Abstract
Introduction
Schistosomiasis is one of the most prevalent parasitic infections in developing countries. Although chemotherapy is one of the main strategies in controlling the disease, it is less effective in reversal of schistosome-induced pathology especially in the chronic and advanced stages of schistosomiasis. New strategies and prospective therapeutic agents with antifibrotic effects are needed. Eugenol has a wide anti-inflammatory effect. In the present study, we investigated the possible antischistosomal effect of eugenol on Schistosoma mansoni.
Materials and methods
The murine model of S. mansoni was established in three groups of adult male Balb-c mice; group I (infected non-treated group) and groups II and III (infected groups) treated orally with eugenol and praziquantel (PZQ), respectively. The expression of the sensitive immunohistochemical marker α-smooth muscle actin (α-SMA) in schistosome-infected tissues was determined. In addition, parasitological, biochemical, and histological parameters that reflect disease severity and morbidity were examined.
Results
Eugenol treatment showed significant reduction in total worm burden by 19.2%; however, the oogram pattern showed no marked difference compared to that of the PZQ group. Yet, eugenol significantly reduced the serum levels of hepatic enzymes: aspartate aminotransferase and alanine aminotransferase. Histopathological examination revealed a significant reduction in both numbers and diameters of hepatic granulomata, which was consistent with reduction in collagen fiber deposition. Additionally, the antifibrotic effect of eugenol was validated by its considerable reduction in the expression of the sensitive marker α-SMA in both eugenol- and PZQ-treated groups.
Conclusion
Although eugenol could not totally eradicate adults of S. mansoni, the significant amelioration of liver enzymes and hepatic fibrosis potentiate eugenol’s role as a promising antifibrotic and a complementary antischistosomal agent.
Keywords: eugenol, Schistosoma mansoni, praziquantel, liver enzymes, hepatic stellate cells, anti-inflammatory
Introduction
Schistosomiasis is one of the high-priority neglected tropical diseases recognized by the WHO.1 This disease remains one of the most prevalent helminthic infections worldwide, as the number of people with active schistosome infections being likely between 391 and 587 million in 78 countries where schistosomiasis is endemic, with an estimated 12,000–200,000 deaths reported annually.2,3 Schistosomiasis is also a major cause of morbidity as it is responsible for causing anemia and significant growth retardation and has educational and nutritional effects not only among children but also among adults living in endemic areas.4
Schistosomiasis is caused by blood flukes belonging to the genus Schistosoma.5 The major Schistosoma species that infect humans are S. haematobium, S. mansoni, and S. japonicum. S. mansoni inhabits the intestinal venules (in close contact with host humoral and cellular cytotoxic factors) and primarily affects the liver and gut.6–8
The main pathological lesions of hepatic schistosomiasis result from a granulomatous reaction against schistosome egg deposition at the acute stage of infection, followed by advanced liver fibrosis in chronic infection.9 The dynamic process of hepatic fibrogenesis in schistosomiasis is a result of massive deposition of extracellular matrix in the periportal spaces, leading to blockage of the portal veins, which results in a series of complications, such as portal hypertension, splenomegaly, portacaval shunting, and gastrointestinal varices.10 One of the main mesenchymal cells that have an important role in schistosomal granulomatous reaction are hepatic stellate cells (HSCs) that are considered the main source of collagen deposition in liver schistosomiasis.11 These cells are normally located within the liver sinusoid in the following two forms: quiescent and activated. Quiescent HSCs are responsible for storage of vitamin A in normal liver tissue, whereas activated HSCs exhibit proliferative, contractile, fibrogenic, and myofibroblastic activities during liver fibrosis.12 Several studies have shown that HSCs can be activated by different means, such as viral infection, parasitic infestation, autoimmune deficiencies, and dietary or chemical causes.13 Eventually, the persistent fibrosis of chronic schistosomiasis may be one of the risk factors for hepatic cirrhosis and/or hepatic carcinoma with a high mortality rate.14
Although chemotherapy effectively eliminates adult worms and prevents egg deposition, few drugs have been developed to reverse existing hepatic fibrosis. Surgical intervention may be the only choice for patients at the chronic and advanced stages of schistosomiasis.15 Meanwhile, praziquantel (PZQ) is the drug of choice for the management of schistosomiasis, and significant limitations have been developed recently with reports of the emergence of drug-resistant strains of the parasite in some endemic areas such as Kenya and Egypt.16,17 Therefore, many medicinal plants have been studied for antischistosomal potency in addition to antifibrotic effects to be as alternatives for chemotherapy.
The essential oil eugenol, which is the main constituent of clove (Syzygium aromaticum), has been widely used as a flavoring agent for food. This substance also exhibits many pharmacological activities, including antimicrobial, anti-inflammatory, fungicidal, antioxidant, anticar-cinogenic, antiallergic, antimutagenic, and insecticidal properties.18–27
Several studies have studied the anti-inflammatory role of eugenol and the effect of eugenol on pro-inflammatory cytokine production by macrophages.28 Studies have shown an inhibitory effect of this compound on prostaglandin synthesis and neutrophil/macrophage chemotaxis in lipopolysaccharide-induced lung injury, preventing nuclear factor-κB (NF-κB) activation and collagen deposition in the lung parenchyma.29 Additionally, eugenol exhibited reduction in inflammation by decreasing TNF-α levels and neutrophil infiltration during pulmonary infection in animals.30
Furthermore, eugenol has high potential as an anti-parasitic agent that can be incorporated into the treatment of many parasitic infections. In vitro studies on eugenol have suggested that this oil has antigiardial, antileishmanial, trypanocidal, and antimalarial activities at high concentrations. Eugenol can be used in combination with standard drugs for increased efficacy, especially against drug-resistant strains. Eugenol has the advantage of being a natural essential oil with fewer side effects than synthetic therapeutics, whereas its activity is concentration-dependent.30
To date, the antihelminthic properties of eugenol have not been well studied; hence, the present work aims to evaluate the antischistosomal, anti-inflammatory, and antifibrotic effects of eugenol on S. mansoni-infected mice.
Materials and methods
Animals and experimental design
Twenty-four adult male Balb-c mice, weighing 18–20 g each, were obtained from the Schistosome Biological Supply Program at Theodor Bilharz Research Institute, Imbaba, Giza, Egypt. Mice were infected percutaneously with approximately 100±2 S. mansoni cercariae by the paddling method.31 The animals were given access to water and a standard diet, and the health status of the animals was monitored daily.
Experimental design
The mice were divided into three groups, with eight mice per group as follows: group I was a positive control (infected with S. mansoni cercariae but non-treated); group II was infected with S. mansoni cercariae and treated with eugenol; and group III was infected with S. mansoni cercariae and treated with PZQ. All mice were sacrificed at the end of the eighth week postinfection.
Treatment of mice
Eugenol was purchased from Sonwu Biotech Co., Ltd. (Xi ’an, Shaanxi, China) The compound was orally administered at a dose of 500 µg/kg/day from the fifth week postinfection until the end of the experiment. PZQ was administered orally to mice in the seventh week postinfection at a dose of 1,000 mg/kg on two successive days.32
Laboratory workup
Parasitological studies Recovery of adult worms
On day 56 postinfection, 24 hours after the final treatment, the mice were euthanized by decapitation. Blood samples were collected for serum analysis, and worms were recovered from the portal and mesenteric veins via vascular perfusion.33
Oogram pattern determination
For each sacrificed mouse, small fragments of the small intestine were processed to calculate the percentage of live immature and mature ova and dead ova.34,35
Intestinal egg count
After perfusion, a piece of the intestine of each mouse was obtained and processed to determine the number of eggs per gram of tissue.36,37
Biochemical measurement
Serum samples were collected to assess the effects of eugenol on mouse livers in comparison to PZQ. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were measured using commercial kits (Human, Wiesbaden, Germany).
Histopathological examination
Liver tissue specimens were obtained from all groups and immediately fixed in 10% buffered formalin for 24 hours and then dehydrated in increasing concentrations of ethanol and processed for paraffin sectioning. Sections (4 µm thick) were deparaffinized, stained with H&E and Masson’s trichrome stain, and examined using an Olympus light microscope. Stained sections were examined to evaluate the area of granuloma formation to assess the extent of hepatic fibrosis and the associated histopathological changes. The number and sizes of the granulomas in different groups were determined at 100× magnification using a digital image analysis system (Leica Qwin 500; Leica Microsystems, Wetzlar, Germany). Only lobular granulomas around schistosome ova or ovum shells in each section were measured. Counting of the granulomas of each specimen was carried out in five successive fields (100×) of different sections that were more than 250 µm apart.
Immunohistochemical staining
Immunohistochemical staining was performed using the avidin–biotin immunoperoxidase method. Sections (4 µm thick) were taken from previously prepared paraffin-embedded tissue blocks and mounted on glass slides. Sections were then dewaxed and rehydrated with a graded ethanol series descending to distilled water. Endogenous peroxidase activity was blocked using 6% hydrogen peroxide for 7 minutes. For epitope retrieval, sections were microwaved in citrate buffer (pH 6) for a total of 12 minutes. Sections were incubated with a primary antibody against α-smooth muscle actin (α-SMA) (clone 17H19L35; Thermo Fisher Scientific, Waltham, MA, USA, diluted at 1/100) for 1 hour at room temperature. Secondary staining kits were used according to the manufacturer’s instructions (Thermo Fisher Scientific). Counterstaining was performed with hematoxylin, and then, the sections were examined by light microscopy. Smooth muscle was used as an external positive control. Negative controls were obtained by omitting the primary antibody.
Statistical analysis
The results were analyzed using the statistical software package SPSS version 16.The data are expressed as mean ± SD or standard error of the mean. Differences between groups were determined using ANOVA and the non-parametric Mann–Whitney test to compare the mean values between treated and control groups for the different variables in the present study. P-values <0.05 were regarded as statistically significant.
Ethical statement
Experiments on animals were performed in accordance with the ethical animal guidelines and regulations set by the animal care committee of the Faculty of Medicine, South Valley University, and also were in accordance with the internationally accepted principles for laboratory animal use and care. Ethical approval was granted by the Research and Ethics committee of the Faculty of Medicine, South Valley University.
Results
Parasitological parameters
Analysis of the parasitological parameters (worm burden, egg count per gram of tissue, and oogram pattern) at the end of the eighth week postinfection showed moderate anti-schitosomal effects of eugenol compared to PZQ. As shown in Table 1, mice treated with PZQ yielded no live worms, whereas administration of eugenol significantly reduced the mean worm output upon perfusion by 19.2% compared to that of infected non-treated mice (P-value <0.05).
Table 1.
Statistical analysis of the parasitic and histopathological effects of eugenol and praziquantel on Schistosoma mansoni-infected mice
| Control group | Eugenol | Praziquantel | ||
|---|---|---|---|---|
| Mean worm burden | Male | 3.2±1.9 | 3±1.4 | 0.0±0.0 |
| Female | 0±0 | 0±0 | 0.0±0.0 | |
| Couple | 4.7±1.03 | 3±1.7 | 0.0±0.0 | |
| Total | 7.8±2.3 | 6.3±2.4 | 0.0±0.0 | |
| Total worm burden reduction (%) | 19.2 | 100 | ||
| Number of ova/gram, mean ± SEM | Intestine | 8,929±1,614.3 | 7,886.6±504.2 | 354.7±64.9 |
| Reduction of ova count in tissues (%) | 11.7* | 96* | ||
| Egg developmental stages, mean ± SEM | Immature eggs | 50±3.6 | 51±1.7 | 0±0 |
| Mature eggs | 42±2.5 | 43±1 | 12±2 | |
| Dead ova | 6.3±1.2 | 6±1 | 88±2 | |
| GD (µm) | Granuloma in liver tissue | 273.25±14.81 | 203.47±8.08 | 239.77±6.58 |
| Reduction in GD (%) | 25.54* | 12.26 | ||
| Granuloma number | Granuloma in liver tissue | 21.38±0.62 | 13.76±0.26 | 9.79±0.58 |
| Reduction in granuloma number (%) | 35.65* | 54.21* |
Note:
Significant result (P<0.05).
Abbreviations: GD, granuloma diameter; SEM, standard error of the mean.
Egg deposition in the intestinal wall was also affected, where the ova count per gram of intestine was reduced in the mice treated with eugenol and PZQ in comparison with the non-treated control mice by 11.7% and 96%, respectively, which was statistically significant (P value < 0.05).
On the other hand, the oogram patterns of mice treated with eugenol showed no marked difference compared to the control group, which received no treatment, indicating that eugenol had no effect on egg development (Table 1).
Biochemical examination
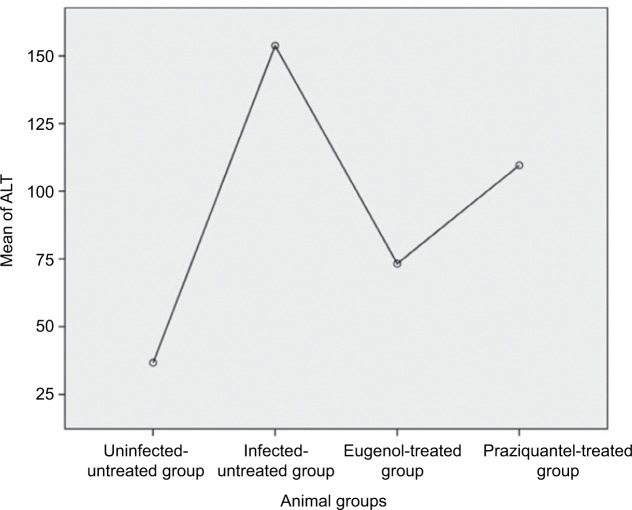
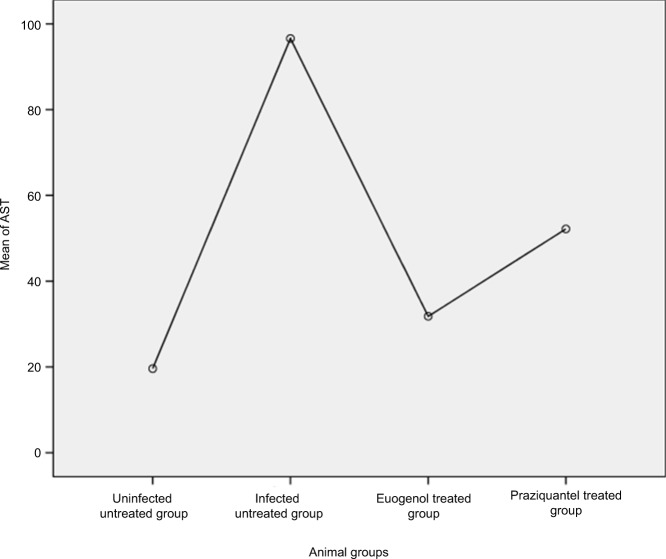
Data are presented as mean ± SD of at least eight independent measurements. Table 2 shows the percentage change due to treatment with eugenol or PZQ relative to the infected non-treated control group (Table 2). The results showed a significant increase in ALT and AST activities in the sera of infected non-treated mice. However, the infected treated groups showed significant reduction in liver enzyme levels, especially in the eugenol-treated group (Figures 1 and 2). The measurements of these biochemical markers in different groups were statistically significant (P value <0.05).
Table 2.
Statistical analysis of the biochemical parameters of the effects of eugenol and praziquantel on Schistosoma mansoni-infected mice
| Examined group | Min | Max | Mean+ Std. Deviation | P-value | |
|---|---|---|---|---|---|
| ALT | Uninfected untreated group | 17 | 36 | 22.60+7.701 | 0.043 |
| Infected untreated group | 123 | 212 | 153.80+38.564 | ||
| Euogenol treated group | 33 | 107 | 80.00+29.198 | ||
| Praziquantel treated group | 38 | 163 | 109.60+51.699 | ||
| AST | Uninfected untreated group | 12 | 29 | 19.60+6.580 | 0.020 |
| Infected untreated group | 52 | 145 | 96.60+36.624 | ||
| Euogenol treated group | 19 | 67 | 31.80+19.842 | ||
| Praziquantel treated group | 15 | 111 | 52.20+35.478 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; min, minimum; max, maximum.
Figure 1.
Mean level of ALT in different mice groups.
Abbreviation: ALT, alanine aminotransferase.
Figure 2.
Mean level of AST in different mice groups.
Abbreviation: AST, aspartate aminotransferase.
Evaluation of pathological changes using H&E-stained sections
Microscopic examination of sections from control livers showed normal cellular organization of uninfected hepatic lobules, with the typical distribution of hepatic cords around central veins to the periphery of the lobule. The cell cords were separated by narrow blood sinusoids lined by endothelial cells (Figure 1).
Histopathological examination of liver sections from the infected non-treated control group showed multiple chronic granulomatous lesions in the hepatic parenchyma. These granulomas were composed of numerous bilharzial ova containing miracidia, which were surrounded by infiltrates of chronic inflammatory cells such as epithelioid cells, lymphocytes, plasma cells, and eosinophils. Granulomas were marked by concentric fibrosis, with many fibroblasts surrounding the trapped ova. Peri-granulomatous hepatocytes showed hydropic degeneration and foci of necrosis. Brownish-black bilharzial pigmentation was also observed in nearby Kupffer cells and sinusoids. Portal tracts exhibited dilated portal veins with some trapped adult worms, chronic inflammatory cell infiltrate, and periportal fibrosis (Figure 3).
Figure 3.
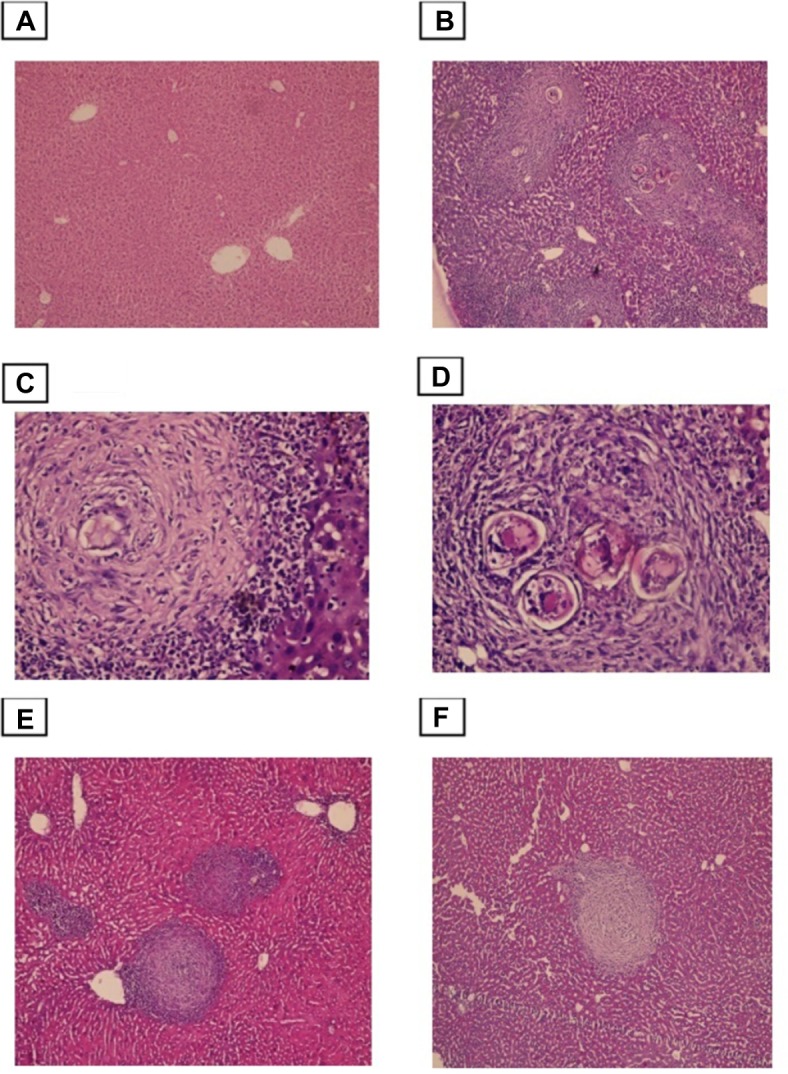
Photomicrographs of the liver stained with H&E.
Notes: (A) control group, showing normal portal triad along with a normal hepatocytes and the central vein (×100). (B)Infected untreated group, showing numerous granulomas with bilharzial ova surrounded by numerous chronic inflammatory cells (×100). (C and D) Higher power view showed numerous bilharzial ova containing meracedium and brownish black bilharzial pigmentation(straight arrow) and hydropic degeneration in hepatocytes (curved arrow) (×400). (E) PZQ treated group. (F) Eugenol treated group, showing reduced size and number of granuloma and decreased amount of bilharzial ova and the chronic inflammatory cells with absence of hydropic changes in hepatocytes (×100).
Histological liver sections from both groups, treated with PZQ and eugenol, showed noticeable improvement compared with the infected non-treated group. This improvement was represented by a reduction in granuloma number and size (Table 1). In addition, these treated groups showed variable degrees of reduction in the number of bilharzial ova, extent of hepatic fibrosis, and amount of chronic inflammatory cells infiltrate.
Our results showed that the reduction in the mean number of granulomas in both the eugenol- and PZQ-treated groups was significant (P<0.05) (Table 1). On the other hand, the reduction in the mean diameter of granulomas was significant only in the eugenol-treated group (Table 1).
Evaluation of the degree of liver fibrosis using Masson’s trichrome-stained sections
Liver fibrosis was evaluated by Masson’s trichrome staining, with the collagen fibers stained blue and hepatocytes stained red. Sections from control mice showed normal collagen deposition, with a thin layer of collagen fibers in the walls of the central vein and portal tracts (Figure 4). However, the amount of collagen fiber was significantly increased in the infected non-treated group, which was characterized by marked periportal fibrosis, portal–portal fibrous bridging, and fibrosis around granulomas. Compared with infection alone both the PZQ- and eugenol-treated groups exhibited decreased collagen deposition in the portal tracts and within the granulomas (Figure 4).
Figure 4.
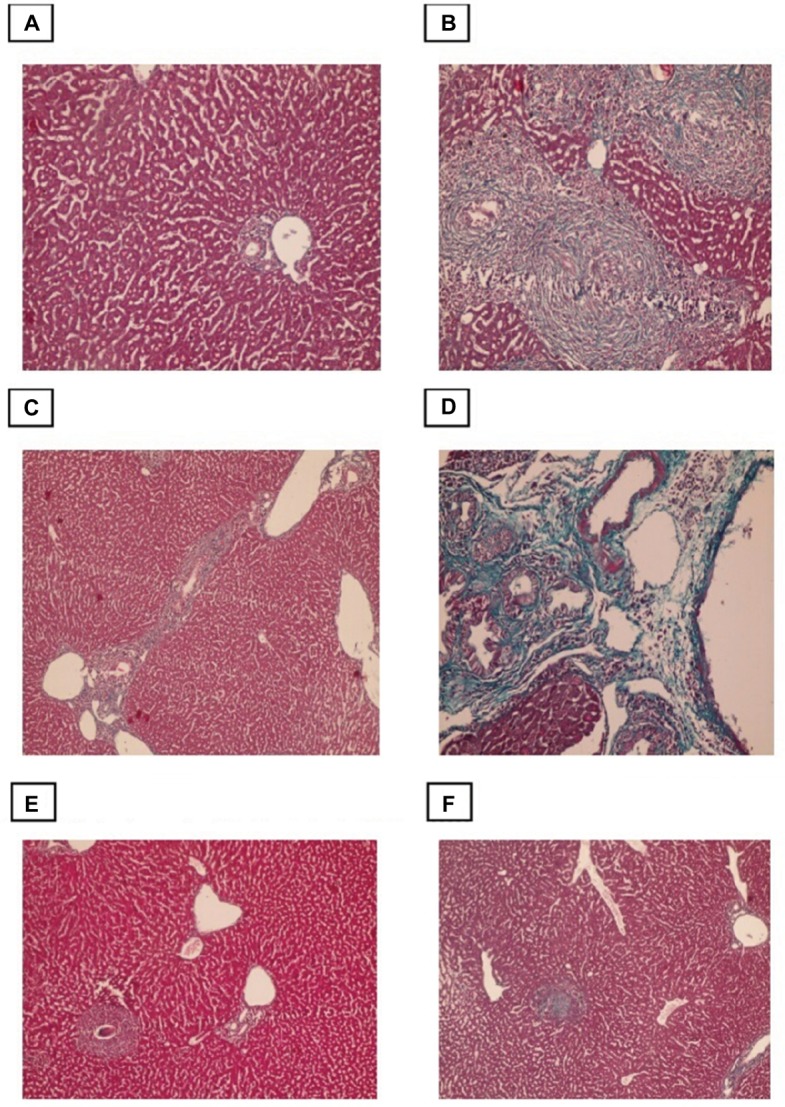
Photomicrographs of the liver stained with Masson trichrome.
Notes: (A) Control group, showing normal (typical) distribution of connective tissue (×100). (B) Infected untreated group, showing granulomatous lesions with condensed fibrous connective tissue in the granuloma and portal tracts (×200). (C) Portal-portal bridging fibrosis (×100). (D) Portal tract that showing marked dilatation of portal vein and excess fibrosis (×200). (E) PZQ treated group. (F) Eugenol treated group, showing reduced size and number of granuloma with less fibrous tissue in the granuloma and portal tracts (×100).
Immunohistochemical evaluation of α-SMA expression
Conversion of HSCs into fibroblasts is the key event in the process of liver fibrosis. The expression of α-SMA is commonly used as a hallmark of activated HSCs. In this study, we found that α-SMA was expressed in HSCs, which exhibited a dark brown cytoplasmic staining pattern. The control group exhibited negative expression of α-SMA or expression that was limited to the walls of the central vein. In the infected non-treated control group, intense α-SMA immunostaining was observed in the central and portal tract areas. Both the PZQ- and eugenol-treated groups showed reduced amounts of α-SMA compared with the infected non-treated control group, which was consistent with the degree of fibrosis, indicating inhibition of HSC activation (Figure 5).
Figure 5.
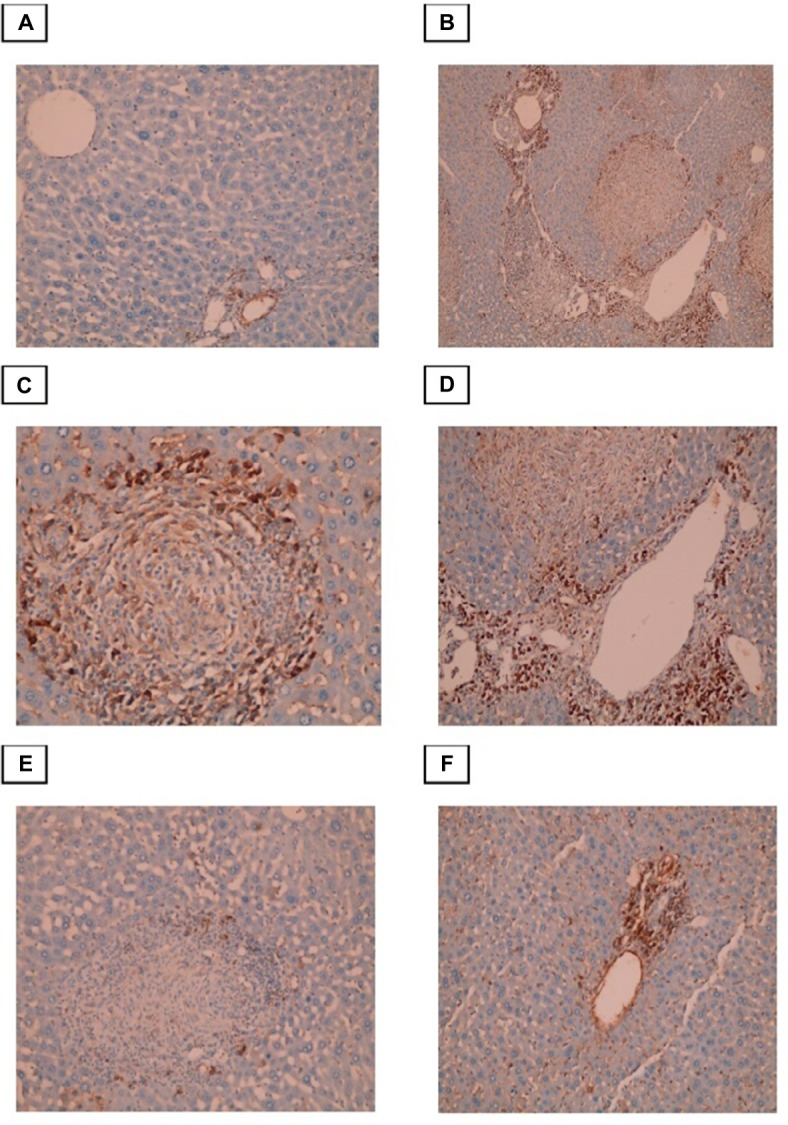
Photomicrographs of the liver stained with Masson trichrome.
Notes: (A) Control group, showing normal (typical) distribution of connective tissue (×100). (B) Infected untreated group, showing granulomatous lesions with condensed fibrous connective tissue in the granuloma and portal tracts (×200). (C)Portal-portal bridging fibrosis (×100). (D)-Portal tract that showing marked dilatation of portal vein and excess fibrosis (×200). (E) PZQ treated group. (F) Eugenol treated group, showing reduced size and number of granuloma with less fibrous tissue in the granuloma and portal tracts (×100).
Discussion
Chemotherapy is the main strategy in the management of schistosomiasis, and PZQ is considered the drug of choice in treatment.38 However, it has been observed that the occurrence of reinfection is common due to the increased resistance of the larval stages of S. mansoni to schistoso-micide drugs, which is an impending danger.39,40 Therefore, the improvement of potential alternatives for controlling schistosomiasis is very important.41
In the present work, we studied the antischistosomal effect of eugenol on S. mansoni infection in a mouse model. The eugenol-treated group exhibited reduction in total worm recovery by 19.2% compared to the infected non-treated group. In addition, there was a reduction in egg density in the intestinal walls in the mice groups treated with eugenol and PZQ by 11.7% and 96%, respectively, compared with the control non-treated group. The reduction in egg count may be attributed to a simultaneous reduction in worm burden.42 However, the oogram patterns of mice treated with eugenol showed no marked difference from the infected non-treated group, indicating that eugenol had no effect on the fecundity of adult worms or on egg development.
Few studies have investigated the effect of eugenol as an antiparasitic agent with potential lethal effects on the growth, viability, and morphology of different parasites, such as Giardia lamblia, Leishmania donovani, and Trypanosoma cruzi.43–46 Eugenol also exhibited antimalarial activity against the chloroquine-resistant strain Plasmodium falciparum.47 Furthermore, eugenol showed high toxicity against adult Fasciola gigantica in all the tested exposure periods in an in vitro study performed by Kumar and Singh.48 Such potent antihelminthic effects have also been reported against Haemonchus contortus, with maximum eclodibility inhibition.49
Similar results have been obtained by previous studies that evaluated the effects of different traditional medicinal plants and essential oils on S. mansoni infection in vivo. Prophylactic administration and treatment of infected mice with garlic (Allium sativum) extract or allicin significantly reduced the mean worm burden (21.88% and 20.33%, respectively) compared to the positive control. Additionally, the oogram patterns and total ova counts in the tissues, compared to the positive control, exhibited significant reductions of 12.59% and 11.42%, respectively.42 These results were consistent with those of Metwalley50 and Mahmoud et al,51 who studied the antischistosomal effects of A. sativum and Nigella sativa oil. These authors reported moderate antischistosomal effects of these medicinal plants and attributed these results mainly to the anti-inflammatory and immunomodulatory effects of the plants rather than a direct effect on the parasites.
In fact, biochemical changes such as hepatic enzyme activities, including serum ALT and AST, are considered to be good biomarkers for the assessment of hepatic cell damage caused by heavy schistosome egg deposition and impaired cell membrane permeability.52,53 In the present study, eugenol exhibited considerable anti-inflammatory activities, because it significantly reduced serum hepatic enzyme levels (AST and ALT) comparable to infected non-treated control. These findings are consistent with those reported by Mahmoud et al, 51 Abdel-Hafeez et al,54 and Metwally et al.42 These studies reported increased serum ALT, AST, and GGT levels in schistosome-infected patients and experimental animals in the non-treated infected groups. Subsequently, reduction in serum liver enzyme levels has been occurred in the treated groups. The reduction in serum transaminase levels can be explained by either restoration of the oxidant/antioxidant balance due to the administration of antioxidants or anti-inflammatory agents or by reduction in hepatic granuloma size and fibrosis as well as amelioration in necrotic liver tissue in infected treated mice.53
In humans, the regulation of liver fibrosis during schistosomiasis may be highly complex, with multiple mediators regulating disease progression. Patients with severe fibrosis express elevated tumor necrosis factor alpha (TNF-α), IL-5, IL-10, and IL-13 levels, whereas patients with mild fibrosis express high levels of IFN-γ.55,56 On the other hand, macrophages may play important role in the immunopathogenesis of schistosomiasis. Actually, macrophages may have dual effects; as initial inflammatory cells, macrophages may support egg sequestration and granuloma development, and at later stages, these cells may assume anti-inflammatory functions during chronic infection by indirect suppression of other inflammatory cells, decreasing the granuloma volume.57 Therefore, there is need for a therapeutic agent that has immunomodulatory effects that shift the cytokine profile from T helper 2 (Th2)-lymphocyte-mediated immune responses, which are responsible for granuloma formation, to Th1-lymphocyte-mediated immune responses, which are responsible for immune resistance.42
The data in the present study showed that the amount of collagen fiber was significantly increased in the infected non-treated group, which was characterized by marked peri-portal fibrosis, portal–portal fibrous bridging, and fibrosis around granulomas, as observed in previous studies.42,58 Meanwhile, infected groups treated with PZQ or eugenol exhibited decreased collagen deposition in the portal tract and within the granulomas, which significantly reduced the granuloma volume, especially in the eugenol-treated group. These results are similar to those of previous studies that attributed this reduction in collagen deposition to decreased infiltration of circulating fibroblasts into the granulomata, affecting collagen synthesis and reducing the levels of procollagen type III, which is responsible for granuloma formation.42,59 To further investigate the cellular mechanism underlying the antifibrotic activity of eugenol, we focused on HSCs, which are liver-resident cells and play an important role in liver fibrosis.13 Their action started after liver damage, where they activated, proliferated, and underwent a series of transformation process into myofibroblast-like cells that deposit large amounts of connective tissue components, including collagens I and III and α-SMA.60,61 Some studies have highlighted the role of activated HSCs in the process of fibrogenesis in both murine and human schistosomiasis as well as demonstrated the regulatory effect of schistosome antigens on this transdifferentiation process.12,62
In this study, immunohistochemical analysis of α-SMA was conducted because this protein is a sensitive marker that increases significantly during liver fibrosis.51 In the infected non-treated control group, intense α-SMA immunostaining was observed in the central and portal tract areas, and the HSCs that expressed this marker were located mainly at the peripheries of granulomatous lesions, which was consistent with other studies on human and mouse models of S. japonicum infection.11,58 Both the PZQ- and eugenol-treated groups showed reduction in α-SMA levels compared with the infected non-treated control group, which was consistent with the degree of fibrosis, indicating the inhibition of HSC activation. These data suggest anti-inflammatory and antifibrotic effects of eugenol and PZQ.
Conclusion
Based on the data described herein, the present study clarified that eugenol has considerable anti-inflammatory and antifibrotic activities with moderate antihelminthic effects against S. mansoni infection compared to the effect of PZQ. There are many factors that influence the effects of eugenol in experimental animal models of schistosomiasis, including the effects on immune host cells, cytotoxicity, routes of administration, and absorption rates of this essential oil. There are many activities and properties of eugenol that remain undiscovered and need to be further investigated to elucidate the antihelminthic properties of eugenol, both in vivo and in vitro.
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
he authors report no conflicts of interest in this work.
References
- 1.Quansah E, Sarpong E, Karikari TK. Disregard of neurological impairments associated with neglected tropical diseases in Africa. eNeurologicalSci. 2016;3:11–14. doi: 10.1016/j.ensci.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lustigman S, Prichard RK, Gazzinelli A, et al. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6(4):e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwinzi PN, Montgomery SP, Owaga CO, et al. Integrated community-directed intervention for schistosomiasis and soil transmitted helminths in Western Kenya - a pilot study. Parasit Vectors. 2012;5(1):182. doi: 10.1186/1756-3305-5-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirst SI, Stapley LA. Parasitology: the dawn of a new millennium. Parasitol Today. 2000;16(1):1–3. doi: 10.1016/s0169-4758(99)01589-6. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S. Immunoregulation and parasitic infections. FEMS Immunol Med Microbiol. 1997;18(4):319–324. doi: 10.1111/j.1574-695X.1997.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheever AW, Yap GS. Immunologic basis of disease and disease regulation in schistosomiasis. Chem Immunol. 1997;66:159–176. doi: 10.1159/000058669. [DOI] [PubMed] [Google Scholar]
- 8.Loverde PT. Do antioxidants play a role in schistosome host-parasite interactions? Parasitol Today. 1998;14(7):284–289. doi: 10.1016/s0169-4758(98)01261-7. [DOI] [PubMed] [Google Scholar]
- 9.Cheever AW. Differential regulation of granuloma size and hepatic fibrosis in schistosome infections. Mem Inst Oswaldo Cruz. 1997;92(5):689–692. doi: 10.1590/s0074-02761997000500024. [DOI] [PubMed] [Google Scholar]
- 10.Gryseels B, Polman K, Clerinx J, Kestens L. Human Schistosomiasis. Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 11.He X, Pu G, Tang R, Zhang D, Pan W. Activation of nuclear factor kappa B in the hepatic stellate cells of mice with Schistosomiasis japonica. PLoS One. 2014;9(8):e104323. doi: 10.1371/journal.pone.0104323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthony B, Allen JT, Li YS, Mcmanus DP. Hepatic stellate cells and parasite-induced liver fibrosis. Parasit Vectors. 2010;3(1):60. doi: 10.1186/1756-3305-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemura Y, Kikuchi S, Inaba Y. Epidemiologic study of the relationship between schistosomiasis due to Schistosoma japonicum and liver cancer/cirrhosis. Am J Trop Med Hyg. 1998;59(4):551–556. doi: 10.4269/ajtmh.1998.59.551. [DOI] [PubMed] [Google Scholar]
- 15.Andersson KL, Chung RT. Hepatic schistosomiasis. Curr Treat Options Gastroenterol. 2007;10(6):504–512. doi: 10.1007/s11938-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 16.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans R Soc Trop Med Hyg. 2002;96(5):465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 17.Mwangi IN, Sanchez MC, Mkoji GM, et al. Praziquantel sensitivity of Kenyan Schistosoma mansoni isolates and the generation of a laboratory strain with reduced susceptibility to the drug. Int J Parasitol Drugs Drug Resist. 2014;4(3):296–300. doi: 10.1016/j.ijpddr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catherine AA, Deepika H, Negi PS. Antibacterial activity of eugenol and peppermint oil in model food systems. J Essent Oil Res. 2012;24:481. [Google Scholar]
- 19.Riella KR, Marinho RR, Santos JS, et al. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol. 2012;143(2):656–663. doi: 10.1016/j.jep.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Divya Nair V, Gopi R, Mohankumar M, Kavina J, Panneerselvam R. Effect of triadimefon: a triazole fungicide on oxidative stress defense system and eugenol content in Ocimum tenuiflorum L. Acta Physiol Plant. 2012;34(2):599–605. [Google Scholar]
- 21.Undeğer U, Başaran A, Degen GH, Başaran N. Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chem Toxicol. 2009;47(8):2037–2043. doi: 10.1016/j.fct.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Zheng GQ, Kenney PM, Lam LK. Sesquiterpenes from clove (Euge-nia caryophyllata) as potential anticarcinogenic agents. J Nat Prod. 1992;55(7):999–1003. doi: 10.1021/np50085a029. [DOI] [PubMed] [Google Scholar]
- 23.Kim HM, Lee EH, Hong SH, et al. Effect of Syzygium aromaticum extract on immediate hypersensitivity in rats. J Ethnopharmacol. 1998;60(2):125–131. doi: 10.1016/s0378-8741(97)00143-8. [DOI] [PubMed] [Google Scholar]
- 24.Corrêa MFP, Melo GO, Costa SS. Substâncias de origem vegetal poten-cialmenteúteisnaterapia da Asma. Rev Bras Farmacogn. 2008;18:785–797. [Google Scholar]
- 25.Miyazawa M, Hisama M. Suppression of Chemical Mutagen-Induced SOS Response by Alkylphenols from Clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J Agric Food Chem. 2001;49(8):4019–4025. doi: 10.1021/jf0103469. [DOI] [PubMed] [Google Scholar]
- 26.Ogata M, Hoshi M, Urano S, Endo T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem Pharm Bull. 2000;48(10):1467–1469. doi: 10.1248/cpb.48.1467. [DOI] [PubMed] [Google Scholar]
- 27.Park IK, Lee HS, Lee SG, Park JD, Ahn YJ. Insecticidal and fumigant activities of Cinnamomum Cassia bark-derived materials against Mecho-ris ursulus (Coleoptera: attelabidae) J Agric Food Chem. 2000;48(6):2528–2531. doi: 10.1021/jf9904160. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues TG, Fernandes A, Sousa JPB, Bastos JK, Sforcin JM. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat Prod Res. 2009;2310(4):319–326. doi: 10.1080/14786410802242679. [DOI] [PubMed] [Google Scholar]
- 29.Magalhães CB, Riva DR, Depaula LJ, et al. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J Appl Physiol. 2010;108(4):845–851. doi: 10.1152/japplphysiol.00560.2009. [DOI] [PubMed] [Google Scholar]
- 30.Charan Raja MR, Srinivasan V, Selvaraj S, Mahapatra SK. Versatile and synergistic potential of eugenol: a review. Pharm Anal Acta. 2015;6:367. [Google Scholar]
- 31.Frandsen F. Cultivation of schistosomes for chemotherapeutic studies. Acta Pharmacol Toxicol. 1981;49(13):118–122. doi: 10.1111/j.1600-0773.1981.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 32.Muchirah PN, Yole D, Kutima H, Rebecca Waihenya R, Kuria MK, Mokua J. Determination of effective praziquantel dose in different mouse strains: BALB/c and Swiss mice in treatment of Schistosoma mansoni. J Clin Immnunol Immunopathol Res. 2012;4(2):12–21. [Google Scholar]
- 33.Duvall RH, Dewitt WB. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino J, Oliveira CA, Faria J. The oogram in the study of relapse in experimental chemotherapy of schistosomiasis mansoni. J Parasitol. 1963;49(3):365–370. [Google Scholar]
- 35.Pellegrino J, Faria J. The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg. 1965;14(3):363–369. doi: 10.4269/ajtmh.1965.14.363. [DOI] [PubMed] [Google Scholar]
- 36.Cheever AW. Quantitative comparison of the intensity of Schistosoma mansoni infections in man and experimental animals. Trans R Soc Trop Med Hyg. 1969;63(6):781–795. doi: 10.1016/0035-9203(69)90122-9. [DOI] [PubMed] [Google Scholar]
- 37.Cheever AW, Anderson LA. Rate of destruction of Schistosoma mansoni eggs in the tissues of mice. Am J Trop Med Hyg. 1971;20(1):62–68. doi: 10.4269/ajtmh.1971.20.62. [DOI] [PubMed] [Google Scholar]
- 38.Cioli D, Valle C, Angelucci F, Miele AE. Will new antischistosomal drugs finally emerge? Trends Parasitol. 2008;24(9):379–382. doi: 10.1016/j.pt.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Kabatereine NB, Kemijumbi J, Ouma JH, et al. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at lake Albert, Uganda. Trans R Soc Trop Med Hyg. 2003;97(5):599–603. doi: 10.1016/s0035-9203(03)80044-5. [DOI] [PubMed] [Google Scholar]
- 40.Silva LM, Menezes RMC, Oliveira Sade, Andrade ZA. Chemotherapeutic effects on larval stages of Schistosoma mansoni during infection and re-infection of mice. Rev Soc Bras Med Trop. 2003;36(3):335–341. doi: 10.1590/s0037-86822003000300004. [DOI] [PubMed] [Google Scholar]
- 41.Utzinger U, Richards-Kortum RR. Fiber optic probes for biomedical optical spectroscopy. J Biomed Opt. 2003;8(1):121–147. doi: 10.1117/1.1528207. [DOI] [PubMed] [Google Scholar]
- 42.Metwally DM, Al-Olayan EM, Alanazi M, Alzahrany SB, Semlali A. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in vivo. BMC Complement Altern Med. 2018;18(1):1–11. doi: 10.1186/s12906-018-2191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado M, Dinis AM, Salgueiro L, Custódio JB, Cavaleiro C, Sousa MC. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp Parasitol. 2011;127(4):732–739. doi: 10.1016/j.exppara.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Islamuddin M, Chouhan G, Tyagi M, Abdin MZ, Sahal D, Afrin F. Leishmanicidal activities of Artemisia annua leaf essential oil against visceral leishmaniasis. Front Microbiol. 2014;5:626. doi: 10.3389/fmicb.2014.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Morais SM, Vila-Nova NS, Bevilaqua CM, et al. Thymol and euge-nol derivatives as potential antileishmanial agents. Bioorg Med Chem. 2014;22(21):6250–6255. doi: 10.1016/j.bmc.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santoro GF, Cardoso MG, Guimarães LG, Mendonça LZ, Soares MJ. Trypanosoma cruzi: activity of essential oils from Achille amillefolium L, Syzygium aromaticum L and Ocimum basilicum L on epimastigotes and trypomastigotes. Exp Parasitol. 2007;116(3):283–290. doi: 10.1016/j.exppara.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Vanzyl Rl, Seatlholo ST, van Vuuren SF, Viljoen AM. The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res. 2006;18:129–133. [Google Scholar]
- 48.Kumar P, Singh VK. Activity of Allium sativum, Ferula asafoetida and Syzygium aromaticum against Fasciola gigantica. J Biol Earth Sci. 2014;4(1):57–65. [Google Scholar]
- 49.Pessoa LM, Morais SM, Bevilaqua CM, Luciano JH. Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vet Parasitol. 2002;109(1–2):59–63. doi: 10.1016/s0304-4017(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 50.Metwalley KM. Assessment of the antischistosomal activity of some plant extracts against Schistosoma mansoni infection. World J Med Sci. 2015;12(2):162–169. [Google Scholar]
- 51.Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79(1):1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 52.Soliman MFM, El-Shenawy NS. Evaluation of the protective effect of two antioxidative agents in mice experimentally infected with Schistosoma mansoni: haematological and histopathological aspects. Pak J Biol Sci. 2003;6(10):887–897. [Google Scholar]
- 53.Hamed MA, Hetta MH. Efficacy of citrus reticulata and Mirazid in treatment of Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2005;100(7):771–778. doi: 10.1590/s0074-02762005000700017. [DOI] [PubMed] [Google Scholar]
- 54.Abdel-Hafeez EH, Ahmad AK, Abdulla AM, Aabdel-Wahab S, Mosalem FA. Therapeutic effect of alpha lipoic acid combined with praziquantel on liver fibrosis induced by Schistosoma mansoni challenged mice. Parasitol Res. 2012;111(2):577–586. doi: 10.1007/s00436-012-2871-4. [DOI] [PubMed] [Google Scholar]
- 55.Henri S, Chevillard C, Mergani A, et al. Cytokine regulation of periportal fibrosis in humans infected with Schistosoma mansoni: IFN-gamma is associated with protection against fibrosis and TNF-alpha with aggravation of disease. J Immunol. 2002;169(2):929–936. doi: 10.4049/jimmunol.169.2.929. [DOI] [PubMed] [Google Scholar]
- 56.Booth M, Mwatha JK, Joseph S, et al. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol. 2004;172(2):1295–1303. doi: 10.4049/jimmunol.172.2.1295. [DOI] [PubMed] [Google Scholar]
- 57.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85(2):148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang YJ, Luo J, Yuan Q, et al. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One. 2011;6(5):e20247–14. doi: 10.1371/journal.pone.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mostafa OM, Eid RA, Adly MA. Antischistosomal activity of ginger (Zingiber officinale) against Schistosoma mansoni harbored in C57 mice. Parasitol Res. 2011;109(2):395–403. doi: 10.1007/s00436-011-2267-x. [DOI] [PubMed] [Google Scholar]
- 60.Karsenty G, Park RW. Regulation of type I collagen genes expression. Int Rev Immunol. 1995;12(2–4):177–185. doi: 10.3109/08830189509056711. [DOI] [PubMed] [Google Scholar]
- 61.Chatterjee S, Mbaye A, de Man JG, van Marck EA. Does the neuropeptide somatostatin have therapeutic potential against schistosomiasis? Trends Parasitol. 2002;18(7):295–298. doi: 10.1016/s1471-4922(02)02294-8. [DOI] [PubMed] [Google Scholar]
- 62.Chang D, Ramalho LN, Ramalho FS, Martinelli AL, Zucoloto S. Hepatic stellate cells in human Schistosomiasis mansoni: a comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006;97(3):318–323. doi: 10.1016/j.actatropica.2005.12.006. [DOI] [PubMed] [Google Scholar]