Abstract
The 1976 Viking Labeled Release (LR) experiment was positive for extant microbial life on the surface of Mars. Experiments on both Viking landers, 4000 miles apart, yielded similar, repeatable, positive responses. While the authors eventually concluded that the experiment detected martian life, this was and remains a highly controversial conclusion. Many believe that the martian environment is inimical to life and the LR responses were nonbiological, attributed to an as-yet-unidentified oxidant (or oxidants) in the martian soil. Unfortunately, no further metabolic experiments have been conducted on Mars. Instead, follow-on missions have sought to define the martian environment, mostly searching for signs of water. These missions have collected considerable data regarding Mars as a habitat, both past and present. The purpose of this article is to consider recent findings about martian water, methane, and organics that impact the case for extant life on Mars. Further, the biological explanation of the LR and recent nonbiological hypotheses are evaluated. It is concluded that extant life is a strong possibility, that abiotic interpretations of the LR data are not conclusive, and that, even setting our conclusion aside, biology should still be considered as an explanation for the LR experiment. Because of possible contamination of Mars by terrestrial microbes after Viking, we note that the LR data are the only data we will ever have on biologically pristine martian samples. Key Words: Extant life on Mars—Viking Labeled Release experiment—Astrobiology—Extraterrestrial life—Mars. Astrobiology 16, 798–810.
1. Introduction
The Viking Labeled Release (LR) experiment (Levin, 1972; Levin and Straat, 1976a, 1976b) was an experiment in radiorespirometry whereby 14C-labeled organics were injected onto a soil sample in a test chamber and continuously monitored for the subsequent evolution of radioactive gas. The experiment was designed to test for the presence of life on the surface of Mars by monitoring for metabolism. The positive results obtained (Levin and Straat, 1976a, 1976b) are consistent with biology but have been challenged by alternate hypotheses that include a variety of nonbiological active agents and by doubts that putative martian organisms could exist in the harsh environmental conditions. However, recent findings of water availability, complex organic molecules possibly of biological significance, and the periodic appearance of methane warrant a reevaluation of the potential for life on Mars whether or not the LR experiment detected it.
2. The Viking Labeled Release Experiment
The Viking LR experiment (Levin, 1972; Levin and Straat, 1976a, 1976b) was one of three life-detection metabolic tests on the 1976 Viking mission to Mars. The question is whether the positive results of this experiment detected extant life or some chemical agent in the regolith. The experiment was designed to look for metabolism whereby one or more simple organic compounds are consumed and one or more carbon-based gases are evolved. The other two life-detection experiments were the Gas Exchange (GEx) experiment (Oyama, 1972; Klein et al., 1976; Oyama and Berdahl, 1977), which tested for microbial life exposed first to humidity only, then to water, and then to immersion in a complex “chicken soup” of organic nutrients and supplements, and the Pyrolytic Release (PR) experiment (Horowitz et al., 1972, 1977; Klein et al., 1976), which was designed to test for microorganisms that photosynthetically incorporated 14CO2 and/or 14CO in a simulated martian atmosphere and environment with and without the addition of water. A fourth Viking experiment, the Gas Chromatograph–Mass Spectrometer (GCMS), was included to analyze organics expected to be widely present on the surface of Mars (Biemann et al., 1977). While not a life-detection experiment per se, it was believed that, if life existed on the planet, organics would be detected by the GCMS and serve as confirmation of life.
The LR experiment was based on the belief that early Mars and Earth possessed similar primordial environments, each of which produced Miller-Urey-type organic compounds that were then available for the genesis of life and its subsequent metabolism and evolution. In the LR experiment, a nutrient solution consisting of Miller-Urey compounds tagged with 14C was added to a sample of martian soil, and the mixture was continuously monitored for evolution of radioactive gas. Early laboratory experiments showed the technology to be very rapid and extremely sensitive, detecting as few as 30 cells in a sample (Levin et al., 1956). The sensitivity of the method reflects the fact that this is a metabolic experiment that utilizes radioactive isotopes and does not depend on culturing, at least initially, thereby eliminating the lag typically observed in classical microbiological methods. The method can thus detect microbial metabolism even for those soil microorganisms that defy culturing, which may explain why soils found “sterile” by classical microbiological methods tested positive by the LR method. The sensitivity is further enhanced by the radioisotopic methodology, which permits the use of low substrate concentrations and thereby reduces potential toxicity to any alien organisms.
The LR nutrient substrates were sodium formate, sodium lactate, glycine, alanine, and calcium glycolate (Levin and Straat, 1976a), all Miller-Urey compounds. Both left-handed and right-handed isomers of alanine and of lactate were included to provide for a possible different chirality with martian life. Concentrations of the selected compounds were only 2.5 × 10−4 molar each to minimize toxicity, with each carbon uniformly labeled with 14C at 2 μCi/m. The experimental procedure consisted of adding 0.115 mL of nutrient solution to 0.5 cc soil sample in a 3.5 cc cylindrical test chamber that had a 2 cm diameter. This provided a moisture gradient starting with the injected liquid at the center of the sample and progressing to minimal moisture at the periphery of the sample. This “moist” mode greatly decreased the response time because no water reservoir had to be saturated with gas before the gas could evolve from the liquid. Other environmental conditions chosen for the experiment were a temperature of 10°C ± 2°C to guarantee liquidity of the nutrient, and martian atmosphere with a helium overpressure of 85 mbar to assure liquidity in the event the martian atmosphere was below the triple point. A major advantage of this simple experiment is that the radioactive end-product is gaseous, easily separating itself from the radioactive liquid nutrient solution on the soil and thereby eliminating any interfering noise from the injected nutrient.
Terrestrial microbial utilization of the substrates in the LR nutrient involves mostly decarboxylation, although other carbon atoms can be metabolized as well. Because metabolic pathways on Mars are unknown, each carbon in each LR substrate was tagged with 14C, and the specific radioactivity was the same for each carbon. This permitted quantification of the amount of carbon gas released, regardless of the carbon atom from which it was derived. There were seven substrates in the LR nutrient for a total of 17 carbons, all present at the same concentration and the same specific radioactivity.
The LR technique was first developed in 1956 for the rapid detection of microorganisms (Levin et al., 1956) before its selection in 1970 as one of the Viking life-detection experiments. Over the entire 20-year development period for this experiment, thousands of LR tests were performed on a wide variety of soils, many of which were provided by NASA as bonded samples from harsh environments around the world (Levin and Straat, 1976a). Field tests were also made in which working models of the instrument were taken to extreme environments, for example, Antarctica, White Mountain above the timberline, Salton Sea flats, Death Valley sands, all of which responded positively. With the exception of three naturally sterile soils, discussed below, an active response was obtained from every soil tested, which was then eliminated in a duplicate sample by application of a control regime to destroy any life present.
This control regime was an essential part of the experiment to confirm that the initial response was biological, not chemical. Extensive laboratory experiments established that the most effective yet practical sterilization regime was heating the soil sample at 160°C for 3 h, followed by cooling before testing with the 14C nutrient. It was thought that this treatment would be sufficient to kill any martian microbial life and thus confirm that an active first response was evidence for life. If an active response were caused by chemistry instead of biology, the heat control response would be expected to be virtually the same as the active response; by definition, then, a positive response is one in which an active response is verified by a negative control. Three naturally sterile soils (Moon, Surtsey, and one Antarctic sample) that tested negative (i.e., the active and control sequences were essentially the same) showed the validity of the LR in not giving false positives (Levin and Straat, 1976b).
Pure cultures tested during the development of the LR experiment (Straat and Levin, 1972) included not only heterotrophs (algae, fungi, actinomycetes, aerobic bacteria, strict and facultative anaerobic bacteria), but also phototrophs and autotrophs. Heterotrophs included Mucor rouxii, Fusarium oxysporum, Candida albicans, Waksmania rosea, Mycobacterium smegmatis, Chlamydomonas reinhardi, Halobacter halobium, Desulfovibrio desulfuricans, and Streptosporangia rosea. Active responses were obtained from these heterotrophs as confirmed by their controls. Phototrophs included Chlorella vulgaris, Oscillatoria sp., and Chromatium sp., all producing positive responses. The results of these studies with pure cultures serve to emphasize the versatility of the LR in detecting microbial metabolism (Straat and Levin, 1972).
The LR flight instrument has been described elsewhere (Levin and Straat, 1976a). Essentially, it contained four incubation cells in a carousel that rotated such that each cell could be positioned to receive a soil sample delivered from the lander sample distribution box. Once soil was received, that test cell was rotated under a head-end and sealed. Any evolved gas following nutrient injection passed through a 13-inch “swan neck” tube (to preclude carryover of radioactive aerosol or dust particles) to a detector chamber where radioactivity measurements were made. The head-end of the test cell contained two heaters and a temperature sensor for controlling and recording temperature, both during the test runs and during heat sterilization of the control soil samples. The second heater boosted the temperature of the soil sample to the desired 160°C.
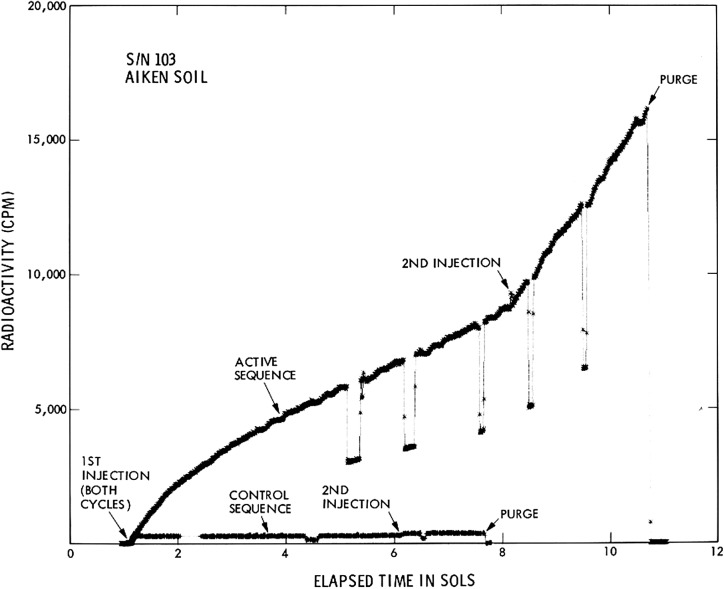
A Test Standards Module (TSM) of the instrument, duplicating all critical flight instrument parameters and experimental conditions, was constructed and tested at TRW, Inc., at Redondo Beach, California, and later operated at the NASA Ames Research Center. This instrument was used to test the various developmental stages of the experiment and create a library of responses. In addition, an actual flight instrument, called SN103, was used to conduct a final preflight test of the LR experiment on a California soil (“Aiken” soil) under martian conditions (Fig. 1). The soil, which contained approximately 105 aerobic microorganisms per gram, was maintained for 3 days under these harsh conditions before being injected with the nutrient. Despite this severe pretreatment, the soil remained viable, as shown by the strong active response following both first and second injection, confirmed by the negative response of the heated control.
FIG. 1.
LR results from California (“Aiken”) soil under martian conditions in the SN103 flight instrument. Nutrient was added either to untreated soil (active sequence) or to soil that had been preheated for 3 h at 160°C (control sequence). A second nutrient injection was added to the active and control samples approximately 7 and 5 sols, respectively, after first injection. Brief intervals where counts are reduced by approximately half reflect times when only one of the two beta detectors were utilized (single channel counting mode). [Reprinted from Levin, G.V. and Straat, P.A. (1977b) Life on Mars? The Viking Labeled Release Experiment. Biosystems, Vol 9/2–3, pp165–174, September 1977; with permission from Elsevier.]
3. The Viking LR Data
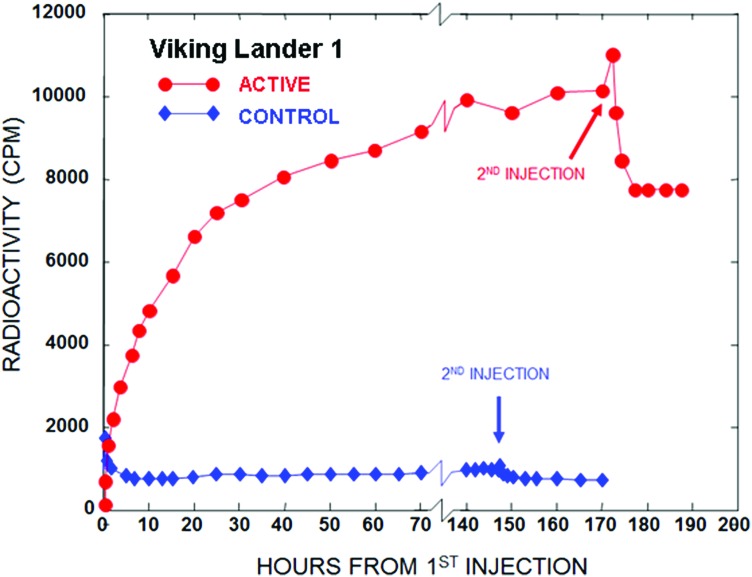
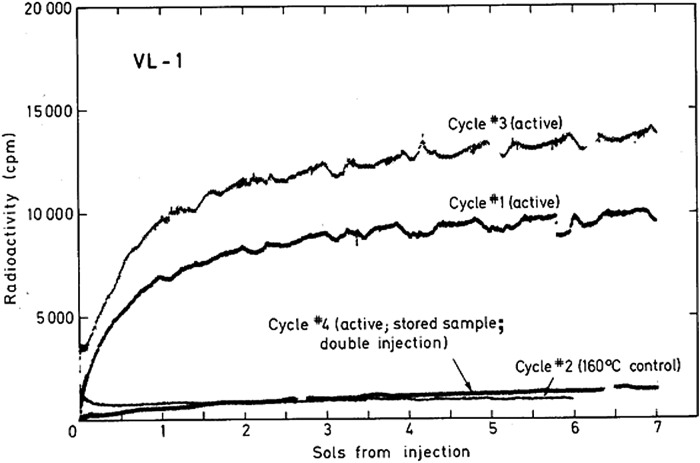
Vikings 1 and 2 were launched August 20 and September 9, 1975, and landed safely on Mars July 20 and September 3, 1976, respectively. On July 30, 1976, Sol 10, the first LR run was conducted on a martian soil sample (Viking 1, cycle 1; i.e., VL1-1). The soil sample had been collected on Sol 8 by the sampling arm, which dredged to a depth of about 4 cm. The soil was stored in the sample distribution box at approximately 10°C for the two intervening sols. As shown in Fig. 2, upon nutrient injection, the LR response was immediate and strongly active, within the lower portion of the range of responses (Fig. 3) from terrestrial viable soils (Levin and Straat, 1976a; Levin, 2006). After the run was complete, the critical 160°C control was performed on a duplicate sample of the same soil (VL1-2). The response was negative (Fig. 2). Taken together, the results from VL1-1 and VL1-2 are consistent with a life response (Levin and Straat, 1976b).
FIG. 2.
LR response to first and second nutrient injection in VL1 cycle 1 (active) and VL1 cycle 2 (160°C control). [Adapted from Levin and Straat, 1976b]
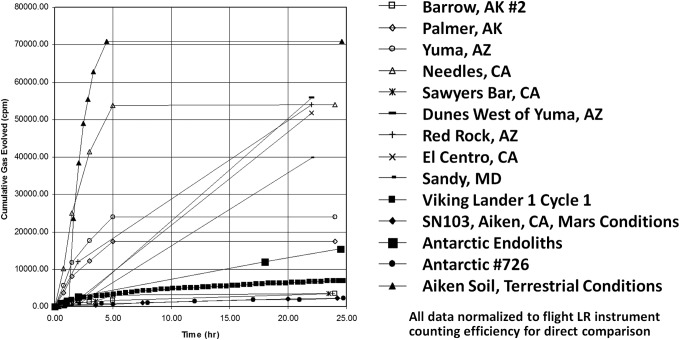
FIG. 3.
Comparison of Viking LR response with those from several viable terrestrial soil samples. Experiments with terrestrial soils were conducted by the “getter” technique, except for the SN103 data, which were obtained with a flight instrument. All data have been normalized to flight LR instrument counting efficiency for direct comparison. The Viking response (VL1-1) is shown by heavy black squares with frequent time intervals toward the bottom of the figure. [Reprinted from Levin, G.V. Modern myths of Mars. Proc. Instruments, Methods, and Missions for Astrobiology, Proc. of SPIE 6309, 1–15, 2006; with permission from SPIE Proceedings; figure compiled from Levin and Straat, 1976a, 1976b.]
Gas evolution in VL1-1 began to level off after around 2 sols, at a level corresponding either to total utilization of only one carbon in the nutrient or partial utilization of several or all of the carbon substrates, and had nearly plateaued 7 sols after the first nutrient injection. This plateau could indicate either running out of substrate or “death” of the active agent, or both. An additional injection of nutrient on Sol 7 failed to produce additional gas evolution, indicating that the plateau resulted from lack of active agent rather than lack of substrate. Instead, there was an immediate depletion in the headspace gas, attributed to reabsorption of CO2, by the wetted alkaline soil (Levin and Straat, 1976b).
Most terrestrial soils responded positively to a second injection of nutrient, as demonstrated in Fig. 1. The failure of the martian sample to respond positively to the addition of more substrate indicated that the active agent was no longer active. Reabsorption of gas following second injection was therefore attributed to a physicochemical reaction involving a carbon dioxide-bicarbonate-water-soil equilibrium in the test cell. In support of this conclusion, laboratory studies in the TSM demonstrated 14CO2 uptake following water injections in the presence of two Mars analog soils as well as in the absence of soil (Levin and Straat, 1979b).
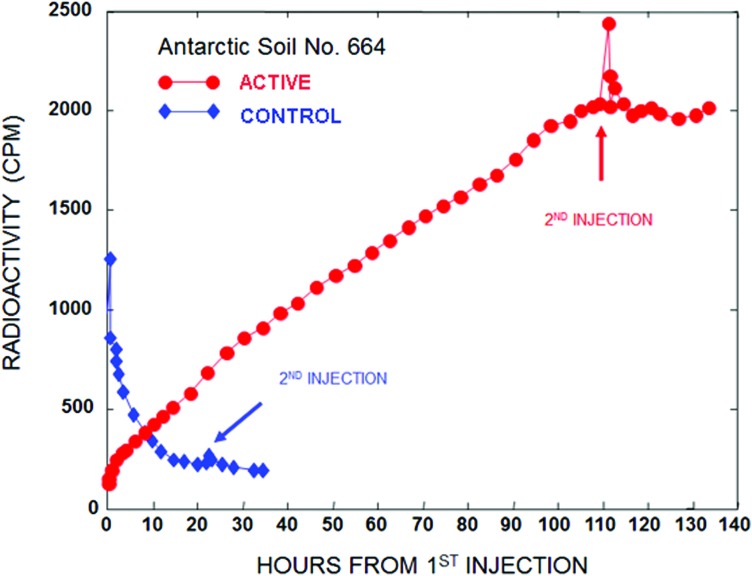
As seen in Fig. 4, Antarctic soil #664 acted similarly in the TSM when a second injection of the LR nutrient was made, although the percentage drop was not nearly as great as in the martian samples (Levin and Straat, 1986). This was a NASA-bonded test soil with a pH of 8.1. Several other Antarctic soils, one naturally sterile and the others with a sparse microbial population, were also examined in our TSM test program. Soil #664 was the only one that showed reabsorption following second injection, and the only one at high pH; this reabsorption is most likely reflecting a physicochemical reaction, as proposed for the martian sample.
FIG. 4.
LR response to first and second nutrient injection added to Antarctic soil #664 in the TSM (active). A duplicate run was conducted on soil presterilized at 160°C (control). [Reprinted from Levin, G.V. and Straat, P.A. (1986) A reappraisal of life on Mars. Figure presented at The NASA Mars Conference in conjunction with the American Astronautical Society (AAS) held July 21–23, 1986, Washington, D.C., U.S.A. Published in The NASA Mars Conference, edited by D.B. Reiber, AAS Science and Technology Series, © 1988, v. 71, pp 187–207; with permission from Univelt, Inc.]
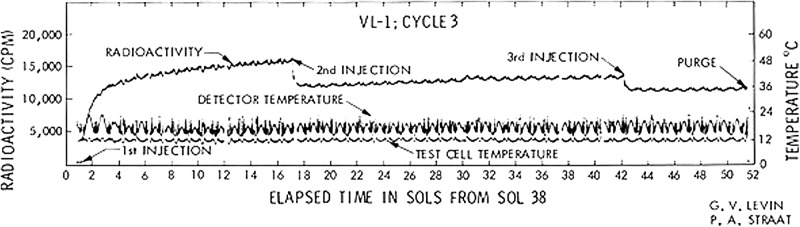
Scientific protocol dictated that the third run, VL1-3, verify or contest the positive result of VL1-1. A fresh sample of the soil was taken from the same area. The response was again positive (Fig. 5), validating the positive response from the first cycle. Second and third injections produced the same reabsorption of gas seen with VL1 cycle 1, followed by a slow linear re-evolution of radioactive gas.
FIG. 5.
Complete VL1 cycle 3. A fresh sample was used in this active cycle. Data show times of first, second, and third injections. [Reprinted from Levin, G.V. and Straat, P.A. (1977b) Life on Mars? The Viking Labeled Release Experiment. Biosystems, Vol 9/2–3, pp165–174, September 1977; with permission from Elsevier.]
The fourth and final LR run at Viking site 1 (VL1-4) was on a sample collected from the same area that had produced the positive response in VL1-1. However, at the time of the injection for this run, the soil had been stored in the sample distribution box, in the dark, open to the martian atmosphere, but maintained at temperatures ranging between 10°C and 26°C for 141 sols. Two nutrient injections were made about 3 h apart, which allowed time to observe a response from the first injection before administering the second. Each injection resulted in a null response (Fig. 6). Apparently, long-term storage of an active soil at 10–26°C inactivates an initially active sample. This, again, is consistent with biology. We would not expect to see such losses in terrestrial analog soils because these temperatures are within normal range for most terrestrial soils, even over long periods of time. On Mars, however, although soil may temporarily see 10°C or even higher temperatures during a normal diurnal cycle, at least during the warmer times of the martian year, the daily exposure to such elevated temperature would be brief; martian organisms would therefore be expected to be much more likely to succumb to long-term storage at 10–26°C than would terrestrial microorganisms. Thus, possibly stressed by long-term isolation from their natural environment at elevated temperatures, the putative martian organisms may have died.
FIG. 6.
All first injection cycles of VL1. A fresh sample was used for the active sequences of cycles 1 and 3, whereas the sample used for active cycle 4 was stored for approximately 141 sols at 10–26°C prior to use. For cycle 2, a stored portion of the same sample used for cycle 1 was heated for 3 h at 160°C prior to nutrient injection. All data have been corrected for background counts observed prior to nutrient injection. [Reprinted from Levin, G.V. and Straat, P.A. (1979a) Completion of the Viking Labeled Release Experiment on Mars. J Mol Evol 14:167–183, 1979; Journal Molecular Evolution © by Springer-Verlag, 1979; with permission of Springer.]
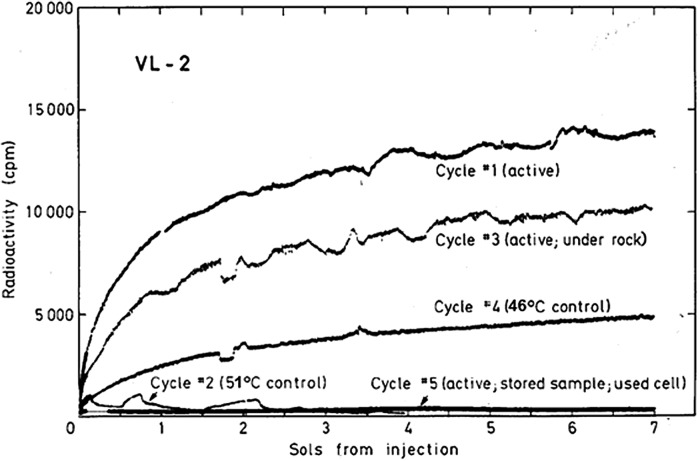
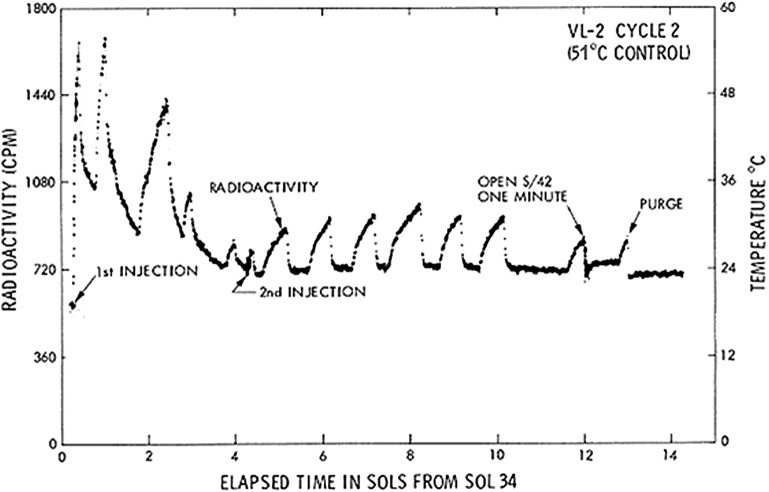
All Viking site 1 test and control responses for first injections for the first 7 sols are shown in Fig. 6 (Levin and Straat, 1979a). The LR first injection results at Viking site 2, some 4000 miles distant, are shown in Fig. 7 (Levin and Straat, 1979a). The first sample on the second lander (VL2-1) produced a positive response, essentially duplicating the positive responses of VL1 cycles 1 and 3. During the mission, because some scientists questioned the 160°C control as evidence of life, the Viking Biology Team unanimously agreed that preheating a sample to 50°C for 3 h was more likely to distinguish between chemistry and biology; few oxidizing chemicals are affected by such a low temperature, whereas martian organisms, acclimated to extremely low ambient temperatures, might be adversely affected. Calculations confirmed that activation of only the booster heater in the head-end should result in soil temperatures around 50°C, although this had never been attempted in advance of the mission. Thus, for VL2-2, a fresh sample was acquired from the same location that supplied the positive sample and heated by activating only the booster heater. The soil temperature achieved during this “low-temperature sterilization” was determined by extrapolation of the thermal signal to be 51°C. As shown in Fig. 8, the initial response following first injection was greatly reduced but not eliminated (Levin and Straat, 1977a). A series of small peaks, not seen in any previous or subsequent runs in either the flight instrument or the TSM, was evolved. The peculiar kinetics suggested a possible instrument anomaly, but on careful investigation, the engineers could find no problem with the instrument, nor was any detected over the remaining course of the mission over which it functioned properly. Comparing only the first peak to the amount of gas evolved in VL2-1 during an equivalent time period showed that only about 15% as much gas was evolved; in total, significantly less gas was produced over the remaining sols than seen in VL2-1.
FIG. 7.
All first injection cycles of VL2. A fresh sample was used for all sequences except cycle 5, which used the same sample as for cycle 4 but was stored for approximately 84 sols before nutrient injection. All soil samples were taken from the same site except the sample for cycle 3, which was taken from under Notch Rock. All data have been corrected for background counts observed prior to nutrient injection. [Reprinted from Levin, G.V. and Straat, P.A. (1979a) Completion of the Viking Labeled Release Experiment on Mars. J Mol Evol 14:167–183, 1979; Journal Molecular Evolution © by Springer-Verlag, 1979; with permission of Springer.]
FIG. 8.
VL2 cycle 2 LR responses to first and second injections. After correcting for background, the cumulative radioactivity evolved in the first peak following first nutrient injection was approximately 15% of that evolved during VL2 cycle 1 in the same time period; little else evolved over the subsequent sols. Following second injection, the peaks represent diurnal movement of gas back and forth from the test cell chamber to the detector chamber as determined by turning off the thermal electric coolers for one sol between Sols 10 and 12, which eliminated the fluctuation. [Reprinted from Levin, G.V. and Straat, P.A. (1977a) Recent Results from the Viking Labeled Release Experiment on Mars. J Geophys Res 82(28):4663–4667. ©1977 by the American Geophysical Union; with permission by Wiley.]
The third cycle at VL2 was a positive run to ensure the integrity of the instrument. At this point, a new possibility was raised: the LR positive responses could be attributed to “activation” of the soil by the intense flux of UV light impacting the martian surface. Activated soil components, it was contended, could react nonbiologically with the nutrients to evolve radioactive gas. To test the UV activation theory, the soil sample for VL2-3 was collected just at dawn by moving a rock (Notch Rock) with the sampling arm and obtaining a sample that had been shielded from UV light by the rock for eons. After 2 days in the test cell, the soil was injected with the nutrient solution. The strong positive VL2-3 response (Fig. 7) dispelled the UV theory and confirmed instrument integrity.
An attempt was then made to clarify the singular results of VL2-2 by repeating that experiment in VL2-4. A fresh soil sample, taken from the same location as the fresh samples for VL2 cycles 1 and 2, was preheated, aiming for 50°C as before. This time, however, the temperature attained in the soil was extrapolated to be 46°C. The LR VL2-4 response (Fig. 7) produced essentially the same kinetics as the positive VL2-1 and VL2-3 samples, but with only about 30% of the amplitude. Clearly the low-temperature treatment had reduced the magnitude of the active response, a result strongly in support of biology; few chemical oxidants would be expected to show such temperature sensitivity. Although the unusual kinetics of VL2-2 are unexplained, the one clear conclusion that can safely be made is that the magnitude of the initial several hours of reaction was greatly reduced by the low-temperature sterilization, in agreement with VL2-4. As for the effect of the unintended 5° temperature difference between the two low-temperature sterilizations, while it hints of biology in that small temperature differences could affect microbial survival, no conclusion can be made in the absence of further data.
Viking 2, cycle 4 (VL2-4) used the last of the four LR test chambers. Since nutrient solution was still available, an additional run was improvised whereby stored soil collected for VL2-4 was added on top of a previously tested soil sample. This VL2-5 soil was taken from the same area as that for VL2-4, but the soil had been held in the sample distribution box for 84 sols at approximately 10°C before the onset of VL2-5. As seen in Fig. 7, long-term storage of the sample used for VL2-5 totally inactivated the soil, producing a negative response.
In summary of the Viking LR data, characteristics determined for the active agent are listed in Table 1. Each of these characteristics is reminiscent of responses by a compendium of terrestrial microorganism species, including the initial positive responses, the 160°C and 50°C heat controls, the reabsorption of evolved gas upon second injection of nutrient, and death from isolated long-term storage. Further, the Viking LR data show similarity to the SN103 data (Levin and Straat, 1977b) obtained with California soil held under martian conditions for 3 days prior to testing (Fig. 1), and are within the lower range of responses from other viable terrestrial soils both in amplitude and kinetics (Fig. 3), including those from Barrow and Palmer, Alaska, and Antarctic soil 726. All in all, the results of the Viking LR experiment are consistent with a biological explanation.
Table 1.
Characteristics of Active Agent Detected by Viking LR Experiment
| (1) Produced positive response when injected with nutrient solution, similar in kinetics and amplitude to responses produced by LR test of a number of terrestrial viable soils. |
| (2) Inactivated by preheating to 160°C for 3 h, similar to terrestrial tests of active soils. |
| (3) Significantly reduced when preheated to approximately 50°C for 3 h. |
| (4) Inactivated when stored approximately 2 months in the dark in the soil distribution box at approximately 10°C. |
| (5) Activation of soil not caused by UV exposure. |
| (6) A second injection of nutrient solution to positively responding soil caused approximately 25% of gas already evolved to disappear from detector cell (probably reabsorbed into soil), gradually to re-evolve. Active agent no longer available at time of second injection. |
| (7) Widespread distribution of active agent (Viking sites 1 and 2 are 4000 miles apart). |
4. Nonbiological Explanations for the LR Data
Despite the positive LR results, the scientific community has remained skeptical of the biological interpretation. Doubt increased greatly when the GCMS reported failure to detect organic matter in the martian soil or atmosphere (Biemann et al., 1977). In the years since Viking, many attempts have been made to explain the LR results nonbiologically, but so far, no nonbiological explanation has met all the criteria. Zent and McKay (1994) reviewed most of the earlier oxidant theories, finding them unconvincing, and offered their theory that atmospheric photochemically produced oxidants react with the martian regolith to create complexes that caused the Viking biology results. However, none of the theoretical oxidants satisfy the Viking LR thermal control data.
One study frequently cited in explaining the Viking LR data is that of Navarro-González et al. (2003), who conducted LR-type reactions in situ in the extremely arid Atacama Desert. They claimed that the soil tested contained only trace organics and extremely low levels of culturable bacteria. LR-type reactions were conducted with 13C-labeled alanine and glucose enantiomers. When results from a mixture of L-alanine and D-glucose were essentially the same as those from a mixture of D-alanine and L-glucose, the soil sample was considered abiotic. Experiments were also conducted with 13C-labeled formate; active responses resulting from the addition of formate to Atacama soil were attributed to an unidentified nonbiological oxidant rather than biology because the chiral experiments with alanine and glucose had indicated that the soil was sterile. While these authors do not specifically claim to have resolved the Viking LR results, the implication is that the Viking results are most likely caused by a nonbiological soil oxidant.
While those Atacama results superficially resemble the Viking LR results, the conditions of the two studies are drastically different. The most critical part of the LR experiment is verifying a positive response by conducting an identical experiment on a 160°C heat-treated sample of the same soil. This would have been especially important in the Atacama study with formate. Formate is known (Straat and Levin, 1971) to react nonbiologically with certain terrestrial soils, particularly those of low pH; presterilization of the soil with heat at 160°C does not eliminate this reaction, showing that the LR technology readily distinguished this chemical reaction from a life response. Had a sterilization control been conducted at the Atacama site, the reaction with formate may have been the same as with the nonsterilized reaction, unlike the Mars result. Another consideration is that the formate concentration in the Atacama studies was 2000 times greater than that used on Mars and 10 times greater than the concentrations of the alanine and glucose isomers used in the Atacama experiments. Thus, while the Atacama studies may provide an important model for testing experiments designed for future Mars missions, they cannot, as these authors themselves concluded, be used to interpret the Viking LR results.
The discovery during the Phoenix mission (Hecht et al., 2009) that perchlorates were present on Mars at approximately 0.5 wt % levels provided yet another candidate for the oxidation of the LR nutrients. Perchlorates have since been found at several Curiosity sites by Sample Analysis at Mars (SAM) (Glavin et al., 2013), implying that perchlorates may be widespread on the surface of Mars. However, perchlorates cannot account for the Viking LR data because perchlorates are stable under all thermal conditions of the Viking LR experiment, including the 160°C control (Quinn et al., 2013).
Quinn et al. (2013) proposed that products from perchlorate exposed to ionizing radiation could account for the LR positive results. They showed that perchlorate exposed to 60Co in a carbon dioxide environment produces hypochlorite, oxygen, and chlorine dioxide. Hypochlorite added to 13C-labeled LR nutrients produced 13CO2. Based on their results, they claimed that galactic cosmic rays and radiation particles from the Sun can produce hypochlorite from perchlorate, which can then interact with 14C-labeled amino acids, especially alanine (present in the Viking LR nutrient solution), and release radioactive gas. They further showed that hypochlorite is destroyed by heating for 3 h at 160°C, meeting that requirement of the Viking LR control cycle.
While tantalizing as a nonbiological explanation for the Viking LR results, there are some problems with this hypothesis. Whether perchlorate or its irradiation products were present at the VL1 and VL2 sites has not been established, although Navarro-González et al. (2010) conducted studies that led them to imply perchlorate may have been present. The Quinn et al. (2013) data also do not address two critical aspects of the LR experiment: First, the active LR agent on Mars is adversely affected by heating at approximately 50°C, and, second, is destroyed by long-term storage in the dark at 10°C, both of which are strong plusses for biology; these critical controls have yet to be performed with irradiation products of perchlorate. Nonetheless, despite deficiencies, this hypothesis remains perhaps the most attractive of the nonbiological explanations because of the likely widespread distribution of perchlorate and, especially, because of the sensitivity of hypochlorite to 160°C.
5. Discussion and Conclusions
The general consensus at the time of Viking was that the LR positive response was nonbiologically caused. However, since then, information from later missions to Mars has called the basis of that consensus into question, and it is timely to reconsider a biological explanation for the LR results. On the side of biology, the LR active results were strongly positive and obliterated by heat treatment at 160°C. Perhaps the strongest experimental evidence for biology is that pretreatment of the soil around 50°C partially destroyed the active agent, and that long-term storage around 10°C completely destroyed it. In our pre-mission testing program, we showed (Straat and Levin, 1970) that a 4 h, 90°C preheat treatment significantly reduced terrestrial soil activity; martian microbes, acclimated to lower environmental temperatures, would be expected to be even more temperature-sensitive. Few chemical agents that would oxidize any of the LR nutrients are affected by such low temperatures, which is why it is so important that candidate nonbiological agents be subjected to those temperature regimes as a control prior to testing for activity. The Mars active result was also similar in magnitude and kinetics to pre-mission tests of a California soil in a flight instrument (Fig. 1) and to results from a variety of terrestrial soils tested either in the field or in the TSM (Fig. 3). Against the biological hypothesis are the candidate nonbiological hypotheses, some of which come tantalizingly close to approximating the LR results (Navarro-González et al., 2003; Quinn et al., 2013). However, none as yet have completely replicated the LR thermal data, mainly because the critical 50°C and 10°C controls are lacking.
The harsh environment of Mars has also been cited as unfavorable to a biological interpretation, particularly because of the lack of liquid water. Yet findings from recent missions provide strong evidence that liquid water is available, albeit in low amounts, either in pure or absorbed form (Levin, 1997; Rennó et al., 2009; Ming et al., 2014), as frost (such as seen at the Viking 2 landing site, Fig. 9), or as briny saltwater (Martin-Torres et al., 2015; Ojha et al., 2015). The temperature of the atmosphere immediately above the surface of large regions of Mars frequently reaches the 20°C range (Gomez-Elvira et al., 2014; Mischna et al., 2014), which is sufficient to provide liquid water from near-surface ice since atmospheric pressure has never been recorded below the triple point, except at high elevations. Considering the ability of terrestrial extremophiles to adapt to harsh arid environments, it would seem that, if life were ever present on Mars, it could have adapted and evolved as Mars transformed to cold conditions where water was scarce. Life may therefore still exist, if only in a cryptobiotic state, subject to resuscitation whenever water becomes available. Overall, the studies of water on Mars do not preclude extant life, whether or not the LR detected it. While the availability of water at the Viking sites was unknown at the time the LR was performed, the availability of water on Mars generally favors a biological interpretation of the LR.
FIG. 9.
Frost at the Viking 2 Lander site. Image acquired May 18, 1979, almost one martian year after the Viking Lander 2 first detected frost at this site. [Image credit: NASA; Viking 2 Lander image P-21873 (other ID 21I093/960) available online at http://nssdc.gsfc.nasa.gov/imgcat/html/object_page/v12_p21873.html.]
Lack of biologically relevant organic molecules on the surface of Mars has also been considered a major detriment to extant life. Recent reports of complex organics, possibly of biological importance, are encouraging (Freissinet et al., 2015), although analyses are ongoing and details are not yet available. Simple organic substrates, such as amino acids or other organics present in the LR nutrient, have not yet been detected on Mars, although the putative martian microbes that produced the LR reaction were demonstrating heterotrophic metabolism by utilizing such organics as substrates. While autotrophs seem a more likely possibility for martian microbial life because of the apparent scarcity of simple organics, it is possible that martian autotrophs would be facultative and able to utilize simple organics whenever they are available, such as through meteoritic fallout or other nonbiological processes.
The sporadic appearance of methane (Mumma et al., 2009; Webster et al., 2015) suggests methanogens as possible martian life-forms. Mumma et al. (2009) stated that methane may be biologically accumulated underground where water may be available, and that geological activity, such as seasonal fissuring, might cause periodic release. Because Mumma et al. (2009) also proposed that a sink was needed to explain the overly rapid disappearance of released methane, Levin and Straat (2009) postulated an anaerobic methanogen-methanotroph ecology on the martian surface where methane-consuming methanotrophs could provide such a sink. Halophiles have also been suggested as candidates for martian life-forms (Leuko et al., 2010). In addition to their ability to tolerate salinity extremes, many terrestrial halophiles withstand damaging radiation, temperature extremes, and low-oxygen atmosphere (DasSarma, 2006). Some terrestrial halophiles are also methanogenic (Ollivier et al., 1994; Oren, 2002). However, Martin-Torres et al. (2015) and Ojha et al. (2015) indicated that water activity in the postulated perchlorate salt brines would be too low for habitability by terrestrial microbes, although this would not preclude halophilic activity at lower salt concentrations. Perchlorates, first discovered on Mars in 2009 (Hecht et al., 2009) and apparently widespread on the surface (Glavin et al., 2013), are toxic to many terrestrial flora and fauna, although several perchlorate-utilizing terrestrial microorganisms have been isolated and described (Coates and Achenbach, 2004; Waller et al., 2004; Bardiya and Bae, 2011). Other extant chemoautotrophic life-forms may yet be defined as candidates for extant martian life.
The LR experiment was designed to monitor microbial metabolism whereby simple organic molecules are consumed to yield carbon dioxide or other carbon gases derived from the LR substrates. The flight instrument was not capable of identifying the labeled gas (or gases) evolved. Laboratory simulations have shown that the primary gaseous end-product of the LR reaction was carbon dioxide (Levin and Straat, 1979b), although other carbon-based gas would also have been detected. Several methanogenic species are known (Miyamoto, 1997; Chaban et al., 2006) that can utilize formate, one of the LR substrates, or other organics in the generation of methane. Thus, while methanogens could not be responsible for the entire active LR response, the LR soil sample could have included methanogens, and the evolved gas could have included methane as well as carbon dioxide. Also, as previously discussed, the LR experiment dropped a small amount of liquid nutrient on a relatively large soil sample, creating a water gradient from wet, where the nutrient landed, to dry or almost dry at the periphery of the soil sample. It is entirely possible that the liquid added to the LR soil sample reactivated cryptobiotic martian microorganisms.
We have long proposed a chiral metabolic experiment as a follow-on to the Viking LR experiment (e.g., Levin, 1998). Chemical reactions cannot distinguish between stereoisomers, whereas all known terrestrial life-forms preferentially utilize L-amino acids and D-carbohydrates. Preferential utilization of one or the other such isomer on Mars would be indicative of a life response. However, some biological utilization of D-amino acids and L-carbohydrates does occur in terrestrial microorganisms. For example, D-alanine is a known constituent of bacterial cell walls (Newton, 1970; Lam et al., 2009), and the toxicity of a Chinese mushroom has been attributed to two novel unsaturated D-amino acids (Stone, 2012). Martinez-Rodriguez et al. (2010) reported incorporation of D-amino acids into peptide antibiotics. Moazeni et al. (2010) reported utilization of both D-lactate and L-lactate by microbial communities, although L-lactate is consumed only after a lag. Zhang and Sun (2014) provided evidence that bacteria use D-amino acids as a source of nitrogen by running enzymatic racemization in reverse. While such reactions may occur in nature, they are rare and do not negate the concept of using chiral compounds to differentiate chemical from metabolic reactions, because it would be expected that the rates of biological utilization of the two isomers would differ, whereas kinetics for chemical reactions of both should be the same. But, in any event, the cogent use of controls could readily distinguish between a chemical and a biological response.
In summary, in the absence of a nonbiological agent that satisfies all Viking findings, and in view of environmental evidence that Mars may well be able to support extant life, it seems prudent that the scientific community maintain biology as a viable explanation of the LR experimental results. It seems inevitable that astronauts will eventually explore Mars. In the interest of their health and safety, biology should be held in the forefront of possible explanations for the LR results. Plans for any Mars sample return mission should also take into account that such a sample may contain viable, even if dormant, alien life. Protected “Special Regions” on Mars, deemed possibly habitable by terrestrial hitch-hikers (Rummel et al., 2014), may even need to be redefined, especially based on the liquid water findings by Martin-Torres et al. (2015) and by Ojha et al. (2015). It is noted, however, that Mars may already have been infected by the many spacecraft that have landed there; although Viking was heat-treated to reduce microbial counts, no other spacecraft have been similarly treated. The Viking LR experiment is thus in the unique position of being the only pristine life-detection experiment that we will ever have. We strongly recommend that life-seeking experiments be considered for future missions. These should include the continued search for organic molecules of biological importance (e.g., amino acids, simple carbohydrates, lipids, DNA, protein); the conduct of further metabolic experiments, including a search for chiral preference in metabolism; the close examination of any tantalizing surface features; and perhaps even microscopic examination of martian soil with and without the addition of water or water vapor, reminiscent of the experiments of Antony van Leeuwenhock, who discovered the phenomenon of cryptobiosis approximately 300 years ago (Clegg, 2001).
Abbreviations Used
- GCMS
gas chromatograph–mass spectrometer
- LR
Labeled Release
- SN103
a flight instrument used for tests prior to the mission
- TSM
Test Standards Module
- VL1
Viking Lander 1
- VL2
Viking Lander 2
Acknowledgment
The Viking Labeled Release results reported herein were supported by Contracts NAS1-9690, NASW-3162, and NASW-3249 from the National Aeronautics and Space Administration.
Author Disclosure Statement
No competing financial interests exist.
References
- Bardiya N. and Bae J.H. (2011) Dissimilatory perchlorate reduction: a review. Microbiol Res 166:237–254 [DOI] [PubMed] [Google Scholar]
- Biemann K., Oró J., Toulmin P., III, Orgel L.E., Nier A.O., Anderson D.M., Simmonds P.G., Flory D., Diaz A.V., and Rushneck D.R. (1977) The search for organic substances and inorganic volatile compounds on the surface of Mars. J Geophys Res 82:4641–4658 [DOI] [PubMed] [Google Scholar]
- Chaban B., Ng S.Y.M., and Jarrall K.F. (2006) Archael habitats—from the extreme to the ordinary. Can J Microbiol 52:73–116 [DOI] [PubMed] [Google Scholar]
- Clegg J.S. (2001) Cryptobiosis—a peculiar state of biological organization. Comp Biochem Physiol B Biochem Mol Biol 128:613–624 [DOI] [PubMed] [Google Scholar]
- Coates J.D. and Achenbach L.A. (2004) Microbial perchlorate reduction: rocket-fuelled metabolism. Nat Rev Microbiol 2:569–580 [DOI] [PubMed] [Google Scholar]
- DasSarma S. (2006) Extreme halophiles are models for astrobiology. Microbe 1:120–126 [Google Scholar]
- Freissinet C., Glavin D.P., Mahaffy P.R., Miller K.E., Eigenbrode J.L., Summons R.E., Brunner A.E., Buch A., Szopa C., Sracher P.D., Jr., Franz H.B., Atreya S.K., Brincherhoff W.B., Cabane M., Coll P., Conrad P.G., Des Marais D.J., Dworkin J.P., Fairen A.G., François P., Grotzinger J.P., Kashyap S., ten Kate I.L., Leshin L.A., Malspin C.A., Martin M.G., Martin-Torres F.J., McAdam A.C., Ming D.W., Navarro-González R., Pavlov A.A., Prats B.D., Squyres S.W., Steele A., Stern J.C., Sumner D.Y., Sutter B., Zorano M.P., and the MSL Science Team. (2015) Organic molecules in the Sheepbed mudstone, Gale Crater, Mars. J Geophys Res: Planets 120:495–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin D.P., Freissinet C., Miller K.E., Eigenbrode J.L., Brunner A.E., Buch A., Sutter B., Archer P.D., Jr., Atreya S.K., Brinckerhoff W.B., Cabane M., Coll P., Conrad P.G., Coscia D., Dworkin J.P., Franz H.B., Grotzinger J.P., Leshin L.A., Martin M.G., McKay C., Ming D.W., Navarro-González R., Pavlov A., Steele A., Summons R.E., Szopa C., Teinturier S., and Mahaffy P.R. (2013) Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J Geophys Res: Planets 118:1955–1973 [Google Scholar]
- Gomez-Elvira J., Armiens C., Carrasco I., Genzer M., Gomez F., Haberle R., Hamilton V.E., Harri A.-M., Kahanpaa H., Kemppinen O., Lepinette A., Soler J.M., Martin-Torres J., Martinez-Frias J., Mischna M., Mora L., Navarro S., Newman C., de Pablo M.A., Peinado V., Polkko J., Rafkin S.C.R., Ramos M., Rennó N.O., Richardson M., Rodriguez-Manfredi J.A., Romeral Planello J.J., Sebastian E., de la Torre Juarez M., Torres J., Urqui R., Vasavada A.R., Verdasca J., and Zorzano M.-P. (2014) Curiosity's Rover Environmental Monitoring Station: overview of the first 100 sols. J Geophys Res: Planets 119:1680–1688 [Google Scholar]
- Hecht M.H., Kounaves S.P., Quinn R.C., West S.J., Young S.M.M., Ming D.W., Catling D.C., Clark B.C., Boynton W.V., Hoffman J., DeFlores L.P., Gospodinova K., Kapit J., and Smith P.H. (2009) Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 325:64–67 [DOI] [PubMed] [Google Scholar]
- Horowitz N.H., Hubbard J.S., and Hobby G.L. (1972) The carbon-assimilation experiment: the Viking Mars lander. Icarus 16:147–152 [Google Scholar]
- Horowitz N.H., Hobby G.L., and Hubbard J.S. (1977) Viking on Mars: the carbon assimilation experiments. J Geophys Res 82:4659–4662 [Google Scholar]
- Klein H.P., Horowitz N.H., Levin G.V., Oyama V.I., Lederberg J., Rich A., Hubbard J.S., Hobby G.L., Straat P.A., Berdahl B.J., Carle G.C., Brown F.S., and Johnson R.D. (1976) The Viking biological investigations: preliminary results. Science 194:99–105 [DOI] [PubMed] [Google Scholar]
- Lam H., Oh D.-C., Cava F., Takacs C.N., Clardy J., de Pedro M.A., and Waldor M.K. (2009) D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuko S., Rothschild L.J., and Burns B.P. (2010) Halophilic archaea and the search for extinct and extant life on Mars. Journal of Cosmology 5:940–950 [Google Scholar]
- Levin G.V. (1972) Detection of metabolically produced labeled gas: the Viking Mars lander. Icarus 16:153–166 [Google Scholar]
- Levin G.V. (1997) Life after Viking: the evidence mounts. In Mars the Living Planet, edited by Digregorio B.E., Levin G.V., and Straat P.A., Frog, Berkeley, CA, pp 287–312 [Google Scholar]
- Levin G.V. (1998) The future search for life on Mars: an unambiguous martian life detection experiment [abstract 7012]. In Workshop on Martian Meteorites: Where Do We Stand, and Where Are We Going? Lunar and Planetary Institute, Houston [Google Scholar]
- Levin G.V. (2006) Modern myths of Mars. Proc SPIE 6309, doi: 10.1117/12.676304 [DOI] [Google Scholar]
- Levin G.V. and Straat P.A. (1976a) Labeled Release—an experiment in radiorespirometry. Orig Life 7:293–311 [DOI] [PubMed] [Google Scholar]
- Levin G.V. and Straat P.A. (1976b) Viking Labeled Release biology experiment: interim results. Science 194:1322–1329 [DOI] [PubMed] [Google Scholar]
- Levin G.V. and Straat P.A. (1977a) Recent results from the Viking Labeled Release experiment on Mars. J Geophys Res 82:4663–4667 [Google Scholar]
- Levin G.V. and Straat P.A. (1977b) Life on Mars? The Viking Labeled Release experiment. Biosystems 9:165–174 [DOI] [PubMed] [Google Scholar]
- Levin G.V. and Straat P.A. (1979a) Completion of the Viking Labeled Release experiment on Mars. J Mol Evol 14:167–183 [DOI] [PubMed] [Google Scholar]
- Levin G.V. and Straat P.A. (1979b) Laboratory simulations of the Viking Labeled Release experiment: kinetics following second injection and the nature of the gaseous end product. J Mol Evol 14:185–197 [DOI] [PubMed] [Google Scholar]
- Levin G.V. and Straat P.A. (1986) A reappraisal of life on Mars. In The NASA Mars Conference, edited by Reiber D.B., Univelt, San Diego, pp 187–207 [Google Scholar]
- Levin G.V. and Straat P.A. (2009) Methane and life on Mars. Proc SPIE 7441, doi: 10.1117/12.829183 [DOI] [Google Scholar]
- Levin G.V., Harrison V.R., and Hess W.C. (1956) Preliminary report on a one-hour presumptive test for coliform organisms. J Am Water Works Assoc 48:75–80 [Google Scholar]
- Martin-Torres F.J., Zorano M.-P., Valentin-Serrano P., Harri A.-M., Genzer M., Kemppinen O., Rivera-Valentin E.G., Jun I., Wray J., Madsen M.B., Goetz W., McEwen A.S., Hardgrove C., Rennó N., Chevrier V.F., Mischna M., Navarro-González R., Martinez-Frias J., Conrad P., McConnochie T., Cockell C., Berger G., Vasavada A.R., Sumner D., and Vaniman D. (2015) Transient liquid water and water activity at Gale Crater on Mars. Nat Geosci 8:357–361 [Google Scholar]
- Martinez-Rodriguez S., Martinez-Gomez A.I., Rodriguez-Vico F., Clemente-Jimenez J.M., and Las Heras-Vazquez F.J. (2010) Natural occurrence and industrial applications of D-amino acids: an overview. Chem Biodivers 7:1531–1548 [DOI] [PubMed] [Google Scholar]
- Ming D.W., Archer P.D., Jr., Glavin D.P., Eigenbrode J.L., Franz H.B., Sutter B., Brunner A.E., Stern J.C., Freissinet C., McAdam A.C., Mahaffy P.R., Cabane M., Coll P., Campbell J.L., Atreya S.K., Niles P.B., Bell J.F., III, Bish D.L., Brinckerhoff W.B., Buch A., Conrad P.G., Des Marais D.J., Ehlmann B.L., Fairen A.G., Farley K., Flesch G.J., Francois P., Gellert R., Grant J.A., Grotzinger J.P., Gupta S., Herkenhoff K.E., Hurowitz J.A., Leshin L.A., Lewis K.W., McLennan S.M., Miller K.E., Moersch R.V., Morris R.V., Navarro-González R., Pavlov A.A., Perrett G.M., Pradler I., Squyres S.W., Summons R.E., Steele A., Stolper E.M., Summer D.Y., Szopa C., Teinturier S., Trainer G.M., Treiman A.H., Vaniman D.T., Vasavada A.R., Webster C.R., Wray J.J., Yingst R.A., and the MSL Science Team. (2014) Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1245267 [DOI] [PubMed] [Google Scholar]
- Mischna M.A., Gomez-Elvira J., Armiens C., Carrasco I., Genzer M., Gomez F., Haberle R., Hamilton V.E., Harri A.-M., Kahanpaa H., Kemppinen O., Lepinette A., Soler J.M., Martin-Torres J., Martinez-Frias J., Mora L., Navarro S., Newman C., de Pable M.A., Peinado V., Polkko J., Rafkin S.C.R., Ramos M., Rennó N.O., Richardson M., Rodriguez-Manfredi J.A., Romeral Planello J.J., Sebastian E., de la Torre Juarez M., Terres J., Urqui R., Vasavada A.R., Verdasca J., Zorzano M.-P., and the MSL Science Team. (2014) Results from the Rover Environmental Monitoring Station (REMS) on board the Mars Science Laboratory. In 40th COSPAR Scientific Assembly, Moscow, Russia [Google Scholar]
- Miyamoto K., editor. (1997) Renewable Biological Systems for Alternate Sustainable Energy Production, FAO Agricultural Services Bulletin 128, Food and Agriculture Organization of the United Nations, Rome [Google Scholar]
- Moazeni F., Zhang G., and Sun H.J. (2010) Imperfect asymmetry of life: Earth microbial communities prefer D-lactate but can use L-lactate also. Astrobiology 10:397–402 [DOI] [PubMed] [Google Scholar]
- Mumma M.J., Villaneuva G.L., Novak R.E., Hewagama T., Bonev B.P., DiSanti M.A., Mandell A.M., and Smith M.D. (2009) Strong release of methane on Mars in northern summer 2003. Science 203:1041–1045 [DOI] [PubMed] [Google Scholar]
- Navarro-González R., Rainey F.A., Molina P., Bagaley D.R., Hollen B.J., de la Rosa J., Small A.M., Quinn R.C., Grunthaner F.J., Caceres L., Gomez-Silva B., and McKay C.P. (2003) Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science 302:1018–1021 [DOI] [PubMed] [Google Scholar]
- Navarro-González R., Vargas E., de la Rosa J., Raga A.C., and McKay C.P. (2010) Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res 115, doi: 10.1029/2010JE003599 [DOI] [Google Scholar]
- Newton J.W. (1970) Metabolism of D-alanine in Rhodospirillum rubrum and its bacilliform mutants. Nature 228:1100–1101 [DOI] [PubMed] [Google Scholar]
- Ojha L., Wilhelm M.B., Murchie S.L., McEwen A.S., Wray J.J., Hanley J., Masse M., and Chojnacki M. (2015) Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat Geosci 8:829–832 [Google Scholar]
- Ollivier B., Caumette P., Garcia J.-L. and Mah R. (1994) Anaerobic bacteria from hypersaline environments. Microbiol Rev 58:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology and applications. J Ind Microbiol Biotechnol 28:56–63 [DOI] [PubMed] [Google Scholar]
- Oyama V.I. (1972) The Gas Exchange experiment for life detection: the Viking Mars lander. Icarus 161:167–184 [Google Scholar]
- Oyama V.I. and Berdahl B.J. (1977) The Viking Gas Exchange experiment results from Chryse and Utopic surface samples. J Geophys Res 82:4669–4676 [Google Scholar]
- Quinn R.C., Martucci H.F.H., Miller S.R., Bryson C.E., Grunthaner F.J., and Grunthaner P.J. (2013) Perchlorate radiolysis on Mars and the origin of martian soil reactivity. Astrobiology 13:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennó N.O., Bos B.J., Catling D., Clark B.C., Drube L., Fisher D., Goetz W., Hviid S.F., Keller H.U., Kok J.F., Kounaves S.P., Leer K., Lemmon M., Madsen M.B., Markiewicz W.J., Marshall J., McKay C., Mehta M., Smith M., Zorzano M.P., Smith P.H., Stoker C., and Yound S.M.M. (2009) Possible physical and thermodynamical evidence for liquid water at the Phoenix landing site. J Geophys Res 114, doi: 10.1029/2009JE003362 [DOI] [Google Scholar]
- Rummel J.D., Beaty D.W., Jones M.A., Bakermans C., Barlow N.G., Boston P.J., Chevrier V.F., Clark B.C., de Vera J.-P.P., Gough R.V., Hallsworth J.E., Head J.W., Hipkin V.J., Kieft T.I., McEwen A.S., Mellon M.T., Mikucki J.A., Nicholson W.L., Omelon C.R., Peterson R., Roden E.E., Lollar B.S., Tanaka R.L., Viola D., and Wray J.J. (2014) A new analysis of Mars “special regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14:887–968 [DOI] [PubMed] [Google Scholar]
- Stone R. (2012) Heart-stopping revelation about how Chinese mushroom kills. Science 335, doi: 10.1126/science.335.6074.1293 [DOI] [PubMed] [Google Scholar]
- Straat P.A. and Levin G.V. (1970, September 18) Monthly Progress Report: Participation in the Science Planning for the Viking 1975 Missions in the Area of Biology, Contract No. NASI-9690 prepared for NASA Langley Research Center, Hampton, VA [Google Scholar]
- Straat P.A. and Levin G.V. (1971, July 30) Quarterly Progress Report: Participation in the Science Planning for the Viking 1975 Missions in the Area of Biology, Contract No. NASI-9690 prepared for NASA Langley Research Center, Hampton, VA [Google Scholar]
- Straat P.A. and Levin G.V. (1972, July 11) Quarterly Progress Report: Participation in the Science Planning for the Viking 1975 Missions in the Area of Biology, Contract No. NASI-9690 prepared for NASA Langley Research Center, Hampton, VA [Google Scholar]
- Waller A.S., Cox E.E., and Edwards E.A. (2004) Perchlorate-reducing microorganisms isolated from contaminated sites. Environ Microbiol 6:517–527 [DOI] [PubMed] [Google Scholar]
- Webster C.R., Mahaffy P.R., Atreya S.K., Flesch G.J., Mischna M.A., Meslin P.-Y., Farley K.A., Conrad P.G., Christensen L.E., Favlov A.A., Martin-Torres J., Zorzano M.-P., McConnochie T.H., Owen T., Eigenbrode J.L., Glavin D.P., Steele A., Malespin C.A., Archer P.D., Jr., Sutter B., Coll P., Freissinet C., McKay C.P., Moores J.E., Schwenzer S.P., Lemmon M.T., and the MSL Science Team. (2015) Mars methane detection and variability at Gale Crater. Science 347:415–417 [DOI] [PubMed] [Google Scholar]
- Zent A.P. and McKay C.P. (1994) The chemical reactivity of the martian soil and implications for future missions. Icarus 108:146–157 [Google Scholar]
- Zhang G. and Sun H.J. (2014) Racemization in reverse: evidence that D-amino acid toxicity on Earth is controlled by bacteria with racemases. Plos One 9, doi: 10.1371/journal.pone.0092101 [DOI] [PMC free article] [PubMed] [Google Scholar]