Abstract
Objective:
To characterize the incidence and clinical characteristics of neurotoxicity in the month following CTL019 infusion in children and young adults, to define the relationship between neurotoxicity and cytokine release syndrome (CRS), and to identify predictive biomarkers for development of neurotoxicity following CTL019 infusion.
Methods:
We analyzed data on 51 subjects, 4–22 years-old, who received CTL019, a chimeric antigen receptor-modified T-cell therapy against CD19, between 1/1/2010–12/1/2015 through a safety/feasibility clinical trial (NCT01626495) at our institution. We recorded incidence of significant neurotoxicity (encephalopathy, seizures, and focal deficits) and CRS, and compared serum cytokine levels in the first month post-infusion between subjects who did and did not develop neurotoxicity.
Results:
Neurotoxicity occurred in 23/51 subjects (45%; 95% CI 31–60%), was positively associated with higher CRS grade (p<0.0001), but was not associated with demographic characteristics or prior oncologic treatment history. Serum IL-2, IL-15, sIL-4, and HGF concentrations were higher in subjects with neurotoxicity than those with isolated CRS. Differences in peak levels of select cytokines including IL-12 and sTNFR-1 within the first three days were seen in subjects with neurotoxicity.
Interpretation:
Neurotoxicity is common after CTL019 infusion in children and young adults, and is associated with higher CRS grade. Differences in serum cytokine profiles between subjects with neurotoxicity and those with isolated CRS suggest unique pathophysiological mechanisms. Serum cytokine profiles in the first three days post-infusion may help identify at-risk children and young adults for neurotoxicity, and may provide a foundation for investigation into potential mitigation strategies
INTRODUCTION
Chimeric antigen receptor (CAR)-modified T-cell therapy against CD19 is a promising new therapy that has transformed the treatment of B-cell malignancies in adults and children1–4,12. Multiple CD19 CAR T-cell products are in clinical trials, many with distinct designs and signaling domains; two are approved for clinical use in the United States. Efficacy of these therapies is dependent upon T-cell activation and expansion, often resulting in a systemic pro-inflammatory cytokine release syndrome (CRS). CRS typically causes fever and flu-like symptoms, but in more severe cases, may cause multi-organ failure15,21. Neurotoxicity, including encephalopathy, seizures, aphasia, and other focal neurologic deficits, has been reported following various CD19 CAR products10,13,14. A recent phase II study of one CD19 CAR product with a 4–1BB costimulatory domain, tisagenlecleucel (also called CART-19 or CTL019), reported neurologic events in 30/75 (40%) children and young adults in the eight weeks following infusion10. Risk factors and neuropathogenesis of these symptoms in children and young adults, however, is not known5,14.
Some investigators have hypothesized that neurotoxicity following CD19 CAR infusion is a direct result of CRS5,14,15. In the above tisagenlecleucel study, most neurologic events occurred during CRS or just after its resolution. In another report, Gust and colleagues14 found 53/133 (40%) adults who received another CD19 CAR product with a CD28 costimulatory domain, JCAR015 (Juno Therapeutics), experienced a neurologic adverse event (AE) after infusion, ranging from a mild symptom (e.g. transient delirium, headache) to death. They reported 48/53 (91%) of adults with any neurologic AE also had CRS; among the 5/53 adults without CRS, neurologic AEs were mild. Younger age (age 18–40) was one risk factor for neurologic AE. Those adults with severe neurologic AEs demonstrated evidence of endothelial activation and systemic capillary leak, which the authors postulated may represent an increase in blood-brain barrier permeability, allowing passive transfer of inflammatory mediators of CRS into the central nervous system (CNS). However, neurologic AEs were not prevented by aggressive CRS therapy with IL-6-receptor blockade (tocilizumab) and/or dexamethasone, suggesting that while related, there may be partially independent mechanisms for neurotoxicity and CRS. There are no comparable data in pediatrics11,16.
A better understanding of the risk factors for neurotoxicity in CD19 CAR therapy across the age span is critically important in order to later identify age-specific potential neurotoxicity mitigation strategies in this highly-effective cancer treatment, and to ensure that neurotoxicity is not ultimately a limiting factor of effective CAR T-cell therapies14,17,18. We therefore investigated the neurotoxicity of CTL019, a CD19 CAR product initially designed and tested by the University of Pennsylvania and The Children’s Hospital of Philadelphia (CHOP), in a pediatric and young adult sample. Tisagenlecleucel/CTL019 (Kymriah, Novartis) now is an FDA-approved treatment for patients up to age 25 with B-cell precursor acute lymphoblastic leukemia (B-ALL) that is refractory or in second or greater relapse. We aimed to (1) characterize the incidence and clinical characteristics of neurotoxicity following CTL019 infusion; (2) define the relationship between neurotoxicity and CRS; and (3) identify predictive biomarkers for the development of neurotoxicity following CTL019 infusion.
METHODS
Study Design and Setting.
We performed a retrospective chart review using data from 51 subjects who received CTL019 infusion at CHOP between 4/1/2012 and 5/1/2015 as a part of a safety/feasibility clinical trial (NCT01626495). Details on the phase I/IIa clinical trial design and CTL019 are described elsewhere16,9,19. The cohort included patients between the ages of 1–24 years old, who had at least two months of clinical follow up post-infusion, and serial serum cytokine concentrations measured within the first one month post-infusion. Subjects with active CNS involvement of their leukemia (defined as CNS lymphoblasts in a cerebrospinal fluid (CSF) sample with >5 leukocytes per microliter, or clinical signs of CNS leukemia) or with encephalopathy attributed to other non-infusion-related medical causes, such as sepsis and electrolyte derangements, were excluded.
Data Collection
Data collection for NCT01626495 was approved by the CHOP institutional review board in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects or their legal guardians. Data were obtained through comprehensive medical evaluations and collection of blood samples. All data for the present sub-study were obtained through the NCT01626495 investigators and additional review of subjects’ electronic medical records (EMR) at CHOP. Patient data from their EMR, including oncologic treatment history prior to CTL019 infusion, neurologic and developmental history, hospital course following infusion, pre-infusion and post-infusion neuroimaging, and post-infusion EEG records (when available) were recorded onto a standardized data collection form. All data were decoded and maintained in secure databases.
Study Definitions
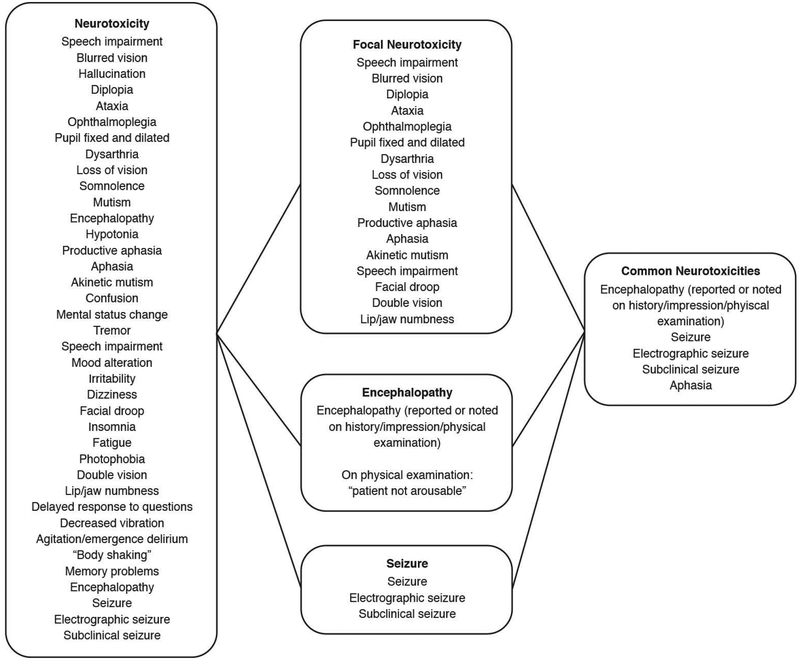
CRS was defined as previously described, and graded on the Penn grading scale, a standard interval adverse event rating scale (range 0–5, 0 representing no reaction and 5 representing death)20. Neurotoxicity was defined as any new and clearly defined neurologic symptom documented in the medical record in the first 60 days following first CTL019 infusion, specifically attributed to this infusion. Examples of these symptoms include encephalopathy, seizures, aphasia, and other focal neurologic deficits. Headache, hallucinations, and isolated delirium were excluded from this definition as these can often be seen as result of high fever or systemic illness and may not represent a primary neurologic pathology. As above, these neurologic symptoms were collected retrospectively. In an effort to reduce potential classification bias based on inconsistent documentation of neurologic symptoms in clinical records, we included an additional outcome measure of “common neurotoxicities” (encephalopathy, seizure, and aphasia only), defined a priori, that were previously reported in children and adults and that were consistently clearly and objectively reported in the medical record (Figure 1).
Figure 1:
Neurotoxicity Classification. Electronic medical records were abstracted for the terms below to define overall neurotoxicity and the specified subgroups.
Neurologic comorbidities present prior to CTL019 infusion, including a history of seizure, need for anticonvulsant medication, or pre-existing neurologic deficit were recorded.
Laboratory Assessments.
Collection protocol and techniques for serum cytokine, chemokine and soluble cytokine receptor quantification in the NCT01626495 study are described elsewhere16. Briefly, serum was collected serially for each subject on approximately 1, 4, 5, 9, 10, 14, 17, 21, 28, and 35 days post-CART-19 infusion and processed in the Translational and Correlative Studies Laboratory at the University of Pennsylvania within two hours of sample draw. Cytokine markers were additionally measured on the serum samples from 10 healthy volunteers16. Serum was isolated by centrifugation, aliquoted, and stored at −80°C. Serum cytokine and soluble cytokine receptor levels were later quantified in batch analysis on thawed samples using a Luminex bead array and a FlexMAP 3D system (Luminex, Austin, TX). Data acquisition and analysis was performed with xPONENT software (Luminex).
Data Analysis and Statistical Methods:
Baseline characteristics of subjects were summarized overall and by neurotoxicity outcome (presence/absence). Outcome associations with continuous variables were tested using the Wilcoxon rank-sum test, and with binary variables using the Fisher exact test. The associations between CRS grade and neurotoxicity outcomes were evaluated using the Cochran Armitage test for trend. Unless otherwise stated, statistical tests were two-sided and done at a 0.05 level.
Serial cytokine measurements were summarized as the peak measurement over the first three days post-infusion (3-day peak), as well as the peak measurement over the first 35 days post-infusion (35-day peak) in order to capture early and overall trends of these biomarkers during the period when CTL019 recipients experience neurotoxicity. Values less than the lower limit of detection were recorded as half the lower limit.
In univariate analysis, the association between each 3-day or 35-day peak values and the occurrence of either any significant neurotoxicity or the common neurotoxicities, was tested using the Wilcoxon rank-sum test. For each hypothesized association (e.g. 3-day peak cytokine level and common neurotoxicities) a Holm’s adjustment for multiple comparisons was applied to the significance level to account for the 43 different cytokines tested22. Results were organized into those cytokines elevated in both CRS and neurotoxicity, and those associated with either CRS or neurotoxicity alone.
Models to predict the incidence of common neurotoxicities over the first 35-days post-infusion were then created using multivariate analyses. Two modeling approaches were considered. First, a regularized regression elastic net procedure23, which accounts for the high dimension of the predictors to prevent overfitting, was applied to fit a logistic model for the development of common neurotoxicities (yes/no) from the candidate predictors: the 3-day peak value for each of the 43 cytokines measured and the baseline clinical characteristics (age, sex, history of seizure, and prior neurologic deficit). Two subjects were excluded in this portion of the analysis due to inadequate cytokine measurements. Forward selection logistic regression modeling using Akaike information criteria (AIC) was then performed among the selected predictors to examine a further reduced model. In a second approach, prediction models for the same outcome and candidate predictors were fit using a classification tree method. Area under the curve, sensitivity, specificity and positive predictive values were assessed for the fitted prediction models. Seven-fold and leave-one-out cross-validation (CV) methods were also used to assess predictive accuracy for the elastic net and tree procedures24. For 7-fold CV, the elastic net and tree models were each fit on a random subset of 6/7 the data (42 subjects) and then predictive accuracy was obtained on the remaining 7 subjects and then averaged across 1000 random subsets. Leave-one-out CV evaluated test error for each subject using a model fit without that subject’s data and averaged the error across subjects.
Data were analyzed using Stata version 14.0 (StataCorp, College Station, Texas, 2015), and R version 3.4.0 (R Core Team, Vienna, Austria 2017). Tree models were fit with the tree package in R, applying the default deviance split method; elastic net models were fit with the glmnet R package.
Standard protocol approvals, registrations, and patient consents.
The Children’s Hospital of Philadelphia institutional review board approved this sub-study of NCT01626495 data, with a waiver of informed consent (5/18/2016). The protocol itself was IRB-approved in 2012.
RESULTS
Cohort demographics and key clinical characteristic of the overall cohort and neurotoxicity subgroups are summarized in Table 1. Among the 51 participants examined, 49% percent were male, with a median age of 11.5 years (range 4–22). Of the 51 total subjects, 50 had B-cell ALL and one had T-cell ALL with CD19 expression. Amongst the total subjects, 61% (31/51) had no prior known history of CNS involvement, 31% (16/51) had known prior CNS involvement, and 8% (4/51) were unknown. All participants had relapsed/refractory ALL, with a median of 2 relapses prior to CART-19 infusion, prior stem cell or bone marrow transplantation in 34/51 (67%), and a history of brain radiation in 13/48 (27%). Neurologic comorbidities present prior to CTL019 infusion, including provoked seizures (16%), need for short-course or standing anti-epileptic drugs (6%), and pre-existing static or resolved neurologic deficits (20%; including cranial neuropathies [4/51], motor deficits, cerebellar abnormalities, and cognitive impairment), were also common. There may have been further neurologic comorbidities present prior to infusion that were not captured or reported prior to treatment, such as prior CNS toxicity from methotrexate.
Table 1:
Demographics and Clinical Characteristics of the Study Population
| All* | By Any Neurotoxicity | By Common Neurotoxicities* | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | (n=51) n(%) |
No (n=28) n(%) |
Yes (n=23) n(%) |
p-value# | No (n=30) n (%) |
Yes (n=21) n (%) |
p-value# |
| Male sex | 25 (49.0) | 15 (53.6) | 10 (43.5) | 0.58 | 16(53.3) | 9 (42.9) | 0.57 |
| Age | 11.5 (8, 15) | 11.5 (8.75,15) | 9 (4,15) | 0.95 | 12 (9, 15) | 9 (8, 15) | 0.66 |
| Oncologic History: | |||||||
| Malignancy: | |||||||
| B-cell ALL CNS- | 33 (63.6) | 19 (67.9) | 14 (60.9) | 0.65 | 19 (63.3) | 14 (66.7) | 0.74 |
| B-cell ALL CNS+ | 17 (34.5) | 9 (32.1) | 8 (34.8) | 10 (36.7) | 7 (38.1) | ||
| T-cell ALL | 1(1.8) | 0 (0.0) | 1(4.3) | 0 (0.0) | 1 (4.8) | ||
| Number of relapses | 2 (2, 3) | 2.5 (2,3) | 2 (2,3) | 0.80 | 2.5 (2, 3) | 2 (2, 3) | 0.17 |
| History of transplant | 34 (66.7) | 21 (75.0) | 13 (56.5) | 0.23 | 22 (73.3) | 12 (57.1) | 0.25 |
| History of blinatumomab | 4 (7.8) | 0 (0.0) 0.11 | 4 (17.4) | 0.11 | 1 (3.3) | 3 (14.3) | 0.33 |
| History of brain radiation | 13 (25.5) | 6 (21.4) | 7 (30.4) | 0.53 | 7 (23.3) | 6 (28.6) | 0.75 |
| Neurologic Comorbidities: | |||||||
| History of seizures | 8 (15.7) | 4 (14.3) | 4 (17.4) | 1 | 4 (13.3) | 4 (19.0) | 0.70 |
| Pre-existing neurologic deficit | 10 (19.6) | 3 (10.7) | 7 (30.4) | 0.15 | 2 (6.7) | 8 (38.1) | 0.01 |
| History of AED use | 3 (5.9) | 1 (3.6) | 2 (8.7) | 0.58 | 1 (3.3) | 2 (9.5) | 0.56 |
Categorical variables are described using n (%). Continuous variables are described using median (IQR).
p-values comparing those with and without neurotoxicity were calculated using Fisher’s exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables
CNS +: history of CNS involvement by leukemia
Neurotoxicity following CTL019 infusion was common in this pediatric and young adult sample, occurring in 23/51 (45%; 95% CI, 31–60%) subjects, 21/23 of these cases were classified into the a priori “common neurotoxicities” panel (Table 2). The most common type of neurotoxicity was encephalopathy, seen in 19/23 (83%) subjects with neurotoxicity, followed by any focal deficits (14/23, 61%), and seizures (4/23, 17%). Types of focal deficits observed included aphasia (6/23, 26%), and less commonly, vision change (n=5/23, 22%), facial droop (n=2/23, 9%), and others. Of note, there were no known cases of posterior reversible encephalopathy syndrome (PRES), or of cerebral edema; each of which are severe sequelae reported in CD19 CAR treated adults.5 19/23 (83%) of patients with neurotoxicity had neuroimaging (CT MRI) obtained, but mild cases may have been missed because neuroimaging was not routinely obtained. No one in this cohort died in the 2-month follow-up time period following CTL019 infusion. There were no significant differences in demographics, oncologic history, or neurologic comorbidities prior to CTL019 infusion between subjects that developed any neurotoxicity post-infusion and those who did not (Table 1). However, a history of pre-existing neurologic deficit prior to CTL019 infusion was associated specifically with development of the common neurotoxicities post-infusion (p=0.01) (Table 1). More detailed sub-analyses of these findings could not be performed given limited treatment history available (most patients had received prior oncologic care at outside institutions)
Table 2:
Incidence of Neurotoxicity over 35-days post CART-19 infusion (n=51)
| Number (%)* | |
|---|---|
| Any Neurotoxicity | 23 (45) |
| Common Neurotoxicities | 21 (41) |
| Encephalopathy | 19 (37) |
| Focal Deficit | 14 (28) |
| Aphasia | 6 (12) |
| Seizures | 4 (8) |
Percentages do not add up to 100%; some children had more than one type of neurotoxicity
CRS was very common, as previously reported16, occurring in 47/51 (92%; 95% CI, 81–98%) subjects at a median of three days after CTL019, infusion, ranging in grade from 1–4 (Figure 2). This is a consistent point estimate of incidence and timing as has now been reported in adult cohorts1,15,16,19,21,25. Neurotoxicity onset occurred at a median of six days post-infusion lagging behind CRS by a median of three additional days. Most neurotoxicity was brief, self-limited and did not require treatment, including steroids, other than antiepileptics; encephalopathy persisted the longest at a median of four days (IQR 3–9.5 days). There was a positive association between incidence of neurotoxicity and increasing grade of CRS (p<0.0001) (Figure 2).
Figure 2:
(A) Distribution of CRS grades. (B) Distribution of CRS grade by neurotoxicity. Incidence of neurotoxicity is positively correlated with increasing CRS grade (*p<0.05, Cochran-Armitage test for trend).
Serial 35-day cytokine profiles in patients who developed any neurotoxicity or common neurotoxicities were examined in order to understand the biology of the elevated cytokines. After the Holm adjustment for multiple comparisons, the 35-day peak levels of 28/43 cytokines were significantly higher in subjects who developed any neurotoxicity compared with those who did not. A different pattern of 28/43 cytokines had significantly elevated peak levels in those who developed common neurotoxicities compared to those that did not (Supplemental table 1). While many of these cytokines were elevated in both neurotoxicity and CRS, interleukin 2 (IL-2), soluble interleukin 4 receptor (sIL-4R), hepatocyte growth factor (HGF), and interleukin 15 (IL-15) were uniquely elevated in subjects with any neurotoxicity alone (Figure 3, Table 3).
Figure 3:
Select 35-day peak cytokines were significantly and specifically elevated in subjects with neurotoxicity.
Table 3:
35-day peak cytokines associated with neurotoxicity subtype
| Cytokine | CRS | Any Neurotoxicities | Common Neurotoxicities | Encephalopathy | Focal Deficit | Seizures |
|---|---|---|---|---|---|---|
| IL-2 | - | ↑ | ↑ | ↑ | - | - |
| sIL-4R | - | ↑ | ↑ | ↑ | - | - |
| HGF | - | ↑ | ↑ | ↑ | - | - |
| IL-15 | - | ↑ | ↑ | ↑ | - | - |
We next evaluated cytokines in the first three days after infusion of CTL019, but prior to the development of neurotoxicity. The purpose was to determine if early rise of certain cytokines could predict the development of later neurotoxicity, as we had previously done to develop a predictive model for CRS16. After adjustment for multiple comparisons, these 3-day peak cytokine levels were largely not significantly different between children and young adults with and without any neurotoxicity. Of the 43 cytokines, chemokines, and soluble receptors tested, only the 3-day peak soluble tumor necrosis factor receptor-1 (sTNFR-1) concentration was significantly higher in those who developed encephalopathy compared with those who did not. We next fit predictive models to identify potential biomarkers for common neurotoxicities. Starting with baseline clinical characteristics and 3-day peak cytokine levels of the 43 cytokines measured, the elastic net procedure identified 22 3-day peak cytokines that perfectly predicted development of common neurotoxicities (Figure 4). Using this model, there was perfect prediction of those who developed common neurotoxicities i.e. AUC=1, a sensitivity and specificity of 100%. Seven-fold cross-validation estimated an overall predictive accuracy of 75% for this model, with a 82% specificity and 67% sensitivity. Because k-fold cross-validation error can be pessimistic in small samples, the cross validation (CV) error was also re-estimated with leave-one-out cross validation, which maximizes the size of the training set. Leave-one-out CV estimated an overall accuracy of 82%, with a 86% specificity and 75% sensitivity.24. When incorporating these 22 cytokines into the forward selection regression prediction model, 6 cytokines (interleukin-12 (IL-12), soluble glycoprotein 130 (sgp130), soluble receptor for advanced glycation end-products (sRAGE), soluble tumor necrosis factor 1 (sTNFR-1), and soluble vascular endothelial growth factors 1 and 2 (sVEGFR-1 andsVEGFR-2) were selected. Using the alternate regression tree-based predictive strategy, 3-day peak levels of sTNFR-1 and sCD30 had a positive predictive value of 0.89, a sensitivity of 85% and a specificity of 93% (Figure 5). For this model, 7-fold cross-validation estimated a lower predictive accuracy than for the elastic net model, with an overall predictive accuracy of 66%, a 61% specificity and 68% sensitivity and leave-one-out CV was similar.
Figure 4:
(A) Tree modeling for common neurotoxicities; (B) Receiver Operating Characteristic curve for Tree model for common neurotoxicities.
DISCUSSION
We observed a 45% (95% CI 31–60%) incidence of significant neurotoxicity in this pediatric and young adult cohort with no fatal outcomes, which appears comparable to studies in adults (40–42%)10,13,14. This incidence point estimate is similar to broad JCAR015 experience (a CD19 CAR with a CD28 costimulatory domain); the Juno ROCKET trial demonstrated severe neurotoxicity in 56%.
The incidence of life-threatening neurotoxicity was lower than adult cohorts, especially those testing CD28 CD19 CAR T cell products, where there were five fatal cases of cerebral edema26. There were no known cases of cerebral edema in this cohort, no patients died of neurotoxicity and all patients had neurologic recovery.. In addition, specific clinical presentations of neurotoxicity differed in these children and young adults compared to some adult studies. For example, aphasia was noted in only 6 (12%; 95% CI 4–24%) subjects in this cohort, which is lower than the incidence of previously described language disturbance in adults (34%)14. Proposed treatment algorithms for CD28-based CD19 CARs require prophylactic admission and use of steroids in many cases of neurotoxicity, often at high doses27,. Conversely, our patients were infused in the outpatient setting, and did not require high-dose corticosteroids28.
There are several possible explanations for these differences in incidence and phenotypes. First, there may be differing underlying ontogenetic vulnerabilities to development of neurotoxicity of CAR T-cell infusions in the developing pediatric brain compared to that of the adult. Second, this pediatric and young adult cohort may have had differing neurologic co-morbidities prior to CTL019 infusion compared to adults, which may have impacted development of neurotoxicity. For example, pediatric ALL treatment protocols routinely include greater that 20 intrathecal administrations of methotrexate in frontline therapy. Finally, specific CAR T-cell products may have differing neurotoxicity profiles. Further study of neurotoxicity following CAR T-cell infusions must therefore examine age-specific epidemiology and pathogenesis of neurotoxicity specific to each CAR T-cell product.
Some investigators have postulated that neurotoxicity following CD19 CAR is directly attributable to CRS. One possible mechanism is based on the observation that individuals with severe neurotoxicity have evidence of endothelial activation and increased blood-brain barrier (BBB) permeability compared to those without14, allowing for passive transfer of inflammatory cytokines into the CNS. Our data may support this concept, with specifically elevated endothelial factors (VEGF, VEGFR) in both neurotoxicity and CRS, and one elevated endothelial factor (HGF) in neurotoxicity alone. Multiple other endothelial activation factors (sRAGE, VEGFR-1 and VEGFR-2) were selected as predictive of neurotoxicity in our elastic net model. However, this mechanism does not explain why the 35-day peak cytokine profiles differ between children and young adults with CRS alone and those with neurotoxicity. Moreover, not everyone with CRS, even high grade CRS, develops neurotoxicity, nor does everyone with neurotoxicity have CRS. Finally, neurotoxicity is not clearly mitigated by CRS treatment strategies13,14. Whether the latter observation is because CRS treatment is initiated after neuropathogenesis has already begun, CRS treatment does not effectively cross the blood brain barrier, or if a separate mechanism is at play, remains unknown.
One possible independent mechanism of neurotoxicity suggested by our data is a role of natural killer (NK) cell-mediated inflammation, given selectively elevated IL-2 and IL-15 in those with neurotoxicity. IL-2 and IL-15 are structurally related cytokines that provide signaling for NK cells, T cells, and B cells. Addition of either cytokine to human peripheral blood monocytes results in selective expansion of NK cells and T cells expressing various NK receptors (CD16, CD161, CD158a, CD158b, KIR3DL1, and CD94)29. While the role of NK cells in the periphery is well described, there are few data on the role of NK cells in the CNS. In one model of murine experimental autoimmune encephalomyelitis, NK cells in the CNS produced large amounts of CCL2, a chemoattractant for microglia30–32. In human in vitro models, activated NK cells appear to lyse resting microglia but spare activated microglia31,33,34. If higher levels of IL-2 and IL-15 are activating NK cells in some CD19 CAR recipients, NK cells may then shift CNS immune homeostasis in favor of activated microglial subtypes, and thus make affected individuals more susceptible to neurologic injury35–38. Interestingly, the Juno analysis of the JCAR015 trial also identified IL-15 as being associated with neurotoxicity26.
Alternatively, some patients may have a selective vulnerability to neurotoxicity. We found that a history of pre-existing neurologic deficit prior to CTL019 infusion was associated with incidence of common neurotoxicities post-infusion. However, clinical history was not a significant factor in later predictive models. The analyses may be discrepant because they were underpowered. This finding is important because it poses challenges for future identification of children at risk for neurotoxicity.
Identifying who is at risk for neurotoxicity before it occurs will be critical to the ongoing clinical care of these children. Our elastic net predictive model identified 3-day peak cytokines that collectively had a positive predictive value of one for later development of severe neurotoxicity. However, this high degree of accuracy should be interpreted with some caution in the context of a relatively small sample size. Though regularized regression controls over-fitting through the use of a tuning parameter and cross validation, there likely still remains some degree of over-fitting to the data due to small sample sizes, as evidenced by the 80% predictive accuracy estimated by leave-one out cross validation. The alternate tree-based predictive strategy identified a two-cytokine 3-day peak (high sTNFR-1 and low sCD30) model. sCD30 is a member of the TNF receptor superfamily and a marker of Th2 polarization39. Low levels of sCD30 and high levels of the classic, pro-inflammatory sTNFR-1 may therefore represent a shift towards a more inflammatory Th1 immune response that may be neurotoxic40,41,30,42,43. Validation and replication of these findings in a future prospective cohort will be critical. If elevations in sTNFR-1 and lower sCD30 are actually causative of neurotoxicity, the TNF pathway may be targeted with existing FDA-approved immunomodulatory therapy.
While this study provides a comprehensive analysis of cytokine profiles associated with neurotoxicity, it has several limitations. First, the sample size was 51 pediatric and young adult patients treated with CTL019 at our institution. Therefore, analyses may be underpowered, and truly significant associations may therefore be underestimated. Second, information regarding clinical history, neurotoxicity incidence, and management was identified retrospectively, rather than through a prospective standardized neurologically-focused assessment. Some historical features may have been missed or misclassified, and some outcome measures may have been misclassified if not documented clearly in medical records. We did apply stringent definitions to our neurotoxicity outcomes, excluding more minor symptoms that may have been included in prior studies and capturing those that are objectively documented in medical records (encephalopathy, seizure, and aphasia). Thus, it is more likely that we are missing cases, rather than misclassifying, which could have biased results towards the null hypothesis if the reasons for misclassification were unassociated with outcomes. Third, cytokine data was limited to set time points, so true 3-day and 35-day peak values may have been missed by this sampling. However, any bias generated by this method should be non-differential across subjects. The predictive models for common neurotoxicities were fit on a small sample size and their generalizability and predictive accuracy need further study and validation in an independent cohort. It is also possible that different types of neurotoxicity have distinct pathophysiologies. We therefore may have obscured significant findings in specific subgroups of neurotoxicity. Finally, because each currently approved CAR T-cell product has distinctive signaling domains (e.g. CD28 vs. 4–1BB), it is also possible that the neurotoxicity profiles may be specific to each product. Thus, caution should be used in generalizing these results describing neurotoxicity of CTL019 to all CD19 CAR products.
In summary, we have demonstrated that neurotoxicity is common following CTL019 infusion in pediatric and young adult patients. While this incidence is positively associated with higher grade of CRS, differing cytokine profiles between children and young adults with neurotoxicity and those with isolated CRS suggest an independent pathophysiology in neurotoxicity that may not be targeted by CRS therapy alone. The novel identification of 3-day peak levels of sTNFR-1 and sCD30 predicting neurotoxicity in these children and young adults should be validated in other cohorts. The ability to predict which patients will develop severe neurotoxicity is enormously important as cellular immunotherapies continue to expand. If future studies confirm the biologic importance of sTNFR-1 in the development of neurotoxicity, this will have therapeutic implications as sTNFR-1 is targetable. Future studies will determine whether such targeted therapy against the TNF pathway can reduce the risk of neurotoxicity, analogous to the dramatic clinical benefit of anti-IL6 therapy for CRS. These data provide the foundation for future pediatric investigation into targeted therapy to treat or prevent neurotoxicity following CTL019 therapy, and more broadly frame questions to be addressed with other CAR T-cell therapies as well.
Acknowledgement:
The initial clinical trial was supported by Novartis clinical trial support and the CHOP Cancer Immunotherapy Frontier Program. This project was supported by the National Institutes of Health (NS094069). This manuscript was written in memory of Jessica Panzer, MD PhD.’
Footnotes
Potential Conflicts of Interest: Nothing to report.
References:
- 1.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DW, Barrett DM, Mackall C, et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res 2012;18(10):2780–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial [Internet]. Lancet 2015;385(9967):517–528.Available from: http://www.sciencedirect.com/science/article/pii/S0140673614614033%5Cnhttp://www.sciencedirect.com/science/article/pii/S0140673614614033/pdfft?md5=740845b29a86193acb1334f97f35cd72&pid=1-s2.0-S0140673614614033-main.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett DM, Grupp SA, June CH. Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street. J. Immunol 2015;195(3):755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davila ML, Sauter C, Brentjens R. CD19-Targeted T Cells for Hematologic Malignancies: Clinical Experience to Date. Cancer J 2015;21(6):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med 2018;378(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med 2013;5(177):177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santomasso B, Park JH, Riviere I, et al. Neurotoxicity Associated with CD19-specific Chimeric Antigen Receptor T cell Therapy for Adult Acute Lymphoblastic Leukemia (B-ALL) (S23.008) [Internet]. Neurology 2018;90(15 Supplement)Available from: http://n.neurology.org/content/90/15_Supplement/S23.008.abstract [Google Scholar]
- 14.Gust J, Hay KA, Hanafi L-A, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 2014;20(2):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia Clinical Description of Patients 2016;(June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3(4):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadelain M From the guest editor: The rise of CAR therapy: the CD19 paradigm, and beyond. Introduction. Cancer J 2014;20(2):105–106. [DOI] [PubMed] [Google Scholar]
- 19.Grupp S a., Kalos M, Barrett D, et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia [Internet]. N. Engl. J. Med 2013;368(16):1509–1518.Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013;121(26):5154–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm S Board of the Foundation of the Scandinavian Journal of Statistics 2013;6(2):65–70. [Google Scholar]
- 23.Zou H, Hastie T. Regularization and variable selection via the elastic net 2005;301–320.
- 24.Friedman J, Tibshirani R, Hastie T. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer Ser. Stat 2001; [Google Scholar]
- 25.Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr. Opin. Pediatr 2014;26(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deangelo DJ, Ghobadi A, Park JH, et al. Clinical Outcomes for the Phase 2, Single-Arm, Multicenter Trial of JCAR015 in Adult B- ALL (ROCKET Study) 2017;2017.
- 27.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T - cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol 2017;15(January):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’ [Internet]. Nat. Publ. Gr 2018;15(4):1.Available from: 10.1038/nrclinonc.2018.19 [DOI] [PubMed] [Google Scholar]
- 29.Dunne J, Lynch S, O’Farrelly C, et al. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J. Immunol 2001;167(6):3129–3138. [DOI] [PubMed] [Google Scholar]
- 30.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol 2011;11(11):762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi F-D, Ljunggren H-G, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat. Rev. Immunol 2011;11(10):658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao J, Liu R, Piao W, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med 2010;207(9):1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lunemann A, Lunemann JD, Roberts S, et al. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J. Immunol 2008;181(9):6170–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunemann JD, Munz C. Do natural killer cells accelerate or prevent autoimmunity in multiple sclerosis? Brain 2008;131(Pt 7):1681–1683. [DOI] [PubMed] [Google Scholar]
- 35.Durafourt BA, Moore CS, Zammit DA, et al. Comparison of Polarization Properties of Human Adult Microglia and Blood-Derived Macrophages. Glia 2012;727(January):717–727. [DOI] [PubMed] [Google Scholar]
- 36.Cherry JD, Olschowka JA, Banion MKO. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 2014;11(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.London A, Cohen M, Schwartz M. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell. Neurosci 2013;7(April):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orihuela R, Mcpherson CA, Harry GJ. polarization and metabolic states 2016;649–665. [DOI] [PMC free article] [PubMed]
- 39.Shinoda K, Sun X, Oyamada A, et al. CD30 ligand is a new therapeutic target for central nervous system autoimmunity. J. Autoimmun 2015;57:14–23. [DOI] [PubMed] [Google Scholar]
- 40.Wang W-Y, Tan M-S, Yu J-T, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med 2015;3(10):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson NG, Wieggel WA, Chen J, et al. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J. Immunol 1999;163(7):3963–3968. [PubMed] [Google Scholar]
- 42.Downen M, Amaral TD, Hua LL, et al. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia 1999;28(2):114–127. [PubMed] [Google Scholar]
- 43.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc. Brain Metab. Rev 1994;6(4):341–360. [PubMed] [Google Scholar]