Abstract
Responding appropriately to stress is essential for survival, yet in pathological states, these responses can develop into debilitating conditions such as post-traumatic stress disorder and generalized anxiety. While genetic models have provided insight into the neurochemical and neuroanatomical pathways that underlie stress, little is known about how evolutionary processes and naturally occurring variation contribute to the diverse responses to stressful stimuli observed in the animal kingdom. The Mexican cavefish is a powerful system to address how altered genetic and neuronal systems can give rise to altered behaviors. When introduced into a novel tank, surface fish and cavefish display a stereotypic stress response, characterized by reduced exploratory behavior and increased immobility, akin to “freezing”. The stress response in cave and surface forms is reduced by pharmacological treatment with the anxiolytic drug, buspirone, fortifying the notion that behavior in the assay represents a conserved stress state. We find that cave populations display reduced behavioral measures of stress compared to surface conspecifics, including increased time in the top half of the tank and fewer periods of immobility. Further, reduced stress responses are observed in multiple independently derived cavefish populations, suggesting convergence on loss of behavioral stress responses in the novel tank assay. These findings provide evidence of a naturally occurring species with two drastically different forms in which a shift in predator-rich ecology to one with few predators corresponds to a reduction in stress behavior.
Keywords: Astyanax, Anxiety, Buspirone, Convergent evolution, Novel tank, Bottom dwelling
1. Introduction
Stereotyped responses to aversive cues are found ubiquitously among animals, and function to increase alertness and aid in escaping threatening and potentially harmful stimuli (Campos et al., 2013; Cockrem, 2013). Normal stress responses are essential to the survival of an organism (Nesse et al., 2016), yet, in pathological states, their dysregulation can be severely debilitating, as in the case of anxiety disorders such as post-traumatic stress disorder and generalized anxiety (Sherin and Nemeroff, 2011; Tovote et al., 2015). Despite the widespread prevalence of such disorders, there exists a poor understanding of the genetic and neuronal underpinnings of these conditions, though several studies suggest a complex relationship between environmental conditions and genetic factors (Cornelis and Nugent, 2010; Etkin and Wager, 2007; Koenen et al., 2008).
Throughout the animal kingdom, stress responses vary both within (Lacey and Lacey, 1958; Schneiderman et al., 2005) and between species (Campos et al., 2013; Cockrem, 2013), yet little is known about how and why these differences evolve. Differences in ecology, such as robust changes in temperature, humidity, food availability or altered threat of predation result in different stress responses (Bateson et al., 2011; Howarth, 1993). Recent studies have explored this phenomenon by taking groups of individuals of the same species and subjecting them to different levels of stressors. These studies demonstrate that animals from environments with varying levels of predation have different stress responses (Adamo et al., 2013; Archard et al., 2012; Brown et al., 2005; Mateo, 2007). Though much is known regarding the influence of ecology and experience on stress responses, the evolutionary basis for varying stress responses have not been well addressed.
The Mexican blind cavefish, Astyanax mexicanus, has emerged as a powerful model to investigate how evolution and environment can shape behaviors in an organism with independently derived variants. Blind A. mexicanus are found in at least 29 distinct populations, which have all evolved from ancestral surface dwelling forms (Borowsky, 2008a; Jeffery, 2008; Mitchell et al., 1977). Multiple cave populations have evolved independent of each other in different migratory events (Bradic et al., 2013; Ornelas-García et al., 2008), making the system a powerful model for exploring both parallel and convergent evolution. The combination of a rich phylogeographic and phylogenetic history (Bradic et al., 2013; Mitchell et al., 1977; Ornelas-García et al., 2008), a sequenced genome (McGaugh et al., 2014), and vast differences in behaviors (Duboué et al. 2011; Kowalko et al., 2013b; Yoshizawa et al., 2010) makes A. mexicanus a unique system to interrogate how genes and environment can influence behavioral traits in naturally evolved variants.
Relative to the epigean environment of surface fish, A. mexicanus cavefish have a near absence of predators in the caves of Northeast Mexico where they live; there are few organisms that inhabit the caves, most of which are invertebrates (Mitchell et al., 1977; Reddell and Elliott, 1973). The drastic differences in predator abundance that exist between cave and surface ecologies suggest that stress responses between the two forms may be different (Mateo, 2007). Many stress assays are highly reliant on visual cues, which provides a confounding factor for blind cavefish. We therefore tested stress using an assay that would be less dependent on eye loss, the novel tank test (Levin et al., 2007), which is commonly used to measure the innate response to stressors in fishes (Cachat et al., 2010; Heinen-Kay et al., 2016; Levin et al., 2007; Thompson et al., 2016). When initially placed in an unfamiliar tank, adults prefer to spend more time in the bottom half of the chamber, yet over time they begin exploring both halves with near-equal frequency (Cachat et al., 2010; Levin et al., 2007). Pharmacological agents that reduce stress in humans cause a preference for exploring the top half, whereas drugs that induce stress result in the individuals remaining at the bottom, even in an acclimated adult (Bencan et al., 2009; Cachat et al., 2010; Levin et al., 2007). Therefore, the amount of time spent at the bottom is a reliable readout of innate stress. Here, we report reduced stress in multiple populations of A. mexicanus cavefish relative to surface fish conspecifics. Pretreatment with an anxiolytic drug abolished the differences seen between the two forms. Moreover, the differences in innate stress responses were independent of visual cues, and evolved in at least three separate lineages of cavefish. Together, our findings reveal convergence on a reduced stress response in cave A. mexicanus, providing a model to investigate the genetic and evolutionary basis for stress behaviors.
2. Materials and methods
2.1. Animal maintenance
Animal care was performed as previously described (Borowsky, 2008b; Jaggard et al., 2017, 2018). Adult A. mexicanus were housed on a circulating filtration system in 18–37 liter tanks. Water temperatures were maintained at 21 ± 1 °C. Fish were housed in a room on a 14:10 light: dark cycle, with the light intensity maintained at 25–40 lux. Fish were fed a mixture of food, which included California black worms (Aquatic Foods) and TetraMin® fish flakes. The fish used in this study were laboratory-born Pachón, Tinaja, and Molino cavefish and surface conspecifics from the Texas or Rio Choy lineages. All experiments in this study were approved by the Institutional Animal Care and Usage Committee (IACUC) at Florida Atlantic University, protocol numbers A17–21 and A15–32.
2.2. Behavioral assessment of stress response
All behavioral assays were performed on adult fish, ranging from 24 to 36 months of age, and were done between 10:00 a.m. and 6:00 p.m. On the morning of experimentation, adult fish to be tested were transferred from their home tanks on the fish system into a dedicated behavioral room, and allowed to acclimate to the room for at least 1 h. After behavioral recording, the fish were transferred to a dedicated holding chamber, and, at the completion of the day’s experiments, placed back into their home tanks. For all experiments, the sex of the fish was documented.
Fish were tested in a rectangular plastic tank with dimensions 28 × 18 × 18 cm (L×W×H). The tank was positioned in front of a high-frame-rate cMOS camera (PointGrey, FLIR Integrated Imaging Systems, Inc.) with custom designed infrared Light Emitting Diodes lights (LED; 880 nm). Records of each trial were captured using FlyCap2 Software (version 2.11, PointGrey, FLIR Integrated Imaging Systems, Inc.). Naïve adults were removed from their holding tank and placed in the apparatus, and video acquisition was started. Recordings were captured at 30 frames-per-second for a 10-min period.
The center position of each fish over the recording period was tracked offline after the experiment using Ethovision XT13 (version 13.0, Noldus, Inc., Leesburg, VA) (Noldus et al., 2001), and the x-y displacement was calculated across all frames. Each frame was then manually inspected for inaccurate tracking, and, when appropriate, tracking for respective frames was corrected manually. Additionally, an imaginary line was drawn in Ethovision at the midline of the tank, dividing the top and bottom halves of the chamber. Total duration of time spent in the top of the recording chamber, distance, velocity, and immobility were measured for each fish. Calibrating to the length of the tank, distance between the center of the fish from one frame to the next was calculated, and total distance and velocity was obtained for the 10-min recording. The program accurately tracked the entire silhouette of the individual using background subtraction, therefore, whenever the silhouette occupied above or below the dividing imaginary line, the program calculated the duration it spent in the sector. Immobility of the fish was defined as any point where the change in silhouette pixels between one frame and the next was less than 5%.
2.3. Pharmacological treatment
The pharmacological 5-HT1A agonist buspirone (Sigma, St. Louis, MO; cat. no. B7148) was used as an anxiolytic drug, in accordance with published protocols established in zebrafish (Bencan et al., 2009; Facchin et al., 2015). Adult fish were placed into an unfamiliar tank and recorded for a 10-min period. After recording a baseline, fish were transferred to a 500-mL beaker filled with buspirone-treated system water (12.5 mg/L). A control group of fish went through a similar procedure except instead of being transferred to a 500-mL beaker of buspirone in system water, they were transferred to a beaker with system water only. Fish were kept in the presence of the drug for 10-min. After drug treatment, adult fish were placed in fresh system water for 10 min, and then transferred to a different testing chamber than was used without drug treatment, and behavior was recorded for an additional 10-min period.
2.4. Constant darkness experiments
For experiments in which behavior was compared between lighted and dark conditions, two behavioral rooms, one with the lights on and another with the lights off, were used. Each room housed identical apparatus. Apparatus and procedures were the same as those described in Section 2.2. Groups of surface or Pachón adults were habituated in the respective rooms 1 h before testing. Each fish was recorded in one condition first, placed in a holding tank for 1 h, and then recorded in the other condition. Trials were randomized such that some fish were recorded in lighted conditions first and then subsequently in constant darkness, whereas others were recorded first in darkness and then after in a lighted setting.
2.5. Statistics
All statistical analyses were performed in GraphPad Prism software (version 7, Graphpad Inc.). In cases where comparisons between two un-paired groups were performed, the Mann-Whitney U test was used. In cases where comparisons between two paired groups were performed, the paired t-test was used. In cases where comparisons between multiple groups were done, the Kruskal-Wallis ANOVA was performed, and, in cases where significance was found, a post-hoc Dunn’s test with correction for multiple comparisons was done. For time-series analysis in Fig. 1B where each minute of a 10-min trial was compared to the first minute, a Friedman test for repeated-measures was used followed by a post-hoc Dunn’s multiple comparison test. For all statistical tests, significance was determined to be below α = 0.05.
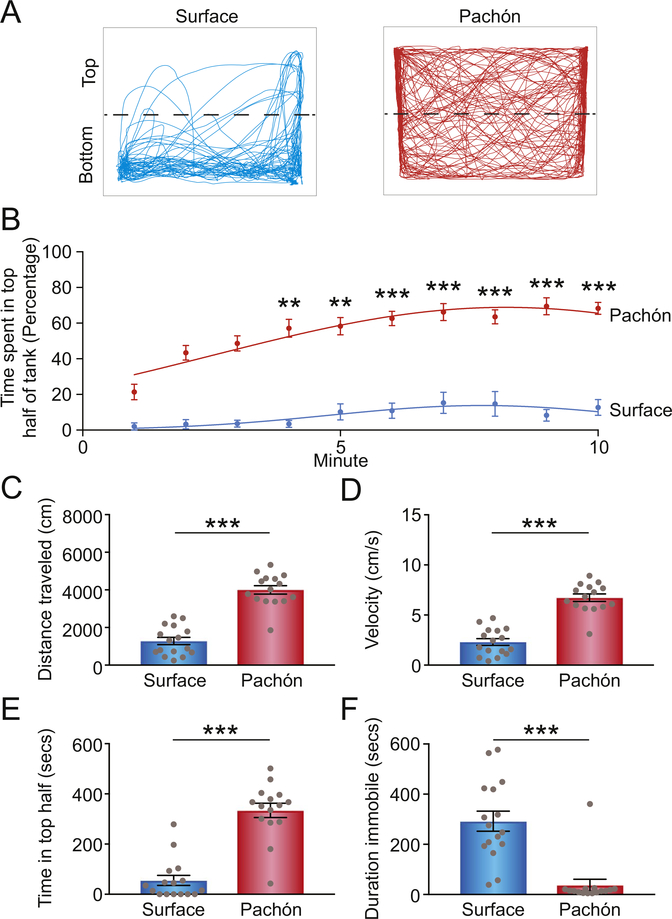
Fig. 1. Reduced stress-related behaviors in Pachón cavefish compared to surface populations.
(A) Representative swimming trajectories of a surface and Pachón fish. (B) Analysis of each minute over a 10-min trial showing percentage of the minute spent exploring the top half of the novel tank. Statistical significances represent comparisons to the first minute. Data analyzed by a Friedman test followed by Dunn’s multiple comparison test: ** indicates P < 0.005; *** indicates P < 0.0001 (Pachón: Minute 1 vs. 4 – P = 0.0038; Minute 1 vs. 5 – P = 0.0017). (C-F) Total distances traveled (C), velocity (D), time spent in top half (E), and duration immobile (F) for surface and Pachón cavefish in the 10-min trial. C-F analyzed by Mann-Whitney test: *** indicates P < 0.0001. (Surface: n = 16; Pachón: n = 15) Error bars ± s.e.m.
3. Results
3.1. Pachón cavefish exhibit reduced stress responses relative to surface conspecifics
To determine whether evolution in a cave environment results in altered stress responses, we tested A. mexicanus in the novel tank diving paradigm, which is commonly used to measure innate stress behavior in fish (Cachat et al., 2010; Levin et al., 2007). Surface and Pachón cave adults were placed individually in an unfamiliar tank, and the duration spent in the top half of the tank, duration spent immobile, distance traveled, and velocity were measured over the 10-min recording (Fig. 1A). Both surface and Pachón cave forms displayed a stereotypic stress response to the novel tank, revealed by an initial preference for the bottom half of the tank (Fig. 1B). Pachón cavefish showed a gradual progression to explore the top half of the tank, and by 3–4 min after the trial began, they were exploring both halves with near-equal frequency (Fig. 1B). By contrast, surface fish displayed a consistent preference for the bottom half of the tank over the entire duration of the recording (Fig. 1B). We next quantified locomotion over the entire 10-min recording period. On average, cavefish were significantly more active, measured by total distance traveled and average velocity over the 10-min recording (Fig. 1C, D). When the total duration in the top half of the tank was measured, the Pachón population spent significantly more time in the top portion relative to the surface population (Fig. 1E). Additionally, surface individuals spent more time immobile, akin to “freezing”, which is typically taken as a measure of anxiety (Cachat et al., 2010) (Fig. 1F). The results suggest that the stereotyped stress response in the novel tank assay is reduced in Pachón cavefish compared to surface fish.
In zebrafish, some stress responses show sex-dependent differences (Rambo et al., 2017). We therefore reanalyzed total distance traveled and duration immobile taking sex into account. For both males and females, Pachón cavefish traveled more distance and spent less time immobile than surface conspecifics. However, for both cave and surface fish, there were no differences between males and females for time spent in the top half (male vs. female surface: 71.97 ± 94.3 s vs. 27.36 ± 41.41 s, p = 0.99; male vs. female Pachón cave: 308.43 ± 141.3 s vs. 363.43 ± 57.5 s, p = 0.99). Similarly, no sex-dependent differences were observed for time immobile between males and females (male vs. female surface: 285.43 ± 188.0 s vs. 303.52 ± 110.5 s, p = 0.99; male vs. female Pachón cave: 56.37 ± 123.1 s vs. 15.89 ± 8.6 s, p = 0.99).
These data suggest no sex-dependent differences in bottom dwelling for A. mexicanus, and thus data from males and females was pooled for all subsequent experiments. Taken together, these data suggest that cavefish evolved reductions in their behavioral response to stress.
3.2. Differences in stress between surface and Pachón cavefish are stable across trials
A common feature of innate stress responses in zebrafish is habituation, which is defined as the dampening of stress responses after repeated exposure to the aversive cue (Wong et al., 2010). To address whether cave and surface fish habituated to this assay, we performed the bottom dwelling test in groups of adults across several days. We performed these experiments across three consecutive days to account for short-term habituation, and five days later to account for long-term habituation (Supplemental Fig. 1A-D). As before, Pachón cavefish spent more time in the top half and spent less time immobile compared to surface fish. Importantly, these measures were constant across all days measured for both cave and surface fish, revealing that neither form habituated to the novel tank (Supplemental Fig. 1A-B). Cavefish begin exploring top and bottom halves of the tank with near-equal frequency at approximately the fourth minute of recording (Fig. 1B), and thus quantifying behavior over the entire 10-min recording period could mask the effect of possible habituation. We therefore quantified time spent in the top and duration of immobility in only the first three minutes of recording over all days. These data revealed no significant differences in behavior across different days (Supplemental Fig. 1C-D), suggesting that, indeed, no habituation occurred. Lastly, because habituation can occur in shorter time scales, as is the case with Medaka (Matsunaga and Watanabe, 2010), we recorded groups of adult cave and surface fish at different time periods on the same day. Neither time spent in the top half or duration of immobility differed across time points for either the entire 10-min recording (Supplemental Fig. 1E-F) or the first three minutes only (Supplemental Fig. 1G-H).
3.3. Reduced bottom dwelling in cavefish is indicative of diminished stress
In order to confirm that the differences we observed between the surface and Pachón cavefish were indicative of altered stress responses, we pre-treated both forms with an anxiolytic drug, buspirone. Buspirone is a serotonin 5-HT1A agonist that inhibits stress and anxiety in many vertebrates including humans and zebrafish (Bencan et al., 2009; Facchin et al., 2015; Gammans et al., 1989; Jacobson et al., 1985). Adult surface fish and Pachón cavefish were tested in the novel tank diving test without treatment to establish a baseline behavior. After the trial, each individual fish was treated in either a dose of buspirone or control solvent. Then, the fish was retested in a second novel tank test following a short rest period (Fig. 2A). In the absence of drug, Pachón cavefish spent more time in the top half of the tank compared to surface forms. However, after a brief 10-min exposure to buspirone, both surface and cavefish explored the top half significantly more than they did in the drug’s absence (Fig. 2B, C). When compared, there were no significant differences in duration swam in the top half or in immobility between surface and cave populations treated with buspirone (Fig. 2C, D, Dunn’s post-test, P > 0.99) for both. Buspirone had a moderately significant effect on total activity in cavefish (p = 0.045) and no effect on surface morphs (p = 0.293), suggesting that buspirone impacts locomotion only marginally (Supplemental Fig. 2A, B). The anxiolytic effect indicated by increased exploration of the top half of the tank was not observed when non-drug treated fish were subjected to the second trial, fortifying the notion that reductions in stress response are due to the effect of buspirone treatment (Fig. 2). Thus, these experiments confirm that the reduced bottom-dwelling behavior observed in Pachón cavefish is indicative of a diminished stress response.
Fig. 2. Pre-treatment with an anxiolytic drug, buspirone, reduces bottom dwelling behavior in surface and cavefish alike.
(A) Diagram of experimental workflow. (B) Representative trajectories of a surface and Pachón adult in a novel tank before and after drug treatment. (C) Quantifications of total duration spent exploring the top half of the novel tank in 10-min trials pre- and post-treatment. Striped bars - Surface(−) vs. Surface(+): P = 0.019; Pachón(−) vs. Pachón(+): P = 0.0074. (D) Quantification of total duration that individuals were immobile. Striped bars - Pachón(−) vs. Pachón(+): P = 0.010. Surface(−) vs. Pachón(−): P = 0.0069. (Surface without buspirone treatment: n = 5; Surface with buspirone treatment: n = 4; Pachón without buspirone treatment: n = 5; Pachón with buspirone treatment: n = 5) Darker-colored bars denote baseline measurements, while lighter-colored bars denote second trials. (−) indicates system water, (+) indicates 12.5 mg/L buspirone dissolved in system water. Striped bars indicate sets of groups that undergo buspirone treatment, whereas solid bars indicate groups that were treated with system water only. Data analyzed by paired t-test: * indicates P < 0.05; ns indicates not significant. Error bars ± s.e.m.
3.4. Differences in stress behavior in the novel tank is independent of vision
Reduced eye size and blindness in A. mexicanus cavefish have been shown to affect other behavioral traits such as shoaling and aggression (Burchards et al., 1985; Elipot et al., 2013; Espinasa et al., 2005; Gregson and De Perera, 2007; Kowalko et al., 2013b). We next asked whether the differences in stress responses observed in cave and surface forms could be attributed to differences in vision. To address this question, we performed the novel tank test on groups of adults in both lighted conditions as described before, as well as in constant darkness. We randomized the conditions such that some adults were recorded in light first and then re-recorded in darkness, and others were recorded in darkness first and then re-tested in an illuminated room. As was the case for studies done in light, surface fish adults recorded in constant darkness explored less of the tank, spent less time in the top half, and had longer periods of immobility relative to Pachón cavefish (Fig. 3A-C). Additionally, surface fish covered less distance and exhibited reduced velocity (Supplemental Fig. 3A-B). Importantly, we found no significant differences between trials done in light and trials done in constant darkness (Fig. 3A-C; Supplemental Fig. 3A-B). Together, these data indicate that the differences in stress behavior observed between the surface fish and Pachón cavefish were not influenced by differences in vision.
Fig. 3. Differences in stress responses between surface and cavefish are not due to differences in vision.
(A) Representative swim paths of a surface and Pachón individual during a 10-min trial in the novel tank assay done in lighted conditions (white background) or in the dark (shaded background). (B) Quantification of the total duration spent in the top half of the tank during the 10-min recording period, in lighted conditions (L) and in the dark (D). Surface (L) vs. Pachón (L): P = 0.0018. Surface (D) vs. Pachón (D): P = 0.00030. (C) Quantification of total duration spent immobile in L and in D. Surface (D) vs. Pachón (D): P = 0.0028. Data analyzed by Kruskal-Wallis test, followed by Dunn’s posthoc: * * indicates P < 0.005; ns indicates not significant. (Surface: n = 8; Pachón: n = 7) Error bars ± s.e.m.
3.5. Convergence of diminished stress in independently evolved cave populations
The multiple, independently evolved cave populations gave us the opportunity to explore whether dampened stress was specific to adults from the Pachón cave, or whether these changes had evolved in other cave populations. To address this, we performed the novel tank test on Molino and Tinaja cavefish populations (Fig. 4A). These populations were chosen because they represent models of convergent evolution and are genetically distinct from each other (Borowsky, 2008c). Similar to the Pachón population, Molino and Tinaja cave forms spent significantly more time in the top half of the tank as compared to the surface form (Fig. 4A-C). Both Molino and Tinaja cavefish were also significantly more active, covering larger distances and exhibiting higher velocity than the surface adults in the 10-min trials (Supplemental Fig. 4A, B). The results demonstrate convergence on diminished stress responses among multiple populations of Mexican cavefish, and raise the possibility that reduced stress response may be a common trait to cave life.
Fig. 4. Convergent evolution of reduced innate stress responses in the Molino and Tinaja cave populations.
(A) Representative swim paths for surface, Molino, and Tinaja adults during the novel tank diving test. (B) Quantification of the total duration spent in the top half of the tank during the 10-min observation period. Surface vs. Molino: P = 0.00090. Surface vs. Tinaja: P = 0.023. (C) Quantification of total duration spent immobile. Surface vs. Molino: P = 0.011. Surface vs. Tinaja: P = 0.0035. (Surface: n = 6; Molino: n = 7; Tinaja: n = 8) Data analyzed by Kruskal-Wallis test followed by Dunn’s multiple comparison test: * indicates P < 0.05; ** indicates P < 0.005; ns indicates not significant. Error bars ± s.e.m.
4. Discussion
A. mexicanus cavefish populations have evolved numerous morphological and physiological traits. In addition to traits such as loss of eyes and pigmentation, behaviors have also been shown to have evolved. Previous work, for example, has demonstrated evolved differences in sleep, circadian rhythms, shoaling, aggression, and feeding (Duboué et al., 2011; Kowalko et al., 2013b; Yoshizawa et al., 2010). Here, we find that multiple cavefish populations exhibit reduced behavioral stress responses in the novel tank assay, adding to the number of evolved behavioral traits that have been identified in cavefish.
4.1. Validation of reduced stress in cave-dwelling A. mexicanus
Various stimuli such as predators, osmotic stress, and thermal alterations induce behavioral measures of stress, and lead to reduced exploration, prolonged immobility or freezing, and reduced social interaction (Facchin et al., 2015; Levin et al., 2007; Mateo, 2007; Ryu and De Marco, 2017; vom Berg-Maurer et al., 2016; Yeh et al., 2013). In fish, several behavioral assays exist for measuring stress in adults. Two popular assays are the open field test and light/dark preference test (Champagne et al., 2010; Maximino et al., 2010; Schnörr et al., 2012; Shams et al., 2015). A tendency to follow the walls (thigmotaxis) of an open arena and a preference for dark compartments (scototaxis) are typically taken as measures of stress. Thigmotaxis has been investigated in adult A. mexicanus, and, although stress was not the primary focus of these studies, it was demonstrated that cavefish exhibit strong thigmotaxis (Patton et al., 2010; Sharma et al., 2009). Thigmotaxis is influenced by a number of factors, including sensitivity of the lateral line (Abdel-Latif et al., 1990; Patton et al., 2010), and dramatic differences in the lateral line exist between surface and cave A. mexicanus (Montgomery et al., 2001; Teyke, 1990), making assessing stress behaviors challenging using this assay. In the scototaxis test, surface fish have a strong preference for the dim compartment, whereas cavefish have no significant preference (Kowalko et al., 2013b). Similarly, the results are difficult to assess with respect to stress since cavefish are blind, and presumably, adults cannot sense changes in illumination as surface forms can.
We therefore turned to an assay that would be less dependent on lateral line function and visual input, the novel tank test, and demonstrate that compared to cavefish, surface fish have a stronger preference for the bottom half. These results are consistent with those suggesting higher levels of a stress hormone, cortisol, in surface fish following one stressor, acute confinement in a restricted space (Gallo and Jeffery, 2012), as well as those showing that surface fish have an innate preference for the bottom half of a tank compared to cavefish when recorded over a 3–5 day period (Carlson and Gross, 2017; Jaggard et al., 2018). Our data now demonstrate that the strong innate preference for the bottom half of a tank in surface fish is likely caused by higher levels of stress, as treatment with the anxiolytic 5-HT1A agonist (Hamik et al., 1990), buspirone abolishes these differences, and causes both forms to prefer the top portion of the test tank. In support of the notion that this assay is independent of visual input, we found that surface forms had a greater degree of bottom dwelling and more time spent immobile in both lighted and darkened conditions.
4.2. Functional significance of reduced stress in cavefish
Caves throughout the world have similarities with one another, including perpetual darkness, cooler and more consistent water temperatures, and a lack or reduction in the number of primary producers (Poulson and White, 1969). As such, many cave animals have converged on a suite of behavioral modifications such as loss of sleep and altered locomotor rhythms (Duboué and Borowsky, 2012; Duboué et al., 2011; Hervant et al., 2000; Jegla and Poulson, 1968), altered feeding (Dorigo et al., 2017; Kowalko et al., 2013a; Mammola and Isaia, 2017), loss of social behavior (Almeida-Silva et al., 2009; Kowalko et al., 2013b; Yap et al., 2011), and reduced aggression (Elipot et al., 2013; Manenti et al., 2015; Stritih and Kosi, 2017). The functional significance of reduced stress in cavefish is not clear, yet it may be related to the lack of predators in the cave environment.
While there exists great variability in stress responses both within and between species, the ecological factors that contribute to these differences have been explored only modestly. In the mosquitofish, Gambusia hubbsi, populations from high-predator troughs have higher behavioral measures of stress relative to same-species controls obtained from environments with few predators (Heinen-Kay et al., 2016). Similarly, fecal sampling of stress hormones in Belding’s ground squirrels demonstrates that individuals in the wild with myriad predators have higher levels of circulating cortisol compared to same-species controls that live in ecological environments with few predators (Mateo, 2007). These studies suggest that there is a positive correlation between predator threat and stress response. Cavefish also seem to follow this trend, where the populations with high predatory pressure have increased stress responses while those with fewer predators correspond to lower responses. It is likely that these traits became fixed in the cave populations over time, and continue to be retained in laboratory-bred animals. These data suggest that low predation can direct evolution causing animals to have lower stress responses.
4.3. Altered stress and neuroanatomical differences between cave and surface fish
Robust differences in neuroanatomy have been described in cave and surface fish, and these differences may point to the brain regions underlying differences in stress responses. One difference between the two forms is that the embryonic blind cavefish have a midline expansion of sonic hedgehog (shh), a morphogen that functions to establish, among other things, the dorsal-ventral axis of animals. Expanded shh signaling in cavefish is hypothesized to contribute to loss of eyes, and also secondarily is thought to cause an enlargement of the hypothalamus and the subpallium of the ventral telencephalon (Menuet et al., 2007).
Stress responses in mammals are driven by diverse brain regions (Tovote et al., 2015), but two of the most important in fish are pallial and subpallial areas of the telencephalon (analogous to the amygdala and extended amygdala in mammals) and the hypothalamus (Mueller et al., 2011; Portavella et al., 2004a, 2004b; Steenbergen et al., 2012; vom Berg-Maurer et al., 2016). In mammals, the central nucleus of the amygdala has both stress-inducing and stress-suppressing neuronal populations (Ciocchi et al., 2010; Li et al., 2013; Tye et al., 2011; Wilensky et al., 2006). The hypothalamus is a central regulator of stress responses, largely through the activation of neurons expressing corti-cotropin-releasing hormone (Cachat et al., 2010; Steenbergen et al., 2012; Yeh et al., 2013). It could therefore be that neuronal changes in either of these areas cause a global shift in the neurobiology underlying stress states, leading to the altered responses we observe. Future experiments using functional imaging should help to resolve which neuronal systems are responsible for the altered stress responses of cavefishes.
4.4. Cavefish as a model for studying evolutionary factors underlying stress response
Stress throughout development or at early life stages can have drastic impacts on stress states in adult forms. For example, in mammals, maternal deprivation or neglect in early life can lead to aberrant behaviors in adults such as increased aggression and increased anxiety. Moreover, the effect of environmental stressors on stress responses follows a characteristic inverted U-shaped curve, where both too much and too little stress in the environment can have debilitating effects in later life (Russo et al., 2012; Ryu and De Marco, 2017). The neuronal systems that lead to this environmental relationship are not well understood, but they are thought to involve synaptic plasticity of the forebrain and epigenetic modifications of the crh gene (Elliott et al., 2010; Murgatroyd et al., 2009). In cavefish, adaptation to cave life would have been influenced by a near absence of natural stressors such as predators and fluctuations in water temperature and pH (Mitchell et al., 1977). We hypothesize that these reduced stress pressures put the cave at the left side of the “U”, and we suspect that without evolutionary modifications, cavefish would have debilitating stress states in adult forms.
The Mexican cavefish has emerged as a powerful model to study how neural circuits regulating diverse behaviors including stress responses are altered through evolution. Whether modifications in stress responses serve a functional purpose, and whether they are generalizable to cave life, is unclear. Further studies into stress responses of this valuable model may shed insight into the neuronal and genetic mechanisms that underlie the observed differences.
Supplementary Material
Acknowledgements
JSRC, ACK and ERD conceived the experiments and wrote the paper with input from all authors. JSRC, CEG, PMA, EL, BAS and JBJ performed the experiments. JSRC and CEG analyzed the data. This work was funded by a startup fund to ERD and by a National Science Foundation Award IOS-125762 to ACK. JSRC and PMA are supported by the Jupiter Life Science Initiative.
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2018.05.009.
References
- Abdel-Latif H, Hassan ES, von Campenhausen C, 1990. Sensory performance of blind Mexican cave fish after destruction of the canal neuromasts. Naturwissenschaften 77, 237–239. 10.1007/BF01138492. [DOI] [PubMed] [Google Scholar]
- Adamo S, Kovalko I, Mosher B, 2013. The behavioral effect of predator-induced stress responses in the cricket (Gryllus texensis): the upside of the stress response. J. Exp. Biol 216, 4608. [DOI] [PubMed] [Google Scholar]
- Almeida-Silva LM, Brescovit AD, Dias SC, 2009. A new species of Goeldia (Araneae: Titanoecidae) with notes on its natural history. Zool. (Curitiba, Impresso) 26, 363–368. 10.1590/S1984-46702009000200021. [DOI] [Google Scholar]
- Archard GA, Earley RL, Hanninen AF, Braithwaite VA, 2012. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol 26, 637–645. 10.1111/j.13652435.2012.01968.x. [DOI] [Google Scholar]
- Bateson M, Brilot B, Nettle D, 2011. Anxiety: an evolutionary approach. Can. J. Psychiatry 10.1177/070674371105601202. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED, 2009. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. (https://doi.org/)Pharmacol. Biochem. Behav. 94, 75–80. 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R, 2008a. Astyanax mexicanus, the blind Mexican cave fish: a model for studies in development and Morphology. Cold Spring Harb. Protoc 3 10.1101/pdb.emo107. [DOI] [PubMed] [Google Scholar]
- Borowsky R, 2008b. Handling Astyanax mexicanus eggs and fry. Cold Spring Harb. Protoc 3 10.1101/pdb.prot5093. [DOI] [PubMed] [Google Scholar]
- Borowsky R, 2008c. Restoring sight in blind cavefish. Curr. Biol. 10.1016/j.cub.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Bradic M, Teotónio H, Borowsky RL, 2013. The population genomics of repeated evolution in the blind cavefish astyanax mexicanus. Mol. Biol. Evol 30, 2383–2400. 10.1093/molbev/mst136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Gardner C, Braithwaite VA, 2005. Differential stress responses in fish from areas of high- and low-predation pressure. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol 175, 305–312. 10.1007/s00360-005-0486-0. [DOI] [PubMed] [Google Scholar]
- Burchards H, Dölle A, Parzefall J, 1985. Aggressive behaviour of an epigean population of Astyanax mexicanus (Characidae, Pisces) and some observations of three subterranean populations. Behav. Process 11, 225–235. 10.1016/0376-6357(85)90017-8. [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, DiLeo J, Frank K, Chang K, Utterback E, Hart P, Kalueff AV, 2010. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc 5, 1786–1799. 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Campos AC, Fogaça MV, Aguiar DC, Guimarães FS, 2013. Animal models of anxiety disorders and stress. Rev. Bras. Psiquiatr 35 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Gross JB, 2017. Characterization and comparison of activity profiles exhibited by the cave and surface morphotypes of the blind Mexican tetra, Astyanax mexicanus. Comp. Biochem. Physiol. Part - C. Toxicol. Pharmacol. 10.1016/j.cbpc.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK, 2010. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav. Brain Res 214, 332–342. 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A, 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cockrem JF, 2013. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 10.1016/j.ygcen.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Nugent NR, 2010. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr. Psychiatr. Rep 12, 313–326. 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo L, Squartini A, Toniello V, Dreon AL, Pamio A, Concina G, Simonutti V, Ruzzier E, Perreau M, Engel AS, Gavinelli F, Martinez-Sañudo I, Mazzon L, Paoletti MG, 2017. Cave hygropetric beetles and their feeding behavior: a comparative study of Cansiliella servadeii and Hadesia asamo (Coleoptera, Leiodidae, Cholevinae, Leptodirini). Acta Carsol. 46, 317–328. [Google Scholar]
- Duboué ER, Borowsky RL, 2012. Altered rest-activity patterns evolve via circadian independent mechanisms in cave adapted balitorid loaches. PLoS One 7 10.1371/journal.pone.0030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboué ER, Keene AC, Borowsky RL, 2011. Evolutionary convergence on sleep loss in cavefish populations. Curr. Biol 21, 671–676. 10.1016/j.cub.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Duboué ER, Borowsky RL, Keene AC, 2011. β-adrenergic signaling regulates evolutionarily derived sleep loss in the mexican cavefish. Brain. Behav. Evol 80, 233–243. 10.1159/000341403. [DOI] [PubMed] [Google Scholar]
- Elipot Y, Hinaux H, Callebert J, Rétaux S, 2013. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Curr. Biol 23, 1–10. 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A, 2010. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci 13, 1351–1353. 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Espinasa L, Yamamoto Y, Jeffery WR, 2005. Non-optical releasers for aggressive behavior in blind and blinded Astyanax (Teleostei, Characidae). Behav. Process 70, 144–148. 10.1016/j.beproc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchin L, Duboué ER, Halpern ME, 2015. Disruption of epithalamic left-right asymmetry increases anxiety in Zebrafish. J. Neurosci 35 10.1523/JNEUROSCI.2593-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo ND, Jeffery WR, 2012. Evolution of space dependent growth in the teleost Astyanax mexicanus. PLoS One 7 10.1371/journal.pone.0041443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammans RE, Westrick ML, Shea JP, Mayol RF, LaBudde JA, 1989. Pharmacokinetics of buspirone in elderly subjects. J. Clin. Pharmacol 29, 72–78. 10.1002/j.1552-4604.1989.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Gregson J, De Perera TB, 2007. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. J. Fish. Biol 70, 1615–1619. 10.1111/j.1095-8649.2007.01430.x. [DOI] [Google Scholar]
- Hamik A, Oksenberg D, Fischette C, Peroutka SJ, 1990. Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol. Psychiatry 28, 99–109. 10.1016/0006-3223(90)90627-E. [DOI] [PubMed] [Google Scholar]
- Heinen-Kay JL, Schmidt DA, Stafford AT, Costa MT, Peterson MN, Kern EMA, Langerhans RB, 2016. Predicting multifarious behavioural divergence in the wild. Anim. Behav 121, 3–10. 10.1016/j.anbehav.2016.08.016. [DOI] [Google Scholar]
- Hervant F, Mathieu J, Durand JP, 2000. Metabolism and circadian rhythms of the European blind cave salamander Proteus anguinus and a facultative cave dweller, the Pyrenean newt (Euproctus asper). Can. J. Zool 78, 1427–1432. 10.1139/cjz-78-8-1427. [DOI] [Google Scholar]
- Howarth FG, 1993. High-stress subterranean habitats and evolutionary change in cave-inhabiting arthropods. Am. Nat 142, S65–S77. 10.1086/285523. [DOI] [PubMed] [Google Scholar]
- Jacobson AF, Dominguez RA, Goldstein BJ, Steinbook RM, 1985. Comparison of Buspirone and Diazepam in Generalized Anxiety DisorderPharmacother. J. Hum. Pharmacol. Drug Ther 5, 290–296. 10.1002/j.18759114.1985.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Jaggard J, Robinson BG, Stahl BA, Oh I, Masek P, Yoshizawa M, Keene AC, 2017. The lateral line confers evolutionarily derived sleep loss in the Mexican cavefish. J. Exp. Biol 220, 284–293. 10.1242/jeb.145128. [DOI] [PubMed] [Google Scholar]
- Jaggard JB, Stahl BA, Lloyd E, Prober DA, Duboue ER, Keene AC, 2018. Hypocretin underlies the evolution of sleep loss in the Mexican cavefish. Elife 7, e32637 10.1101/122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, 2008. Emerging model systems in evo-devo: cavefish and microevolution of development. Evol. Dev. 10.1111/j.1525-142X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegla TC, Poulson TL, 1968. Evidence of circadian rhythms in a cave crayfish. J. Exp. Zool 168, 273–282. 10.1002/jez.1401680213. [DOI] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter AB, 2008. Gene-environment interaction in posttraumatic stress disorder: review, strategy and new directions for future research. Eur. Arch. Psychiatry Clin. Neurosci. 10.1007/s00406007-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalko J, Rohner N, Linden T, Rompani S, Warren W, Borowsky R, Tabin C, Jeffery W, Yoshizawa M, 2013a. Convergence in feeding posture occurs through different genetic loci in independently evolved cave populations of Astyanax mexicanus. Proc. Natl. Acad. Sci 110, 16933–16938. 10.1073/pnas.1317192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalko J, Rohner N, Rompani S, Peterson B, Linden T, Yoshizawa M, Kay E, Weber J, Hoekstra H, Jeffery W, Borowsky R, Tabin C, 2013b. Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr. Biol. 23, 1874–1883. 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey JI, Lacey BC, 1958. Verification and extension of the principle of autonomic response-stereotypy. Am. J. Psychol. 71, 50–73. 10.2307/1419197. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT, 2007. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav 90, 54–58. 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B, 2013. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339. 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammola S, Isaia M, 2017. Spiders in caves. Proc. R. Soc. Lond. B 284, 20170193 10.1098/rspb.2017.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R, Pennati R, Ficetola GF, 2015. Role of density and resource competition in determining aggressive behaviour in salamanders. J. Zool 296, 270–277. 10.1111/jzo.12241. [DOI] [Google Scholar]
- Mateo JM, 2007. Ecological and hormonal correlates of antipredator behavior in adult Belding’s ground squirrels (Spermophilus beldingi). Behav. Ecol. Sociobiol 62, 37–49. 10.1007/s00265-007-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga W, Watanabe E, 2010. Habituation of medaka (Oryzias latipes) demonstrated by open-field testing. Behav. Process 85, 142–150. 10.1016/j.beproc.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Maximino C, Marques de Brito T, Dias CAG, Gouveia A, Morato S, 2010. Scototaxis as anxiety-like behavior in fish. Nat. Protoc 5, 209–216. 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- McGaugh SE, Gross JB, Aken B, Blin M, Borowsky R, Chalopin D, Hinaux H, Jeffery WR, Keene A, Ma L, Minx P, Murphy D, O’Quin KE, Retaux S, Rohner N, Searle SMJ, Stahl BA, Tabin C, Volff JN, Yoshizawa M, Warren WC, 2014. The cavefish genome reveals candidate genes for eye loss. Nat. Commun. 5, 5307 10.1038/ncomms6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Alunni A, Joly J-S, Jeffery WR, Rétaux S, 2007. Expanded expression of Sonic Hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development 134, 845–855. 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Russell WH, Elliott WR, 1977. Mexican Eyeless Characin Fishes, Genus Astyanax: Environment, Distribution, and Evolution.
- Montgomery JC, Coombs S, Baker CF, 2001. The mechanosensory lateral line system of the hypogean form of Astyanax fasciatus. Environ. Biol. Fishes. 10.1023/A:1011873111454. [DOI] [Google Scholar]
- Mueller T, Dong Z, Berberoglu MA, Guo S, 2011. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res. 1381, 95–105. 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OFX, Spengler D, 2009. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci 12, 1559–1566. 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Bhatnagar S, Ellis B, 2016. Evolutionary Origins and Functions of the Stress Response System. In: Stress: Concepts, Cognition, Emotion, and Behavior: Handbook of Stress pp. 95–101. 10.1016/B978-0-12-800951-2.00011-X. [DOI] [Google Scholar]
- Noldus LPJJ, Spink AJ, Tegelenbosch RAJ, 2001. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods, Instrum., Comput 33, 398–414. 10.3758/BF03195394. [DOI] [PubMed] [Google Scholar]
- Ornelas-García CP, Domínguez-Domínguez O, Doadrio I, 2008. Evolutionary history of the fish genus astyanax baird & Girard (1854) (Actinopterygii, Characidae) in mesoamerica reveals multiple morphological homoplasies. BMC Evol. Biol 8 10.1186/1471-2148-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton P, Windsor S, Coombs S, 2010. Active wall following by Mexican blind cavefish (Astyanax mexicanus). J. Comp. Physiol. A Neuroethol. Sens., Neural, Behav. Physiol 196, 853–867. 10.1007/s00359-010-0567-8. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C, 2004a. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci 24, 2335–2342. 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C, Papini MR, 2004b. Lesions of the medial pallium, but not of the lateral pallium, disrupt spaced-trial avoidance learning in goldfish (Carassius auratus). Neurosci. Lett 362, 75–78. 10.1016/j.neulet.2004.01.083. [DOI] [PubMed] [Google Scholar]
- Poulson TL, White WB, 1969. The cave environment. Science (80-.) 165, 971–981. 10.1126/science.165.3897.971. [DOI] [PubMed] [Google Scholar]
- Rambo CL, Mocelin R, Marcon M, Villanova D, Koakoski G, de Abreu MS, Oliveira TA, Barcellos LJG, Piato AL, Bonan CD, 2017. Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav 171, 50–54. 10.1016/j.physbeh.2016.12.032. [DOI] [PubMed] [Google Scholar]
- Reddell J, Elliott WR, 1973. A checklist of the cave fauna of Mexico. V. Additonal records from the Sierra de Guatemala, Tamaulipas. Assoc. Mex. Cave Stud., Bull 5, 181–190. [Google Scholar]
- Russo SJ, Murrough JW, Han M, Charney DS, Nestler EJ, 2012. Neurobiology of resilience. Nat. Neurosci 15, 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, De Marco RJ, 2017. Performance on innate behaviour during early development as a function of stress level. Sci. Rep 7, 7840 10.1038/s41598-017-08400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD, 2005. Stress and Health: psychological, Behavioral, and Biological Determinants. Annu Rev. Clin. Psychol 1, 607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnörr SJ, Steenbergen PJ, Richardson MK, Champagne DL, 2012. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res 228, 367–374. 10.1016/j.bbr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Shams S, Chatterjee D, Gerlai R, 2015. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav. Brain Res 292, 283–287. 10.1016/j.bbr.2015.05.061. [DOI] [PubMed] [Google Scholar]
- Sharma S, Coombs S, Patton P, De Perera TB, 2009. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax). J. Comp. Physiol. A Neuroethol. Sens., Neural, Behav. Physiol 195, 225–240. 10.1007/s00359-008-0400-9. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB, 2011. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialog-. Clin. Neurosci 13, 263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen PJ, Metz JR, Flik G, Richardson MK, Champagne DL, 2012. Methods to quantify basal and stress-induced cortisol response in larval zebrafish., Zebrafish protocols for neurobehavioral research. Neuromethods 66... [Google Scholar]
- Stritih N, Kosi AŽ, 2017. Olfactory signaling of aggressive intent in male-male contests of cave crickets (Troglophilus neglectus; Orthoptera: rhaphidophoridae). PLoS One 12 10.1371/journal.pone.0187512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T, 1990. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain. Behav. Evol 35, 23–30. 10.1159/000115853. [DOI] [PubMed] [Google Scholar]
- Thompson RRJ, Paul ES, Radford AN, Purser J, Mendl M, 2016. Routine handling methods affect behaviour of three-spined sticklebacks in a novel test of anxiety. Behav. Brain Res 306, 26–35. 10.1016/j.bbr.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A, 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331. 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-YY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K, 2011. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362. 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Berg-Maurer CM, Trivedi CA, Bollmann JH, De Marco RJ, Ryu S, 2016. The severity of acute stress Is represented by increased synchronous activity and recruitment of hypothalamic CRH neurons. J. Neurosci 36, 3350–3362. 10.1523/JNEUROSCI.3390-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE, 2006. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of pavlovian fear conditioning. J. Neurosci 26, 12387–12396. 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan J, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu N, Cachat J, Kalueff AV, 2010. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res 208, 450–457. 10.1016/j.bbr.2009.12.023 [DOI] [PubMed] [Google Scholar]
- Yap L-MYL, Norma-Rashid Y, Liu F, Liu J, Li D, 2011. Comparative biology of cave-dwelling spitting spiders (Araneae: Scytodidae): Parental care, cooperative prey-capture, cannibalism, natal dispersal and reproductive behaviour. Raffles Bull. Zool. 59, 269–284. [Google Scholar]
- Yeh CM, Glöck M, Ryu S, 2013. An optimized whole-body cortisol quantification method for assessing stress levels in larval zebrafish. PLoS One 8 10.1371/journal.pone.0079406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Gorički Š, Soares D, Jeffery WR, 2010. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol 20, 1631–1636. 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.