Abstract
Rapid advances in neuroimaging and network science have produced powerful tools and measures to appreciate human brain organization at multiple spatial and temporal scales. It is now possible to obtain increasingly meaningful representations of whole-brain structural and functional brain networks and to formally assess macroscale principles of network topology. In addition to its utility in characterizing healthy brain organization, individual variability, and life span-related changes, there is high promise of network neuroscience for the conceptualization and, ultimately, management of brain disorders. In the current review, we argue for a science of the human brain that, while strongly embracing macroscale connectomics, also recommends awareness of brain properties derived from meso- and microscale resolutions. Such features include MRI markers of tissue microstructure, local functional properties, as well as information from nonimaging domains, including cellular, genetic, or chemical data. Integrating these measures with connectome models promises to better define the individual elements that constitute large-scale networks, and clarify the notion of connection strength among them. By enriching the description of large-scale networks, this approach may improve our understanding of fundamental principles of healthy brain organization. Notably, it may also better define the substrate of prevalent brain disorders, including stroke, autism, as well as drug-resistant epilepsies that are each characterized by intriguing interactions between local anomalies and network-level perturbations.
Keywords: connectome, network neuroscience, neuroimaging, MRI, pathoconnectomics
Introduction
Neuroscience is moving beyond a modular apprehension of brain organization, which emphasizes the relevance of individual regions, toward an approach that prioritizes the wiring and interactions between areas (Schroter et al., 2017; van den Heuvel et al., 2015). In the study of the healthy human brain, the last decade has witnessed major advances in characterizing large-scale structural and functional networks, and in relating network descriptions to interindividual variability, brain development and aging, skill learning and plasticity, as well as cognition and affect (Misic and Sporns, 2016). While the shift from region-centric to network-based analysis is welcome and truly exciting, the increased focus toward high-level network models should not undermine relevant information embedded in regional structural and functional properties as well as local circuits.
This review argues for a description of the human brain that, while embracing network-level paradigms, should also take advantage of the increasingly detailed information on individual nodes and edges that can be gathered with modern neuroimaging. Such an approach offers a rich description of the graph's individual elements, including microstructural properties, local structural markup, and the embedding of a region within large-scale networks. Addressing interactions between these domains may offer utility in understanding fundamental principles of brain organization and structure/function correspondence. Moreover, fusing these different levels of analyses may serve the study of brain disorders, in which anomalies may vary in location, size, and which have been shown to differentially affect whole-brain networks.
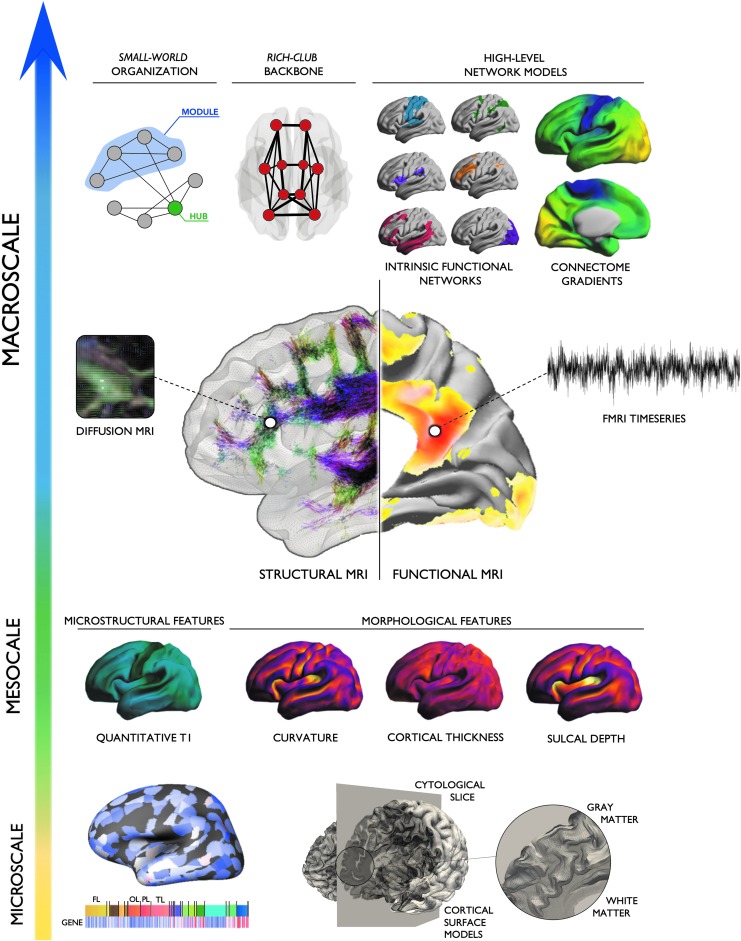
In what follows, we overview contributions of network neuroscience to the understanding of macroscale brain organization, developmental and aging-related processes, and the study of individual variations. We briefly outline parallel advances in high-resolution neuroimaging of local morphology, tissue microstructure, and function. Bridging the gap between network-level descriptions and these increasingly detailed markers of local properties promises a comprehensive characterization of the brain [see Fig. 1; for additional reviews, see Bullmore and Sporns (2009); Stam (2014)]. Furthermore, recent years have witnessed increased efforts to enrich neuroimaging analysis with nonimaging data, including information on gene expression, protein synthesis, and cytoarchitecture. In addition to discussing the promise of such multiscale paradigms to study healthy brains, we illustrate the potential of microstructure-informed connectomics in the study of diseases. We specifically focus on three broad classes of disorders, namely acquired lesions (such as strokes), neurodevelopmental disorders mainly characterized by network-level imbalances (such as autism), and finally, drug-resistant epilepsies that show an intriguing interplay between focal lesions and network-level anomalies.
FIG. 1.
Enriched connectomics. Multiscale connectomics integrate various aspects of brain properties located along a continuum between microscale (gene expression, cytoarchitecture), mesoscale (MRI-derived markers of intracortical microstructure and morphology), and macroscale topology (small-worldness, core-periphery, modularity, connectome gradients). Leveraging parallel advances in high-resolution neuroimaging, detailed markers of local properties can be used to inform structural and functional network models, and vice versa. Fusing these different levels of analyses may shed light on fundamental principles of structure/function correspondence and unravel a more precise characterization of healthy and diseased brain organization.
Modeling Brain Networks
Over the past decade, the neuroimaging and neuroscience communities have witnessed a paradigm shift away from the focus on single regions and toward models that emphasize connectivity (Bassett and Bullmore, 2009; Bassett and Sporns, 2017; Bullmore and Sporns, 2009; Craddock et al., 2013). Increased availability of high-definition and multimodal neuroimaging, the maturation of data coregistration and integration processing pipelines, as well as advances in complex systems analytics have all contributed to an explosion of interest in the field. With respect to network-level approximations, diffusion MRI tractography has made it possible to approximate fiber tracts and derive structural connectomes in individual subjects (Jbabdi et al., 2015). In addition, analysis of statistical dependencies of functional MRI (fMRI) signals between different regions, especially in “resting” brains not engaged by any specific and controlled task, has been used to target multiple intrinsic functional networks using a single, preferably long acquisition (Biswal et al., 1995; Fox et al., 2005; Friston, 1994). Finally, structural MRI covariance analysis can tap into patterns of coordinated maturational trajectories in populations (Evans, 2013) and, more recently, in single individuals (Seidlitz et al., 2017).
The field of network science has offered formal analytics to parameterize whole-brain networks. Notable examples include decomposing the whole brain into a set of smaller communities (Girvan and Newman, 2002; Meunier et al., 2009; Newman and Girvan, 2004) and mapping network properties back to individual nodes, for example, in terms of overall connectivity degree or regional routes of communication efficiency (Sporns et al., 2007; van den Heuvel and Sporns, 2013). In parallel, graph-theoretical metrics can capture macroscale topology derived from fMRI (He et al., 2009; Honey et al., 2009; Salvador et al., 2005; Watts and Strogatz, 1998), diffusion MRI (Gong et al., 2009; Hagmann et al., 2008; Iturria-Medina et al., 2007), and MRI covariance analysis (Bassett et al., 2008; Chen et al., 2008; He et al., 2007), together with metabolic uptake data (Hu et al., 2015) as well as meta-analytical activations (Crossley et al., 2013). Collectively, these studies have shown that the human brain is neither organized purely randomly nor regularly, but rather according to principles that incorporate high clustering within segregated communities together with short paths between them. This small-world organization enables functional specialization and integration (Sporns et al., 2004), and closely relates to modularity, that is, network decomposability (Bullmore and Sporns, 2009). Modularity may offer adaptability and robustness to changing environmental conditions (Meunier et al., 2010), help to segregate externally oriented from internal processing (Vidaurre et al., 2017), and promote diverse functional dynamics (Gu et al., 2017; Kaiser and Hilgetag, 2010). Brain networks also seem to adhere to principles of hierarchy; connectomes have been subdivided into core and peripheral regions, a finding sometimes dubbed the rich-club principle, where a set of high-degree nodes are excessively interconnected (van den Heuvel and Sporns, 2011). Interestingly, the rich-club subnetworks seem to accumulate most long-range connections, indicating their role as a backbone for cross-module connectivity (van den Heuvel and Sporns, 2011) and functional diversity (Senden et al., 2014). By counting connections of a region (or alternatively the number of efficient paths passing through), network properties can be mapped back to individual nodes (Buckner et al., 2009; Hagmann et al., 2008; Zuo et al., 2012). Such “centrality mapping” can help to visualize connectome organization and accordingly identify hub regions that play key roles in network organization, communication, and dynamics. As an illustration of how different levels of network organization can be integrated, centrality mapping techniques can be enriched by modularity information, classifying hubs into those with provincial roles (mediating primarily connectivity within modules) versus connector hubs mediating between-module cross talk (Sporns et al., 2007). Conversely, it is also possible to simulate random failures as well as targeted attacks on specific nodes or edges to assess the relevance of specific network elements on global topology and community decomposition (He et al., 2008).
In addition to providing descriptors of human brain network organization, connectome properties may be useful markers of interindividual variability, life span- and experience-related changes, structure/function relationships, and ultimately behavior. Indeed, despite consistency of core network features across subjects (e.g., small-word organization, rich-club backbone, the layout of large-scale communities), there is also considerable variability across individuals (Smith et al., 2015; Tavor et al., 2016) and even within a given subject over time (Poldrack et al., 2015). Functional connectivity mapping has indeed demonstrated that some brain regions show more consistent connectivity profiles across individuals, while others have high variability (Mueller et al., 2013). Variable regions are often found in “multimodal association” cortices and, interestingly, correspond to those having undergone marked evolutionary expansion (Mueller et al., 2013). In the study of individual differences, recent work predicted subject identity using connectome “fingerprints” (Finn et al., 2015; Miranda-Dominguez et al., 2014; Xia and He, 2017), while others have used multivariate techniques to associate connectomes with cognitive/affective phenotypes (Smith et al., 2015; Tavor et al., 2016). These results illustrate significant promise in connectome-level studies to move beyond population-level inferences to inferences about single subjects, exploiting the uniqueness in network organization and the relationship to behavior. Accordingly, network neuroscience approaches have begun to address macroscopic plasticity as a result of experience and skill learning (Bassett and Mattar, 2017; Telesford et al., 2017). Finally, with ongoing efforts to make large-scale data sets available (Van Essen et al., 2012), often already in preprocessed and curated form, findings in young adults are increasingly complemented by targeted connectome analyses across the life span, covering developing (Satterthwaite et al., 2014) as well as aging cohorts (Zhou et al., 2012).
Enriching Connectomics with Local Information
In addition to the adoption of connectomics as one of the prevailing paradigms to study large-scale organization, there is also promise as well as need in refining the information from local and microstructural scales (Weiskopf et al., 2015). By guiding and enriching the definition of nodes and edges, the building blocks of connectomes, advances in microscale definition may improve biological validity of connectome models. Moreover, they lend novel opportunities in addressing interactions between microstructural and macroscale properties and ultimately increase the explanatory power of connectomics to address mechanistic as well as generative principles of brain organization.
At the scale of individual nodes, there is already considerable diversity in definition, a situation relating to the long-standing debate of what exactly constitutes a meaningful element or brain region. Postmortem anatomy has emphasized cytoarchitectural criteria, including lamination and cellular composition, for parcellations (Amunts et al., 2007). Alternative approaches build parcellations from in vivo measures directly. Features previously studied include anatomical landmarks and sulco-gyral patterns derived from cortical surface morphometry, and increasingly detailed markers of tissue microstructure obtained from quantitative MRI, including measurement of T1 relaxation times that may reflect intracortical myelin content (Waehnert et al., 2016). Structural markers are complemented with task-based functional activations as well as resting-state functional or structural connectivity (Eickhoff et al., 2015). Leveraging the Human Connectome Project data set, a recent study integrated spatial patterns in resting-state connectivity, task-based activations, image intensity, and cortical thickness measurements to parcellate the neocortex into 360 areas (Glasser et al., 2016). Although initial parcellations were generated at the group level, the authors notably devised an algorithm that parcellates an individual brain in native space, sidestepping potential problems relating to spatial normalization and between-subject averaging in a stereotaxic space (Braga and Buckner, 2017). Using spectral clustering of diffusion tractograms, another group identified 210 cortical/subcortical parcels (Fan et al., 2016), emphasizing that parcellations based on different input features may not necessarily converge toward equivalent solutions. Stability across algorithm choices and spatial scales will become increasingly relevant, as will be the definition of formal criteria to fuse methods. Recently, parcellations incorporated formal consensus procedures to maximize consistency across modalities, subjects, and data sets (Kelly et al., 2012). The continued move toward quantitative neuroimaging and increased access to nonimaging data in MRI-compatible reference frames provides new possibilities for parcellation enrichment and validation.
As for the nodes, the characterization of network edges has benefited from developments in MRI acquisition and modeling. Moving beyond early studies that were predominantly based on binary graphs, analytics is increasingly incorporating edge weighting when calculating network degrees, clustering, and efficiency (Rubinov and Sporns, 2010). Structural connectome studies based on diffusion MRI tractography have, for example, initially weighted edge strength through streamline counts, connectivity probabilities, and diffusion tensor metrics such as fractional anisotropy. In recent years, a microstructure-informed connectomic framework has become increasingly adopted, through which weights are derived from quantitative MRI markers of myelin, axon diameter (Assaf et al., 2013), and g-ratios (Mancini et al., 2017). In contrast to binary edge definitions or a weighting based on algorithm-dependent parameters, such as the number of streamlines, incorporating microstructural parameters into connectomics may provide more direct measures of conduction velocity (Mancini et al., 2017). Furthermore, the use of more sophisticated diffusion models resolving crossing fibers may reduce the number of false positive/negative connections, ultimately resulting in a better map of white matter connectivity (Jbabdi et al., 2015; Maier-Hein et al., 2017). Finally, enhancing the definition of structural connections will be of vital use in building predictive models of structure/function relationships (Daducci et al., 2016) and also in determining appropriate measures of functional connectivity itself (Buckner et al., 2013).
In addition to leveraging increasingly detailed data on individual nodes and edges based on advanced neuroimaging technique, there have been emerging efforts to enrich analyses with neurobiological information that is not neuroimaging derived. Examples include the Allen Institute transcriptional gene atlas (Hawrylycz et al., 2012; Sunkin et al., 2013), recently cross-referenced against morphological (Whitaker et al., 2016), functional connectivity (Krienen et al., 2016), and structural connectivity data (Romme et al., 2017). Other studies have related in vivo connectomics with atlas-based information on regional cytoarchitecture (Hilgetag et al., 2016) or chemoarchitecture (Turk et al., 2016). Interestingly, regions with similar microscale properties seem to preferentially interconnect, supporting homophily as an important generative principle of brain network formation (Betzel and Bassett, 2017; Betzel et al., 2016). By guiding and enriching the definition of nodes and edges, such multiscale advances may improve the biological validity of connectome representations and further increase the explanatory power of connectomics.
The Interplay of Local and Distributed Disturbances in Brain Disorders
In addition to its promise in typifying healthy brain organization, emerging work supports the potential of microstructure-enriched connectomics in the study of prevalent brain disorders. Three broad classes of disorders where local- and network-level anomalies interact in intricate ways are stroke, autism, and drug-resistant epilepsies. Stroke was chosen as an example of an acquired focal lesion that may lead to widespread network perturbations. Autism spectrum disorders (ASD) were selected as neurodevelopmental conditions primarily associated with connectome miswiring, but generally without consistently localizable substrate (i.e., lesional finding or brain abnormality typically detectable on an MRI). Finally, drug-resistant epilepsies often show a complex interplay between a focal lesion and widespread network anomalies, likely due to interacting developmental- and disease-related processes.
Stroke
Ischemic stroke is a leading cause of adult disability, with as many as 40% of stroke survivors classified as chronically disabled, being dependent on others for activities of daily living (Krueger et al., 2015). In addition to functional impairments, costs related to poststroke rehabilitation and treatment of morbidity and mortality further contribute to its economic burden worldwide (Mittmann et al., 2012; Rosamond et al., 2008).
Current models of stroke recovery based on functional and structural connectivity analyses continue to explain more and more of the variance in motor and cognitive recovery (Krueger et al., 2015; Stinear et al., 2012). Nonetheless, these have not been fully translated into clinical practice, that is, stroke survivors are not yet stratified into different subpopulations based on their residual network profile, and rehabilitation interventions continue to use a “one-size-fits-all” approach (Ward, 2017). A key impediment to the development of effective, individualized treatment may lie in the lack of a clear understanding of the dynamic interactions between local/regional circuit properties and macroscale functional reconfiguration that actively promote regeneration and recovery both at the lesion site and in remote areas after stroke. Previous studies have suggested that investigation of the underlying response that mediates the spread of pathology after an infarct is likely to yield a better understanding of the mechanisms that drive brain reorganization after an infarct (Carrera and Tononi, 2014; Rossini et al., 2003). This concept dates back to 1914 when Constantin von Monakow coined the term “diaschisis,” referring to a transient metabolic or functional dysfunction in areas that are remote to the primary site of injury (von Monakow, 1914). Although diaschisis is a well-established phenomenon after stroke, network analyses have become instrumental to further extend this concept. Resting-state fMRI experiments in patients with heterogeneous lesions, for instance, have shown that brain anomalies often extend beyond the primary lesion site but remain nevertheless constrained and predictable from connectivity patterns (Nomura et al., 2010). Likewise, computational models have shown that focal lesions have widespread dynamical consequences on system-wide integrative processes (Lariviere et al., 2018), which are influenced, to some extent, by the topological characteristics of the areas harboring the lesion (Honey and Sporns, 2008).
The connectivity between brain regions depends on axonal projections within white matter, a compartment highly susceptible to ischemic injury (Matute et al., 2013). Indeed, the white matter is less vascularized than gray matter (Fisher, 2011) and its anaerobic resistance declines with aging (Hamner et al., 2011). Furthermore, neuronal loss leads to Wallerian degeneration of axonal projections and contributes to disconnections of spared cortical areas, thus producing additional clinical deficits (Jones et al., 2013; Kuhn et al., 1989; Thomalla et al., 2005). The degree of disconnection is not always appreciable on MRI and brain damage is often underestimated, leading to less accurate models of poststroke impairments and potential for recovery.
Considering that a structural lesion resulting from a stroke may disturb the balance between local and large-scale connectivity, the concept of diaschisis can now be understood with respect to three categories: focal diaschisis (local changes in metabolism and neural activity), connectional diaschisis (changes between nodes of a same network), and connectome diaschisis (changes affecting the structural or functional connectome, including disconnections and reorganizations of subnetworks) (Carrera and Tononi, 2014). While the former often contributes predominantly to clinical deficits observed after subcortical lesions (e.g., thalamic strokes), network alterations seen in “connectional” and “connectomal” diaschisis appear to relate more consistently to clinical findings when lesions affect cortical areas.
Strikingly, investigations of multiscale, multimodal, or cross-network interactions in stroke research have so far been largely neglected. Influences of the microstructural state of the corticospinal tract on large-scale anomalies and motor performance have only been investigated sporadically (Borich et al., 2014; Carter et al., 2010; Schulz et al., 2015; Wu et al., 2015). One study, for instance, reported that interhemispheric functional connectivity significantly related to motor impairment only in patients with intact corticospinal tract, whereas those with greater damage did not show an association (Carter et al., 2012). While the interplay between structure and function at specific time points during stroke recovery has not yet been systematically studied, understanding cross-network interactions may be critical to move rehabilitation research forward. In fact, data based on microscale properties and macroscale organization may provide a rationale for patient stratification in addition to providing a neurobiological basis for rehabilitation interventions that target the optimal circuits for effective, individually tailored treatments. More specifically, network modeling could provide important information about the impact of focal lesions on overall network profiles. These models may extend standard connectomic frameworks, which generally focus on edge associations between two regions, by also incorporating indirect routes of information spread through a third region. In other words, information spread can be modeled as a cascading process through multiple paths and walks. A recent study leveraged these models based on structural connectome information in stroke, and demonstrated gains in predicting the degree of language impairments in individual patients [Fig. 2; Del Gaizo et al. (2017)]. Importantly, while many connections are lost in strokes, indirect pathways may still exist and may represent viable neurobiological targets to promote recovery.
FIG. 2.
Dynamic network modeling in stroke (Del Gaizo et al., 2017). (A) The poststroke lesion overlaid in blue. The superior temporal gyrus is seeded (red). Based on the weight of structural connectivity between the superior temporal gyrus and the whole-brain connectome, there is a spreading cascade of network involvement (step-wise progression shown by arrows). The dynamic model is an individual map of network activation with the color bar illustrating the normalized number of steps taken to reach each region. (B) Brain regions (left) and connections (right) color coded according to their weighted influence on the connectivity model used to predict severity of aphasia (assessed using WAB-AQ). Findings suggest that ensemble models, which combined information on regional damage, connectome link weights, and connectivity dynamics, can better predict aphasia severity (WAB-AQ) compared with unimodal approaches. The histograms illustrate how often the ensemble method was superior to the unimodal approaches. The x-axes in the histograms denote the difference between the Pearson correlation coefficient obtained from the ensemble method minus the one obtained from the unimodal approach: regional damage (“regions”) and connectome links (“connections”). A value of >0 indicates a higher Pearson correlation coefficient in the ensemble method. WAB-AQ, Western Aphasia Battery aphasia quotient.
Autism
ASD is among the most common developmental conditions, affecting currently around 1 in 70 children. With an onset in early childhood, autism spectrum conditions manifest with rather heterogeneous behavioral symptoms, but generally, deficits in communication and social cognition, often with repetitive behaviors/interests as well as sensory anomalies. While frequently assessed with neuroimaging, the literature has so far provided a rather complex pattern of alterations when comparing cohorts with autism with controls, dampening optimism that a single and consistent brain pattern can be identified.
At the level of brain structure, cortical thickness and voxel-based morphometric findings have indeed suggested both increases in gray matter, often in frontal and temporal regions (Ecker et al., 2013a; Valk et al., 2015; van Rooij et al., 2017), and also decreases (Wallace et al., 2010), null results (Haar et al., 2014), or mixed patterns (Ecker et al., 2012). Findings seem to vary with age of the cohorts studied, with increases in thickness being more consistently found in group-level analysis of children than in adults (Valk et al., 2015), suggesting an interaction between the disease and developmental trajectories (Raznahan et al., 2010; Smith et al., 2016). Earlier observations using MRI volumetry have indicated aberrant brain growth in early age and altered maturation in late childhood and adolescence (Courchesne et al., 2003). Likewise, histological reports have pointed to anomalies in prenatal stages, showing the occurrence of ectopic neurons in the white matter as well as cortical blurring, possibly reflecting incomplete neuronal migration (Avino and Hutsler, 2010). Recently, increased cortical blurring in autism has been confirmed in vivo using surface-based group-level analysis of MRI contrast (Andrews et al., 2017; Hong et al., 2017c). Furthermore, atypical cortical organization in autism was suggested using multidimensional cortical profiling, showing distorted surface area, curvature, and geodesic distance (Ecker et al., 2013a,b).
Challenges in the exact localization of structural anomalies across the spectrum could relate to a malformative process that variably affects network nodes and their communication patterns, ultimately contributing to the broader autism phenotype. In this scenario, anomalies in the formation and organization of large-scale networks may differentially contribute to impairments in higher order cognition or sensory integration. Accordingly, this may also be associated with some of the inconsistencies reported in the autism-related resting-state functional imaging literature, among which the majority has focused on mapping connectivity anomalies. Indeed, findings appear somewhat heterogeneous in location and even direction, with some studies reporting scattered functional connectivity increases in addition to prevailing and most frequently reported connectivity reductions (Deen and Pelphrey, 2012; Heinsfeld et al., 2018; Keown et al., 2013; Uddin, 2015; Yahata et al., 2016). Inconsistent differences at the group level may propagate down to the individual subject level, and classifiers designed to dissociate ASD from controls have provided rather variable accuracies, often below behavioral criteria (Heinsfeld et al., 2018; Plitt et al., 2015). Collectively, these results suggest that the ASD phenotype might arise from broader imbalances in functional architecture across individuals, which may not necessarily be localizable to specific regions but rather may reflect overarching principles of network-level organization, a finding also supported by higher variability in autism functional connectivity patterns than in controls (Nunes et al., 2018).
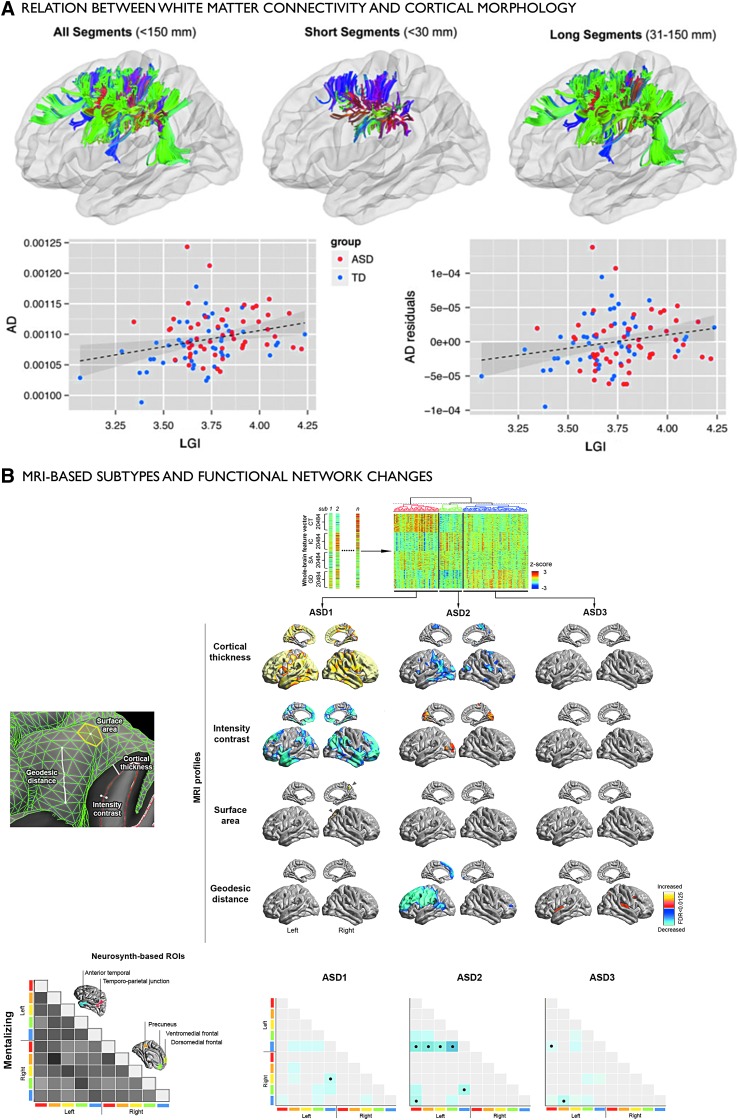
In addition to consolidating previous findings, for example, by providing more biologically plausible metrics of connectivity, enriched connectomics may be useful to assess the link between local anomalies and large-scale network perturbations in ASD (Fig. 3). Of interest will be to clarify the association between changes in local cortical organization (e.g., cortical thickness increases, cortical interface blurring, and laminar alterations) and structural and functional connectivity. Diffusion MRI group-level analyses have shown alterations in the microstructure and architecture of deep as well as superficial white matter tracts related to default-mode, auditory, and visual cortices (Barnea-Goraly et al., 2004; Fletcher et al., 2010; Sundaram et al., 2008). Analyzing diffusion MRI as well as cortical morphology, a recent study observed abnormalities in cortical folding together with white matter microstructure, suggesting coupled effects in both compartments (Ecker et al., 2016).
FIG. 3.
Findings in ASD showing interactions between local- and network-level anomalies. (A) Top: Findings from a previous combined diffusion MRI tractography and MRI morphology study (Ecker et al., 2016). Length distribution of short- and long-range white matter tract classes. Bottom: Findings indicate a significant positive relationship between AD in short-distance tracts and the LGI across groups. (B) Multidimensional MRI clustering of autism heterogeneity, and relation to large-scale networks (Hong et al., 2017c). Top: Several MRI-derived morphological features (cortical thickness, intensity contrast, surface area, geodesic distance) were used to identify three data-driven ASD subtypes (ASD-I, -II, -III), which were subsequently compared to typically developing controls (increases/decreases in red/blue). Bottom: Functional connectivity between regions of interest derived from a meta-analysis of previous studies related to mentalizing. Connectivity decreases relative to controls were observed in ASD-II and ASD-III, primarily in temporal and medial frontal cortices. Findings reproduced with permissions. AD, axial diffusivity; ASD, autism spectrum disorder; LGI, local gyrification index.
Epilepsy
Around 30% of epileptic patients suffer from seizures that cannot be controlled by antiepileptic mediation and are therefore generally considered surgical candidates, given the established benefits of surgery for seizure control and quality life in epileptic patients (Engel et al., 2012; Wiebe et al., 2001). Neuroimaging of drug-resistant epilepsies has traditionally focused on the detection of focal lesions considering its relevance in surgical planning. Indeed, the MRI detection and subsequent resection of a lesion are currently the best predictors of postsurgical seizure freedom (Bernasconi et al., 2011). In temporal lobe epilepsy, the most prevalent syndrome, patients commonly present with mesiotemporal sclerosis, a lesion characterized by variable cell loss and gliosis in the hippocampal formation and adjacent structures (Blumcke et al., 2013; Thom et al., 2009). Frontal lobe epilepsy, another prevalent surgically remediable syndrome, often relates to cortical dysplasia, a malformation characterized by laminar and cytological anomalies. Surface-based structural MRI features of morphology as well as tissue contrast and intensity have provided an increasingly detailed in vivo characterization of these lesions and have been useful in guiding algorithms for automated lesion detection (Hong et al., 2014). Furthermore, these markers can capture interpatient variability and have been statistically related to histological subtypes (Bernhardt et al., 2015b, 2016; Hong et al., 2017b).
In addition to its utility for targeted local studies, MRI has also been instrumental in demonstrating distributed pathological networks in both syndromes [Fig. 4; Bernhardt et al. (2015a); Gleichgerrcht et al. (2015); Richardson (2012)]. In temporal lobe epilepsy, converging evidence from voxel-wise and surface-based morphometry (Bernhardt et al., 2010; Bonilha et al., 2004; Keller and Roberts, 2008; McDonald et al., 2008), quantitative longitudinal relaxation time mapping (Bernhardt et al., 2017), and diffusion imaging (Concha et al., 2012; Keller et al., 2017; Liu et al., 2016) has revealed anomalies beyond mesiotemporal regions and temporo-limbic tracts, often extending into a broad cortical/subcortical territory. In dysplasia-related frontal lobe epilepsy, cortical thickness and curvature analyses also emphasized widespread anomalies (Hong et al., 2016), complemented by diffusion MRI studies suggesting architectural and microstructural alterations of intrahemispheric fibers as well as the corpus callosum (Campos et al., 2015; Fonseca Vde et al., 2012). In both syndromes, structural data are increasingly complemented by resting-state fMRI studies suggesting an imbalance in connectivity between several intrinsic functional systems, predominantly temporo-limbic and default-mode networks (Bettus et al., 2010; Doucet et al., 2013; Maccotta et al., 2013; Morgan et al., 2011). In addition, graph theoretical network analyses consistently described large-scale alterations in global topology across several modalities, emphasizing system-level compromise in syndromes traditionally defined as focal (Bernhardt et al., 2011; Bonilha et al., 2012; Liu et al., 2014; Wang et al., 2014; Yasuda et al., 2015).
FIG. 4.
Findings in drug-resistant epilepsy suggesting an interplay between regional anomalies and large-scale network phenotypes. (A) Resting-state functional connectivity analysis in patients with temporal lobe epilepsy and different degrees of hippocampal sclerosis, suggesting dysconnectivity between the hippocampus and default-mode hubs in patients with cell loss and gliosis, but only subthreshold changes in patients with isolated astrogliosis (Bernhardt et al., 2016). (B) Combined structural covariance and functional connectivity analysis in patients with drug-resistant extratemporal epilepsy and different malformations of cortical development (FCD-II, a malformation of early proliferative stages; HET, related to atypical neuronal migration; PMG, a disruption of postmigratory cortical organization) (Hong et al., 2017a). Findings suggest a gradual effect of malformative timing on networks, with late-stage PMG having the largest impact on structural networks and structure/function coupling. Findings reproduced with permissions. FCD-II, focal cortical dysplasia Type-2; HET, heterotopia; PMG, polymicrogyria.
To study interactions between whole-brain phenotypes and microscopic variations of specific regions, several studies addressed connectome anomalies in patients with postoperative histopathological lesion grading. In temporal lobe epilepsy, patients with marked hippocampal sclerosis have been shown to present with perturbed resting-state functional connectivity to default-mode hubs compared with those with isolated gliosis (Bernhardt et al., 2016). Using subfield-level volumetric and T2 intensity analysis of the hippocampal formation as a bridge, the study furthermore correlated degrees of functional connectivity decreases to structural alterations, supporting a parametric modulation of network anomalies by degrees of structural damage (Bernhardt et al., 2016). A separate analysis of the same group reported a similar modulation at the level of white matter microstructure, with patients presenting with severe hippocampal volume loss also showing more marked anomalies than patients with normal hippocampal volumes (Liu et al., 2016). By showing that microscopically different patterns of hippocampal pathology may result in rather variable perturbations of macroscopic functional and structural networks, these findings lend important evidence for multiscale interactions in pathological networks.
In extratemporal lobe epilepsy related to cortical malformations, a recent analysis showed a graded pattern of structural and functional network rearrangements across different lesion types, with severe alterations in patients with malformations believed to occur late during corticogenesis, while patients with malformation due to disruptions of early proliferative stages showed only modest disruptions (Hong et al., 2017a). Overall, these findings suggest that time of insult during corticogenesis impacts the severity of topological network reconfiguration.
As a surgically amenable disorder, epilepsy provides several opportunities to validate and inform neuroimaging and connectome approaches, for example, to develop and validate models for surgical outcome prediction. In temporal lobe epilepsy, several recent studies have combined machine learning techniques of different MRI and network features, and supported prognostic yield of limbic tract-wise diffusion tensor imaging assessment (Keller et al., 2017), extrahippocampal gray matter surface shape analysis (Bernhardt et al., 2015b), as well as large-scale diffusion connectomes in predicting outcome in individual patients (Bonilha et al., 2013, 2015). While independence of some of these features from hippocampal pathology (also a recognized predictor of outcome) still needs to be established, the literature is overall on a promising path toward predictive platforms, integrating local- and network-level information (Bonilha et al., 2013, 2015).
Conclusions
Neuroimagers and network neuroscientists live in exciting times, where a rich catalog of powerful measures of tissue microstructure and connectivity has become accessible and integrative analysis practical. Multivariate techniques, such as canonical correlation and partial least squares, provide promising formalisms to relate one multidimensional space to another. Accordingly, these models can capture complex relationships, including those between structural and functional domains, between macro- and microstructural properties, as well as those between brain and behavioral measures, with the latter characterizing observable phenotypic variability (Misic and Sporns, 2016). An emerging class of formalisms to integrate information in the structural and functional domain and between different scales is connectome-informed computational models, such as those predicting functional dynamics and interactions from structural connectome data (Bettinardi et al., 2017; Deco et al., 2013, 2017; Sanz Leon et al., 2013). In more clinically driven neuroimaging research settings, these in silico models have been used to assess network-level consequences of focal brain lesions, promising potential utility as prognostic tools (Alstott et al., 2009; Hutchings et al., 2015). Finally, the increasing dimensionality and complexity of multimodal and multiscale approaches justify the use of supervised as well as unsupervised statistical learning techniques that can be used to identify salient features, assess feature interdependencies, as well as to project the data into informative lower dimensional subspaces that facilitate integrative investigations (Bzdok et al., 2017).
While we have advocated for reconciling micro-, meso-, and macroscale information into a unified framework, challenges remain in fusing different levels of analysis. Indeed, despite continued efforts to integrate multiscale data into a common reference frame, technical and conceptual limitations remain to be addressed to improve interpretability. On the one hand, conducting multiscale analyses at high spatial resolutions (e.g., at the level of individual vertices or voxels) poses computational challenges. On the other hand, although aggregation of individual nodes into parcels or regions of interest has become a viable alternative (Fan et al., 2016; Glasser et al., 2016; Schaefer et al., 2017; Tzourio-Mazoyer et al., 2002), only few specific guidelines and formal procedures exist as to which parcellation scheme(s) should be chosen. Another challenge imminent to high-dimensional data and multivariate analysis techniques more generally lies in the increased risk of overfitting and false positive results, mandating the use of conservative cross-validation and reproducibility analyses aiming to control for false positive findings and to ensure replicability.
Despite these challenges, we are nevertheless confident that the near future brings promising new developments in enriched connectomics, a multidisciplinary and multiscale enterprise that will not only advance our understanding of fundamental aspects of healthy brain organization but also of the neurobiological underpinnings of prevalent and often detrimental diseases.
Acknowledgments
S.L. acknowledges funding from Fonds de la Recherche du Québec–Santé (FRQS). S.-J.H. was funded by the Canadian League Against Epilepsy and the Canadian Institutes of Health Research (CIHR). N.B. was funded by CIHR and received salary support from FRQS. A.B. acknowledges funding from CIHR, National Science and Engineering Research Council of Canada (NSERC), and the Quebec Bioimaging Network (QBIN). L.B. receives research support from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (DC014021, DC011739, and DC014664) and from the American Heart Association (SFDRN26030003). B.C.B. acknowledges research support from NSERC (Discovery-1304413), CIHR (FDN-154298), Azrieli Center for Autism Research (ACAR), SickKids Foundation (NI17-039), an MNI-Cambridge collaboration grant, and received salary support from FRQS (Chercheur Boursier Junior 1).
Author Disclosure Statement
No competing financial interests exist.
References
- Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. 2009. Modeling the impact of lesions in the human brain. PLoS Comput Biol 5:e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Zilles K. 2007. Cytoarchitecture of the cerebral cortex—more than localization. NeuroImage 37:1061–1065; discussion 66–68 [DOI] [PubMed] [Google Scholar]
- Andrews DS, Avino TA, Gudbrandsen M, Daly E, Marquand A, Murphy CM, et al. 2017. In vivo evidence of reduced integrity of the gray-white matter boundary in autism spectrum disorder. Cereb Cortex 27:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Alexander DC, Jones DK, Bizzi A, Behrens TE, Clark CA, et al. 2013. The CONNECT project: combining macro- and micro-structure. Neuroimage 80:273–282 [DOI] [PubMed] [Google Scholar]
- Avino TA, Hutsler JJ. 2010. Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res 1360:138–146 [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. 2004. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry 55:323–326 [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. 2009. Human brain networks in health and disease. Curr Opin Neurol 22:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Mattar MG. 2017. A network neuroscience of human learning: potential to inform quantitative theories of brain and behavior. Trends Cogn Sci 21:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Sporns O. 2017. Network neuroscience. Nat Neurosci 20:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi A, Bernasconi N, Bernhardt BC, Schrader D. 2011. Advances in MRI for ‘cryptogenic’ epilepsies. Nat Rev 7:99–108 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi A, Liu M, Hong SJ, Caldairou B, Goubran M, et al. 2016. The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Ann Neurol 80:142–153 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. 2010. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 74:1776–1784 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bonilha L, Gross DW. 2015a. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav 50:162–170 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. 2011. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21:2147–2157 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Fadaie F, Vos de Wael R, Hong SJ, Liu M, Guiot MC, et al. 2017. Preferential susceptibility of limbic cortices to microstructural damage in temporal lobe epilepsy: a quantitative T1 mapping study. Neuroimage. DOI: 10.1016/j.neuroimage.2017.06.00 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Hong SJ, Bernasconi A, Bernasconi N. 2015b. Magnetic resonance imaging pattern learning in temporal lobe epilepsy: classification and prognostics. Ann Neurol 77:436–446 [DOI] [PubMed] [Google Scholar]
- Bettinardi RG, Deco G, Karlaftis VM, Van Hartevelt TJ, Fernandes HM, Kourtzi Z, et al. 2017. How structure sculpts function: unveiling the contribution of anatomical connectivity to the brain's spontaneous correlation structure. Chaos 27:047409. [DOI] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, et al. 2010. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154 [DOI] [PubMed] [Google Scholar]
- Betzel RF, Avena-Koenigsberger A, Goni J, He Y, de Reus MA, Griffa A, et al. 2016. Generative models of the human connectome. Neuroimage 124:1054–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Bassett DS. 2017. Multi-scale brain networks. Neuroimage 160:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. 2013. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54:1315–1329 [DOI] [PubMed] [Google Scholar]
- Bonilha L, Helpern JA, Sainju R, Nesland T, Edwards JC, Glazier SS, Tabesh A. 2013. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 81:1704–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Jensen JH, Baker N, Breedlove J, Nesland T, Lin JJ, et al. 2015. The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology 84:1846–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A. 2012. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry 83:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM. 2004. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 61:1379–1384 [DOI] [PubMed] [Google Scholar]
- Borich MR, Brown KE, Boyd LA. 2014. Motor skill learning is associated with diffusion characteristics of white matter in individuals with chronic stroke. J Neurol Phys Ther 38:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Buckner RL. 2017. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron 95:457–471e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. 2013. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832–837 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29:1860–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Krzywinski M, Altman N. 2017. Points of Significance: machine learning: a primer. Nat Methods 14:1119–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos BM, Coan AC, Beltramini GC, Liu M, Yassuda CL, Ghizoni E, et al. 2015. White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia 56:125–132 [DOI] [PubMed] [Google Scholar]
- Carrera E, Tononi G. 2014. Diaschisis: past, present, future. Brain 137:2408–2422 [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. 2010. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67:365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, et al. 2012. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair 26:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. 2008. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex 18:2374–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. 2012. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology 79:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. 2003. Evidence of brain overgrowth in the first year of life in autism. JAMA 290:337–344 [DOI] [PubMed] [Google Scholar]
- Craddock RC, Jbabdi S, Yan CG, Vogelstein JT, Castellanos FX, Di Martino A, et al. 2013. Imaging human connectomes at the macroscale. Nat Methods 10:524–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vertes PE, Winton-Brown TT, Patel AX, Ginestet CE, et al. 2013. Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci U S A 110:11583–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Dal Palu A, Descoteaux M, Thiran JP. 2016. Microstructure informed tractography: pitfalls and open challenges. Front Neurosci 10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML, Jirsa VK, Ritter P. 2017. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci Rep 7:3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Mantini D, Romani GL, Hagmann P, Corbetta M. 2013. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J Neurosci 33:11239–11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pelphrey K. 2012. Perspective: brain scans need a rethink. Nature 491:S20. [DOI] [PubMed] [Google Scholar]
- Del Gaizo J, Fridriksson J, Yourganov G, Hillis AE, Hickok G, Misic B, et al. 2017. Mapping language networks using the structural and dynamic brain connectomes. eNeuro 4:e0204–17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI. 2013. Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connect 3:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Andrews D, Dell'Acqua F, Daly E, Murphy C, Catani M, et al. 2016. Relationship between cortical gyrification, white matter connectivity, and autism spectrum disorder. Cereb Cortex 26:3297–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC, et al. 2013a. Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry 70:59–70 [DOI] [PubMed] [Google Scholar]
- Ecker C, Ronan L, Feng Y, Daly E, Murphy C, Ginestet CE, et al. 2013b. Intrinsic gray-matter connectivity of the brain in adults with autism spectrum disorder. Proc Natl Acad Sci U S A 110:13222–13227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, et al. 2012. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry 69:195–209 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Thirion B, Varoquaux G, Bzdok D. 2015. Connectivity-based parcellation: critique and implications. Hum Brain Mapp 36:4771–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr., McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. 2012. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307:922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. Neuroimage 80:489–504 [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. 2016. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 26:3508–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. 2015. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci 18:1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM. 2011. Lacunes: small, deep cerebral infarcts. Neurology 77:2104. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, et al. 2010. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 51:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca Vde C, Yasuda CL, Tedeschi GG, Betting LE, Cendes F. 2012. White matter abnormalities in patients with focal cortical dysplasia revealed by diffusion tensor imaging analysis in a voxelwise approach. Front Neurol 3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. 1994. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 2:56–78 [Google Scholar]
- Girvan M, Newman ME. 2002. Community structure in social and biological networks. Proc Natl Acad Sci U S A 99:7821–7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature 536:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Kocher M, Bonilha L. 2015. Connectomics and graph theory analyses: novel insights into network abnormalities in epilepsy. Epilepsia 56:1660–1668 [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. 2009. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19:524–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Betzel RF, Mattar MG, Cieslak M, Delio PR, Grafton ST, et al. 2017. Optimal trajectories of brain state transitions. Neuroimage 148:305–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Berman S, Behrmann M, Dinstein I. 2014. Anatomical abnormalities in autism? Cereb Cortex 26:1440–1452 [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MA, Moller T, Ransom BR. 2011. Anaerobic function of CNS white matter declines with age. J Cereb Blood Flow Metab 31:996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. 2012. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. 2008. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci 28:4756–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. 2007. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex 17:2407–2419 [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, et al. 2009. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 4:e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsfeld AS, Franco AR, Craddock RC, Buchweitz A, Meneguzzi F. 2018. Identification of autism spectrum disorder using deep learning and the ABIDE dataset. Neuroimage 17:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Medalla M, Beul SF, Barbas H. 2016. The primate connectome in context: principles of connections of the cortical visual system. Neuroimage 134:685–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O. 2008. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 29:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Bernhardt BC, Schrader DV, Bernasconi N, Bernasconi A. 2016. Whole-brain MRI phenotying of dysplasia-related frontal lobe epilepsy. Neurology 86:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Kim H, Bernasconi N, Bernhardt BC, Bernasconi A. 2014. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology 83:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Bernhardt BC, Bernasconi N, Bernasconi A. 2017a. The spectrum of structural and functional network anomalies across the spectrum of malformations of cortical development. Brain 140:2133–2143 [DOI] [PubMed] [Google Scholar]
- Hong SJ, Bernhardt BC, Caldairou B, Hall JA, Guiot MC, Schrader D, et al. 2017b. Multimodal MRI profiling of focal cortical dysplasia type II. Neurology 88:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Valk SL, Di Martino A, Milham MP, Bernhardt BC. 2017c. Multidimensional neuroanatomical subtyping of autism spectrum disorder. Cereb Cortex [Epub ahead of print]; DOI: 10.1093/cercor/bhx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xu Q, Shen J, Li K, Zhu H, Zhang Z, Lu G. 2015. Small-worldness and gender differences of large scale brain metabolic covariance networks in young adults: a FDG PET study of 400 subjects. Acta Radiol 56:204–213 [DOI] [PubMed] [Google Scholar]
- Hutchings F, Han CE, Keller SS, Weber B, Taylor PN, Kaiser M. 2015. Predicting surgery targets in temporal lobe epilepsy through structural connectome based simulations. PLoS Comput Biol 11:e1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Canales-Rodriguez EJ, Melie-Garcia L, Valdes-Hernandez PA, Martinez-Montes E, Aleman-Gomez Y, Sanchez-Bornot JM. 2007. Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. Neuroimage 36:645–660 [DOI] [PubMed] [Google Scholar]
- Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE. 2015. Measuring macroscopic brain connections in vivo. Nat Neurosci 18:1546–1555 [DOI] [PubMed] [Google Scholar]
- Jones KC, Hawkins C, Armstrong D, Deveber G, Macgregor D, Moharir M, Askalan R. 2013. Association between radiographic Wallerian degeneration and neuropathological changes post childhood stroke. Dev Med Child Neurol 55:173–177 [DOI] [PubMed] [Google Scholar]
- Kaiser M, Hilgetag CC. 2010. Optimal hierarchical modular topologies for producing limited sustained activation of neural networks. Front Neuroinf 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Glenn GR, Weber B, Kreilkamp BA, Jensen JH, Helpern JA, et al. 2017. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain 140:68–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N. 2008. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49:741–757 [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. 2012. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 61:1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keown Christopher L, Shih P, Nair A, Peterson N, Mulvey Mark E, Müller R-A. 2013. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep 5:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Ge T, Buckner RL, Sherwood CC. 2016. Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A 113:E469–E478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger H, Koot J, Hall RE, O'callaghan C, Bayley M, Corbett D. 2015. Prevalence of individuals experiencing the effects of stroke in Canada. Stroke 46:2226–2231 [DOI] [PubMed] [Google Scholar]
- Kuhn MJ, Mikulis DJ, Ayoub DM, Kosofsky BE, Davis KR, Taveras JM. 1989. Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology 172:179–182 [DOI] [PubMed] [Google Scholar]
- Lariviere S, Ward NS, Boudrias MH. 2018. Disrupted functional network integrity and flexibility after stroke: relation to motor impairments. Neuroimage Clin 19:883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Bernhardt BC, Hong SJ, Caldairou B, Bernasconi A, Bernasconi N. 2016. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain 139:2431–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Chen Z, Beaulieu C, Gross DW. 2014. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia 55:674–682 [DOI] [PubMed] [Google Scholar]
- Maccotta L, He BJ, Snyder AZ, Eisenman LN, Benzinger TL, Ances BM, et al. 2013. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin 2:862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, et al. 2017. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun 8:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Giulietti G, Dowell N, Spano B, Harrison N, Bozzali M, Cercignani M. 2017. Introducing axonal myelination in connectomics: a preliminary analysis of g-ratio distribution in healthy subjects. Neuroimage [Epub ahead of print]; DOI: 10.1016/j.neuroimage.2017.09.018 [DOI] [PubMed] [Google Scholar]
- Matute C, Domercq M, Perez-Samartin A, Ransom BR. 2013. Protecting white matter from stroke injury. Stroke 44:1204–1211 [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Jr., Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, et al. 2008. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 49:794–803 [DOI] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, Bullmore ET. 2010. Modular and hierarchically modular organization of brain networks. Front Neurosci 4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, Fornito A, Ersche KD, Bullmore ET. 2009. Hierarchical modularity in human brain functional networks. Front Neuroinf 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Carpenter SD, Grant KA, Kroenke CD, Nigg JT, Fair DA. 2014. Connectotyping: model based fingerprinting of the functional connectome. PLoS One 9:e111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B, Sporns O. 2016. From regions to connections and networks: new bridges between brain and behavior. Curr Opin Neurobiol 40:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Coté R, et al. 2012. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci 39:793–800 [DOI] [PubMed] [Google Scholar]
- Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou-Khalil B. 2011. Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52:1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, et al. 2013. Individual variability in functional connectivity architecture of the human brain. Neuron 77:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME, Girvan M. 2004. Finding and evaluating community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys 69:026113. [DOI] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D'Esposito M. 2010. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci 107:12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes AS, Peatfield N, Vakorin V, Doesburg SM. 2018. Idiosyncratic organization of cortical networks in autism spectrum disorder. Neuroimage [Epub ahead of print]; DOI: 10.1016/j.neuroimage.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Plitt M, Barnes KA, Martin A. 2015. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. Neuroimage Clin 7:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen MY, et al. 2015. Long-term neural and physiological phenotyping of a single human. Nat Commun 6:8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. 2010. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex 20:1332–1340 [DOI] [PubMed] [Google Scholar]
- Richardson MP. 2012. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 83:1238–1248 [DOI] [PubMed] [Google Scholar]
- Romme IA, de Reus MA, Ophoff RA, Kahn RS, van den Heuvel MP. 2017. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol Psychiatry 81:495–502 [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. 2008. Heart disease and stroke statistics—2008 update. Circulation 117:e25–e146 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron J-C. 2003. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol 2:493–502 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069 [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Schwarzbauer C, Bullmore E. 2005. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci 360:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Leon P, Knock SA, Woodman MM, Domide L, Mersmann J, McIntosh AR, Jirsa V. 2013. The virtual brain: a simulator of primate brain network dynamics. Front Neuroinf 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. 2014. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 86:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. 2017. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter M, Paulsen O, Bullmore ET. 2017. Micro-connectomics: probing the organization of neuronal networks at the cellular scale. Nat Rev Neurosci 18:131–146 [DOI] [PubMed] [Google Scholar]
- Schulz R, Koch P, Zimerman M, Wessel M, Bönstrup M, Thomalla G, et al. 2015. Parietofrontal motor pathways and their association with motor function after stroke. Brain 138:1949–1960 [DOI] [PubMed] [Google Scholar]
- Seidlitz J, Váša F, Shinn M, Romero-Garcia R, Whitaker KJ, Vértes PE, et al. 2017. Morphometric similarity networks detect microscale cortical organisation and predict inter-individual cognitive variation. Neuron 97:231–247.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senden M, Deco G, de Reus MA, Goebel R, van den Heuvel MP. 2014. Rich club organization supports a diverse set of functional network configurations. Neuroimage 96:174–182 [DOI] [PubMed] [Google Scholar]
- Smith E, Thurm A, Greenstein D, Farmer C, Swedo S, Giedd J, Raznahan A. 2016. Cortical thickness change in autism during early childhood. Hum Brain Mapp 37:2616–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, et al. 2015. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 18:1565–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. 2004. Organization, development and function of complex brain networks. Trends Cogn Sci 8:418–425 [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kotter R. 2007. Identification and classification of hubs in brain networks. PLoS One 2:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ. 2014. Modern network science of neurological disorders. Nat Rev Neurosci 15:683–695 [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. 2012. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 135:2527–2535 [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. 2008. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex 18:2659–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, et al. 2013. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucl Acids Res 41:D996–D1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S. 2016. Task-free MRI predicts individual differences in brain activity during task performance. Science 352:216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford QK, Ashourvan A, Wymbs NF, Grafton ST, Vettel JM, Bassett DS. 2017. Cohesive network reconfiguration accompanies extended training. Hum Brain Mapp 38:4744–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Eriksson S, Martinian L, Caboclo LO, McEvoy AW, Duncan JS, Sisodiya SM. 2009. Temporal lobe sclerosis associated with hippocampal sclerosis in temporal lobe epilepsy: neuropathological features. J Neuropathol Exp Neurol 68:928–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Weiller C, Rother J. 2005. Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry 76:266–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E, Scholtens LH, van den Heuvel MP. 2016. Cortical chemoarchitecture shapes macroscale effective functional connectivity patterns in macaque cerebral cortex. Hum Brain Mapp 37:1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Uddin LQ. 2015. Idiosyncratic connectivity in autism: developmental and anatomical considerations. Trends Neurosci 38:261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, Di Martino A, Milham MP, Bernhardt BC. 2015. Multicenter mapping of structural network alterations in autism. Hum Brain Mapp 36:2364–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Scholtens LH, Feldman Barrett L, Hilgetag CC, de Reus MA. 2015. Bridging cytoarchitectonics and connectomics in human cerebral cortex. J Neurosci 35:13943–13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2011. Rich-club organization of the human connectome. J Neurosci 31:15775–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci 17:683–696 [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage 62:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, et al. 2017. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results From the ENIGMA ASD Working Group. Am J Psychiatry 175:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D, Smith SM, Woolrich MW. 2017. Brain network dynamics are hierarchically organized in time. Proc Natl Acad Sci U S A 114:12827–12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Monakow C. 1914. Brain localization and functional disturbances caused by lesions of the cortex [Die Lokalisation im Grosshirn und der Abbau der Funktion durch kortikale Herde]. Wiesbaden, Germany: Verlag von JF Bergmann [Google Scholar]
- Waehnert MD, Dinse J, Schafer A, Geyer S, Bazin PL, Turner R, Tardif CL. 2016. A subject-specific framework for in vivo myeloarchitectonic analysis using high resolution quantitative MRI. Neuroimage 125:94–107 [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. 2010. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain 133:3745–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qiu S, Xu Y, Liu Z, Wen X, Hu X, et al. 2014. Graph theoretical analysis reveals disrupted topological properties of whole brain functional networks in temporal lobe epilepsy. Clin Neurophysiol 125:1744–1756 [DOI] [PubMed] [Google Scholar]
- Ward NS. 2017. Restoring brain function after stroke—bridging the gap between animals and humans. Nat Rev Neurol 13:244–255 [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. 1998. Collective dynamics of ‘small-world’ networks. Nature 393:440–442 [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Mohammadi S, Lutti A, Callaghan MF. 2015. Advances in MRI-based computational neuroanatomy: from morphometry to in-vivo histology. Curr Opin Neurol 28:313–322 [DOI] [PubMed] [Google Scholar]
- Whitaker KJ, Vertes PE, Romero-Garcia R, Vasa F, Moutoussis M, Prabhu G, et al. 2016. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A 113:9105–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. 2001. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318 [DOI] [PubMed] [Google Scholar]
- Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, et al. 2015. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain 138:2359–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, He Y. 2017. Functional connectomics from a “big data” perspective. Neuroimage 160:152–167 [DOI] [PubMed] [Google Scholar]
- Yahata N, Morimoto J, Hashimoto R, Lisi G, Shibata K, Kawakubo Y, et al. 2016. A small number of abnormal brain connections predicts adult autism spectrum disorder. Nat Commun 7:11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda CL, Chen Z, Beltramini GC, Coan AC, Morita ME, Kubota B, et al. 2015. Aberrant topological patterns of brain structural network in temporal lobe epilepsy. Epilepsia 56:1992–2002 [DOI] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. 2012. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73:1216–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP. 2012. Network centrality in the human functional connectome. Cereb Cortex 22:1862–1875 [DOI] [PubMed] [Google Scholar]