Abstract
Mild traumatic brain injury (mTBI) is a significant cause of disability, especially when symptoms become chronic. This chronicity is often linked to oculomotor dysfunction (OMD). To our knowledge, this is the first prospective study to localize aberrations in brain function between mTBI cohorts, by comparing patients with mTBI with OMD with an mTBI control group without OMD, using task and resting-state functional magnetic resonance imaging (fMRI). Ten subjects with mTBI who had OMD (OMD group) were compared with nine subjects with mTBI who had no findings of OMD (control group). These groups were determined by a developmental optometrist using objective testing for OMD. The (convergence) task fMRI data demonstrated significantly decreased brain activity, measured as decreases in the blood oxygen level dependent (BOLD) signal, in the OMD group compared with the control group in three brain regions: the left posterior lingual gyrus, the bilateral anterior lingual gyrus and cuneus, and the parahippocampal gyrus. When doing a seed-based resting state fMRI analysis in the lingual/parahippocampal region, a large cluster covering the left middle frontal gyrus and the dorsolateral pre-frontal cortex (Brodmann areas 9 and 10), with decreased functional correlation in the OMD group, was identified. Together these observations provide evidence for neural networks of interactions involving the control of eye movement for visual processing, reading comprehension, spatial localization and navigation, and spatial working memory that appear to be decreased in mTBI patients with OMD compared with mTBI patients without OMD. The clinical symptomatology associated with post-traumatic OMD correlates well with these MRI findings.
Keywords: functional magnetic resonance imaging, oculomotor dysfunction, post-traumatic visual dysfunction, traumatic brain injury
Introduction
Mild traumatic brain injury (mTBI) comprises 80% of the 3.65 million brain injuries in the United States and costs an estimated $17 billion annually.1–4 Mild traumatic brain injury (mTBI) is a challenging diagnosis and can be as disabling as a severe TBI when chronic symptoms develop.4–7 In the past several years, researchers have found that a somewhat silent majority of patients with mTBI in whom lasting symptoms develop have concurrent oculomotor dysfunction (OMD).8,9 This cohort of patients with post-traumatic OMD, regardless of mechanism of injury, seem to demonstrate more significant and lasting disability and poorer overall outcome than other TBI cohorts, yet these correlations have not been studied adequately.10,11 In addition, the exact neurostructural and biological basis of OMD after mTBI is unknown.
The goal of this study was to identify brain areas underlying post-traumatic visual differences in subjects with acute mTBI with versus without OMD, using task and resting-state functional magnetic resonance imaging (fMRI).
Methods
Twenty subjects treated for mTBI were entered into a prospective clinical cohort study to localize fMRI differences specifically associated with mTBI with OMD using whole brain task and resting-state fMRI. Mild TBI subjects who had OMD (OMD group) were compared with mTBI subjects who had no findings of OMD (control group). These groups were determined by a developmental optometrist using the following objective testing for OMD: positive fusional vergence, near point convergence (NPC), accommodation amplitude, positive relative accommodation (PRA), negative relative accommodation (NRA), smooth pursuits, and saccadic movement. All subjects had sustained mild TBI as defined by a Glasgow Coma Scale (GCS) score of ≥13 and no findings on either a computed tomography (CT) or a clinical MRI scan.
Subjects were possible candidates for the study if they met all inclusion/exclusion criteria listed in Table 1 and, for controls, those who also had no visual findings. They were entered into the study within nine months of their injury. After eligibility was established, informed consent was obtained from either the subject or the subjects' legally authorized representative. The human subjects protocol for this study was approved by the Institutional Review Boards from Hennepin County Medical Center (HCMC) and the University of Minnesota. This clinical study was registered with clinicaltrials.gov; NCT # 02771106.
Table 1.
Study Inclusion and Exclusion Criteria
| Criteria | Description |
|---|---|
| Inclusion | • Mild TBI (GCS score ≥13, PTA <24 h) |
| • No brain CT or MRI findings | |
| • Diagnosed with OMD by developmental optometrist | |
| • Age >16 years and <55 years | |
| • Injury between 1–9 months | |
| • Informed consent obtained | |
| Exclusion | • Any type of bio-implant activated by mechanical, electronic, or magnetic means |
| • Any type of ferromagnetic bio-implant that could potentially be displaced | |
| • Significant anxiety and/or claustrophobia | |
| • Known ocular problems | |
| • Any vision rehabilitation | |
| • Near point convergence >25 cm or sustained diplopia | |
| • Cranial nerve II, III, IV, or VI palsy | |
| • Pregnancy | |
| • Severe mental retardation or prior severe brain injury or stroke | |
| • Age <16 and >55 years | |
| • GCS score <13 | |
| • Time since TBI >9 months |
TBI, traumatic brain injury; GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; CT, computed tomography; MRI, magnetic resonance imaging; OMD, oculomotor dysfunction.
Vision testing
Potential subjects were identified by physicians in the TBI Outpatient program at HCMC and referred to the developmental optometrist who performed objective testing to confirm whether they did or did not have OMD related to the mTBI, as well as the severity of their OMD if they were found to have this diagnosis. Ten subjects with mTBI were found to have significant OMD (OMD group), and 10 subjects with mTBI had no OMD findings (control group). One control subject was withdrawn from the study because of incidental findings on the MRI. An assessment for pre-injury OMD symptomatology as well as a Post-Trauma Vision Survey were used to screen for undiagnosed or subclinical vision problems.12
The primary indicators of convergence insufficiency are the presence of a receded NPC and a decreased positive fusional vergence. The NPC was measured with the fixation stick (Gulden Ophthalmics, Elkins Park, PA). The fixation stick has a single letter (20/80 equivalent at 40 cm). This letter size was selected to accommodate presbyopic subjects who would not have their spectacle correction during the task fMRI, ensuring their ability to see the near target and accurately report any diplopia. The fixation stick was slowly brought toward the subject, and the distance at which either the subject reported diplopia of the letter or the distance at which the developmental optometrist observed an eye drift was recorded. If an eye drift was observed and no diplopia was reported, suppression was recorded. Suppression was also evaluated with the Worth four dot (W4D). The W4D was held 16 inches away from subjects while they are wearing red and green glasses. If the subject saw four dots, there was not suppression, and if they reported two or three dots, then there was suppression.
The positive fusional vergence was measured using a single column of letters on a fixation stick (20/30 equivalent at 40 cm) and a horizontal prism bar (HLB-15 Astron International, Naples, FL). This measurement was performed in free space, which allowed for more peripheral visual input because mTBI patients have decreased peripheral visual processing. A phoropter may restrict the TBI subject's peripheral vision when performing vergence tasks. Free space vergences have been shown to have good repeatability.13 The fixation stick was held by the subject 40 cm from the nose. The developmental optometrist then presented an increasing amount of base out prism in front of the subject's right eye until the subject report sustained diplopia or when an eye drift was observed.
The two primary indicators of accommodative insufficiency are a reduced amplitude of accommodation and a decreased PRA.14 The amplitude of accommodation was measured using the Reichart phoropter (model 11625B, Depew, NY), and the subject viewed a single horizontal line of letters (20/30 at 40 cm) with one eye occluded. The amount of minus lens needed to blur the letters was recorded as the amplitude of accommodation measurement. This test was repeated for the left eye. The PRA was measured in the phoropter; both eyes were open for this test. The amount of minus lens needed to completely blur the letters was recorded as the PRA. This test was repeated with plus lenses presented in front of both eyes, the amount of plus lens needed to completely blur the letters was recorded as the NRA.
The smooth pursuit and saccadic eye movement testing was performed with a Wolff wand (Bernell, Mishawaka, IN). For the smooth pursuits testing, the wand was held in front of the subject and moved in a large circular pattern several times. For the saccades testing, two wands were held in front of the subject who was asked to move the eyes back and forth between the two wands when prompted. The developmental optometrist observed the accuracy of the subject's eye movements and graded the accuracy and speed of the eye movements on a scale of 1–4, with 4 being normal. The objective measurements used to test for OMD are listed in Table 2.15–17
Table 2.
Oculomotor Measurements with Normal Values and Purpose
| Measurement | Normal | Purpose |
|---|---|---|
| Near point of convergence (break/recovery) | 5 cm | Ability for both eyes to maintain single binocular vision with a near target |
| Positive fusional vergence (prism bar) | 30 prism diopters | Additional measurement of convergence |
| Worth Four Dot | Flat fusion | Determines if there is suppression of an eye during testing |
| Smooth pursuit (using Wolff wand) | Smooth and full | Ability for eyes to accurately follow a moving target |
| Saccadic eye movement (using Wolff wand) | No under or overshoot | Ability for eyes to accurately move from one target to another |
| Near relative accommodation (in phoropter) | -2.50 diopters | Ability for both eyes to focus while maintaining single vision |
| Amplitude of accommodation (minus lens method in phoropter) | Age dependent | To determine the maximum amount of accommodative focus that can be exerted |
There is no validated objective test for diagnosing deficits of ambient visual processing. The diagnosis is determined by the developmental optometrist who assesses the subject by asking a very specific symptomatology list. These symptoms are ones that other OMD diagnoses do not cause and can include nausea or dizziness with reading and up close tasks, as well as difficulty scrolling on computers, walking through narrow hallways, driving with windshield wipers on, and/or looking at busy patterns on the floor.
MRI
Within three weeks of the objective visual testing, MRI data were acquired using a Siemens Magnetom Prisma 3T scanner, including T1-weighted anatomical (MPRAGE, voxel size: 0.94 × 0.94 × 0.9 mm3) and fMRI (TR/TE = 1000/36 msec, voxel size: 2 × 2 × 2 mm3) scans.
During the task fMRI acquisitions, subjects held their gaze at a near target, presented 10 cm from the nose, for 10 sec and attempted to maintain fusion. Next, they relaxed their gaze to a far target that was 110 cm from the nose for 30 sec. The far target stimuli were generated using PsychoPy and were presented on a rear-projection screen visible through a mirror.18,19 This cycle repeated for about 6 min. For resting-state fMRI, subjects were scanned “at rest” for 6 min and 40 sec with their eyes closed.
Data processing and analyses were performed using programs from the AFNI and FSL analysis packages.20,21 Pre-processing steps include motion correction, distortion correction, co-registration to the T1-weighted anatomical images and spatial smoothing.22 The data were smoothed conservatively (to preserve spatial resolution) with a 3 mm full width at half maximum (FWHM) Gaussian kernel.
Each subject's task fMRI data were statistically analyzed with a general linear model, with the time course of the blood oxygen level dependent (BOLD) signal serving as the dependent variable in each voxel. The regressor (effect) of interest was coded as a boxcar function, modeling whether the subject's focus was on the near or far target, convolved with a gamma variate to account for the gradual rise and fall of the hemodynamic response. Additional regressors were also included to account for head movement and drift in the baseline MR signal over time. At each voxel and for each subject, a beta weight for near versus far target was estimated as the effect of eye convergence (independent variable).
For the group analysis (OMD group vs. control group), these betas were used in a voxel-wise two-sample t test comparing the two groups. Based on the results of this t test, voxels were thresholded at an uncorrected p value <0.01. The correction for multiple testing was achieved by determining the minimum significant cluster size. Taking into account increasing concerns over the risk of inflated false positives with voxel-wise group comparisons, as reported by Eklund and associates,23 a non-parametric permutation test was used. This permutation test randomized the signs of the residuals of the model among subjects, per voxel, and then performed a t test, with these steps iterated 10,000 times, to determine the probability that, if each voxel has a 1% chance of displaying a false positive group difference, clusters of a given size would occur by chance. Based on these probabilities, clusters (in the original voxel-thresholded group-difference map) that were smaller than those that would occur by chance more than 5% of the time were filtered out of the results to achieve a cluster-level α = 0.05.
After similar pre-processing for the resting-state fMRI data, each of the (three) identified clusters of activation differences between groups, found with task fMRI, was treated as a “seed” region. Resting-state fMRI time courses across voxels for each region were averaged to generate representative seed time courses. These seed time courses were then correlated with all other voxels' time courses throughout the brain in each subject. Correlation coefficients were Fisher z-transformed and treated as the dependent variable in a t test comparing groups. Results were thresholded and corrected for multiple testing as in the task analysis.
Correlation analyses of vision measures and fMRI data
To study possible correlations between objective oculomotor measures and fMRI data, task fMRI activation maps (beta weights) were averaged, for each subject, within brain areas showing significant response to our task using only the control group. (Defining the regions by significant contrast between the groups would result in inflated correlations because of selection bias of voxels sensitive to group differences).24 With this approach, six brain clusters were identified, which include the lingual gyrus (bilateral), middle occipital gyrus (bilateral), cuneus (right) and tuber of vermis (right).
Statistical methods
Comparisons between the OMD group and the control group were performed with the Fisher exact test for categorical variables and with the Wilcoxon test for continuous variables. The fMRI task activation (beta weights averaged for each subject over voxels in each of the clusters defined by where controls had significant activation) was associated with continuous vision metrics using the Spearman correlation. The non-parametric tests and correlations were used because of the small sample sizes and skewness in some measures. The statistical significance level is taken as 0.05 throughout.
Results
Demographic characteristics
The demographic characteristics including age, sex, attention deficit disorder diagnosis, learning disability, presence of recurrent TBIs, loss of consciousness with TBI, and time from injury to MRI (range 26–200 days) were compared between the OMD and control groups (Table 3). There were no statistically significant differences found (p > 0.05), except for the presence of recurrent TBIs in the OMD group compared with the control group, which almost reached statistical significance (p = 0.069). Only one OMD subject was found to have abnormal formal vestibular testing. No subject in either the OMD or control group reported any pre-injury symptomology on the Post-Trauma Vision Survey.
Table 3.
Summary of Demographic Characteristics of Patients with Mild Traumatic Brain Injury with Oculomotor Dysfunction and without Control
| Variable | OMD | Control |
|---|---|---|
| Number of patients | 10 | 9 |
| M:F ratio | 4:6 | 5:4 |
| Mean age (yrs) ± standard error | 31.0 ± 2.7 | 31.1 ± 3.6 |
| Mean time in days from TBI to MRI ± standard error | 89 ± 16 | 66 ± 9 |
| N (%) with attention deficit disorder | 1 (10%) | 1 (11%) |
| N (%) with learning disability (1 control missing data) | 0 (0%) | 0 (0%) |
| N (%) with recurrent TBIa | 7 (70%) | 2 (22%) |
| N (%) with loss of consciousness after TBI (2 control, 1 OMD missing data) | 2 (20%) | 3 (33%) |
OMD, oculomotor dysfunction; TBI, traumatic brain injury; MRI, magnetic resonance imaging.
There is no statistically significant difference between groups in any comparison (p > 0.05), although the group difference in recurrent TBI almost reached statistical significance (p = 0.069). The p values are from two-sample Wilcoxon test (continuous measures) or Fisher exact test (category measures). Percents assume the unknowns are not “yes” for that characteristic.
OMD
No subjects in the control group had an OMD diagnosis by design. All subjects in the OMD group received a diagnosis of convergence insufficiency, accommodative insufficiency, and ambient visual processing disorder by the developmental optometrist (group difference. p < 0.001). No suppression was found in either the OMD group or the control group. Thirty percent of subjects had deficits of smooth pursuits and 30% had deficits of saccadic movement. The objective measurements used to diagnose OMD, including NPC, positive fusional vergence, accommodation amplitude, PRA, and NRA shown in Table 4, were compared between the OMD group and control group and found to be significantly different except for NRA and PRA that almost reached statistical significance (p = 0.067). Negative relative accommodation (focus at far) is typically normal in patients with OMD.
Table 4.
Objective Oculomotor Dysfunction Measurements of Patients with Mild Traumatic Brain Injury with Oculomotor Dysfunction and without Control
| Measurement | OMD | Control | p |
|---|---|---|---|
| Near point of convergence (cm) | 5.84 ± 0.66 | 0.78 ± 0.36 | 0.002 |
| Positive fusional vergence (diopters) | 16.30 ± 2.43 | 27.78 ± 1.47 | 0.009 |
| Near positive relative accommodation (diopters) | 2.08 ± 0.29 | 3.00 ± 0 | 0.067 |
| Near negative relative accommodation (diopters) | 2.65 ± 0.18 | 3.00 ± 0 | 0.33 |
| Accommodation amplitude right eye (diopters) | 5.50 ± 0.53 | 8.83 ± 0.17 | 0.05 |
| Accommodation amplitude left eye (diopters) | 5.50 ± 0.49 | 8.67 ± 0.33 | 0.05 |
OMD, oculomotor dysfunction.
Values are expressed as the mean ± standard error. Near negative relative accommodation (far focus) would not be expected to change with OMD. p-values are from two-sample Wilcoxon test.
The common symptoms associated with OMD were observed significantly more often in the OMD group than the control group, except for diplopia (p = 0.21) as shown in Table 5. The ability for the subjects in the OMD group to read (mean 34 min) or use the computer (mean 24 min) before becoming symptomatic, compared with the control group (unlimited time) was significantly different (p < 0.007). During the MRI scan, no control subjected reported diplopia during the convergence task, but of the seven OMD patients asked, four reported diplopia.
Table 5.
Oculomotor Dysfunction Symptomatology of Patients with Mild Traumatic Brain Injury with Oculomotor Dysfunction and without Control
| Measurement | OMD | Control | p |
|---|---|---|---|
| N (%) with diplopia | 3 (30%) | 0 (0%) | 0.21 |
| N (%) with blurry vision while reading | 6 (60%) | 0 (0%) | 0.01 |
| N (%) with difficulty shifting from near to far distances (3 control, 2 OMD missing data) | 6 (60%) | 0 (0%) | 0.017 |
| N (%) with difficulty with retaining what they read (2 OMD missing data) | 7 (70%) | 0 (0%) | 0.0001 |
| N (%) with difficulty losing track of where they are when reading or words would move on page | 9 (90%) | 1 (11%) | 0.001 |
| N (%) with ambient visual processing symptoms such as dizziness, loss of balance, difficulty in busy visual environment | 10 (100%) | 0 (0%) | 0.0001 |
OMD, oculomotor dysfunction.
p values are from Fisher exact test. Percents assume the unknowns are not “yes” for that symptom.
MRI
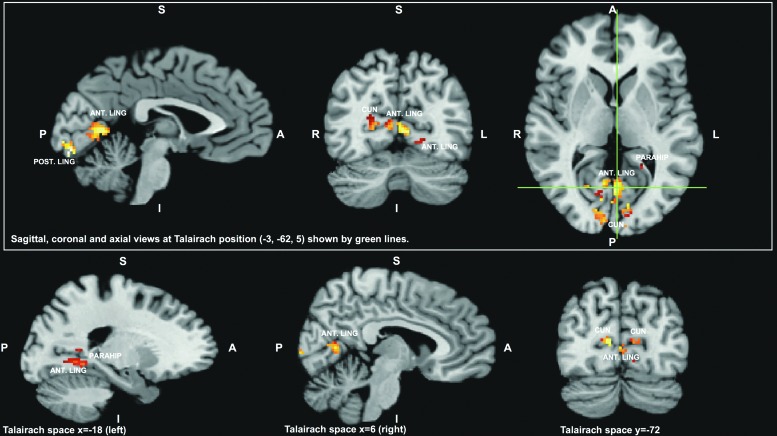
The convergence task (near vs. far) fMRI data demonstrated significantly decreased BOLD responses in the OMD group, compared with the control group, in three clusters in the following areas: the left posterior lingual gyrus, the bilateral anterior lingual gyrus and cuneus, and the parahippocampal gyrus as depicted in Figure 1 (p ≤ 0.01 for each).
FIG. 1.
The brain areas identified as significantly different, between the mild traumatic brain injury (mTBI) group with oculomotor dysfunction (OMD) (OMD group, n = 10) and the mTBI without OMD group (control group, n = 9), in functional magnetic resonance imaging activation maps obtained using the convergence (near vs. far) task. The results are shown in normalized Talairach space. The green lines indicate locations of the sagittal and coronal slices in the upper part of the figure. In the lower part, additional views are provided. Three regions showed decreased blood oxygen level dependent response during the convergence task in the mTBI OMD group compared with the control group. The significant clusters of voxels consisted of a posterior left lingual gyrus (POST. LING) region, a more anterior bilateral region that included lingual gyrus (ANT. LING) and cuneus (CUN), and a still more anterior region that included lingual gyrus and parahippocampal (PARAHIP) gyrus.'
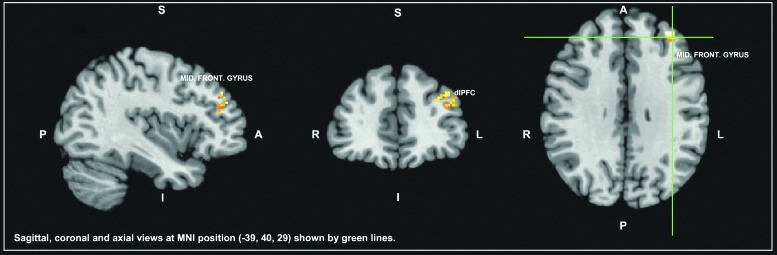
For the resting-state analysis, the left middle frontal gyrus, including the dorsolateral pre-frontal cortex (Brodmann areas [BA] 9, 10, and 46) were found to be more strongly and significantly (p ≤ 0.05) correlated with the lingual/parahippocampal seed for the control group than for the OMD group as depicted in Figure 2. The other two seeds did not show differences in functional connectivity between groups.
FIG. 2.
The brain areas identified as significantly less correlated with the lingual/parahippocampal seed in the mild traumatic brain injury (mTBI) group with oculomotor dysfunction (OMD) (OMD group, n = 10) and the mTBI without OMD group (control group, n = 9), using resting-state functional magnetic resonance imaging. The results are shown in normalized MNI space. The green lines indicate locations of the sagittal and coronal slices. A left middle frontal gyrus region (including the dorsolateral pre-frontal cortex - dlPFC) showed reduced connectivity with the seed area in the OMD group compared with the control group. MNI, Montreal Neurological Institute 152 template.
Correlation analyses of vision measures and fMRI data
Using data from the OMD group only, we compared the vision measures with the average task (convergence) fMRI activation within each of the six clusters of activation (and within those six clusters pooled to form one region of interest) found for the control subjects performing the convergence task (as described in the Methods section). Our hypothesis was that reading and computer time, as well as NPC and positive fusional vergence, may correlate with fMRI activations in the OMD group. Within the aggregate cluster, a moderately high Spearman correlation was found with positive fusional vergence (r = 0.68, p = 0.03) but not with NPC (r = 0.02, p = 0.87). We note, however, that one of the six clusters (the right cuneus) showed a correlation of 0.76 (p = 0.01) with positive fusional vergence.
Discussion
This is the first report of a prospective cohort clinical study to identify brain areas underlying post-traumatic visual changes in acute mTBI subjects using whole brain task and resting-state fMRI. Unlike here, where we compare data from different mTBI cohorts, previous studies compared mTBI subjects with healthy controls.9,25–29 Injury at any level of the complex oculomotor system undermines the efficiency of cognitive abilities involved in reading comprehension, as well as other visuocognitive tasks.30 There was significantly decreased fMRI responses in the visual areas during the convergence task in the OMD cohort compared with the control cohort, which correlates well to the clinical symptomatology that patients with OMD experience.
The cortical areas that showed decreased activation in the OMD group play an important role in vision and eye movement. Specifically, the lingual gyrus and calcarine sulcus comprise visual areas in the occipital cortex, involved in processing visual information and eye movement.31 Most symptoms of convergence insufficiency, accommodative insufficiency, and saccadic dysfunction are associated with reading or other close-range work.32 Convergence insufficiency symptoms include the loss of reading comprehension over time, a pulling sensation in the eyes while reading, impaired concentration and memory for reading material, slowed processing, impaired visual attention, and the movement of the print.8,33,34 Seventy percent of subjects in the OMD group in this study had similar symptoms with reading comprehension.
The cuneus gyrus is part of the primary visual area and processes information from the retina by way of the lateral geniculate and optic radiation pathway. Positron emission tomography studies suggest that the medial cuneus is involved in pursuit and saccadic eye movements.35 A more recent study by Schraa-Tam and colleagues36 demonstrates the involvement of the cuneus in optokinetic reflexive eye movements to stabilize images on the retina. Functional deficits of the cuneus, therefore, would lead to alterations in eye movement.
Convergence and accommodative insufficiency can cause eyestrain and headaches after short periods of reading, blurred vision, and diplopia. Common complaints of saccadic dysfunction include frequent loss of place, omission of words, skipping lines, slow reading speed, poor comprehension, short attention span, and symptoms related to school tasks such as taking standardized tests.37 Ninety percent of our OMD subjects lost track of where they were while reading, or would perceive words as moving on the page. Accommodative insufficiency symptoms include difficulty changing focus from one distance to another.38 More than 60% of our OMD subjects had difficulty with this symptom.
The parahippocampal gyri are those areas of brain that surround the hippocampal formation. They include the entorhinal cortex and perirhinal cortex. These areas of the brain provide input to the hippocampus, which utilizes spatial and visual information for navigation.39 Lesions of the parahippocampal areas of the brain result in spatial learning and memory deficiencies.40 The dorsolateral pre-frontal cortex (attributed anatomically to BA 8, 9, 10, and 46) plays an important role in spatial memory and navigation,41 like the hippocampal formation that also sends axons to BA 9.42 BA 10 is interconnected with limbic areas of the brain involved in working memory.43 Patients who have ambient visual processing disorder have difficulty with orientation in space, balance, movement, coordination, and posture.34,44–46 All of our OMD subjects reported these types of symptoms.
The parahippocampal region also plays an important role in the acquisition and retention of story content. Patients with lesions in this region of the brain exhibit difficulty in remembering the content of short prose passages.47 In our study, 70% of the OMD subjects have deficits in remembering what they read as opposed to none of the subjects in the control group. A possible explanation for this reading comprehension deficit exhibited by the OMD group may be decreases in the correlation of activity between the parahippocampal gyrus and other areas of the brain as revealed by resting state fMRI.
A limitation of this study is the relatively small number of subjects, which limits the generalizability, power, and significance level of some of our findings. Therefore, the results need to be interpreted with caution and point toward the need for a much larger study. Another is that the general population can have undiagnosed or subclinical vision difficulties. Although the subjects were screened for pre-clinical vision difficulties and symptomatology, a validated measure was not used, which would be valuable in future studies. Finally, there currently is no validated objective test for diagnosing deficits of ambient visual processing, so only subjective reporting can be used. One future direction would be a longitudinal study to demonstrate the effectiveness of neurovision rehabilitation on OMD, initiated after mTBI, by normalizing objective visual measurements as well as fMRI findings.
Conclusion
The results of this study can be summarized by the following key points: (1) This is the first prospective cohort clinical study to identify brain areas underlying post-traumatic visual changes in acute mTBI, comparing mTBI patients with OMD with an mTBI control group, rather than healthy controls, using whole brain task and resting-state fMRI. (2) The OMD group had significantly different objective measurements during the developmental optometrist's examination compared with the control group. (3) The convergence task fMRI data demonstrated significantly decreased brain activation in the OMD group, compared with the control group in three brain areas: the left posterior lingual gyrus, the bilateral anterior lingual gyrus and cuneus, and the parahippocampal gyrus. (4) The resting-state fMRI identified a large cluster in the left middle frontal gyrus and dorsolateral prefrontal cortex with significantly decreased correlation with the lingual/parahippocampal area in the OMD group compared with the control group.
(5) Together, these observations provide evidence for neural networks of interaction involving the control of eye movement for visual processing, reading comprehension, spatial localization and navigation, and spatial working memory that are affected in mTBI patients with OMD compared with mTBI patients without OMD. (6) The clinical symptomatology associated with post-traumatic OMD is in agreement with these MRI findings. (7) Future studies should include a larger number of subjects and determine whether interventional procedures in mTBI patients with OMD result in any corresponding recoveries in task and/or resting state fMRI signals in the associated regions.
Acknowledgments
This work was supported by the Minnesota Spinal Cord Injury and Traumatic Brain Injury Annual Research Grant Program: Neuroimaging and Neurorehabilitation of Oculomotor Dysfunction in Mild Traumatic Brain Injury Grant Contract No. 105001 as well as the Minneapolis Medical Research Foundation. It was also partly supported by NIH grants P41 EB015894 and P30 NS076408.
Diane Hutter, R.N. and Julie Emanuel, Cot, provided invaluable assistance in the coordination and scheduling of the patients. Sey Lee provided assistance for data collection and entry into the database.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Center for Disease Control (2003). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2. Center for Disease Control (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths 2002–2006. www.cdc.gov/TraumaticBrainInjury (Last accessed August8, 2018)
- 3. Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Inj. Prev. 16, A268 [Google Scholar]
- 4. National Institutes of Health Consensus Development Panel. (1999). Rehabilitation of persons with traumatic brain injury. JAMA 282, 974–983 [PubMed] [Google Scholar]
- 5. Humphreys I., Wood R.L., Phillips C.J., and Macey S. (2013). The costs of traumatic brain injury: a literature review. Clinicoecon. Outcomes Res. 5, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McAllister T.W., and Arciniegas D. (2002). Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation 17, 265–283 [PubMed] [Google Scholar]
- 7. Mealings M., Douglas J., and Olver J. (2012). Considering the student perspective in returning to school after TBI: a literature review. Brain Inj. 26, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 8. Thiagarajan P., Ciuffreda K.J., and Ludlam D.P. (2011). Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic Physiol. Opt. 31, 456–468 [DOI] [PubMed] [Google Scholar]
- 9. Tyler C.W., Likova L.T., Mineff K.N., and Nicholas S.C. (2015). Deficits in the activation of human oculomotor nuclei in chronic traumatic brain injury. Front Neurol. 6, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraus M.F., Little D.M., Donnell A.J., Reilly J.L., Simonian N., and Sweeney J.A. (2007). Oculomotor function in chronic traumatic brain injury. Cogn. Behav. Neurol. 20, 170–178 [DOI] [PubMed] [Google Scholar]
- 11. Lynch J.M., Anderson M., Benton B., and Green S.S. (2015). The gaming of concussions: a unique intervention in post-concussion syndrome. J. Athl. Train. 50, 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang A., Ritter S.E., and Yu X.X. (2016). Neurovision Rehabilitation Guide. 1st ed. CRC Press Taylor & Francis Group: Boca Raton, FL [Google Scholar]
- 13. Antona B., Barrio A., Barra F., Gonzalez E., and Sanchez I. (2008). Repeatability and agreement in the measurement of horizontal fusional vergences. Ophthalmic Physiol. Opt. 28, 475–491 [DOI] [PubMed] [Google Scholar]
- 14. Scheiman M., and Wick B. (2014). Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders. 4th ed. Lippincott Williams & Wilkins Publishing: Philadelphia [Google Scholar]
- 15. Cooper J.S., Burns C.R., Cotter S.A., Daum K.M., Griffin J.R., and Scheiman M.M. (2010). Care of the patient with accommodative and vergence dysfunction, in: Optometric Clinical Practice Guidelines. American Optometric Association: St. Louis, MO [Google Scholar]
- 16. Green W., Ciuffreda K.J., Thiagarajan P., Szymanowicz D., Ludlam D.P., and Kapoor N. (2010). Accommodation in mild traumatic brain injury. J. Rehabil. Res. Dev. 47, 183–199 [DOI] [PubMed] [Google Scholar]
- 17. Goodrich G.L., Flyg H.M., Kirby J.E., Chang C.Y., and Martinsen G.L. (2013). Mechanisms of TBI and visual consequences in military and veteran populations. Optom. Vis. Sci. 90, 105–112 [DOI] [PubMed] [Google Scholar]
- 18. Peirce J.W. (2007). PsychoPy—psychophysics software in python. J. Neurosci. Methods 162, 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peirce J.W. (2009). Generating stimuli for neuroscience using psychopy. Front. Neuroinform. 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 [DOI] [PubMed] [Google Scholar]
- 21. Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., and Smith S.M. (2009). Bayesian analysis of neuroimaging data in FSL. Neuroimage. 45, S173–S186 [DOI] [PubMed] [Google Scholar]
- 22. Andersson J.L., Skare S., and Ashburner J. (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 [DOI] [PubMed] [Google Scholar]
- 23. Eklund A., Nichols T.E., and Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 113, 7900–7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vul E., Harris C., Winkielman P., and Pashler H. (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 4, 274– 290 [DOI] [PubMed] [Google Scholar]
- 25. Johnson B., Zhang K., Hallett M., and Slobounov S. (2015). Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 9, 564–573 [DOI] [PubMed] [Google Scholar]
- 26. Johnson B., Hallett M., and Slobounov S. (2015). Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology 85, 1163–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarez T.L., Vicci V.R., Alkan Y., Kim E.H., Gohel S., Barrett A.M., Chiaravalloti N., and Biswal B.B. (2010). Vision therapy in adults with convergence insufficiency: clinical and functional magnetic resonance imaging measures. Optom. Vis. Sci. 87, E985–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang K., Johnson B., Pennell D., Ray W., Sebastianelli W., and Slobounov S. (2010). Are functional deficits in concussed individuals consistent with white matter structural alterations: combined fMRI & DTI study. Exp. Brain Res. 204, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astafiev S.V., Shulman G.L., Metcalf N.V., Rengachary J., MacDonald C.L., Harrington D.L., Maruta J., Shimony J.S., Ghajar J., Diwakar M., Huang M.X., Lee R.R., and Corbetta M. (2015). Abnormal white matter blood-oxygen-level-dependent signals in chronic mild traumatic brain injury. J. Neurotrauma 32, 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciuffreda K.J., Han Y., Kapoor N., and Ficarra A.P. (2006). Oculomotor rehabilitation for reading in acquired brain injury. NeuroRehabilitation 21, 9–21 [PubMed] [Google Scholar]
- 31. Corbetta M., Akbudak E., Conturo T.E., Snyder A.Z., Ollinger J.M., Drury H.A., Linenweber M.R., Petersen S.E., Raichle M.E., Van Essen D.C., and Shulman G.L. (1998). A common network of functional areas for attention and eye movements. Neuron 21, 761–773 [DOI] [PubMed] [Google Scholar]
- 32. Borsting E., Rouse M., and Chu R. (2005). Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry 76, 588–592 [DOI] [PubMed] [Google Scholar]
- 33. Norn M.S. (1966). Convergence insufficiency: incidence in ophthalmic practice—results of orthoptics treatment. Acta Ophthalmol. 44, 132–138 [Google Scholar]
- 34. Scheiman M. and Scheiman M. (2011). The inter-relationship model, in: Understanding and Managing Vision Deficits: A Guide for Occupational Therapists. SLACK Incorporated Publishing: Thorofare, NJ, pps. 339-344 [Google Scholar]
- 35. O'Driscoll G.A., Wolff A.L., Benkelfat C., Florencio P.S., Lal S., and Evans A.C. (2000). Functional neuroanatomy of smooth pursuit and predictive saccades. Neuroreport. 11, 1335–1340 [DOI] [PubMed] [Google Scholar]
- 36. Schraa-Tam C.K., van der Lugt A., Smits M., Frens M.A., van Broekhoven P.C., and van der Geest J.N. (2009). Differences between smooth pursuit and optokinetic eye movements using limited lifetime dot stimulation: a functional magnetic resonance imaging study. Clin. Physiol. Funct. Imaging 29, 245–254 [DOI] [PubMed] [Google Scholar]
- 37. Lieberman S., Cohen A.H., and Rubin J. (1983). NYSOA K-D test. J. Am. Optom. Assoc. 54, 631–637 [PubMed] [Google Scholar]
- 38. Daum K.M. (1983). Accommodative dysfunction. Doc. Ophthalmol. 55, 177–198 [DOI] [PubMed] [Google Scholar]
- 39. Burgess N. and O'Keefe J. (1996). Neuronal computations underlying the firing of place cells and their role in navigation. Hippocampus 6, 749–762 [DOI] [PubMed] [Google Scholar]
- 40. Olton D.S. (1977). The function of septo-hippocampal connections in spatially organized behaviour. Ciba Found. Symp. 58, 327–349 [DOI] [PubMed] [Google Scholar]
- 41. Slotnick S.D. and Moo L.R. (2006). Prefrontal cortex hemispheric specialization for categorical and coordinate visual spatial memory. Neuropsychologia 44, 1560–1568 [DOI] [PubMed] [Google Scholar]
- 42. Goldman-Rakic P.S., Selemon L.D., and Schwartz M.L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12, 719–743 [DOI] [PubMed] [Google Scholar]
- 43. Gilbert S.J., Spengler S., Simons J.S., Steele J.D., Lawrie S.M., Frith C.D., and Burgess P.W. (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 18, 932–948 [DOI] [PubMed] [Google Scholar]
- 44. Zelinsky D.G. (2010). Brain injury rehabilitation: cortical and subcortical interfacing via retinal pathways. PM. R. 2, 852–857 [DOI] [PubMed] [Google Scholar]
- 45. Leibowitz H.W., Shupert C.L., and Post R.B. (1984). Two modes of processing visual information: implications for spatial orientation, in: Peripheral Vision Horizon Display (PVHD). NASA Conference Publication. Dryden Flight Research Center: California, pps. 41–44 [Google Scholar]
- 46. Padula W.V., Capo-Aponte J.E., Padula W.V., Singman E.L., and Jenness J. (2017). The consequence of spatial visual processing dysfunction caused by traumatic brain injury (TBI). Brain Inj. 31, 589–600 [DOI] [PubMed] [Google Scholar]
- 47. Frisk V. and Milner B. (1990). The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 28, 349–359 [DOI] [PubMed] [Google Scholar]