Abstract
Background
Melanocortin 3 and 4 receptors (MC3R and MC4R) are known to play an essential role in hypothalamic weight regulation. In addition to these two G-protein-coupled receptors (GPCRs), a huge number of other GPCRs are expressed in hypothalamic regions, and some of them are involved in weight regulation. So far, homodimerization was shown for a few of these receptors. Heterodimerization of unrelated receptors may have profound functional consequence but heterodimerization of GPCRs involved in weight regulation was not reported yet.
Methods
A selective number of hypothalamically expressed GPCRs were cloned into a eukaryotic expression vector. Cell surface expression was demonstrated by an ELISA approach. Subcellular distribution was investigated by confocal laser microscopy. A sandwich ELISA and fluorescence resonance energy transfer (FRET) were used to determine protein-protein interaction.
Results
Via sandwich ELISA and FRET approach we could demonstrate a robust interaction of the MC4R with GPR7, both of which are expressed in the hypothalamic nucleus paraventricularis. Moreover, we determined a strong interaction of MC3R with the growth hormone secretagogue receptor expressed in the nucleus arcuatus.
Conclusion
Identification GPCR heterodimerization adds to the understanding of the complexity of weight regulation and may provide important information to develop therapeutic strategies to treat obesity.
Key Words: Dimerization, Weight regulation, Arcuate nucleus, GPCR, Hypothalamus, Paraventricular nucleus
Introduction
Oligomerization or dimerization as the smallest interaction unit of G-protein-coupled receptors (GPCRs) is now an accepted structural and functional feature of the largest class of integral membrane receptors. Since the first reports on heterodimerization of GABA B receptors and its importance for GABA B receptor function [1–3] were published, a huge number of GPCRs was reported to form or function as homo- or heterodimer [4–6].
The pharmaceutical industry develops drugs that modulate GPCR function since GPCRs are involved in nearly every physiological process [5,7]. Homo- or heterodimerization could influence trafficking to the cell surface, ligand binding, G-protein coupling, and agonist-induced internalization [8]. Knowledge of the existence of tissue-specific and functionally relevant GPCR dimers is of interest when targeting a GPCR as treatment option.
The new obesity epidemic [9] is an increasing health problem worldwide. Lifestyle changes and currently available drugs on the market have only little effects on weight reduction and maintenance of a reduced body weight. Therefore, treatment options are needed that specifically interfere with the disturbed balance of food intake and energy expenditure.
The leptin-melanocortin pathway is the most important pathway in the regulation of body weight [10]. Leptin represses the expression of orexigenic neuropeptides (agouti-related protein and neuropeptide Y) and enhances the expression of anorexigenic peptides proopiomelanocortin (POMC) in first-order neurons of the hypothalamic arcuate nucleus (ARC). POMC is processed to the peptides α- and β-MSH, which in turn modulate second-order neurons in the paraventricular nucleus (PVN) by activating the melanocortin 4 receptor (MC4R), thereby influencing food intake and energy expenditure. In the ARC α-, β- as well as γ-MSH activate the melanocortin 3 receptor MC3R) on first-order neurons establishing an inhibitory feedback loop [11]. Intracerebroventicular injection of highly potent MC4R agonists based structurally on α-MSH leads to a reduction of food intake in mice [12]. However, long-term treatment with the MC4R agonist leads to erections in mice because MC4R is also expressed in penis tissue [13]. Activation of the MC4R by artificial ligands is now investigated as a treatment option for sexual dysfunction [14] but makes it unfavorable for obesity treatment, especially in children. Within the leptin-melanocortin pathway a number of GPCRs are expressed that are known to be involved in weight regulation.
MC4R mutations represent the most frequent monogenic cause of obesity to date [15,16]. Mutations in the MC3R are a rare cause of obesity [17,18]. For two mutations of the MC4R a dominant-negative effect was shown, leading to the identification of MC4R dimerization [19–21]. Besides the MC4R only homooligomerization of MC3R [22] was reported as well as heterodimerization of MC1R and MC3R which are co-expressed on human monocytes [22].
Up to now, there is a lack of information on heterodimerization of MC4R which is mainly expressed in the hypothalamic PVN and of MC3R which is expressed in the ARC. To develop efficient treatment options that specifically interfere with disturbed hypothalamic weight regulation investigation of the dimerization partner of GPCR involved in energy homeostasis is one important prerequisite.
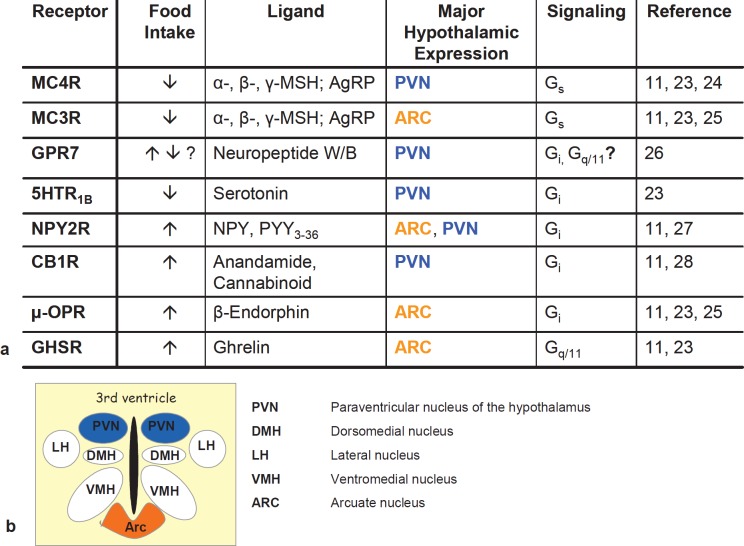
The brain and especially areas of the hypothalamus involved in energy homeostasis like ARC and PVN are the regions with the highest expression of GPCRs [11]. In addition to melanocortin receptors a total of more than 40 GPCRs [23] expressed in hypothalamic nuclei are involved in energy homeostasis. For most of these receptors, their role in energy homeostasis is not fully understood. In this study we investigated the interaction of MC4R or MC3R with other GPCRs potentially expressed in the PVN or ARC. We chose GPCRs that have an overlapping expression pattern like MC4R or MC3R and are involved in weight regulation as detected by knockout models or intracerebroventricular injection of specific ligands (fig. 1) [11, 23–28]. We tested heterooligomerization of MC4R with G-protein-coupled receptor 7 (GPR7), which was recently deorphanized to be the receptor for the neuropeptides W (NPW) and B (NPB), the cannabinoid 1 receptor (CB1R), and the serotonin 1B receptor (5HTR1B). Furthermore, we determined the potential protein-protein interaction of MC3R with the growth hormone secretagogue receptor (GHSR) which is the receptor for the stomach-derived satiety hormone ghrelin, the µ-opioid receptor (µ-OPR), and the neuropeptide Y2 receptor (NPY2R) (fig. 1).
Material and Methods
Cloning of Hypothalamically Expressed GPCRs
MC3R, MC4R, GPR7 and 5HTR1B were cloned from genomic DNA, CB1R was cloned from human brain cDNA. GHSR and µ-OPR cDNAs were purchased from UMR cDNA Resource Center, Rolla, MO, USA. For the cloning and aminoterminal HA tagging of all receptors, constructs were amplified by PCR using a tag- and restriction site-encoding sense primer and a restriction site-encoding antisense primer. All constructs were cloned into the eucaryotic expression vector pcDps via KpnI/SpeI or AatII/SpeI. For carboxyterminally FLAG tagging the melanocortin receptors, an epitope-coding antisense primer was used, and the construct was cloned into the pcDps using KpnI/SpeI sites. To fuse different fluorophores to the C-terminus of the receptors, PCR was performed based on the existing constructs. A restriction site-encoding sense primer and a stop codon-lacking antisense primer were used. MC3R, MC4R, GPR7, NPY2R, 5HTR1B and CB1R cDNAs were cloned 5’ into pECFP-N1 or pEYFP-N1 (Clontech, Palo Alto, CA, USA) preserving the reading frame, using EcoRI/KpnI sites. In the case of µ-OPR we used PstI/KpnI sites.
Cell Culture and Transfection
COS-7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Sigma, Deisenhausen, Germany) supplemented with 10% fetal calf serum (FCS) and 20 mmol/l glutamin. Cells were incubated at 37 °C in humidified air containing 5% CO2. Transfections were carried out using Metafectene™ (Biontex, Munich, Germany) according to the manufacturer's protocol. For the sandwich ELISA approach 1 × 106 cells were seeded in 6 cm dishes and transfected with a total of 3 µg DNA and 4 µl Metafectene. Cell surface expression assays were performed in 48 well plates (4 × 104 cells/well), and cells were transfected with 0.25 µg DNA/well and 1 µl Metafectene/well.
For investigation of receptor-receptor interaction in living cells by fluorescence resonance energy transfer (FRET) and for confocal laser scanning microscopy, HEK293 cells were grown on poly-L-lysine-treated cover slips and were transfected with 2 µg DNA and 4 µl FuGene6 (Roche, Grenzach-Whylen, Germany).
For ELISA approaches COS-7 cells were chosen due to their high adherence which is necessary to pass multiple wash steps. HEK293 cells were used for FRET experiments because their smaller size allowed measuring more cells in one field of view.
Investigation of Cell Surface Expression
To investigate cell surface expression of single or co-expressed GPCRs, these were N-terminally HA-tagged and tested in cell surface ELISA studies. 48 h after transfection (Metafectene) into COS-7 cells, a fixation with paraformaldehyde was performed before the extracellular HA epitope was probed with a biotin-labelled antibody (Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany). Bound anti-HA antibody was detected by peroxidase-labeled streptavidin (Dianova, Hamburg, Germany) in a substrate/chromogen reaction as described previously [29].
Determination of Subcellular Localization
HEK293 cells for imaging were cultured on glass cover slips, which were treated with 0.01 mg/ml poly-L-lysine (Biochrom AG, Berlin, Germany). For single and co-expression studies we used YFP- or CFP- and YFP-fused GPCR constructs. Cells were overlaid with Hepes-buffered solution (138 nmol/l NaCl, 6 mmol/l KCl, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 5.5 mmol/l glucose, 2 mg/ml bovine serum albumin, and 10 mmol/l Hepes, pH 7.5) and imaged by using a confocal laser scanning microscope (LSM 510 META; Carl Zeiss, Jena, Germany) with a Plan-Fluar 100x/1.45-objective. Microscopy pictures were generated using a 458 nm and a 488 nm laser for excitation and a LP505 emission filter.
Investigation of Protein-Protein Interaction: A Sandwich ELISA and a FRET Approach
For investigation of receptor dimerization we detected the constitutive dimers of an aminoterminal HA-tagged (N-HA) and carboxyterminal FLAG-tagged (C-FLAG) GPCR by an ELISA approach. Sandwich ELISAs were performed as previously described [19]. Briefly, COS-7 cells were co-transfected with differentially tagged GPCR constructs, solubilized overnight and incubated in FLAG antibody-coated 96-well plates. Dimerization was measured via detection of the N-terminally tagged HA epitope as increase in optical density.
To demonstrate the interaction of GPCR in living cells, we used FRET as previously described [19]. Briefly, C-terminally YFP-tagged MC3 or MC4 receptors were co-transfected with C-terminally CFP-fused GPCRs in HEK293 cells. 24 h later YFP was photobleached at 512 nm, and the increase of CFP-emission was measured at excitation at 410 nm. FRET efficiency was calculated as follows: E = (FCFPmax – FCFPmin)/FCFPmax[30]. Cells with a molar ratio (YFP:CFP) of less than 1 and greater than 4 or exhibiting unacceptable signal-to-noise ratios (linear regression analysis giving R2 values < 0.6) were omitted. Stabile dimerizations are defined by FRET efficiency between 8 and 25%.
Determination of Total Expression
To determine total expression of GPCRs, COS-7 cells were transfected with N-terminally HA and C-terminally FLAG double-tagged receptor constructs. An ELISA was performed as described for the sandwich ELISA.
Results
To investigate the interaction of MC4R in the PVN and of MC3R in the ARC with other GPCRs potentially expressed in the same nuclei, we chose GPCRs that have an overlapping expression pattern like MC4R or MC3R and are involved in weight regulation. We investigated the interaction of MC4R with GPR7, CB1R, and the 5HTR1B. Furthermore, we determined protein-protein interaction of MC3R with the GHSR, µ-OPR, and NPY2R (fig. 1).
Investigation of Cellular Localization and Cell Surface Expression
Subcellular localization was investigated by transient transfection of fluorescence-tagged receptors in HEK293 cells (fig. 2a). We determined considerable amounts of GHSR, µ-OPR, MC4R, 5HTR1B and CB1R in the endoplasmatic reticulum, whereas only a weak signal from plasma membrane was observed. In contrast, NPY2R expressed in HEK293 cells was localized predominantly at the plasma membrane. For GPR7 we observed an intracellular accumulation in vesicular structures.
For investigation of the cell surface expression all N-terminally HA-tagged receptors were transiently expressed into COS-7 cells, and a cell surface ELISA was performed. All investigated receptors displayed cell surface expression to different degrees (fig. 2b). The low amount of GPR7 on the cell surface expected from confocal images could be confirmed. Comparable to the positive control, the rat muscarinic receptor (rM3R), the expression of NPY2R, GHSR, and 5HTR1Bon the surface were 3- to 4-fold reduced. This reduction did not occur due to reduction of total receptor expression levels, which was equal for NPY2R, GHSR, 5HTR1B, and the melanocortin receptors (data not shown). However, MC3R, MC4R, µ-OPR, and CB1R are strongly expressed at the plasma membrane.
Determination of Protein-Protein Interaction
To identify the interaction partners of MC4R and MC3R, we applied a biochemical method which enables the detection of dimers by differential epitope tagging. Sandwich ELISA was performed in transiently co-transfected COS-7 cells. Moreover, to prove our results and to visualize the dimerization of GPCRs in living HEK293 cells, C-terminal fluorophore-tagged receptors were analyzed by FRET.
Using sandwich ELISA, we determined the interaction of MC4R with GPR7 and 5HTR1B which was comparable to the formation of MC4R homodimers (positive control). However, no interaction was found for the phylogenetically closely related CB1R (fig. 3b). For investigation of the interaction partner of MC3R, CB1R served as negative control. We demonstrated heterodimerization of MC3R with GHSR, NPY2R, and µ-OPR to nearly identical degrees as MC3R homodimer formation (fig. 3a).
To verify the results obtained by sandwich ELISA and to prove heterodimer formation in the cell membrane of intact HEK293 cells, we performed photobleaching FRET which showed a strong interaction of MC4R with GPR7, with a FRET efficiency of 19.6 ± 2.6%, thereby slightly exceeding the efficiency of the MC4R homodimer (17.3 ± 2.4%). Compared to the MC4R homodimer, the FRET efficiency of the 5HTR1B-MC4R heterodimer was significantly reduced to 9.3 ± 0.4%. The low FRET efficiency of 5.9 ± 1.2% (fig. 4b) confirmed the lack of interaction for MC4R and CB1R.
FRET showed a strong interaction of MC3R with GHSR, with an efficiency of 16.1 ± 0.9% which was within the range of the MC3R homodimer (15.2 ± 1.3%). Moreover, we found a significant reduction of FRET efficiencies for the interaction of MC3R with µ-OPR (9.7 ± 3.0%) and NPY2R (8.3 ± 1.4%) (fig. 4a)
Localization of Co-Expressed Receptors
Intracellular co-localization of expressed receptors is a necessity for interaction. Therefore, we tested co-localization of differentially fluorophore-tagged receptors after transient transfection into HEK293 cells by confocal laser microscopy. We found co-localization of all tested receptor pairs (fig. 5). Coexpression of MC4R and GPR7 resulted in a shift of GPR7 to the plasma membrane.
Discussion
The function of most of hypothalamically expressed GPCRs in energy homeostasis is not fully understood. For this reason some of these receptors were investigated in this study for their role in heterodimerization: GPR7, 5HTR1B, and CB1R for interaction with MC4R and µ-OPR, NPY2R, and GHSR for interaction with MC3R.
In this study we showed for the first time homo- and heterodimerization of GPCRs to be involved in hypothalamic weight regulation. We investigated heterodimer formation in HEK293 and COS-7 cells with two different methods. We demonstrated two different pairs of heterodimers, MC4R-GPR7 and MC3R-GHSR, which show robust dimer formation with both methods. The sandwich ELISA additionally demonstrated heterodimer formation for MC3R with µ-OPR and NPY2R. However, both of them display significantly reduced heterodimer formation compared with the FRET methods. Reasons for this could probably be methodical problems like sterical hindrance due to large CFP/YFP tags, which are excluded by use of smaller tags for sandwich ELISA approach. Also cell line differences which implicate different endogenously expressed chaperons and GPCRs are involved in interaction behavior. Another difference between both methods is that for the FRET experiments plasmids were transfected to express a stable acceptor:donor ratio of 1:1–3, whereas for the sandwich ELISA the plasmids were transfected in the same amount ignoring the expression levels of each receptor.
Interestingly, we found no interaction of MC4R with the phylogenetically closely related CB1R. However, also CB1R homodimer formation (data not shown) was not observed. This could be due to the usage of heterologous cell systems as homodimerization of CB1R was demonstrated via a dimer-specific antibody [31] in neuronal cells, indicating that additional proteins like the recently reported cannabinoid receptor interacting protein are involved in CB1R interaction [32]. Up to now, no heterologous CB1R homodimer formation could be shown using FRET, BRET, or immunoprecipitation. We used that lack of heterodimer formation of MC4R and CB1R as negative control for both cell systems and methods to ensure that the results that we obtained were not due to the used overexpression system.
Heterodimerization of GPCRs can result in a structural and functional unit that is completely different to homodimeric or monomeric GPCRs. Recently, it was shown that ghrelin is able to amplify signal transduction properties of the dopamine 1 receptor due to heterooligomerization of both receptors. Moreover, this heterodimerization results in a shift in G-protein coupling from Gq/11 to Gi for the GHSR [33]. In contrast the activation of one heterodimer partner can result in inactivation of the other as it was demonstrated for the interaction of the µ-OPR and the α2A adrenergic receptor [6].
Interaction of GPCRs was shown to influence cell surface expression. In this study we found no effect on cell surface expression after co-transfection of GHSR and MC3R. However, the long isoform of GHSR was shown to heterodimerize with the prostaglandin E2 receptor and the thromboxane A2 receptor, and this heterodimerization influences the constitutive activity of the GHSR and the cellular distribution [34]. Whereas GPR7 is only weakly expressed at the cell surface, major amounts of the receptors are trapped in the endoplasmatic reticulum (fig. 2), indicating that possibly an accessory protein is necessary for proper cell surface expression. Recently it was shown for CB1R that high constitutive activity is responsible for receptor internalization [35]. So far signal transduction properties of the GPR7 are uncertain. Therefore, also high constitutive activity of the receptor may be responsible for constitutive endocytosis. However, co-expression of GPR7 with MC4R resulted in significant overlap at the plasma membrane and strong co-localization on internal membrane structures as well. Thus, maybe trafficking and cell surface localization of GPR7 are regulated by interaction with MC4R.
The identification of MC4R and MC3R interaction with other GPCRs that are also involved in weight regulation (GPR7 for MC4R and GHSR for MC3R) add a new piece to the complex puzzle of weight regulation. After demonstrating heterodimerization of GHSR with MC3R and MC4R with GPR7, the next step is the elucidation of functional properties of the determined heterodimer pairs. To date MC4R is the obesity-related candidate gene in which most of its mutations were identified in obese patients. The vast majority of these mutations occur in the heterozygous state. In accordance with data from heterozygous knockout mice, a gene-dosage effect seems to be the underlying mechanism [36]. However, for two MC4R mutations a dominant-negative effect was shown [19,20], leading to the identification of MC4R dimerization [20]. So far no human MC4R mutation was identified that interrupts the MC4R homodimer formation. To completely characterize functional characteristics of naturally occurring MC4R mutations, their role in homo- or heterodimer formations have to be taken into account. The knowledge of a MC4R heterodimer formation offers a new and specific starting point for the development of therapeutic agents for obesity treatment.
Disclosure
The authors declared no conflict of interest.
Fig. 1.
a Influence on food intake, expression and G-protein coupling of hypothalamic GPCRs involved in body weight regulation. b Schematic illustration of major nuclei of the hypothalamus
Fig. 2.
Cellular localization and cell surface expression of the analyzed GPCRs a HEK293 cells were transiently transfected with expression plasmids encoding MC3R, GHSR, NPY2R, µ-OPR or MC4R, GPR7, 5HTR1B, CB1R carboxyterminally fused with yellow fluorescent protein (YFP). Cells were imaged by confocal laser scanning microscopy 24 h after transfection. Excitation and emission wavelengths for YFP were 488 nm and 515–550 nm. Confocal images of typical cells are shown. b To investigate cell surface expression COS-7-cells were transiently transfected by N-terminal HA-tagged GPCRs. 48 h after transfection the extracellular HA-epitope was detected with a biotin-labelled antibody and peroxidase-labelled streptavidin. Values are mean ± SEM of at least four independent experiments each performed in hexapletts. One way ANOVA and Dunnett tests were conducted for the difference of the positive control rM3R (rat muscarinic receptor, rM3R) and the different GPCRs (* p < 0.01).
Fig. 3.
Investigation of dimerization of a MC3R or b MC4R with other GPCRs by sandwich-ELISA. COS-7 cells were co-transfected with differentially tagged GPCR constructs, solubilized overnight and incubated in FLAG-antibody-coated 96-well plates. Dimerization was measured via the N-terminally tagged HA epitope as increase in optical density. MC3R-C-Flag was co-transfected with GHSR-, NPY2R – or µ-OPR-N-HA (orange), while MC4R-C-Flag was co-transfected with GPR7- or 5HTR1B (blue). As a negative control CB1R was co-expressed with equal amounts of MC3R- and MC4R-C-Flag (beige). The homomeric combination of MC3R-C-Flag and MC3R-N-HA like MC4R-C-Flag and MC4R-N-HA served as positive control (open bars). The mean absorption (492 nm / 620 nm) is shown as percentage of the FLAG-tagged MC3R or MC4R alone of three independent experiments. One way ANOVA and Dunnett tests were performed to determine the significance of the heterodimerization in contrast to the MC3R or MC4R homodimer (**p < 0.01, *p < 0.05).
Fig. 4.
FRET analysis of living HEK293 cells transiently co-transfected with a MC3R and GHSR, NPY2R or µ-OPR, or b MC4R and GPR7 or 5HTR1B. HEK293 cells were co-transfected with plasmids encoding MC3R(oronge)- or MC4R(blue)-YFP and GHSR- NPY2R-, µ-OPR-, GPR7- or 5HTR1B-CFP. Cotransfection of CB1R with even amounts of MC3R or MC4R served as negative control (beige). As positive control the homodimer of MC3R and MC4R was used (open bars). To prove the significance of heterodimerization compared to the MC3R or MC4R homodimer one way ANOVA and Dunnett tests were performed (**p < 0.01, *p < 0.05). Emission light of a characteristic wavelength is detectable. FRET efficiencies (E) were calculated from the relative increase in CFP emission and the decrease in YFP emission during selective photobleaching at 512 nm. The depicted data represent means ± SEM of 4–6 single cells of one representative measurement.
Fig. 5.
Subcellular localization after cotransfection HEK293 cells were transiently co-transfected with expression plasmids encoding MC3R-YFP or MC4R-YFP and GHSR, NPY2R, µ-OPR or MC4R, GPR7, 5HTR1B, CB1R coupled to CFP. Cells were imaged by confocal laser scanning microscopy 24 h after transfection. CFP-tagged receptors were visualized by excitation at 458 nm, whereas YFP-tagged GPCRs were excitated at 488 nm. Emission wavelengths were detected by using a LP505 filter. Representative cells are shown.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 577 TP A9, the graduate college 1208, TP1, and the Bundesministerium für Bildung und Forschung (BMBF) 01GS0825.
References
- 1.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386((6622)):239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 2.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396((6712)):679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 3.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396((6712)):674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 4.Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2((4)):274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 5.George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1((10)):808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 6.Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4((2)):126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 7.Lim WK. GPCR drug discovery: novel ligands for CNS receptors. Recent Patents on CNS Drug Discovery. 2007;2((2)):107–112. doi: 10.2174/157488907780832689. [DOI] [PubMed] [Google Scholar]
- 8.Terrillon S, Barberis C, Bouvier M. Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc Natl Acad Sci U S A. 2004;101((6)):1548–1553. doi: 10.1073/pnas.0305322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopelman PG. Obesity as a medical problem. Nature. 2000;404((6778)):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 10.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8((5)):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 11.Bagnol D. G protein-coupled receptors in hypothalamic circuits involved in metabolic diseases. Curr Opin Drug Discov Devel. 2004;7((5)):665–682. [PubMed] [Google Scholar]
- 12.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385((6612)):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 13.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99((17)):11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7((4)):307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- 15.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20((2)):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 16.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20((2)):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 17.Lee YS, Poh LK, Loke KY. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab. 2002;87((3)):1423–1436. doi: 10.1210/jcem.87.3.8461. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y X, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89((8)):3936–3942. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 19.Tarnow P, Rediger A, Brumm H, Ambrugger P, Rettenbacher E, Widhalm K, Hinney A, Kleinau G, Schaefer M, Hebebrand J, Krause G, Gruters A, Biebermann H. A heterozygous mutation in the third transmembrane domain causes a dominant-negative effect on signalling capability of the MC4R. Obes Facts. 2008;1((3)):155–162. doi: 10.1159/000138251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52((12)):2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 21.Nickolls SA, Maki RA. Dimerization of the melanocortin 4 receptor: a study using bioluminescence resonance energy transfer. Peptides. 2006;27((2)):380–387. doi: 10.1016/j.peptides.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Mandrika I, Petrovska R, Wikberg J. Melanocortin receptors form constitutive homo- and heterodimers. Biochem Biophys Res Commun. 2005;326((2)):349–354. doi: 10.1016/j.bbrc.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Schioth HB. G protein-coupled receptors in regulation of body weight. CNS Neurol Disord Drug Targets. 2006;5((3)):241–249. doi: 10.2174/187152706777452263. [DOI] [PubMed] [Google Scholar]
- 24.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268((20)):15174–15179. [PubMed] [Google Scholar]
- 25.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411((6836)):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 26.Ishii M, Fei H, Friedman JM. Targeted disruption of GPR7, the endogenous receptor for neuropeptides B and W leads to metabolic defects and adult-onset obesity. Proc Natl Acad Sci U S A. 2003;100((18)):10540–10545. doi: 10.1073/pnas.1334189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broberger C, Landry M, Wong H, Walsh JN, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66((6)):393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 28.De Jesús ML, Sallés J, Meana JJ, Callado LF. Characterization of CB1 cannabinoid receptor immunoreactivity in postmortem human brain homogenates. Neuroscience. 2006;140((2)):635–643. doi: 10.1016/j.neuroscience.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Schulz A, Bruns K, Henklein P, Krause G, Schubert M, Gudermann T, Wray V, Schultz G, Schoneberg T. Requirement of specific intrahelical interactions for stabilizing the inactive conformation of glycoprotein hormone receptors. J Biol Chem. 2000;275((48)):37860–37869. doi: 10.1074/jbc.M006709200. [DOI] [PubMed] [Google Scholar]
- 30.Amiri H, Schultz G, Schaefer M. FRET-based analysis of TRPC subunit stoichiometry. Cell Calcium. 2003;33((5–6)):463–470. doi: 10.1016/s0143-4160(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 31.Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005;77((14)):1667–1673. doi: 10.1016/j.lfs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Niehaus JL, Liu Y, Wallis KT, Egertová M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, Elphick MR, Lewis DL. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72((6)):1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20((8)):1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- 34.Chow KB, Leung PK, Cheng CH, Cheung WT, Wise H. The constitutive activity of ghrelin receptors is decreased by co-expression with vasoactive prostanoid receptors when over-expressed in human embryonic kidney 293 cells. Int J Biochem Cell Biol. 2008;40((11)):2627–2637. doi: 10.1016/j.biocel.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 2006;26((12)):3141–53. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88((1)):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]