Abstract
Objective
A subgroup of patients with Parkinson disease (PD) develops impulse control disorders (ICD) associated with their dopamine replacement therapy. Patients and their families may be reluctant to report ICD symptoms or unaware these symptoms are related to PD medication, which can make detecting an ICD difficult for clinicians. Ideally, a behavioral measure that is sensitive to ICD could be employed to ensure that patients with these behaviors are identified and treated. The Iowa Gambling Task (IGT), a standardized decision-making task, has proven sensitive in other populations with impulse control problems. We hypothesized that the IGT would differentiate between PD patients with and without ICD.
Methods
We compared IGT performance and disease variables in 24 PD patients with ICD and 24 PD patients without ICD. Patient groups were matched in terms of age, sex, and duration of PD.
Results
There were no significant differences in IGT scores between PD groups. IGT performance declined with increasing age, but the majority of patients performed within normal limits based on published age- and education-corrected normative data.
Conclusions
The IGT did not distinguish between PD patients with and without ICD. Increasing age negatively impacted performance in both groups. Other studies have found that IGT performance may decline in normal aging. Our results suggest that the IGT lacks the sensitivity and specificity needed to differentiate between age-related deficits and disruption in frontal-subcortical circuits underlying ICD associated with PD medications. Therefore, the IGT is not an appropriate behavioral measure for ICD in PD patients.
Keywords: Parkinson’s disease, Elderly/geriatrics/aging, Executive functions
Introduction
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases in the world, second only to Alzheimer’s disease (Aarsland, Bronnick, & Fladby, 2011). Although PD is diagnosed on the basis of the cardinal motor symptoms—bradykinesia, tremors, rigidity, and postural instability—there is also a consistent array of non-motor, cognitive symptoms, and side effects. Among the most disruptive are Impulse Control Disorders (ICD) such as pathological gambling, hypersexuality, binge eating, and compulsive shopping. ICD occur in approximately 17% of patients with Parkinson disease (PD). Pathological gambling is the most common ICD, affecting 2.3%–9.3% of patients, followed by hypersexuality which affects 0.9%–13% of patients and compulsive buying/eating which affects 0.4%–5.7% of patients (Evans, Strafella, Weintraub, & Stacy, 2009; Vitale et al., 2011; Voon, Hassan, Zurowski, de Souza et al., 2006a; Voon, Hassan, Zurowski, Duff-Canning et al., 2006b; Voon, Potenza, & Thomsen, 2007; Weintraub et al., 2006). These reward-based behaviors are typically the result of excess stimulation of mesolimbic dopamine receptors by dopamine-replacement therapy intended to correct dopamine depletion in the nigrostriatal dopamine system (Santangelo et al., 2009; Tessitore et al., 2017; Vitale et al., 2011; Weintraub et al., 2010; Weintraub, 2009; Zhang et al., 2016).
Dopamine agonists (DA) like pramipexole and ropinirole are the most frequent triggers of ICD in PD, although levodopa has also been associated with some cases (Weintraub et al., 2006). DAs act more selectively on the D3 dopamine receptors prevalent in the mesolimbic reward pathway (Brewer & Potenza, 2008; Broen, Duits, Visser-Vandewalle, Temel, & Winogrodzka, 2011; Evans et al., 2009; Gerlach et al., 2003; Tessitore et al., 2017; Weintraub et al., 2006, 2010). The mesolimbic system is less affected by pathological changes in PD and therefore DA use can lead to hyper stimulation which impairs reversal learning, increases impulsivity, and increases sensitivity to reward while simultaneously decreasing sensitivity to punishment (Cools, Altamirano, & D’Esposito, 2006; Cools, Barker, Sahakian, & Robbins, 2003; Evans et al., 2009; Kubler, Schroll, Buchert, & Kuhn, 2017). PD patients with an impulsive personality or history of impulsive behavior, earlier onset PD, prior substance abuse, male sex, or certain psychiatric conditions such as bipolar disorder are all at higher risk for ICD (Ceravolo, Frosini, Rossi, & Bonuccelli, 2009; Weintraub, 2009), potentially due to prior sensitization of the mesolimbic dopamine system. The typical strategy to reduce ICD symptoms is to reduce the patient’s dopaminergic medication, which can lead to poorer control over the motor symptoms. However, an ICD can be even more disabling than motor symptoms as the financial, psychosocial, or functional costs have the potential to become catastrophic.
ICDs can be difficult to detect. Health providers must rely on patient and caregiver reports of impulsive behaviors, either through interview or questionnaire responses, because ICD is typically inconspicuous in the examination room. The Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (Weintraub et al., 2009) is a useful tool for this purpose. Unfortunately, the disadvantages of using self-report methods when attempting to measure negative behaviors have long been recognized in the field of psychology (Edwards, 1957). Patients with PD who have ICD (Thiel et al., 2003) often have reduced insight into impulsive behaviors or may be ashamed to discuss aberrant behaviors that they do not realize are likely a side effect of the DA medication. Likewise, caregivers may not feel at liberty to “tell on” or contradict the patient.
This becomes particularly problematic in the context of candidacy evaluation for Deep Brain Stimulation (DBS) treatment. Patients and families may under-report problem behaviors out of fear that they will be denied surgery. However, identifying ICD is crucial for determining candidacy and risk profiles for DBS therapy given that DBS, particularly targeting the subthalamic nucleus, may exacerbate or cause de novo ICD symptoms (Lim et al., 2009; Castrioto et al., 2015; Moum et al., 2012). A review examining patient behavioral outcomes found that patients with an older age of onset (≥56 years old) were most at risk for worsening or inducing an ICD after STN-DBS. Thus, a patient’s risk for developing or worsening an ICD must be assessed carefully before a decision to pursue DBS is made which can be very difficult when relying on patient or caregiver report. A standardized behavioral measure sensitive to ICDs that does not rely on subjective report would be invaluable.
One existing measure with the potential to fulfill that need is the Iowa Gambling Task (IGT), a computerized measure that was originally developed to evaluate defective decision-making observed in patients with damage to the ventromedial (VM) prefrontal cortex (Bechara, Damasio, Damasio, & Anderson, 1994). These patients often engaged in risky or impulsive behavior during their daily lives and seemed unable to learn from the consequences of their poor judgment, but perform normally on neuropsychological measures of executive function (Bechara et al., 1994; Bechara, Tranel, Damasio, & Damasio, 1996). The IGT simulates a card game in which the player tries to win “money” by choosing cards from four decks. Each deck contains cards that award money but may also take away money. Two of the decks are advantageous to the player, yielding lower rewards but also imposing lower penalties. The other two decks are disadvantageous, yielding higher rewards but also imposing higher penalties. Successful performance requires intact decision-making, reversal learning, impulse control, mental flexibility, and reward/punishment sensitivity (Fellows & Farah, 2005; Mimura, Oeda, & Kawamura, 2006; Thiel et al., 2003). Over time the players ideally learn that choosing from the two advantageous decks will maximize their winnings, but players with damage to the ventromedial prefrontal cortex persist in choosing from the disadvantageous decks with the higher rewards even as their losses mount (Bechara et al., 1994, 1996). Bechara and colleagues referred to this neglect of future consequences and single-minded focus on the present outcome desired as “myopia for the future” (Bechara, Tranel, & Damasio, 2000; Fellows & Farah, 2005). In addition to the original population of VM lesion patients, the IGT has also demonstrated sensitivity to poor decision-making skills in other patient groups with impulsive behaviors, including substance abuse patients and pathological gamblers. These non-lesion groups are typically not as severely impaired and eventually developed some preference for the advantageous decks (Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2005; Rossi et al., 2010).
Although the IGT is a popular measure in the cognitive neuroscience literature, it has not been widely adopted in clinical practice (Rabin, Barr, & Burton, 2005). In the movement disorders realm, this may reflect the fact that research using the IGT in PD patients has produced mixed results. Several studies have found that PD patients score lower on the IGT and show a marked preference for disadvantageous decks relative to healthy controls (Gescheidt et al., 2012; Ibarretxe-Bilbao et al., 2009; Kobayakawa, Koyama, Mimura, & Kawamura, 2008; Mapelli, Di Rosa, Cavalletti, Schiff, & Tamburin, 2014; Mimura et al., 2006).Other studies have demonstrated that PD patients will eventually develop a preference for the advantageous decks (Czernecki et al., 2002; Stout, Rodawalt, & Siemers, 2001). There are even fewer studies evaluating PD patients with ICD and those findings are also mixed. One small study in seven patients found that PD patients with pathological gambling achieved poorer scores on the IGT than PD patients without pathological gambling (Rossi et al., 2010). However, a second study of 17 PD patients with mixed ICDs did not find any difference in IGT performance relative to a matched PD control sample (Bentivoglio, Baldonero, Ricciardi, De Nigris, & Daniele, 2013). Given the importance of issue, the potential utility of the IGT should be further evaluated. If the IGT can detect a response strategy indicative of reward hypersensitivity, punishment insensitivity, and impaired decision-making in older PD patients, then it could be used to screen DBS candidates and limit the risk of worsening an existing ICD.
Methods and Materials
The study was approved by the Cleveland Clinic Institutional Review Board.
Subjects
Forty-eight patients diagnosed with idiopathic PD (24 patients with ICD; 24 patients without ICD matched for age, gender, education, and PD duration) were retrospectively identified from an IRB approved patient registry including patients who completed clinical neuropsychological evaluations as part of a comprehensive candidacy evaluation for DBS. Exclusion criteria included dementia, non-native English speakers, and any prior neurosurgical procedures. PD-related impulse control problems (pathological gambling N = 6, compulsive spending N = 10, hypersexuality N= 17) were identified through separate clinical interviews with the patient and their spouse or caregiver using criteria conforming to Voon and Fox (2007). In most cases, the ICD had been treated with medication reductions, but all patients reported continued behaviors associated with ICD. Based on collateral report, eight patients continued to demonstrate significant behaviors associated with ICD (i.e., creating financial, legal, or marital problems) at the time of their neuropsychological evaluation. No patients had a history of ICD prior to onset of PD. Non-ICD PD patients had no known history of impulse control problems. The demographic, neuropsychological, and clinical characteristics of the sample are presented in Table 1. All patients completed the IGT while ON their regular medications.
Table 1.
Sample characteristics
| ICD | PD | t | p | Cohen’sd | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||||
| Age | 61.2 | 8.3 | 60.5 | 7.8 | 0.3 | 0.775 | 0.09 | ||
| Sex (#male) | 21 | 21 | |||||||
| Education | 15.2 | 3.8 | 15.8 | 2.4 | −0.7 | 0.499 | 0.20 | ||
| PD duration (yrs) | 13.2 | 7.1 | 11.9 | 7.1 | 0.6 | 0.544 | 0.18 | ||
| # on Agonist | 16 | 17 | |||||||
| Agonist LEDD | 179.8 | 164.8 | 166.0 | 185.8 | 0.3 | 0.787 | 0.08 | ||
| Total LEDD | 1582.3 | 898.6 | 1006.3 | 552.4 | 2.7 | 0.010 | 0.77 | ||
| UPDRS-III Off | 17 | 41.3 | 11.3 | 19 | 37.2 | 14.8 | 0.9 | 0.360 | 0.31 |
| UPDRS-III On | 23 | 21.6 | 9.9 | 20 | 18.3 | 10.5 | 1.0 | 0.301 | 0.32 |
| DRS-II | 138.0 | 3.5 | 136.5 | 5.9 | 1.1 | 0.278 | 0.32 | ||
| WASI Full Scale IQ* | 105.9 | 12.6 | 109.0 | 12.2 | −0.9 | 0.395 | 0.25 | ||
| WRAT Reading* | 22 | 98.5 | 15.5 | 101.5 | 16.7 | −0.6 | 0.527 | 0.19 | |
| Boston Naming | 53.7 | 5.1 | 56.2 | 2.0 | −2.3 | 0.027 | 0.66 | ||
| Phonemic Fluency | 36.8 | 11.2 | 40.4 | 10.1 | −1.1 | 0.257 | 0.33 | ||
| Semantic Fluency | 40.0 | 7.8 | 47.1 | 7.7 | −3.2 | 0.003 | 0.92 | ||
| Digit Span** | 23 | 9.6 | 1.9 | 23 | 11.7 | 3.3 | −2.7 | 0.010 | 0.79 |
| LNS** | 9.3 | 2.5 | 23 | 11.1 | 3.3 | −2.1 | 0.043 | 0.61 | |
| JOLO | 23.6 | 6.6 | 23.8 | 4.4 | −0.1 | 0.939 | 0.02 | ||
| SDMT | 43.0 | 12.9 | 23 | 44.8 | 11.4 | −0.5 | 0.618 | 0.15 | |
| WCST Persev Err* | 86.3 | 13.4 | 86.8 | 12.3 | −0.1 | 0.893 | 0.04 | ||
| WCST Categories | 2.5 | 2.3 | 3.7 | 2.2 | −1.9 | 0.059 | 0.56 | ||
| Logical Memory I** | 9.0 | 2.6 | 10.1 | 2.6 | −1.6 | 0.126 | 0.45 | ||
| Logical Memory II** | 9.8 | 2.3 | 10.8 | 2.6 | −1.3 | 0.204 | 0.37 | ||
| RAVLT Immediate | 37.9 | 10.5 | 43.1 | 9.1 | −1.8 | 0.074 | 0.53 | ||
| RAVLT Delay | 6.3 | 4.0 | 7.2 | 4.2 | −0.7 | 0.485 | 0.20 | ||
| BDI-II | 11.2 | 6.8 | 8.5 | 5.3 | 1.6 | 0.125 | 0.45 | ||
| BAI | 14.4 | 10.8 | 10.9 | 6.4 | 1.4 | 0.172 | 0.40 | ||
N = 24 & df = 46 unless otherwise noted; values are raw scores unless otherwise noted.
Higher scores indicated better performance for: Mattis Dementia Rating Scale-second Edition (DRS-II) (Jurica, Leitten, & Mattis, 2001), and measures of general intelligence (Wechsler Abbreviated Scale of Intelligence, WASI Full Scale), premorbid verbal abilities (Wide Range Achievement Test-fourth Edition Reading Subscale, WRAT) naming (Boston Naming Test, BNT) (Kaplan, Goodglass, & Weintraub, 1983), phonemic fluency (Psychological Corporation, 1999), oral processing speed (Symbol Digit Modalities Test, SDMT) (Smith, 1982), attention span (Wechsler Memory Scale-third Edition Digit Span), working memory (Wechsler Memory Scale-third Edition Letter Number Sequencing, LNS), (visuospatial ability, Judgment of Line Orientation, JOLO) (Benton, Sivan, Hamsher, Varney, & Spreen, 1983), problem solving (Wisconsin Card Sorting Test Categories completed, WCST Categories), Immediate and Delayed List Memory (Trials 1–5 recall Rey Auditory Verbal Learning Test, RAVLT immediate; RAVLT Delay) (Lezak, 1983), and immediate and delayed story recall (Wechsler Memory Scale-third Edition Logical Memory I and II Subtests) (Wechsler, 1997). Lower scores indicated better performance on measures of perseverative tendencies (Wisconsin Card Sorting Test, Perseverative errors) (Heaton, 1981), depression symptoms (Beck Depression Inventory-second Edition, BDI-II), and anxiety symptoms (Beck Anxiety Inventory, BAI).
*Standard Score.
**Scaled Score.
Iowa Gambling Task
The IGT format used was a standardized computer-administered test (Bechara et al., 1994) wherein the examinee makes 100 selections from among the four decks of cards displayed in a horizontal array. After each selection, the computer displays an associated abstract monetary reward and, occasionally, a monetary punishment. Two decks (A&B) are the disadvantageous decks that provide large rewards but even larger punishments resulting in a net loss over time. The other two decks (C&D) are the advantageous decks that provide small rewards but even smaller punishments resulting in a net gain over time. Examinees are told in advance that there are good and back decks and they should try to avoid the bad decks and select from the good decks to accrue as much “money” as possible. The primary dependent variable is the number of advantageous selections minus disadvantageous selections in each block of 20 trials [(C + D) – (A + B)].
Statistical Analysis
Independent samples t-tests were used to compare groups on baseline characteristics. IGT performance (number of advantageous selections) was examined across five blocks (20 trials each) and compared across groups using repeated measures General Linear Modeling. Similar analyses were repeated for the first and second blocks alone to examine decision making under ambiguity as well as the third through fifth blocks to examine decision making under risk. Pearson correlations were used to evaluate associations between total IGT performance and patient characteristics. Effect sizes were calculated using partial eta squared (ηp2) or Cohen’s d. 95% confidence intervals are provided for correlation analyses. Corrected alpha of .01 was used for all analyses to offset multiple comparisons.
Results
Sample Characteristics
Group comparisons are provided in Table 1. The ICD group was on a higher levodopa-equivalent daily dose but the number of patients taking dopamine agonists were similar in both groups. Patients with ICD scored lower on tests of semantic fluency and verbal attention span. The groups did not differ on any other demographic or clinical variable.
IGT
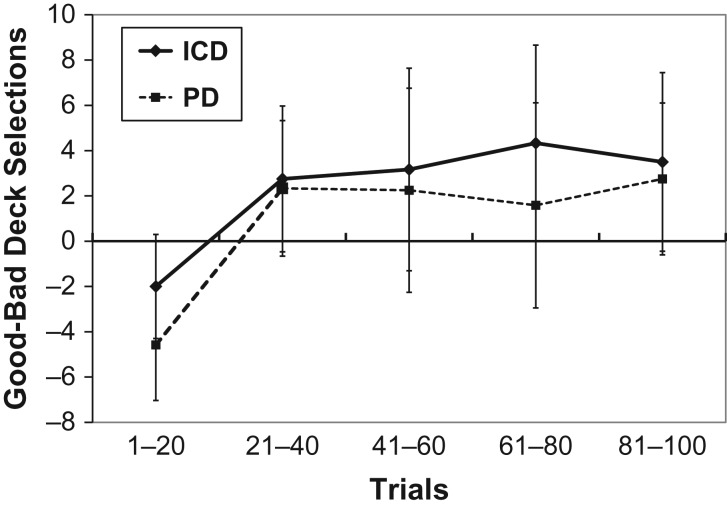
IGT performance for the two groups is displayed in Fig. 1. There was a significant main effect of block [F(4,184) = 6.7, p < .001, ηp2 = .31] but no main effect or interaction involving group on IGT performance with small effect sizes [F < 1, ηp2 = .02–.04]. When a subset of eight patients with severe, functionally impairing ICD symptoms were selected and compared with their matched control patients, the effect of group remained non-significant [F < 1, ηp2 = .09], indicating that the null result is not attributable to variability in ICD “severity.” There was also no relation between the character/number of ICD symptoms (i.e., hypersexuality, pathological gambling, and compulsive spending) and IGT performance.
Fig. 1.
The total number of cards selected from “Good” decks minus the number of cards selected from “Bad” decks for patients with ICD (diamond symbol) and without ICD (square symbol). Response bins represent 20 consecutive selections. Error bars represent 2 standard errors of the mean.
Decision-making Context
To examine whether ICD was associated with difficulty making decisions under ambiguous conditions, we compared group performance on the initial 40 trials of the IGT. As noted above, there was a significant shift away from disadvantageous decks beginning with the second blocks of 20 trials [F(1,46) = 14.2, p < .001, ηp2 = .24]. There was no main effect or interaction involving group on IGT performance [F < 1.6, p > .2, ηp2 = .01–.03].
We also explored whether ICD groups differed in decision-making under risk (the last 60 trials). In this analysis, there was no effect of block, group, or interaction [all F < 1, all ηp2 < .01].
Normative Performance
According to the age- and education-corrected normative sample reported in the test manual, mean T-scores for total performance (Total selections from C&D minus total selections from decks A&B) fell in the average range for both groups. The proportion of patients demonstrating impaired total performance (T < 40) per the normative data was equivalent for both groups (four ICD patients, seven PD patients; χ2 = 2.26, p = .32). Likewise, mean T-scores for the final block of 20 trials also revealed average range performance for both patient groups, with no difference in the proportion of subjects that demonstrated impaired performance (five ICD, 7 PD; χ2 = 0.5, p = .5).
IGT Associations
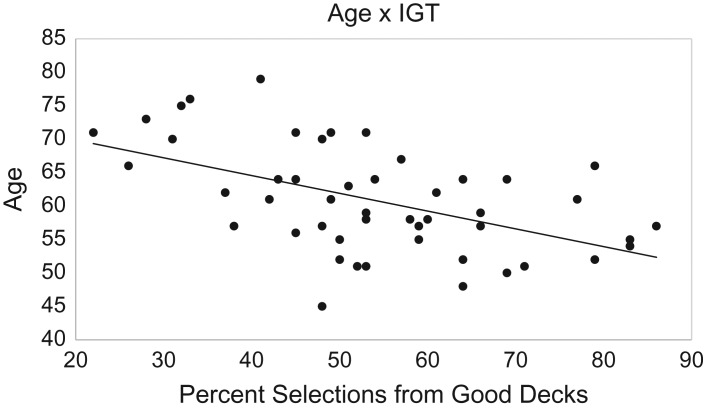
To better understand the variables associated with performance on the IGT, we calculated Pearson correlations between IGT and demographic and clinical variables across the entire sample. Better IGT performance was associated with younger age (r = .52, p < .001; 95% CI = .28–.7), faster processing speed (r = .44, p = .002; 95% CI = .18–.64), and higher scores on the Mattis Dementia Rating Scale (r = .47, p = .001; 95% CI = .21–.66) (see Fig. 2). There was no relationship between IGT performance and any other measured characteristic including education, disease duration, DA agonist dosage, Levodopa-equivalent daily dosage, or Unified Parkinson Disease Rating Scale-III scores on or off medications.
Fig. 2.
Scatterplot of the relationship between patient age and overall percentage of selections from “good” decks.
Discussion
The goal of this study was to evaluate the utility of a standardized decision-making test, the Iowa Gambling Task, to distinguish PD patients with an ICD stemming from DRT from patients without an ICD. We found that the IGT did not differentiate between Parkinson patients with and without ICD symptoms, consistent with the findings of Bentivoglio and colleagues (2013). This was true even for the subgroup of patients with the most severe ICD symptoms. The type or number of impulse control problems was also not related to IGT performance. Thus, the discrepant findings of Rossi cannot be attributed to the fact that their sample consisted only of pathological gamblers (Rossi et al., 2010). There were no indications that our sample of ICD patients was atypical. Our patient group was fairly consistent with other (Voon & Fox, 2007; Weintraub et al., 2010) descriptions of PD patients with ICD: predominately male patients with young-onset PD treated with dopamine agonists However, our sample consisted of patients with moderate to severe disease based on UPDRS-3 motor scores (on-medication), which did diverge from some prior reports of impaired IGT scores in less impaired PD-ICD patients (Fellows & Farah, 2005). It is unclear what influence disease severity might have on the IGT, although our results found no correlation between UPDRS-3 scores and performance. Future research will examine this possible interaction.
The only significant variable found to affect the IGT T-score was age; IGT performance declined with age in PD patients irrespective of ICD group. The influence of age on IGT performance is also reflected in the published norms which indicate that, for ages 60 and above, total scores indicative of a slight preference for the disadvantageous decks (i.e., up to 60% of disadvantageous selections) still fall within the normal range. Indeed, the majority of our PD sample, with or without ICD, performed within normal limits based on published normative data. Age may also be an important variable in understanding discrepant results in prior studies of ICD in PD. Our sample was similar in age to the Bentivoglio and colleagues (2013) sample who showed no influence of ICD on IGT but older than that of the Rossi and colleagues (2010) sample who did demonstrate IGT differences. It seems likely, therefore, that the IGT is not specific enough to detect and differentiate between an ICD in older PD patient or normal age-related changes in decision-making skills. Older individuals appear less sensitive to the game’s negative consequences and less able to learn over time, compared to younger subjects (Di Rosa et al., 2017; Fein, McGillivray, & Finn, 2007). The frontal aging hypothesis would suggest that variations in IGT scores among healthy older adults is due to decreased functionality in the frontal lobe but it is not clear that the neurological underpinnings of performance in aging and ICD in PD are similar.
A direct comparison of our data and prior studies of other non-PD ICD groups revealed very similar performance levels. As seen with substance dependent individuals (Bechara, & Martin, 2004) and pathological gamblers (Goudriaan et al., 2005), our sample of PD patients developed a slight preference for the two advantageous decks. This contrasts starkly with the performance of patients with known ventromedial prefrontal damage where patients maintained a clear preference for the two disadvantageous decks (Bechara et al., 1994). This suggests that there may be important differences in the neuroanatomical bases of impulsive behaviors associated with ICD in PD and addiction compared to those that arise in patients with ventromedial frontal lobe damage. While the ventral striatum has been shown to be active during the IGT task (Tanabe et al., 2007), function in this region may not influence performance to the same extent as frontal lobe damage. In other words, the IGT may index frontal lobe function more than subcortical function. This will clearly limit its usefulness in populations with subcortical disruption such as PD. The relative importance of frontal lobe dysfunction would also account for the salient age effects in the IGT; age-related frontal lobe dysfunction leads to worsening of IGT performance. As such, if the patient or the control/comparison population has some degree of frontal lobe dysfunction, this could obscure any performance changes associated with subcortical dysfunction.
A more detailed analysis examining responses by block also failed to detect significant differences between the groups after the first block. Some have proposed that the first 40 trials (blocks 1 and 2) represent decision making under ambiguity while the last 60 trials (blocks 3, 4, & 5) represent decision-making under risk (Brand, Heinze, Labudda, & Markowitsch, 2008). To determine whether these types of decision making are differentially affected by ICD in PD, we performed separate analyses for these trials. Our results indicate that presence of ICD is not a factor in either type of decision making. Both groups of PD patients undergoing DBS evaluation show a shift from disadvantageous preferences to advantageous decks when making decisions under ambiguity, and show a stable preference for advantageous desks when making decisions under risk. Unfortunately, this means that even a more detailed analysis of strategy on the IGT is not likely to be useful in identifying PD patients with an ICD.
Despite the difficulty in identifying ICDs in our sample using the IGT, the general approach of using a risk-based task has ecological validity. The cognitive neuroscience literature, where the IGT was first developed, contains many other paradigms that could have utility for this purpose. There are several tasks that involve decisions under risky conditions including the Cambridge Gambling task (Rogers et al., 1999), the Toronto Gambling task (Floden, Alexander, Kubu, Katz, & Stuss, 2008), or the Balloon Analogue Risk Task (Lejuez et al., 2002). In particular, although it has not yet been standardized for clinical populations, the Balloon Analogue Risk Test has also been used to examine ICD in patients with PD. Claassen and colleagues (2011) compared BART scores of PD patients with and without ICD while they were on and off their DA medications. There was no difference between the groups when patients were off their medications. However, when patients were on their medications, risk-taking behavior increased only in the PD patients with an active ICD. This suggests that future research in the utility of the BART for clinical application is warranted. Alternatively, other paradigms that investigate sensitivity to reward value (i.e., delayed discounting) or reward-based learning (i.e., reversal learning tasks) may be fruitful avenues to understand and identify ICDs in patients with PD.
There are a few limitations of the current study that should be acknowledged. First, the sample studied here was fairly small (N = 24 patients with ICD) and therefore, there could be concerns about generalizability or power. However, it should be noted that the effect sizes for the main comparisons were small, suggesting that lack of power does not fully account for the negative findings. Moreover, the size of our sample is 40% larger than the largest published study on IGT in PD with ICD. Second, our study is retrospective which may lead to sampling bias. We took particular care to match control patients and both groups are similar to our larger sample of PD patients seeking DBS at our center. Nonetheless, a prospective design with consecutive enrollment would be preferable. Third, although our method of identifying participants with ICD using clinical interviews with patients and families is the current gold standard, it is not without weaknesses as previously discussed. Finally, most of the patients with ICD were being treated at the time of the study. It is possible that another study examining patients with untreated, fulminant ICD might obtain different results. However, since an ICD may worsen after DBS surgery, it is important to detect even the milder cases that our sample represents.
Our data indicate that the IGT is not sufficiently sensitive to identify ICD in PD patients who are seeking DBS treatment and that any relative impairments detected may be ascribed to performance changes with normal aging. Clinicians seeking an objective method to screen for ICD in the context of DBS candidacy evaluations are encouraged to look to alternative techniques that are sensitive to reward processing deficits but relatively impervious to normal aging.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke [1K23NS091344 to D.P.F.]; and the National Institute of Mental Health [R01MH114853 to C.S.K.]. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of interest
None declared.
References
- Aarsland D., Bronnick K., & Fladby T. (2011). Mild cognitive impairment in Parkinson’s disease. Current Neurology and Neuroscience Reports, 11, 371–378. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A. R., Damasio H., & Anderson S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A., & Martin E. M. (2004). Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology, 18, 152–162. [DOI] [PubMed] [Google Scholar]
- Bechara A., Tranel D., & Damasio H. (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain, 123, 2189–2202. [Erratum appears in Brain. 2009 Jul;132(Pt 7):1993]. [DOI] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H., & Damasio A. R. (1996). Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex, 6, 215–225. [DOI] [PubMed] [Google Scholar]
- Bentivoglio A. R., Baldonero E., Ricciardi L., De Nigris F., & Daniele A. (2013). Neuropsychological features of patients with Parkinson’s disease and impulse control disorders. Neurological Sciences, 34, 1207–1213. [DOI] [PubMed] [Google Scholar]
- Benton A. L., Sivan A. B., Hamsher K., de S., Varney N. R., & Spreen O. (1983). Judgment of line orientations. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Brand M., Heinze K., Labudda K., & Markowitsch H. J. (2008). The role of strategies in deciding advantageously in ambiguous and risky situations. Cognitive Processing, 9, 159–173. [DOI] [PubMed] [Google Scholar]
- Brewer J. A., & Potenza M. N. (2008). The neurobiology and genetics of impulse control disorders: Relationships to drug addictions. Biochemical Pharmacology, 75, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broen M., Duits A., Visser-Vandewalle V., Temel Y., & Winogrodzka A. (2011). Impulse control and related disorders in Parkinson’s disease patients treated with bilateral subthalamic nucleus stimulation: A review. Parkinsonism & Related Disorders, 17, 413–417. [DOI] [PubMed] [Google Scholar]
- Castrioto A., Funkiewiez A., Debu B., Cools R., Lhommee E., Ardouin C. … Krack P. (2015). Iowa gambling task impairment in Parkinson’s disease can be normalised by reduction of dopaminergic medication after subthalamic stimulation. Journal of Neurology, Neurosurgery & Psychiatry, 86, 186–190. [DOI] [PubMed] [Google Scholar]
- Ceravolo R., Frosini D., Rossi C., & Bonuccelli U. (2009). Impulse control disorders in Parkinson’s disease: Definition, epidemiology, risk factors, neurobiology and management. Parkinsonism Related Disorders, 15, S111–S115. [DOI] [PubMed] [Google Scholar]
- Claassen D. O., van den Wildenberg W. P., Ridderinkhof K. R., Jessup C. K., Harrison M. B., Wooten G. F., et al. (2011). The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behavioral Neuroscience, 125, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., Altamirano L., & D’Esposito M. (2006). Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia, 44, 1663–1673. [DOI] [PubMed] [Google Scholar]
- Cools R., Barker R. A., Sahakian B. J., & Robbins T. W. (2003). L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia, 41, 1431–1441. [DOI] [PubMed] [Google Scholar]
- Czernecki V., Pillon B., Houeto J. L., Pochon J. B., Levy R., & Dubois B. (2002). Motivation, reward, and Parkinson’s disease: Influence of dopatherapy. Neuropsychologia, 40, 2257–2267. [DOI] [PubMed] [Google Scholar]
- Di Rosa E., Mapelli D., Arcara G., Amodio P., Tamburin S., & Schiff S. (2017). Aging and risky decision-making: New ERP evidence from the Iowa Gambling Task. Neuroscience Letters, 640, 93–98. [DOI] [PubMed] [Google Scholar]
- Edwards A. L. (1957). The social desirability variable in personality assessment and research. New York: Dryden. [Google Scholar]
- Evans A. H., Strafella A. P., Weintraub D., & Stacy M. (2009). Impulsive and compulsive behaviors in Parkinson’s disease. Movement Disorders, 24, 1561–1570. [DOI] [PubMed] [Google Scholar]
- Fein G., McGillivray S., & Finn P. (2007). Older adults make less advantageous decisions than younger adults: Cognitive and psychological correlates. Journal of the International Neuropsychological Society, 13, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows L. K., & Farah M. J. (2005). Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex, 15, 58–63. [DOI] [PubMed] [Google Scholar]
- Floden D., Alexander M. P., Kubu C. S., Katz D., & Stuss D. T. (2008). Impulsivity and risk-taking behavior in focal frontal lobe lesions. Neuropsychologia, 46, 213–223. [DOI] [PubMed] [Google Scholar]
- Gerlach M., Double K., Arzberger T., Leblhuber F., Tatschner T., & Riederer P. (2003). Dopamine receptor agonists in current clinical use: Comparative dopamine receptor binding profiles defined in the human striatum. Journal of Neural Transmission, 110, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Gescheidt T., Czekoova K., Urbanek T., Marecek R., Mikl M., Kubikova R., et al. (2012). Iowa Gambling Task in patients with early-onset Parkinson’s disease: Strategy analysis. Neurological Sciences, 33, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., de Beurs E., & van den Brink W. (2005). Decision making in pathological gambling: A comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cognitive Brain Research, 23, 137–151. [DOI] [PubMed] [Google Scholar]
- Heaton R. (1981). A manual for the Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Tolosa E., Marti M. J., Valldeoriola F., Bargallo N., et al. (2009). Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. European Journal of Neuroscience, 30, 1162–1171. [DOI] [PubMed] [Google Scholar]
- Jurica P.J., Leitten C.L., & Mattis S. (2001). Dementia rating scale-2: Professional manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Kaplan E., Goodglass H., & Weintraub S. (1983). Boston naming test. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Kobayakawa M., Koyama S., Mimura M., & Kawamura M. (2008). Decision making in Parkinson’s disease: Analysis of behavioral and physiological patterns in the Iowa gambling task. Movement Disorders, 23, 547–552. [DOI] [PubMed] [Google Scholar]
- Kubler D., Schroll H., Buchert R., & Kuhn A. A. (2017). Cognitive performance correlates with the degree of dopaminergic degeneration in the associative part of the striatum in non-demented Parkinson’s patients. Journal of Neural Transmission, 124, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lejuez C. W., Read J. P., Kahler C. W., Richards J. B., Ramsey S. E., Stuart G. L., et al. (2002). Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). The Journal of Experimental Psychology: Applied, 8, 75–84. [DOI] [PubMed] [Google Scholar]
- Lezak MD. (1983). Neuropsychological assessment (2nd ed.). New York: Oxford University Press. [Google Scholar]
- Lim S. Y., O’Sullivan S. S., Kotschet K., Gallagher D. A., Lacey C., Lawrence A. D., et al. (2009). Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. Journal of Clinical Neuroscience, 16, 1148–1152. [DOI] [PubMed] [Google Scholar]
- Mapelli D., Di Rosa E., Cavalletti M., Schiff S., & Tamburin S. (2014). Decision and dopaminergic system: An ERPs study of Iowa gambling task in Parkinson’s disease. Frontiers in Psychology, 5, 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura M., Oeda R., & Kawamura M (2006). Impaired decision-making in Parkinson’s disease. Parkinsonism Related Disorders, 12, 169–175. [DOI] [PubMed] [Google Scholar]
- Moum S. J., Price C. C., Limotai N., Oyama G., Ward H., Jacobson C., et al. (2012). Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PLoS One, 7, e29768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. (1999). Wechsler Abbreviated Scale of Intelligence (WASI) manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Rabin L. A., Barr W. B., & Burton L. A. (2005). Assessment practices of clinical neuropsychologists in the United States and Canada: A survey of INS, NAN, and APA Division 40 members. Archives of Clinical Neuropsychology, 20, 33–65. [DOI] [PubMed] [Google Scholar]
- Rogers R. D., Owen A. M., Middleton H. C., Williams E. J., Pickard J. D., Sahakian B. J., et al. (1999). Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience, 19, 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M., Gerschcovich E. R., de Achaval D., Perez-Lloret S., Cerquetti D., Cammarota A., et al. (2010). Decision-making in Parkinson’s disease patients with and without pathological gambling. European Journal of Neurology, 17, 97–102. [DOI] [PubMed] [Google Scholar]
- Santangelo G., Vitale C., Trojano L., Verde F., Grossi D., & Barone P. (2009). Cognitive dysfunctions and pathological gambling in patients with Parkinson’s disease. Movement Disorders, 24, 899–905. [DOI] [PubMed] [Google Scholar]
- Smith A. (1982). Symbol Digit Modalities Test (SDMT) manual (revised). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Stout J. C., Rodawalt W. C., & Siemers E. R. (2001). Risky decision making in Huntington’s disease. Journal of the International Neuropsychological Society, 7, 92–101. [DOI] [PubMed] [Google Scholar]
- Tanabe J., Thompson L., Claus E., Dalwani M., Hutchison K., & Banich M. T. (2007). Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping, 28, 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A., Santangelo G., De Micco R., Giordano A., Raimo S., Amboni M., et al. (2017). Resting-state brain networks in patients with Parkinson’s disease and impulse control disorders. Cortex, 94, 63–72. [DOI] [PubMed] [Google Scholar]
- Thiel A., Hilker R., Kessler J., Habedank B., Herholz K., & Heiss W. D. (2003). Activation of basal ganglia loops in idiopathic Parkinson’s disease: A PET study. Journal of Neural Transmission, 110, 1289–1301. [DOI] [PubMed] [Google Scholar]
- Vitale C., Santangelo G., Trojano L., Verde F., Rocco M., Grossi D., et al. (2011). Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson’s disease. Movement Disorders, 26, 830–836. [DOI] [PubMed] [Google Scholar]
- Voon V., & Fox S. H. (2007). Medication-related impulse control and repetitive behaviors in Parkinson disease. Archives of Neurology, 64, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Voon V., Hassan K., Zurowski M., de Souza M., Thomsen T., Fox S., et al. (2006. a). Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology, 67, 1254–1257. [DOI] [PubMed] [Google Scholar]
- Voon V., Hassan K., Zurowski M., Duff-Canning S., de Souza M., Fox S., et al. (2006. b). Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology, 66, 1750–1752. [DOI] [PubMed] [Google Scholar]
- Voon V., Potenza M. N., & Thomsen T. (2007). Medication-related impulse control and repetitive behaviors in Parkinson’s disease. Current Opinion in Neurology, 20, 484–492. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997). Wechsler Memory Scale-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weintraub D. (2009). Impulse control disorders in Parkinson’s disease: Prevalence and possible risk factors. Parkinsonism & Related Disorders, 15, S110–S113. [DOI] [PubMed] [Google Scholar]
- Weintraub D., Hoops S., Shea J. A., Lyons K. E., Pahwa R., Driver-Dunckley E. D., et al. (2009). Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Movement Disorders, 24, 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D., Koester J., Potenza M. N., Siderowf A. D., Stacy M., Voon V., et al. (2010). Impulse control disorders in Parkinson disease: A cross-sectional study of 3090 patients. Archives of Neurology, 67, 589–595. [DOI] [PubMed] [Google Scholar]
- Weintraub D., Siderowf A. D., Potenza M. N., Goveas J., Morales K. H., Duda J. E., et al. (2006). Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of Neurology, 63, 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Dissanayaka N. N., Dawson A., O’Sullivan J. D., Mosley P., Hall W., et al. (2016). Management of impulse control disorders in Parkinson’s disease. International Psychogeriatrics, 28, 1597–1614. [DOI] [PubMed] [Google Scholar]