Abstract
Purpose: Brimonidine is a selective alpha-2 adrenergic agonist used to reduce intraocular pressure and it has been shown to have some neuroprotective effects. Hydroquinone (HQ) is a toxicant present in cigarette smoke, and other sources. In this study, we investigated the cyto-protective effects in vitro of Brimonidine on human retinal pigment epithelium cells (ARPE-19) and human retinal Müller cells (MIO-M1) that had been treated with HQ.
Methods: Cells were pretreated for 6 h with different doses of Brimonidine tartrate 0.1% (1/2×, 1×, 5×, 10×), followed by a 24-h exposure to 100 μM of HQ, while the Brimonidine was still present. Assays were used to measure cell viability, mitochondrial membrane potential (ΔΨm), reactive oxygen species (ROS) production, and lactate dehydrogenase (LDH) release.
Results: Brimonidine increased the cell viability at all concentrations studied in both cell lines studied. ΔΨm also improved at all Brimonidine doses in ARPE-19 cells and in the 5× and 10× dosages MIO-M1 cells. The ROS levels decreased at 1×, 5×, and 10× doses of Brimonidine in ARPE-19 but only at 10× on MIO-M1 cells. The 10×-Brimonidine ARPE-19 cells had decreased LDH release, but no LDH changes were observed on MIO-M1 cells.
Conclusion: HQ-induced toxicity is mediated through mitochondrial damaging, oxidative stress-related and necrosis-related pathways; Brimonidine significantly prevented the mitochondrial damaging and oxidative stress-related effects but had little effect on blocking the necrosis component of HQ-toxicity. Brimonidine protective effects differ between the different retinal cell types and high concentrations of Brimonidine (10×) have minimal damaging effects on human retinal cells.
Introduction
Brimonidine is a selective alpha-2 adrenergic agonist, commonly used topically to lower the intraocular pressure (IOP) in primary open-angle glaucoma and ocular hypertension. The mechanisms of action for alpha-2 agonists are to inhibit adenylate cyclase, decrease intracellular cAMP, and cause ciliary vasoconstriction, which reduces aqueous production in the ciliary body and increases uveoscleral outflow, leading to IOP reduction.1–3
Several studies have proved the safety and efficacy of Brimonidine alone or combined with other glaucoma treatments in lowering IOP.1,4 Optic neuroprotection is commonly referred to the protection of retinal ganglion cells (RGCs) from different optic nerve injuries.5 Authors have proposed that to be considered neuroprotective, a drug should accomplish 4 criteria: (1) it must target receptors on its target tissue; (2) it must show pharmacological levels of penetration into the vitreous and retina; (3) it must induce intracellular changes in neurons that impede apoptosis or increase neuronal resistance to the insult; and (4) similar efficacy must be shown in clinical trials.6
Several in vitro and animal models studies have suggested a neuroprotective effect of Brimonidine to different optic nerve insults, including glaucomatous optic neuropathy, ischemic neuropathies, and other optic nerve disorders.7–9 Brimonidine has also been suggested to protect photoreceptors from phototoxicity.10 Brimonidine transport has been described in retinal pigment epithelium (RPE) cells in vitro and in animal models11; and alpha-2 adrenergic receptors have been found in Müller cells in vitro and in vivo.12 Furthermore, in increased IOP and normal tension glaucoma animal models, a role of Müller cells in neuroprotection of RGCs has been suggested.13,14
Smoking is an important risk factor for age-related macular degeneration (AMD).15 In 2000, the Age-Related Eye Disease Study (AREDS), a large epidemiologic study, found association between smoking and advanced AMD, including geographic atrophy and neovascularization.16 The 10-year follow-up of the initial AREDS subjects, showed that current smoking is also a risk factor for progression to neovascular AMD.17 Oxidative stress and inflammation are believed to be possible mechanisms for this association.18,19
Cigarette smoke contains thousands of toxic and carcinogenic chemicals,20,21 which can be divided into 2 categories: the gas-phase smoke components and substances retained in the filter (referred to tar). The most common component in cigarette tar is hydroquinone (HQ), semiquinone mixture.22 Up to 155 μg of HQ has been reported per cigarette.23 HQ is also used as a reducing agent in the petrochemical, rubber, and polymers industries. HQ is found in black and white photography paper and is used in X-ray film processing.24 Topical cream HQ formulations are commercialized for bleaching hyperpigmented skin, but with concerns from Food and Drug Administration (FDA).25
HQ is rapidly absorbed after inhalation or oral ingestion, the absorption is slower after dermal exposition. The oral lethal dosage of HQ is between 300 and 1,300 mg/kg.26 HQ can be also found in natural sources including food. Increased plasma and urine HQ levels were reported to be found after ingestion of wheat products, pears, coffee, and tea; even in higher levels than after smoking 4 cigarettes in 30 min.27 Previous in vitro studies with retinal cells have shown toxicity from HQ, nicotine, and benzo(e)pyrene (BeP), each a key cigarette smoke compound.28–32
HQ has also been found in vitro and in animal models to affect gene expression of oxidative stress-related mediators and genes related to AMD pathogenesis.33–35 HQ can reduce the activity of matrix metalloproteinase 2 (MMP-2) in vitro,33 downregulate the monocyte chemoattractant protein 1 (MCP1), upregulate vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF) expression in vitro and in mice34, and upregulate the expression of heat shock protein 27 (Hsp-27) in mice and in vitro models.35 In a rat model, it has been reported that after exposition to cigarette smoke or HQ supplemented in the rat's diet, the trial animals developed sub RPE deposits and Bruch's membrane thickening and deposits.36
The human retina integrates different types of cells and neurons with the photoreceptors. The RPE, which overlies Bruch's membrane, is located between the retina and choriocapillaris and constitutes the blood-retinal barrier. The RPE functions include light absorption and protection to photo-oxidation, transepithelial transport between choriocapillaris and photoreceptors, ion buffering and homeostasis in the subretinal space, phagocytosis of the outer segments of photoreceptors, and secretion of platelet-derived growth factor and VEGF.37 Loss of RPE cells is one of the earliest changes in AMD.
Müller cells, the most common glial cell in the retina, provide structural and metabolic support to retinal neurons, by regulating cellular homeostasis, including pH, and modulating neurotransmitter recycling.38,39 They also contribute to the blood-retinal barrier by surrounding retinal capillaries with glial processes38,39 and act as light collectors by directing light to photoreceptors.39,40 Müller cells secrete and regulate VEGF and PEDF, playing a role in wound healing, retinal regeneration, and neuroprotection.38,39,41
The purpose of this study was to evaluate the in vitro protective effects of different Brimonidine concentrations on an immortalized cell line of human RPE cells (ARPE-19) and an immortalized cell line of retinal Müller cells (MIO-M1) treated with HQ.
Methods
Cell culture
ARPE-19 cells were obtained from ATCC, Manassas, VA; and then cultured in Dulbecco's modified Eagle's medium (DMEM) mixture 1:1 Ham's F-12 medium (Corning–Cellgro, Mediatech, Manassas, VA), containing 10% fetal bovine serum (FBS), penicillin G 100 U/mL, streptomycin sulfate 0.1% mg/mL, gentamicin 10 μg/mL, and amphotericin B 2.5 μg/mL. Serum-free medium was used after cells reached monolayer confluence.
Human retinal Müller cells (MIO-M1), obtained from the Department of Cell Biology of the University College, London,38 were grown in Dulbecco's modified medium 1× with high glucose (DMEM+GlutaMAX; Gibco, Carlsbad, CA). Initially, cells were cultured in 10% FBS and penicillin G 100 U/mL, streptomycin sulfate 0.1 mg/mL, but once established, the culture media were changed to 2% FBS. This simulates the natural human Müller cells to remain in a nonproliferating phase. Both cell lines were kept under standard incubating conditions: 37°C and 5% carbon dioxide.
Pretreatment with brimonidine
Brimonidine tartrate 0.1% was obtained from an ophthalmic solution (Alphagan P; Allergan, Irvine, CA) containing Purite as a vehicle. Several dilutions were made to reach 4 different concentrations, 1× (which is defined as the clinical dose 50 μL in 4 mL of solution), 1/2×, 5×, and 10×. The highest concentration (10×) of Brimonidine alone, only dissolved in dimethyl sulfoxide (DMSO) without HQ, was used as a control.
Exposure to HQ
Commercially available HQ powder was solubilized into DMSO and 100 μM solution prepared. This concentration of HQ was taken from previous studies showing that this concentration could stress the cells without being toxic.28,29 Cells were pretreated with Brimonidine in the different concentrations for 6 h. Then, cells were exposed to 100 μM HQ in culture media and incubated at 37°C for 24 h. Brimonidine was kept in the medium throughout the culture period. Equivalent amount of DMSO was used as a control.
Controls
Three different controls were used in this study: (1) cells treated with 10× Brimonidine alone, (2) cells treated with 100 μM DMSO (the maximum amount of DMSO used to solubilize the HQ), and (3) untreated cells.
Cell viability assay
About 5 × 105 cells per well were plated in 6-well plates for 24 h and treated as mentioned above. Cells were harvested using trypsin-EDTA 0.2% for 5 min. Cells were centrifuged for 5 min at 1,000 rpm, and then resuspended in 1 mL of culture medium. Cell viability was assessed by trypan blue dye-exclusion with an automated ViCell cell viability analyzer (Beckman Coulter, Inc., Fullerton, CA).
Mitochondrial membrane potential (ΔΨm)
Detection of ΔΨm values was performed using the JC-1 mitochondrial membrane potential detection kit (Biotium, Hayward, CA). JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine-iodide) is a cationic dye that accumulates in the mitochondrial membranes of healthy cells resulting in red fluorescence (590 nm). Stressed or damaged cells have reduced ΔΨm and the JC-1 dye accumulates in the cytosol instead of the mitochondria, resulting in green fluorescence (530 nm). The ratio of red to green fluorescence is calculated to obtain the changes in ΔΨm. The fluorescent signals were measured with the fluorescent image scanning unit FMBio III (Hitachi Solutions America, San Bruno, CA) set to detect green (excitation [EX] 485 nm, emission [EM] 535 nm) and red (EX 550 nm, EM 600 nm) emissions.
Reactive oxygen species assay
Reactive oxygen species (ROS) production was assessed using the fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen-Molecular Probes, Eugene, OR).42 The fluorescent signal was measured using the fluorescent image scanning unit FMBio III (Hitachi Solutions America) with EX filter in 550 nm and EM filter in 600 nm. This assay detects hydrogen peroxide, peroxyl radicals, and peroxynitrite anions.
Lactate dehydrogenase cytotoxicity assay
The lactate dehydrogenase (LDH) assay was performed using the LDH Cytotoxicity Assay Kit II (BioVision, Inc., Mountain View, CA) according to the manufacturer's protocol. The amount of LDH released by the cells can be used as an index of necrosis. This kit uses the WST (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) reagent for detection of LDH released from the damaged cells. LDH oxidizes lactate to generate NADH, reacting with WST, which can be quantified at 450 nm optical density with a BioTek ELx808 absorbance plate reader (BioTek, Winooski, VT).
Statistical analyses
Data were analyzed with unpaired t-test and one-way ANOVA using the GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com). P < 0.05 (*) statistically significant; P < 0.01 (**) very significant; P < 0.001 (***) extremely significant. Error bars in the graphs represent SEM with experiments performed in triplicate.
Results
Cell viability studies
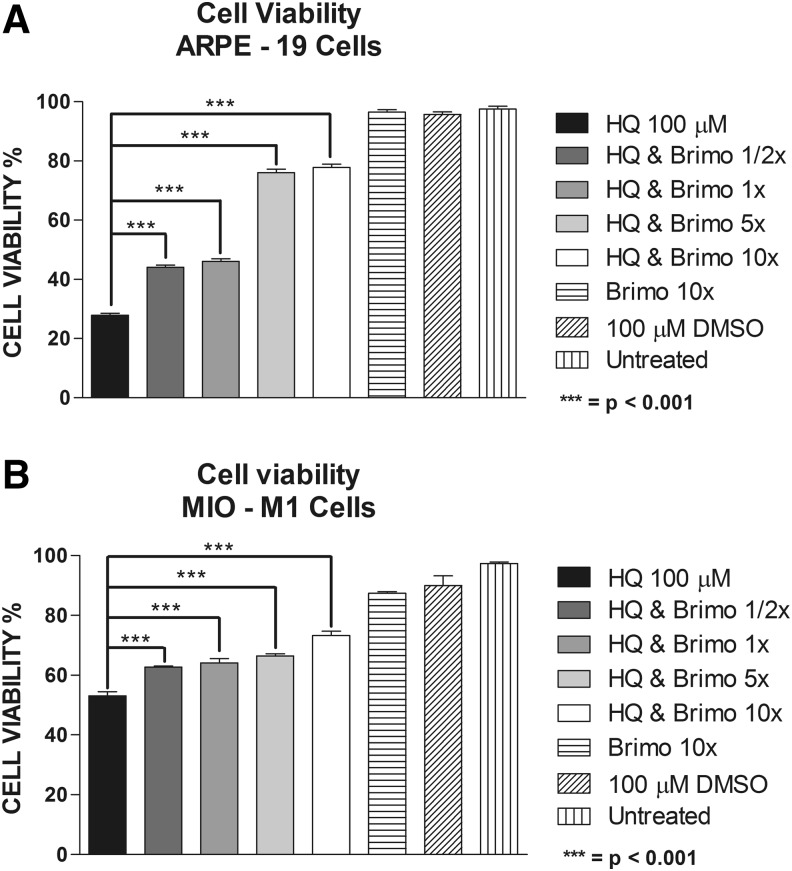
Mean percentage cell viability for ARPE-19 cells exposed to 100 μM of HQ was 27.92 ± 0.66 compared to the DMSO-equivalent of 95.73 ± 0.84 (P < 0.0001) and for MIO-M1 cells was 53.1 ± 1.34 compared to the DMSO-equivalent of 89.95 ± 3.27 (P < 0.0001). At 1/2×, 1×, 5×, and 10× of the Brimonidine clinical dose, the mean cell viability for the ARPE-19 cells was 44.03 ± 0.74 (P < 0.0001), 46.07 ± 0.89 (P < 0.0001), 76.02 ± 1.18 (P < 0.0001), and 77.80 ± 1.05 (P < 0.0001), respectively. For the MIO-M1, the cell viability values were 62.67 ± 0.41 (P < 0.0001), 64.15 ± 1.38 (P < 0.0001), 66.49 ± 0.64 (P < 0.0001), and 73.27 ± 1.42 (P < 0.0001) for the 1/2×, 1×, 5×, and 10× Brimonidine doses, respectively. Cell viability for Brimonidine alone 10× and untreated cells was 96.48 ± 0.77 and 97.56 ± 0.86 for ARPE-19, respectively; and 87.38 ± 0.49 and 97.41 ± 0.48 for MIO-M1, respectively (Fig. 1a, b).
FIG. 1.
Cell viability assay. (A) ARPE-19 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to hydroquinone (HQ) 100 μM. (B) MIO-M1 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM.
Mitochondrial membrane potential
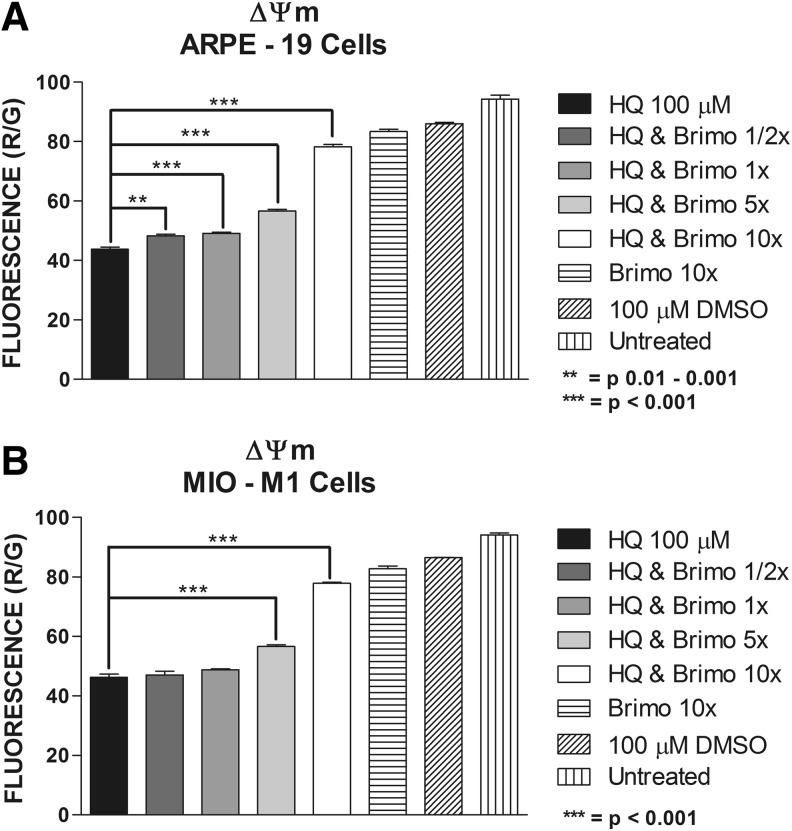
The ΔΨm fluorescence ratios in the ARPE-19 cells exposed to 100 μM HQ were 43.8 ± 0.74 (P < 0.0001) compared to 100 μM DMSO-equivalent dose of 86.08 ± 0.39 and for MIO-M1 were 46.33 ± 1.07 (P < 0.0001) compared to the 100 μM DMSO-equivalent of 86.55 ± 0.08. At 1/2×, 1×, 5×, and 10× Brimonidine co-treated groups, the ARPE-19 ΔΨm fluorescence ratios were 48.25 ± 0.58 (P = 0.0033), 49.13 ± 0.41 (P = 0.0008), 56.65 ± 0.58 (P < 0.0001), and 78.18 ± 0.90 (P < 0.0001), respectively (Fig. 2a). For MIO-M1 cells, only at 5× Brimonidine dose (56.05 ± 0.45, P = 0.0001) and 10× Brimonidine dose (77.85 ± 0.31, P < 0.0001) the ΔΨm was significantly changed after treatment with HQ (Fig. 2b). ΔΨm fluorescence ratios for Brimonidine 10× alone and untreated cells were 83.4 ± 0.76 and 94.25 ± 1.38, respectively, for ARPE-19 cells; and 82.80 ± 0.92 and 94.10 ± 0.69, respectively, for MIO-M1 cells.
FIG. 2.
Mitochondrial membrane potential (ΔΨm) assay. (A) ARPE-19 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM. (B) MIO-M1 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM.
ROS assay
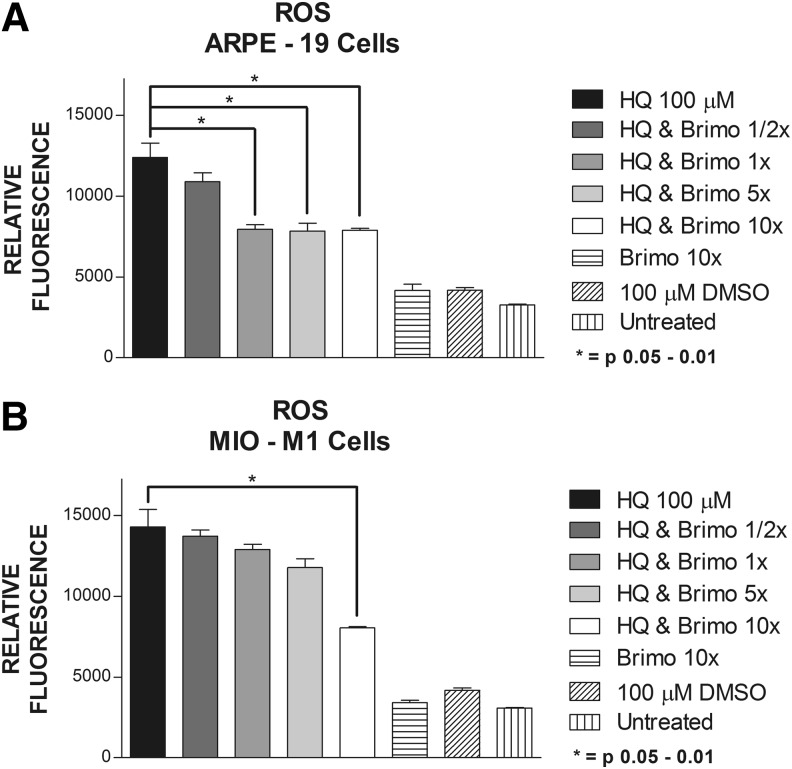
ROS levels of the ARPE-19 cells were increased with 100 μM of HQ with relative fluorescence values of 12,400 ± 878. The ROS values were lowered after treatment with Brimonidine at doses of 1× (7,944 ± 290, P = 0.041), at 5× dose (7,830 ± 495, P = 0.045), and at 10× dose (7,887 ± 111, P = 0.0364) (Fig. 3a). MIO-M1 cultures had increased ROS levels with 100 μM HQ (14,300 ± 1,078) and the ROS levels were decreased only at 10× dose Brimonidine (8,049 ± 72.5, P < 0.0286) (Fig. 3b). The ROS levels for Brimonidine alone groups were 4,159.5 ± 395 and 3,410 ± 144 for ARPE-19 and MIO-M1 cells, respectively. The results for 100 μM DMSO groups were 4,177 ± 156 for ARPE-19 cells and 4,173 ± 152 for MIO-M1 cells. Untreated ARPE-19 cells showed ROS levels of 3,267 ± 46 and MIO-M1 cells of 3,069 ± 43.5.
FIG. 3.
Reactive oxygen species (ROS) production. (A) ARPE-19 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM. (B) MIO-M1 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM.
LDH release assay
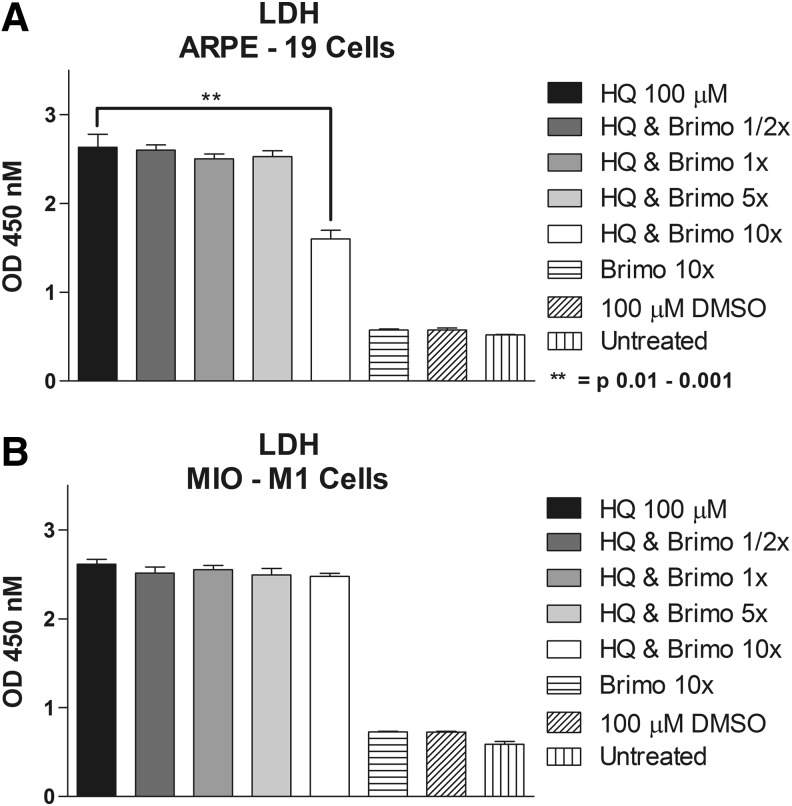
Conditioned media of ARPE-19 cells pretreated with 10× Brimonidine showed significant lower LDH levels compared with 100 μM HQ alone (1.6 ± 0.1 vs. 2.63 ± 0.15, P = 0.0042). There was no significant protective effect for 1/2× Brimonidine (2.60 ± 0.058, P = 0.84), 1× dose (2.5 ± 0.058, P = 0.44), and 5× dose (2.5 ± 0.065, P = 0.54) (Fig. 4a). In the MIO-M1 conditioned media, LDH levels did not decrease at any of the studied concentrations: 2.516 ± 0.057 (P = 0.32), 2.554 ± 0.046 (P = 0.46), 2.494 ± 0.074 (P = 0.27), and 2.479 ± 0.0337 (P = 0.11) for 1/2×, 1×, 5×, and 10× Brimonidine doses, respectively, compared to 2.614 ± 0.0574 for 100 μM HQ-treated alone cells (Fig. 4b). In ARPE-19 conditioned media low LDH values were found for Brimonidine 10× alone, 100 μM DMSO and untreated groups (0.57 ± 0.01, 0.57 ± 0.02, and 0.52 ± 0.007, respectively). Similar findings were observed for MIO-M1 conditioned media with LDH release of 0.73 ± 0.004, 0.73 ± 0.007, and 0.59 ± 0.032, for Brimonidine 10× alone, 100 μM DMSO, and untreated groups, respectively.
FIG. 4.
Lactate dehydrogenase (LDH) release. (A) ARPE-19 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM. (B) MIO-M1 cells treated with 1/2×, 1×, 5×, and 10× Brimonidine and exposed to HQ 100 μM.
Discussion
Previous studies have shown HQ toxicity in vitro in human RPE cells (ARPE-19), human Müller retinal cells (MIO-M1), human microvascular endothelial cells, and rat neurosensory retinal cells (R-28).28,29 In this study the HQ at 100 μM concentration caused more loss of cell viability in the ARPE-19 cells compared with the MIO-M1 cells (27.92% vs. 53.1%). In both cell types the mechanisms involved were similar, reducing ΔΨm, and increasing significantly the ROS production and LDH release.
The goal of the study was to determine whether Brimonidine might prevent the harmful effects of the HQ exposure. Studies have shown that Brimonidine, apart from the IOP lowering effect, has a neuroprotective effect for glaucomatous neuropathy and other optic neuropathies and injuries.5,6,8,9 This is important because RGC loss is a key event in glaucomatous neuropathy and vision loss; however, reducing the IOP is not enough to prevent the RGC loss.4,7,8 In addition, Brimonidine neuroprotective effects have been observed in vitro and in animal models, but not in large controlled trials.7 Another factor that makes Brimonidine a possible cyto-protectant for the retina is that after topical instillation of Brimonidine, good concentrations have been found in the retina,7 and both RPE and retinal Müller cells have alpha-2 adrenergic receptors.11,12,14
Our study shows that Brimonidine improved CV of both cell lines and the Brimonidine protective effects were dose dependent: the higher the Brimonidine dose, the higher the CV in both cell lines. This effect was more pronounced in the ARPE-19 cells, where CV improved from 27.92% to 77.8% in Brimonidine 10× group; while in the MIO-M1 the improvement was from 53.1% CV HQ alone to 73.27% at 10×. Also for ARPE-19 cells, the improvement in 10× group is higher than Brimonidine 5× group but there is no statistically significant difference between them.
Clinically, it is important to identify cyto-protective drugs for human RPE cells as they are damaged in AMD, the most common cause of vision loss in the elderly. In ARPE-19 cultures, the 10× dose Brimonidine increased CV by 50% compared with the HQ-treated alone. In addition, Brimonidine is a drug that has been used in glaucoma patients for many years and has an excellent safety record. This makes Brimonidine an attractive candidate for cyto-protection in early AMD or high-risk patients.
While Brimonidine can protect both ARPE-19 and MIO-M1 cells, the mechanisms for action appear to differ. In the ARPE-19 cells, all of the concentrations of Brimonidine improved the ΔΨm in HQ-treated cultures but in the MIO-M1 cells it was only the Brimonidine 5× and 10× concentration that were protective. The 10× Brimonidine was the most effective concentration to increase the mitochondrial membrane potential in both cell lines, and although it caused mild decrease in ΔΨm compared to DMSO control, the reversing effects to the HQ-induced toxicity were very significant. It should be noted that even at 10× Brimonidine, the ΔΨm values were still lower than in the DMSO control.
Similar differential cyto-protective effects for Brimonidine were also found with respect to ROS protection. Brimonidine significantly decreased ROS production on ARPE-19 cells at the 1×, 5×, and 10× concentrations but not in a dose-dependent fashion. In contrast, only the 10× Brimonidine concentrations were able to significantly reduce ROS production in the MIO-M1 cell line. These findings suggest that the Brimonidine cyto-protective effects for the ARPE-19 cells are through an oxidative stress pathway, which is generating ROS, while this pathway may be less involved in the MIO-M1 cells. Alternatively, it may be that more ROS are produced by Müller cells than ARPE-19 cells and this necessitates higher doses of the drug to have a significant effect.
Increase in LDH release is typically an indicator of cell damage and necrosis. In our study, for both ARPE-19 and MIO-M1 cells, HQ exposure resulted in significantly elevated levels of LDH, and only the 10× Brimonidine significantly decreased LDH release for the ARPE-19 cells compared to HQ-treated alone. Although there was a significant reduction of LDH levels in 10× Brimonidine group, it was still 2.8 times higher than in DMSO control group. Interestingly, no changes in LDH levels were found in MIO-M1 cells in any Brimonidine concentrations, suggesting that the necrosis pathway was not involved.
Studies have shown that in glaucoma neuropathy and induced optic nerve ischemia models, apoptosis is responsible of the progressive RCG loss, and that Brimonidine can protect RCG from that apoptosis process43–45 Previous studies in ARPE-19 and MIO-M1 cells have shown that HQ did not induce apoptosis (no changes in caspase activities were shown), but in MIO-M1 cells, autophagy markers were found.28,29 In our study, Brimonidine treatment was nevertheless able to increase the CV, suggesting that Brimonidine may be protecting ARPE-19 cells by blocking an oxidative stress (ROS producing) pathway, while the cell viability of the MIO-M1 cells may be protected by a non-apoptosis/non-necrosis pathway (eg, autophagy).
Our findings suggest that high Brimonidine doses (10×) can significantly block the HQ-induced toxicity in immortalized human cell lines of RPE and retinal Müller cells. Brimonidine can improve the cell viability and mitochondrial membrane potential and also partially reduce the ROS formation in both cell lines. However, Brimonidine had minimal to no effects on the LDH production, which represents necrosis.
Our findings indicate that (1) HQ-induced toxicity is mediated through mitochondrial-damaging, oxidative stress-related and necrosis-related pathways; (2) while Brimonidine can significantly prevent mitochondrial damaging and oxidative stress-related effects in the ARPE-19 cells, other cyto-protective agents should be identified that can efficiently block the necrosis component of HQ-toxicity; (3) the protective effects of Brimonidine differ between the different retinal cell types (eg, RPE cells vs. Müller cells); and (4) human retinal cells can safely be exposed to high concentrations of Brimonidine (10×) with minimal damaging effects.
As in any study with immortalized cell lines grown under stringent culture conditions, it should be acknowledged that in situ or primary RPE or retinal Müller cultures may have different responses to the Brimonidine protection. This supports that further testing in primary in vitro or in vivo models should be performed.
In summary, although the HQ toxicity may not be too frequent among the general population, and the amount of HQ acquired by smoking may not be high, these studies provide novel information regarding cell protection by Brimonidine in human RPE cells and retinal Müller cells. The fact that Brimonidine can improve mitochondrial health, reduce the oxidative stress damage, and confer cell protection, in in vitro and animal models in RGC,5–9,13,44,46 and now in RPE and Müller cells in vitro, opens the possibility of use for several clinical entities. This also opens the possibility that Brimonidine may not only be a neuroprotectant (in relation to RGC) but also a retinal-protectant. Of course, further studies are needed to clarify its role in vitro, in vivo, and in clinical trials.
As mentioned above, RGC neuroprotection has not been shown yet in clinical trials,7,47 but there are ongoing clinical trials using a Brimonidine implant for geographic atrophy, a final irreversible stage in AMD.48 As AMD has such a large impact on vision loss in the aging populations, more basic science research and clinical trials are needed to identify cyto-protective agents for this disease, along with other age-related ocular disorders.
Acknowledgments
This research was done with the support of the Discovery Eye Foundation, Iris and B. Gerald Cantor Foundation, Henry L. Guenther Foundation, Polly and Michael Smith Foundation, and Lincy Foundation.
Author Disclosure Statement
C.R., J.C., J.C., M.T.M., M.C., G.A.L., and M.C.K. have no financial interests to disclose related to this research. J.C.d.C. is the David and Julianna Pyott Pan-American Retina Research Fellow and has no financial interests to disclose related to this research. B.D.K. is a consultant to the following companies: AcuFocus, Alcon, Allegro, Allergan, Ampio, Aqua Therapeutics, Genentech, Glaukos, Neurotech, Novartis, Ophthotech, Regeneron, SecondSight, Staar Surgical, Teva, and ThromboGenics. Additionally, Dr. B.D.K. has received grants/research support from Alcon, Allegro, Allergan, Genentech, Glaxo Smith Kline, Neurotech, Ophthotech, Regeneron, and ThromboGenics.
References
- 1.Arthur S., and Cantor L.B. Update on the role of alpha-agonists in glaucoma management. Exp. Eye Res. 93:271–283, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Toris C.B., Gleason M.L., Camras C.B., and Yablonski M.E. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch. Ophthalmol. 113:1514–1517, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Reitsamer H.A., Posey M., and Kiel J.W. Effects of a topical alpha2 adrenergic agonist on ciliary blood flow and aqueous production in rabbits. Exp. Eye Res. 82:405–415, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer N., Lamparter J., Gericke A., Grus F.H., Hoffmann E.M., and Wahl J. Neuroprotection of medical IOP-lowering therapy. Cell Tissue Res. 353:245–251, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Weinreb R.N. Is neuroprotection a viable therapy for glaucoma? Arch. Ophthalmol. 117:1540, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Wheeler L.A., Gil D.W., and WoldeMussie E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv. Ophthalmol. 45 Suppl 3:S290–S294; discussion S295–S296, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Saylor M., McLoon L.K., Harrison A.R., and Lee M.S. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: an evidence-based review. Arch. Ophthalmol. 127:402–406, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bessero A.C., and Clarke P.G. Neuroprotection for optic nerve disorders. Curr. Opin. Neurol. 23:10–15, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Danesh-Meyer H.V. Neuroprotection in glaucoma: recent and future directions. Curr. Opin. Ophthalmol. 22:78–86, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Ortin-Martinez A., Valiente-Soriano F.J., Garcia-Ayuso D., Alarcon-Martinez L., Jimenez-Lopez M., Bernal-Garro J.M., Nieto-Lopez L., Nadal-Nicolas F.M., Villegas-Perez M.P., Wheeler L.A., and Vidal-Sanz M. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS One. 9:e113798, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang N., Kannan R., Okamoto C.T., Ryan S.J., Lee V.H., and Hinton D.R. Characterization of brimonidine transport in retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 47:287–294, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Harun-Or-Rashid M., Lindqvist N., and Hallbook F. Transactivation of EGF receptors in chicken Muller cells by alpha2A-adrenergic receptors stimulated by brimonidine. Invest. Ophthalmol. Vis. Sci. 55:3385–3394, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Semba K., Namekata K., Kimura A., Harada C., Mitamura Y., and Harada T. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 5:e1341, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidal L., Diaz F., Villena A., Moreno M., Campos J.G., and Perez de Vargas I. Reaction of Muller cells in an experimental rat model of increased intraocular pressure following timolol, latanoprost and brimonidine. Brain Res Bull. 82:18–24, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Solberg Y., Rosner M., and Belkin M. The association between cigarette smoking and ocular diseases. Surv. Ophthalmol. 42:535–547, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. Ophthalmology. 107:2224–2232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew E.Y., Clemons T.E., Agron E., Sperduto R.D., Sangiovanni J.P., Davis M.D., and Ferris F.L., 3rd; Age-Related Eye Disease Study Research Group. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. 132:272–277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascella R., Ragazzo M., Strafella C., Missiroli F., Borgiani P., Angelucci F., Marsella L.T., Cusumano A., Novelli G., Ricci F., and Giardina E. Age-related macular degeneration: insights into inflammatory genes. J. Ophthalmol. 2014:582842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiras D., Kitsos G., Petersen M.B., Skalidakis I., and Kroupis C. Oxidative stress in dry age-related macular degeneration and exfoliation syndrome. Crit. Rev. Clin. Lab. Sci. 52:12–27, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Talhout R., Schulz T., Florek E., van Benthem J., Wester P., and Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 8:613–628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. The Scientific Basis of Tobacco Product Regulation, Second Report of a WHO Study Group—WHO Technical Repor Series 951. Available at www.who.int/tobacco/global_interaction/tobreg/publications/9789241209519.pdf 2008. (accessed Jan. 29, 2015)
- 22.Pryor W.A. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect. 105 (Suppl 4):875–882, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton J.L., Trush M.A., Penning T.M., Dryhurst G., and Monks T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 13:135–160, 2000 [DOI] [PubMed] [Google Scholar]
- 24.EPA. Hydroquinone hazard summary. In: Agency E.P., ed. Environmental Protection Agency—Technology Transfer Network. Available at www.epa.gov/airtoxics/hlthef/hydroqui.html revised January 2000. (accessed March5, 2015)
- 25.FDA. Hydroquinone Studies Under The National Toxicology Program (NTP). Available at http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm203112.htm (accessed March5, 2015)
- 26.WHO. International Programme on Chemical Safety—Health and Safety Guide No. 101—Hydroquinone, Health and Safety Guide. In: Programme UNE, ed. Available at www.inchem.org/documents/hsg/hsg/hsg101.htm (Section Number: 2.2) 1996 (accessed Jan. 29, 2015)
- 27.Deisinger P.J., Hill T.S., and English J.C. Human exposure to naturally occurring hydroquinone. J. Toxicol. Environ. Health. 47:31–46, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Sharma A., Patil J.A., Gramajo A.L., Seigel G.M., Kuppermann B.D., and Kenney C.M. Effects of hydroquinone on retinal and vascular cells in vitro. Indian J. Ophthalmol. 60:189–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez C., Pham K., Franco M.F., Chwa M., Limb A., Kuppermann B.D., and Kenney M.C. Hydroquinone induces oxidative and mitochondrial damage to human retinal Muller cells (MIO-M1). Neurotoxicology. 39:102–108, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Sharma A., Neekhra A., Gramajo A.L., Patil J., Chwa M., Kuppermann B.D., and Kenney M.C. Effects of Benzo(e)Pyrene, a toxic component of cigarette smoke, on human retinal pigment epithelial cells in vitro. Invest. Ophthalmol. Vis. Sci. 49:5111–5117, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Patil A.J., Gramajo A.L., Sharma A., Chwa M., Seigel G.M., Kuppermann B.D., and Kenney M.C. Effects of benzo(e)pyrene on the retinal neurosensory cells and human microvascular endothelial cells in vitro. Curr. Eye Res. 34:672–682, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Patil A.J., Gramajo A.L., Sharma A., Seigel G.M., Kuppermann B.D., and Kenney M.C. Differential effects of nicotine on retinal and vascular cells in vitro. Toxicology. 259:69–76, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Alcazar O., Cousins S.W., and Marin-Castano M.E. MMP-14 and TIMP-2 overexpression protects against hydroquinone-induced oxidant injury in RPE: implications for extracellular matrix turnover. Invest. Ophthalmol. Vis. Sci. 48:5662–5670, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pons M., and Marin-Castano M.E. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS One. 6:e16722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pons M., Cousins S.W., Csaky K.G., Striker G., and Marin-Castano M.E. Cigarette smoke-related hydroquinone induces filamentous actin reorganization and heat shock protein 27 phosphorylation through p38 and extracellular signal-regulated kinase 1/2 in retinal pigment epithelium: implications for age-related macular degeneration. Am. J. Pathol. 177:1198–1213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinosa-Heidmann D.G., Suner I.J., Catanuto P., Hernandez E.P., Marin-Castano M.E., and Cousins S.W. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest. Ophthalmol. Vis. Sci. 47:729–737, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 85:845–881, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Limb G.A., Salt T.E., Munro P.M., Moss S.E., and Khaw P.T. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1). Invest. Ophthalmol. Vis. Sci. 43:864–869, 2002 [PubMed] [Google Scholar]
- 39.Reichenbach A., and Bringmann A. New functions of Muller cells. Glia. 61:651–678, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Franze K., Grosche J., Skatchkov S.N., Schinkinger S., Foja C., Schild D., Uckermann O., Travis K., Reichenbach A., and Guck J. Muller cells are living optical fibers in the vertebrate retina. Proc. Natl. Acad. Sci. U. S. A. 104:8287–8292, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia M., and Vecino E. Role of Muller glia in neuroprotection and regeneration in the retina. Histol. Histopathol. 18:1205–1218, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Chwa M., Atilano S.R., Reddy V., Jordan N., Kim D.W., and Kenney M.C. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest. Ophthalmol. Vis. Sci. 47:1902–1910, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Tatton W.G., Chalmers-Redman R.M., and Tatton N.A. Apoptosis and anti-apoptosis signalling in glaucomatous retinopathy. Eur. J. Ophthalmol. 11(Suppl 2):S12–S22, 2001 [PubMed] [Google Scholar]
- 44.Aktas Z., Gurelik G., Akyurek N., Onol M., and Hasanreisoglu B. Neuroprotective effect of topically applied brimonidine tartrate 0.2% in endothelin-1-induced optic nerve ischaemia model. Clin. Experiment. Ophthalmol. 35:527–534, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Aktas Z., Gurelik G., Gocun P.U., Akyurek N., Onol M., and Hasanreisoglu B. Matrix metalloproteinase-9 expression in retinal ganglion cell layer and effect of topically applied brimonidine tartrate 0.2% therapy on this expression in an endothelin-1-induced optic nerve ischemia model. Int. Ophthalmol. 30:253–259, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Lee K.Y.C., Nakayama M., Aihara M., Chen Y.N., and Araie M. Brimonidine is neuroprotective against glutamate-induced neurotoxicity, oxidative stress, and hypoxia in purified rat retinal ganglion cells. Mol. Vis. 16:246–251, 2010 [PMC free article] [PubMed] [Google Scholar]
- 47.Sena D.F., and Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2:CD006539, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NIH. A Safety and Efficacy Study of Brimonidine Intravitreal Implant in Geographic Atrophy Secondary to Age-related Macular Degeneration (BEACON) NCT02087085. Available at https://clinicaltrials.gov/ct2/show/NCT02087085 2014. (accessed March12, 2015)