Abstract
A series of new derivatives was prepared by derivatisation of the 7-amino moiety present in 7-amino-3,4-dihydroquinolin-2(1H)-one, a compound investigated earlier as CAI. The derivatisation was achieved by: i) reaction with arylsulfonyl isocyanates/aryl isocyanates; (ii) reaction with fluorescein isothiocyanate; (iii) condensation with substituted benzoic acids in the presence of carbodiimides; (iv) reaction with 2,4,6-trimethyl-pyrylium tetrafluoroborate; (v) reaction with methylsulfonyl chloride and (vi) reaction with maleic anhydride. The new compounds were assayed as inhibitors of four carbonic anhydrases (CA, EC 4.2.1.1) human (h) isoforms of pharmacologic relevance, the cytosolic hCA I and II, the membrane-anchored hCA IV and the transmembrane, tumour-associated hCA IX. hCA IX was the most inhibited isoform (KIs ranging between 243.6 and 2785.6 nm) whereas hCA IV was not inhibited by these compounds. Most derivatives were weak hCA I and II inhibitors, with few of them showing KIs < 10 µm. Considering that the inhibition mechanism with these lactams is not yet elucidated, exploring a range of such derivatives with various substitution patterns may be useful to identify leads showing isoform selectivity or the desired pharmacologic action.
Keywords: Carbonic anhydrase, inhibitor, coumarin, dihydroquinolinone, sulfonamide
Introduction
CO2, bicarbonate and protons are essential molecules/ions in important physiologic processes in the three life kingdoms (Bacteria, Archaea and Eukarya), and for this reason, relatively high amounts of the enzymes carbonic anhydrases (CAs, EC 4.2.1.1) are present in different tissues/cell compartments of most investigated organisms1–11. The α-CAs are present in vertebrates, protozoa, algae and cytoplasm of green plants and in some Bacteria1–19, the β-CAs are predominantly found in Bacteria, algae and chloroplasts of both mono- as well as dicotyledons, but also in many fungi and some Archaea1–11. The γ-CAs were found in plants, Archaea and Bacteria1–11, whereas the δ-, ζ- and θ-CAs seem to be present only in marine diatoms11. The η-CA class has been discovered in protozoa such as those belonging to the genus Plasmodium20. In many organisms, these enzymes are involved in crucial physiological processes connected with respiration and transport of CO2/bicarbonate, pH and CO2 homeostasis, electrolyte secretion in a variety of tissues/organs, biosynthetic reactions (e.g. gluconeogenesis, lipogenesis and ureagenesis), bone resorption, calcification, tumourigenicity and many other physiologic or pathologic processes (thoroughly studied in vertebrates)1–11,21–26, whereas in algae, plants and some bacteria they play an important role in photosynthesis and other biosynthetic reactions8,11. In diatoms δ- and ζ-CAs play a crucial role in carbon dioxide fixation11. Many such enzymes from vertebrates, fungi and bacteria are well-known drug targets, with inhibitors and activators possessing various pharmacologic applications23–42.
Sulfonamides are the most important class of CA inhibitors CAIs1,4–12, with several compounds in clinical use for many years, as diuretics1,26,28, antiglaucoma agents1,27,33, antiepileptics30–34 and more recently as anticancer agents1,2,12. Although a large number of isoform-selective sulfonamide CAIs were reported ultimately, mostly by using the tail approach for their synthesis16–23,26, a large variety of other chemotypes were investigated for their interaction with these enzymes, which led to the development of a large number of non-classic CAIs, belonging to various classes14,33. Here, we report a new series of such derivatives which incorporate the 7-amino-3,4-dihydroquinolin-2(1H)-one scaffold43.
Materials and methods
Chemistry
Anhydrous solvents and all reagents were purchased from Sigma-Aldrich (Milan, Italy). All reactions involving air- or moisture-sensitive compounds were performed under a nitrogen atmosphere using dried glassware and syringes techniques to transfer solutions. Nuclear magnetic resonance (1H-NMR, 13C-NMR) spectra were recorded using a Bruker Advance III 400 MHz spectrometer in DMSO-d6. Chemical shifts are reported in parts per million (ppm) and the coupling constants (J) are expressed in Hertz (Hz). Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; dd, double of doublet. The assignment of exchangeable protons (OH and NH) was confirmed by the addition of D2O.
General procedure for the preparation of compounds 2–20
A solution of 7-amino-3,4-dihydroquinolin-2(1H)-one (1) in dry dimethylformamide (3–5 ml) was treated with a stoichiometric amount of appropriate isocyanates/isothiocyanate. The mixture was stirred at room temperature until the consumption of starting materials (TLC monitoring). The reaction was quenched with a 1.0 M aqueous solution of HCl to give a precipitate that was washed with diethyl ether (3 × 5 ml), filtered and dried under vacuum (compounds 2–19) or extracted with ethyl acetate (3 × 15 ml), the combined organic layers were washed with H2O (3 × 15 ml), dried over Na2SO4, filtered, and concentrated (compound 20) to afford the title compounds 2–20.
N-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)carbamoyl)benzenesulfonamide (2)
Beige solid, yield 89%; m.p.: 272–273 °C; silica gel TLC Rf = 0.16 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.42 (2H, d, J 6.8), 2.81 (2H, d, J 6.8), 6.84 (1H, dd, J 2.0, 8.4), 7.02 (1H, d, J 2.0), 7.06 (1H, d, J 8.4), 7.67 (2H, t, J 8.0), 7.73 (1H, t, J 8.0), 8.00 (2H, d, J 8.0), 8.91 (1H, s, exchange with D2O, NH), 10.03 (1H, s, exchange with D2O, NH), 10.70 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.7, 106.2, 112.9, 117.2, 127.7, 128.3, 129.1, 131.9, 139.2, 140.4, 145.2, 171.2; m/z (ESI negative) 344.0 [M − H]−.
4-Methyl-N-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)carbamoyl)benzenesulfonamide (3)
White solid, yield 60%; m.p.: 260–261 °C; silica gel TLC Rf = 0.16 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.43 (5H, m), 2.81 (2H, t, J 7.8), 6.84 (1H, dd, J 2.0, 8.0), 7.01 (1H, d, J 2.0), 7.06 (1H, d, J 8.0), 7.46 (2H, d, J 8.4), 7.87 (2H, d, J 8.4), 8.82 (1H, s, exchange with D2O, NH), 10.03 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 22.0, 25.1, 31.5, 106.8, 113.4, 119.3, 128.4, 128.8, 130.4, 137.9, 138.1, 139.5, 144.8, 150.1, 171.1; m/z (ESI negative) 358.0 [M − H]−.
2-Methyl-N-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)carbamoyl)benzenesulfonamide (4)
White solid, yield 79%, m.p.: 285–286 °C; silica gel TLC Rf = 0.43 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.42 (2H, d, J 7.6), 2.66 (3H, s), 2.80 (2H, t, J 7.6), 6.81 (1H, d, J 8.0), 7.05 (2H, m), 7.47 (2H, m), 7.61 (1H, m), 8.01 (1H, d, J 7.6), 8.69 (1H, s, exchange with D2O, NH), 10.02 (1H, s, exchange with D2O, NH), 10.58 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 20.6, 25.1, 31.5, 106.7, 113.3, 119.3, 127.2, 128.8, 131.0, 133.3, 134.3, 137.6, 137.8, 138.8, 139.6, 149.8, 171.1; m/z (ESI negative) 358.0 [M − H]−.
4-Chloro-N-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)carbamoyl)benzenesulfonamide (5)
White solid, yield 67%; m.p.: 253–254 °C; silica gel TLC Rf = 0.35 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.43 (2H, t, J 6.8), 2.81 (2H, t, J 6.8), 6.85 (1H, dd, J 2.0, 8.4), 7.01 (1H, d, J 2.0), 7.06 (1H, d, J 8.4), 7.75 (2H, d, J 8.8), 8.01 (2H, d, J 8.8), 8.94 (1H, s, exchange with D2O, NH), 10.03 (1H, s, exchange with D2O, NH), 10.81 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.1, 31.5, 107.0, 113.5, 119.5, 128.8, 130.1, 130.4, 137.8, 139.2, 139.6, 139.8, 150.1, 171.1; m/z (ESI negative) 378.0 [M − H]−.
4-Fluoro-N-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)carbamoyl)benzenesulfonamide (6)
White solid, yield 68%; m.p.: 245–246 °C; silica gel TLC Rf = 0.23 (Ethyl acetate/n-hexane 80% v/v); δH (400 MHz, DMSO-d6) 2.42 (2H, t, J 7.6), 2.81 (2H, t, J 7.6), 6.85 (1H, dd, J 1.8, 8.1), 7.02 (1H, d, J 1.8), 7.06 (1H, d, J 8.1), 7.51 (2H, m), 8.06 (2H, m), 8.92 (1H, s, exchange with D2O, NH), 10.04 (1H, s, exchange with D2O, NH), 10.77 (1H, brs, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.1, 31.5, 106.9, 113.4, 117.2 (d, 2JC–F 23), 119.4, 128.8, 131.6 (d, 3JC–F 10), 137.2 (d, 4JC–F 3), 137.8, 139.5, 150.1, 165.6 (d, 1JC–F 250), 171.1; δF (376 MHz, DMSO-d6) −105.1 (1 F, s); m/z (ESI negative) 362.0 [M − H]−.
1-(2-Oxo-1,2,3,4-tetrahydroquinolin-7-yl)-3-phenylurea (7)
White solid, yield 85%; m.p.: 255–256 °C (dec.); silica gel TLC Rf = 0.65 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, d, J 7.6), 2.83 (2H, d, J 7.6), 6.99 (2H, m), 7.08 (2H, m), 7.31 (2H, d, J 7.9), 7.47 (2H, d, J 7.9), 8.60 (1H, s, exchange with D2O, NH), 8.66 (1H, s, exchange with D2O, NH), 10.09 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.7, 106.1, 112.7, 117.9, 119.0, 122.7, 128.8, 129.7, 139.5, 139.6, 140.6, 153.3, 171.2; m/z (ESI positive) 282.0 [M + H]+.
1-(2-Oxo-1,2,3,4-tetrahydroquinolin-7-yl)-3-(p-tolyl)urea (8)
White solid, yield 88%; m.p.: 276–277 °C; silica gel TLC Rf = 0.48 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.28 (3H, s), 2.46 (2H, t, J 7.6), 2.83 (2H, t, J 7.6), 7.00 (1H, dd, J 2.0, 8.4), 7.09 (4H, m), 7.35 (2H, d, J 8.4), 8.48 (1H, s, exchange with D2O, NH), 8.60 (1H, s, exchange with D2O, NH), 10.07 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 21.2, 25.1, 31.6, 106.0, 112.6, 117.7, 119.1, 128.7, 130.0, 131.5, 138.0, 139.5, 139.7, 153.3, 171.2; m/z (ESI positive) 296.0 [M + H]+.
1-(2-Oxo-1,2,3,4-tetrahydroquinolin-7-yl)-3-(o-tolyl)urea (9)
White solid, yield 90%; m.p.: > 300 °C; silica gel TLC Rf = 0.47 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.27 (3H, s), 2.46 (2H, t, J 6.8), 2.83 (2H, t, J 6.8), 6.97 (1H, t, J 7.2), 7.07 (3H, m), 7.18 (2H, m), 7.89 (2H, m, 1H exchange with D2O, NH), 9.01 (1H exchange with D2O, NH), 10.11 (1H exchange with D2O, NH); δC (100 MHz, DMSO-d6) 18.8, 25.1, 31.7, 105.9, 112.5, 117.7, 121.6, 123.4, 127.1, 128.1, 128.8, 131.1, 138.4, 139.5, 139.8, 153.4, 171.2; m/z (ESI positive) 296.0 [M + H]+.
1-(4-Chlorophenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (10)
White solid, yield 97%; m.p.: 249–250 °C; silica gel TLC Rf = 0.55 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.6), 2.83 (2H, t, J 7.6), 7.00 (1H, dd, J 2.0, 8.4), 7.08 (2H, m), 7.35 (2H, d, J 9.2), 7.50 (2H, d, J 9.2), 8.08 (1H, s, exchange with D2O, NH), 8.88 (1H, s, exchange with D2O, NH), 10.09 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.7, 106.2, 112.8, 118.0, 120.5, 126.1, 128.8, 129.5, 139.5, 139.5, 139.7, 153.3, 171.2; m/z (ESI positive) 316.0 [M + H]+.
1-(2-Chlorophenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (11)
White solid, yield 83%; m.p.: > 300 °C; silica gel TLC Rf = 0.50 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, d, J 7.2), 2.84 (2H, t, J 7.2), 7.08 (4H, m), 7.33 (1H, t, J 8.0), 7.49 (1H, d, J 8.0), 8.20 (1H, d, J 8.0), 8.30 (1H, s, exchange with D2O, NH), 9.41 (1H, s, exchange with D2O, NH), 10.14 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.1, 31.6, 106.0, 112.6, 118.2, 122.0, 122.7, 124.1, 128.5, 128.9, 130.1, 136.9, 139.3, 139.6, 152.9, 171.2; m/z (ESI positive) 316.0 [M + H]+.
1-(4-Fluorophenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (12)
White solid, yield 98%; m.p.: 257–258 °C; silica gel TLC Rf = 0.59 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.45 (2H, t, J 7.8), 2.83 (2H, t, J 7.8), 7.00 (1H, dd, J 2.0, 8.8) 7.08 (2H, m), 7.14 (2H, m), 7.48 (2H, m), 8.62 (1H, s, exchange with D2O, NH), 8.64 (1H, s, exchange with D2O, NH), 10.08 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.6, 106.2, 112.8, 116.1 (d, 2JC–F 22), 117.9, 120.7 (d, 3JC–F 8), 128.8, 137.0 (q, JC–F 2), 139.5, 139.6, 153.4, 158.5 (d, 1JC–F 237), 171.2; δF (376 MHz, DMSO-d6) −121.5 (1 F, s); m/z (ESI positive) 300.0 [M + H]+.
1-(4-Fluoro-3-methylphenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (13)
White solid, yield 89%; m.p.: > 300 °C; silica gel TLC Rf = 0.47 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.24 (3H, d, J 1.5), 2.45 (2H, t, J 7.6), 2.82 (2H, t, J 7.6), 7.00 (1H, dd, J 2.0, 8.10), 7.07 (3H, m), 7.27 (1H, m), 7.38 (1H, m), 8.55 (1H, exchange with D2O, NH), 8.64 (1H, s, exchange with D2O, NH), 10.07 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 15.3 (d, JC–F 3), 25.2, 31.7, 106.2, 112.8, 115.8 (d, 2JC–F 23), 117.9, 118.2 (d, 3JC–F 8), 122.1 (d, 3JC–F 4), 125.1 (d, 2JC–F 18), 128.8, 136.6 (d, 4JC–F 3), 139.5, 139.7, 153.5, 157.0 (d, JC–F 236), 171.3; δF (376 MHz, DMSO-d6) −125.9 (1 F, s); m/z (ESI positive) 314.0 [M + H]+.
1-(2,4-Difluorophenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (14)
White solid, yield 95%; m.p.: 240–241 °C; silica gel TLC Rf = 0.42 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.8), 2.83 (2H, t, J 7.8), 7.07 (4H, m), 7.34 (1H, m), 8.13 (1H, m), 8.47 (1H, s, exchange with D2O, NH), 9.03 (1H, s, exchange with D2O, NH), 10.11 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.7, 104.7 (t, 2JC–F 24), 106.0, 111.9 (dd, 2JC–F 4, 22), 112.6, 118.1, 122.7, (dd, 3JC–F 3.0, 9.0), 125.1 (dd, 3JC–F 3.0, 10.0), 128.9, 139.4, 139.6, 153.1 (dd, 1JC–F 12.0, 244.0), 153.2, 157.7 (dd, 1JC–F 12.0, 240.0), 171.3; δF (376 MHz, DMSO-d6) −124.3 (1 F, d, J 3.0), −118.2 (1 F, d, J 3.0); m/z (ESI positive) 318.0 [M + H]+.
1-(2-Oxo-1,2,3,4-tetrahydroquinolin-7-yl)-3-(perfluorophenyl)urea (15)
White solid, yield 88%; m.p.: 297–298 °C; silica gel TLC Rf = 0.8 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.45 (2H, d, J 7.2), 2.83 (2H, t, J 7.2), 7.00 (1H, dd, J 2.0, 8.0), 7.09 (2H, m), 8.41 (1H, s, exchange with D2O, NH), 9.07 (1H, s, exchange with D2O, NH), 10.10 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.6, 106.5, 113.1, 115.0 (m, JC–F 15), 118.5, 128.8, 138.1 (m, JC–F 245), 139.1, 139.3 (m, JC–F 245), 139.6, 143.9 (m, JC–F 245), 152.8, 171.3; δF (376 MHz, DMSO-d6) –164.3 (1 F, t, J 22), −159.9 (2 F, t, J 23), −146.4 (2 F, d, J 20); m/z (ESI negative) 370.0 [M − H]−.
1-(2-Oxo-1,2,3,4-tetrahydroquinolin-7-yl)-3-(4-(trifluoromethyl)phenyl)urea (16)
White solid, yield 72%; m.p.: 284–285 °C; silica gel TLC Rf = 0.55 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.6), 2.84 (2H, t, J 7.6), 7.02 (1H, dd, J 2.0, 8.0), 7.10 (2H, d, J 8.0), 7.67 (4H, m), 8.79 (1H, s, exchange with D2O, NH), 9.01 (1H, s, exchange with D2O, NH), 10.09 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.1, 31.6, 106.3, 112.9, 118.3, 118.7, 122.6 (q, 2JC–F 32), 125.4 (q, 1JC–F 270), 126.9 (q, 3JC–F 4), 128.8, 139.1, 139.5, 144.3 (q, 4JC–F 1), 153.0, 171.1; δF (376 MHz, DMSO-d6) −60.1 (3 F, s); m/z (ESI positive) 350.0 [M + H]+.
1-(2-Chloro-4-(trifluoromethyl)phenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (17)
White solid, yield 85%; m.p.: > 300 °C; silica gel TLC Rf = 0.58 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.47 (2H, t, J 7.2), 2.85 (2H, t, J 7.2), 7.10 (3H, m), 7.71 (1H, dd, J 1.6, 8.8), 7.91 (1H, d, J 1.6), 8.51 (1H, d, J 8.8), 8.61 (1H, s, exchange with D2O, NH), 9.61 (1H, s, exchange with D2O, NH), 10.16 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.6, 106.2, 112.8, 118.6, 121.0, 122.2, 123.7 (q, JC–F 4), 124.6 (q JC–F 271), 125.7 (q, JC–F 4), 127.2 (q, JC–F 4), 129.0, 138.9, 139.6, 140.8 (q, JC–F 40), 152.5, 171.2; δF (376 MHz, DMSO-d6) −60.4 (3 F, s); m/z (ESI positive) 384.0 [M + H]+.
1-(2-Fluoro-5-(trifluoromethyl)phenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (18)
White solid, yield 15%; m.p.; 253–254 °C; silica gel TLC Rf = 0.50 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.47 (2H, t, J 7.2), 2.84 (2H, t, J 7.2), 7.05 (1H, dd, J 2,8), 7.12 (2H, m), 7.42 (1H, m), 7.53 (1H, m), 8.66 (1H, m), 9.18 (1H, exchange with D2O, NH), 9.61 (1H, s, exchange with D2O, NH), 10.09 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.6, 106.1, 112.6, 117 (d, JC–F 21), 117.3 (t, JC–F 3), 118.5, 120.0 (m), 123.5, 126.3 (td, JC–F 3, 32), 128.9, 129.7 (d, JC–F 11), 138.9, 139.6, 152.9, 154.3 (d, JC–F 248), 171.2; δF (376 MHz, DMSO-d6) −60.7 (3 F, s), −124.5 (1 F, s); m/z (ESI positive) 368.0 [M + H]+.
1-(3,5-Bis(trifluoromethyl)phenyl)-3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)urea (19)
White solid, yield 30%; m.p.; 278–279 °C; silica gel TLC Rf = 0.70 (Ethyl acetate 100% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.5), 2.85 (2H, t, J 7.5), 7.05 (2H, m), 7.17 (1H, m), 7.67 (1H, s), 8.16 (2H, s), 9.00 (1H, s, exchange with D2O, NH), 9.32 (1H, s, exchange with D2O, NH), 10.08 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.2, 31.6, 106.8, 113.3, 115.2 (m), 118.6, 118.8 (m), 124.2 (q, 1JC–F 270), 128.8, 131.6 (q, 2JC–F 32), 138.9, 139.6, 142.8, 153.2, 171.2; δF (376 MHz, DMSO-d6) −61.7 (6 F, s); m/z (ESI negative) 416.0 [M − H]−.
2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-(3-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)thioureido)benzoic acid (20)
Red solid, yield 75%; m.p.: 189–190 °C; silica gel TLC Rf = 0.23 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.49 (2H, t, J 7.6), 2.89 (2H, t, J 7.6), 6.62 (4H, m), 6.71 (2H, d, J 2.0), 7.04 (2H, m), 7.18 (1H, d, J 8.4), 7.24 (1H, d, J 8.4), 7.86 (1H, dd, J 2.0, 8.4), 8.22 (1H, d, J 2.0), 10.09 (1H, s, exchange with D2O, NH), 10.12 (1H, s, exchange with D2O NH), 10.16 (2H, s, exchange with D2O, OH), 10.17 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.4, 31.4, 103.2, 110.6, 111.7, 113.6, 118.4, 118.5, 121.2, 124.8, 127.4, 128.7, 130.0, 131.4, 138.9, 139.4, 142.3, 152.8, 160.5, 169.4, 171.1, 180.5; m/z (ESI negative) 550.0 [M − H]−.
2-((2,3-Dimethylphenyl)amino)-N-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)benzamide (21)
A solution of 1 (1.2 mmol) was treated with mefenamic acid (2.4 mmol) in dry N,N-Dimethylformamide (DMF) (5 ml) then N,N′-Dicyclohexylcarbodiimide (DCC) (2.0 equiv.) and catalytic amount of 4-(Dimethylamino)pyridine (DMAP) were added to reaction mixture. The reaction continued until the consumption of starting materials (TLC monitoring), quenched with 1 M aqueous HCl solution and extracted with ethyl acetate (3 × 15 ml). The combined organic layers were washed with H2O (3 × 15 ml), dried over Na2SO4, filtered, and concentrated to obtain a residue which was purified by silica gel column chromatography eluting with ethyl acetate/n-hexane 50% v/v to afford titled compound.
White solid, yield 20%; m.p.: 220–221 °C; silica gel TLC Rf = 0.18 (Ethyl acetate/n-hexane 50% v/v); δH (400 MHz, DMSO-d6) 2.15 (3H, s), 2.31 (3H, s), 2.48 (2H, t, J 7.6), 2.87 (2H, t, J 7.6), 6.87 (2H, m), 6.98 (1H, m), 7.13 (3H, m), 7.23 (1H, dd, J 2.0, 8.0), 7.34 (1H, td, J 2.0, 7.8), 7.42 (1H, d, J 2.0), 7.81 (1H, dd, J 2.0, 8.0), 9.15 (1H, s, exchange with D2O, NH), 10.16 (1H, s, exchange with D2O, NH), 10.32 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 14.5, 21.2, 25.3, 31.5, 108.9, 115.1, 115.5, 117.9, 118.8, 120.0, 120.8, 126.2, 126.8, 128.5, 130.3, 130.4, 133.2, 138.6, 138.7, 139.3, 140.1, 147.1, 168.8, 171.2; m/z (ESI negative) 384.0 [M − H]−.
2′,4′-Difluoro-4-hydroxy-N-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)-[1,1′-biphenyl]-3-carboxamide (22)
A solution of 1 (1.0 mmol) was treated with diflunisal (1.0 mmol) in dry N,N-Dimethylacetamide (DMA) (4 ml) then N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI) (2.0 mmol), 1-Hydroxy-7-azabenzotriazole (HOAT) (2.0 mmol), N,N-Diisopropylethylamine (DIPEA) (3.0 mmol) were added to reaction mixture. The reaction continued until the consumption of starting materials (TLC monitoring), quenched with 1 M aqueous HCl solution and extracted with ethyl acetate (3 × 15 ml). The combined organic layers were washed with H2O (3 × 15 ml), dried over Na2SO4, filtered, and concentrated to obtain a residue which was purified by silica gel column chromatography eluting with ethyl acetate/n-hexane 50% v/v to afford titled compound.
White solid, yield 15%, m.p.: 281–282 °C; silica gel TLC Rf = 0.23 (Ethyl acetate/n-hexane 50% v/v); δH (400 MHz, DMSO-d6) 2.49 (2H, d, J 7.8), 2.89 (2H, t, J 7.8), 7.12 (1H, d, J 7.6), 7.23 (3H, m), 7.41 (2H, m), 7.65 (2H, m), 8.13 (1H, m), 10.18 (1H, s, exchange with D2O, NH), 10.47 (1H, s, exchange with D2O, NH), 12.04 (1H, s, exchange with D2O, OH); δC (100 MHz, DMSO-d6) 25.3, 31.5, 105.4 (t, JC–F 26), 108.9, 112.9 (dd, JC–F 4, 21), 115.6, 118.5 (d, JC–F 19), 120.5, 125.0 (dd, JC–F 4, 14), 126.0 (d, JC–F 1), 128.7, 130.0 (d, JC–F 2), 132.6 (dd, JC–F 5, 10), 134.8 (d, JC–F 3), 137.9, 139.5, 158.7 (d, JC–F 12), 159.1, 161.1 (dd, JC–F 3, 12), 163.6 (d, JC–F 12), 167.1, 171.2; δF (376 MHz, DMSO-d6) −113.8 (1 F, d, J 7), −111.5 (1 F, d, J 7); m/z (ESI negative) 393.0 [M − H]−.
(Z)-4-oxo-4-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)amino)but-2-enoic acid (23)
A solution of compound 1 (1.0 mmol) was treated with maleic anhydride (1.05 mmol) in dry DMF then heated up to 150 °C. The reaction continued until the consumption of starting materials, quenched with 1 M aqueous HCl solution to obtain a precipitate which was washed with Et2O (3 × 5 ml) and dried under vacuum to obtain desired product.
White solid, yield 30%; m.p.: > 300 °C; δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.6), 2.86 (2H, t, J 7.6), 6.67 (1H, d, J 15.3), 7.16 (2H, m), 7.23 (1H, dd, J 1.8, 8.0), 7.34 (1H, d, J 1.8), 10.20 (1H, s, exchange with D2O, NH), 10.51 (1H, s, exchange with D2O, NH), 13.03 (1H, s, exchange with D2O, OH); δC (100 MHz, DMSO-d6) 25.2, 31.4, 107.3, 113.9, 120.2, 128.8, 131.4, 138.1, 138.4, 139.5, 162.3, 167.1, 171.1; m/z (ESI positive) 261.0 [M + H]+.
N-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)methanesulfonamide (24)
Compound 1 (1.2 mmol) was treated with methanesulfonyl chloride (1.01 mmol) in dry THF (3.0 ml) followed by addition of Et3N (1.1 mmol). The reaction continued until the consumption of starting materials (TLC monitoring) then quenched with 1 M aqueous HCl solution. Excess of solvents were removed under reduced pressure to obtain a residue which was filtered, washed with Et2O (3 × 5 ml) and dried under vacuum to afford titled compound.
White solid, yield 57%; m.p.: 236–237 °C; silica gel TLC Rf = 0.37 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.46 (2H, t, J 7.6), 2.85 (2H, t, J 7.6), 2.98 (3H, s), 6.79 (1H, dd, J 2.4, 8.0), 6.84 (1H, d, J 2.4), 7.14 (1H, d, J 2.4), 9.66 (1H, s, exchange with D2O, NH), 10.13 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.1, 31.4, 39.9, 108.0, 114.5, 120.2, 129.2, 138.2, 139.9, 171.1; m/z (ESI negative) 239.0 [M − H]−.
N-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)methanesulfonamide (25)
A solution of 24 (0.4 mmol) was treated with iodomethane (0.4 mmol) in dry DMF (3.0 ml) at 0 °C, followed by addition of K2CO3 (0.4 mmol) then warmed up to rt. The reaction continued until the consumption of starting materials and quenched with slush, acidified with 1 M aqueous HCl solution to obtain a precipitate which was collected, washed with Et2O (3 × 5 ml) and dried under vacuum to obtain desired product.
White solid; 80% yield; m.p.: 226–227 °C; silica gel TLC Rf = 0.59 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.49 (2H, t, J 7.6), 2.90 (2H, t, J 7.6), 2.97 (3H, s), 3.22 (3H, s), 6.91 (1H, d, J 2.2), 7.01 (1H, dd, J 2.2, 8.0), 7.24 (1H, d, J 8.0), 10.15 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 25.3, 31.1, 35.8, 38.8, 114.6, 119.9, 123.5, 129.1, 139.7, 141.5, 171.0; m/z (ESI positive) 255.0 [M + H]+.
2,4,6-Trimethyl-1-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yl)pyridin-1-ium perchlorate (26)
A solution of 1 (2.0 mmol) was treated with 2,4,6-trimethylpyrylium tetrafluoroborate (2.4 mmol) in dry methanol (10 ml) then the solution was refluxed for 5 h. Solvent was partially removed, the mixture was cooled down to room temperature and treated with a 1.0 M aqueous solution of HClO4 (3.0 equiv.). The precipitate formed was collected by filtration, and crystallised from water to afford the desired product.
Pale yellow solid, yield 40%; m.p.: 280–281 °C; silica gel TLC Rf = 0.10 (MeOH/DCM 10% v/v); δH (400 MHz, DMSO-d6) 2.37 (6H, s), 2.59 (2H, d, J 7.8), 2.63 (3H, s), 3.06 (2H, t, J 6.8), 6.97 (1H, d, J 2.4), 7.13 (1H, dd, J 2.4, 8.0) 7.56 (1H, d, J 8.0), 7.94 (2H, s), 10.50 (1H, s, exchange with D2O, NH); δC (100 MHz, DMSO-d6) 22.2, 22.4, 25.5, 30.8, 112.6, 119.8, 127.6, 128.1, 130.9, 138.0, 141.3, 155.6, 159.8, 171.1; m/z (ESI positive) 267.0 [M]+.
CA assay
A stopped-flow method44 has been used for assaying the CA catalysed CO2 hydration activity with Phenol red as an indicator, working at the absorbance maximum of 557 nm, following the initial rates of the CA-catalysed CO2 hydration reaction for 10–100 s. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.01 mm) were prepared in distilled-deionised water with 5% DMSO and dilutions up to 0.1 nm were done thereafter with the assay buffer. Enzyme and inhibitor were incubated for 6 h45–48. The inhibition constant (KI) was obtained by considering the classical Michaelis–Menten equation which has been fitted by using non-linear least squares with PRISM 3 (La Jolla, CA). All CA isozymes used in the experiments were purified, recombinant proteins obtained as reported earlier by our group49–59.
Results and discussion
Chemistry
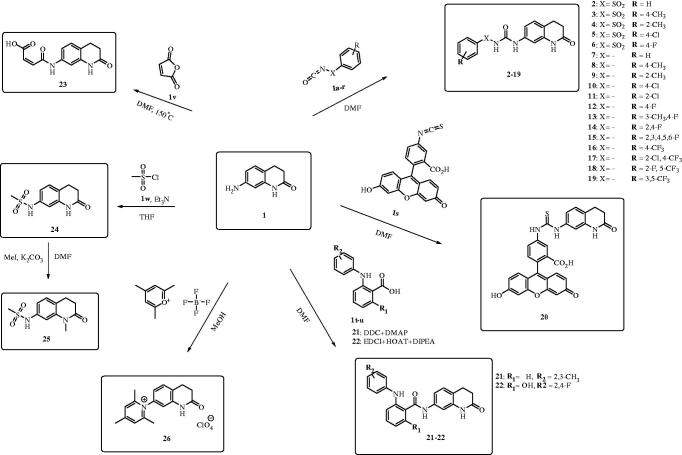
In a previous report from this group43, we showed that 7-amino-3,4-dihydroquinolin-2(1H)-one (1) (Scheme 1) possesses interesting CA inhibitory properties against many human isoforms such as hCA VII, IX, XII and XIV, some of which are important drug targets for various applications of the CAIs. The lactam 1 was investigated as a CAI due to its structural similarity with the coumarins, a class of CAIs reported by this group45–48. Indeed, unlike other classes of such pharmacological agents, the coumarins act as prodrug inhibitors, being hydrolysed by the CA esterase activity to substituted 2-hydroxy-cinnamic acids, which thereafter bind at the entrance of the active site cavity, far away from the catalytic Zn(II) ion with which most CAIs interact13,45. That region is the most variable among the 15 human CAs, and this explains why coumarins and their derivatives are among the most isoform-selective CAIs reported so far1,13,45–48. In fact, a large number of substitution patterns at the coumarin ring, isosteric replacements or various other modifications were done on this chemotype, leading to a large number of CAIs possessing interesting properties13,45–48. Thus, the rationale of this work was to derivatise the 7-amino moiety of the lead 1, by reacting it with a variety of agents used earlier for the design of sulfonamide or dithiocarbamate CAIs (Scheme 1)13–16,22–25,35–37,60,61.
Scheme 1.
Synthesis of compounds 2–26.
As shown in Scheme 1, a multitude of derivatisations of the amino moiety of compound 1 were achieved, such as: (i) reaction with arylsulfonyl isocyanates (leading to arylsulfonylureido derivatives 2–6); (ii) reaction with isocyanates, leading to ureas 7–19; (iii) reaction with fluoresceine isothiocyanate, leading to the fluorescent thiourea 20; (iv) condensation with substituted benzoic acids in the presence of carbodiimides, leading to the amides 21 and 22; (v) reaction with 2,4,6-trimethyl-pyrylium tetrafluoroborate, leading to the pyridinium salt 26; (vi) reaction with methylsulfonyl chloride leading to the secondary sulfonamide 24, which was subsequently methylated with methyl iodide, leading to the 1-N-methyl derivative 25, and (vii) reaction with maleic anhydride leading to the monoamide 23 (Scheme 1). All these compounds were thoroughly characterised by physicochemical procedures which confirmed their structures (see “Materials and methods” for details).
CA inhibition
Compounds 2–26 were assayed for their CA inhibitory activity by a stopped-flow, CO2 hydrase method44 against four isoforms of pharmacologic relevance, the cytosolic human (h) hCA I and II, the membrane-anchored hCA IV and the transmembrane, tumour-associated hCA IX (Table 1). The following structure-activity relationship can be observed from the inhibition data of Table 1:
Table 1.
Inhibition data of hCA I, hCA II, hCA IV, hCA IX with compounds 2–26 reported here and the standard sulfonamide inhibitor acetazolamide (AAZ) by a stopped-flow CO2 hydrase assay.
|
KI (nm) |
||||
|---|---|---|---|---|
| Cmp | hCA I | hCA II | hCA IV | hCA IX |
| 2 | 8241.0 | 7467.6 | >10,000 | 2133.3 |
| 3 | 6813.4 | 6966.7 | >10,000 | 1461.0 |
| 4 | 3690.4 | 6852.2 | >10,000 | 1051.8 |
| 5 | >10,000 | 6379.1 | >10,000 | 2234.6 |
| 6 | 3202.4 | 4437.9 | >10,000 | 1688.2 |
| 7 | >10,000 | >10,000 | >10,000 | 2420.3 |
| 8 | >10,000 | >10,000 | >10,000 | >10,000 |
| 9 | >10,000 | >10,000 | >10,000 | 2267.5 |
| 10 | >10,000 | >10,000 | >10,000 | >10,000 |
| 11 | >10,000 | >10,000 | >10,000 | 1158.3 |
| 12 | >10,000 | >10,000 | >10,000 | 2489.6 |
| 13 | >10,000 | >10,000 | >10,000 | 2105.0 |
| 14 | >10,000 | >10,000 | >10,000 | 1373.1 |
| 15 | >10,000 | 7883.8 | >10,000 | 243.6 |
| 16 | >10,000 | 5724.1 | >10,000 | >10,000 |
| 17 | 5328.9 | 4973.1 | 3801.4 | 2165.2 |
| 18 | 8749.6 | 5490.4 | >10,000 | 1524.5 |
| 19 | >10,000 | >10,000 | >10,000 | 2386.7 |
| 20 | >10,000 | 3378.5 | >10,000 | 1941.1 |
| 21 | >10,000 | >10,000 | >10,000 | >10,000 |
| 22 | >10,000 | >10,000 | >10,000 | 2516.7 |
| 23 | >10,000 | >10,000 | >10,000 | 1473.3 |
| 24 | >10,000 | >10,000 | >10,000 | 292.8 |
| 25 | >10,000 | >10,000 | >10,000 | 2758.6 |
| 26 | >10,000 | >10,000 | >10,000 | 2658.3 |
| AAZ | 250 | 12 | 74 | 25 |
Errors were in the range of ±5–10% of the reported data, from three different assays.
hCA I was poorly inhibited by most derivatives 2–26, with only seven of them showing KIs in the micromolar range (i.e. 3.20–8.75 µm), the remaining ones having KIs > 10 µm (Table 1). The more effective inhibitors were 2–4, 6, 17 and 18, which incorporate arylsulfonylureido and ureido moieties. The other substitution patterns led to compounds with much weaker hCA I inhibitory activity.
hCA II, the dominant cytosolic isoform was generally also poorly inhibited by these derivatives (KIs > 10 µm) except the arysulfonylureido ones 2–6 (KIs of 4.43–7.46 µm) the ureas 15–18 (KIs of 4.97–7.88 µm) and the thiourea 20 (KI of 3.37 µm), which was the best hCA II inhibitor in the series.
hCA IV was the least sensitive isoform to these compounds with only one of them (17, KI of 3.80 µm) having an activity <10 µm (Table 1). It is rather difficult to explain this result considering that the inhibition mechanism with these lactams is not yet elucidated.
The tumour-associated hCA IX was the most inhibited isoform among the four investigated ones, with KIs ranging between 243.6 and 2758.6 nm (Table 1). Only four derivatives (8, 10, 16 and 21) had KIs > 10 µm, whereas the best hCA IX inhibitors were 15 and 24 with KIs of 243.6–292.8 nm. These compounds rather different as the first one is a urea incorporating a pentafluorophenyl moiety, whereas the second one has the secondary sulfonamide functionality. It should be noted that small variations in the structures of such derivatives (as the N1-methylation of 24 leading to 25) or the reduction of the number of fluorine atoms on the phenyl ring, as in 14, led to a rather important reduction of the hCA IX inhibitory power compared to 24 and 15, respectively. Generally, all other arylsulfonylureas/ureas 2–19 (except the two compounds mentioned above as weak inhibitors and 15 which is one of the best) showed a similar behaviour of medium potency hCA IX inhibitors with KIs of 1.05–2.48 µm.
All the derivatives reported here showed much weaker CA inhibitory activity compared to the clinically used sulfonamide acetazolamide AAZ (Table 1).
Conclusions
A series of derivatives was prepared by derivatisation of the 7-amino moiety of 7-amino-3,4-dihydroquinolin-2(1H)-one, a compound investigated earlier as CAI. The derivatisation was achieved by: (i) reaction with arylsulfonyl isocyanates (ii) reaction with aryl isocyanates; (iii) reaction with fluoresceine isothiocyanate; (iv) condensation with substituted benzoic acids in the presence of carbodiimides; (v) reaction with 2,4,6-trimethyl-pyrylium tetrafluoroborate; (vi) reaction with methylsulfonyl chloride and (vii) reaction with maleic anhydride. The new compounds were assayed as inhibitors of four CA human isoforms of pharmacologic relevance, the cytosolic hCA I and II, the membrane-anchored hCA IV and the transmembrane, tumour-associated hCA IX. hCA IX was the most inhibited isoform (KIs ranging between 243.6 and 2658.3 nm) whereas hCA IV was not inhibited by these compounds. Most derivatives were weak hCA I and II inhibitors, with few of them showing KIs < 10 µm. Considering that the inhibition mechanism with these lactams is not yet elucidated, exploring a large range of derivatives with various substitution patterns may be useful to identify leads showing isoform selectivity.
Acknowledgements
This work was financed in part by a Distinguished Scientist Fellowship Program (DSFP) of King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
One author (CTS) declares conflict of interest, being author of several patents in the field of CA inhibitors/activators. The other authors do not declare conflict of interest.
References
- 1.Supuran CT.Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. [DOI] [PubMed] [Google Scholar]
- 2.Neri D, Supuran CT.. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77. [DOI] [PubMed] [Google Scholar]
- 3.Capasso C, Supuran CT.. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. [DOI] [PubMed] [Google Scholar]
- 4.Supuran CT.Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72. [DOI] [PubMed] [Google Scholar]
- 5.Supuran CT.Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74. [DOI] [PubMed] [Google Scholar]
- 6.Supuran CT.Bacterial carbonic anhydrases as drug targets: towards novel antibiotics? Front Pharmacol 2011;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Prete S, Vullo D, De Luca V, et al. Biochemical characterization of recombinant beta-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J Enzyme Inhib Med Chem 2015;30:366–70. [DOI] [PubMed] [Google Scholar]
- 8.Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30. [DOI] [PubMed] [Google Scholar]
- 9.Capasso C, Supuran CT.. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin Ther Pat 2013;23:693–704. [DOI] [PubMed] [Google Scholar]
- 10.Capasso C, Supuran CT.. Sulfa and trimethoprim-like drugs – antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87. [DOI] [PubMed] [Google Scholar]
- 11.Supuran CT.Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 12.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76. [DOI] [PubMed] [Google Scholar]
- 13.Supuran CT.How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. [DOI] [PubMed] [Google Scholar]
- 14.Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 15.Angiolella L, Carradori S, Maccallini C, et al. Targeting Malassezia species for novel synthetic and natural antidandruff agents. Curr Med Chem 2017. [Epub ahead of print]. DOI: 10.2174/0929867324666170404110631 [DOI] [PubMed] [Google Scholar]
- 16.Scozzafava A, Menabuoni L, Mincione F, Supuran CT.. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long lasting, topical intraocular pressure lowering properties. J Med Chem 2002;45:1466–76. [DOI] [PubMed] [Google Scholar]
- 17.Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;27:138–47. [DOI] [PubMed] [Google Scholar]
- 18.Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 2012;55:1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois L, Lieuwes NG, Maresca A, et al. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumor model. Radiother Oncol 2009;92:423–8. [DOI] [PubMed] [Google Scholar]
- 20.Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum – the η-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96. [DOI] [PubMed] [Google Scholar]
- 21.Winum JY, Scozzafava A, Montero JL, Supuran CT.. Therapeutic potential of sulfamides as enzyme inhibitors. Med Res Rev 2006;26:767–92. [DOI] [PubMed] [Google Scholar]
- 22.Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;46:8371–3. [DOI] [PubMed] [Google Scholar]
- 23.Carta F, Garaj V, Maresca A, et al. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: solution and X-ray crystallographic studies. Bioorg Med Chem 2011;19:3105–19. [DOI] [PubMed] [Google Scholar]
- 24.Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg Med Chem Lett 2004;14:5427–33. [DOI] [PubMed] [Google Scholar]
- 25.Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors. Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumor-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;15:3102–8. [DOI] [PubMed] [Google Scholar]
- 26.Carta F, Scozzafava A, Supuran CT. Sulfonamides: a patent review (2008 - 2012). Expert Opin Ther Pat 2012;22:747–58. [DOI] [PubMed] [Google Scholar]
- 27.Masini E, Carta F, Scozzafava A, Supuran CT.. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:705–16. [DOI] [PubMed] [Google Scholar]
- 28.Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008 - 2013)). Expert Opin Ther Pat 2013;23:737–49. [DOI] [PubMed] [Google Scholar]
- 29.Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005 - 2013). Expert Opin Ther Pat 2013;23:681–91. [DOI] [PubMed] [Google Scholar]
- 30.Supuran CT.Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs 2012;17:11–15. [DOI] [PubMed] [Google Scholar]
- 31.Scozzafava A, Supuran CT, Carta F.. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35. [DOI] [PubMed] [Google Scholar]
- 32.Supuran CT.The safety and clinical efficacy of acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 2015;15:851–6. [DOI] [PubMed] [Google Scholar]
- 33.Supuran CT.Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. [DOI] [PubMed] [Google Scholar]
- 34.De Simone G, Alterio V, Supuran CT.. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810. [DOI] [PubMed] [Google Scholar]
- 35.Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: is the tail more important than the ring? J Med Chem 1999;42:2641–50. [DOI] [PubMed] [Google Scholar]
- 36.Borras J, Scozzafava A, Menabuoni L, et al. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: is the tail more important than the ring? Bioorg Med Chem 1999;7:2397–406. [DOI] [PubMed] [Google Scholar]
- 37.Winum JY, Supuran CT.. Recent advances in the discovery of zinc binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2015;30:321–4. [DOI] [PubMed] [Google Scholar]
- 38.Briganti F, Pierattelli R, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Part 37. Novel classes of carbonic anhydrase inhibitors and their interaction with the native and cobalt-substituted enzyme: kinetic and spectroscopic investigations. Eur J Med Chem 1996;31:1001–10. [Google Scholar]
- 39.Supuran CT.Carbonic anhydrase inhibitors In: Puscas I, ed. Carbonic anhydrase and modulation of physiologic and pathologic processes in the organism. Timisoara: Helicon; 1994:29–111. [Google Scholar]
- 40.Clare BW, Supuran CT.. Carbonic anhydrase activators. Part 3. Structure-activity correlations for a series of isozyme II activators. J Pharm Sci 1994;83:768–73. [DOI] [PubMed] [Google Scholar]
- 41.Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J Enzyme Inhib Med Chem 2016;31:894–9. [DOI] [PubMed] [Google Scholar]
- 42.Kalinin S, Supuran CT, Krasavin M.. Multicomponent chemistry in the synthesis of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2016;31:185–99. [DOI] [PubMed] [Google Scholar]
- 43.Vullo D, Isik S, Bozdag M, et al. 7-Amino-3,4-dihydro-1H-quinolin-2-one, a compound similar to the substituted coumarins, inhibits α-carbonic anhydrases without hydrolysis of the lactam ring. J Enzyme Inhib Med Chem 2015;30:773–7. [DOI] [PubMed] [Google Scholar]
- 44.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 45.Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62. [DOI] [PubMed] [Google Scholar]
- 46.Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44. [DOI] [PubMed] [Google Scholar]
- 47.Maresca A, Scozzafava A, Supuran CT.. 7,8-disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 2010;20:7255–8. [DOI] [PubMed] [Google Scholar]
- 48.Carta F, Maresca A, Scozzafava A, Supuran CT.. 5- and 6-membered (thio)lactones are prodrug type carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2012;22:267–70. [DOI] [PubMed] [Google Scholar]
- 49.Yamali C, Gul HI, Sakagami H, Supuran CT.. Synthesis and bioactivities of halogen bearing phenolic chalcones and their corresponding bis Mannich bases. J Enzyme Inhib Med Chem 2016;31:125–31. [DOI] [PubMed] [Google Scholar]
- 50.Mollica A, Locatelli M, Macedonio G, et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J Enzyme Inhib Med Chem 2016;31:1–6. [DOI] [PubMed] [Google Scholar]
- 51.Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31:60–3. [DOI] [PubMed] [Google Scholar]
- 52.Mishra CB, Kumari S, Angeli A, et al. Design, synthesis and biological evaluation of N-(5-methyl-isoxazol-3-yl/1,3,4-thiadiazol-2-yl)-4-(3-substitutedphenylureido) benzenesulfonamides as human carbonic anhydrase isoenzymes I, II, VII and XII inhibitors. J Enzyme Inhib Med Chem 2016;31:174–9. [DOI] [PubMed] [Google Scholar]
- 53.Diaz JR, Fernández Baldo M, Echeverría G, et al. A substituted sulfonamide and its Co (II), Cu (II), and Zn (II) complexes as potential antifungal agents. J Enzyme Inhib Med Chem 2016;31:51–62. [DOI] [PubMed] [Google Scholar]
- 54.Supuran CT, Kalinin S, Tanç M, et al. Isoform-selective inhibitory profile of 2-imidazoline-substituted benzene sulfonamides against a panel of human carbonic anhydrases. J Enzyme Inhib Med Chem 2016;31:197–202. [DOI] [PubMed] [Google Scholar]
- 55.Federici C, Lugini L, Marino ML, et al. Lansoprazole and carbonic anhydrase IX inhibitors sinergize against human melanoma cells. J Enzyme Inhib Med Chem 2016;31:119–25. [DOI] [PubMed] [Google Scholar]
- 56.Chohan ZH, Scozzafava A, Supuran CT.. Unsymmetrical 1,1′-disubstituted ferrocenes: synthesis of Co(ii), Cu(ii), Ni(ii) and Zn(ii) chelates of ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with antimicrobial properties. J Enzyme Inhib Med Chem 2002;17:261–6. [DOI] [PubMed] [Google Scholar]
- 57.Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902. [DOI] [PubMed] [Google Scholar]
- 58.Supuran CT, Scozzafava A, Mastrolorenzo A.. Bacterial proteases: current therapeutic use and future prospects for the development of new antibiotics. Expert Opin Ther Pat 2001;11:221–59. [Google Scholar]
- 59.Supuran CT, Barboiu M, Luca C, et al. Carbonic anhydrase activators. Part 14. Synthesis of mono- and bis- pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole, and their interaction with isozyme II. Eur J Med Chem 1996;31:597–606. [Google Scholar]
- 60.Nocentini A, Ceruso M, Carta F, Supuran CT.. 7-Aryl-triazolyl-substituted sulfocoumarins are potent, selective inhibitors of the tumor-associated carbonic anhydrase IX and XII. J Enzyme Inhib Med Chem 2016;31:1226–33. [DOI] [PubMed] [Google Scholar]
- 61.Supuran CT, Mincione F, Scozzafava A, et al. Carbonic anhydrase inhibitors. Part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem 1998;33:247–54. [Google Scholar]