Abstract
Background:
This study was designed to compare the prevention of emergence agitation (EA) of sevoflurane anesthesia by an intraoperative bolus or low-dose infusion of dexmedetomidine in pediatric patients undergoing lower abdominal surgeries.
Materials and Methods:
Forty-eight patients, aged 2–12 years, undergoing lower abdominal surgeries with sevoflurane anesthesia were enrolled in this study. Patients were randomly assigned to receive either intravenous bolus over 10 min. 0.4 μg/kg dexmedetomidine (Group I, n = 24) or low-dose infusion 0.4 μg/kg/h of dexmedetomidine (Group II, n = 24) after intubation. Heart rate and mean arterial pressure were recorded before induction, at induction and every 5 min after induction. Observational pain scores (OPS), pediatric anesthesia emergence delirium (PAED) scores, and Ramsay sedation scores (RSS) were recorded on arrival to the postanesthesia care unit and at 5, 10, 15, 30, 45, 60 min thereafter. Extubation time, emergence time, and time to reach Aldrete score ≥9 were recorded.
Results:
OPS and PAED scores and percentage of patients with OPS ≥4 or PAED scale ≥10 were significantly higher in Group II as compared to Group I. RSS score, extubation time, emergence time, and time to reach Aldrete score ≥9 did not show any significant difference.
Conclusion:
Both bolus or low-dose infusion of dexmedetomidine was effective for the prevention of EA with sevoflurane anesthesia, but bolus dose of dexmedetomidine was more effective.
Keywords: Dexmedetomidine, emergence agitation, emergence delirium, pediatric patients, sevoflurane
INTRODUCTION
Emergence agitation (EA) has also been referred to as emergence delirium or emergence excitement, and it may be associated with physical injury as well as negative postoperative behaviors in children. EA is often witnessed with sevoflurane anesthesia with an incidence of approximately 10%–80%[1,2] and the patient exhibits nonpurposeful restlessness and agitation, thrashing, crying or moaning, disorientation, and incoherence.[3,4] When present, EA occurs within the first 30–45 min of recovery from anesthesia, is usually self-limiting but can last up to 2 days. It has the risks of self-injury, requires extra nursing care, family dissatisfaction, and increased cost.[5] EA is not only a major source of dissatisfaction for parents and caregivers postoperatively, but it also may lead to some complications such as increased bleeding from the operative site and pulling out an intravenous (IV) catheter. It may require pharmacological intervention which itself may result in a prolonged postanesthesia care unit (PACU) stay. While emergence delirium remains a poorly understood phenomenon, a variety of potential etiologies including pain, stressful induction, hypoxemia, rapid awakening in hostile environment, and physical stimulation (noise) have been implicated.[5,6]
Sevoflurane is the most popular inhaled anesthetic agent in pediatric anesthesia because it is characterized with rapid onset and offset of anesthesia and causes less irritation to the airway.[7] It is a smooth induction drug, which allows a safe and pleasant mask induction and causes less impairment of cardiovascular function when compared with other potent inhaled anesthetics.
Several drugs have been used to treat postsevoflurane agitation which include propofol,[8] midazolam,[9,10] ketamine,[11] and α-2 agonists such as clonidine[12] and dexmedetomidine.[13,14,15] However, these medications may increase sedation after anesthesia, cause slow awakening, and in some cases are associated with undesirable side effects, such as nausea and vomiting.[16]
Dexmedetomidine, a highly selective α-2 receptor agonist and is commonly used in adult anesthesia and intensive care.[6,17,18] Dexmedetomidine is also beneficial in the perioperative period of pediatric patients. It is anxiolytic, sedative, and analgesic properties are beneficial for EA control.[5,19] It is a recent drug used to prevent postsevoflurane agitation. However, due to the variation in the dose used in various studies, till date, there has been no consensus on the dose of dexmedetomidine used for the prevention of EA.[13,14,15] Doses of 0.5 μg/kg and above have been effectively used to reduce postsevoflurane agitation but were associated with increased incidence of side effects such as reduction in heart rate (HR), blood pressure, delayed emergence, and extubation.[14]
This study was designed to evaluate the efficacy of dexmedetomidine bolus or infusion for prevention of EA with sevoflurane anesthesia in pediatric patients undergoing lower abdominal surgery.
MATERIALS AND METHODS
This prospective, randomized, double-blind, comparative study conducted over a period of 1 year in the Department of Anaesthesiology and Paediatric Surgery, King George's Medical University, Lucknow, Uttar Pradesh, India. After obtaining approval from the Ethics Committee of the university, a written informed consent from the parents/guardians was obtained. Patients of ASA Physical Status I–II, aged 2–12 years of either sex with ± 20% of ideal body weight, were enrolled in this prospective, randomized, double-blind study. Patients with a history of developmental delay, congenital airway problems, cardiac disorders, psychological disorders, epilepsy, and allergy to study medications were excluded from the study.
Children fasted according to established guidelines and received 0.5 mg/kg oral midazolam approximately 30 min before separation from the parents. An electrocardiogram, pulse oximeter, and noninvasive arterial blood pressure monitor were attached. General anesthesia was induced with 8% inspired sevoflurane in 100% oxygen by facemask. After establishing IV access, rocuronium 0.6 mg/kg and fentanyl 1 μg/kg were given. Orotracheal intubation was performed, and anesthesia was maintained with 60% nitrous oxide in oxygen, supplemented by an end-tidal concentration of 2%–3% sevoflurane with controlled ventilation, to maintain an end-tidal CO2 of 4.6 ± 0.5 kPa (35 ± 4 mmHg).
Patients were assigned to one of two groups according to a computer-generated random number table. After intubation patients in Group-I received bolus dexmedetomidine 0.4 μg/kg over 10 min and the patients in Group-II received a continuous infusion of dexmedetomidine 0.4 μg/kg/h throughout the procedure. HR, mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) were recorded before induction (baseline), at induction and every 5 min after induction during the procedure.
For the maintenance of anesthesia, patients received 2% sevoflurane in 60% N2O and 40% oxygen with controlled ventilation to maintain normocapnia. In both groups, 25% increase in HR and MAP with respect to the baseline value before anesthesia induction and sustained for 5 min was considered as tachycardia and hypertension was treated with 0.5 mcg/kg fentanyl. Likewise, 25% decrease in HR and MAP is defined as bradycardia and hypotension, respectively. Atropine 0.01 mg/kg intravenously for bradycardia and 10 ml/kg Ringer's lactate solution for hypotension was administered to the patients. Intraoperative dexamethasone 0.5 mg/kg (maximum 10 mg), ondansetron 0.1 mg/kg, and antibiotics were administered to all patients according to institutional practice. Ringer's lactate solution was administered for fluid management to all the patients.
After completion of surgery, when hemostasis was achieved, anesthetic gases were discontinued, and neuromuscular blockade was reversed with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg IV. The trachea was extubated once appropriate extubation criteria were met, including eye-opening, purposeful movement, and response to command and thereafter patients were transferred to PACU. The duration between the termination of anesthetic gases and the extubation was defined as “extubation time.”
In PACU, the intensity of pain was assessed by using a modified Hannallah pain score[19] [Table 1] – an observational pain score (OPS). Moreover, pediatric anesthesia emergence delirium (PAED) scale[20] [Table 2], Ramsay sedation score (RSS) (0 = paralyzed, unable to evaluate, 1 = awake, 2 = lightly sedated, 3 = moderately sedated, follows simple commands, 4 = deeply sedated, responds to nonpainful stimuli, 5 = deeply sedated, responds only to painful stimuli, and 6 = deeply sedated, unresponsive to painful stimuli),[21] HR, MAP, and SpO2 were measured and recorded on arrival to PACU and 5, 10, 15, 30, 45, 60 min thereafter. Besides these, any adverse effects such as vomiting, airway obstruction, laryngospasm or bronchospasm, bradycardia, hypotension, and sedation were also recorded. All these measurements and recordings were done by the anesthetist or nurse who was blind to the group of the patient.
Table 1.
Observational pain score - observational pain scores[19]
| Observation | Criteria | Points |
|---|---|---|
| Arterial pressure | +10% preoperative | 0 |
| >20% preoperative | 1 | |
| >30% preoperative | 2 | |
| Crying | No crying | 0 |
| Crying responded to TLC | 1 | |
| Crying not responding to TLC | 2 | |
| Movement | None | 0 |
| Restless | 1 | |
| Thrusting | 2 | |
| Agitation | Asleep/calm | 0 |
| Mild | 1 | |
| Hysterical | 2 | |
| Posture | No special posture | 0 |
| Flexing legs and thighs | 1 | |
| Holding groin | 2 | |
| Complains of pain | Asleep/state no pain | 0 |
| Cannot localize | 1 | |
| Can localize pain | 2 |
TLC=Tender loving care
Table 2.
Pediatric anesthesia emergence delirium scale[20]
| Scale | Not at all | Just a little | Quite a bit | Very much | Extremely |
|---|---|---|---|---|---|
| Makes eye contact with caregiver | 4 | 3 | 2 | 1 | 0 |
| Actions are purposeful | 4 | 3 | 2 | 1 | 0 |
| Aware of surroundings | 4 | 3 | 2 | 1 | 0 |
| Restless | 0 | 1 | 2 | 3 | 4 |
| Inconsolable | 0 | 1 | 2 | 3 | 4 |
The number of patients who presented with Modified Hannallah Pain Score (OPS) ≥4 or PAED scale items 4 “the child is restless” or 5 “the child is inconsolable” with an intensity of 3 (very much) or 4 (extremely) were recorded and 15 mg/kg paracetamol was administered as rescue analgesic to these patients. Patients who had an Aldrete score ≥9 without vomiting were discharged from PACU to surgical ward. The duration between admission to PACU and discharge from PACU to surgical ward was defined as time to reach Aldrete score ≥9 [Table 3].
Table 3.
Aldrete discharge scoring system
| 2 | 1 | 0 | |
|---|---|---|---|
| Activity | Able to move 4 extremities voluntarily or on command | Able to move 2 extremities voluntarily or command | Able to 0 extremities voluntarily command |
| Respiration | Able to take breath and cough | Dyspnea/shallow breathing | Apnea |
| Circulation | BP±20 mmHg preoperative | BP±20-50 mmHg preoperative | BP±50 mmHg preoperative |
| Oxygen saturation | Maintains >92% on room air | Needs oxygen inhalation to maintain oxygen saturation >90% | Saturation <90% even with supplemented oxygen |
| Consciousness | Fully awake | Arousable on calling | Not responding |
BP=Blood pressure
The duration of surgery and anesthesia, extubation time, need for supplemented analgesic, the incidence of vomiting during the 60 min period, and time to reach Aldrete score ≥9 was recorded.
Sample size estimation
The sample size was calculated on the basis of the proportion of incidence of EA in case of using dexmedetomidine by some previous study.[22] A sample of 24 patients in each group was calculated (α = 0.05 and β = 0.8).
Statistical analysis
All the statistical analyses were performed using SPSS 16.0 windows software (Chicago, Inc., USA). The comparison of quantitative variables between the study groups was done using unpaired Student's t-test when the data were normally distributed and Mann–Whitney rank sum (when indicated). For comparing categorical data, Chi-square test was performed. Continuous variables are presented as mean ± standard deviation, and categorical data are presented as numbers and frequencies. All the categorical data were compared by using Chi-square test. P < 0.05 was considered as statistically significant.
RESULTS
Both groups were comparable regarding age, sex, weight, duration of anesthesia and surgery. Hemodynamically, patients in both groups were stable during our observation period [Figures 1 and 2]. Time to extubation and emergence showed no significant difference between the groups [Figure 3]. Pain recorded on OPS scale showed statistically significantly better scores in Group I (P < 0.05) [Figure 4]. Similarly, PAEDS score was significantly lower in Group I as compared to Group II [Figure 5]. Although after 30 min, RSS score was statistically significant among the two groups, it was clinically not relevant (Data not shown). Time to reach Aldrete score ≥9 was comparable among the two groups, but one patient in Group I and three patients in Group II did not achieve Aldrete score ≥9 till 60 min postoperatively.
Figure 1.
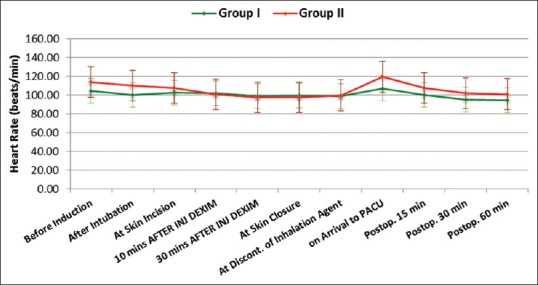
Intergroup comparison of heart rate
Figure 2.
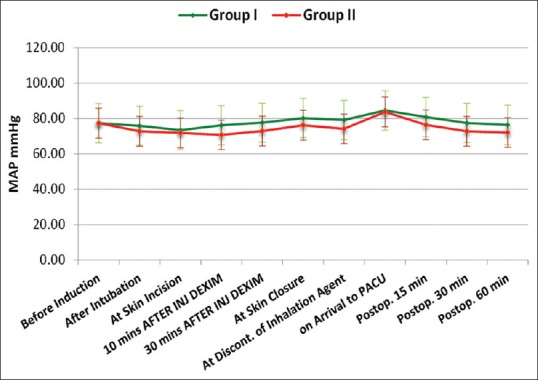
Intergroup comparison of mean arterial pressure
Figure 3.
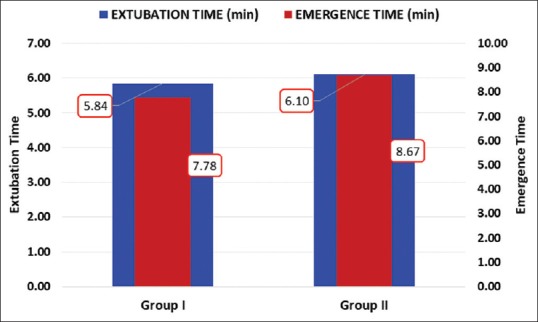
Intergroup comparison of extubation and emergence time
Figure 4.
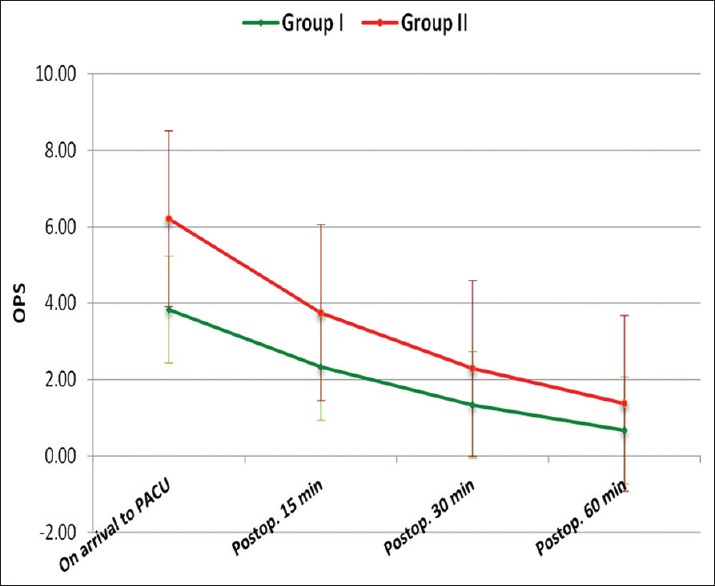
Intergroup comparison of observational pain score between the groups
Figure 5.
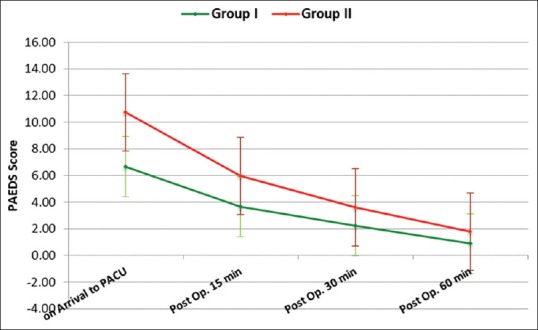
Intergroup comparison of PAEDS score between the groups
DISCUSSION
The present study was performed to evaluate and compare whether dexmedetomidine bolus or continuous infusion was able to prevent EA in children aged 2–12 years after sevoflurane anesthesia undergoing lower abdominal surgery. The present findings indicated that dexmedetomidine bolus 0.4 μg/kg was more effective for the prevention of EA as compared to continuous infusion of dexmedetomidine 0.4 μg/kg/h. However, both the regimens did not alter the time to extubation and emergence or the recovery time.
Hemodynamic instability did not occur in any of the patients, and vital signs remained within 20% of baseline in all patients. HR, systolic blood pressure, and diastolic blood pressure were similar in both groups. Deutsch and Tobias[23] reported that an IV administration of 0.5 μg/kg of dexmedetomidine over 5 min caused an approximate 10% and 25% reduction in blood pressure and HR, respectively, in pediatric patients undergoing general anesthesia. In addition, Patel et al.[13] reported that mean HR and mean systolic blood pressure were significantly reduced after administration of 0.7 μg/kg/h dexmedetomidine following a 2 μg/kg loading dose. We were concerned about the hemodynamic effects of dexmedetomidine, thus we have reduced the bolus and infusion dose to almost half of the recommended dose without an initial loading dose. Despite the relatively low dose used in our study, we found that 0.4 μg/kg/h was sufficient to prevent postoperative severe EA in pediatric patients without compromising hemodynamics. Although a higher dose of dexmedetomidine might have further reduced the incidence of EA, it may have also caused delayed emergence.
We used PAED score ≥10 as the indicator of agitation. A score of ≥10 on the PAED scale has been defined as the best discriminator between the presence and absence of clinical agitation as reported by Bong and Ng.[24] The present study showed that the incidence of EA was 20% in Group 1 and 50% in Group 2. The results of our study demonstrated that both bolus and low-dose infusion prevented EA, but bolus dose of dexmedetomidine was better as compared to low-dose infusion. Our findings are in consistance with the study done by Hauber et al.[25] They noted that a rapid (2–3 s) bolus (0.5 μg/kg) injection of dexmedetomidine administered 5 min before the end of the surgery significantly reduced the incidence of EA without causing hemodynamic instability or delayed PACU length of stay at the main hospital. In addition, they also found that patients receiving dexmedetomidine required less postoperative supplemental opioids, and have fewer postoperative adverse events.
Kim et al.[26] study indicated that continuous intraoperative infusion of low-dose dexmedetomidine (0.2 μg/kg/h) can reduce the incidence of EA following desflurane anesthesia in pediatric patients undergoing strabismus surgery. Furthermore, low-dose dexmedetomidine reduced postoperative pain without any hemodynamic compromise or delayed emergence.
In the study of Boku et al.[27] dexmedetomidine was administered in a loading dose of 6 μg/kg/h, followed by an infusion at 0.4 μg/kg/h. HR and MAP after extubation were significantly lower in the dexmedetomidine group than in the saline group, but no serious circulatory depression was observed after the administration of dexmedetomidine. A recent meta-analysis revealed a lower risk for EA following dexmedetomidine in comparison with placebo.[7] However, there were large differences in dexmedetomidine regimen (low dose: 0.15 μg/kg and high dose: 4 μg/kg) between studies. Shukry et al.[22] also reported that dexmedetomidine was used successfully as continuous infusion (0.2 μg/kg/h) for 15 min in the postoperative period to prevent or reduce EA in children. On the other hand, Guler et al.[14] and Ibacache et al.[15] reported that a single dose of dexmedetomidine (0.5 μg/kg) 5 min before the end of surgery and 0.3 μg/kg after induction of anesthesia reduced EA without significant hemodynamic effects, respectively. Thus, the administration of dexmedetomidine at a slow rate may contribute to hemodynamic stability.
EA generally occurs within the first 30 min after anesthesia is over and generally self-limiting, with a mean duration of 5–15 min.[1,28,29] In our study, we also found that EA occurred within the first 30 min. However, agitation and regressive behavior lasting up to 2 days have also been described.[29]
EA is a complex phenomenon, the etiology of which is multifactorial. The wide variability in the incidence of agitation in the different studies on EA may be due to the criteria used to define this phenomenon and the time in the PACU when EA was measured.[29] We did repeated measurements at frequent time intervals, because a single measurement may not reflect the true incidence of EA.[30]
Pain has been the most important confounding variable while assessing a child's behavior upon emergence because of the overlapping clinical picture with EA; a reliable, valid, and simple scoring scale to measure postoperative pain and EA is very important in PACU. Unfortunately, none of the scales has been tested to differentiate EA from pain. Preemptive analgesia suggests that pain maybe its major source.[29,31] However, EA has also been reported in patients undergoing nonpainful procedures.[32] It can be postulated that pain during emergence in some patients may cause EA, but it may not be the sole etiology.
We used OPS score ≥4 as the indicator of pain, in our study, patients in both groups received similar pain relief, however, there was a higher incidence of EA in Group II as compared to Group I. We did find a positive correlation between agitation and pain; Group II had higher pain and EA scores than did Group I. Results on the OPS, and PAED showed a very similar trend in both groups; scores were highest on arrival in the PACU and decreased over the period of time.
In the analysis of Zhu et al.,[33] dexmedetomidine significantly increased emergence time (weighted mean difference [WMD] 1.16; 95% confidence interval [CI] 0.72–1.60) and extubation time (WMD 0.61; 95% CI 0.27–0.95) in children undergoing sevoflurane anesthesia in PACU. This might be due to the excessive sedation associated with dexmedetomidine and contribute to the decreased incidence of EA postanesthesia. However, time to discharge from PACU in the dexmedetomidine group was also significantly increased compared with the placebo group in this study (WMD 2.67; 95% CI 0.95–4.39). In our study, we used RSS score as indicator for sedation, and we found only one patient in each group with RSS score = 3 which was not significant.
We found no significant difference in the average time to reach Aldrete score ≥9 in both groups during our observation period, but one patient in Group I and three patients in Group II did not achieve Aldrete score ≥9 till 60 min postoperatively which could be attributed to poor pain control. Patients were discharged from the PACU once deemed safe, stable, and had met all discharge criteria. We did not follow patients once they were discharged from the PACU.
We did not observe any complication in PACU such as PONV, apneic spells, and desaturation episodes (SpO2 <90%) during our observation period (1 h). As in the previous study compared to placebo, dexmedetomidine decreased significantly the incidence of the occurrence of postoperative nausea and vomiting in children undergoing sevoflurane anesthesia (RR 0.57; 95% CI 0.38–0.85).
Limitation of study
There were few limitations in our study as follows: (1) small sample size, (2) control group is not included in this study, (3) postoperative follow-up time was very short (1 h), (4) we did not use MAC value and bispectral index for the depth of anesthesia, and (5) multiple observers have recorded the findings which could have a subjective bias. It was important that only one blinded observer should have graded all scores employed in the study.
CONCLUSION
In summary, intraoperative bolus or low dose continuous infusion of dexmedetomidine is effective for reducing the incidence of EA without clinically significant hemodynamic deterioration or delay of the recovery time in pediatric patients undergoing lower abdominal surgery with sevoflurane anesthesia. However, the bolus dose of dexmedetomidine is more effective in reducing EA.
It is important to note that we studied relatively healthy (ASA I-II) children (2–12 years) and excluded patients with a history of airway problems because dexmedetomidine required in the study protocol may subject these children to unacceptably greater risks for postoperative airway complications. Further studies focusing on obstructive airway complications due to dexmedetomidine in children with Robin sequence and/or Treacher Collins syndrome are needed. Rapid awakening in a hostile environment can frighten the children and may provoke agitation. To eliminate this, further studies should be conducted to compare the effect of parental presence or absence on EA in children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
We would like to thank the Department of Paediatric Surgery, K.G.M.U., Lucknow, Uttar Pradesh, India.
REFERENCES
- 1.Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625–30. doi: 10.1213/01.ANE.0000062522.21048.61. [DOI] [PubMed] [Google Scholar]
- 2.Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: A comparison with halothane. Paediatr Anaesth. 2000;10:419–24. doi: 10.1046/j.1460-9592.2000.00560.x. [DOI] [PubMed] [Google Scholar]
- 3.Olympio MA. Postanesthetic delirium: Historical perspectives. J Clin Anesth. 1991;3:60–3. doi: 10.1016/0952-8180(91)90209-6. [DOI] [PubMed] [Google Scholar]
- 4.Constant I, Seeman R. Inhalational anesthetics in pediatric anesthesia. Curr Opin Anaesthesiol. 2005;18:277–81. doi: 10.1097/01.aco.0000169235.83561.3a. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 6.Mantz J, Josserand J, Hamada S. Dexmedetomidine: New insights. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 7.Lerman J. Inhalation agents in pediatric anaesthesia – An update. Curr Opin Anaesthesiol. 2007;20:221–6. doi: 10.1097/ACO.0b013e32811e16e7. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Shahwan I. Effect of propofol on emergence behavior in children after sevoflurane general anesthesia. Paediatr Anaesth. 2008;18:55–9. doi: 10.1111/j.1460-9592.2007.02376.x. [DOI] [PubMed] [Google Scholar]
- 9.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–54. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 10.Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children: A comparison with halothane. Paediatr Anaesth. 1999;9:299–304. doi: 10.1046/j.1460-9592.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen JY, Jia JE, Liu TJ, Qin MJ, Li WX. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth. 2013;60:385–92. doi: 10.1007/s12630-013-9886-x. [DOI] [PubMed] [Google Scholar]
- 12.Ghai B, Ram J, Chauhan S, Wig J. Effects of clonidine on recovery after sevoflurane anaesthesia in children undergoing cataract surgery. Anaesth Intensive Care. 2010;38:530–7. doi: 10.1177/0310057X1003800319. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Davidson M, Tran MC, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:1004–10. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 14.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15:762–6. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibacache ME, Muñoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]
- 16.Beskow A, Westrin P. Sevoflurane causes more postoperative agitation in children than does halothane. Acta Anaesthesiol Scand. 1999;43:536–41. doi: 10.1034/j.1399-6576.1999.430508.x. [DOI] [PubMed] [Google Scholar]
- 17.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud M, Mason KP. Dexmedetomidine: Review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115:171–82. doi: 10.1093/bja/aev226. [DOI] [PubMed] [Google Scholar]
- 19.Hannallah RS, Broadman LM, Rice LJ. Testing the validity of an objective pain scale for infants and children. Anesthesiology. 1988;69:A770. [Google Scholar]
- 20.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100:1138–45. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2005;15:1098–104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 23.Deutsch E, Tobias JD. Hemodynamic and respiratory changes following dexmedetomidine administration during general anesthesia: Sevoflurane vs.desflurane. Paediatr Anaesth. 2007;17:438–44. doi: 10.1111/j.1460-9592.2006.02139.x. [DOI] [PubMed] [Google Scholar]
- 24.Bong CL, Ng AS. Evaluation of emergence delirium in Asian children using the pediatric anesthesia emergence delirium scale. Paediatr Anaesth. 2009;19:593–600. doi: 10.1111/j.1460-9592.2009.03024.x. [DOI] [PubMed] [Google Scholar]
- 25.Hauber JA, Davis PJ, Bendel LP, Martyn SV, McCarthy DL, Evans MC, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121:1308–15. doi: 10.1213/ANE.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Kim SY, Lee JH, Kang YR, Koo BN. Low-dose dexmedetomidine reduces emergence agitation after desflurane anaesthesia in children undergoing strabismus surgery. Yonsei Med J. 2014;55:508–16. doi: 10.3349/ymj.2014.55.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boku A, Hanamoto H, Oyamaguchi A, Inoue M, Morimoto Y, Niwa H, et al. Effectiveness of dexmedetomidine for emergence agitation in infants undergoing palatoplasty: A randomized controlled trial. Rev Bras Anestesiol. 2016;66:37–43. doi: 10.1016/j.bjan.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Valley RD, Ramza JT, Calhoun P, Freid EB, Bailey AG, Kopp VJ, et al. Tracheal extubation of deeply anesthetized pediatric patients: A comparison of isoflurane and sevoflurane. Anesth Analg. 1999;88:742–5. doi: 10.1097/00000539-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Bortone L, Ingelmo P, Grossi S, Grattagliano C, Bricchi C, Barantani D, et al. Emergence agitation in preschool children: Double-blind, randomized, controlled trial comparing sevoflurane and isoflurane anesthesia. Paediatr Anaesth. 2006;16:1138–43. doi: 10.1111/j.1460-9592.2006.01954.x. [DOI] [PubMed] [Google Scholar]
- 30.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 31.Davis PJ, Greenberg JA, Gendelman M, Fertal K. Recovery characteristics of sevoflurane and halothane in preschool-aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesth Analg. 1999;88:34–8. doi: 10.1097/00000539-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Uezono S, Goto T, Terui K, Ichinose F, Ishguro Y, Nakata Y, et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000;91:563–6. doi: 10.1097/00000539-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Wang H, Zhu A, Niu K, Wang G. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: Different administration and different dosage. PLoS One. 2015;10:e0123728. doi: 10.1371/journal.pone.0123728. [DOI] [PMC free article] [PubMed] [Google Scholar]


