trends, rifampicin resistance was significantly higher in patients with prior TB treatment (14.5%) than in naïve patients (2.0%). These percentages are slightly below the global averages, but nonetheless suggest the need for continued vigilance. Extending the use of Xpert testing should strengthen the surveillance of DR-TB in Burkina Faso.
Keywords: tuberculosis, survey, Xpert MTB/RIF, rifampicin resistance, prevalence, Burkina Faso
Introduction
The problem of tuberculosis (TB) is global. According to the World Health Organization (WHO), 10.4 million cases occurred in 2016 and 1.7 million (including 0.4 million who also have HIV) died [1]. In countries with limited resources, tuberculosis is a major concern for the public health services. In sub-Saharan Africa, the situation is even more alarming because of the impacts of economic difficulties, malnutrition, HIV infection, and the spread of multidrug-resistant tuberculosis (MDR-TB) [2, 3]. Traditional methods of diagnosing MDR-TB are slow and require infrastructure that is often lacking in the laboratories of resource-limited countries. To bypass these limitations, the WHO has recommended the use of the Xpert Mycobacterium tuberculosis/rifampicin (MTB/RIF) test (Cepheid, Sunnyvale, CA, USA) for the rapid diagnosis of TB and the detection of rifampicin-resistant mutations [4].
In Burkina Faso, among 18 million inhabitants, 5594 were diagnosed with TB in 2015 (31.01 cases per 100,000 inhabitants). When this is compared to the 2331 cases in 2000 (20.08 cases per 100,000 inhabitants), it suggests an annual increase in diagnosed tuberculosis cases of 14.5%. In addition, the annual activity reports show MDR-TB increasing year by year in previously treated patients. Currently, the National Tuberculosis Program (NTP) has implemented an algorithm to screen for MDR-TB in the following patient categories: patients who were in contact with MDR-TB patients, patients who are culture- or smear-positive after the 3rd month of treatment with first line drugs, relapsed patients, and patients receiving repeated treatment after non-adherence and treatment failure. The WHO recommends that the National Tuberculosis Program (NTP) should periodically survey the prevalence of resistance to provide essential information for effective planning of TB control and management of TB patients. A survey of MDR-TB in new TB smear-positive patients has not been undertaken in Burkina Faso since the last study performed from April 2005 to September 2006 [5]. This previous study, published in 2010, found MDR-TB to be 3.4% and 50.5% among new cases and previously treated patients (PTPs), respectively [5]. The recent installation of 15 GeneXpert MTB/RIF instruments in Burkina Faso, as well as the implementation of the GenoType MTBDRplus v2.0 and GenoType MTBDRsl v2.0 tests (Hain Lifescience, Nehren, Germany) in the National Reference Laboratory of TB (NRL-TB) in Ouagadougou, has made it possible to conduct a second national survey to provide data on the current burden of drug-resistant TB (DR-TB).
Population and Methods
Study Design and Population
A cross-sectional survey was conducted from November 2016 to April 2017. Patients were enrolled in all of the 86 Diagnostic and Treatment Centers (DTCs) in the 13 national health regions. The study population was composed of new bacteriologically confirmed pulmonary TB patients and previously treated smear-positive patients. After the patients provided a written consent, a questionnaire was used to record their sociodemographic, clinical, biological, and behavioral characteristics and whether they had been previously treated for TB.
Sample Size Calculation of New Patients
In calculating the sample size [6], we based our calculations on the number (N = 3722) of smear-positive patients registered in Burkina Faso in 2014, the 1.7% rifampicin resistance prevalence estimated by the WHO, the 1.96 z-value-associated 95% confidence interval (CI = 1.3–2.1), and the absolute precision of d = 0.8%. These calculations suggested that a sample size of at least 790 smear-positive patients would be required to detect a 1.7% resistance level in 3722 pulmonary TB patients. To compensate for potential loss to follow-up, which was estimated at 15%, we estimated that 930 patients were required for the study to yield statistically robust results. Enrollment of patients in DTCs was done consecutively until the desired sample size was reached, and patient recruitment period of five and a half months was predicted.
Sample Size Calculation of Previously Treated Patients
The inclusion of consecutive previously treated smear-positive patients was carried out concomitantly with the recruitment of new patients and discontinued as soon as the recruitment goal of new patients had been reached.
Classification of Patients
The data from the questionnaire and the results of the bacteriological examinations made it possible to classify the pulmonary tuberculosis patients into new and previously treated patients, according to the following definitions:
new patients were bacteriologically confirmed pulmonary TB patients who had no previous history of ever receiving anti-tuberculosis medication;
previously treated patients were patients who had received antituberculosis treatment in the past for at least one month and had experienced relapse, treatment failure, a positive smear, or culture after three months on first line treatment or were resuming treatment after discontinuation.
Included Patients
New and previously treated patients found to be smear-positive, and Xpert-positive patients were included in this study after providing a written consent.
Excluded Patients
Patients without Xpert tests or with negative or invalid Xpert results were excluded, as well as those whose rifampicin resistance result was indeterminate. Those patients who could not be unequivocally classified as either new or previously treated were also excluded.
Laboratory Procedures
Sputum from presumed TB patients and those already on treatment were analyzed in the DTC laboratories using the Ziehl Neelsen hot method. Two sputa from each TB patient were transported in triple packaging to one of 15 Xpert site laboratories. Sputum from each patient was tested by the GeneXpert MTB/RIF kit (Cepheid, Sunnyvale, CA, USA) according to the manufacturer's instructions. If the test gave a positive result for Mycobacterium tuberculosis with resistance to rifampicin (MTB + RIF+), the second paired sputum from the same patient was treated with OMNIgene•SPUTUM (DNA Genotek, Ottawa, Canada) and sent to the NRL-TB for further resistance testing using GenoType MTBDRplus v2.0 test and Genotype MTBDRsl v2 (Hain Lifescience GmbH, Nehren, Germany). DRplus detects both Mycobacterium tuberculosis complex DNA and mutations conferring resistance to rifampicin and isoniazid. DRsl detects Mycobacterium tuberculosis complex DNA and mutations conferring resistance to the fluoroquinolones, ethambutol, and the injectable second-line drugs kanamycin, amikacin, and capreomycin. Extensively drug-resistant (XDR) TB is defined to be in vitro resistant to isoniazid and rifampin and also resistant to any fluoroquinolone and at least one injectable drug (capreomycin, kanamycin, or amikacin). Pre-extensively drug-resistant (pre-XDR) TB is defined to be in vitro resistant to isoniazid and rifampin with additional resistance to any fluoroquinolone or to any injectable drug.
The DRplus and DRsl tests always included positive internal quality controls, as well as negative controls. The rpoB, katG, inhA, gyrA, gyrB, rrs, and eis gene loci each have a control, the presence of which is mandatory, interpreting the results according to the product insert.
For external quality evaluation, 10 samples were sent to the Supra National Reference Laboratory of Cotonou, Benin, for DRplus and DRsl testing.
HIV antibody testing was performed with patient consent at the DTCs or TB clinics according to WHO/UNAIDS [7] Strategy II, using the Determine HIV1/2 combination test and the SD Bioline HIV1/2 3.0 discriminant test (Standard Diagnostics). The statistical analysis included only tests from consenting patients that yielded clear positive or negative HIV results.
Statistical Analyses
The data were analyzed with IBM SPSS Statistics 20.0. The age variable was recoded. We analyzed the distribution of different variables (type of patient, sex, age, HIV status, health regions, history of smoking, alcohol consumption, and previous imprisonment) according to their different modalities. Univariate and multivariate logistic regression was performed to determine the factors associated with rifampicin resistance. Odds ratios (ORs) with 95% confidence intervals were calculated to evaluate the association of possible risk factors with rifampicin resistance. Probability values of p < 0.25 for a univariate and p < 0.05 for a multivariate analysis were considered statistically significant.
Ethics
The informed and voluntary consent of the patients was obtained prior to their participation in the study. The study received approval from the health ethics committee of Burkina Faso.
Results
Patients Inclusion
As shown in Figure 1, of the 1250 eligible smear-positive pulmonary TB patients, Xpert test was performed for 1228 of them. Of these, 1141 were positive for Mycobacterium tuberculosis complex. The concordance between smear microscopy and GeneXpert for bacteriological confirmation was 93.2%). According to our methodology, 110 patients (Xpert not performed: 22; Mycobacterium tuberculosis detected/rifampicin-indeterminate: 01; no result: 01; Mycobacterium tuberculosis not detected: 86) did not meet the inclusion criteria and were excluded from the study.
Figure 1.
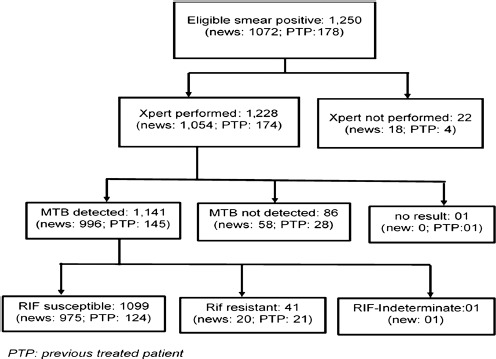
Flow chart of the patients enrolled in the study
Among the 1140 remaining patients, 995 were new cases and 145 were PTPs. Among the 145 PTPs, 21 (14.5%) were smear-positive in the 3rd month on first line treatment, 49 (33.8%) were treatment failures, 58 (40.0%) were relapses, and 17 (11.7%) were patients who were resuming treatment after discontinuation. Table 1 shows the patients' characteristics. The median patient age was 36.5 years (range: 2–95), with 57.1% younger than 40 years old, and 74.1% were men. Among the 857 new patients for whose results were available, 69 (8.1%) were HIV-positive. The geographic distribution of patients ranged from 359 living in the Central area to 115 residing in the Eastern region. The principal behavioral risk factors in the patients were the use of alcohol (28.3%) and tobacco (28.3%) and previous incarceration (4.7%).
Table 1.
Baseline characteristics of the included patients in the study
| Characteristics | New patients | previously treated | All patients | |||
|---|---|---|---|---|---|---|
| N1 | % | N2 | % | n | % | |
| Gender | ||||||
| Men | 725 | 72.9 | 120 | 82.8 | 845 | 74.1 |
| Women | 270 | 27.1 | 25 | 17.2 | 295 | 25.9 |
| Age group in years | ||||||
| 2-39 | 581 | 58.4 | 70 | 48.3 | 651 | 57.1 |
| ≥40 | 414 | 41.6 | 75 | 51.7 | 489 | 42.9 |
| HIV statusa | ||||||
| Positive | 69 | 8.1 | 6 | 4.6 | 75 | 7.6 |
| Negative | 788 | 91.9 | 124 | 95.4 | 912 | 92.4 |
| Geographical areas | ||||||
| Western area | 285 | 28.6 | 36 | 24.83 | 321 | 28.2 |
| Central area | 311 | 31.3 | 48 | 33.10 | 359 | 31.5 |
| Northem area | 293 | 29.4 | 52 | 35.86 | 345 | 30.3 |
| Eastern area | 106 | 10.7 | 9 | 6.21 | 115 | 10.1 |
| History of smoking, | ||||||
| Yes | 269 | 27.0 | 54 | 37.2 | 323 | 28.3 |
| No | 726 | 73.0 | 91 | 62.8 | 817 | 71.7 |
| Alcohol consumption | ||||||
| Yes | 288 | 28.9 | 46 | 31.7 | 334 | 29.3 |
| No | 707 | 71.1 | 99 | 68.3 | 806 | 70.7 |
| Previous imprisonmentb | ||||||
| Yes | 44 | 4.5 | 8 | 5.7 | 52 | 4.7 |
| No | 932 | 95.5 | 133 | 94.3 | 1065 | 95.3 |
a153 (13.4%) missing values.
b23 (2.02%) missing values.
- Western area: Health region of Boucle du Mouhoun, Hauts Bassins and Sud-Ouest;
- Central area: Health region of Centre, Plateau central, Centre-Ouest and Centre-Sud;
- Northern area: Health region of Sahel, Centre-Nord and Nord;
- Eastern area: Health region of Est and Centre-Est.
Drugs Susceptibility Testing Results
The MDR/ rifampicin-resistant TB (MDR/RR-TB), pre-XDR-TB, or XDR-TB status for the patients is shown in Table 2. Xpert MTB/RIF detected 41 RR-TB patients - 20 newly treated and 21 PTPs. The overall prevalence of rifampicin resistance was 3.6% (2.5–4.7), with 2.0% (1.1–2.9) in newly treated patients and 14.5% (8.7–20.2) in PTPs. Among the 21 RR-TB PTPs, 14.3% (3/21) had positive sputum after 3 months of therapy, 2.4% (10/49) were treatment failure cases in the 5th month and 13.8% (8/58) were relapsed cases.
Table 2.
Prevalence or proportion of M. tuberculosis drug resistance in TB patients by TB treatment history as determined by Xpert MTB/RIF and GenoType (DRplus, DRsl) (PTP: previous treated patient; RIF: rifampicin; INH: isoniazid; No: number; (%) = percentage; Pre-XDR-TB: Pre-Extensively Drug-Resistant Tuberculosis; XDR-TB: Extensively Drug-Resistant Tuberculosis)
| Tests | Resistance type | No. of patients/total no. of patients (%) 95% CI | p-valuea | |
|---|---|---|---|---|
| New patients | PTP | |||
| Xpert | RIF-resistant | 20/995 (2.0), 1.1–2.9 | 21/145 (14.5) 8.8–20.2 | <0.0001 |
| DRplus | INH-resistant | 17/20 (85.0), 69.4–100.6 | 19/21 (90.5), 77.9–103.0 | 0.298 |
| RIF-resistant | 15/20 (75.0), 56.0–94.0 | 19/21 (90.5), 77.9–103.0 | 0.188 | |
| MDR | 15/20 (75.0), 56.0–94.0 | 19/21 (90.5), 77.9–103.0 | 0.188 | |
| Any resistance | 17/20 (85.0), 69.4–100.6 | 19/21 (90.5), 77.9–103.0 | 0.298 | |
| DRsl | Pre-XDR-TB | 0 | 0 | – |
| XDR-TB | 0 | 0 | – | |
aKhi-deux de Pearson.
DRplus and DRsl were performed only on the 41 samples with rifampicin resistance detected by Xpert MTB/RIF. Of these, 34 (15 for new cases and 19 for PTPs) were confirmed to have resistance to rifampicin and isoniazid, and thus MDR. The concordance between GeneXpert and DRplus v2.0 for rifampicin resistance was 83%. Of the 7 samples with discordant results, one was sensitive to both rifampicin and isoniazid, 2 were resistant to isoniazid but sensitive to rifampicin, one was rifampicin-indeterminate but resistant to isoniazid, and Mycobacterium tuberculosis was not detected in 3 samples. The DRsl test found that none were resistant to either fluoroquinolones or injectable drugs.
A quality external evaluation of our DRplus and DRsl tests was performed by the Supra National Reference Laboratory of Cotonou in Benin and confirmed our results in all 10 samples tested.
Risk Factors for Rifampicin Resistance
As shown in Table 3, the only variable significantly associated with rifampicin resistance was the previous tuberculosis treatment, with an odds ratio of 8.2 (95% CI: 4.3–15, p < 0.0001) compared to new patients. The percentage of RR-TB among the HIV-positive patients (5.3%) was not significantly different from the percentage in HIV-negative patients (4.1%).
Table 3.
Multivariate analysis of associated factors with prevalence of rifampicin resistance in pulmonary TB patients (RR-TB: rifampicin-resistant tuberculosis; Ref: reference)
| Associated factors | Total number | RR-TB prevalence | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | % | OR | 95% CI | P | OR | 95% CI | P | ||
| Previously treated | |||||||||
| Yes | 145 | 21 | 14.5 | 8.2 | [4.3–15.6] | <0.0001 | 8.01 | [4.2-15.2] | <0.0001 |
| No | 995 | 20 | 2.0 | Ref | Ref | ||||
| Gender | |||||||||
| Men | 845 | 34 | 4.0 | 1.7 | [0.7–3.9] | 0.195 | 1.4 | [0.60-3.2] | 0.422 |
| Women | 295 | 7 | 2.4 | Ref | Ref | ||||
| Age group in years | |||||||||
| 2-39 | 651 | 22 | 3.4 | 0.85 | [0.4–1.6] | 0.664 | – | – | – |
| ≥40 | 470 | 19 | 3.9 | Ref | |||||
| HIV statusa | |||||||||
| Positive | 75 | 4 | 5.3 | 1.3 | [0.4–3.8] | 0.594 | – | – | – |
| Negative | 912 | 37 | 4.1 | Ref | |||||
| Geographical area | |||||||||
| Western area | 321 | 14 | 11.6 | 1.7 | [0.4–6.03]. | 0.410 | – | – | – |
| Central area | 359 | 13 | 3.6 | 1.4 | [0.3–5.01] | 0.602 | – | – | – |
| Northem area | 345 | 11 | 3.2 | 1.2 | [0.3–4.4] | 0.754 | – | – | – |
| Eastern area | 115 | 3 | 2.6 | Ref | |||||
| History of smoking | |||||||||
| Yes | 266 | 12 | 3.3 | 1.04 | [0.5–2.08] | 0.892 | – | – | – |
| No | 874 | 29 | 3.3 | Ref | |||||
| Alcohol consumption | |||||||||
| Yes | 334 | 12 | 3.6 | 0.99 | [0.5–1.9] | 0.997 | – | – | – |
| No | 806 | 29 | 3.6 | Ref | |||||
| Previous imprisonmentb | |||||||||
| Yes | 52 | 2 | 3.8 | 1.05 | [0.2–4.4] | 0.943 | – | – | – |
| No | 1065 | 39 | 3.7 | Ref | |||||
a153 (13.4%) missing values.
b23 ((2.02%) missing values.
Discussion
The current survey differs from the previous 2005–2006 survey in the sampling method, the number of sites, and the time of realization, although both were representative which may explain why we obtained different results. The TB patients in this study were predominantly males (74.1%) as has been reported in several other studies [8–13] and were mostly below age 40 (57.1%).
Rifampicin-resistance TB is a marker for MDR-TB [14]. The rate of 14.5% found among PTPs in this study is similar to what was found in Botswana [1] (13%). Higher levels were reported in the nearby countries of Democratic Republic of the Congo (17%) [1], Ivory Coast (22%) [15], and in Ghana (27.7%) [16]. The prevalence of RR-TB was highest among patients failing a category I regimen and patients with TB relapse. The 20.4% MDR prevalence that we found in patients with treatment failure may reflect the quality of care or poor patient compliance.
Although the prevalence that we found of 2% rifampicin resistance in TB new cases is similar to the 1.7% estimated by the WHO [6] and lower than the global average of 3.5%, it nevertheless remains important. As a comparison, a systematic review revealed a pooled prevalence of 2.1% MDR-TB in new patients in sub-Saharan Africa [17], with lower levels reported in Kenya (1.3%) [18] and Uganda (1.9%) [19], and higher levels in Somalia (5.2%) [20], Lagos (17.6%) [21], and northwest Ethiopia (3.8%) [22]. The prevalence of 14.5% RR-TB among PTB patients in our study is also lower than the 18% global estimate of RR among PTPs, but is still quite high.
Prior to 2013, Burkina Faso had difficulties managing MDR-TB for various reasons: the non-adherence of some MDR-TB patients to the long treatment regimen, insufficient numbers of hospital beds in two MDR-TB in-patient treatment sites (Ouagadougou and Bobo-Dioulasso), no outpatient treatment site, and delayed diagnosis of drug-resistant tuberculosis related to the difficulties and time required for sending strains for traditional drug susceptibility tests (DSTs) at the Supra National Reference Laboratory of the San Raffaele Scientific Institute of Milan, Italy. All of these factors could have allowed ongoing transmission of drug-resistant strains in Burkina Faso. It is therefore encouraging that we did not detect pre-XDR-TB or XDR-TB in any of the RR-TB found in the current survey. All cases of RR-TB we identified have been put on the shorter-course MDR-TB treatment regimen of 9–12 months [23].
In a 2010 report of 34 cases of MDR-TB in Burkina Faso, 5.9% (2/34) were XDR-TB [24], while the current survey found no cases of XDR or pre-XDR-TB. The difference between the findings of the two studies was probably because the previous study analyzed a selected population that included patients already receiving treatment with second-line drugs. The authors of that study recognized that due to the selected population studied, they may have overestimated the prevalence of XDR-TB cases among all MDR-TB patients, which is also suggested by the agreement of our results with the data from Gehre et al. [25]. Since 2014, Burkina Faso's NTP has focused on improving adherence to the anti-TB drug regimens in both susceptible and MDR-TB patients. This focus, together with a decreasing prevalence of HIV infection in Burkina Faso could have had a positive impact on the circulation of drug-resistance M. tuberculosis strains.
Currently, there is ongoing investment in strengthening the MDR-TB control program through the implementation of Xpert testing throughout the country, the planned acquisition of additional Xpert instruments, and an emphasis on appropriate MDR/RR-TB treatment regimens administered for MDR/ RR-TB cases at the two MDR-TB treatment sites. Beginning in 2018, the National Tuberculosis Guidelines have recommended the use of the Xpert test for all new bacteriologically confirmed patients to assure early detection and effective treatment of all RR-TB cases.
Using univariate and multivariate analysis, we found that the odds of having rifampicin resistance are much higher (OR = 8.2) in patients who have previously received anti-TB treatment, which corroborates with the results of other studies [20, 21]. Our finding of no relationship between rifampicin resistance and sex, age, or HIV status is in agreement with the previous study in Burkina Faso [5]. Other studies examining the role of HIV infection as a risk factor of MDR-TB have shown varying results [26–29].
Our survey has some limitations. First, 22 sputum smear-positive patients without Xpert results were excluded. Second, the DRplus identified fewer patients as RR-TB than the Xpert, but it was impossible to calculate a Kappa score to compare the tests because DRplus was only performed on samples found to be rifampicin-resistant with the Xpert. Our statistics were based on the Xpert results and included those that were discordant with the DRplus, and we were unable to pursue further studies to resolve these discordant results. Third, phenotypic DST was not performed. Although it is possible that we missed some MDR-TB cases with the Xpert, this seems unlikely, as the DRplus results suggest that it was more likely that through a lack of specificity, the Xpert overdiagnosed rifampicin resistance. Also, the prevalence of RR-TB in this study is similar to the WHO estimates for Burkina Faso.
Conclusion
Rifampicin resistance was higher in patients with prior TB treatment (14.5%) than that in naïve patients (2.0%), and all samples confirmed to be rifampicin-resistant by DRplus were also isoniazid-resistant, and thus MDR-TB. These results have shown that the burden of RR/MDR-TB in Burkina Faso is not negligible and requires continual vigilance. The expansion of Xpert testing is essential for the rapid detection of all RR/ MTDR-TB cases so that they can be cured with appropriate therapy and thereby prevent transmission. The TB control program should continue to focus on improving resistance detection, implementation of appropriate drug regimens, and patient adherence to the anti-TB therapy, including nutritional support to encourage the successful completion of the MDR-TB treatment.
Acknowledgments
We thank the patients whose data were used in this study and the health professionals in all 86 diagnostic and treatment centers who participated in this survey. We thank Irith De Baetselier, Institute of tropical Medicine, Antwerp and Howard Takiff, at the Pasteur Institute of Paris for valuable comments. We thank Anna Dean of World Health Organization, Global TB Programme, Geneva, Switzerland, and Ricardo Alagna and Daniela Cirillo of WHO Collaborating Centre for TB Laboratory Strengthening San Raffaele Scientific Institute Milano, Italy, for their support.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors.
Funding Sources
This survey was funded by The Global Fund to fight against tuberculosis.
Authors’ Contributions
SD, IZ, TS, AD, and SK contributed to the study conception, data analysis and manuscript. BG, AC, SG, AZ, and SMO contributed to the study conception. LTS and NB contributed to GenoType MTBDRplus and DRsl tests. LS contributed to the study conception, manuscript writing and study supervision.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization. Global tuberculosis report 2017. WHO/HTM/TB/2017.23. Geneva, Switzerland; 2017. [Google Scholar]
- 2.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? a systematic review. PLoS One. 2009;4: e5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blöndal K. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. Bull World Health Organ. 2007;85:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3. Geneva, Switzerland; 2010. [Google Scholar]
- 5.Sangaré L, Diandé S, Badoum G, Dingtoumda B, Traoré AS. Anti-tuberculosis drug resistance in new and previously treated pulmonary tuberculosis cases in Burkina Faso. Int J Tuberc Lung Dis. 2010;14:1424–9. [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis 5th ed. WHO/HTM/TB/2015.13 [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO. Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81–7. [PubMed] [Google Scholar]
- 8.Rasaki SO, Jibola AA, Musa SA, et al. Rifampicin Resistant Tuberculosis in a secondary Health institution in Nigeria, West Africa. J infect Dis Ther. 2014;2:139 doi: 10.4172/2332-0877.1000139. [Google Scholar]
- 9.Coovadia YM, Mahomed S, Pillay M, Werner L, Mlisana K. Rifampicin Mono Resistance in Mycobacterium tuberculosis in Kwazulu Natal, South Africa: A significant phenomenon in high prevalence TB/HIV Region. PLos One. 2013;8:e77712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otu A, Umoh V, Habib A, Ameh S, Lawson L, Ansa V. Drug Resistance among Pulmonary Tuberculosis patients in Calabar, Nigeria. Pulm Med. 2013;1-6. doi: 10.1155/2013/235190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH. The Social Determinants of Tuberculosis: From Evidence to Action. Am J Public Health. 2011;101:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipruto H, Mung’atu J, Ogila K, et al. The epidemiology of tuberculosis in Kenya, a high TB/HIV burden country (2000-2013). Int J Public Health Epidemiol Res. 2015;1:002–13. [Google Scholar]
- 13.Santos MLSG, Vendramini SHF, Gazetta CE, Oliveira SAC, Villa TCS. Poverty: socioeconomic characterization at tuberculosis. Rev Latino-am Enfermagem. 2007;15:762–7. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Sahrin M, Afrin S, et al. Comparison of Xpert MTB/RIF Assay and GenoType MTBDRplus DNA Probes for Detection of Mutations Associated with Rifampicin Resistance in Mycobacterium tuberculosis. PLoS One. 2016;11:e0152694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.N’Guessan KR, Ouassa T, Dean SA, et al. Multidrug-Resistant Tuberculosis in Côte d’Ivoire from 1995 to 2016: Results of National Surveys. Eur J Microbiol Immunol. 2018;8:91-4. doi: 10.1556/1886.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forson A, Kwara A, Kudzawu S, et al. A cross-sectional study of tuberculosis drug resistance among previously treated patients in a tertiary hospital in Accra, Ghana: public health implications of standardized regimens. BMC Infect Dis. 2018;18:149-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musa BM, Adamu AL, Galadanci NA, Zubayr B, Odoh CN, Aliyu MH. Trends in prevalence of multi drug resistant tuberculosis in sub-Saharan Africa: A systematic review and meta-analysis. PLoS One. 2017;12:e0185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitienei J, Kimenye K, Wahogo J, et al. 4th national anti-tuberculosis drug resistance survey in Kenya. J Health Sci. 2017;5:282-91. [Google Scholar]
- 19.Lukoye D, Adatu F, Musisi K, et al. Anti-Tuberculosis Drug Resistance among New and Previously Treated Sputum Smear-Positive Tuberculosis Patients in Uganda: Results of the First National Survey. PLoS One. 2013;8: e70763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sindani I, Fitzpatrick Ch, Falzon D, et al. Multidrug-Resistant Tuberculosis, Somalia, 2010-2011. Emerg Infect Dis. 2013;19:478-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yetunde A, Kuyinu FMCPH, Babatunde A, et al. Characteristics of Mycobacterium tuberculosis Positive Patients Screened for Drug-Resistant Tuberculosis at a Tertiary Health Facility in Lagos, Nigeria. J Nat Med Ass. 2018;110:88–91. [DOI] [PubMed] [Google Scholar]
- 22.Adane K, Ameni G, Bekele Sh, Abebe M, Aseffa A. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Public Health. 2015;15:572 doi: 10.1186/s12889-015-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49 doi: 10.1183/13993003.02308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleri N, Badoum D, Ouedraogo M, et al. Extensively Drug-Resistant Tuberculosis, Burkina Faso,. 2010, Emerging Infect Dis. 2010;16:840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehre F, Jacob Otu J, Kendal L, et al. The emerging threat of pre-extensively drug-resistant tuberculosis in West Africa:preparing for large-scale tuberculosisresearch and drug resistance surveillance. BMC Med. 2016;14:160 doi: 10.1186/s12916-016-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workicho A, Kassahun W, Alemseged F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist. 2017;10:91-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haar CH, Cobelens FGJ, Kalisvaart NA, van der Have JJ, Gerven JHJvan, Soolingen Dvan. Tuberculosis drug resistance and HIV infection, the Netherlands. Emerg Infect Dis. 2007;13:776–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diandé S, Sangaré L, Kouanda S, et al. Risk Factors for Multidrug-Resistant Tuberculosis in Four Centers in Burkina Faso, West Africa 2009. Microb Drug Resist. 2009;15:217-21. doi: 10.1089=mdr.2009.0906. [DOI] [PubMed] [Google Scholar]
- 29.Weyer K, Brand J, Lancaster J, Levin J, Van der Walt M. Determinants of multidrug-resistant tuberculosis in South Africa: results from a national survey. S Afr Med J. 2007;97:1120–8. [PubMed] [Google Scholar]


