Abstract
Extensive pre-clinical studies suggest that sex steroids are neuroprotective in experimental traumatic brain injury (TBI). However, clinical trials involving sex hormone administration have not shown beneficial results, and our observational cohort studies show systemic estradiol (E2) production to be associated with adverse outcomes. Systemic E2 is produced via aromatization of testosterone (T) or reduction of estrone (E1). E1, also produced via aromatization of androstenedione (Andro) and is a marker of T-independent E2 production. We hypothesized that E1 would be (1) associated with TBI-related mortality, (2) the primary intermediate for E2 production, and (3) associated with adipose tissue-specific aromatase transcription. We assessed 100 subjects with severe TBI and 8 healthy controls. Serum levels were measured on days 0–3 post-TBI for key steroidogenic precursors (progesterone), aromatase pathway intermediates (E1, E2, T, Andro), and the adipose tissue-specific aromatase transcription factors cortisol, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). E1 was elevated after TBI versus controls. High E1 was associated with higher progesterone, cortisol, and IL-6 (p < 0.05). Multivariable logistic regression demonstrated that those in the highest E1 tertile had increased odds for mortality (adjusted OR = 5.656, 95% CI = 1.102–29.045, p = 0.038). Structural equation models show that early serum E2 production is largely T independent, occurring predominantly through E1 metabolism. Acute serum E1 functions as a mortality marker for TBI through aromatase-dependent E1 production and T-independent E2 production. Further work should evaluate risk factors for high E2 production and how systemic E2 and its key intermediate E1 contribute to the extracerebral consequences of severe TBI.
Keywords: aromatase, estradiol, estrone, inflammation, TBI
Introduction
Traumatic brain injury (TBI) contributes to ∼235,000 hospitalizations and 50,000 deaths annually in the United States.1 The complex and multifaceted nature of secondary damage following the primary insult that occurs with TBI is a major issue that has made identifying efficacious treatments difficult.2,3 In addition, the systemic pathophysiological responses to TBI, as well as concomitant extracerebral injury and non-neurological organ dysfunction (NNOD), have been relatively underappreciated for their role in affecting TBI outcome.4–8 Further, the autonomic nervous system response to injury may be central to at least a portion of the systemic pathology associated with NNOD after TBI, including the initiation of an innate inflammatory response,8–10 which we posit has some influence on other systemic response pathways such as injury-induced steroidogenesis.11
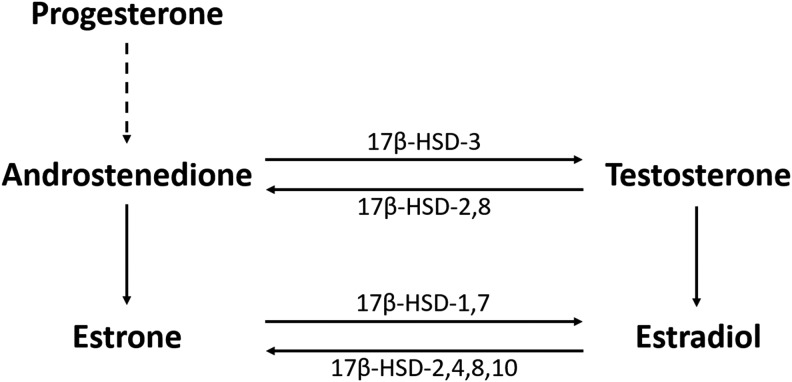
Multiple animal studies suggest that sex hormones such as progesterone and estradiol (E2) mitigate damage in models of TBI12–15 and stroke.15–18 However, systemic E2 production has been associated with poor outcomes and higher mortality rates in critical illness,19,20 major trauma,21,22 sepsis,23–26 nontraumatic subarachnoid hemorrhage,27 and severe TBI.11 The steroidogenesis of estrogens begins with progesterone conversion to the sex steroid androstenedione (Andro), which then may be metabolized further in multiple tissue types, including adipose tissue, to E2 via aromatization and reduction pathways using testosterone (T) and estrone (E1) as steroidogenic precursors (Fig. 1). Andro is converted to E2 through two major steroidogenic pathways. The first is a T-dependent pathway in which Andro is reduced predominantly via 17β-hydroxysteroid dehydrogenase-3 (17β-HSD-3) to T and then aromatized via the aromatase enzyme to E2.28,29 The second is a T-independent pathway requiring aromatization to E1 followed by reduction to E2 using enzymatic reductase isoforms 1 and 7 (17β-HSD-1,7).28,29
FIG. 1.
Sex hormone steroidogenesis from progesterone. Dashed line represents conversion of progesterone to 17α-hydroxyprogesterone and subsequently to androstenedione via cytochrome P450c17. The main isoforms of 17β-hydroxysteroid dehydrogenase (17β-HSD) catalyzing oxidative and reductive pathways are shown.
T has a much lower affinity for aromatase than Andro;30 therefore, recent reports suggest that estrogenic steroidogenesis, particularly in extragonadal tissues, favors biosynthetic pathways that do not consume T.30 In addition to elevated serum E2 levels in both men and women with major illnesses, concurrent T consumption is observed specifically in men and postmenopausal women.20,23,24,31,32 Increased systemic aromatization in response to post-injury stress may occur via multiple transcription factors that include tumor necrosis factor-alpha (TNF-α),33,34 cortisol,35 and interleukin-6 (IL-6),36 and may support both T and E1 consumption for E2 production.37 Therefore, we hypothesize that E1 may be a prognostic biomarker for mortality after severe TBI that also reflects/contributes to NNOD, along with other factors such as hypothalamic–pituitary axis (HPA) activation and injury-induced initiation of the systemic inflammatory response.
Regardless of whether T-dependent or T-independent aromatization occurs, serum E2 levels are associated with higher mortality risk in the setting of acute stressors such as critical illness and severe trauma.19,22,38 Higher serum E2 and T levels acutely after TBI have both been associated with acute mortality and poor 6 month Glasgow Outcome Scale (GOS) scores in patients with severe TBI.11 Further, T pretreatment in animal models of TBI can increase histological damage.39
Despite being a T-independent precursor to E2, little is known about E1 effects in brain injury pathology and outcome. Similar to other E2 administration studies involving experimental central nervous system (CNS) injury models,40–44 rat TBI models and cellular neuronal injury models suggest that supraphysiological E1 administration can be neuroprotective.45–47 Akin to clinical studies evaluating endogenous systemic E2 levels, clinical studies in septic shock and subarachnoid hemorrhage draw a sharp contrast to these experimental findings of E1-associated neuroprotection by showing that increased endogenous systemic E1 levels are associated with worse outcomes.27,48 E1 associations with septic shock are interesting when considered in the context of TBI, given that NNOD following TBI can lead to systemic inflammation49 and immune dysregulation.50 Further, inflammatory mediators are associated with increased aromatase activity,36,51 which may act to increase E1 production.30 Together, E1's association with outcomes in other types of brain injury, along with its role in sex hormone steroidogenesis, make it a potential systemic biomarker of injury when predicting outcomes following TBI and when understanding possible contributions to NNOD associated with TBI. Therefore, the goals of this study are to (1) identify if E1 is elevated in TBI, (2) determine if E1 can predict outcomes following TBI, and (3) assess E1's association with other biomarkers and sex hormones known to be dysregulated after TBI.
Methods
Study design and subjects
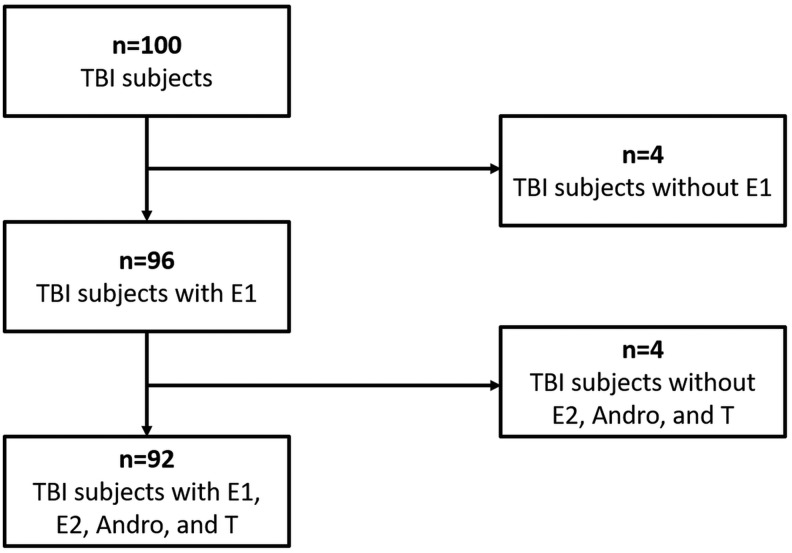
This study was approved by the University of Pittsburgh's Institutional Review Board. We enrolled 100 unique individuals admitted with severe TBI, defined as admission Glasgow Coma Scale (GCS) ≤8, at a level 1 trauma center. Figure 2 shows how our cohort was used for analysis: 96 patients had E1 data from days 0–3, and 92 of these individuals had E1, T, E2, and Andro data from samples collected on days 0–3 post-injury. Inclusion criteria were as follows: (1) age between 16 and 75 years, (2) positive findings on head CT, (3) insertion of extraventricular drainage catheter (EVD) for intracranial pressure (ICP) monitoring, and (4) signed consent for enrollment from appropriate proxy. Individuals with penetrating brain injury, documented history of prolonged cardiac or respiratory arrest, history of endocrine tumor, history of breast cancer requiring chemotherapy or tamoxifen, history of prostate cancer with orchiectomy or luteinizing hormone suppressing agents, or untreated thyroid disease were excluded from this study. Also, eight healthy controls were recruited as a reference group for serum hormone measurements. Healthy controls did not have previous endocrine pathology, were not pregnant, and were not using oral contraceptives, hormone replacement therapy, or hormone-modifying supplements. Those with a history of TBI, neurological disorder, or bleeding disorder were also excluded as healthy controls.
FIG. 2.
Divisions of cohort used for analyses. Our 100 patient cohort had 96 individuals with data on E1 and 92 individuals with data on estrone (E1), estradiol (E2), androstenedione (Andro), and testosterone (T).
Critical care management of individuals with TBI was consistent with The Guidelines for the Management of Severe Head Injury.52 Initial interventions included targeted temperature management, EVD placement, central venous catheterization, arterial catheterization, and monitoring via pulse oximetry. ICP and cerebral perfusion pressure (CPP) were managed to target (< 20 mm Hg and >60 mm Hg, respectively). Mean arterial pressure was maintained at least at >90 mm Hg. Two patients were enrolled in a trial evaluating moderate hypothermia in TBI, and five patients were involved in the Citicoline Brain Injury Treatment (COBRIT) study. The details of these trials are as described previously.53
Study variable description
The clinical and demographic characteristics of the individuals with E1 data (n = 96) were assessed in relation to survival status and day 0–3 E1 levels. Demographic variables (age, sex, race, body mass index [BMI]) were recorded from admission hospital records. We obtained best in 24 h GCS, given that it more reliably predicts cognitive outcome than does admission GCS.54,55 Additional clinical variables included Injury Severity Score (ISS), non-head ISS, length of hospital stay (LOS), mechanism of injury (MOI), total hospital complications (e.g., pulmonary, cardiac), and injury type on head CT. The vast majority of subjects (or proxy respondents) could not provide accurate information regarding hormone supplementation/replacement at the time of injury.
The GCS is a measure of the degree of impaired consciousness in patients, with lower scores representing more severe deficits.56 The ISS combines scores from the most severe injuries in distinct anatomical locations, resulting in a positive association with injury severity.57 We additionally calculated a non-head ISS, excluding brain injuries, using the same method to represent extracerebral trauma severity. Injury types on head CT included subdural hemorrhage (SDH), subarachnoid hemorrhage (SAH), diffuse axonal injury (DAI), epidural hemorrhage (EDH), contusion, intraventricular hemorrhage (IVH), intracerebral hemorrhage (ICH), and other injury not classified elsewhere. CT injury types associated with mortality in bivariate analysis (p < 0.2) were incorporated into a neurological burden score (NBS). NBS was calculated by summing the total number of these injuries observed on CT in each patient. Presence of SDH, SAH, and contusion were summed to calculate the NBS. Presence of sepsis, splenic injury, and other abdominal injury was also obtained using records on International Statistical Classification of Disease Injury codes version 9 (ICD-9). Presence of sepsis, splenic injury, and other abdominal injury were summed to generate a score (SSA) to assess concomitant extracerebral inflammatory burden. We measured mortality at 1 month given that the majority of deaths after TBI occurred in the 1st month in our cohort and a previously reported mean acute hospital stay of 3–5 weeks following severe TBI.58–60
Serum sample collection and measurements
Blood samples were collected at ∼7:00 a.m. daily during days 0–3 post-injury. Samples were centrifuged, aliquoted, and stored at −80°C until used for assay. Samples from control subjects were drawn at 7:00 am, processed, and stored as described. Serum samples were measured for E2 (n = 272 samples), T (n = 272 samples), and progesterone (n = 281 samples), using radioimmunoassays via the COAT-A-COUNT® In-vitro Diagnostic Test Kit (#TKE2, #TKTT, and #TKPG, respectively; Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Assay sensitivities were as follows: E2, 8 pg/mL; T, 4 ng/mL; and progesterone, 0.03 ng/mL. Serum samples (n = 212) were measured for Andro using competitive enzyme-linked immunosorbent assay (ELISA) kits (sensitivity 0.021 ng/mL; #EIA-3265; DRG International, Springfield, NJ). Also, serum samples (n = 202) were measured for E1 concentrations using competitive ELISA kits (sensitivity 2.21 pg/mL; #EIA-4174; DRG International, Springfield, NJ).
Given the known effects of cortisol and inflammatory cytokines as aromatase gene transcription factors within the steroidogenesis pathway,35,36,51 we assessed cortisol, TNF-α, and IL-6 levels measured in this population. Serum samples were measured for cortisol (n = 281 samples) using RIA via the COAT-A-COUNT® In-vitro Diagnostic Test Kit (sensitivity 20 ng/mL; #TKCO; Siemens Healthcare Diagnostics Inc., Tarrytown, NY). Serum samples (n = 201) were measured for TNF-α, and IL-6 concentrations using a Luminex™ bead array assay (#HSCYTO-60SK; Millipore, Billerica, MA). Sensitivities for the cytokines were as follows: TNF-α, 0.05 pg/mL; and IL-6, 0.1 pg/mL.
Statistical analysis
Statistical analyses were completed using SPSS (v23.0; IBM, Armonk, NY) and SAS (v9.4; SAS Institute, Cary, NC). Summary statistics of means, standard error of means (SE), medians, and interquartile ranges (IQR) were calculated for continuous variables. Data were assessed for normality using the Shapiro–Wilk test, and nonparametric tests (Mann–Whitney's test, Kruskal–Wallis's test, and Spearman's rank correlation) were used as appropriate to compare variables when data did not meet normality assumptions. Categorical variables were summarized with frequencies and percentages. Associations between categorical variables were determined using the χ2 test, or the Fisher's exact test as appropriate.
For all individuals with TBI and serum E1, averages were calculated. Restricted cubic spline (RCS) analysis was conducted to assess departures from linearity in the effect of E1 on mortality over the range of E1 levels in the study population. The RCS procedure uses piecewise functions of low-order polynomials over specified intervals, known as knots, to discern points of departure from linearity.61 After inspecting these data, we applied tertile cut points for E1 to discriminate the dose-response relationship between E1 and mortality. Subjects were accordingly assigned to E1 low, medium, and high groups based on having E1 levels in the 0th–25th, 25th–75th, and 75th–100th percentiles.
Binary logistic multivariate regression was performed to adjust for the effects of demographic and injury variables in assessing the association between E1 tertile and 1 month mortality. Covariates were tested in the model if they demonstrated a trend with mortality (p < 0.2). To assess potential variation between hormone values by sex, interaction terms were generated between sex and E1 level.
Day 0–3 averages for individuals with Andro, T, E2, and E1 were calculated for 92 patients. Mean hormone levels in relation to mortality status were assessed as well as average Andro, T, and E2 levels for each E1 tertile. In order to assess relationships between serum steroids in the aromatization pathway via causal inferences, a structural equations model (SEM) was fitted using the covariance analysis of linear structural equations (CALIS) procedure in SAS. The data for the four serum hormones were first z-score standardized to have a mean of 0 and a standard deviation of 1 to adjust for inherent differences between hormone marker concentrations. An SEM was run among all individuals, and a post-hoc exploratory analysis was conducted among survivors and non-survivors respectively. Standardized β coefficients and standard errors were reported for each path, and significant paths (p < 0.05) were indicated.
Results
Characteristics of cohort
Demographic and clinical characteristics by survival status of the 96 enrolled subjects with data on days 0–3 are described in Table 1. Mean age of the cohort was 37.3 ± 1.7 years, and mean BMI was 25.8 ± 0.5. The majority of the cohort were men (83.3%) and white (92.7%). The primary mechanism of injury was motor vehicle collision (50%) followed by motorcycle accidents (18.8%) and falls (16.7%). Mean LOS was 19.8 ± 1.3 days, and median total number of hospital complications was 1 (IQR 1–2).
Table 1.
Population Characteristics by Survivorship at 1 Month
| Total (n = 96) | Survivors (n = 67) | Non-survivors (n = 29) | p value | |
|---|---|---|---|---|
| Age, mean (SE) | 37.3 (1.7) | 33.6 (1.9) | 45.9 (3.2) | <0.001a |
| Sex, men (%) | 80 (83.3) | 58 (86.6) | 22 (75.9) | 0.196 |
| Race, n (%) | 0.794 | |||
| Black | 6 (6.3) | 4 (6.0) | 2 (6.9) | |
| White | 89 (92.7) | 62 (92.5) | 27 (93.1) | |
| Other | 1 (1.0) | 1 (1.5) | 0 (0.0) | |
| BMI, Mean (SE) | 25.8 (0.5) | 25.6 (0.6) | 26.4 (1.1) | 0.480 |
| GCS (best in 24 h), median (IQR) | 6 (5–7) | 7 (5–8) | 6 (4.5–7) | 0.187 |
| ISS, Mean (SE) | 33.9 (1.0) | 32.9 (1.2) | 36.3 (1.8) | 0.230 |
| Non-head ISS, mean (SE) | 11.7 (1.0) | 11.2 (1.2) | 12.9 (2.0) | 0.600 |
| Hospital length of stay, mean (SE) | 19.8 (1.3) | 24.2 (1.5) | 9.8 (1.4) | <0.001a |
| Mechanism of injury, n (%) | b | |||
| Motor vehicle accident | 48 (50.0) | 36 (53.7) | 12 (41.4) | |
| Motorcycle | 18 (18.8) | 14 (20.9) | 4 (13.8) | |
| Falls | 16 (16.7) | 6 (9.0) | 10 (34.5) | |
| Assault | 5 (5.2) | 4 (6.0) | 1 (3.4) | |
| Other | 6 (6.3) | 5 (7.5) | 1 (3.4) | |
| Unknown | 3 (3.1) | 2 (3.0) | 1 (3.4) | |
| Injury type, n (%) | ||||
| SDH | 61 (63.5) | 39 (58.2) | 22 (75.9) | 0.099c |
| SAH | 71 (74.0) | 45 (67.2) | 26 (89.7) | 0.021a |
| DAI | 33 (34.4) | 27 (40.3) | 6 (20.7) | 0.063c |
| EDH | 17 (17.7) | 12 (17.9) | 5 (17.2) | 0.937 |
| Contusion | 44 (45.8) | 25 (37.3) | 19 (65.5) | 0.011a |
| IVH | 31 (32.3) | 26 (38.8) | 5 (17.2) | 0.038a |
| ICH | 33 (34.4) | 24 (35.8) | 9 (31.0) | 0.650 |
| Other | 4 (4.2) | 3 (4.5) | 1 (3.4) | 1.000 |
| NBS, median (IQR) | 2 (1–3) | 2 (1–2) | 2 (2–3) | <0.001a |
| Total hospital complications, median (IQR) | 1 (1–2) | 1 (1–2) | 1 (0–3) | 0.941 |
| Abdominal injury, n (%) | 19 (19.8) | 13 (19.4) | 6 (20.7) | 0.884 |
| Splenic laceration, n (%) | 12 (12.5) | 9 (13.4) | 3 (10.3) | 1.000 |
| Sepsis, n (%) | 18 (18.8) | 14 (20.9) | 4 (13.8) | 0.413 |
| SSA, median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.541 |
Comparisons that are statistically significant (p < 0.05).
Insufficient data in each category to determine statistically significant comparisons.
Comparisons that demonstrate a trend (p < 0.10).
BMI, body mass index; GCS, Glasgow Coma Scale; IQR, interquartile range; ISS, Injury Severity Score; SDH, subdural hemorrhage; SAH, subarachnoid hemorrhage; DAI, diffuse axonal injury; EDH, epidural hemorrhage; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; NBS, neurological burden score; SSA, sepsis, splenic injury, and other abdominal injury score.
In our cohort, 29 subjects (30.2%) did not survive past 1 month. Non-survivors were on average 12.3 years older (mean age 45.9, p < 0.001) and had a shorter LOS by 14.4 days (mean 9.8, p < 0.001). Presence of SDH, SAH, and contusion were summed to calculate the NBS. Of injury types on CT, SAH and contusions were more frequent among non-survivors (89.7%, p = 0.021 and 65.5%, p = 0.011, respectively). There also was a trend for SDH to occur more frequently among non-survivors as well (p = 0.099). Sex, race, BMI, best in 24 h GCS, ISS, and non-head ISS were not associated with 1 month mortality status. In addition, sepsis, splenic laceration, abdominal injury, and SSA score were not associated with mortality.
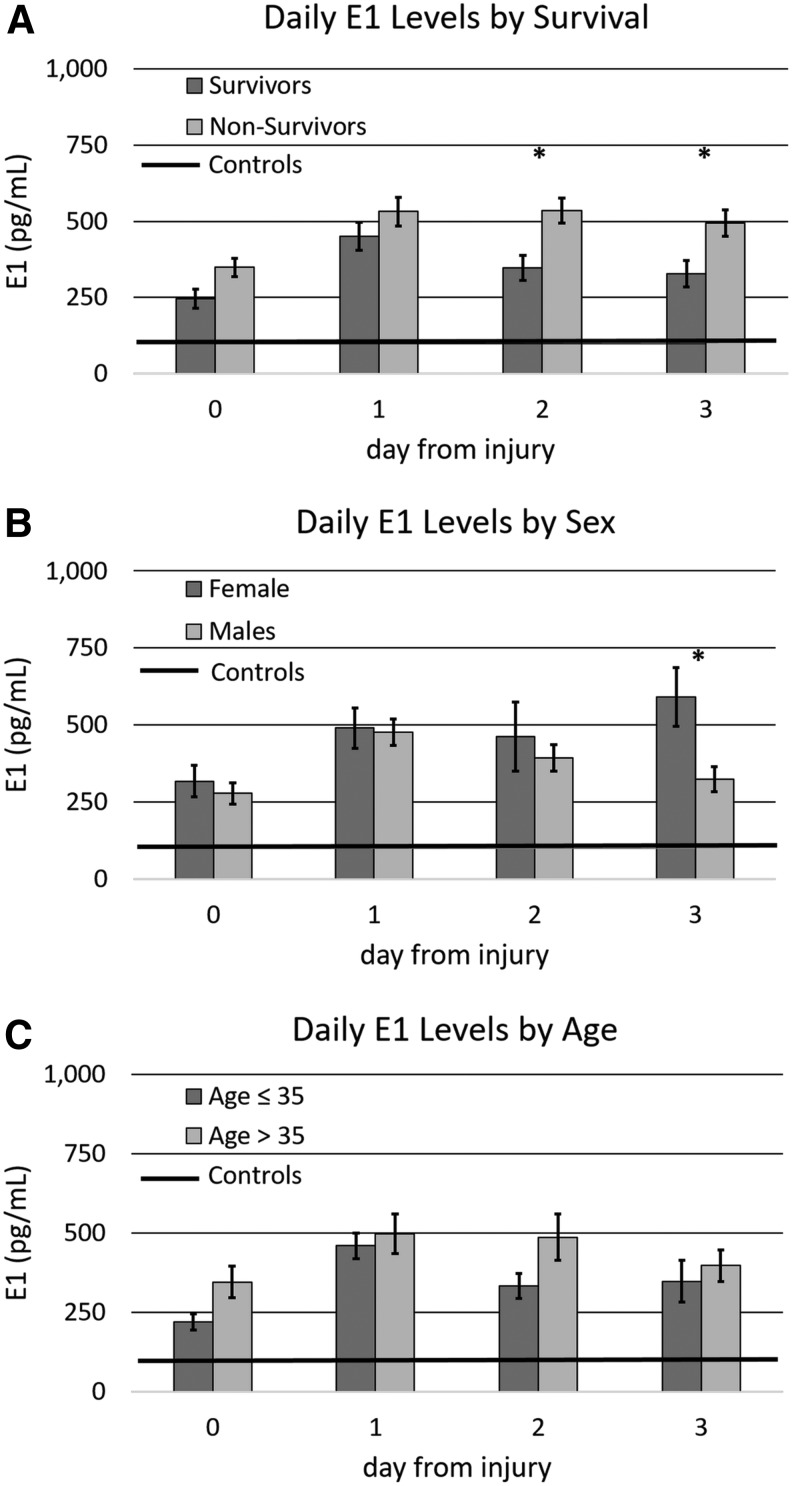
Daily E1 levels by mortality status, sex, and age
Average daily E1 levels (for days 0–3) were compared between subjects surviving at least 1 month and those who did not survive 1 month following injury (Fig. 3A). E1 was significantly elevated compared to control values for both survivors and non-survivors (p < 0.02 all comparisons). E1 was elevated on days 2 and 3 in non-survivors compared with survivors (p < 0.05 in both comparisons). Daily E1 levels were also examined by sex (Fig. 3B). E1 also was elevated in both men and women with TBI compared with controls (p < 0.02 both comparisons). Whereas values for women rose over time, values for men decreased after day 1 such that pairwise analysis showed that E1 levels were significantly higher by day 3 in women compared to in men (p < 0.02). Daily E1 levels were also examined by age, comparing subjects ≤35 to those >35 years of age (Fig.3C). Age 35 was chosen as the cutoff to reflect the median age of our cohort, and E1 levels were higher in TBI subjects than in controls regardless of age (p < 0.05 all comparisons). However, no significant differences in daily E1 levels by age were noted (p > 0.05 all comparisons).
FIG. 3.
Daily serum estrone (E1) levels after injury by mortality at 1 month, sex, and age with comparison to control levels. (A) E1 levels by survival. (B) E1 levels by sex. (C) E1 levels by age. Means with ±1 SE error bars are shown. *Days with significant differences in E1 by mortality, sex, or age.
Associations of demographic variables, clinical characteristics, and mortality with E1
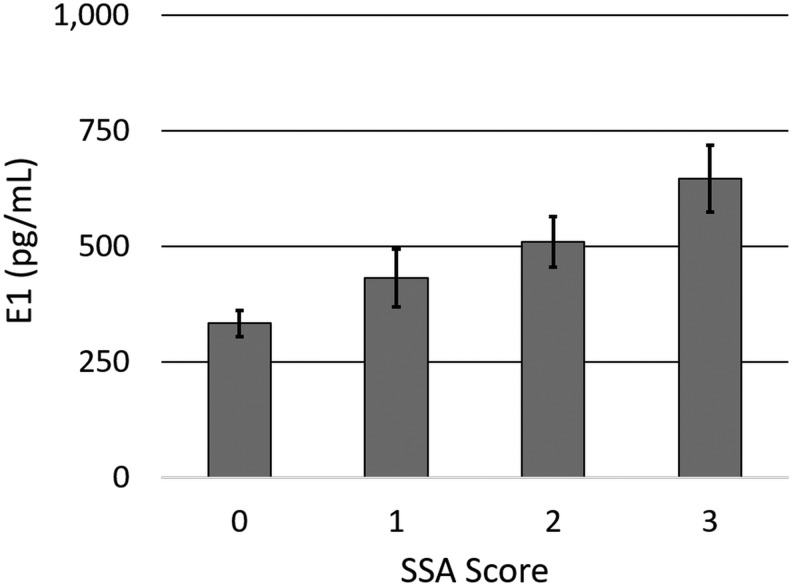
The cohort was broken down into tertiles based on cubic spline analysis (E1 low [E1-L], middle [E1-M], and high [E1-H] groups) to represent day 0–3 average levels in the 0th–25th, 25th–75th, and 75th–100th percentiles by using E1 cut points 200.9 pg/mL and 558.0 pg/mL. Clinical and demographic characteristics by E1 tertile are shown in Table 2. There was a trend for GCS to be lower (indicating more severe neurological injuries) among patients in higher E1 tertiles (p = 0.094). Individuals in higher E1 tertiles also had higher rates of abdominal injury (E1-H 37.5%, E1-M 18.8%, El-L 4.2%; p = 0.015). Although presence of sepsis was significantly associated with the E1 tertile (p = 0.025), there was a U pattern such that higher rates of sepsis were observed in the E1-H (33.5%) and E1-L (25%) groups compared to the E1-M (8.3%) group. Individuals in higher E1 tertiles also tended to have higher rates of splenic lacerations (p = 0.084). Overall, the SSA score increased as E1 tertile increased (p = 0.012) (see Fig. 4). Mortality frequency at 1 month was significantly associated with E1 tertile (p = 0.018), with rates of 50.0% in E1-H, 29.2% in E1-M, and 12.5% in E1-L. Age, sex, race, BMI, ISS, non-head ISS, and injury types on CT were not associated with 1 month mortality status.
Table 2.
Characteristics of TBI Cohort by E1 Tertiles (Low, Medium, High)
| E1-L (n = 24) | E1-M (n = 48) | E1-H (n = 24) | p value | |
|---|---|---|---|---|
| Age, mean (SE) | 35.1 (3.1) | 36.5 (2.4) | 41.0 (3.7) | 0.505 |
| Sex, men (%) | 21 (87.5) | 42 (87.5) | 17 (70.8) | 0.165 |
| Race, n (%) | 0.367 | |||
| Black | 1 (4.2) | 5 (10.4) | 0 (0.0) | |
| White | 23 (95.8) | 42 (87.5) | 24 (100.0) | |
| Other | 0 (0.0) | 1 (2.1) | 0 (0.0) | |
| BMI, mean (SE) | 25.7 (1.2) | 25.5 (0.7) | 26.7 (1.3) | 0.906 |
| GCS (best in 24 h), median (IQR) | 7 (6–8) | 7 (5–7) | 6 (3.5–7) | 0.094a |
| ISS, mean (SE) | 32.1 (1.9) | 33.1 (1.5) | 37.5 (2.1) | 0.218 |
| Non-head ISS, mean (SE) | 10.5 (1.5) | 10.9 (1.5) | 14.6 (2.2) | 0.269 |
| Hospital length of stay, mean (SE) | 24.0 (2.5) | 19.2 (1.7) | 16.9 (3.1) | 0.068a |
| Mechanism of injury, n (%) | b | |||
| Motor vehicle accident | 12 (50.5) | 23 (47.9) | 13 (54.2) | |
| Motorcycle | 4 (16.7) | 10 (20.8) | 4 (16.7) | |
| Falls | 3 (12.5) | 8 (16.7) | 5 (20.8) | |
| Assault | 0 (0.0) | 4 (8.3) | 1 (4.2) | |
| Other | 3 (12.5) | 2 (4.2) | 1 (4.2) | |
| Unknown | 2 (8.3) | 1 (2.1) | 0 (0.0) | |
| Injury type, n (%) | ||||
| SDH | 15 (62.5) | 33 (68.8) | 13 (54.2) | 0.476 |
| SAH | 16 (66.7) | 34 (70.8) | 21 (87.5) | 0.203 |
| DAI | 9 (37.5) | 18 (37.5) | 6 (25.0) | 0.524 |
| EDH | 3 (12.5) | 10 (20.8) | 4 (16.7) | 0.675 |
| Contusion | 11 (45.8) | 20 (41.7) | 13 (54.2) | 0.604 |
| IVH | 9 (37.5) | 15 (31.3) | 7 (29.2) | 0.807 |
| ICH | 10 (41.7) | 18 (37.5) | 5 (20.8) | 0.256 |
| NBS | 2 (1–2) | 2 (1–3) | 2 (1.25–2.75) | 0.620 |
| Total hospital complications, median (IQR) | 1 (1–2) | 1 (0–2) | 2 (0–3) | 0.444 |
| Abdominal injury, n (%) | 1 (4.2) | 9 (18.8) | 9 (37.5) | 0.015c |
| Splenic Laceration, n (%) | 1 (4.2) | 5 (10.4) | 6 (25.0) | 0.084a |
| Sepsis, n (%) | 6 (25.0) | 4 (8.3) | 8 (33.3) | 0.025c |
| SSA, median (IQR) | 0 (0–1) | 0 (0–1) | 2 (1–2.5) | 0.012c |
| Mortality, n (%) | 3 (12.5) | 14 (29.2) | 12 (50.0) | 0.018c |
Comparisons that demonstrate a trend (p < 0.10).
Insufficient data in each category to determine statistically significant comparisons.
Comparisons that are statistically significant (p < 0.05).
TBI, traumatic brain injury; E1, estrone; BMI, body mass index; GCS, Glasgow Coma Scale; IQR, interquartile range; ISS, Injury Severity Score; SDH, subdural hemorrhage; SAH, subarachnoid hemorrhage; DAI, diffuse axonal injury; EDH, epidural hemorrhage; IVH, intraventricular hemorrhage; ICH, intracerebral hemorrhage; NBS, neurological burden score; SSA, sepsis, splenic injury, and other abdominal injury score.
FIG. 4.
Estrone (E1) levels by SSA score. E1 significantly increases with SSA score (p = 0.012). Means with ±1 SE error bars are shown.
Multivariate regression modeling: E1 association with mortality
E1 tertiles were assessed as a 1 month mortality predictor (Table 3). In addition to age, additional demographic and injury characteristics associated with mortality (p < 0.2) were explored in the regression model. An interaction between sex and E1 tertile was explored, but did not significantly contribute to this model. The final model showed that when adjusting for the covariates of age, sex, best in 24 h GCS, and NBS, individuals in the E1-H group, but not the E1-M group, had increased odds for mortality compared with the E1-L group (adjusted OR = 5.656, 95% CI = 1.102–29.045, p = 0.038).
Table 3.
Multivariate Odds Ratio Estimates for 1 Month Mortality
| Independent variable | Odds ratio | 95% CI | p value | |
|---|---|---|---|---|
| Age | 1.044 | 1.012 | 1.077 | 0.007* |
| Sex – men | 0.586 | 0.159 | 2.158 | 0.421 |
| GCS (best in 24 h) | 0.919 | 0.735 | 1.150 | 0.461 |
| NBS | 2.720 | 1.354 | 5.464 | 0.005* |
| E1-Ma | 2.753 | 0.623 | 12.165 | 0.182 |
| E1-Ha | 5.656 | 1.102 | 29.045 | 0.038* |
E1-L was the reference category.
Comparisons that are statistically significant (p < 0.05).
GCS, Glasgow Coma Scale; NBS, neurological burden score; E1, estrone; M, middle; H, high; L, low.
T-dependent and T-independent sex hormone steroidogenesis
Mean levels of E2, Andro, and T over the first 72 h post-TBI were assessed by E1 tertile (Table 4). E2 and Andro were positively associated with E1 tertile (p < 0.001 and p = 0.001, respectively). Average E1, E2, Andro, and T levels are provided in relation to mortality for each hormone and are displayed in Table 5. Non-survivors, compared with survivors, had elevated levels of E1, E2, Andro, and T (p < 0.05, all comparisons).
Table 4.
Hormones and Cytokines by E1 Tertiles
| n | E1-L | E1-M | E1-H | p-value | |
|---|---|---|---|---|---|
| TNF-α, pg/mL (SE) | 80 | 9.4 (0.9) | 16.3 (5.2) | 13.8 (1.6) | 0.051a |
| IL-6, pg/mL (SE) | 80 | 252.4 (52.6) | 249.4 (62.2) | 618.5 (210.5) | 0.033b |
| Progesterone, ng/mL (SE) | 95 | 1.9 (0.4) | 2.4 (0.4) | 4.6 (1.1) | 0.003b |
| Cortisol, ng/mL (SE) | 95 | 191.2 (12.0) | 209.5 (10.8) | 296.5 (26.2) | 0.006b |
| E2, pg/mL (SE) | 92 | 36.1 (3.6) | 73.6 (6.3) | 227.0 (45.0) | <0.001b |
| Andro, ng/mL (SE) | 92 | 1.3 (0.2) | 2.2 (0.2) | 2.6 (0.4) | 0.001b |
| T, ng/mL (SE) | 92 | 1.0 (0.2) | 1.3 (0.2) | 1.3 (0.2) | 0.329 |
Comparisons that demonstrate a trend (p < 0.10).
Comparisons that are statistically significant (p < 0.05).
E1, estrone; TNF, tumor necrosis factor; IL, interleukin; E2, estradiol; Andro, androstenedione; T, testosterone.
Table 5.
Serum E1, E2, Andro, and T Average Day 0–3 Levels by 1 Month Mortality
| Total (n = 92) | Survivors (n = 64) | Non-survivors (n = 28) | p value | |
|---|---|---|---|---|
| E1, pg/mL (SE) | 384.3 (25.7) | 337.7 (27.6) | 490.8 (51.1) | 0.008a |
| E2, pg/mL (SE) | 104.6 (14.4) | 85.8 (16.8) | 147.7 (26.3) | 0.001a |
| Andro, ng/mL (SE) | 2.1 (0.2) | 1.7 (0.1) | 2.9 (0.4) | 0.003a |
| T, ng/mL (SE) | 1.3 (0.1) | 1.1 (0.1) | 1.6 (0.3) | 0.039a |
Significant differences by mortality status at 1 month (p < 0.05).
E1, estrone; E2, estradiol; Andro, androstenedione; T, testosterone.
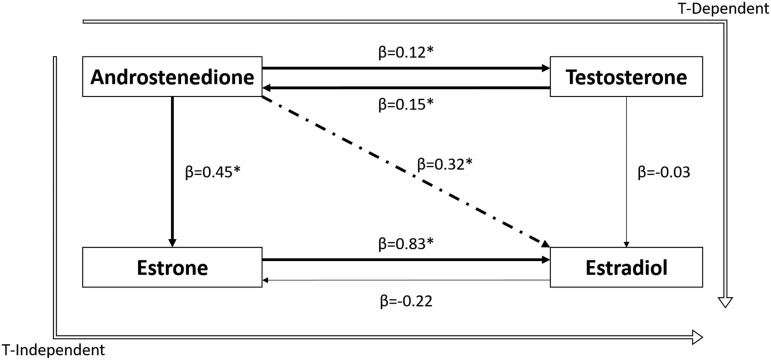
The SEM exploring causal relationships between serum hormones in the aromatization pathway (n = 92) is provided in Figure 5. The strongest association in the pathway was the relationship from E1 to E2 (β = 0.83, SE = 0.18, p < 0.001). Other relationships in the pathway that were significant included (Andro to E1 [β = 0.45, SE = 0.20, p = 0.02], Andro to T [β = 0.12, SE = 0.05, p = 0.02] and the reverse: T to Andro [β = 0.15, SE = 0.05, p = 0.005]). T was not a significant contributor to E2, potentially because of its significant reverse pathway relationships to Andro. Moreover, the indirect pathway from Andro to E2, which reflects overall E2 production and considers all relationships in the aromatization pathway, was significant (β = 0.32, SE = 0.10, p < 0.001). Interestingly, post-hoc exploratory SEM analysis (not shown) demonstrated that among survivors, the indirect pathway representing all pathways of E2 production was not significant (β = 0.13, SE = 0.12, p = 0.278). In non-survivors, the indirect pathway from Andro to E2 was significant (β = 0.43, SE = 0.16, p = 0.007), suggesting that larger injury-induced increases in what is primarily E1-mediated E2 production occurs in the setting of mortality.
FIG. 5.
Sex hormone structural equations model (SEM) in all subjects (n = 92), Androstenedione (Andro)/Estrone (E1), E1/Estradiol (E2), and forward and reverse pathways for testosterone (T)/Andro were significant. The indirect Andro to E2 path was significant as well. Weighted lines represent significant associations in the pathway (p < 0.05). The dotted line represents the indirect Andro to E2 path. Unfilled lines represent the T-independent and T-dependent pathways of E2 production from Andro. *Significant paths (p < 0.05).
Steroidogenesis pathway modifier hormones and cytokines: Associations with E1
Average TNF-α, IL-6, progesterone, and cortisol by E1 tertile are provided in Table 4. Progesterone levels increased as E1 tertile increased (p = 0.003), suggesting that high progesterone levels may facilitate or enhance steroidogenesis. The aromatase gene transcription factor and serum mortality marker, TNF-α,62 tended to be positively associated with E1 tertile (p = 0.056). Mean IL-6 and cortisol serum levels significantly increased with E1 tertiles (p = 0.033 and p = 0.006, respectively), suggesting the possibility of elevated aromatase gene transcription among those with higher E1 tertiles.
Discussion
Understanding injury-induced alterations in gonadal and extragonadal steroidogenesis is important in order to understand their role in the systemic response to TBI and in sex hormone associations with TBI outcome. This study is the first to characterize serum E1 levels in a clinical population with severe TBI and to show how these levels relate to other hormones in the steroidogenesis pathway, to relevant demographic and clinical variables, and to mortality status. Notable comparisons with demographic variables include no significant association of E1 with either age or BMI. However, our data do show that TBI results in elevated serum E1 levels compared with control subjects. Additionally, progesterone levels were positively associated with E1 tertiles, suggesting that progesterone levels facilitate E1 production. In a multivariate model controlling for age, sex, best in 24 h GCS, and NBS, the highest tertile of E1 was associated with an increased odds of mortality by 1 month. We also assessed the aromatization pathway and inter-relationships among serum Andro, T, E1, and E2 in the acute period after TBI, including both T-dependent and T-independent pathways for E2 production. It is of note that all four of these hormones were elevated on days 0–3 post-injury in non-survivors compared with survivors, possibly indicating increased sex hormone steroidogenesis in more severe states of systemic stress. These findings are consistent with our prior studies in this cohort, demonstrating worse outcomes in patients with acute elevations in T and E2.11
There are limited studies investigating the link among E1, brain injury pathology, and outcomes. One study shows that pharmacological E1 administration to murine cortical neurons subjected to oxidative stress and excitotoxicity significantly reduces neuronal injury.46,47 Similar to the neuroprotective effects observed with E2 and progesterone administration using in vivo models of experimental TBI, E1 therapy also was shown to reduce apoptotic cell death and ischemia following experimental TBI.45 However, the clinical relationship between E1 and TBI observed in our study parallels the relationship between E1 and insult severity as well as 3 month mortality clinically after aneurysmal SAH.27 Also, our findings with E1 and mortality are consistent with our previous report showing that higher E2 levels are associated with 6 month mortality and poor global outcome.11 Together with the current report, the data suggest that endogenous systemic estrogen associations represent unique systemic responses to injury that are independent of the neuroprotective sex hormone (progesterone/E2) effects observed with pharmacological dosing in animal models of isolated CNS injury, in which extracerebral trauma, systemic response to injury, and complications including sepsis and critical illness are not a part of model preparation.23,48 It is of note that we have previously found that individuals with higher E2 to T ratios in cerebrospinal fluid (CSF) following TBI have better global outcome, suggesting that traditional neuroprotective benefits of E2 production and/or T consumption are observed in the CNS.53 Similarly, future CSF analysis of E1 relationships to outcome after TBI is warranted.
Mechanistic links underlying E1 associations with poor outcomes following TBI have not yet been described, but may have to do with the non-neurological consequences of TBI such as hypotension and sepsis,4,50,63,64 which are commonly associated with mortality among critically ill individuals. Studies have identified significant associations between elevated E1 with septic shock and vasodilation.23,48 Given our previous reports of HPA activation after TBI,11 the innate systemic inflammatory response that occurs after TBI,49 and the prevalence of sepsis and other major infections,4 it may be that the elevated inflammatory mediators that accompany these responses such as TNF-α,65 cortisol,66 and IL-667 also amplify aromatase activity in peripheral adipose tissues, where the literature suggests that there is a preferential increase in Andro aromatization to E1.30,33,34
Increased TNF-α in adipose tissue is associated with upregulation of promoter I.4 responsible for the expression of the aromatase gene.51,68,69 Upstream from this promoter, exists a glucocorticoid response element necessary for gene expression.35 Further upstream is a γ interferon-activating sequence that responds to activation of the Jak tyrosine kinase and signal transducer and activator of transcription (STAT) factor pathway.36 Studies show that IL-6 can activate the Jak/STAT pathway to increase aromatase expression in adipose tissue, provided that soluble IL-6 receptor is available.36 The cytokine and hormonal milieu following both septic and non-septic inflammation following TBI may therefore contribute to increased aromatase activity and consequently elevated E1 production. Indeed, our data show that mean levels of TNF-α, cortisol, and IL-6 are associated with higher E1 tertiles. Therefore, E1 may be a common marker of poor outcomes in both TBI and sepsis, in part because of its association with more severe inflammation.
It is not surprising that E1 levels in our cohort do not reflect the established norms (that postmenopausal aging women show an increase in E170,71 and aging men reflect the opposite trend72,73) given the hormonal dysregulation observed as a consequence of TBI.11 The lack of positive association between E1 and age in our cohort is interesting in the context of our prior work demonstrating elevated acute levels of other sex hormones (progesterone and E2) in older patients following TBI,11 but may be the result (in part) of differences in data presentation in each report (e.g., mean value vs. trajectory group membership). However, our previous work in this same published report shows no age differences in T, which like E1, is an immediate precursor for E2.11 When taken together, evidence from the previous and this current report show that neither immediate precursor for E2 (i.e., E1 and T) were affected by age. Regardless of age, previous work suggests that aromatization in extragonadal tissues is a significant source of estrogen production. As such, typical age effects on neuroendocrine production are less likely to influence the acute hormone profiles that we have reported in this cohort.
Multiple studies have linked elevated E1 levels to obesity because of the increased aromatization occurring in adipose tissue.70,71,74 Given that acute elevations in progesterone are a consequence of TBI11 and that progesterone can be utilized by adipose tissue for Andro and, subsequently, E1 production,75 it is somewhat surprising increased BMI is not associated with increased E1 production following injury. However, BMI is neither the most accurate marker for obesity nor does it accurately predict physiological consequences of increased adiposity,76–78 factors which may explain this lack of association. Future studies may benefit from examining more sensitive measures of adiposity, such as waist circumference.78
Interestingly, our data show that although not associated with 1 month mortality, sepsis was associated with higher E1 levels after TBI. This finding may support the idea that sepsis burden may be a unique contributor to systemic injury-induced steroidogenesis apart from the innate inflammatory response associated with severe TBI in general. We observed a similar positive association between rates of abdominal injury and E1. Given that abdominal trauma can induce inflammatory responses that includes IL-6 cytokine production,79,80 its association with E1 may be a consequence of increased inflammatory cytokine induced aromatization. Further, those with splenic laceration tended to have higher E1 production, which is interesting, given that early splenectomy has been shown in one report to reduce TBI-associated mortality and improve cognitive performance in an animal model of severe TBI.81 Taken together, the SSA score, representing the sum of the presence of sepsis, splenic laceration, and other abdominal injury, demonstrated significant positive association with E1 levels and E1 tertiles in our population, further reinforcing that extracerebral inflammatory burden may contribute to elevated E1 levels.
Serum E1 and E2 associations with mortality may be as much a consequence of a more severe clinical disease state as it is a direct result of E1 and E2 effects on systemic physiology. Reports suggest that 40% of single-cause acute mortality associated with TBI results from the systemic response to TBI and/or concurrent extracerebral trauma known as NNOD, and that 85% of mortality is in part caused by non-neurological conditions/complications.5 The case for estrogen-driven systemic injury and NNOD in response to TBI and its associated injury complex is plausible. Experimental studies demonstrate direct actions of E2 in sepsis and vasodilation, which are potential non-neurologic complications of TBI. In addition to E1 vasodilatory effects via Ca2+ channel receptors and NOS activation,82,83 E2 induces vasodilation through both nitric oxide- dependent and hydrogen sulfide-dependent pathways.84–87 A significant portion of TBI mortality is related to vascular system compromise that leads to NNOD complications, including arrhythmias, myocardial ischemia, neurogenic pulmonary edema, congestive heart failure, and acute kidney injury,4–6 much of which can arise from sympathetic nervous system (SNS) overactivity and dysfunction.9,88,89 SNS-innervated peripheral lymphoid tissue can perpetuate the inflammatory response, including ongoing IL-6 and TNFα production,8,9 which fuels E1 production from Andro. As the metabolic product of E1, E2 production can have direct effects on SNS-mediated inflammation by blocking adrenergic reuptake,9,90 potentiating the sympathetic response to injury, and indirect effects through TNFα that have potent vasodilator effects on systemic vasculature.91,92 Together, estrogen effects on inflammation and vasodilation are viable mechanisms by which elevated systemic estrogen production may contribute to NNOS and worsen TBI outcomes.
Structural equations modeling, a causal inference framework, was used to derive estimates regarding the magnitude and direction of the causal relationships among Andro, T, E1, and E2. The proposed model, with the T-dependent and T-independent paths, is highly grounded in known hormone biology. The structural model displays the interrelationships among a set of observed hormone values as a succession of structural models, which is parallel to running a series of regression equations.93 The effects estimated from the equation represent causal relationships in instances for which the key assumptions of causality are met, which include temporality, normality, linearity, no unmeasured confounding, or common effects. In all patients, independent of survivorship, A→E1, E1→ E2, T→Andro, Andro→T, and the indirect A→E2 were significant pathways. Both forward and reverse pathways in the Andro/T interconversion had similar effect size, indicating relative equilibrium between the oxidative and reductive pathways between these hormones. Further, overall, T-independent aromatization to E2 via Andro→E1 and E1→E2 was significant, as was Andro→E2, which is a measure of “all-cause aromatization.” That all-cause aromatization to E2 was associated with mortality is consistent with prior studies in major illness indicating increased estrogen production through androgen consumption is associated with worse outcomes.20,23,24,31 The significant conversion of E1 to E2 in non-survivors compared with survivors may suggest a change in the enzyme activity responsible for their interconversion, 17β-HSD. Little is known regarding 17β-HSD function in relation to trauma, inflammation, or sepsis. Isoforms of 17β-HSD have preferential activity toward the oxidative or reductive pathway.94 Thus, these enzymes do not drive steroid flux in one direction, but instead, support functional equilibria among intact cells, reflecting thermodynamically driven steroid distributions.28,94 One potential interpretation of the relationships described is that injury is associated with an equilibrative shift away from T-dependent aromatization, and mortality is associated with a stronger equilibrative shift of this enzyme toward oxidative pathways that facilitate T-independent estrogen (E1 and E2) production.
These data on systemic aromatization associations with mortality may help explain clinical trial failures examining progesterone therapy following TBI.95,96 It is of note that previous explanations for these failures have been proposed and include: inappropriate classification of TBI with oversimplified unidimensional scoring systems, non-optimized treatment dosing and duration, and heterogeneity of patient population and injury characteristics.97 However, we propose that physiological mechanisms may exist for progesterone's clinical failure. Indeed, prior characterization of endogenous sex hormone steroidogenesis following TBI demonstrates that progesterone has a significant negative impact on mortality through its effect on E2.11 We demonstrate that systemic production of E2 from Andro in all patients is elevated following TBI, and that this occurs predominantly through the T-independent pathway. Exogenous progesterone administration could amplify T-independent E2 production and increase systemic E2 and T levels, both of which are associated with poor outcomes following brain injury.11,39 Although rodent models of TBI do not recapitulate the extracerebral trauma complex, the critical illness, and the period of coma observed clinically after severe TBI, recent studies in a rodent weight drop model do show elevated plasma progesterone levels for males and an transient increase in E2 among female rats acutely after injury,98,99 findings that are consistent with clinical literature and suggestive that peripheral steroidogenesis in acute response to TBI may be a mechanistic consideration for future study in animal models of TBI that include other concomitant injury as a part of the model. We hypothesize that given progesterone's success in isolated experimental TBI studies, patients with TBI who have a less-pronounced systemic amplification of the aromatization pathway may be more likely to benefit from progesterone as a neuro-therapeutic. Further examination of sex hormone steroidogenesis in patients from prior clinical trials utilizing progesterone in TBI would be useful in exploring this hypothesis.
Study limitations include the lack of generalizability of results. Given known variation in predilection for E1 production in aging men and postmenopausal women,71–73 E1 patterns following TBI may represent different magnitudes of association with outcome based on demographic characteristics. Although this was not demonstrated in our study, subpopulations based on these characteristics were too small to draw definitive conclusions from. However, recent trends suggest increased age, and associated comorbidity with TBI, which may influence NNOD, complications, and systemic biomarkers reflecting these patient characteristics.100,101
Another limitation is that sepsis was defined using ICD-9 codes, in contrast to using a state of the art prospective case-based definition.102 Also, prospective temporal assessment of sex steroids and infection type/treatment, systemic inflammatory response syndrome (SIRS), shock, and pressor requirement would be a useful future direction. Finally, the study is limited in that we did not delineate the cause of death in this cohort and whether or not mortality was primarily the result of neurological causes or related to extracerebral injury complex or response to the TBI.
Conclusion
E1 is as an intriguing mortality marker after TBI because it may directly impact the non-neurologic consequences of TBI and be an indicator of a T-independent pathway to E2 production. Although laboratory studies indicated that E1 may improve TBI outcome,45,46 this study demonstrated that E1 is associated with worse outcomes clinically, further reinforcing the paradox observed in pre-clinical and clinical data in TBI. Future work conducting a clinical CSF analysis of E1 may inform differences between pre-clinical and clinical data. Further, future research into the therapeutic potential of aromatase inhibitors in TBI should be explored, given the improved immune response in experimental models of trauma and hemorrhage observed with these drugs103,104 and the negative association between TBI outcomes and estrogen production outlined here. Injury severity associations with E1 could also then be explored in this model.
Acknowledgments
The authors acknowledge the University of Pittsburgh Medical Center (UPMC) Trauma Registry team for their role in clinical data collection. This work was supported in part by National Institutes of Health (NIH) 5P01NS030318 and TL1 TR0001858, Centers for Disease Control (CDC) R49 Consumer Confidence Report (CCR) 323155, Department of Defense (DOD) W81XWH-071-0701, and National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) 90DP0041.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Centers for Disease Control and Prevention (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention: Atlanta [Google Scholar]
- 2. Corps K.N., Roth T.L., and McGavern D.B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 4. Corral L., Javierre C.F., Ventura J.L., Marcos P., Herrero J.I., and Mañez R. (2012). Impact of non-neurological complications in severe traumatic brain injury outcome. Crit. Care Lond. Engl. 16, R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kemp C.D., Johnson J.C., Riordan W.P., and Cotton B.A. (2008). How we die: the impact of nonneurologic organ dysfunction after severe traumatic brain injury. Am. Surg. 74, 866–872 [PubMed] [Google Scholar]
- 6. Ley E.J., Clond M.A., Bukur M., Park R., Chervonski M., Dagliyan G., Margulies D.R., Lyden P.D., Conti P.S., and Salim A. (2012). β-adrenergic receptor inhibition affects cerebral glucose metabolism, motor performance, and inflammatory response after traumatic brain injury. J. Trauma Acute Care Surg. 73, 33–40 [DOI] [PubMed] [Google Scholar]
- 7. Anthony D.C., and Couch Y. (2014). The systemic response to CNS injury. Exp. Neurol. 258, 105–111 [DOI] [PubMed] [Google Scholar]
- 8. Kenney M.J., and Ganta C.K. (2014). Autonomic nervous system and immune system interactions. Compr. Physiol. 4, 1177–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elenkov I.J., Wilder R.L., Chrousos G.P., and Vizi E.S. (2000). The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638 [PubMed] [Google Scholar]
- 10. Nance D.M., and Sanders V.M. (2007). Autonomic innervation and regulation of the immune system (1987–2007). Brain. Behav. Immun. 21, 736–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagner A.K., McCullough E.H., Niyonkuru C., Ozawa H., Loucks T.L., Dobos J.A., Brett C.A., Santarsieri M., Dixon C.E., Berga S.L., and Fabio A. (2011). Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 28, 871–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein D.G. (2001). Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24, 386–391 [DOI] [PubMed] [Google Scholar]
- 13. Djebaili M., Guo Q., Pettus E.H., Hoffman S.W., and Stein D.G. (2005). The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J. Neurotrauma 22, 106–118 [DOI] [PubMed] [Google Scholar]
- 14. He J., Evans C.-O., Hoffman S.W., Oyesiku N.M., and Stein D.G. (2004). Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 189, 404–412 [DOI] [PubMed] [Google Scholar]
- 15. Roof R.L., and Hall E.D. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 [DOI] [PubMed] [Google Scholar]
- 16. Liu F., Benashski S.E., Xu Y., Siegel M., and McCullough L.D. (2012). Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J. Neuroendocrinol. 24, 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCullough L.D., Blizzard K., Simpson E.R., Oz O.K., and Hurn P.D. (2003). Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 23, 8701–8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manwani B., Bentivegna K., Benashski S.E., Venna V.R., Xu Y., Arnold A.P., and McCullough L.D. (2015). Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J. Cereb. Blood Flow Metab. 35, 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. May A.K., Dossett L.A., Norris P.R., Hansen E.N., Dorsett R.C., Popovsky K.A., and Sawyer R.G. (2008). Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit. Care Med. 36, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spratt D.I., Longcope C., Cox P.M., Bigos S.T., and Wilbur-Welling C. (1993). Differential changes in serum concentrations of androgens and estrogens (in relation with cortisol) in postmenopausal women with acute illness. J. Clin. Endocrinol. Metab. 76, 1542–1547 [DOI] [PubMed] [Google Scholar]
- 21. Dossett L.A., Swenson B.R., Evans H.L., Bonatti H., and May A.K. (2008). Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg. Infect. 9, 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dossett L.A., Swenson B.R., Heffernan D., Bonatti H., Metzger R., Sawyer R.G., and May A.K. (2008). High levels of endogenous estrogens are associated with death in the critically injured adult. J. Trauma 64, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fourrier F., Jallot A., Leclerc L., Jourdain M., Racadot A., Chagnon J.L., Rime A., and Chopin C. (1994). Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ. Shock 43, 171–178 [PubMed] [Google Scholar]
- 24. Christeff N., Benassayag C., Carli-Vielle C., Carli A., and Nunez E.A. (1988). Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J. Steroid Biochem. 29, 435–440 [DOI] [PubMed] [Google Scholar]
- 25. Angstwurm M.W.A., Gaertner R., and Schopohl J. (2005). Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit. Care Med. 33, 2786–2793 [DOI] [PubMed] [Google Scholar]
- 26. Tsang G., Insel M.B., Weis J.M., Morgan M.A.M., Gough M.S., Frasier L.M., Mack C.M., Doolin K.P., Graves B.T., Apostolakos M.J., and Pietropaoli A.P. (2016). Bioavailable estradiol concentrations are elevated and predict mortality in septic patients: a prospective cohort study. Crit. Care 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crago E.A., Sherwood P.R., Bender C., Balzer J., Ren D., and Poloyac S.M. (2015). Plasma estrogen levels are associated with severity of injury and outcomes after aneurysmal subarachnoid hemorrhage. Biol. Res. Nurs. 17, 558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mindnich R., Möller G., and Adamski J. (2004). The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 218, 7–20 [DOI] [PubMed] [Google Scholar]
- 29. Miller W.L., and Auchus R.J. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luu-The V. (2013). Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steroid Biochem. Mol. Biol. 137, 176–182 [DOI] [PubMed] [Google Scholar]
- 31. Van den Berghe G., Weekers F., Baxter R.C., Wouters P., Iranmanesh A., Bouillon R., and Veldhuis J.D. (2001). Five-day pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic-pituitary-gonadal defects underlying profound hypoandrogenism in men with prolonged critical illness. J. Clin. Endocrinol. Metab. 86, 3217–3226 [DOI] [PubMed] [Google Scholar]
- 32. Spratt D.I., Cox P., Orav J., Moloney J., and Bigos T. (1993). Reproductive axis suppression in acute illness is related to disease severity. J. Clin. Endocrinol. Metab. 76, 1548–1554 [DOI] [PubMed] [Google Scholar]
- 33. Simpson E.R., Mahendroo M.S., Means G.D., Kilgore M.W., Jo Corbin C., and Mendelson C.R. (1993). Tissue-specific promoters regulate aromatase cytochrome P450 expression. J. Steroid Biochem. Mol. Biol. 44, 321–330 [DOI] [PubMed] [Google Scholar]
- 34. Simpson E.R., Zhao Y., Agarwal V.R., Michael M.D., Bulun S.E., Hinshelwood M.M., Graham-Lorence S., Sun T., Fisher C.R., Qin K., and Mendelson C.R. (1997). Aromatase expression in health and disease. Recent Prog. Horm. Res. 52, 185–213; discussion 213–214 [PubMed] [Google Scholar]
- 35. Zhao Y., Mendelson C.R., and Simpson E.R. (1995). Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Mol. Endocrinol. 9, 340–349 [DOI] [PubMed] [Google Scholar]
- 36. Zhao Y., Nichols J.E., Bulun S.E., Mendelson C.R., and Simpson E.R. (1995). Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. J. Biol. Chem. 270, 16,449–16,457 [DOI] [PubMed] [Google Scholar]
- 37. Spratt D.I., Morton J.R., Kramer R.S., Mayo S.W., Longcope C., and Vary C.P.H. (2006). Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am. J. Physiol. Endocrinol. Metab. 291, E631–E638 [DOI] [PubMed] [Google Scholar]
- 38. Zolin S.J., Vodovotz Y., Forsythe R.M., Rosengart M.R., Namas R., Brown J.B., Peitzman A.P., Billiar T.R., and Sperry J.L. (2015). The early evolving sex hormone environment is associated with significant outcome and inflammatory response differences after injury. J. Trauma Acute Care Surg. 78, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herson P.S., Koerner I.P., and Hurn P.D. (2009). Sex, sex steroids and brain injury. Semin. Reprod. Med. 27, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green P.S., Bishop J., and Simpkins J.W. (1997). 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. J. Neurosci. 17, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dubal D.B., Shughrue P.J., Wilson M.E., Merchenthaler I., and Wise P.M. (1999). Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 19, 6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hawk T., Zhang Y.Q., Rajakumar G., Day A.L., and Simpkins J.W. (1998). Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 796, 296–298 [DOI] [PubMed] [Google Scholar]
- 43. Toung T.J., Traystman R.J., and Hurn P.D. (1998). Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29, 1666–1670 [DOI] [PubMed] [Google Scholar]
- 44. Simpkins J.W., Rajakumar G., Zhang Y.Q., Simpkins C.E., Greenwald D., Yu C.J., Bodor N., and Day A.L. (1997). Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 87, 724–730 [DOI] [PubMed] [Google Scholar]
- 45. Gatson J.W., Liu M.-M., Abdelfattah K., Wigginton J.G., Smith S., Wolf S., Simpkins J.W., and Minei J.P. (2012). Estrone is neuroprotective in rats after traumatic brain injury. J. Neurotrauma 29, 2209–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regan R.F., and Guo Y. (1997). Estrogens attenuate neuronal injury due to hemoglobin, chemical hypoxia, and excitatory amino acids in murine cortical cultures. Brain Res. 764, 133–140 [DOI] [PubMed] [Google Scholar]
- 47. Kajta M., Lasoń W., Bień E., and Marszał M. (2002). Neuroprotective effects of estrone on NMDA-induced toxicity in primary cultures of rat cortical neurons are independent of estrogen receptors. Pol. J. Pharmacol. 54, 727–729 [PubMed] [Google Scholar]
- 48. Christeff N., Carli A., Benassayag C., Bleichner G., Vaxelaire J.-F., and Nunez E.A. (1992). Relationship between changes in serum estrone levels and outcome in human males with septic shock. Circ. Shock 36, 249–255 [PubMed] [Google Scholar]
- 49. Lu J., Goh S.J., Tng P.Y.L., Deng Y.Y., Ling E.-A., and Moochhala S. (2009). Systemic inflammatory response following acute traumatic brain injury. Front. Biosci. (Landmark Ed.) 14, 3795–3813 [DOI] [PubMed] [Google Scholar]
- 50. Das M., Mohapatra S., and Mohapatra S.S. (2012). New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao Y., Nichols J.E., Valdez R., Mendelson C.R., and Simpson E.R. (1996). Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 10, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 52. Brain Trauma Foundation, American Association of Neurological Surgeons, and Congress of Neurological Surgeons (2007). Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24, Suppl. 1, S1–106 [DOI] [PubMed] [Google Scholar]
- 53. Garringer J.A., Niyonkuru C., McCullough E.H., Loucks T., Dixon C.E., Conley Y.P., Berga S., and Wagner A.K. (2013). Impact of aromatase genetic variation on hormone levels and global outcome after severe TBI. J. Neurotrauma 30, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Udekwu P., Kromhout-Schiro S., Vaslef S., Baker C., and Oller D. (2004). Glasgow Coma Scale score, mortality, and functional outcome in head-injured patients. J. Trauma 56, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 55. Cifu D.X., Keyser-Marcus L., Lopez E., Wehman P., Kreutzer J.S., Englander J., and High W. (1997). Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch. Phys. Med. Rehabil. 78, 125–131 [DOI] [PubMed] [Google Scholar]
- 56. Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 57. Baker S.P., O'Neill B., Haddon W., and Long W.B. (1974). The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 14, 187–196 [PubMed] [Google Scholar]
- 58. Fakhry S.M., Trask A.L., Waller M.A., Watts D.D., and IRTC Neurotrauma Task Force. (2004). Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J. Trauma 56, 492–500 [DOI] [PubMed] [Google Scholar]
- 59. Moein H., Khalili H.A., and Keramatian K. (2006). Effect of methylphenidate on ICU and hospital length of stay in patients with severe and moderate traumatic brain injury. Clin. Neurol. Neurosurg. 108, 539–542 [DOI] [PubMed] [Google Scholar]
- 60. Hawkins M.L., Lewis F.D., and Medeiros R.S. (2005). Impact of length of stay on functional outcomes of TBI patients. Am. Surg. 71, 920–930 [PubMed] [Google Scholar]
- 61. Desquilbet L., and Mariotti F. (2010). Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 29, 1037–1057 [DOI] [PubMed] [Google Scholar]
- 62. Kumar R.G., Ritter A.C., Kochanek P.M., Berga S.L., and Wagner A.K. (2015). Abstract: Serum tumor necrosis factor-a association with mortality six months after TBI: mechanistic relationships with estradiol. J. Neurotrauma 32, A92 [Google Scholar]
- 63. Selassie A.W., Fakhry S.M., and Ford D.W. (2011). Population-based study of the risk of in-hospital death after traumatic brain injury: the role of sepsis. J. Trauma Inj. Infect. Crit. Care 71, 1226–1234 [DOI] [PubMed] [Google Scholar]
- 64. Santarsieri M., Kumar R.G., Kochanek P.M., Berga S., and Wagner A.K. (2015). Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain. Behav. Immun. 45, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Spooner C.E., Markowitz N.P., and Saravolatz L.D. (1992). The role of tumor necrosis factor in sepsis. Clin. Immunol. Immunopathol. 62, S11–17 [DOI] [PubMed] [Google Scholar]
- 66. Ho J.T., Al-Musalhi H., Chapman M.J., Quach T., Thomas P.D., Bagley C.J., Lewis J.G., and Torpy D.J. (2006). Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J. Clin. Endocrinol. Metab. 91, 105–114 [DOI] [PubMed] [Google Scholar]
- 67. Harbarth S., Holeckova K., Froidevaux C., Pittet D., Ricou B., Grau G.E., Vadas L., Pugin J., and Geneva Sepsis Network. (2001). Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 164, 396–402 [DOI] [PubMed] [Google Scholar]
- 68. Irahara N., Miyoshi Y., Taguchi T., Tamaki Y., and Noguchi S. (2006). Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer. Int. J. Cancer 118, 1915–1921 [DOI] [PubMed] [Google Scholar]
- 69. Simpson E.R. (2004). Aromatase: biologic relevance of tissue-specific expression. Semin. Reprod. Med. 22, 11–23 [DOI] [PubMed] [Google Scholar]
- 70. Cleland W.H., Mendelson C.R., and Simpson E.R. (1985). Effects of aging and obesity on aromatase activity of human adipose cells. J. Clin. Endocrinol. Metab. 60, 174–177 [DOI] [PubMed] [Google Scholar]
- 71. Bulun S.E., Zeitoun K., Sasano H., and Simpson E.R. (1999). Aromatase in aging women. Semin. Reprod. Endocrinol. 17, 349–358 [DOI] [PubMed] [Google Scholar]
- 72. Simon D., Preziosi P., Barrett-Connor E., Roger M., Saint-Paul M., Nahoul K., and Papoz L. (1992). The influence of aging on plasma sex hormones in men: The Telecom Study. Am. J. Epidemiol. 135, 783–791 [DOI] [PubMed] [Google Scholar]
- 73. Feldman H.A., Longcope C., Derby C.A., Johannes C.B., Araujo A.B., Coviello A.D., Bremner W.J., and McKinlay J.B. (2002). Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 87, 589–598 [DOI] [PubMed] [Google Scholar]
- 74. Morris P.G., Hudis C.A., Giri D., Morrow M., Falcone D.J., Zhou X.K., Du B., Brogi E., Crawford C.B., Kopelovich L., Subbaramaiah K., and Dannenberg A.J. (2011). Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. (Phila.) 4, 1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cadagan D., Khan R., and Amer S. (2014). Female adipocyte androgen synthesis and the effects of insulin. Mol. Genet. Metab. Rep. 1, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Swainson M.G., Batterham A.M., Tsakirides C., Rutherford Z.H., and Hind K. (2017). Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PloS One 12, e0177175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tanamas S.K., Permatahati V., Ng W.L., Backholer K., Wolfe R., Shaw J.E., and Peeters A. (2015). Estimating the proportion of metabolic health outcomes attributable to obesity: a cross-sectional exploration of body mass index and waist circumference combinations. BMC Obes. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Janssen I., Katzmarzyk P.T., and Ross R. (2004). Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 79, 379–384 [DOI] [PubMed] [Google Scholar]
- 79. Gregorić P.D., Bajec D.D., Sijacki A.D., and Karadzić B.A. (2003). Relation between cytokine IL-6 levels and the occurrence of systemic complications in patients with multiple injuries and blunt abdominal trauma [in Serbian]. Srp. Arh. Celok. Lek. 131, 118–121 [DOI] [PubMed] [Google Scholar]
- 80. Taniguchi T., Koido Y., Aiboshi J., Yamashita T., Suzaki S., and Kurokawa A. (1999). The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. Am. J. Emerg. Med. 17, 548–551 [DOI] [PubMed] [Google Scholar]
- 81. Li M., Li F., Luo C., Shan Y., Zhang L., Qian Z., Zhu G., Lin J., and Feng H. (2011). Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J. Trauma 71, 141–147 [DOI] [PubMed] [Google Scholar]
- 82. Li J., Li W.-Q., and Yao Y. (2016). Vasorelaxation Effect of Estrone Derivate EA204 in Rabbit Aorta. Scientifica 2016, 7405797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Selles J., Polini N., Alvarez C., and Massheimer V. (2005). Novel action of estrone on vascular tissue: regulation of NOS and COX activity. Steroids 70, 251–256 [DOI] [PubMed] [Google Scholar]
- 84. Tep-areenan P., Kendall D.A., and Randall M.D. (2003). Mechanisms of vasorelaxation to 17beta-oestradiol in rat arteries. Eur. J. Pharmacol. 476, 139–149 [DOI] [PubMed] [Google Scholar]
- 85. Zhou K., Gao Q., Zheng S., Pan S., Li P., Suo K., Simoncini T., Wang T., and Fu X. (2013). 17β-estradiol induces vasorelaxation by stimulating endothelial hydrogen sulfide release. Mol. Hum. Reprod. 19, 169–176 [DOI] [PubMed] [Google Scholar]
- 86. Garbán H.J., Buga G.M., and Ignarro L.J. (2004). Estrogen receptor-mediated vascular responsiveness to nebivolol: a novel endothelium-related mechanism of therapeutic vasorelaxation. J. Cardiovasc. Pharmacol. 43, 638–644 [DOI] [PubMed] [Google Scholar]
- 87. Egami R., Tanaka Y., Nozaki M., Koera K., Okuma A., and Nakano H. (2005). Chronic treatment with 17beta-estradiol increases susceptibility of smooth muscle cells to nitric oxide. Eur. J. Pharmacol. 520, 142–149 [DOI] [PubMed] [Google Scholar]
- 88. Tahsili-Fahadan P., and Geocadin R.G. (2017). Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ. Res. 120, 559–572 [DOI] [PubMed] [Google Scholar]
- 89. van der Jagt M., and Miranda D.R. (2012). Beta-blockers in intensive care medicine: potential benefit in acute brain injury and acute respiratory distress syndrome. Recent Pat. Cardiovasc. Drug Discov. 7, 141–151 [DOI] [PubMed] [Google Scholar]
- 90. Salt P.J., and Iverson L.L. (1972). Inhibition of the extraneuronal uptake of catecholamine in the isolated rat heart by cholesterol. Nat. New Biol. 238, 91–92 [DOI] [PubMed] [Google Scholar]
- 91. Zuckerman S.H., Bryan-Poole N., Evans G.F., Short L., and Glasebrook A.L. (1995). In vivo modulation of murine serum tumour necrosis factor and interleukin-6 levels during endotoxemia by oestrogen agonists and antagonists. Immunology 86, 18–24 [PMC free article] [PubMed] [Google Scholar]
- 92. Cid M.C., Kleinman H.K., Grant D.S., Schnaper H.W., Fauci A.S., and Hoffman G.S. (1994). Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J. Clin. Invest. 93, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schreiber J.B., Nora A., Stage F.K., Barlow E.A., and King J. (2006). Reporting structural equation modeling and confirmatory factor analysis results: a review. J. Educ. Res. 99, 323–338 [Google Scholar]
- 94. Khan N., Sharma K.K., Andersson S., and Auchus R.J. (2004). Human 17beta-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch. Biochem. Biophys. 429, 50–59 [DOI] [PubMed] [Google Scholar]
- 95. Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., and Stocchetti N. (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 96. Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., and others. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stein D.G. (2015). Embracing failure: what the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 29, 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lopez-Rodriguez A.B., Acaz-Fonseca E., Spezzano R., Giatti S., Caruso D., Viveros M.-P., Melcangi R.C., and Garcia-Segura L.M. (2016). Profiling neuroactive steroid levels after traumatic brain injury in male mice. Endocrinology 157, 3983–3993 [DOI] [PubMed] [Google Scholar]
- 99. Lopez-Rodriguez A.B., Acaz-Fonseca E., Giatti S., Caruso D., Viveros M.-P., Melcangi R.C., and Garcia-Segura L.M. (2015). Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology 56, 1–11 [DOI] [PubMed] [Google Scholar]
- 100. Jha R.M., and Kochanek P.M. (2017). Adding insight to injury: a new era in neurotrauma. Lancet Neurol. 16, 578–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kumar R.G., Juengst S.B., Wang Z., Dams-O'Connor K., Dikmen S.S., O'Neil-Pirozzi T.M., Dahdah M.N., Hammond F.M., Felix E.R., Arenth P.M., and Wagner A.K. (2018). Epidemiology of comorbid conditions among adults 50 years and older with traumatic brain injury. J. Head Trauma Rehabil. 33, 15–24 [DOI] [PubMed] [Google Scholar]
- 102. Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.-L., and Angus D.C. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Schneider C.P., Nickel E.A., Samy T.S., Schwacha M.G., Cioffi W.G., Bland K.I., and Chaudry I.H. (2000). The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock (Augusta, Ga.) 14, 347–353 [DOI] [PubMed] [Google Scholar]
- 104. Schneider C.P., Schwacha M.G., Samy T.S.A., Bland K.I., and Chaudry I.H. (2003). Androgen-mediated modulation of macrophage function after trauma-hemorrhage: central role of 5alpha-dihydrotestosterone. J. Appl. Physiol. 1985 95, 104–112 [DOI] [PubMed] [Google Scholar]