Abstract
The treatment of B-cell malignancies by adoptive cell transfer (ACT) of anti-CD19 chimeric antigen receptor T cells (CD19 CAR-T) has proven to be a highly successful therapeutic modality in several clinical trials.1–6 The anti-CD19 CAR-T cell production method used to support initial trials relied on numerous manual, open process steps, human serum, and 10 days of cell culture to achieve a clinical dose.7 This approach limited the ability to support large multicenter clinical trials, as well as scale up for commercial cell production. Therefore, studies were completed to streamline and optimize the original National Cancer Institute production process by removing human serum from the process in order to minimize the risk of viral contamination, moving process steps from an open system to functionally closed system operations in order to minimize the risk of microbial contamination, and standardizing additional process steps in order to maximize process consistency. This study reports a procedure for generating CD19 CAR-T cells in 6 days, using a functionally closed manufacturing process and defined, serum-free medium. This method is able to produce CD19 CAR-T cells that are phenotypically and functionally indistinguishable from cells produced for clinical trials by the previously described production process.
Keywords: : anti-CD19 CAR, transduction, expansion, cryopreservation, GMP, closed system
Introduction
The incidence of b-cell malignancies continues to rise, with approximately 84,000 patients being newly diagnosed in 2012.1 Even though there have been substantial advances in treatment options for these cancers, including chemotherapy, either with or without monoclonal antibodies, >22,000 patients succumb to their disease yearly.1–4 Clearly, there is a need for new therapeutic options for patients who are failed by the frontline therapies for these B-cell malignancies. One promising treatment option involves the adoptive transfer of T cells that target molecules on the surface of B-cell cancers. CD19 is an attractive target for such adoptive cell transfer immunotherapies, as it is expressed on the surface of most B-cell malignancies, with a normal tissue expression pattern restricted to B lymphocytes.5 A number of groups have developed chimeric antigen receptors (CARs) that target CD19 expressing B cells.6–16 CARs comprise an antigen-binding motif, typically a single chain variable fragment (scFv) region, a transmembrane domain that links the scFv to activation elements inside the cell, and an intracellular domain responsible for activating the T cell upon binding to the target cell. Unlike traditional T-cell receptors (TCRs), CARs do not require the presentation of their antigen within the context of a major histocompatibility complex molecule (MHC). Consequently, all patients, irrespective of their MHC restriction, can be treated with a single CAR if their tumor expresses the target antigen.17 The results from several clinical trials using CD19 CAR-T cells have been encouraging, with ∼80% of patients exhibiting objective clinical responses at the National Cancer Institute (NCI).10,13–16,18
One strategy for production of CAR-modified T cells involves the isolation of peripheral blood mononuclear cells (PBMCs) followed by their ex vivo genetic modification and expansion to therapeutically useful numbers. The method previously used to generate CD19 CAR-T cells at the Surgery Branch of the NCI consisted of a 10-day process that utilized open-tissue culture vessels and required human serum (HS).11,19–21 As anti- CD19 CAR-T cell therapy moves through clinical development, a more controlled system to modify genetically, expand, and harvest T cells in the absence of serum will be required. This process will need to be a robust, simple, and GMP-compliant production platform to scale out manufacturing, allowing multiple patient treatments to be produced simultaneously in a single facility. This article describes such a functionally closed system for producing CD19 CAR-T cells reproducibly in 6 days.
Methods
Clinical retroviral vector production
A plasmid encoding the CD19 CAR consisting of the mouse stem-cell virus gamma-retroviral backbone engineered to express an ScFv derived from the mouse anti-CD19 hybridoma, FMC63, fused to intracellular domains from human CD28 and CD3 zeta was used for retroviral vector production. Clinical grade retroviral vector was manufactured in accordance with current good manufacturing practices (cGMP) for Phase I by the Surgery Branch Vector Production Facility, NCI, National Institutes of Health.22,23 All studies were approved by the Institutional Review Board of the National Institutes of Health.
Cell culture medium
As part of a medium optimization strategy, four different media and one serum-free supplement were evaluated during the process optimization: OpTmizer™ CTS, AIM V (Life Technologies, Grand Island, NY), X-VIVO 15 (Lonza, Walkersville, MD), and TexMACS GMP (Miltenyi Biotec, Cambridge, MA). Individual media were evaluated with or without 2.5–5% T-cell serum replacement (TCSR), later renamed CTS™ ImmuneCellSR (Life Technologies). Where indicated, AIM V medium containing either 1% or 5% HS was used as positive controls. All media contained the following: Glutamax-1 (100 × ; Life Technologies), Pen/strep (100 × ; Lonza), and hIL-2 (300 IU/mL; Prometheus, San Diego, CA). For activation of T cells with anti-CD3 antibody, OKT3 (Miltenyi Biotech) was added to each medium at a final concentration of 50 ng/mL.
Ficoll isolation of PBMCs and cell wash steps
The Sepax II cell processing device from Biosafe America (Houston, TX) is an automated closed system for processing of blood-derived cell products. Apheresis products from healthy donors and clinical trial subjects were concentrated and their volume reduced to 120 mL using the Sepax PeriCell program and a CS-490.1 kit. PBMCs were isolated from volume-reduced apheresis products using the Sepax NeatCell program with Ficoll (Lonza) density gradient centrifugation and a CS-900.2 kit in accordance with the manufacturer's instructions. Sepax CultureWash program and the CS-600.1 kit was used for cell culture washes after the activation step and for the final cell product wash. Manual processing of apheresis products was carried out as described previously.24
Transduction, expansion, and cryopreservation of CD19 CAR-T cells
PBMCs were collected from the apheresis products of healthy donors and melanoma or lymphoma patients using the Sepax II for automated closed system processing of blood-derived cell products (Biosafe America). Freshly processed PBMCs (day 0) were cultured in PermaLife bags (OriGen Biomedical, Austin, TX) at 1 × 106 cells/mL (1 × 109 cells in 800 mL of Optimizer medium) and activated with anti-CD3 antibody (OKT3; 50 ng/mL) and human IL-2 (300 IU/mL) for 2 days. On day 1, new PermaLife bags were coated with retronectin (GMP-grade recombinant human fibronectin fragment CH296; Takara BioDivision, Shiga, Japan) at 10 μg/mL diluted in phosphate-buffered saline (PBS) and stored at 4°C overnight. The next day, the retronectin was removed and the bags washed once with 2.5% HEPES in HBSS (Lonza). In some experiments, the retronectin was removed and bags were blocked with 2.5% human serum albumin (HSA) in PBS for 30 min prior to the wash. Retroviral vector supernatant was then added to retronectin-coated bags and incubated for 2 h at 37°C and 5% CO2. For untransduced control cells, bags were loaded with medium only. The activated PBMCs were washed using the Sepax II, transferred to the retrovirus loaded bags (0.5 × 106 cells/mL, 2 × 108 cells in 200 mL Optimizer medium), and incubated at 37°C. In the experiments, a second transduction was performed by either flipping the initial transduction bag over or transferring cells to a second vector loaded bag. Cells were transferred on day 3 into new bags for further expansion after which point the cells were washed with 0.9% saline using the Sepax II. The anti-CD19 CAR-T cell product was formulated in CS250 cryostorage bags (OriGen Biomedical) to achieve the target dose in a solution containing 0.9% saline plus 5% HSA, and then diluted 1:1 with Cryostor 10 (BioLife Solutions, Bothell, WA). The cells were frozen in a controlled-rate freezer (Kryosave, Planer, United Kingdom) using the following protocol: (1) 4°C hold for 10 min; (2) cool at −1°C/min to −6°C; (3) cool at −25°C/min to −40°C; (4) warm up at 9°C/min to −12°C; (5) cool at −1°C/min to −40°C; (6) cool at −10°C to −90°C and store in vapor phase liquid nitrogen until further use. On day 6, prior to final formulation, the transduction efficiency and functional potency analysis were assessed by fluorescence-activated cell sorting (FACS) for CD19 CAR expression and interferon gamma (IFN-γ) enzyme-linked immunosorbent assay (ELISA), respectively.
Detection of CD19 CAR-T cells by FACS
To assess the T-cell transduction efficiency, the expression of the anti-CD19 CAR was quantified by flow cytometry. Transduced cells were stained with biotin labeled goat anti-mouse F(ab)2 (anti-Fab; Jackson Immuno Research Laboratories, West Grove, PA). Anti-F(ab)2 binding was detected by staining with phycoerythrin (PE)-labeled streptavidin (BD Biosciences, San Jose, CA). Cells were also stained with anti-CD3 (UCHT-1), anti-CD4 (OKT-4), and anti-CD8 (RPA-T8). The percentage of anti-CD19 CAR+ T cells was calculated by subtracting background staining on CD3+ mock-transduced control T cells from the CD3+ anti-CD19 CAR+ T cells in the transduced cell population (maximum allowable background for a valid assay was ≤5% in the mock-transduced population). Other antibodies used in this study include anti-CD56, CD14, CD19, γδ-TCR, CCR7, and CD45RA from either BD Biosciences or from BioLegend (San Diego, CA). All antibodies were research-grade reagents. Data acquisition was performed using a FACS Canto II (BD Biosciences), and data were analyzed using FlowJo software (FlowJo, Inc., Ashland, OR).
Cytokine release assays
CD19 CAR-T cells (1 × 105 cells) and CD19+ (Toledo, NALM6, and K562-CD19) and CD19− (K562-NGFR and CEM) cell lines (1 × 105 cells) were placed in Dulbecco's modified Eagle's medium (Life Technologies) containing 10% fetal bovine serum for overnight co-culture at 37°C and 5% CO2. Culture supernatants were evaluated for IFN-γ after overnight co-cultivation by ELISA (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis
Where appropriate, data were analyzed by unpaired Student's t-test or one-way analysis of variance followed by Tukey's multiple comparisons analysis between groups using Graphpad Prism software (La Jolla, CA).
Results
Closed system isolation of PBMCs
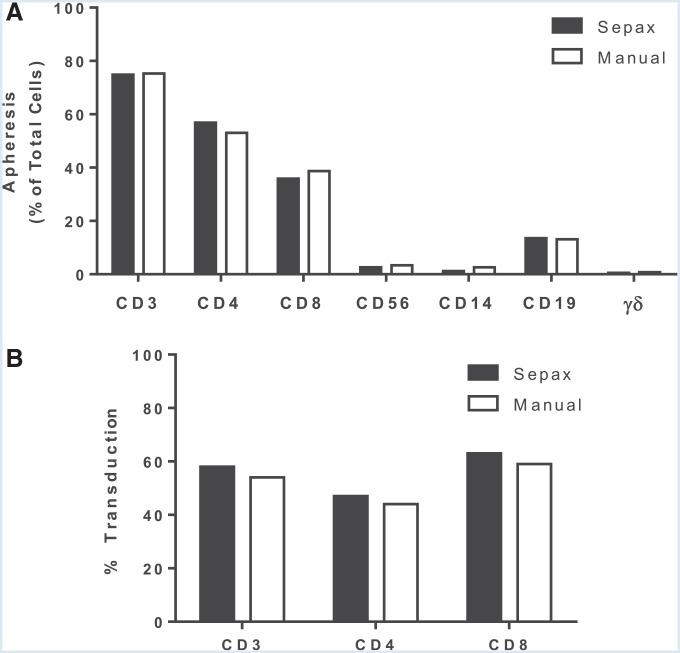
PBMCs have historically been isolated from donor apheresis material by centrifugation on a Ficoll density cushion in centrifuge tubes. The PBMC layer at the density interface is collected manually and platelets removed using two slow-speed centrifugation wash steps. This method involves multiple open processing steps and introduces a risk that PBMCs could become inadvertently contaminated with microorganisms. The Sepax II (Biosafe) isolates PBMCs on Ficoll gradients and performs the downstream washing steps in a closed manner using the propriety NeatCell process.25 To test the comparability of PBMCs isolated using the Sepax II with those collected by the standard method, donor apheresis products were split in half and processed using both methods and the phenotype of the PBMC determined by FACS. There was no significant different in lymphocyte recovery when the apheresis product was processed using the Sepax II compared to a manual Ficoll gradient separation: 56.2 ± 4.7% and 51.4 ± 6.8%, respectively. PBMCs obtained using both methods were indistinguishable in terms of T, B, and natural killer (NK) cells, as well as monocyte content (Fig. 1A). In addition, both methods isolated similar proportions of CD4+ and CD8+ (Fig. 1A), and there was no difference in the transduction efficiency of lymphocytes recovered by both methods (Fig. 1B).
Figure 1.
Comparison of peripheral blood mononuclear cells (PBMCs) isolated from apheresis using Sepax II or manual Ficoll separation. (A) Apheresis from a healthy donor was split equally and processed using the Sepax II density gradient-based preparation with the kit CS-900 from Biosafe or by manual Ficoll separation. The following lymphocyte cell subpopulations were analyzed by fluorescence-activated cell sorting (FACS): total lymphocytes (CD3+), natural killer (NK; CD3−CD56+), CD19+ cells, gd T cells, and, within the CD3+ T cells, the percentages of T helper (CD3+CD4+) and CTL (CD3+CD8+). (B) T cells isolated from both procedures were activated with soluble OKT3 and transduced with PG13-CD19-H3 vector. The vector transduction efficiency was measured by FACS. Data are presented from a single experiment and are representative of at least three independent experiments.
Serum-free culture system
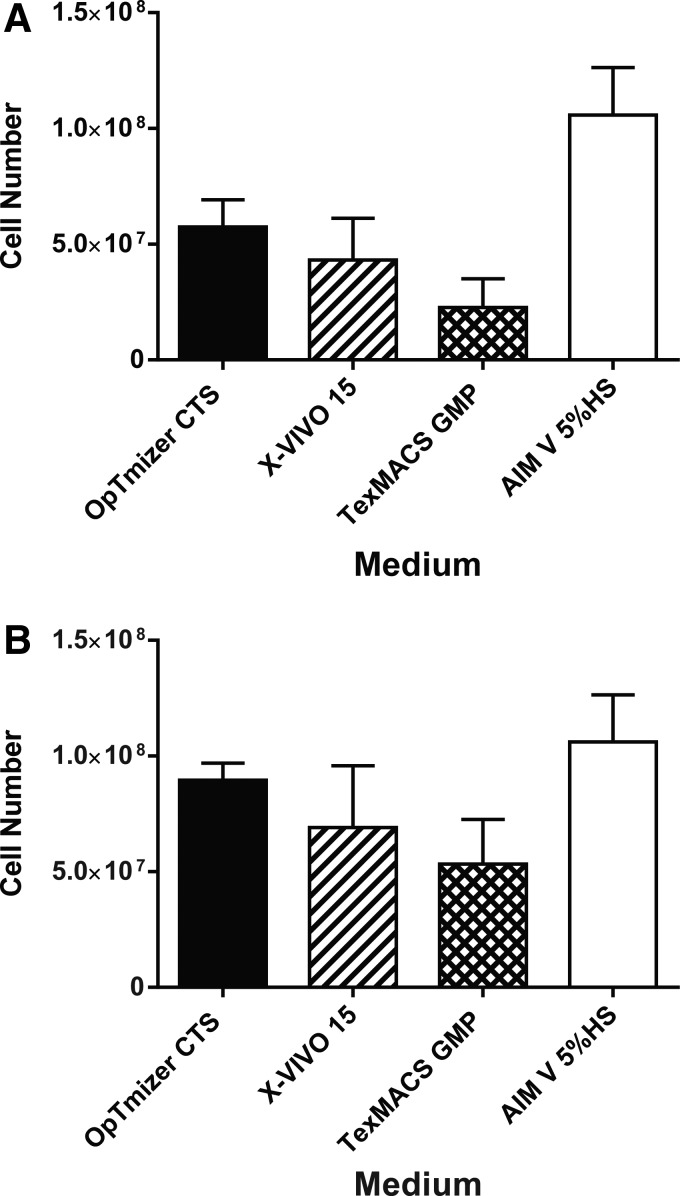
The Surgery Branch at the NCI typically utilized AIM V medium supplemented with 5% HS to manufacture cell therapy products. Of note, individual lots of HS can be highly variable in their ability to support the lymphocyte expansion, which could impact the consistency of manufacturing gene-modified cell products.26 In an effort to remove HS from the cell production process, four media (AIM V, OpTmizer CTS, X-Vivo 15, and TexMACS GMP) were tested for their ability to support lymphocyte expansion in the absence of HS (Fig. 2A). Compared with AIM V + 5% HS, none of the media evaluated was able to support lymphocyte expansion over 6 days in a manner similar to AIM V + 5% HS. Interestingly, supplementation with TCSR resulted in higher levels of lymphocyte growth and expansion for all medium evaluated. However, only OpTmizer CTS-5% TCSR produced comparable expansions to cells grown in AIM V-5% HS (9.0 × 107 ± 0.7 and 1.1 × 108 ± 0.2 cells, respectively; p = 0.78; Fig. 2B). Additional experiments comparing OpTmizer CTS supplemented with either 2.5% or 5% TCSR for lymphocyte expansion showed no difference in cell expansion (data not shown). OpTmizer CTS-2.5% TCSR was therefore selected for T-cell growth and expansion in subsequent studies.
Figure 2.
Optimization of T-cell growth in serum-free medium. Lymphocytes were activated with OKT3 for 2 days and expanded in media containing interleukin-2 (IL-2) for 6 days. During the expansion phase, cells were split to 0.5e6/mL when reaching a density of 2 × 106 cells/mL. (A) The number of total viable cells cultured in the various medium in the absence of serum, or (B) in the same medium supplemented with 5% T-cell serum replacement (TCSR). Data are from three independent experiments represented as the mean ± the standard error of the mean (SEM).
Development of an optimized system for transduction of CD19 CAR-T cells
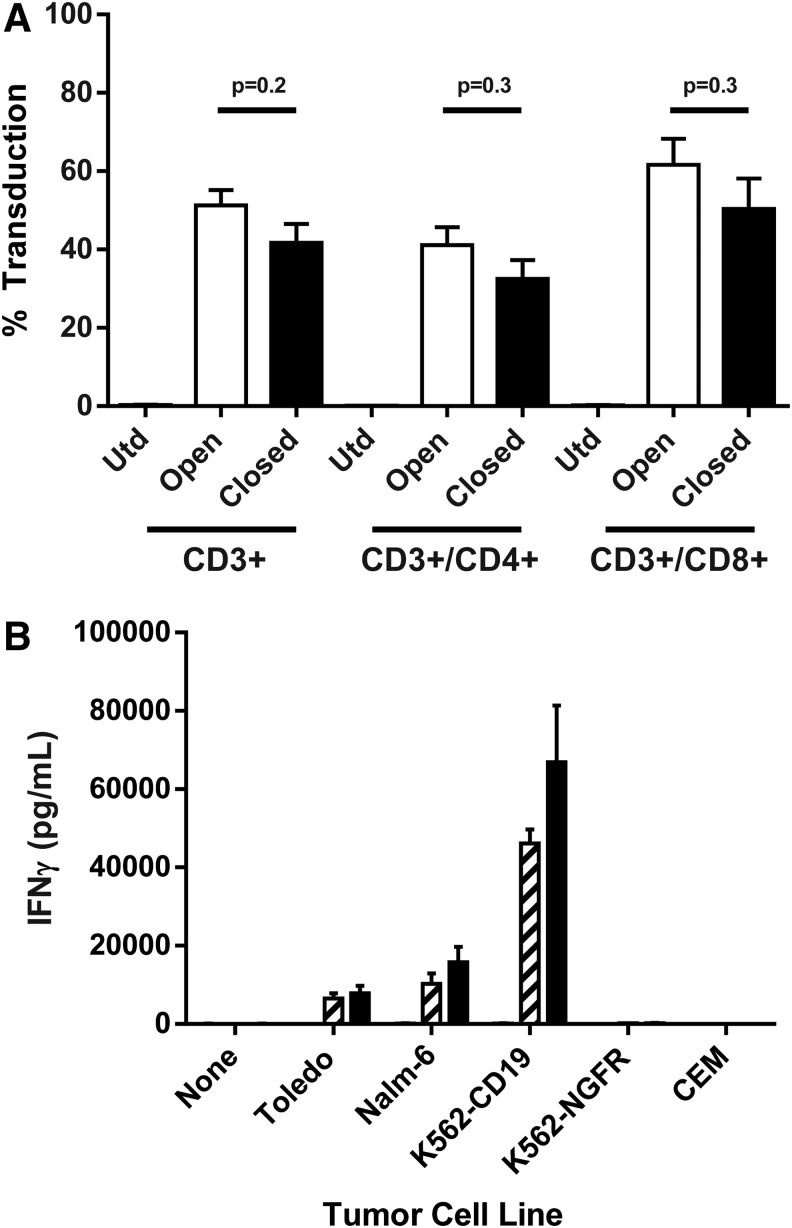
Performing T-cell stimulations and transductions in plates and T-cell expansions in flasks as done in previous clinical trials22 can increase the risk for contamination due to the many open manipulations performed. In order to produce a robust cGMP compliant process that will scale out to meet the demands of multicenter anti-CD19 CAR-T cell clinical trials, this study sought to develop a functionally closed process and close as many of the unit operations as possible for the manufacture of anti-CD19 CAR-T cells. To that end, PermaLife bags were coated with retronectin overnight. Of note, in the absence of retronectin coating of the bags, no observable vector binding to PermaLife bags or tissue culture plates has been observed.27 Following retronectin coating, the bags are washed, blocked, and loaded with retroviral vector and incubated for 2 h at 37°C without spinning. Cells were then added into the bags, mixed with viral supernatants, and placed at 37°C overnight. The following day, cells were transferred from the transduction bag into a new culture bag at a cell density of 0.5 × 106 cells/mL for expansion. The CD19 CAR-T cell transduction efficiency for three separate donors was determined by FACS comparing transduction in plates (spinoculation) with static transduction in bags. While overall transduction efficiency was slightly lower in the closed bag transduction system, there was no significant difference in the ability to transduce CD3+, CD3+/CD4+, or CD3+/CD8+ T cells (p = 0.2, p = 0.3, and p = 0.3, respectively; Fig. 3A). Transduced T cells from both the bag and plate transduction systems were comparable in regards to INF-γ secretion following coculture with CD19+ target cells (Fig. 3B). However, there was a trend of higher levels of IFN-γ secretion from cells transduced and cultured in bags. In addition, there was no significant difference in the ability of the transduced cell manufactured in plates or bags to upregulate CD107a specifically, a marker of lymphocyte degranulation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hgtb).
Figure 3.
Comparison of CD19 chimeric antigen receptor (CAR) T cell transduction by spinoculation or in static bags. Apheresis from three subjects was processed either manually or using the Sepax II, and PBMCs were activated with OKT3 in AIM V medium containing 5% human serum and 300 IU of IL-2 for 2 days. Cells were then transduced with PG13-CD19-H3 Vector by spinoculation in six-well plates (open) or in static culture using Origen PermaLife PL07 bags (closed). (A) Four days post transduction, cells were analyzed by FACS for the percentages of CD3+ and CD4+ or CD8+ (of the CD3+) transduced cells. (B) CD19 CAR-T cells were co-cultured with CD19+ target cells (Toledo and Nalm6) or CD19− (K562-NGFR and CEM). The next day, supernatants were collected, and interferon gamma (IFN-γ) was detected by enzyme-linked immunosorbent assay. The data represent three independent experiments and are presented as mean ± SEM.
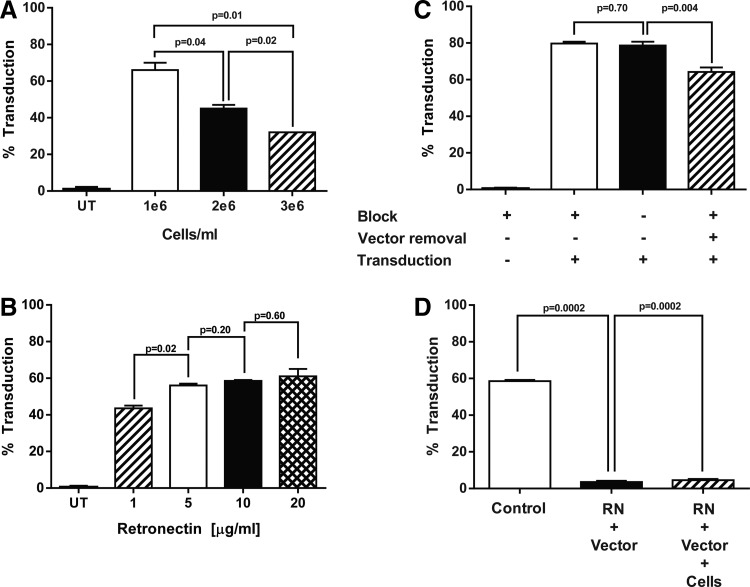
To optimize transduction in a closed bag unit operation further, the following process steps were evaluated: (1) cell density during the OKT3 activation of PBMCs; (2) retronectin concentration for bag coating; (3) post-retronectin blocking step and removal of vector supernatant prior to transduction; (4) dilution of vector supernatant; and (5) T-cell density for transduction. The findings suggest that OKT3 activation at a concentration of 1 × 106 cells/mL was optimal, as there was a significant decrease in transduction efficiency as the cell concentration increased from 1 × 106 to 2 × 106 and 3 × 106 cells/mL (66% vs. 45% and 32%, respectively; Fig. 4A). Varying the concentration of retronectin for loading of the retroviral vector showed that 1 μg/mL of retronectin resulted in a significant decrease in transduction efficiency. However, there was no difference in transduction efficiency when using retronectin concentrations ranging from 5 to 20 μg/mL (Fig. 4B). In the plate-based transduction system, a process referred to as spinoculation was utilized, where wells are coated with retronectin, washed and blocked with has, and loaded with retroviral vector followed by a low-speed centrifugation for 2 h prior to the addition of T cells. In the bag, blocking with HSA has no effect on CD19 CAR-T cell transduction, while the removal of the retroviral vector prior to addition of the T cells decreased transduction (Fig. 4C). In addition, while washing out unbound retronectin was not a critical performance step, combining the addition of retronectin and vector or retronectin, vector, and cells into one step significantly reduced CD19 CAR-T cell transduction, as retronectin likely binds directly to retroviral vector particles, thereby blocking T-cell transduction (Fig. 4D). Lastly, lowering the cell density at the time of transduction from 0.5 × 106 to either 0.1 × 106 or 0.25 × 106 cells/mL had no effect on transduction, nor was there any significant increase in T-cell transduction following a second transduction, either by flipping the retronectin/vector-coated bag or by transferring cells to a second retronectin-coated bag loaded with retroviral vector (data not shown).
Figure 4.
Optimization of closed transduction process for the manufacture of CD19 CAR-T cells. Lymphocytes were isolated from subject apheresis products using the Sepax II and stimulated as previously described with the following changes. (A) After stimulation, PBMCs were seeded at the indicated cell density and transduced in bags as previously described. The transduction efficiency was measured by FACS staining for CD19 CAR expression 4 days post transduction. (B) Origen cell culture bags were coated with retronectin at the indicated concentrations. The next day, retronectin was removed and the bags were blocked with 2.5% human serum albumin (HSA) before vector loading. OKT3 activated-PBMCs from three patients (1 × 106 cells/mL) were added to separate bags, and transduction efficiency was measured by FACS. (C) To evaluate the need for a blocking step following retronectin coating of the bag, bags were coated with 10 μg/mL of retronectin and blocked with either 2.5% HSA or HBSS and then loaded with retroviral vector. No significant difference in transduction was observed by the addition of a blocking step. However, removal of the retroviral vector prior to the T cell transduction did significantly reduce the level of CD19 CAR expression (p = 0.004, stippled bar). (D) In the closed transduction process (control), the bag is coated with retronectin overnight followed by a wash-and-block step prior to vector loading. In an attempt to simplify the process, the block step prior to transduction was removed and the addition of retronectin and vector prior to the addition of cells was combined. In a separate effort, the addition of retronectin, vector, and cells was combined into a single step. All other conditions were the same. All data are representative of at least three independent experiments. Data are presented as mean ± SEM.
Clinical production of CD19 CAR-T cells
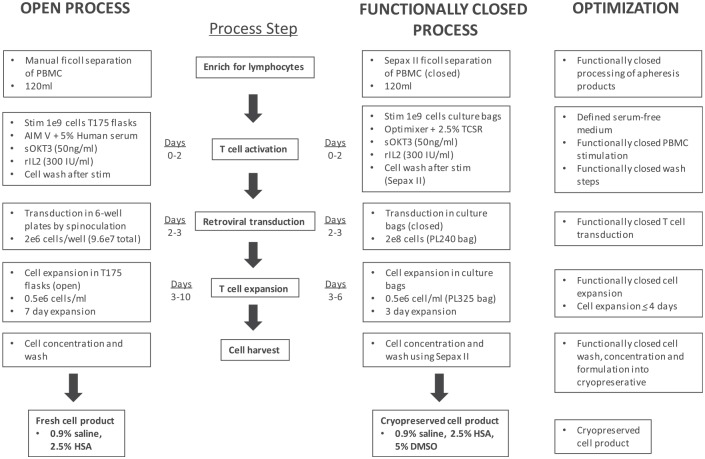
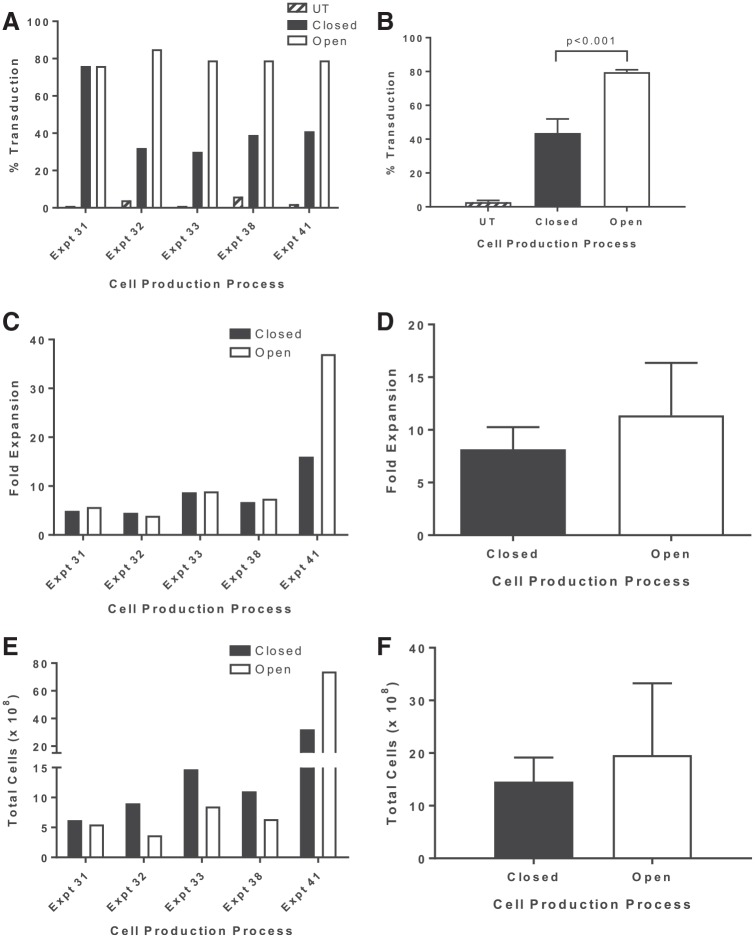
A schematic of the optimized 6-day GMP compliant procedure for the production of CD19 CAR-T cells is shown in Fig. 5. To validate the functionally closed bag–based process, five engineering runs were performed at clinical scale compared to the open plate–based spinoculation process using a split-apheresis approach. Apheresis products from lymphoma subjects enrolled in clinical trial (NCT00924326) were split and processed side by side using the two production methods. The percentage of cells transduced in bags compared with cells transduced in plates was lower: 43.6 ± 8.3% and 79.6 ± 1.5% (n = 5), respectively (p < 0.001; Fig. 6A and B). There was no significant difference in the overall cell expansion over the 6-day culture period, which ranged from 4.5- to 16-fold in bags compared with 3.9- to 37-fold in plates (Fig. 6C and D). In addition, there was no significant difference in the total cell number generated between the bag- and plate-based process: 14.6 ± 4.6 × 108 and 12.0 ± 6.0 × 108, respectively (Fig. 6E and F). Of note, while the fold expansion was generally equivalent between the two processes, with one exception (experiment 41), there was no significant difference in the fold expansion or the total cell numbers generated between the two processes. At the transduction step, the cells were counted and seeded at a fixed concentration of 0.5 × 106 cells/mL. Thus, any difference in fold expansion between the two processes is likely a reflection of the cell recovery following the transduction and input cell concentration at the time of expansion. Thus, even though the fold expansion may differ within an experiment, there is no significant difference in total cell numbers at day 6. These data do make it clear that there is significant patient-to-patient variability in terms of T-cell transduction efficiency and cell growth that needs to be taken into account to ensure sufficient numbers of CD19 CAR-T cells for patient treatment. The functionality of the CD19 CAR-T cells manufactured using both the spinoculation and the functionally closed bag-based process was assessed in co-culture experiments (Table 1). Comparable levels of INF-γ were secreted in response to CD19+ cell lines. The phenotype of the cells produced by both culture systems was assessed by FACS, and typically >95% of the cells are CD3+ T cells. The final product at day 6 may also contain a small percentage of autologous NK and/or NK-T cells (data not shown). The composition of the T-cell subsets can vary greatly between patients. For the engineering runs, the CD19 CAR-T cell products were comprised of comparable percentages of naïve (CCR7+/CD45RA+), Tcm (CCR7+/CD45RA−), Tem (CCR7−/CD45RA−), and Teff (CCR7−/CD45RA+) cell subsets, with the majority of cells being of the Tem phenotype (Table 2 and Supplementary Fig. S2).
Figure 5.
Manufacturing scheme for the functionally closed production of CD19 CAR-T cells. An overview of the 6-day cell production process developed at the Surgery Branch of the National Cancer Institute. The schematic shows a comparison between the 10-day open spinoculation process for the administration of a non-cryopreserved cell product and the new 6-day closed-cell production process followed by cryopreservation of the final cell product. Optimizations achieved at each process step are indicated.
Figure 6.
Comparison of an open versus functionally closed production system for the manufacture of CD19 CAR-T cells. To evaluate the efficacy of the production process with closed-unit operations, five engineering runs were performed at scale and compared directly to cells manufactured using the open spinoculation process. (A and B) The transduction of PBMCs with anti-CD19 CAR viral vectors in a closed bag system (closed) was compared to the previous Surgery Branch (open) plate transduction platform. In both cases, 1 × 109 PBMCs were stimulated with soluble OKT3 for 2 days, followed by transduction of PBMCs at a density of 0.5 × 106 cells/mL with a 2× diluted vector supernatant. Transduction was significantly higher for cell manufactured using the open system (p < 0.001). (C and D) The comparison of the cell expansion at the end of the 6 days. (E and F) The comparison of total cell numbers between open and closed production process over 6 days. The data are representative of five engineering runs at scale. Individual experiments (A, C, and E) are plotted, as well as summary data (B, D, and F) for each parameter evaluated. Summary data are presented as the mean ± SEM.
Table 1.
Functional comparison of CAR-T cells manufactured using open versus closed production platform
| IFN-γ (pg/mL ± SEM) | ||
|---|---|---|
| Cella | Openb | Closedc |
| None | 133.8 ± 66.8 | 42.0 ± 13.6 |
| Toledo | 15169 ± 5494.3 | 27026.2 ± 16501.6 |
| NALM6 | 12773.4 ± 4629.7 | 24241.2 ± 12432.8 |
| CEM | 115.6 ± 54.0 | 21.0 ± 13.7 |
CD19+ cell lines (Toledo, NALM6); CD19− (CEM).
Open: cells transduced by spinoculation.
Closed: cells transduced using closed production process.
CAR-T cells, chimeric antigen receptor T cells; IFN-γ, interferon gamma; SEM, standard error of the mean.
Table 2.
Comparison of final T-cell phenotype from five engineering runs for cells grown in open versus closed production platform
| Opena | Closedb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell | Phenotypec | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Mean ± SEM | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Mean ± SEM |
| Tnaive | CD45RA+/CCR7+ | 3.0 | 9.0 | 1.0 | 7.0 | 60.0 | 16.0 ± 11.1 | 14.0 | 5.0 | 3.0 | 24.0 | 67.0 | 22.6 ± 11.7 |
| TCM | CD45RA−/CCR7+ | 37.0 | 20.0 | 22.0 | 31.0 | 1.9 | 22.4 ± 6.0 | 20.0 | 16.0 | 16.0 | 39.0 | 1.0 | 18.4 ± 6.1 |
| TEM | CD45RA−/CCR7− | 57.0 | 62.0 | 74.0 | 51.0 | 1.0 | 49.0 ± 12.6 | 35.0 | 67.0 | 75.0 | 27.0 | 1.0 | 41.0 ± 13.5 |
| TEMRA | CD45RA+/CCR7− | 3.0 | 9.0 | 3.0 | 12.0 | 37.0 | 12.8 ± 6.3 | 31.0 | 11.0 | 6.0 | 11.0 | 31.0 | 18.0 ± 5.4 |
Open: cells transduced by spinoculation in plates.
Closed: cells transduced in bags using closed production process.
Phenotype shown as percentage of CD3+ lymphocytes.
Discussion
Phase I clinical trials utilizing CD19 CAR-T cells have produced durable, often complete, responses in heavily pretreated patients with advanced B-cell malignancies. Initial methodologies for transducing and expanding T cells included steps in open cell culture or processing vessels, resulting in a complex manufacturing process that was both labor intensive and difficult to scale out. Therefore, a simple, robust production process was developed for the manufacture of CD19 CAR-T cells utilizing a gammaretroviral vector and autologous T lymphocytes in 6 days that removes HS from the process stream and employs functionally closed processing at several key unit operations. This 6-day process shortens the time required to generate a cell product and generates equal or greater number of TNaive and TCM anti-CD19 CAR-T cells compared with the open production process (Table 2). Less differentiated T-cell subsets (TNaive and TCM) may provide more therapeutic benefit following adoptive cell transfer. However, clinical data to support this claim have yet to be generated.28,29 The simplicity of this process is also reflected in the fact that it does not require the addition and subsequent removal of anti-CD3/CD28 beads for T-cell stimulation, nor is there a need to sort out a less differentiated T-cell population physically prior to transduction.
In the development of a functionally closed production process for CD19 CAR-T cells, a media formulation (OpTmizer + 2.5% TCSR) for the expansion of T cells was identified that is free from allogeneic and xenogeneic components. This medium formulation had no detrimental effect on cell growth or CD19 CAR-T transduction efficiency (Figs. 1 and 2). In addition, it is beneficial to utilize a fully defined medium that demonstrates little lot-to-lot variability, allowing for more consistent product manufacturing. For activation of the PBMCs prior to transduction, soluble OKT3 was used, which relies on monocytes and other antigen-presenting cells to bind and present the OKT3, as well as provide CD28 co-stimulation. The co-stimulation of PBMCs using anti-CD3/CD28 beads was not evaluated due to the added process complexity, as well as a potentially skewed CD3+ population toward CD4+ T cells.
One limitation of this new procedure is that the CD19 CAR-T cell transduction efficiency using a retroviral vector encoding the anti-CD19 CAR and detected using an anti-F(ab)2 detection method is lower in bags than the previously described when cells are transduced using the spinoculation method. The decrease in transduction efficiency in bags is likely due to scale up of the number of cells transduced in PL240 bags, as there was no difference in transduction efficiency when transductions were carried out in PL07 bags (Fig. 3). It is possible that removal of the spinoculation step reduces the CD19 CAR-T transduction efficiency. However, given the lower cell numbers required for CAR-T cell therapies, introduction of a bag centrifugation step did not increase the transduction efficiency enough to warrant the added process complexity. Alternatively, use of an indirect method for CD19 CAR-T cell detection may underrepresent the number of CAR receptors on the T-cell surface. More effort will be required to evaluate the ability to detect CD19 CAR on T cells using direct detection methods such as the anti-FMC63 anti-idiotype antibody or conjugated-CD19 protein. While efforts are ongoing to improve the transduction efficiency in bags, our functionally closed-cell production process is capable of generating sufficient numbers of CD19 CAR-T cells for patient treatment (currently 2 × 106 CAR + T cells/kg) with specific and robust recognition of CD19+ tumor cell lines. Importantly, generation of larger numbers CD19 CAR-T cells can be simply achieved by increasing the cell expansion phase beyond 4 days. In the future, it may be possible to automate the gammaretroviral vector transduction of T cells and their expansion similar to that done for lentiviral vector-mediated T cell transduction. However, as of now, that technology is currently not available.30,31
In conclusion, a robust, rapid cell production process has been developed for the administration of a 6-day cryopreserved T-cell product that targets CD19 expressing tumors. The manufacturing process described will allow this promising adoptive cell transfer therapy to enter multicenter clinical trials, which will hopefully facilitate further commercial development.
Supplementary Material
Acknowledgments
This work was supported, in part, by Kite Pharma, Inc., through a collaborative research and development agreement (CRADA) with Surgery Branch, NCI, National Institutes of Health. Thanks also go to David Stroncek and the Department of Transfusion Medicine, National Institutes of Health, for technical input and discussion.
Disclosure Statement
M.B. has employment and equity ownership in Kite Pharma. O.P. is a former KITE Pharma employee with no outstanding financial interests. No competing financial interests exist for the remaining authors.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethedsda, MD: National Cancer Institute, 2016 [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenkre VP, Smith SM. Management of relapsed diffuse large B-cell lymphoma. Curr Oncol Rep 2008;10:393–403 [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:S82–91 [DOI] [PubMed] [Google Scholar]
- 5.Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol 1989;143:712–717 [PubMed] [Google Scholar]
- 6.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 2012;119:2709–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013;19:2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a Phase 1 dose-escalation trial. Lancet 2014;385:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013;122:4129–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139–303ra139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126:2123–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Kochenderfer JN. Personalized cell transfer immunotherapy for B-cell malignancies and solid cancers. Mol Ther 2011;19:1928–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003;26:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman SA, Goff SL, Xu H, et al. Rapid production of clinical-grade gammaretroviral vectors in expanded surface roller bottles using a “modified” step-filtration process for clearance of packaging cells. Hum Gene Ther 2011;22:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 2009;32:689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorg N, Poppe C, Bunos M, et al. Red blood cell depletion from bone marrow and peripheral blood buffy coat: a comparison of two new and three established technologies. Transfusion 2015;55:1275–1282 [DOI] [PubMed] [Google Scholar]
- 26.Stein A. Decreasing variability in your cell culture. Biotechniques 2007;43:228–229 [DOI] [PubMed] [Google Scholar]
- 27.Dodo K, Chono H, Saito N, et al. An efficient large-scale retroviral transduction method involving preloading the vector into a RetroNectin-coated bag with low-temperature shaking. PLOS ONE 2014;9:e86275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinrichs CS, Borman ZA, Gattinoni L, et al. T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 2011;117:808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klebanoff CA, Scott CD, Leonardi AJ, et al. Memory T cell–driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 2016;126:318–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mock U, Nickolay L, Philip B, et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS Prodigy. Cytother 2016;18:1002–1011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.