Abstract
The inflammatory response to moderate-severe controlled cortical impact (CCI) in adult male mice has been shown to exhibit greater glial activation compared with age-matched female mice. However, the relative contributions of resident microglia and infiltrating peripheral myeloid cells to this sexually dimorphic neuroinflammatory responses remains unclear. Here, 12-week-old male and female C57Bl/6 mice were subjected to sham or CCI, and brain samples were collected at 1, 3, or 7 days post-injury for flow cytometry analysis of cytokines, reactive oxygen species (ROS), and phagocytosis in resident microglia (CD45intCD11b+) versus infiltrating myeloid cells (CD45hiCD11b+). Motor (rotarod, cylinder test), affect (open field), and cognitive (Y-maze) function tests also were performed. We demonstrate that male microglia had increased phagocytic activity and higher ROS levels in the non-injured brain, whereas female microglia had increased production of tumor necrosis factor (TNF) α and interleukin (IL)-1β. Following CCI, males showed a significant influx of peripheral myeloid cells by 1 day post-injury followed by proliferation of resident microglia at 3 days. In contrast, myeloid infiltration and microglial activation responses in female CCI mice were significantly reduced. No sex differences were observed for TNFα, IL-1β, transforming growth factor β, NOX2, ROS production, or phagocytic activity in resident microglia or infiltrating cells at any time. However, across these functions, infiltrating myeloid cells were significantly more reactive than resident microglia. Female CCI mice also had improved motor function at 1 day post-injury compared with male mice. Thus, we conclude that sexually dimorphic responses to moderate-severe CCI result from the rapid activation and infiltration of pro-inflammatory myeloid cells to brain in male, but not female, mice.
Keywords: macrophage, microglia, neuroinflammation, sex differences, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality worldwide, affecting more than 1.7 million individuals annually in the United States.1 The overall death rate of TBI is roughly three times higher for males than for females,2 and only recent research has begun to address the complex role that sex has on outcomes after TBI. Clinical data are conflicting regarding sex differences in recovery following head injury. Some clinical studies report better outcomes in females,3,4 whereas others report no difference5,6 or worse outcomes in females compared with males.7–10 Injury severity (concussion/mild vs. severe), age of TBI onset (pediatric, pre- vs. post-menopause, geriatric), and comorbidities can influence brain injury outcomes in a sex-dependent manner,11 yet existing clinical evidence for sex differences in functional outcomes after TBI remains controversial.12
For many years, the majority of experimental TBI research has been carried out in male subjects. Pre-clinical studies that have compared male and female animals report mixed and unclear results with regard to effects of biological sex on secondary injury mechanisms and TBI outcomes.13–21 Microglia and infiltrating myeloid cells (inflammatory monocytes and neutrophils) play central roles in acute neuroinflammatory responses following TBI, and persistent microglial activation can lead to chronic neurodegeneration and loss of neurological function.22–24 However, the role of sex differences in post-traumatic neuroinflammatory responses has been debated and the data are currently conflicted.15,17,25–27 An early study reported that sex did not affect microglial activation status in the hippocampus after moderate-severe TBI.26 In contrast, more recent studies have demonstrated significant sex differences in microglial and astrocyte activation during acute phase responses after moderate-severe TBI.25,28,29 Nevertheless, all studies to date have used immunohistochemical assessments that provide excellent spatial and morphological resolution of post-traumatic neuroinflammation, but are limited in their ability to provide phenotypic and functional assessments of immune cell function in either resident (microglia) or infiltrating (myeloid, lymphocytes) cells in the central nervous system (CNS). Flow cytometry approaches can distinguish microglia from infiltrating myeloid cells in the brain based on the relative expression of the CD45 antigen, and provide the added advantage of assessing functional responses ex vivo.30–32
In the present study, we used advanced flow cytometry approaches to investigate phenotypic and functional responses in microglia and infiltrating myeloid cells following moderate-severe TBI in adult male and female C57Bl/6 mice in order to provide novel insight into sex differences in post-traumatic neuroinflammation. We also performed motor and cognitive function assessments in sham and TBI mice during the acute phase post-injury to correlate changes in post-traumatic neuroinflammation with neurological outcomes. Studies have recently demonstrated that infiltrating myeloid cells promote a robust acute neuroinflammatory response to TBI22,33–36; therefore, we hypothesized that peripherally-derived myeloid cells initiate acute neuroinflammatory responses in the injured brain, which are sexually dimorphic and exaggerated in males compared with female TBI mice. Our data show that although microglia in males and females have similar functional responses following TBI, there are significant sex differences in peripheral immune activation and infiltration after TBI that are associated with transient sex differences in acute neurobehavioral outcomes.
Methods
Animals
Young adult male and female C57BL/6 mice (12 weeks old) from Taconic Biosciences (Germantown, NY) were housed on standard bedding in a specific pathogen free facility (12 h light/dark cycle). All animals had access to chow and water ad libitum. Animal procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals, and approved by the Animal Care Committee of the University of Maryland School of Medicine. The average body weight at the time of injury was 26.3 ± 0.7 g for males and 20.3 ± 0.3 g for females. We used random cycling of female mice to better reflect clinical applicability. We measured 17β-estradiol in the serum of females and males by enzyme-linked immunosorbent assay (ELISA) assay (Cayman Chemicals Corporation; 17β-estradiol; Ann Arbor, MI) according to manufacturer's instructions. Sham and controlled cortical impact (CCI) male and female mice at 1, 3, and 7 days post-injury (randomly selected n = 4/group) were analyzed (Table 1), and males had significantly reduced 17β-estradiol levels than females at each time-point (p < 0.05). Notably, sham and CCI females had equivalent levels of estradiol at each time-point, indicating random cycling and no effect of TBI on 17β-estradiol levels
Table 1.
17β-Estradiol Levels in Sham and CCI Male and Female Mice
| 17β-estradiol in pg/mL ± SEM | ||||
|---|---|---|---|---|
| Sham | 1 dpi | 3 dpi | 7 dpi | |
| Male | 48.3 ± 6.2 | 53.4 ± 5.3 | 53.0 ± 2.4 | 42.8 ± 1.6 |
| Female | 72.3 ± 15.3 | 72.8 ± 13.8 | 64.7 ± 6.7 | 62.03 ± 12.2 |
17β-Estradiol was measured from serum of males and females sham and CCI across each time-point (n = 4 per group). Females mice had significantly higher 17β-estradiol levels compared with male mice (p < 0.05). Levels of 17β-estradiol in females were the same in the sham and CCI groups across each time-point, indicating no injury effect on plasma 17β-estradiol levels (p > 0.05). 17β-estradiol levels are expressed in pg/mL (mean ± SEM).
CCI, controlled cortical impact; SEM, standard error of the mean; dpi, days post-injury.
Controlled cortical impact
Our custom-designed CCI injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip. Briefly, mice were anesthetized with isoflurane evaporated in a gas mixture containing 70% N2O and 30% O2 and administered through a nose mask. Mice were placed on a heated pad and core body temperature was maintained at 37°C. The head was mounted in a stereotaxic frame, a 10-mm midline incision was made over the skull and the skin and fascia were reflected. A 5-mm craniectomy was made on the central aspect of the left parietal bone. The impounder tip of the injury device was then extended to its full stroke distance (44 mm), positioned to the surface of the exposed dura, and reset to impact the cortical surface. Moderate-level CCI was induced using an impactor velocity of 6 m/sec and deformation depth of 2 mm. After injury, the incision was closed with interrupted 6-0 silk sutures, anesthesia was terminated, and the animal was placed into a heated cage to maintain normal core temperature for 45 min post-injury. Sham animals underwent the same procedure as CCI mice except for craniectomy and cortical impact.31
Flow cytometry
Immediately after mice were euthanized using Euthasol (Virbac Corp., Fort Worth, TX), blood (200 μL) was drawn by cardiac puncture with heparinized needles and later spun down for plasma analysis. Following transcardial perfusion with 60 mL of ice-cold sterile PBS, the brains were removed and the ipsilateral hemisphere was processed by mechanical disruption on a 70-μm filter screen and resuspended in a total of 5 mL of RPMI (Lonza Group, Basel, Switzerland). Papain (Sigma-Aldrich, St. Louis, MO), DNase II (Sigma), and Collagenase (Sigma) were added to the brain suspension and incubated on a shaker for 1 h at 37°C for mechanical and enzymatic digestion of the brain tissue. After incubation, leukocytes were separated from other brain cells by a Percoll gradient (GE Healthcare, Little Chalfont, U.K.).
Leukocytes were then washed and blocked with mouse Fc Block (clone 93; eBioscience, San Diego, CA) prior to staining with primary antibody-conjugated fluorophores including CD45-eF450 (30-F11), CD11b-APCeF780 (M1/70), TNF-PE-Cy7 (MP6-XT22), IL-1β-PerCP-eF710 (NJTEN3; eBioscience), transforming growth factor (TGF) β-APC (TW7-16B4; Biolegend, San Diego, CA), NOX2-gp91-A647 (BIOSS, Boston, MA), and H2AX pS139-PE, MACS Miltenyi Biotec (130-107-585; Bergisch Gladbach, Germany). For live/dead cell discrimination, a fixable viability dye, Zombie AquaTM (Biolegend), was dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions and added to cells in a final concentration of 1:50. Data were acquired on a LSRII using FACsDiva 6.0 (BD Biosciences, San Jose, CA) and analyzed using FlowJo (Tree Star, San Carlos, CA). A standardized gating strategy was used to identify microglia (CD45intCD11b+Ly6C-) and infiltrating myeloid (CD45hiCD11b+Ly6C+) populations as shown (Supplementary Fig. 1; see online supplementary material at http://www.liebertpub.com). Hereafter, these cells will be abbreviated and referred to as CD45int microglia and CD45hi infiltrating myeloid cells. Cell-specific fluorescence minus one (FMO) controls were used to determine the positivity of each antibody. The entirety of each sample are run through the cytometer until there are zero events to obtain an absolute cell count/hemisphere.
For intracellular cytokine staining, leukocytes were collected as described above, and 1 μL of GolgiPlug containing brefeldin A (BD Biosciences) was added to 500 μL complete RPMI. Cells were resuspended in Fc Block, stained for surface antigens and washed in 100 μL of fixation/permeabilization solution (BD Biosciences) for 20 min. Cells were then washed twice in 500 μL Permeabilization/Wash buffer (BD Biosciences) and resuspended in an intracellular antibody cocktail of cytokine antibodies and fixed. To measure reactive oxygen species levels, leukocytes were incubated with dihydrorhodamine (DHR) 123 (5mM; 1:500 in RPMI; Ex/Em: 500/536), a cell-permeable fluorogenic probe (Life Technologies, Invitrogen). DHR123 passively diffuses into cells and is oxidized by peroxide and peroxynitrite, causing a reaction that produces a green fluorescence.37 Cells were loaded for 20 min in a 37°C water bath, washed twice with FACS buffer (without NaAz), then stained for surface markers including viability dye and subsequently fixed in paraformaldehyde (PFA). Phagocytic activity was assessed as described before with minor modification.31 Briefly, Texas-red fluorescent carboxylate-modified polystyrene latex beads (0.5 μm mean diameter; Sigma) were added to freshly isolated cells in a final dilution of 1:500 (in RPMI). After 1 h incubation in a 37°C water bath, the cells were washed three times, re-suspended in FACS buffer, stained for surface markers and viability dye, and fixed in PFA. Ex vivo flow cytometry studies were performed by an investigator blinded to sex and surgical condition.
Neurobehavioral testing
Sham and CCI mice underwent a battery of neurological tests, including Rotarod, Cylinder, Open field, and Y-maze tests as outlined below. Investigators were blinded to surgical condition during testing and analysis.
Rotarod
Motor function was assessed in sham and CCI mice at baseline (day 0), and 1, 2, and 3 days post-injury using an accelerating rotarod test (Ugo-Basile, Collegeville, PA).38,39 In brief, in each trial the rotarod accelerated from 4 to 60 RPM over a period of 3 min. Mice were trained on the rotarod test for 2 days prior to sham or CCI surgery, and each mouse had three trials per day. Latency to fall from the rod (or cling to and rotate with the rod for two consecutive rotations) was recorded for three trials on each testing day, allowing 5-10 min of rest with access to food and water between each trial. Scores from the three trials on each testing day were averaged to give a single score for each mouse on each testing day.
Cylinder test
Forearm preference was assessed by the cylinder test on Day 2 post-injury as previously described.40,41 This test evaluates forelimb use asymmetry following acute brain injury. The mouse was placed in a clean and transparent 2-liter glass beaker. A mirror was placed behind the beaker and the mouse was videotaped throughout the test. Forelimb use of the first contact against the wall after rearing and during lateral exploration was recorded. Forelimb use was scored using described criteria: 1) the first forelimb to contact the wall during a full rear was recorded as an independent wall placement for that limb; 2) simultaneous use of both the left and right forelimb by contacting the wall of the cylinder during a full rear and for lateral movements along the wall was recorded as “both” movement; 3) after the first forelimb (for example right forelimb) contacted the wall and then the other forelimb was placed on the wall, but the right forelimb was not removed from the wall, a “right forelimb independent” movement and a “both” movement were recorded; however, if the other (left forelimb) made several contacting movements on the wall, a “right forelimb independent” movement and only one “both” movement was recorded; and 4) when the mouse explored the wall laterally, alternating both forelimbs, it was recorded as a “both” movement.”40 A total of 20 movements were recorded during the 10-min test. The final score = (nonimpaired forelimb movement − impaired forelimb movement)/(nonimpaired forelimb movement + impaired forelimb movement + both movement).
Open field
The open field test was performed on Day 6 post-injury to assess locomotor activity as previously described.42 Sham and CCI mice were individually placed in a corner facing the wall of the open field chamber (22.5 × 22.5 cm), and allowed to freely explore the chamber for 5 min. The distance traveled and average speed was recorded by Any-Maze tracking software (Stoelting Co., Wood Dale, IL).
Y-maze spontaneous alternation
The Y-maze spontaneous alternation behavior test, to assess hippocampal-dependent working (short-term) memory, was performed on Day 5 post-injury, as previously described.32 Briefly, the Y-maze (Stoelting Co.) consisted of three identical arms, each arm 35 cm long, 5 cm wide, and 10 cm high, at an angle of 120° with respect to the other arms. One arm was randomly selected as the “start” arm, and the mouse was placed within and allowed to explore the maze freely for 5 min. Arm entries (arms A-C) were recorded by analyzing mouse activity using ANY-maze tracking software (Stoelting Co.). An arm entry was attributed when all four paws of the mouse entered the arm, and an alternation was designated when the mouse entered three different arms consecutively. The percentage of alternation was calculated as follows: total alternations × 100/(total arm entries −2). If a mouse scored significantly >50% alternations (the chance level for choosing the unfamiliar arm), this was indicative of hippocampal-dependent working memory.
Estradiol measurement
17β-estradiol was measured in plasma from sham and CCI mice at each time-point using an ELISA assay (Cayman Chemical Company, Ann Arbor, MI). Plasma 17β-estradiol levels were determined according to the manufacturer's instructions and expressed as mean ± standard error of the mean (SEM).
Statistical analyses
Data from individual experiments were analyzed for normal distribution and are presented as mean ± SEM. Baseline differences in ex vivo microglial functional responses were determined by unpaired t-test. Behavioral differences on Rotarod were analyzed by two-way analysis of variance (ANOVA) with repeated measures and a Tukey post hoc analysis. Cylinder, Y maze, and Open field tests were analyzed by two-way ANOVA with a Tukey post hoc for multiple comparisons. Myeloid and microglial cell functional responses over time were analyzed by a two-way ANOVA with a Tukey post hoc analysis. Myeloid versus microglial cell comparisons were analyzed per time-point by one-way ANOVA with Tukey post hoc analysis. Raw data (mean fluorescence intensities) were converted into a ratio of CD45himyeloid:CD45intmicroglial functional responses and are presented in Figure 5. Sex differences in plasma 17β-estradiol levels were analyzed by Student's test at each time-point. Statistical analysis was performed using GraphPad Prism Software v. 6.0 (GraphPad Software, Inc., La Jolla, CA), and a p < 0.05 was considered statistically significant.
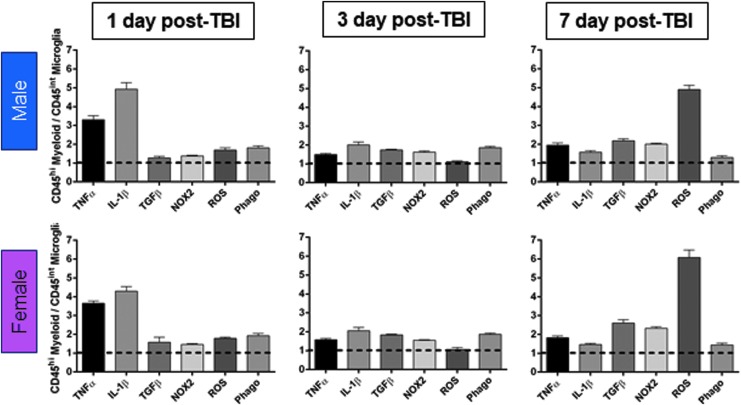
FIG. 5.
Infiltrating peripheral myeloid cells produce high levels of inflammatory cytokines and reactive oxygen species (ROS), and have high phagocytic activity following controlled cortical impact. Comparison of infiltrating myeloid cells (CD45hi) versus resident microglial (CD45int) levels of tumor necrosis factor (TNF) α, interleukin (IL)-1β, transforming growth factor (TGF) β, NOX2, ROS, and phagocytosis at each post-injury time-point. Males and females MFI values for each marker are represented at each time-point (1, 3, and 7 days post-injury) and are expressed as a relative CD45hi myeloid / CD45int microglia levels. After traumatic brain injury (TBI), infiltrating myeloid cells express higher levels of TNFα, IL1β, TGFβ, and NOX2 protein, produce more ROS, and have greater phagocytic ability than resident microglia at each time-point. Student's t-test, p < 0.01 for all comparisons; n = 6-12/group. Color image is available online.
Results
Microglial functional responses differ at baseline between male and female mice
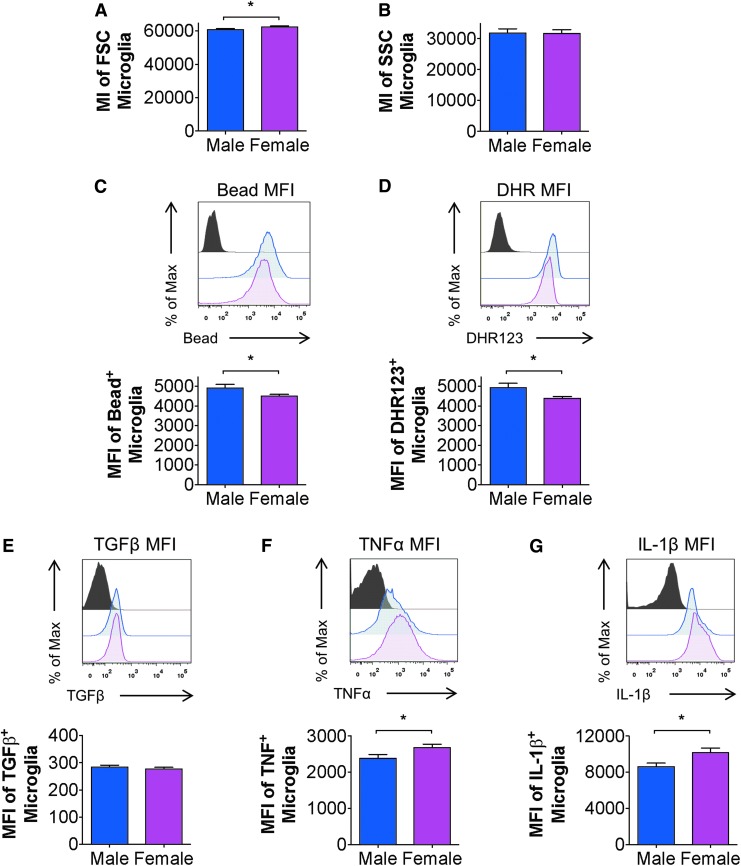
To establish baseline microglial function in adult male and female mice, we used flow cytometry and an established gating strategy for viable microglia (CD45intCD11b+Ly6C-; Supplementary Fig. 1)30,35 to assess microglial morphological features and functional responses. Analysis of microglial size, as defined by forward scatter (FSC), revealed a significant increase in cell size in females compared with males (p < 0.01; Fig. 1A), whereas microglial cellular granularity, defined by side scatter, was not different between male and females at baseline (p > 0.05; Fig. 1B). We then assessed microglial functional responses and their ability to produce ROS and cytokines, as well as phagocytose inert latex beads. Our ex vivo analysis revealed that female microglia had decreased phagocytic activity and lower ROS levels (p < 0.05 and p < 0.01 respectively), compared with male microglia (Fig. 1C, 1D). Analysis of cytokine production in microglia showed that despite no sex difference in anti-inflammatory TGFβ production at baseline (p > 0.05; Fig. 1E), there was a significant increase in pro-inflammatory TNFα and IL-1β production in microglia from females compared with males (p < 0.05; Fig. 1F, 1G). Therefore, our data demonstrate that adult male and female microglia have different baseline characteristics that may reflect sex-specific homeostatic functions. Under homeostatic conditions, male microglia have greater phagocytic potential and higher oxidative stress levels, whereas female microglia are larger in size and exhibit elevated levels of TNFα and IL-1β.
FIG. 1.
Basal microglial function in adult male and female mice. Flow cytometry analysis of adult microglia isolated from male and female brains without any surgical condition demonstrated a significant difference in size as measured by forward size scatter (FSC) (A), but showed no sex differences in granularity as measured by side scatter (SSC) (B). Male microglia demonstrated increased levels of phagocytosis as measured by mean fluorescence intensity (MFI) of 0.5 μm latex bead uptake when compared with female microglia (C). Male microglia exhibited higher reactive oxygen species levels as measured by dihydrorhodamine (DHR) 123 MFI (D). Analysis of intracellular cytokines demonstrated that male and female microglia produced similar levels of transforming growth factor (TGF) β (E). However, female microglia produced more tumor necrosis factor (TNF) α and interleukin (IL)-1β compared with male microglia as measured by MFI (F, G). Student t-test, *p < 0.05; mean ± standard error of the mean; n = 12-15/group. Color image is available online.
Robust proliferation of resident microglia is preceded by early infiltration of peripheral myeloid cells in the injured brain of male mice compared with female mice
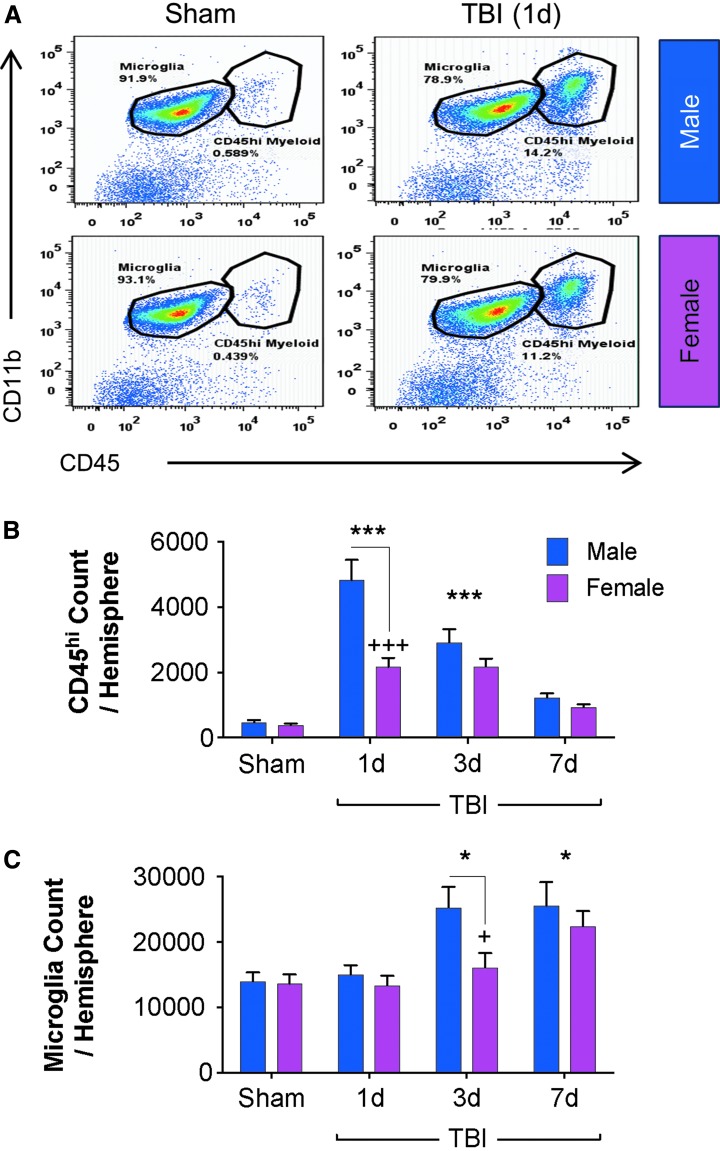
To provide novel insight into sex-dependent effects on the neuroinflammatory response during the acute phase post-injury, we examined the functional dynamics of resident microglia (CD45intCD11b+) and infiltrating myeloid cells (CD45hiCD11b+). We induced moderate-level CCI in adult male and female mice, and euthanized cohorts of injured mice at 1, 3, and 7 days post-injury (dpi) along with time-point matched sham mice (pooled) for phenotypic and functional analyses. Representative dot plots show that under sham conditions, the majority of cells in the brain are resident microglia (> 90% CD45intCD11b+; Fig. 2A). Within one day of TBI, there is a significant influx of peripherally derived CD45hi myeloid cells (14%; Fig. 2A).
FIG. 2.
Time course of peripheral myeloid cell infiltration and microglial activation in male and female mice following controlled cortical impact (CCI). Flow cytometry analysis of brains from male and female mice from sham and CCI through 7 days post-injury. Representative dot plots at 1 day post-injury demonstrated that in male and female traumatic brain injury (TBI), there are similar numbers of microglia (CD45intCD11b+), but more infiltrating myeloid cells (CD45hiCD11b+) in male TBI (A). Both males and females showed a significant injury effect in the number of infiltrating cells in the brain compared with sham at 1 (***) and 3 (***) days post-injury; however, there were significantly more infiltrating myeloid cells in the male TBI brain at 1 day post-injury compared with the female TBI brain (+++) (B). When compared with sex-matched shams, TBI resulted in a significant increase in numbers of microglia both in male and female TBI brains at 3 (*) and 7 (*) days post-injury. Sex and injury effects were analyzed by two-way analysis of variance, and Tukey post hoc analysis was performed for multiple comparisons where *p < 0.05, ***p < 0.01 for Sham vs. TBI, +p < 0.05, +++p < 0.001 for male vs. female. Mean ± standard error of the mean; n = 8-15/group. Color image is available online.
Statistical analysis of CD45hi myeloid cell counts (infiltrating myeloid cells) revealed an effect of TBI [F(3,130) = 8.255; p < 0.0001)] and sex [F(3,130) = 46.84; p < 0.0001]. Specifically, there was a robust and significant increase in the number of peripherally derived CD45hi myeloid cells in the injured brain at 1 and 3 days post-injury (p < 0.001 vs. sham; Fig. 2B). At 7 days post-injury the numbers of CD45hi myeloid cells in the injured brain returned to sham levels. Notably, post-hoc analyses revealed a sex-dependent effect on CD45hi myeloid cell infiltration at 1 day post-injury, with significantly increased numbers of cells in the injured brain of males versus females (p < 0.0001 vs. female TBI 1dpi; Fig. 2B). The levels of infiltrating CD45hi myeloid cells in the brains of males and females were equivalent at 3 and 7 days post-injury.
Statistical analysis of CD45int cell counts (microglia) revealed an effect of TBI [F(3,230) = 9.326; p < 0.0001)] and sex [F(1,230) = 4.842; p = 0.0288]. Specifically, CD45int cell count analysis demonstrated a significant increase in microglial cell number at 3 and 7 days post-injury (p < 0.05 vs. sham; Fig. 2C), indicative of robust proliferative response to injury at these time-points. There was a sex difference on microglial activation at 3 days post-injury, but it failed to reach statistical significance in post hoc analysis (p = 0.0781, male TBI 3 dpi vs. female TBI 3 dpi; Fig. 2C). Thus, our analysis revealed an early and robust CD45hi myeloid cell infiltration response in the brain of male mice compared with female mice at 1 day post-injury, as well as exaggerated microglial proliferation at 3 days post-injury. By 7 days post-injury, the levels of peripheral myeloid cell infiltration and microglial numbers were similar between male and female mice.
Microglial phenotypic or functional responses after TBI are not significantly altered by sex
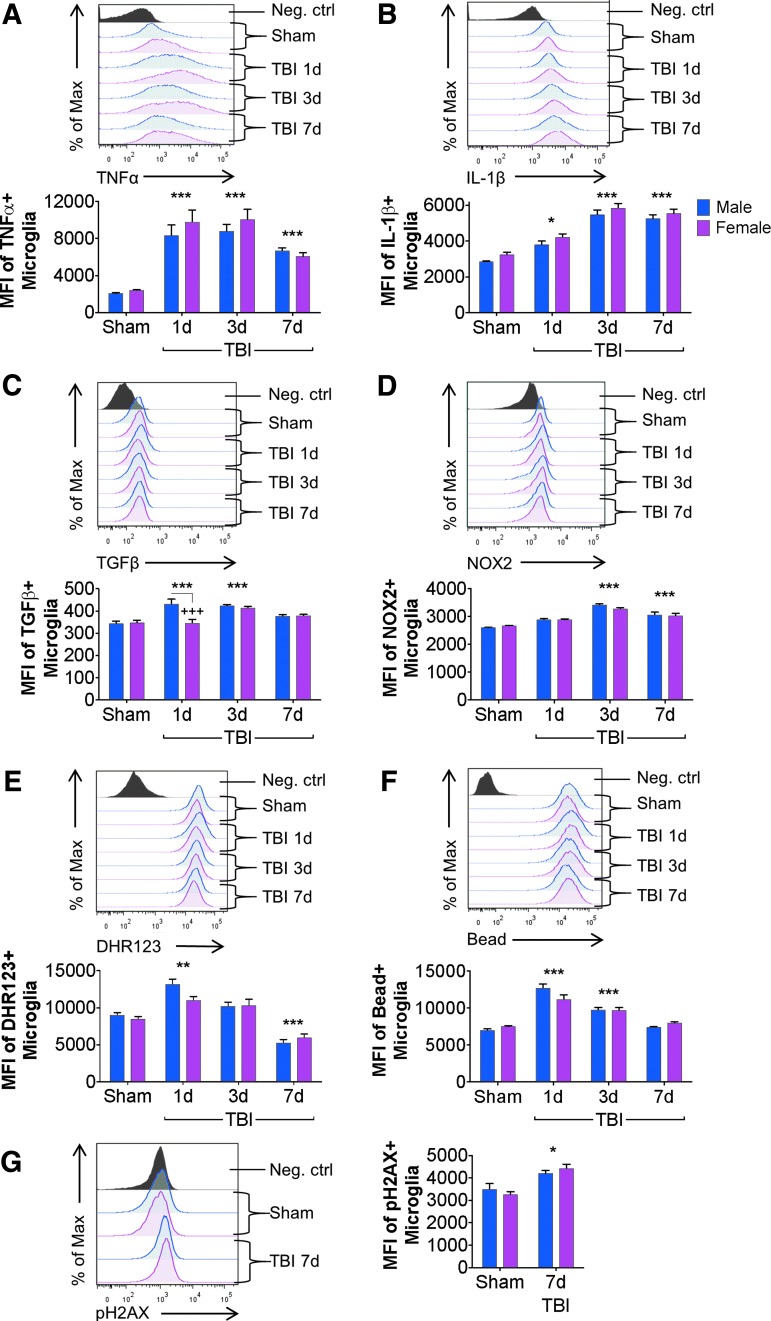
We next evaluated the expression level of various pro- and anti-inflammatory cytokines (TNFα, IL-1β, and TGFβ), as well as the nicotinamide adenine dinucleotide phosphate oxidase subunit NOX2, in microglia from sham and TBI mice. Statistical analysis revealed an effect of TBI for each marker [TNFα: F(3,104) = 51.38, p < 0.0001; IL-1β: F(3,75) = 78.51, p < 0.0001; TGFβ: F(3,74) = 16.26, p < 0.0001; NOX2: F(3,74) = 32.02, p < 0.0001], and an effect of Sex for IL-1β and TGFβ only [TNFα: F(1,104) = 1.607, p = 0.2077; IL-1β: F(1,75) = 5.755, p = 0.0189; TGFβ: F(1,75) = 8.689, p = 0.0043; NOX2: F(1,74) = 0.2727, p = 0.6031]. Specifically, following a moderate-level CCI, microglia up-regulated pro-inflammatory TNFα, IL-1β and anti-inflammatory TGFβ, starting at 1 day post-injury (p < 0.05 – p < 0.0001 vs. sham; Fig. 3A-C), and NOX2 starting at 3 days post-injury (p < 0.0001 vs. sham; Fig. 3D). Injury-induced levels of TNFα, IL-1β, and NOX2 were elevated through 7 days post-injury (p < 0.01 – p < 0.0001 vs. sham); however, TGFβ returned to sham levels by 7 days post-injury. There were no sex differences in microglial TNFα, IL-1β, or NOX2 levels at any time-point (Fig. 3A, 3B, and 3D). However, at 1 day post-injury, there was a significant decrease in TGFβ expression in female microglia (p = 0.0003 vs. male TBI 1 dpi; Fig. 3C); thereafter, female levels normalized to male TGFβ levels at 3 and 7 days post-injury.
FIG. 3.
Microglial functional responses to controlled cortical impact (CCI) are minimally altered by biological sex. Flow cytometry analysis of resident microglia (CD45intCD11b+) in male and female mice from sham and (CCI) through 7 days post-injury. Traumatic brain injury (TBI) significantly increased microglial tumor necrosis factor (TNF) α in males and females at each post-injury time-point (***), but TNFα production was independent of sex (A). In both sexes, microglial interleukin (IL)-1β production was significantly increased at 1 (*), 3 (***), and 7 (***) days post-injury compared with sex-matched shams. Two-way analysis of variance (ANOVA) demonstrated an effect of sex, but post hoc time-point differences were not significant (B). Microglial transforming growth factor (TGF) β was significantly increased at 1 (***) and 3 (***) days post-injury in both males and females; however, male microglia produced significantly more TGFβ than female microglia at 1 day post-injury (+++) (C). Microglial NOX2 was significantly increased at 3 (***) and 7 (***) days post-injury in both males and females; but NOX2 expression in microglia was independent of sex (D). Examination of microglial reactive oxygen species (ROS) production demonstrated a significant increase in ROS production at 1 day post-injury (**), and a decrease in ROS production at 7 days post-injury (***) in both sexes; however, ROS production following TBI was independent of sex (E). Microglial phagocytic activity was significantly increased at 1 (***) and 3 (***) days post-injury compared with sham, but phagocytic activity was independent of sex (F). Levels of microglial phosphorylated H2AX were significantly increased at 7 (*) days post-injury compared with sham, but was independent of sex (G). Sex and injury effects were analyzed by two-way ANOVA, and Tukey post hoc analysis was performed for multiple comparisons where +p < 0.05, +++p < 0.01 for male vs. female, *p < 0.05, **p < 0.01, ***p < 0.001 for Sham vs. TBI. Mean ± standard error of the mean; n = 8-15/group. Color image is available online.
We then assayed injury-induced microglial ROS production and phagocytosis activity in sham and TBI mice. Statistical analysis revealed an effect of TBI [ROS: F(3,74) = 42.74, p < 0.0001; phagocytosis: F(3,60) = 65.11, p < 0.0001], but no effect of sex [ROS: F(1,74) = 1.002, p = 0.3201; phagocytosis: F(1,60) = 0.2543, p = 0.6159]. Specifically, TBI resulted in increased microglial ROS production at 1 day post-injury (p = 0.0036 vs. sham; Fig. 3E) and phagocytic activity at 1 and 3 days post-injury (p < 0.0001 vs. sham; Fig. 3E, 3F), before both functional markers returned to sham levels at 7 days post-injury. There were no sex differences in microglial ROS production or phagocytic function at any time-point (Fig. 3E, 3F). Finally, because elevate oxidative stress levels and pro-inflammatory cytokines can lead to apoptosis and cell death, we measured DNA damage in microglia at 7 days post-injury using phosphorylated (s139) H2AX, a histone complex that marks impaired DNA. Statistical analysis revealed an effect of TBI [F(1,21) = 17.77, p = 0.0004], but no effect of sex [F(1,21) = 4.142e-005, p = 0.9949; Fig. 3G) on H2AX phosphorylation in microglia.
Sex does not alter cytokine, ROS, or phagocytosis levels in infiltrating myeloid cells after TBI
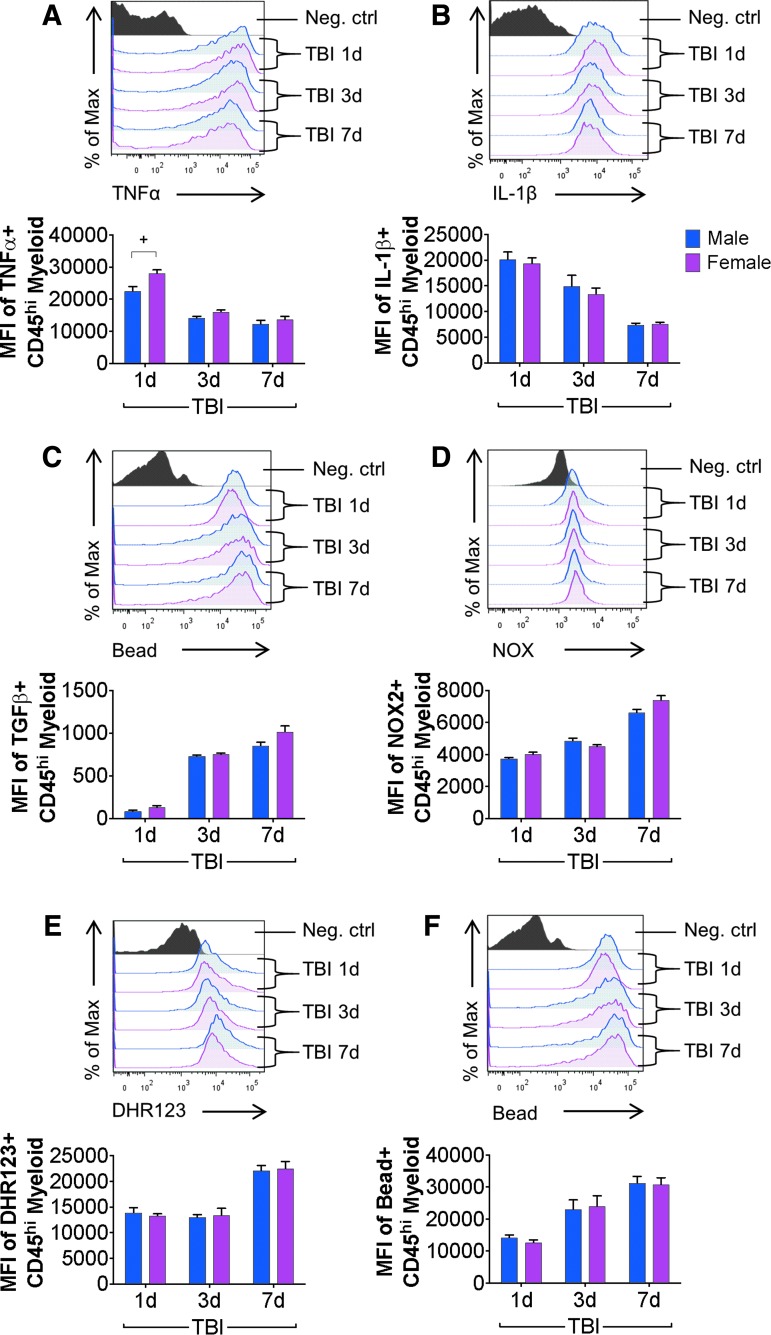
We then evaluated expression levels of TNFα, IL-1β, TGFβ, and NOX2 in peripherally derived CD45hi myeloid cells in the brain of male and female TBI mice at 1, 3, and 7 days post-injury. Sham mice do not accumulate CD45hi myeloid cells in the brain (Fig. 2A) and were not included in this analysis. Statistical analysis revealed an effect of TBI for each marker [TNFα: F(2,71) = 82.35, p < 0.0001; IL-1β: F(2,74) = 53.69, p < 0.0001; TGFβ F(2,51) = 110.6, p < 0.0001; NOX2: F(2,51) = 103, p < 0.0001], and an effect of sex for TNFα only [TNFα: F(1,71) = 12.67, p = 0.0007; IL-1β: F(1,74) = 0.5305, p = 0.4687; TGFβ: F(1,51) = 2.561, p = 0.1157; NOX2: F(1,51) = 1.242, p = 0.2703]. There was a robust up-regulation of TNFα and IL-1β in CD45hi myeloid cells in the brain following TBI, with highest protein expression levels of both pro-inflammatory cytokines at 1 day post-injury, and reduced expression at 3 and 7 days post-injury (TNFα, IL-1β: p < 0.05 – p < 0.0001 for 1 dpi vs. 3 dpi; p < 0.0001 for 1 dpi vs. 7 dpi; Fig. 4A, 4B). A sex difference in TNFα expression in infiltrating CD45hi myeloid cells was observed, with increased TNFα in female TBI compared with male TBI mice at 1 day post-injury (p = 0.0043 vs. male TBI 1 dpi; Fig. 4A), but not at later time-points. There was no sex difference in IL-1β expression across all time-points (Fig. 4B). There was a delayed but robust increase in anti-inflammatory TGFβ in CD45hi myeloid cells in the brain following TBI (Fig. 4C). At 1 day post-injury, there were few CD45hi TGFβ + myeloid cells in the brain, but TGFβ was greatly increased in CD45hi myeloid cells at 3 and 7 days post-injury (p < 0.0001 for 1 dpi vs. 3 dpi; p < 0.0001 for 1 dpi vs. 7 dpi; Fig. 4C); there was no sex difference in TGFβ expression at any time-point.
FIG. 4.
Peripheral myeloid cell functional responses to controlled cortical impact (CCI) are not altered by biological sex. Flow cytometry analysis of infiltrating myeloid cells (CD11b+CD45hi) in male and female mice from sham and CCI through 7 days post-injury. In infiltrating myeloid cells, traumatic brain injury (TBI) increased tumor necrosis factor (TNF)α protein expression in males and females at each post-injury time-point. TNFα was significantly increased in females compared with males at 1 day post-injury (+) (A). TBI increased myeloid cell IL-1β protein at each post-injury time-point in males and females; however, these effects were independent of sex. Myeloid cell IL-1β levels decreased significantly over time (B). TBI increased myeloid cell transforming growth factor (TGF) β protein at each post-injury time-point in males and females; however, these effects were independent of sex (C). TBI increased myeloid cell NOX2 protein at each post-injury time-point in males and females, however, these effects were independent of sex (D). TBI increased myeloid cell reactive oxygen species levels at each post-injury time-point in males and females; however, these effects were independent of sex (E). TBI increased myeloid cell phagocytic activity at each post-injury time-point in males and females; however, these effects were independent of sex (F). Sex and injury effects were analyzed by two-way analysis of variance, and Tukey post hoc analysis was performed for multiple comparisons where +p < 0.05 for male vs. female. Mean ± standard error of the mean; n = 8-15/group. Color image is available online.
NOX2 was highly expressed in CD45hi myeloid cells within 1 day of TBI, and there was a significant increase in NOX2 expression at 7 days post-injury (p < 0.0001 for 1dpi vs. 7 dpi; Fig. 4D). There was no sex difference in NOX2 expression at any time-point. We then assessed ROS production and phagocytic activity in infiltrating CD45hi myeloid cells and statistical analysis revealed an effect of TBI [ROS: F(2,50) = 37.59, p < 0.0001; phagocytosis: F(2,41) = 11.47, p < 0.0001], but no effect of sex [ROS: F(1,50) = 0.0032, p = 0.9549; phagocytosis: F(1,41) = 0.01574, p = 0.9008]. Functional analysis of ROS production in CD45hi myeloid cells demonstrated high ROS levels in peripherally derived cells in the brain at 1 and 3 days post-injury, and peak ROS production in CD45hi myeloid cells was observed at 7 days post-injury (p < 0.001 for 1 dpi vs. 7 dpi; Fig. 4E). There was no sex difference in ROS production at any time-point. Finally, peripherally derived CD45hi myeloid cells were highly phagocytic after TBI, with increased phagocytic activity in infiltrating CD45hi myeloid cells at 1, 3, and 7 days post-injury (p < 0.05 for 1 dpi vs. 7 dpi; Fig. 4F). There was no sex difference in CD45hi myeloid phagocytic activity at any time-point.
Infiltrating myeloid cells are the predominant producers of inflammatory cytokines and ROS, and exhibit high phagocytic activity during acute brain injury
Our flow cytometry analyses demonstrated that despite resident microglia and infiltrating myeloid cells releasing cytokines, producing high levels of ROS, and having enhanced phagocytic activity during the acute phase after TBI, the effect of sex on these functional outcome measures was minor. To determine whether relative numbers of infiltrating myeloid cells with a pro-inflammatory and reactive phenotype could be responsible for a divergent neuroinflammatory response to TBI in males versus female mice, we compared expression levels of cytokines, ROS and phagocytosis in microglia (CD45int) and infiltrating myeloid cells (CD45hi) at 1, 3, and 7 days post-injury (Fig. 5). At 1 day post-injury, peripherally derived CD45hi myeloid cells in the brain were producing 3-5 times more TNFα and IL-1β compared with microglia (TNFα, IL-1β: p < 0.001 CD45hi vs. CD45int; Fig. 5). CD45hi myeloid cells also produced more NOX2 and ROS, and this was associated with increased phagocytic activity when compared with levels in resident CD45int microglia (NOX2, ROS, phagocytosis: p < 0.001 CD45hi vs. CD45int). Peripherally derived CD45hi myeloid cells also produced higher levels of cytokines, NOX2/ROS, and had greater phagocytic activity than microglia at 3 and 7 days post-injury (All: p < 0.001 CD45hi vs. CD45int).
Of note, while there were significantly fewer CD45hi myeloid cells infiltrating the injured brain at 7 days post-injury (p < 0.01 CD45hi 1dpi vs. 7 dpi; Fig. 2B), those that did accumulate in the injured brain at 7 days produced >5 times more ROS than the resident CD45int microglia. Therefore, given sex differences in CD45hi myeloid cell infiltration at 1 day post-injury (Fig. 2B), the influx of CD45hi myeloid cells with an increased pro-inflammatory and ROS phenotype may be responsible for the exaggerated neuroinflammatory responses in male mice compared with female mice acutely after TBI.
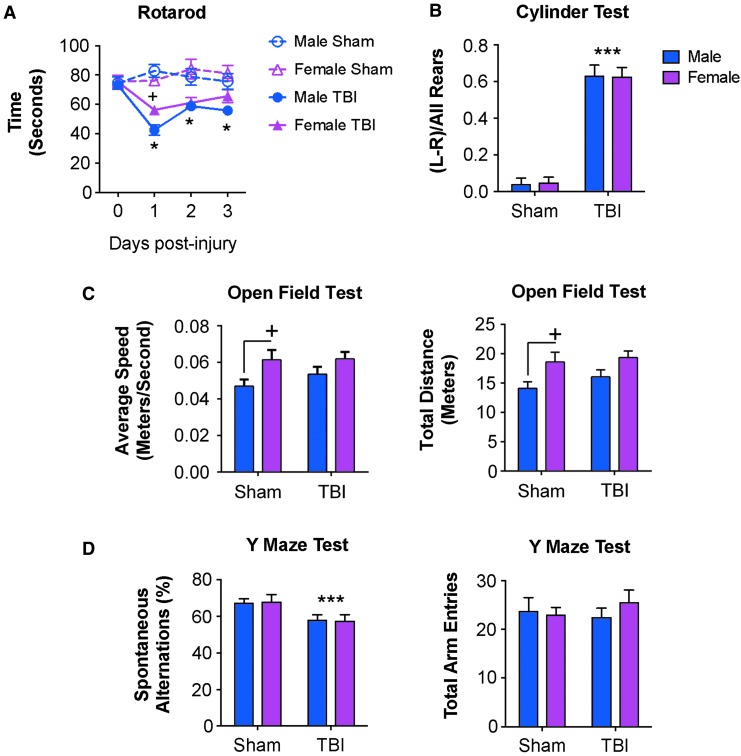
Female mice exhibit reduced motor coordination deficits acutely after TBI
To correlate brain immune function with neurological recovery, sham and CCI mice underwent a battery of neurobehavioral tests to assess motor and cognitive function. Assessment of motor coordination using an accelerating Rotarod test revealed TBI [F(3,114) = 9.152, p < 0.0001] and sex [F(3,38) = 10.15, p < 0.0001] effects on motor function. When compared with sham levels, TBI mice spent significantly reduced time on the accelerating Rotarod on days 1, 2, and 3 post-injury (p < 0.01 – p < 0.0001 vs. sham; Fig. 6A). Notably, female TBI mice performed significantly better than male TBI mice at 1 day post-injury and spent increased time on the Rotarod (p = 0.038 vs. male TBI 1 dpi). Sex differences in acute motor performance were transient because male and female TBI mice had equal Rotarod latencies on Days 2 and 3 post-injury.
FIG. 6.
Acute neurobehavioral outcomes in adult male and female mice following controlled cortical injury. Sham and controlled cortical impact (CCI) male and female mice underwent neurobehavioral testing through 7 days post-injury. (A) In the Rotarod test, traumatic brain injury (TBI) induced impairments in motor function in males and female mice through 3 days post-injury (*) when compared with sham mice. At 1 day post-injury, female TBI mice had reduced motor function deficits when compared with male TBI mice (+). (B) In the cylinder test, both male and female TBI mice showed a significant preference for the ipsilateral paw placement (***) compared with sham mice, indicating a TBI-induced impairment in forelimb function. However, deficits in forelimb function were independent of sex. (C) In the open field test, sham female mice had significantly increased average speed and travelled greater distances than sham male mice (+). There was no effect of TBI in each open field outcome measures. (D) In the Y-maze test, there was a significant TBI effect resulting in decreased spontaneous alterations in male and female TBI mice (***) compared with sham mice. TBI-induced deficits in cognition were independent of sex. Sham and CCI male and female mice had equal numbers of total arm entries in this test. Sex and injury effects were analyzed by two-way analysis of variance (ANOVA), and Tukey post hoc analysis was performed for multiple comparisons where +p < 0.05 for male vs. female, *p < 0.05 and ***p < 0.01 for Sham vs. TBI. Mean ± standard error of the mean; n = 8-15/group. Color image is available online.
We also assessed forelimb function using a Cylinder test on Day 2 post-injury. Statistical analysis revealed a TBI effect [F(1,31) = 87.75, p < 0.0001], but no sex effect [F(1,31) = 0.0005, p = 0.9818] in forelimb function (Fig. 6B). We also assessed locomotor activity in sham and TBI mice at 6 days post-injury using an open field test. Statistical analysis revealed a sex effect in speed [F(1,35) = 7.332, p = 0.0104] and distance travelled [F(1,35) = 7.595, p = 0.0092] in the open field, but no TBI effects [speed: F(1,35) = 1.068, p = 0.3085; distance: F(1,35) = 0.9266, p = 0.3423; Fig. 6C]. We also tested hippocampal-dependent spatial working memory in sham and TBI mice using a Y-maze task on Day 5 post-injury. Statistical analysis revealed a TBI effect [F(1,33) = 7.297, p = 0.0108), but no sex effect [F(1,33) = 0.00133, p = 0.9710; Fig. 6D] in the Y-maze test. Therefore, neurobehavioral testing demonstrated acute and transient improvements in motor coordination in female TBI mice compared with male TBI mice at Day 1 post-injury, and equal performance between sexes in all other motor and cognitive tasks.
Discussion
To our knowledge, this is the first pre-clinical study to directly compare the effect of TBI on infiltrating versus resident macrophage responses in age-matched sexually mature male and female adult mice. Here, using advanced flow cytometry and neurobehavioral testing, we demonstrated that female mice had significant, albeit transient, improvements in acute neurological function after TBI, which were associated with significantly reduced peripheral myeloid cell infiltration into the injured brain at 1 day post-injury and reduced microglial proliferation at 3 days post-injury. Thus, our flow cytometry analysis confirms prior histological studies that demonstrated sexually dimorphic neuroinflammatory responses acutely after TBI, with injured male mice displaying faster and more pronounced microglial/macrophage activation responses following moderate-severe CCI, compared with female mice.29
Although sex differences in leukocyte extravasation and microglial proliferation after TBI were short-lived, our study also demonstrated basal sex differences in microglial function such that male microglia had increased ROS production and greater phagocytic activity, whereas female microglia had an increased ability to produce pro-inflammatory cytokines (TNFα, IL-1β). Despite the observed baseline differences, we did not detect major sex differences in microglial functional responses, including pro- and anti-inflammatory cytokine production, ROS release, or phagocytic activity at any time-point after TBI. However, when we assessed myeloid cell infiltration dynamics following TBI, significantly more peripheral immune cells trafficked to the injured brain of male versus female mice. Importantly, these cells expressed higher levels of pro-inflammatory cytokines, produced more NOX2/ROS, and had greater phagocytic activity than resident microglia at each time-point.
The majority of prior experimental studies that investigated neuroinflammatory responses following TBI have used histological approaches that do not adequately discriminate between resident microglia and infiltrating myeloid cells in the brain, because commonly used antibodies recognize antigens that are expressed on both resident (microglia) and infiltrating myeloid cells (e.g. Iba1). Similar to our study, these histological studies demonstrated sexual dimorphism in neuroinflammatory responses during the acute period post-injury (4-72 h), with both sexes reaching similar levels of glial activation by 3 days post-injury.29 Flow cytometry overcomes these limitations and can provide multi-dimensional phenotypic and functional assessments in resident (microglia) versus infiltrating immune cells (myeloid cells and lymphocytes),30,31,42,43 thereby providing cell-specific functional readouts of neuroinflammation after TBI.
In resident CNS myeloid cells (CD45intCD11b+ microglia), we demonstrated that microglial phagocytic activity and ROS production were robustly increased following TBI and remained elevated through 3 days post-injury. Pro-inflammatory cytokines TNFα and IL-1β also were increased in microglia within 1 day of TBI, and remained elevated through 7 days post-injury. In addition, levels of NOX2, the enzyme complex responsible for ROS production in phagocytes, were significantly increased in microglia at 3 and 7 days post-injury. In comparison to pro-inflammatory cytokine levels, baseline levels of microglial anti-inflammatory TGFβ were reduced, but following TBI there was a significant increase in TGFβ and 1 and 3 days post-injury. However, there were minimal sex differences in microglial functional responses following TBI. Statistical analysis showed a significant sex effect for microglial IL-1β and TGFβ only, with IL-1β levels elevated in female microglia at each post-injury time-point and TGFβ levels significantly reduced in female microglia at 1 day post-injury. These data are consistent with fluorescent in situ hybridization analysis, which identified similar sex differences in IL-1β and TGFβ messenger RNA in microglia/macrophages in the injured cortex at 4 and 24 h post-injury.29
Rapid infiltration of peripheral immune cells, including myeloid and lymphocyte subsets, play a critical role in the brain's inflammatory response to TBI.22,34,35 Moreover, inhibition of chemokine signaling, such as the CCL2/CCR2 signaling axis, reduces peripheral immune cell infiltration, attenuates secondary neuroinflammation and improves long-term cognitive function recovery after TBI.22,23,33,36,44,45 Recent studies from the experimental stroke field implicate peripherally derived immune cells in sex differences for infarct volume, neuronal cell death and secondary neuroinflammation.11,46 For example, adult female mice exhibited a decrease in infiltrating and activated monocytes/microglia in the ischemic brain after transient focal cerebral ischemia (MCAO) compared with males.47 Further, splenectomy prior to MCAO eliminated sex differences in infarct volume and activated brain monocytes/microglia.48
In our TBI model, we also observed a rapid activation and infiltration of peripherally derived myeloid cells in the injured brain of male, but not female mice. There was an early and robust CD45hi myeloid cell infiltration in male compared with female mice at 1 day post-injury, and a non-significant sex difference in infiltration at 3 days post-injury. The acute infiltration response in male mice preceded an exaggerated microglial response at 3 days post-injury that was not seen in females. However, by 7 days post-injury, the levels of peripheral myeloid cell infiltration and microglial activation had equalized between male and female TBI mice. The described peripheral immune cell infiltration dynamics corroborate prior histological studies, which used F4/80 as a marker of peripherally derived macrophages and showed significantly increased infiltration of F4/80+ macrophages in the cortex of male TBI mice at 1 and 3 days post-injury when compared with female TBI mice.29 Moreover, reduced influx of peripheral F4/80+ macrophages in females was associated with reduced neuronal cell death and lesion volume acutely after TBI compared with male mice.29 Here we did not observe major sex differences in infiltrating myeloid cell function at any time-point, except for myeloid TNFα, which was significantly elevated in females compared with males at 1 day post-injury.
However, despite not observing sex differences in myeloid cell function, we detected an increased number of infiltrating myeloid cells in the male brain as compared with the female brain at 1 day post-injury. It follows that because there are more active cells infiltrating the brain globally, there would be a more pro-inflammatory milieu in the injured brains of males compared with females at 1 day post-injury. Moreover, the peripheral myeloid cells that trafficked to the injured brain were highly reactive and the strongest producers of pro-inflammatory and neurotoxic mediators. When compared with levels in resident microglia, the peripheral cells produced very high levels of NOX2/ROS, pro-inflammatory cytokines, TNFα and IL-1β, and had elevated phagocytic activity at all time-points analyzed. Although infiltrating myeloid cell numbers are greatly reduced by 7 days post-injury, the cells that remain are highly reactive and pro-inflammatory. Therefore, our data indicate that peripheral immune cells harbored late within the TBI brain may have an impact on injury resolution and neurological recovery, perhaps by propagating enhanced and sustained microglial activation after TBI that lasts for weeks and months post-injury.24
In neurobehavioral testing, we identified sex differences in locomotor function acutely after TBI using a rotarod test. Male CCI mice had significantly worse rotarod performance at 1 day post-injury compared with female CCI mice, but at later time-points there were no differences in locomotor function between males and females. Sex differences in the number of peripheral myeloid infiltration in the brain at 1 day post-injury coupled with an early microglial response may explain the sexually dimorphic responses in acute locomotor function following moderate-severe TBI. Several prior studies also report significantly worse locomotor measures after TBI in male rodents compared with females,14,20,41,49,50 while others report no sex differences.19,51 In tests of cognitive and anxiety-like function, male and female CCI mice showed similar TBI impairments during the acute phase response 1 day through 7 days post-injury, as previously reported.19,20,41,52 Overall, our neurobehavioral results are similar to what has been reported previously,19,52 where sex differences are present acutely after TBI but are lost at later time-points. This suggests that although there may be acute differences in immune cell infiltration and microglial activation in males versus females following severe TBI, the neuroinflammatory response equilibrates at later time-points such that males and females have equal levels of secondary neuroinflammation, neuronal cell death, lesion expansion, and long-term neurological impairments in the weeks and months post-injury.19,29,52
Although the present study highlights important differences between male and female neuroinflammatory dynamics and functional outcomes following TBI, there are several caveats to note. Firstly, this study did not distinguish between neutrophils and monocytes when analyzing CD45hiCD11b+ infiltrating myeloid cells in the brain. While these two cell types perform different functions after entering the brain,53,54 we found that monocytes greatly outnumbered neutrophils in the injured brain (4:1), and there were no sex differences in the frequencies of neutrophils to monocytes infiltrating in the brain (Supplementary Fig. 1). The majority of neutrophils were detected at 1 day post-injury and represented ∼20% of total infiltrating myeloid cells at this time-point, whereas only ∼5% neutrophils trafficked to the injured brain at 3 days post-injury.
Secondly, we chose to use intact female mice in our study in order to get a better understanding of sex differences in microglial function and secondary neuroinflammatory responses at baseline and during the acute post-traumatic period. Clinical data is currently conflicted as to whether there is a role for ovarian sex hormones in the setting of TBI because age, TBI heterogeneity, and severity are all major confounders in clinical studies.56 Analysis of 17β-estradiol levels in the blood were similar in all female mice tested and across all time-points, indicating that females were in proestrus stage and that estrogen levels did not change with injury (Table 1). As predicted, 17β-estradiol levels were elevated in females compared with males, suggesting that estrogen may be related to the observed sex differences in our studies. Female sex steroids have been extensively studied in pre-clinical TBI models, and exogenous administration of estrogen have documented neuroprotective effects.15,57 In fact, there is evidence from pig TBI and rat repeated mild TBI models that phases of the estrous cycle can impact female neurological outcomes.20,41,55
With regard to post-traumatic neuroinflammation, an early study reported that neither sex nor estrogen manipulation by ovariectomy surgery affected microglial activation in the hippocampus after moderate-severe TBI26; however, more recent studies demonstrated significant sex differences in microglial and astrocyte activation during acute phase responses after moderate-severe TBI25,28,29—effects that can be reversed by ovariectomy surgery.28,58 These later studies support our findings and indicate that endogenous female sex steroids can regulate the neuroinflammatory response to TBI leading to acute neuroprotection in female animals. In our study we also chose to use age-matched male and female C57BL/6 mice with identical injury parameters including impact velocity and depth of CCI, despite a ∼5 g difference in body weight. Conventionally, the thinking is that a smaller body weight with a similar injury would cause more harm.59–61 Notably, we demonstrated that injured female mice did not have worse neurological outcomes or post-traumatic neuroinflammatory responses, but rather showed acute neuroprotection associated with reduced immune cell infiltration and microglial activation, despite having lower body mass.
In summary, we demonstrated that at baseline male and female microglia have different functional activation states during homeostasis, such as differences in cell morphology, cytokine and ROS production, and phagocytosis. Following moderate-severe TBI, we observed minimal sex differences in microglial functional responses. However, male mice had significantly increased numbers of infiltrating peripheral immune cells within the brain compared with female mice at 1 day post-injury that preceded exaggerated microglial activation at 3 days post-injury; acute neuroinflammatory changes correlated with poorer motor function recovery acutely after TBI. Importantly, infiltrating myeloid cells produced higher levels of pro-inflammatory cytokines and NOX2/ROS compared with resident microglia following TBI, indicating that peripherally derived immune cells dictate sex-dependent post-traumatic neuroinflammatory responses that impair neurological outcomes acutely after TBI.
Supplementary Material
Acknowledgments
We would like to acknowledge Victoria Meadows, B.Sc., Nivedita Hegdekar, B.Sc., Nicholas Braganca, B.Sc., and Wesley Shoap, B.Sc., for their technical assistance. We thank Xiaoxuan Fan, Ph.D., and Karen Underwood, B.Sc., of the University of Maryland Greenebaum Comprehensive Cancer Center Flow Cytometry Facility for support with flow cytometry studies. This work was supported by National Institutes of Health grants R01NS082308 (D.J. Loane), R01NS037313 (A.I. Faden), F32NS105355 (R.M. Ritzel), and T32AI095190 (S.J. Doran).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalization, and Deaths. Centers for Disease Control and Prevention; National Center for Injury Prevention and Control: Atlanta, GA, pps. 891–904 [Google Scholar]
- 2. Langlois J.A., Rutland-Brown W., and Thomas K. (2004). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalization, and Deaths. Centers for Disease Control and Prevention; National Center for Injury Prevention and Control: Atlanta, GA, 1–74 [Google Scholar]
- 3. Berry C., Ley E.J., Tillou A., Cryer G., Margulies D.R., and Salim A. (2009). The effect of gender on patients with moderate to severe head injuries. J. Trauma 67, 950–953 [DOI] [PubMed] [Google Scholar]
- 4. Ottochian M., Salim A., Berry C., Chan L.S., Wilson M.T., and Margulies D.R. (2009). Severe traumatic brain injury: is there a gender difference in mortality? Am. J. Surg. 197, 155–158 [DOI] [PubMed] [Google Scholar]
- 5. Baum J., Entezami P., Shah K., and Medhkour A. (2016). Predictors of outcomes in traumatic brain injury. World Neurosurg. 90, 525–529 [DOI] [PubMed] [Google Scholar]
- 6. Moore D.W., Ashman T.A., Cantor J.B., Krinick R.J., and Spielman L.A. (2010). Does gender influence cognitive outcome after traumatic brain injury? Neuropsychol. Rehabil. 20, 340–354 [DOI] [PubMed] [Google Scholar]
- 7. Albrecht J.S., McCunn M., Stein D.M., Simoni-Wastila L., and Smith G.S. (2016). Sex differences in mortality following isolated traumatic brain injury among older adults. J. Trauma Acute Care Surg. 81, 486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farin A., Deutsch R., Biegon A., and Marshall L.F. (2003). Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurgery 98, 32–36 [DOI] [PubMed] [Google Scholar]
- 9. Scholten A.C., Haagsma J.A., Andriessen T.M., Vos P.E., Steyerberg E.W., van Beeck E.F., and Polinder S. (2015). Health-related quality of life after mild, moderate and severe traumatic brain injury: patterns and predictors of suboptimal functioning during the first year after injury. Injury 46, 616–624 [DOI] [PubMed] [Google Scholar]
- 10. Whelan-Goodinson R., Ponsford J.L., Schonberger M., and Johnston L. (2010). Predictors of psychiatric disorders following traumatic brain injury. J. Head Trauma Rehabil. 25, 320–329 [DOI] [PubMed] [Google Scholar]
- 11. Spychala M.S., Honarpisheh P., and McCullough L.D. (2017). Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J. Neurosci. Res. 95, 462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caplan H.W., Cox C.S., and Bedi S.S. (2017). Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 95, 509–517 [DOI] [PubMed] [Google Scholar]
- 13. Bramlett H.M. and Dietrich W.D. (2001). Neuropathological protection after traumatic brain injury in intact female rats bersus males or ovariectomized females. J. Neurotrauma 18, 891–900 [DOI] [PubMed] [Google Scholar]
- 14. O'Connor C.A., Cernak I., and Vink R. (2003). Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J. Neurotrauma 20, 533–541 [DOI] [PubMed] [Google Scholar]
- 15. Roof R.L. and Hall E.D. (2000). Estrogen-related gender difference in survival rate and cortical blood flow after impact acceleration head injury in rats. J. Neurotrauma 17, 1155–1169 [DOI] [PubMed] [Google Scholar]
- 16. Semple B.D., Dixit S., Shultz S.R., Boon W.C., and O'Brien T.J. (2017). Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behav. Brain Res. 319, 48–62 [DOI] [PubMed] [Google Scholar]
- 17. Hall E.D., Gibson T.R., and Pavel K.M. (2005). Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J. Neurotrauma 22, 669–679 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki T., Bramlett H.M., and Dietrich W.D. (2003). The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp. Neurol. 184, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 19. Tucker L.B., Fu A.H., and McCabe J.T. (2016). Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma 33, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner A.K., Willard L.A., Kline A.E., Wenger M.K., Bolinger B.D., Ren D., Zafonte R.D., and Dixon C.E. (2004). Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 998, 113–121 [DOI] [PubMed] [Google Scholar]
- 21. Xiong Y., Mahmood A., Lu D., Qu C., Goussev A., Schallert T., and Chopp M. (2007). Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice. Brain Res. 1185, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., and Nakamura M.C. (2014). CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma 31, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morganti J.M., Riparip L.K., Chou A., Liu S., Gupta N., and Rosi S. (2016). Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J. Neuroinflammation 13, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acaz-Fonseca E., Duran J.C., Carrero P., Garcia-Segura L.M., and Arevalo M.A. (2015). Sex differences in glia reactivity after cortical brain injury. Glia 63, 1966–1981 [DOI] [PubMed] [Google Scholar]
- 26. Bruce-Keller A.J., Dimayuga F.O., Reed J.L., Wang C., Angers R., Wilson M.E., Dimayuga V.M., and Scheff S.W. (2007). Gender and estrogen manipulation do not affect traumatic brain injury in mice. J. Neurotrauma 24, 203–215 [DOI] [PubMed] [Google Scholar]
- 27. Wright D.K., O'Brien T.J., Shultz S.R., and Mychasiuk R. (2017). Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann. Clin. Transl. Neurol. 4, 640–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clevenger A.C., Kim H., Salcedo E., Yonchek J.C., Rodgers K.M., Orfila J.E., Dietz R.M., Quillinan N., Traystman R.J., and Herson P.S. (2018). Endogenous sex steroids dampen meuroinflammation and improve outcome of traumatic brain injury in mice. J. Mol. Neurosci. 64, 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villapol S., Loane D.J., and Burns M.P. (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ritzel R.M., Patel A.R., Grenier J.M., Crapser J., Verma R., Jellison E.R., and McCullough L.D. (2015). Functional differences between microglia and monocytes after ischemic stroke. J. Neuroinflammation 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ritzel R.M., Doran S.J., Barrett J.P., Henry R.J., Ma E.L., Faden A.I., and Loane D.J. (2018). Chronic alterations in systemic immune function after traumatic brain injury. J. Neurotrauma 35, 1419–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar A., Barrett J.P., Alvarez-Croda D.M., Stoica B.A., Faden A.I., and Loane D.J. (2016). NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 58, 291–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Israelsson C., Kylberg A., Bengtsson H., Hillered L., and Ebendal T. (2014). Interacting chemokine signals regulate dendritic cells in acute brain injury. PLoS One 9, e104754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin X., Ishii H., Bai Z., Itokazu T. and Yamashita T. (2012). Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One 7, e41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar A., Alvarez-Croda D.M., Stoica B.A., Faden A.I., and Loane D.J. (2016). Microglial/macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma 33, 1732–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morganti J.M., Jopson T.D., Liu S., Riparip L.K., Guandique C.K., Gupta N., Ferguson A.R., and Rosi S. (2015). CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J. Neurosci. 35, 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jirapongsananuruk O., Malech H.L., Kuhns D.B., Niemela J.E., Brown M.R., Anderson-Cohen M., and Fleisher T.A. (2003). Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J. Allergy Clin. Immunol. 111, 374–379 [DOI] [PubMed] [Google Scholar]
- 38. Simard J.M., Tsymbalyuk O., Keledjian K., Ivanov A., Ivanova S., and Gerzanich V. (2012). Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp. Neurol. 233, 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tosun C., Koltz M.T., Kurland D.B., Ijaz H., Gurakar M., Schwartzbauer G., Coksaygan T., Ivanova S., Gerzanich V., and Simard J.M. (2013). The protective effect of glibenclamide in a model of hemorrhagic encephalopathy of prematurity. Brain Sci. 3, 215–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X., Blizzard K.K., Zeng Z., DeVries A.C., Hurn P.D., and McCullough L.D. (2004). Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp. Neurol. 187, 94–104 [DOI] [PubMed] [Google Scholar]
- 41. Russell K.L., Kutchko K.M., Fowler S.C., Berman N.E., and Levant B. (2011). Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: assessment of sex differences. J. Neurosci. Methods 199, 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bai R., Gao H., Han Z., Ge X., Huang S., Chen F., and Lei P. (2017). Long-term kinetics of immunologic components and neurological deficits in rats following repetitive mild traumatic brain injury. Med. Sci. Monit. 23, 1707–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bai R., Gao H., Han Z., Huang S., Ge X., Chen F., and Lei P. (2017). Flow cytometric characterization of T cell subsets and microglia after repetitive mild traumatic brain injury in rats. Neurochem. Res. 42, 2892–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Semple B.D., Bye N., Rancan M., Ziebell J.M., and Morganti-Kossmann M.C. (2010). Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J. Cereb. Blood Flow Metab. 30, 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Semple B.D., Bye N., Ziebell J.M., and Morganti-Kossmann M.C. (2010). Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol. Dis. 40, 394–403 [DOI] [PubMed] [Google Scholar]
- 46. Dotson A.L. and Offner H. (2017). Sex differences in the immune response to experimental stroke: Implications for translational research. J. Neurosci. Res. 95, 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Banerjee A., Wang J., Bodhankar S., Vandenbark A.A., Murphy S.J., and Offner H. (2013). Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl. Stroke Res. 4, 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dotson A.L., Wang J., Saugstad J., Murphy S.J., and Offner H. (2015). Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J. Neuroimmunol. 278, 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wagner A.K., Kline A.E., Ren D., Willard L.A., Wenger M.K., Zafonte R.D., and Dixon C.E. (2007). Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav. Brain Res. 181, 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner A.K., Kline A.E., Sokoloski J., Zafonte R.D., Capulong E., and Dixon C.E. (2002). Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neuroscience Lett. 334, 165–168 [DOI] [PubMed] [Google Scholar]
- 51. Grossman K.J. and Stein D.G. (2000). Does endogenous progesterone promote recovery of chronic sensorimotor deficits following contusion to the forelimb representation of the sensorimotor cortex? Behav. Brain Res. 116, 141–148 [DOI] [PubMed] [Google Scholar]
- 52. Tucker L.B., Burke J.F., Fu A.H., and McCabe J.T. (2017). Neuropsychiatric symptom modeling in male and remale C57BL/6J mice after experimental traumatic brain injury. J. Neurotrauma 34, 890–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dale D.C., Boxer L., and Liles W.C. (2008). The phagocytes: neutrophils and monocytes. Blood 112, 935–945 [DOI] [PubMed] [Google Scholar]
- 54. Prame Kumar K., Nicholls A.J., and Wong C.H.Y. (2018). Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 371, 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Armstead W.M., Riley J., and Vavilala M.S. (2017). Sex and age differences in epinephrine mechanisms and outcomes after brain injury. J. Neurotrauma 34, 1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Slewa-Younan S., van den Berg S., Baguley I.J., Nott M., and Cameron I.D. (2008). Towards an understanding of sex differences in functional outcome following moderate to severe traumatic brain injury: a systematic review. J. Neurol. Neurosurg. Psychiatry 79, 1197–1201 [DOI] [PubMed] [Google Scholar]
- 57. Khaksari M., Soltani Z., and Shahrokhi N. (2018). Effects of female sex steroids administration on pathophysiologic mechanisms in traumatic brain injury. Transl. Stroke Res. 9, 393–416 [DOI] [PubMed] [Google Scholar]
- 58. Taylor A.N., Tio D.L., Paydar A., and Sutton R.L. (2018). Sex differences in thermal, stress, and inflammatory responses to minocycline administration in rats with traumatic brain injury. J. Neurotrauma 35, 630–638 [DOI] [PubMed] [Google Scholar]
- 59. McFadyen M.P., Kusek G., Bolivar V.J., and Flaherty L. (2003). Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2, 214–219 [DOI] [PubMed] [Google Scholar]
- 60. Meyer M.R., Clegg D.J., Prossnitz E.R., and Barton M. (2011). Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. (Oxf.) 203, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schellinck H.M., Cyr D.P., and Brown R.E. (2010). How many ways can mouse behavioral experiments go wrong? Confounding variables in mouse models of neurodegenerative diseases and how to control them. Adv. Study Behav. 41, 255–366 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.