Abstract
Objective
The study analyses i) the effect of overweight, waist circumference and dietary habits on postprandial (pp) triglyceride (TG) response and compares ii) pp TG response with fasting TG levels and iii) pp TG peak values with TG-AUC (area under curve) with respect to cardiometabolic risk assessment.
Methods
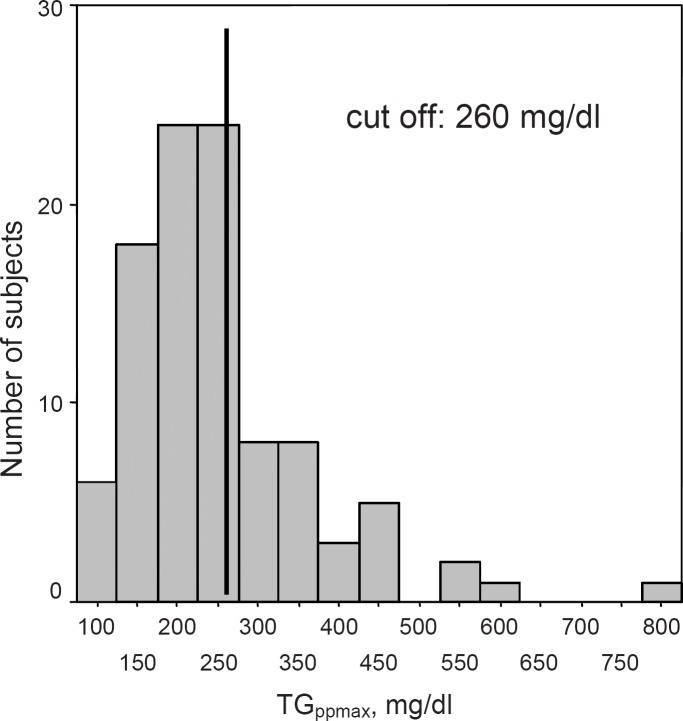
In 100 men (44–68 years) body composition (air-displacement plethysmography), dietary habits, cardiometabolic risk and pp lipid metabolism (standardised lipid load) were assessed. A pp TG peak value of 260 mg/dl was used as a cut-off to classify TG normal- and high-responders.
Results
pp TG response had positive associations with BMI (r = 0.24; p < 0.05), fat mass (r = 0.28; p < 0.01), waist circumference (r = 0.33; p < 0.01), systolic blood pressure (r = 0.21; p < 0.05), fasting (r = 0.29; p < 0.01) and pp glucose (r = 0.40; p < 0.001), fasting (r = 0.38; p < 0.001) and pp insulin levels (r = 0.46; p < 0.001), and inverse associations with HDL-C (r = –0.43; p < 0.001) and intake of dietary fibre (r = –0.31; p < 0.05). Fasting TG levels had a greater contribution to the variance in 12 of 14 cardiometabolic risk factors compared to pp TG response. TG-AUC was highly correlated to cardiometabolic risk.
Conclusion
Overweight, abdominal fat and a low intake of dietary fibre were determinants of increased pp TG response. Contrary to findings in younger normal-weight men, fasting TG levels had a stronger association with cardio metabolic risk compared to pp TG response. This might be explained by differences in fat mass.
Key Words: Postprandial triglyceride response, Postprandial triglyceride peak value, Triglyceride-area under curve, Fasting triglyceride levels, Cardiometabolic risk
Background and Aims
Obesity and its co-morbidities have become a major public health concern. Fasting triglyceride (TG) levels are positively associated with body weight and are considerable risk factors for atherosclerosis [1]. Because TG levels raise in response to dietary intake and TG-rich lipoproteins like VLDL, IDL and chylomicron remnants have a high atherogenic potential, it was proposed to assess postprandial (pp) TG levels after an oral lipid load in order to estimate cardiometabolic risk [2]. Fasting TG levels cannot precisely predict pp TG response (that is the response of TG levels to an oral lipid load and reflects the individual capacity to handle the fat intake) since some fasting normotriglyceridaemics display an exacerbated pp TG response [3]. However, a standardised and reproducible lipid load test as well as a consistent characterisation is so far lacking [4]. Pp lipaemia tests differ regarding types of test meals (e.g. milk shakes, fluids with defined ingredients, mixed meals), meal sizes and compositions (e.g. different fat contents, different amounts of other nutrients like carbohydrates, fatty acids, cholesterol and alcohol which can alter pp TG response) [4]. In addition, test meals with the same amount of nutrient/energy for every subject irrespective of body weight are used [5]. By contrast other test meals are referred to body surface area (BSA) [3] and body weight [6]. Characterisation of pp TG response is performed using pp TG peak values (TGppmax) [2] or TG-AUC (TG area under curve) which reflects occurrence, accumulation and elimination of TG in plasma [3].
Among others, pp TG response is determined by overweight, abdominal obesity [7–9] and nutrition. Pp TG response was found to be associated with cholesterol [10], energy intake [11, 12] as well as the intake of protein [13], fat, alcohol [14] dietary fibre [5] and n-3 fatty acids [6]. Furthermore, cardiometabolic risk factors such as elevated LDL-cholesterol [2], low HDL-cholesterol, elevated glucose and insulin levels [3] as well as a high blood pressure [8] are also related to pp TG response. Taken together, a comprehensive view of pp TG response compared with basal TG levels is missing.
To bring all these points together this study aims to investigate:
i) the influence of overweight, abdominal obesity, body composition and dietary habits on pp TG response,
ii) the importance of pp TG response when compared with fasting TG levels for assessing cardiometabolic risk,
iii) different possibilities to characterise pp TG response (TGppmax vs. TG-AUC).
To follow these questions we have used a standardised lipid load and followed a standardised test protocol.
Material and Methods
Study Design and Population
The Metabolic Intervention Cohort Kiel (MICK) is a prospective as well as an intervention cohort as part of the research network in Kiel (Germany) ‘Fat and Metabolism – Gene Variation, Gene Regulation and Gene Function’. 750 men aged 45 to 65 years were recruited through requisition of the local registration office. Exclusion criteria were endocrinological dysfunction, known disturbances in fat metabolism, diagnosed diabetes mellitus, impaired liver or kidney function, tumours and alcohol dependency. 100 men (60.3 ± 5.6 years) of Caucasian descent participated in this sub-cohort analysis and pp lipid metabolism, anthropometry, body composition and dietary habits as well as cardiovascular risk factors were assessed. The study was approved by the local ethic committee of the medical faculty of Christian-Albrechts-University in Kiel. Written informed consent was obtained from each subject. The study meets the standards of the Declaration of Helsinki in its revised version of 1975 and its amendments of 1983, 1989 and 1996.
Anthropometric Measurements and Body Composition Analysis
Body height was measured with a stadiometer to the nearest 0.5 cm (SECA, Modell 220; Vogel and Halke, Hamburg, Germany). Body weight was determined to the nearest 0.1 kg on an electronic scale coupled to the Bod Pod® system (Bod Pod®; Body Compostion System; Life Measurement Instruments, Concord, CA, USA), and BMI was calculated as ‘body weight (kg) / body height2 (m2)’. Waist circumference was measured midway between the lowest rib and the iliac crest while the subjects were at minimal respiration. Body composition (fat mass (FM) and fat free mass (FFM)) was assessed by air-displacement plethysmography (Bod Pod), which is described elsewhere [15]. Manufacturer’s software was used to calculate body density and % body fat using Siri’s equation [16]. FFM was calculated as ‘body weight (kg) – FM (kg)’.
Assessment of Energy and Nutrient Intake
Subjects were instructed to complete a 7-day dietary record to assess their usual dietary habits. These records were analysed for energy and nutrient intake using the software program Prodi® (PRODI 4.5 LE 2001 Expert; Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany). Underreporting (that is the incomplete declaration of ingested foods) was checked by calculating resting energy expenditure (REE) according to Müller et al. [17]. In 36 subjects REE multiplied with 1.2 showed a higher value than the declared energy intake. These dietary records were discarded because of underreporting. Underreporter (n = 36) were not older than normalreporter (n = 61) (59.9 ± 5.2 years vs. 60.6 ± 5.6 years; p > 0.05), but had a higher BMI (29.6 ± 4.4 kg/m2 vs. 26.5 ± 2.7 kg/m2; p < 0.001). Three dietary records were not send back, leaving 61 7-day dietary records for analysis.
Assessment of Cardiometabolic Risk Factors
Blood pressure was measured with a standard manual sphygmomanometer. After a minimum of 12 h fasting, a blood sample was taken for analysing total-, HDL- and LDL-cholesterol as well as TG levels enzymatically by Konelab 20i Analyzer (Konelab, Espoo, Finland). The intraassay CVs were 1.2% for total-cholesterol, 3.5% for HDL-cholesterol, 2.7% for LDL-cholesterol and 2.5% for triglycerides. Glucose levels were determined enzymatically with Konelabs testkit (Thermo Clinical Labsystems; intra-assay CV < 2.2%). Serum insulin levels were assessed by radioimmunassay (Biochem Immunosystems, Freiburg i. Br., Germany; testkit of Adaltis Dt. GmbH, Freiburg i. Br., Germany; CV < 5.4%). Excluding subjects with a fasting glucose level >7.0 mmol/l (n = 11), insulin resistance was calculated by HOMA-IR (homeostasis model assessment – insulin resistance) and β-cell function by HOMA-bcell (homeostasis model assessment – β-cell function) [18]:
Assessment of pp Lipid Metabolism
After a minimum of 12 h fasting, oral metabolic tolerance test (OMTT) was performed to assess pp lipid metabolism. A catheter was placed into an anticubital vein to allow frequent blood sampling: fasting, 0.5 h, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h and 9 h after ingestion of 500 ml of a fluid test meal, which contained the following ingredients: 4,221 kJ energy, 30 g of protein (11.9% of energy), 75 g of carbohydrate (29.6% of energy; 93% saccharose and 7% lactose), 58 g of fat (51.6% of energy; 65% saturated and 35% unsaturated fatty acids), 10 g of alcohol (6.9% of energy), 600 mg cholesterol and 30,000 IU retinylpalmitate. During examination, subjects were allowed to drink water; food intake or smoking were not allowed. Physical activity was standardised. Pp TG, glucose and insulin levels were used to calculate the AUC.
Characterisation of pp TG Response
TGppmax was used to characterise pp TG response. Applying a cut-off of 260 mg/dl [2], 64 subjects were divided into TG normal-responders (TGppmax < 260 mg/dl) and 36 subjects into TG high-responders (TGppmax ≥ 260 mg/dl) (fig. 1).
Fig. 1.
Frequency distribution of subjects (n = 100) according to TGppmax.
Taking into account differences in the volume of distribution, data were adjusted for ‘ingested fat / FFM (kg)’. FFM was assumed as volume of distribution of ingested fat because TGppmax was reached after 3.4 ± 1.5 h and fat is stored in adipose tissue with temporal delay [19]. It has been shown that after 4 h only one quarter of ingested fat is incorporated into fat mass [20]. Alternative adjustments of TGppmax for ‘ingested fat / weight (kg)’ and ‘ingested fat / BSA (cm2)’ were performed in order to compare different ways to characterise pp TG response. TG-AUC (adjusted for ‘ingested fat / weight (kg)’) was also considered as a parameter of pp TG response. Over a period of 9 h, which reflects TG-AUC, body weight (FM and FFM) was assumed as the volume of distribution of ingested fat.
Statistics
All analyses were performed using SPSS for Windows (Statistical Package for the Social Sciences, version 13.0). For normally distributed parameters, descriptive statistics were given as mean ± standard deviation or standard error (SE) and otherwise (for alcohol intake, fasting TG levels, TGppmax, TG-AUC, fasting insulin levels, insulin-AUC, HOMA-IR and HOMA-bcell) as mean (95% confidence interval).
TG-, glucose- and insulin-AUC were calculated by trapezoidal rule. Analysis of covariance (ANCOVA (Bonferroni’s post-hoc test)) was performed to compare TG normal- and high-responders. Relationships between two variables were tested by Pearson’s correlation coefficient. Partial correlations were used to adjust for covariates. Multiple stepwise regression analysis was used to describe the relationship between one dependent and multiple independent variables. Parameters which showed no normal distribution were log-transformed for correlation and regression analysis. Chi-square test was applied to analyse differences in the frequency distribution of categorical variables. All tests were two-sided with a probability of 5%.
Results
The study population consisted of 100 men aged between 44 and 68 years (60.3 5.6 years), who were on average overweight (BMI 27.7 �} 3.8 kg/m2). 24% were normal weight (BMI 20.24.9 kg/m2), 57% overweight (BMI 25.29.9 kg/m2) and 19% obese (BMI . 30 kg/m2). The subjects had a mean body fat of 28.4 �} 7.2%, a FFM of 61.9 �} 8.0 kg and a waist circumference of 100.6 �} 11.5 cm.
Influence of Overweight, Abdominal Obesity and Body Composition on pp TG Response
After adjusting for �eingested fat / FFM (kg)�f, TG high-responders had a higher%body fat and a higher waist circumference when compared with TG normal-responders. Age, height, body weight, BMI and FFM showed no differences between the groups (table 1). BMI, % body fat and waist circumference were positively associated with pp TG peak values after adjusting for �eingested fat / FFM (kg)�f (table 2).
Influence of Dietary Habits on pp TG Response
Comparison of energy and nutrient intake between TG normal- and high-responders is shown in table 3. After adjusting for �eingested fat / FFM (kg)�f TG high-responders had a higher intake of polyunsaturated fatty acids (PUFA, g/day) when compared with TG normal-responders. Furthermore, the intake of dietary fibre was inversely correlated with pp TG peak values after adjusting for �eingested fat / FFM (kg)�f (r = .0.31; p < 0.05).
Comparison of pp and Fasting TG Levels for Assessment of Cardiometabolic Risk
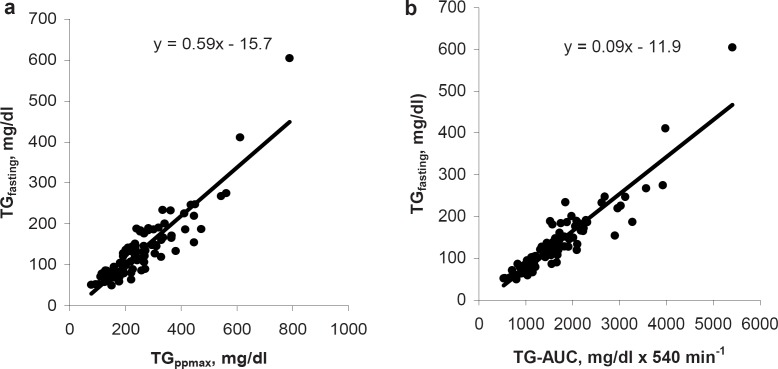
Fasting TG levels were highly correlated with both parameters of pp TG response: TGppmax (r = 0.89; p < 0.001) and TG-AUC (r = 0.93; p < 0.001) (fig. 2).
Comparison of cardiometabolic risk between TG normal- and high-responders is shown in table 4. When compared with TG normal-responders, high-responders showed higher fasting TG, total- and LDL-cholesterol levels as well as a higher glucose- AUC, insulin-AUC, HOMA-IR and systolic blood pressure after adjusting for �eingested fat / FFM (kg)�f. Furthermore, TG high-responders had lower HDL-cholesterol levels and a lower HOMA-bcell index when compared with TG normalresponders.
The prevalence of the metabolic syndrome (according to NCEP-ATPIII criteria [21]) was significantly higher in TG high- vs. normal-responders (41.7 vs. 15.6%; p < 0.01).
After adjusting for �eingested fat / FFM (kg)�f and fasting TG levels, only LDL-cholesterol levels remained significantly different between TG normal- and high-responders (TG normal-responders 128.9 �} 4.0 mg/dl vs. high-responders 151.5 �} 5.7 mg/dl; p < 0.01).
After adjusting for �eingested fat / FFM (kg)�f, pp TG peak values were positively associated with fasting TG and glucose levels, glucose-AUC, fasting insulin levels, insulin-AUC, HOMA-IR as well as systolic blood pressure and inversely associated with HDL-cholesterol as well as HOMA-bcell index (table 2).
Using a multiple stepwise regression analysis with cardiometabolic risk factors as dependent variables and fasting TG levels, pp TG peak values, �eingested fat / FFM (kg)�f and TG-AUC as independent variables, fasting TG levels explained the highest proportion of variance in 12 of 14 examined cardiometabolic risk factors (BMI, waist circumference, % body fat, systolic blood pressure, total cholesterol, HDL-cholesterol, fasting glucose levels, glucose-AUC, log HOMA-IR, log HOMA-bcell, log insulin, log insulin-AUC).
Characterisation of pp TG Response
The association between cardiometabolic risk factors and different opportunities to normalise pp TG response is shown in table 2. TG-AUC (adjusted for �eingested fat / weight (kg)�f) showed the highest correlation coefficients in 10 of 15 examined cardiometabolic risk factors. Comparing different ways to normalise pp TG peak values (adjustments for �eingested fat/ FFM (kg)’, ‘ingested fat / weight (kg)’ and ‘ingested fat / BSA (cm2)’), pp TG peak values adjusted for ‘ingested fat / FFM (kg)’ showed the highest correlation coefficients in 10 of 15 examined cardiometabolic risk factors.
Discussion
Influence of Overweight, Abdominal Obesity and Body Composition on pp TG Response
In agreement with previous studies, BMI and % body fat were positively associated with pp TG response (table 2). Lewis et al. [7] found a higher pp TG response in overweight when compared with normal-weight subjects. This positive association between BMI as well as FM and pp TG response could be due to a hypercaloric diet inducing a higher synthesis of TGs in the liver. Other studies focused on the importance of abdominal FM regarding pp TG response [22, 23]. Waist circumference was significantly higher in TG high- than in TG normal-responders (table 1) and was positively associated with pp TG response (table 2). This might be explained by insulin resistance, higher lipolysis in visceral FM, increased influx of free fatty acids via portal vein to the liver and an overproduction of TG-rich VLDL in abdominal/visceral obesity.
Influence of Dietary Habits on pp TG Response
In agreement with other studies, the intake of dietary fibre was inversely correlated to pp TG response [5, 24]. This might be due to a decreased absorption of fat, an increased clearance of chylomicrons, an elevated absorption of chylomicron remnants or a decreased secretion of VLDL by the liver as well as a higher catabolism of VLDL induced by dietary fibre [5].
TG high-responders had a higher intake of PUFA when compared with normal-responders (table 3). Unfortunately a differentiation of PUFA intake into n-3 fatty acids was impossible from our data. Contrary to our results, other authors found an inverse relationship between the intake of n-3 fatty acids (contained e.g. in fish oils) and pp TG response [6, 12, 25]. This might be due to the inhibition of hepatic VLDL TG synthesis and to the improvement of VLDL catabolism by n-3 fatty acids [26].
Other studies found more associations between dietary habits and pp TG response: an inverse relationship between energy intake and pp TG response [12] as well as positive associations between pp TG response and the intake of protein [13], fat, carbohydrates [27] and alcohol [14] as well as cholesterol and SFA intake [10]. These associations could potentially not be obtained in this study because of 37% underreporting.
Comparison of pp and Fasting TG Levels for Assessment of Cardiometabolic Risk
Fasting TG levels were positively associated with TGppmax and TG-AUC (fig. 2). Considering this, most of the subjects with an elevated pp TG response also had elevated fasting TG levels and vice versa. However, an elevated fasting TG level did not necessarily predict an elevated pp TG response. Verifying this, 4% of the subjects only had elevated fasting TG levels, and 13% only had elevated pp TG levels.
Fig. 2.
Association between fasting triglyceride levels (TGfasting) and a TGppmax as well as b TG-AUC in 100 men.
In agreement with previous studies, pp TG response was associated with a higher prevalence of the metabolic syndrome and elevated cardiometabolic risk factors (table 2). TG high-responders had higher LDL-cholesterol levels when compared with TG normal-responders (table 4; see also [8]). HDL-cholesterol levels were inversely associated with pp TG response [table 2; see also [28]), whereas fasting glucose and insulin levels, HOMA-IR as well as glucose- and insulin-AUC showed a positive association with pp TG response (table 2; see also [22]). A higher systolic blood pressure was also related to a higher pp TG response (tables 2, 4; see also [29]).
After adjusting for ‘ingested fat / FFM (kg)’ and fasting TG levels, the differences in cardiometabolic risk factors between TG normal- and high-responders disappeared; only fasting LDL-cholesterol levels remained higher in TG high-responders when compared with normal-responders. Therefore, fasting TG levels had a higher impact to assess cardiometabolic risk than pp TG response. Verifying this, multiple stepwise regression analysis showed a higher explanation of variance in 12 cardiometabolic risk factors by fasting TG levels when compared with pp TG peak values and TG-AUC. This result is contrary to examinations in younger normal-weight men [2] and might be explained by differences in FM.
To get further insight, a longitudinal assessment of subjects with elevated pp TG levels with regard to the incidence of the metabolic syndrome is necessary. Furthermore, a discrimination of plasma TGs could be of interest. In a study by Carstensen et al. [30], type 2 diabetic males with prior myocardial infarction had higher responses of plasma TGs and retinyl-palmitate-labeled lipoproteins of intestinal origin when compared with a matched group without prior myocardial infarction. However, further analyses of the lipid fractions are impossible in our study, the data are not available.
Another interesting topic is the assessment of the genetic background. As our study is a sub-cohort analysis of the MICK, such analyses have already been published. For example, the study by Rubin et al. [31] dealt with the common functional exon polymorphism in the microsomal TG transfer protein gene, which was associated with type 2 diabetes, impaired glucose metabolism and insulin levels.
Characterisation of pp TG Response
Adjusting for ‘ingested fat / FFM (kg)’, pp TG peak values showed the highest correlations with 10 of 15 cardiometabolic risk factors when compared with the other possibilities of normalisation (‘ingested fat / weight (kg)’ as well as ‘ingested fat / BSA (cm2)’) (table 2). Other arguments in favour of adjusting pp TG peak values for ‘ingested fat / FFM (kg)’ were:
i) Characterisation of pp TG response on the basis of TGppmax could lead to misinterpretation because the volume of distribution of ingested fat is not considered. A higher pp TG response would be expected in subjects with a low volume of distribution.
ii) Adjusting TGppmax for ‘ingested fat / weight (kg)’ might be inappropriate because weight is not the initial volume of distribution of ingested fat [19, 20].
iii) BSA is related to metabolic mass, so it is an indirect parameter of FFM. BSA can be calculated by body height and weight, but in an individual case this is imprecise.
TG-AUC (adjusted for ‘ingested fat / weight (kg)’) had the highest correlations with 10 of 15 cardiometabolic risk factors compared with the other parameters of pp TG response (table 2). Therefore, TG-AUC might be a more precise parameter to characterise pp TG response when compared with TGppmax. In contrast to TGppmax TG-AUC does not only reflect the rise/absorption and accumulation of TGs but also the elimination of TGs in plasma.
Conclusion
Elevated BMI, waist circumference as well as % body fat and a low intake of dietary fibre are associated with a higher pp TG response. When compared with pp TG peak values, TG-AUC is preferred for cardiometabolic risk assessment. There is a positive association between pp TG response and cardiometabolic risk. In contrast to studies in younger normal-weight subjects, fasting TG levels exceeded pp TG response in cardiometabolic risk assessment.
Table 1.
Comparison of age, body composition and anthropometric variables between TG normal- (TGppmax < 260 mg/dl) and high-responders (TGppmax ≥ 260 mg/dl) (adjusted for ‘ingested fat / FFM (kg)’; means ± SE)
| Normal-responders (n = 64) | High-responders (n = 36) | |
|---|---|---|
| Age, years | 60.2 ± 0.7 | 60.5 ± 0.9 |
| Weight, kg | 85.9 ± 1.2 | 89.6 ± 1.6 |
| Height, m | 1.77 ± 0.01 | 1.78 ± 0.01 |
| BMI, kg/m2 | 27.3 ± 0.4 | 28.4 ± 0.6 |
| Body fat, % | 27.2 ± 0.9 | 30.6 ± 1.2* |
| FFM, kg | 61.9 ± 0.2 | 61.8 ± 0.3 |
| Waist circumference, cm | 99.1 ± 1.2 | 103.4 ± 1.7* |
p < 0.05; difference between TG normal- and high-responders; ANCOVA.
Table 2.
Correlations between cardiometabolic risk factors and log TGppmax and log TG-AUC’ in 100 men and accordingly 89 men for HOMA-IR and HOMA-bcell
| log TGppmaxa | log TGppmax adjusted for ‘ingested fat/FFM (kg)’ b | log TGppmax adjusted for ‘ingested fat/weight (kg)’c | log TGppmax adjusted for ‘ingested fat/BSA(cm2)’d | log TG-AUC adjusted for ‘ingested fat/weight (kg)’ c | |
|---|---|---|---|---|---|
| BMI, kg/m2 | 0.15 | 0.24* | 0.16 | 0.19 | 0.26* |
| Body fat, % | 0.29** | 0.28** | 0.30** | 0.31** | 0.35*** |
| Waist circumference, cm | 0.22* | 0.33** | 0.31** | 0.32** | 0.37*** |
| log TG, mg/dl | 0.89*** | 0.89*** | 0.89*** | 0.89*** | 0.93*** |
| Cholesterol, mg/dl | 0.20* | 0.18 | 0.21* | 0.21* | 0.22* |
| LDL-cholesterol, mg/dl | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| HDL-cholesterol, mg/dl | −0.42*** | −0.43*** | −0.41*** | −0.42*** | −0.46*** |
| Glucose, mg/dl | 0.28** | 0.29** | 0.27** | 0.28** | 0.30** |
| Glucose-AUC, mg/dl × 540 min−1 | 0.40*** | 0.40*** | 0.39*** | 0.40*** | 0.45*** |
| log Insulin, µU/ml | 0.32** | 0.38*** | 0.32** | 0.35*** | 0.33** |
| log Insulin-AUC, µU/ml × 540 min−1 | 0.43*** | 0.46*** | 0.44*** | 0.45*** | 0.48*** |
| log HOMA-IR, mmol/l × µU/ml | 0.24* | 0.29** | 0.27* | 0.27* | 0.34** |
| log HOMA-bcell, µU/ml / mmol/l | −0.24* | −0.30** | −0.27* | −0.28** | −0.30** |
| RRsys, mm Hg | 0.17 | 0.21* | 0.16 | 0.18 | 0.18 |
| RRdias, mm Hg | 0.09 | 0.12 | 0.07 | 0.09 | 0.06 |
RRdias = diastolic blood pressure; RRsys = systolic blood pressure.
p < 0.05;
p < 0.01;
p < 0.001.
Pearson’s correlation coefficient.
Partial correlation (adjusted for ‘ingested fat / FFM (kg)’).
Partial correlation (adjusted for ‘ingested fat / weight (kg)’).
Partial correlation (adjusted for ‘ingested fat / BSA (cm2)’).
Table 3.
Comparison of energy and nutrient intake between TG normal- (TGppmax < 260 mg/dl) and high-responders (TGppmax ≥ 260 mg/dl) (adjusted for ‘ingested fat / FFM (kg)’; means ± SE or means (95% confidence interval))
| Normal-responders (n = 40) | High-responders (n = 21) | |
|---|---|---|
| Energy intake, kcal/day | 2468 ± 51.9 | 2573 ± 71.7 |
| Protein intake | ||
| g/day | 88.5 ± 3.0 | 96.3 ± 4.1 |
| % energy intake | 14.6 ± 0.4 | 15.2 ± 0.6 |
| Fat intake | ||
| g/day | 100.4 ± 3.3 | 107.5 ± 4.5 |
| % energy intake | 36.4 ± 0.8 | 37.6 ± 1.1 |
| Carbohydrate intake | ||
| g/day | 267.8 ± 7.6 | 267.2 ± 10.6 |
| % energy intake | 44.2 ± 0.9 | 42.4 ± 1.3 |
| Alcohol intake | ||
| g/day | 15.8 (10.9–20.7) | 17.7 (10.7–24.8) |
| % energy intake | 4.8 (3.4–6.2) | 4.8 (2.8–6.8) |
| Cholesterol intake, mg/day | 346.1 ± 18.8 | 372.5 ± 25.9 |
| SFA | ||
| g/day | 37.8 ± 1.7 | 38.5 ± 2.3 |
| % energy intake | 14.0 ± 0.5 | 13.8 ± 0.7 |
| MUFA | ||
| g/day | 30.9 ± 1.4 | 34.0 ± 1.9 |
| % energy intake | 11.5 ± 0.4 | 12.2 ± 0.6 |
| PUFA | ||
| g/day | 12.1 ± 0.7 | 14.8 ± 0.9* |
| % energy intake | 4.5 ± 0.2 | 5.2 ± 0.3 |
| Dietary fibre, g/day | 26.5 ± 1.3 | 23.8 ± 1.7 |
MUFA = Monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; SFA = saturated fatty acids.
p < 0.05; differences between TG normal- and high-responders; ANCOVA.
Table 4.
Comparison of cardiometabolic risk factors between TG normal- (TGppmax < 260 mg/dl) and high-responders (TGppmax ≥ 260 mg/dl) (adjusted for ‘ingested fat / FFM (kg)’; means ± SE or means (95% confidence interval))
| Normal-responders (n = 64) | High-responders (n = 36) | |
|---|---|---|
| Triglycerides, mg/dl | 98.0 (82.8–113.2) | 192.0 (171.6–212.3)*** |
| Cholesterol, mg/dl | 213.1 ± 5.3 | 232.0 ± 7.0* |
| LDL-cholesterol, mg/dl | 130.8 ± 3.7 | 148.2 ± 4.9** |
| HDL-cholesterol, mg/dl | 56.6 ± 1.7 | 47.6 ± 2.2** |
| Glucose, mg/dl | 101.2 ± 1.9 | 106.1 ± 2.5 |
| Glucose-AUC, mg/dl × 540 min−1 | 498.2 ± 10.9 | 558.1 ± 14.6** |
| Insulin, µU/ml | 14.3 (12.1–16.4) | 16.2 (13.2–19.2) |
| Insulin-AUC, µU/ml × 540 min−1 | 181.0 (147.5–214.4) | 249.6 (205.0–294.2)* |
| HOMA-IR, mmol/l × µU/mla | 3.0 (2.5–35) | 4.0 (3.3–4.7)* |
| HOMA-bcell, µU/ml / mmol/l | 8.2 (6.9–9.6) | 5.0 (3.1–6.9)** |
| RRsys, mm Hg | 126.0 ± 2.1 | 134.1 ± 2.8* |
| RRdias, mm Hg | 79.3 ± 1.4 | 81.9 ± 1.8 |
RRdias = diastolic blood pressure; RRsys = systolic blood pressure.
p < 0.05;
p < 0.01;
p < 0.001; differences between TG normal- and high-responders; ANCOVA.
Comparison between 59 TG normal- and 30 high-responders.
References
- 1.Durrington PN. Triglycerides are more important in atherosclerosis than epidemiology has suggested. Atherosclerosis. 1998;141((suppl 1)):57–62. doi: 10.1016/s0021-9150(98)00219-6. [DOI] [PubMed] [Google Scholar]
- 2.Schrezenmeir J, Keppler I, Fenselau S, Weber P, Biesalski HK, Probst R, Laue C, Zuchhold HD, Prellwitz W. Beyer J: The phenomenon of a high triglyceride response to an oral lipid load in healthy subjects and its link to the metabolic syndrome. Ann N Y Acad Sci. 1993;683:302–314. doi: 10.1111/j.1749-6632.1993.tb35721.x. [DOI] [PubMed] [Google Scholar]
- 3.Couillard C, Bergeron N, Prud’homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP. Postprandial triglyceride response in visceral obesity in men. Diabetes. 1998;47:953–960. doi: 10.2337/diabetes.47.6.953. [DOI] [PubMed] [Google Scholar]
- 4.Lairon D, Lopez-Miranda J. Williams C: Methodology for studying postprandial lipid metabolism. Eur J Clin Nutr. 2007;61:1145–1161. doi: 10.1038/sj.ejcn.1602749. [DOI] [PubMed] [Google Scholar]
- 5.Cara L, Dubois C, Borel P, Armand M, Senft M, Portugal H, Pauli A, Bernard P. Lairon D: Effects of oat bran, rice bran, wheat fiber and wheat germ on postprandial lipemia in healthy adults. Am J Clin Nutr. 1992;55:81–88. doi: 10.1093/ajcn/55.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Ågren J, Hänninen O, Julkunen A, Fogelholm L, Vidgren H, Schwab U, Pynnönen O. Uusitupa M: Fish diet, fish oil and docosahexaenoic acid rich oil lower fasting and postprandial plasma lipid levels. Eur J Clin Nutr. 1996;50:765–771. [PubMed] [Google Scholar]
- 7.Lewis GF, O’Meara NM, Soltys PA, Blackman JD, Iverius PH, Druetzler AF, Getz GS, Polonsky KS. Postprandial lipoprotein metabolism in normal and obese subjects: comparison after the vitamin A fatloading test. J Clin Endocrinol Metabol. 1990;71:1041–1050. doi: 10.1210/jcem-71-4-1041. [DOI] [PubMed] [Google Scholar]
- 8.Kolovou GD, Anagnostopoulou KK, Pavlidis AN, Salpea KD, Iraklianou SA, Tsarpalis K, Damaskos DS, Manolis A, Cokkinos DV. Postprandial lipemia in men with metabolic syndrome, hypertensives and healthy subjects. Lipids Health Dis. Lipids Health Dis;2005:4. doi: 10.1186/1476-511X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couillard C, Bergeron N, Prud’homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP. Gender difference in postprandial lipemia, importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 19:2448–2455. doi: 10.1161/01.atv.19.10.2448. [DOI] [PubMed] [Google Scholar]
- 10.Tremblay AJ, Després JP, Piché ME, Nadeau A, Bergeron J, Alméras N, Tremblay A. Lemieux S: Associations between the fatty acid content of triglyceride, visceral adipose tissue accumulation, and components of the insulin resistance syndrome. Metabolism. 2004;53:310–317. doi: 10.1016/j.metabol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Datillo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 12.Volek JS, Gómez AL, Kraemer MS, Kraemer WJ. Fasting lipoprotein and postprandial triacylglycerol responses to a low-carbohydrate diet supplemented with n-3 fatty acids. J Am Coll Nutr. 2000;19:383–391. doi: 10.1080/07315724.2000.10718935. [DOI] [PubMed] [Google Scholar]
- 13.Van Wijk J, Cabezas MZ, Halkes C, Erkelens DW. Effects of different nutrient intakes on daytime triacylglycerolemia in healthy, normolipemic, free-living men. Am J Clin Nutr. 2001;74:171–178. doi: 10.1093/ajcn/74.2.171. [DOI] [PubMed] [Google Scholar]
- 14.Brewster AC, Lankford HG, Schwartz MG, Sullivan JF. Ethanol and alimentary lipemia. Am J Clin Nutr. 1966;19:255–259. doi: 10.1093/ajcn/19.4.255. [DOI] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A, Danielzik S, Becker C, Geisler C, Onur S, Korth O, Bührens F, Müller MJ. Need for optimal body composition data analysis using air-displacement plethysmography in children and adolescents. J Nutr. 2005;135:2257–2262. doi: 10.1093/jn/135.9.2257. [DOI] [PubMed] [Google Scholar]
- 16.Siri WE, Body composition from fluid spaces and density analysis of methods . Techniques for Measuring Body Composition. In: Brozek JHenschel., editor. National Academy of Sciences. Washington DC: 1961. pp. pp 223–244. [Google Scholar]
- 17.Müller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Lührmann PM, Neuhäuser-Berthold M, Noack R, Pirke KM, Platte P, Selberg O. Steiniger J: World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80:1379–1390. doi: 10.1093/ajcn/80.5.1379. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Summers L, Barnes SC, Fielding BA, Beysen C, Ilic V, Humphreys SM, Frayn KN. Uptake of individual fatty acids into adipose tissue in relation to their presence in the diet. Am J Clin Nutr. 2000;71:1470–1477. doi: 10.1093/ajcn/71.6.1470. [DOI] [PubMed] [Google Scholar]
- 20.Marin P, Rebuffe-Scrive M. Bjorntorp P: Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest. 1990;20:158–165. doi: 10.1111/j.1365-2362.1990.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 22.Schrezenmeir J, Fenselau S, Keppler I, Abel J, Orth B, Laue C, Stürmer W, Fauth U, Halmagyi M. März W: Postprandial triglyceride high response and the metabolic syndrome. Ann N Y Acad Sci. 1997;827:353–368. doi: 10.1111/j.1749-6632.1997.tb51847.x. [DOI] [PubMed] [Google Scholar]
- 23.Mamo JCL, Watts GF, Barrett HR, Smith D. James AP, Pal S: Postprandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression? Am J Physiol Endocrinol Metabol. 2001;281:626–632. doi: 10.1152/ajpendo.2001.281.3.E626. [DOI] [PubMed] [Google Scholar]
- 24.Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault M. Dietary fibre intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82:1185–1194. doi: 10.1093/ajcn/82.6.1185. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988;29:1451–1460. [PubMed] [Google Scholar]
- 26.Stanner S (ed):The Report of the British Nutrition Task Force chaired by Frayn KN. Cardiovascular Disease, Diet, Nutrition and Emerging Risk Factors. Oxford, Blackwell. 2005 [Google Scholar]
- 27.Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr. 2001;73:892–899. doi: 10.1093/ajcn/73.5.892. [DOI] [PubMed] [Google Scholar]
- 28.Kolovou GD, Anagnostopoulou KK, Pilatis N, Kalfaltis N, Sarodila K, Psarros E, Cokkinos DV. Low fasting low high-density lipoprotein and postprandial lipemia. Lipids Health Dis. Lipids Health Dis;2004:3. doi: 10.1186/1476-511X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolovou GD, Daskalova DC, Iraklianou SA, Adamopoulou EN, Pilatis ND, Hatzigeorgiou GC, Cokkinos DV. Postprandial lipemia in hypertension. J Am Coll Nutr. 2003;22:80–87. doi: 10.1080/07315724.2003.10719279. [DOI] [PubMed] [Google Scholar]
- 30.Carstensen M, Thomsen C, Gotzsche O, Holst JJ, Schrezenmeir J, Hermansen K. Differential postprandial lipoprotein responses in type 2 diabetic men with and without clinical evidence of a former myocardial infraction. Rev Diabet Stud. 2004;1:175–184. doi: 10.1900/RDS.2004.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin D, Helwig U, Pfeuffer M, Schreiber S, Boeing H, Fisher E, Pfeiffer E, Freitag-Wolf S, Foelsch U, Doering F, Schrezenmeir J. A common functional exon polymorphism in the microsomal triglyceride transfer protein gene is associated with type 2 diabetes, impaired glucose metabolism and insulin levels. J Hum Genet. 2006;51:567–574. doi: 10.1007/s10038-006-0400-y. [DOI] [PubMed] [Google Scholar]