Abstract
Background: Current pharmacological treatments for Tourette Syndrome (TS), such as antipsychotic agents and α-2 agonists, are moderately effective in the treatment of tics, but have substantial side effects that limit their use. N-acetylcysteine (NAC) modulates glutamatergic systems, and has been used safely as an antioxidant agent with minimal side effects for decades. NAC has been increasingly studied for the treatment of other obsessive-compulsive spectrum disorders. We aim to examine the efficacy of NAC for the treatment of pediatric TS in a double-blind, placebo-controlled, add-on study.
Methods: Thirty-one children and adolescents 8–17 years of age with TS were randomly assigned to receive NAC or matching placebo for 12 weeks. Our primary outcome was change in severity of tics as measured by the Yale Global Tic Severity Scale (YGTSS), Total tic score. Secondary measures assessed comorbid obsessive-compulsive disorder (OCD), depression, anxiety, and attention-deficit/hyperactivity disorder (ADHD). Linear mixed models in SAS were used to examine differences between NAC and placebo.
Results: Of 31 randomized subjects, 14 were assigned to placebo (two females; 11.5 + 2.8 years) and 17 to active NAC (five females; 12.4 + 1.4 years) treatment. No significant difference between NAC and placebo was found in reducing tic severity or any secondary outcomes.
Conclusions: We found no evidence for efficacy of NAC in treating tic symptoms. Our findings stand in contrast to studies suggesting benefits of NAC in the treatment of other obsessive-compulsive spectrum disorders in adults, including OCD and trichotillomania, but are similar to a recent placebo-controlled trial of pediatric trichotillomania that found no benefit of NAC.
Introduction
Tourette Syndrome (TS) is a childhood-onset neuropsychiatric disorder characterized by chronic motor and vocal tics that are often preceded by premonitory urges (Leckman et al. 2013). Once thought to be rare, the current lifetime prevalence estimates for TS range from 0.1% to 1.0% (Robertson et al. 2000; Leckman et al. 2013). In one half to two thirds of cases, tic symptoms improve during adolescence; however, the most severe cases of TS continue into adulthood (Bloch et al. 2006). Although there are currently several effective behavioral (McGuire et al. 2014) and pharmacological (Roessner et al. 2011) treatments for TS, one third of patients with TS do not actually benefit from these first-line treatments (Roessner and Rothenberger 2013). Additionally, several of the most effective medications used to treat tics have significant side effects (Robertson et al. 2000; Roessner and Rothenberger 2013).
Antipsychotic agents, which are primarily D2-receptor antagonists, are the most effective treatment currently available for TS (Weisman et al. 2013). Randomized, double-blind, placebo-controlled trials (RCT) estimate that antipsychotic agents reduce tic severity on average by 20–30% compared with placebo (Dion et al. 2002; Scahill et al. 2003). Nevertheless, these medications have significant side effects, including weight gain, sedation, extrapyramidal effects, depression, and anxiety (Robertson et al. 2000; Roessner and Rothenberger 2013). Alpha-2 agonists reduce tic severity by 5–30% on average compared with placebo in RCT (Scahill et al. 2001), and are often used as first-line treatments in TS because of their milder side effect profile and efficacy in treating comorbid attention-deficit/hyperactivity disorder (ADHD) symptoms (Bloch et al. 2009). Still, α-2 agonists are associated with sedation, dry mouth, hypotension, and rebound hypertension with discontinuation (Minns et al. 2010). Moreover, several behavioral treatments have shown promise in reducing tic severity in TS (McGuire et al. 2014). Even if efficacious, however, the dissemination of these therapies remains a challenge. In this context, novel and safe pharmacological treatments to reduce tics are urgently needed.
N-acetylcysteine (NAC) is a naturally occurring, safe, and inexpensive amino acid supplement that is available over the counter and has been widely used because of its recognized antioxidant and anti-inflammatory properties (Cotgreave 1997; De Rosa et al. 2000; Sommer et al. 2014). NAC is also a demonstrated glutamate modulating agent. NAC is converted to cystine, a substrate for the glutamate/cystine antiporter located on glial cells. The uptake of cystine by glia causes glial release of glutamate into the extra synaptic space, where it appears to stimulate inhibitory metabotropic glutamate receptors on glutamatergic nerve terminals, and thereby reduces the synaptic release of glutamate (Moran et al. 2005). The glutamatergic system has been strongly implicated in the pathogenesis of TS in several neuroimaging (Plessen et al. 2004; DeVito et al. 2005), brain tissue (Anderson et al. 1992), and genetic (Adamczyk et al. 2011; Crane et al. 2011) studies.
Previous trials have suggested that NAC is a safe and potentially effective treatment for a variety of psychiatric conditions including schizophrenia (Berk et al. 2008b), bipolar depression (Berk et al. 2008a), cannabis dependence (Gray et al 2012), and autism (Hardan et al. 2012). Several trials have also suggested that NAC is well tolerated in pediatric populations (Hardan et al. 2012; Bloch et al. 2013). Additionally, NAC has been demonstrated to be effective as an augmentation agent to selective-serotonin reuptake inhibitors (SSRIs) in a double-blind, placebo-controlled trial in adult patients with refractory obsessive-compulsive disorder (OCD) (Afshar et al. 2012). Furthermore, a single RCT suggested that NAC may also be effective for adults with trichotillomania (TTM) (Grant et al. 2009). The benefits of NAC for TTM in the pediatric population, however, were not replicated (Bloch et al. 2013).
The benefits of NAC for OCD and TTM in previous clinical trials led us to examine NAC's effectiveness in TS patients for several reasons. First, TS, TTM, and OCD present with similar phenotypes (e.g., presence of premonitory urges and motor disinhibition), and these disorders are considered part of the obsessive-compulsive (OC) spectrum (McElroy et al. 1994). Second, TS, TTM, and OCD share similar neurobiological abnormalities (e.g., basal ganglia and corticostriatal thalamocortical circuits) (Chamberlain et al. 2009; van Velzen et al. 2014) and genetic (e.g., familial aggregation, common candidate genes) aspects (Bienvenu et al. 2009; Ferrao et al. 2009). Finally, TS, TTM, and OCD present similar responses to treatment with glutamate- and dopamine-modulating agents and specific behavioral treatments (Bloch et al. 2007; Chamberlain et al. 2009; Wetterneck et al. 2010). We conducted a 12 week, double-blind, placebo-controlled, randomized, add-on trial to examine the efficacy of NAC in treating children and adolescents with TS.
Methods
Participants
Children and adolescents 8–17 years of age, with a primary diagnosis of TS or chronic tic disorder and current Yale Global Tic Severity Scale (YGTSS) score ≥22, were recruited through a tertiary TS/OCD Specialty Clinic (Yale Child Study Center) and the Northern California and Hawaii Tourette Syndrome Chapter of the Tourette Syndrome Association. Subjects were also referred through local providers in the community, the Tourette Syndrome Association and clinicaltrials.gov. Subjects were required to be on a stable medication and psychotherapy regimen 4 weeks prior to enrolling in the trial, as well as throughout the duration of the trial. A stable medication regimen was defined as no recent addition, discontinuation, or dosing change in medications that have potential effects on the central nervous system (such as antidepressants, naltrexone, lithium, psychostimulants, anxiolytics, or antipsychotics) in the previous 4 weeks. Subjects were also excluded if they had started behavioral therapy treatment for tics in the prior 4 weeks. Subjects who were already engaging in behavioral treatments for tics (for a period of >4 weeks) were encouraged to continue the behavioral therapy throughout the trial. Further, subjects were excluded if they 1) had significant comorbid psychiatric illness, including bipolar disorder, psychotic disorder, substance use disorder, autism spectrum disorder, or mental retardation according to Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) criteria as diagnosed by the lead study investigator (American Psychiatric Association 1994); 2) had had asthma requiring use of an inhaler in the previous 6 months (case reports have associated asthma exacerbation with given intravenous NAC administration); 3) had previously used NAC (dose >600 mg for >2 weeks); or 4) had a positive urine drug screen or pregnancy test. Subjects provided informed assent and parents provided informed consent under an institutional review board (IRB) approved protocol prior to enrollment in the trial.
Families were made aware of other options for evidence-based treatment of TS before study enrollment. Families received $150 compensation for participation in the trial. Subjects recruited from the Northern California area were evaluated and treated during the study at a separate physician's office in the Palo Alto, California area. However, subjects seen at the Palo Alto site still received all study assessments and procedures from Yale Child Study Center staff (the same physician and research support staff as at Yale). The only protocol difference between subjects treated at the two sites was that additional research staff (A.Y.) helped coordinate study appointments at the Palo Alto site.
Intervention
Subjects were randomized in a ratio of 1:1 to receive treatment with NAC (Swanson Premium Brand N-acetylcysteine from Swanson Health Products) or placebo. NAC is an acetylated form of the amino acid cysteine, which is commonly found in food and synthesized by the body. NAC is generally well tolerated, and has been extensively used in the treatment of acetaminophen overdose/toxicity at high doses for many years (Cotgreave 1997), and has been well tolerated in several previous trials of psychiatric conditions in both adults and children (Berk et al. 2008a; Hardan et al. 2012; Bloch et al. 2013). NAC (or placebo) was titrated up to a maximum dose of 2400 mg over the course of 2 weeks. Subjects were assigned 600 mg twice a day for weeks 1–2, and then were assigned 1200 mg twice a day for the remainder of the 12 week study.
After completion of the study, all subjects receiving placebo were offered NAC treatment. We offered active NAC to all subjects who were randomized to receive placebo, in order to ensure that all subjects had potential access to the active treatment of the trial and had access to our expertise in dosing and managing potential side effects associated with NAC. Subjects, their parents, investigators, and persons performing the assessments remained blind to treatment assignment from the time of randomization until the completion of the study. Randomization assignments were kept strictly confidential by the investigational pharmacist until the time of unblinding, after all study assessments were complete. The identity of the treatment was concealed by the use of study drugs that were identical in packaging, labeling, schedule of administration, appearance, and smell.
Blinding of treatment is particularly challenging in studies of NAC because of its strong, distinctive sulfur odor. Peppermint-scented oil was added to the outside of both NAC and placebo capsules to make them nearly identical in appearance and smell. Additionally, placebo capsules were stored in bottles with active NAC for several weeks prior to dispensation in order to mimic the smell of NAC in the placebo capsules. At the time of dispensation, placebo capsules contained no appreciable trace of active NAC. Adequacy of blinding was assessed by asking both subjects and the treating clinician, at the end of the trial, whether they thought they were assigned to NAC or placebo. Neither subjects (p = 0.92) nor treating clinicians (p = 0.24) were significantly better than chance when determining treatment allocation at study completion. Medication compliance was assessed during each visit by asking the subject and the family whether the subject was still taking the study medication, and estimating the number of medication doses missed since the last visit. We did not perform pill counts to assess compliance.
Assessments
Baseline assessment consisted of a clinical evaluation involving a psychiatric history, mental status examination, and clinical assessment of past medication and behavioral treatment. A medical assessment including vital signs, physical examination, and urine drug screen and pregnancy test (for female adolescents) was performed by the lead study investigator (M.H.B) or another MD (A.L.W.). Standardized assessment included: 1) YGTSS –Total tic score (Leckman et al. 1989), 2) Premonitory Urge for Tics Scale (PUTS) (Woods et al. 2005), 3) Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) (Goodman et al. 1989), 4) ADHD self-report rating scale (ADHD-RS) (Adler et al. 2006), 5) Multidimensional Anxiety Scale for Children (MASC) (March et al. 1997), 6) Children's Depression Inventory (CDI) (Kovacs 1985), and 7) Pediatric Adverse Events Rating Scale (PAERS) (Shapiro et al. 2009). The principal investigator (M.H.B.) or the other MD (A.L.W.) conducted all clinical ratings at baseline weeks 0, 4, 8, and 12. A research assistant or associate researcher (K.E.P., A.L.W., J.M.M.), with extensive experience with TS, observed all study visits and conducted the ratings at weeks 1, 2, 3, and 6 by telephone assessments. The ADHD-RS was completed by a parent, whereas the YGTSS, PUTS, CY-BOCS, CDI, MASC, and PAERS were completed through clinical interview with the subject and family. The same rater conducted ratings of both efficacy and side effects in this trial.
Data analyses
This trial was designed to have 80% power to detect a large effect of NAC (d = 0.9) assuming α = 0.05 and a dropout rate of 10%. A sample size of 38 subjects would give us 80% power to detect a treatment effect size = 0.9, setting α = 0.05 and assuming a dropout rate of 10%. The primary investigator decided to stop enrollment at 31 subjects because of poor recruitment and lack of continued funding. We used the PROC MIXED statement in SAS version 9.2 to analyze all outcomes related to NAC efficacy. We used mixed models with restricted maximum likelihood (REML) solution and an unstructured covariance matrix. We examined treatment, time, and treatment by time interaction in the mixed model. Time was treated as a repeated measure within subject. The treatment by time interaction on the YGTSS total tic score was the main outcome of interest in the trial. As secondary outcomes, we examined the PUTS, CY-BOCS, CDI, MASC, ADHD-RS, PAERS, and Clinical Global Impressions (CGI) scores. Mixed-models analysis uses intention-to-treat principles and accounts for missing data. A χ2 or Fisher's exact test was used to compare dichotomous outcomes, such as proportion of treatment responders and frequency of side effects between the NAC and placebo groups. A partial response was considered at least 25% improvement from baseline in YGTSS total tic score and clinical severity scores on all symptom rating scales in the final study visit. A full response was considered as at least 35% improvement in YGTSS total tic score and CGI = 1 or 2 on the final study visit.
Results
Participants
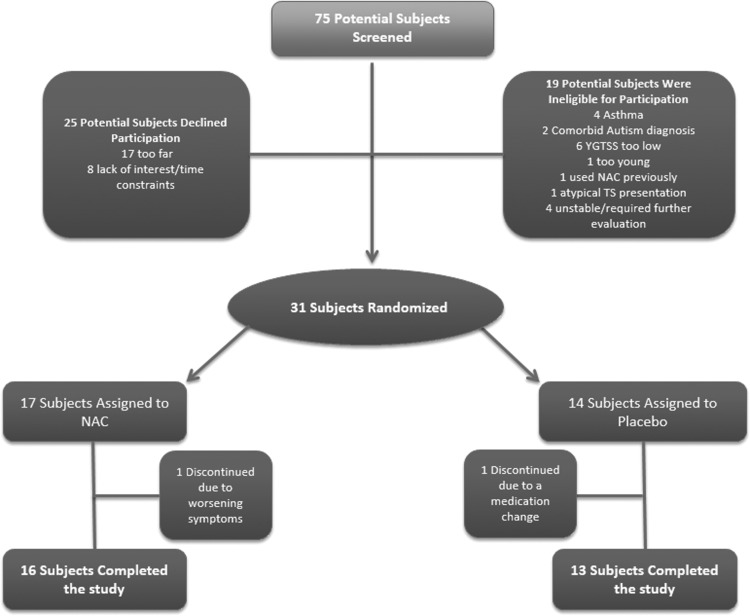
Figure 1 is a Consolidated Standards of Reporting Trials (CONSORT) diagram that describes the flow of subjects through the trial. Table 1 compares the demographic and clinical characteristics of the subjects randomly assigned to NAC and placebo at baseline. All subjects enrolled in the trial met criteria for a diagnosis of TS. Randomized treatment groups were comparable in tic severity at baseline, but subjects in the NAC group had greater anxiety, were more likely to be taking an antipsychotic medication for tics, and had less severe ADHD symptoms (p < 0.05). We had two dropouts (one in each group) during the course of the trial (Figure 1). The subject in the NAC group experienced worsening of tics and depression and discontinued from the protocol after Week 8. The subject in the placebo group required a decrease in stimulant medication for comorbid ADHD at Week 8, and was, therefore, also discontinued from the protocol.
FIG. 1.
Selection of studies and search strategy.
Table 1.
Baseline Characteristics of Subjects
| Demographic characteristic | NAC | Placebo |
|---|---|---|
| Female/Male | 5/12 | 2/12 |
| Age (mean [SD]) | 12.4 (1.4) | 11.5 (2.8) |
| Age of onset (mean [SD]) | 6.8 (1.4) | 6.1 (2.4) |
| Duration of illness (mean [SD]) | 5.6 (3.4) | 5.4 (2.8) |
| Comorbid OCD (n [%]) | 2 (12%) | 4 (29%) |
| Comorbid ADHD (n [%]) | 6 (35%) | 9 (64%) |
| Pharmacological treatment for tics (current/ever) | 7 (41%)/11 (65%) | 6 (43%)/11 (79%) |
| Current antidepressant use (n [%]) | 5 (29%) | 2 (14%) |
| Current antipsychotic use (n [%]) | 7 (41%) | 0 |
| Current α-2 agonist use (n [%]) | 2 (12%) | 5 (36%) |
| Current psychostimulant use (n [%]) | 2 (12%) | 2 (14%) |
| Behavioral treatment for tics (current/ever) | 1 (6%)/3 (18%) | 2 (14%)/5 (36%) |
| YGTSS (mean [SD]) | 27.1 (7.2) | 26.3 (7.7) |
| PUTS (mean [SD]) | 24.4 (4.6) | 23.8 (4.1) |
| CGI – Severity (mean [SD]) | 3.7 (1.1) | 3.9 (0.6) |
| ADHD-RS (mean [SD]) | 17.6 (9.2) | 23.7 (11.8) |
| CYBOCS (mean [SD]) | 6.9 (8.3) | 10.7 (9.0) |
| CDI (mean [SD]) | 25.1 (2.5) | 24.9 (2.7) |
| MASC (mean [SD]) | 41.6 (15.4) | 23.8 (4.1) |
NAC, active N-acetylcysteine treatment; OCD, obsessive-compulsive disorder; ADHD: attention-deficit/hyperactivity disorder; YGTSS, Yale Global Tic Severity Scale; PUTS, Premonitory Urge Tic Scale; CGI, Clinical Global Impressions; ADHD-RS, ADHD Rating Scale; CYBOCS: Children Yale-Brown Obsessive-Compulsive Scale; CDI, Children's Depressive Inventory; MASC, Multidimensional Anxiety Scale for Children.
Tic symptoms
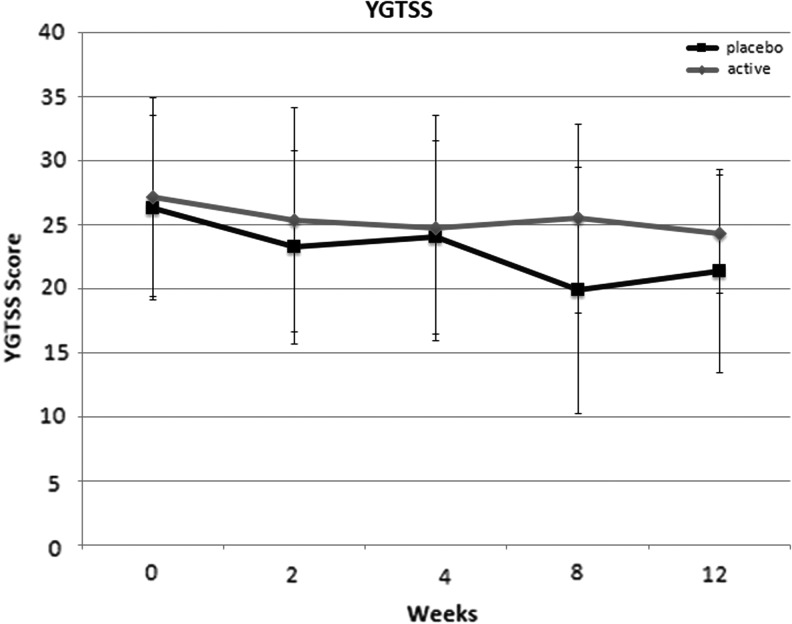
There were no significant differences between NAC and placebo in improving tic severity as measured on the YGTSS. Figure 2 depicts the change in YGTSS over time for both treatment groups. Table 2 presents the results from mixed models analysis of all primary and secondary measures.
FIG. 2.
Effects of N-acetylcysteine (NAC) and placebo on tic severity (Yale Global Tic Severity Scale [YGTSS]). There was no significant effect of NAC on tic severity over time in comparison with placebo.
Table 2.
Clinical Measures Across 12 Week NAC and Placebo Treatment in Children with Tourette Syndrome
| NAC treatment (n = 17) | Placebo treatment (n = 14) | Mixed model | |||||
|---|---|---|---|---|---|---|---|
| Dependent measures | Baseline | Week 12 | Baseline | Week 12 | Treatment | Time | Treatment × time |
| YGTSS | 27.1 (7.2) | 24.3 (7.9) | 26.3 (7.7) | 21.3 (4.6) | −0.6403 (F = 0.06, p = 0.815) | −0.4555 (F = 8.08, p = 0.008) | 0.3921 (F = 3.32, p = 0.079) |
| PUTS | 24.4 (4.6) | 24.7 (4.9) | 23.8 (4.1) | 24.1 (4.8) | 1.0609 (F = 0.54, p = 0.475) | −0.00625 (F = 0.0, p = 0.954) | 0.1750 (F = 1.49, p = 0.232) |
| CGI | 3.7 (1.1) | 3.3 (1.1) | 3.9 (0.6) | 3.4 (1.1) | −0.2289 (F = 0.4, p = 0.533) | −0.05930 (F = 3.33, p = 0.079) | 0.02252 (F = 0.612, p = 0.112) |
| ADHD-RS | 17.6 (9.2) | 16.4 (4.9) | 23.7 (11.8) | 22.7 (8.5) | −10.2914 (F = 10.2, p = 0.03) | −0.3066 (F = 3.4, p = 0.076) | 0.3014 (F = 1.78, p = 0.1923) |
| MASC | 41.6 (15.4) | 33.4 (13.8) | 23.8 (4.1) | 38.4 (14.8) | −4.8276 (F = 0.75, p = 0.3921) | −0.4454 (F = 4.4, p = 0.0444) | 0.05392 (F = 0.04, p = 0.851) |
| CDI | 25.1 (2.5) | 25.7 (1.8) | 24.9 (2.7) | 22.8 (3,5) | 0.04910 (F = 0.00, p = 0.958) | −0.1720 (F = 4.41, p = 0.045) | 0.2337 (F = 4.5, p = 0.042) |
| CYBOCS | 6.9 (8.3) | 4.4 (8.0) | 10.7 (9.0) | 11.5 (9.8) | −3.0345 (F = 1.14, p = 0.295) | 0.07353 (F = 0.47, p = 0.498) | −0.2396 (F = 2.75, p = 0.108) |
NAC, active N-acetylcysteine treatment; YGTSS, Yale Global Tic Severity Scale - Total tic score; PUTS, Premonitory Urge Tic Scale; CGI, Clinical Global Impressions; ADHD-RS Attention-Deficit/Hyperactivity Disorder Rating Scale; MASC: Multidimensional Anxiety Scale for Children; CDI, Children's Depressive Inventory; CYBOCS: Children Yale-Brown Obsessive-Compulsive Scale.
Six of 17 (35.3%) subjects in the NAC group were judged as treatment partial responders compared with 4 of 14 (28.6%) in the placebo group (Fisher p = 0.5). Four of 17 (35.2%) subjects in the NAC group were judged as treatment full responders (>35% and CGI 1 or 2) compared with 3 of 14 (21.4%) in the placebo group (Fisher p = 0.6).
Premonitory urges
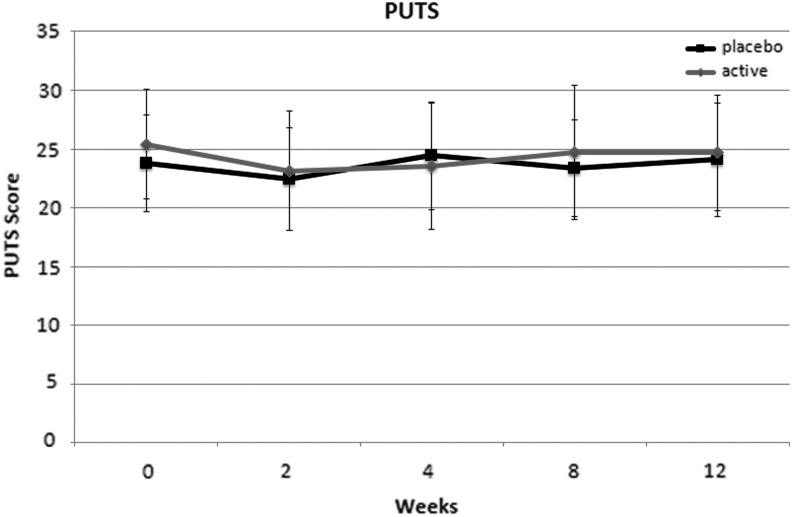
There were no significant differences between NAC and placebo in terms of reducing severity of premonitory urges associated with tic symptoms (PUTS). Figure 3 depicts the change in PUTS severity in the NAC and placebo groups during the trial.
FIG. 3.
Effects of N-acetylcysteine (NAC) and placebo on premonitory urge (Premonitory Urge Tic Scale [PUTS]). There was no significant effect of NAC on premonitory urge ratings over time in comparison with placebo.
Comorbid symptom severity
There were no significant differences between NAC and placebo in reducing severity of comorbid symptoms, including: OCD (CY-BOCS), ADHD (ADHD-RS), and anxiety symptoms (MASC). Depression symptoms improved significantly in the placebo compared with the NAC group (treatment-by-time interaction p = 0.04). Table 2 presents the results of the comparison between NAC and placebo on comorbid symptom severity during the course of the trial. Graphs comparing change in comorbid symptom severity during the course of the trial are contained in Supplementary Figure S1(Supplementary Data are available online at www.liebertpub.com/cap).
Safety and tolerability
Table 3 presents side effects reported in the NAC and placebo groups. There were no significant differences in side effect rates between NAC and placebo. No severe side effects were reported.
Table 3.
Side Effects of Treatment Among Placebo and Active NAC Groups
| PAERS item | NAC | Placebo |
|---|---|---|
| Irritability (1) | 0 | 0 |
| Sadness or depressive mood (3) | 0 | 1 (7%) |
| Fatigue (12) | 0 | 0 |
| Imnsonia (14) | 0 | 0 |
| Decreased appetite (18) | 0 | 0 |
| Chest pain (35) | 0 | 0 |
| Syncope (38) | 0 | 0 |
| Stomach ache (39) | 0 | 2 (14%) |
| Nausea (40) | 0 | 3 (21%) |
| Vomiting (41) | 0 | 0 |
| Headache (43) | 1 (6%) | 1 (7%) |
NAC, active N-acetylcysteine treatment; PAERS, Pediatric Adverse Events Rating Scale.
Discussion
This study is the first randomized, placebo-controlled trial of NAC for the treatment of tics. We observed no benefit to the use of NAC as an add-on treatment for children and adolescents with TS on any primary or secondary clinical measures.
The findings of this trial stand in contrast to several placebo-controlled trials of NAC for the treatment of other psychiatric conditions in both adults and children, which have yielded promising results. Individual trials have suggested significant benefit of NAC for the treatment of schizophrenia (Berk et al. 2008b; Sommer et al 2014) and bipolar depression (Berk et al. 2008a). Likewise, pediatric randomized, placebo-controlled trials have suggested a benefit for autism spectrum disorders (Hardan et al. 2012) and cannabis dependence (Gray et al. 2012). Among subjects with autism spectrum disorders, NAC was demonstrated to significantly reduce motor stereotypies as a secondary outcome in that trial. Similarly designed trials examining NAC for the treatment of other OC spectrum disorders in adults, such as OCD (Afshar et al. 2012) and TTM (Grant et al. 2009) have also suggested a significant benefit of NAC compared with placebo. The benefits of NAC for adult TTM, however, were not demonstrated in a similarly designed pediatric study (Bloch et al. 2013). Consistent with previous trials, NAC was safe and well tolerated in this trial.
There are several possibilities for the discordant results between our pediatric trials of NAC in TS and TTM and other previously published trials described, most specifically adult OCD, TTM, and decreasing motor stereotypies in ASD. 1) NAC may be ineffective in treating tics but effective in these other conditions, 2) developmental stage may play an important moderating effect in the efficacy of NAC in OC spectrum disorders, 3) the discordant results may be a result of statistical chance caused by the limited power of available studies, and/or 4) there may be systematic biases in previous NAC trials that are producing positive results.
It remains quite plausible that NAC affects tic symptoms differently than how it affects the symptoms of other OC spectrum disorders. OCD and TS have several differences in clinical presentation, including gender distribution (TS more commonly affects males), clinical course (TS is more likely to remit in adolescence), and response to treatment (OCD is more responsive to SSRIs and less responsive to antipsychotic monotherapy and α-2 agonists than TS) (Ferrao et al. 2009). TS and TTM share several important clinical similarities (e.g., premonitory urges, response to habit reversal therapy, and possibly response to antipsychotics) (Bloch et al. 2007), but the underlying pathology of TTM remains largely unexplored.
Another possibility is that age has moderating effects on the efficacy of NAC for OC spectrum disorders. It seems that our negative results of NAC treatment to pediatric TTM (Leckman et al. 1989) and pediatric TS are both accurate and reliable. The divergent results regarding the efficacy of NAC for treating these conditions in pediatric and adult populations reinforces the differences in presentation between adult- and childhood-onset OC spectrum disorders. NAC is hypothesized to work in targeting repetitive behaviors (e.g.: TS, OCD, and TTM) by reducing the frequency and intensity of the premonitory urges via glutamate modulation (Grant et al. 2009). This hypothesis derives from the substance abuse literature, in which NAC has been demonstrated to modulate glutamate in the nucleus accumbens and reduce drug-associated cravings (Moussawi et al. 2009; Kupchik et al. 2012). As children tend to engage in more automatic and ego-syntonic repetitive behaviors than adults (Alvarenga et al. 2012), they might have less benefit from NAC. Results of this trial and a similarly designed pediatric TTM trial are consistent with this hypothesis, as neither trials found any significant effect of NAC compared with placebo on any measures that specifically probed the frequency and intensity of, or control over urges. However, arguing against the possibility is the observation that NAC had beneficial effects for the treatment of stereotypies in children with ASD in a previous double-blind, placebo-controlled trial. Children enrolled in this trial had an earlier age of onset than those enrolled in the present study, and stereotypies are typically not associated with premonitory urges, in contrast to tics (Hardan et al. 2012).
Another plausible explanation for the difference in adult and pediatric populations is that NAC's antioxidant (Cotgreave 1997; De Rosa et al. 2000) and anti-inflammatory (Sommer et al. 2014) properties are more effective for adults with longer periods of illness and, consequently, more brain injury and comorbid anxiety and depressive symptoms (Bloch et al. 2006). A recent neuroimaging (proton magnetic spectroscopy) study reported a decrease in metabolite (derived from oxidative stress) concentration in the cingulate cortex of adult patients with depression taking NAC, compared with those taking placebo. This finding supports our hypothesis that the antioxidant properties of NAC might lead to increased efficacy in adults (Das et al. 2013).
Further, the divergent results between NAC trials may be the result of the small sample size and limited statistical power of many of the included trials. Our trial had limited power to detect a significant difference between NAC and placebo. Post-hoc power estimates suggest that we had 68% power to detect an effect size of NAC = 0.9, assuming α = 0.05. Most trials in this area have been similarly powered, although most of the larger, better-powered trials of NAC in the treatment of other psychiatric disorders in adults and children have suggested benefit of this condition. The small sample size of our current NAC trial remains a major limitation; however, there is no evidence or suggestion that NAC is effective for treating tic symptoms. It remains exceedingly unlikely that NAC has even a medium-to-large benefit in improving tic disorders. Currently available pharmacological and behavioral treatments for TS have this demonstrated effect size (Piacentini et al. 2010; Weisman et al. 2014). Other limitations in our trial include the fact that our randomly assigned NAC and placebo groups differed significantly in terms of severity of anxiety and ADHD symptoms and concomitant use of antipsychotic medications. These baseline differences could have affected our ability to detect difference in subject outcomes that were attributable to medication effects. Many of our subjects received concomitant treatments (at stable doses) along with study medications during the course of this trial; this concomitant medication use may influence the measured efficacy of NAC (although NAC was found to be effective as an adjunctive agent to antipsychotic medications in schizophrenia previously) (Berk et al. 2008b; Sommer et al. 2014). Additionally, we did not perform pill counts to assess compliance; therefore, we cannot verify with certainty that the included subjects took all doses of the assigned medication.
Conclusions
Earlier trials of NAC may also have contained systematic bias that could have led to spurious positive findings across a plethora of psychiatric conditions. Poor blinding between NAC and placebo remains a possible candidate as a source of bias. Most trials of NAC conducted to date have utilized tablet formulations of NAC that may contain a sulfur-like odor that makes the agent difficult to blind with placebo trials. We went to great efforts in this trial to try to properly blind the NAC capsules. Methods of achieving proper blinding are often undescribed in earlier trials, and when described, are often difficult to evaluate in terms of adequacy. Although this is a possibility, there are two pieces of evidence that argue against this blinding issue being solely responsible for the positive findings of NAC across psychiatric conditions: 1) Several more recent trials using NAC formulations with identical appearing and smelling placebo controls have produced positive results, and 2) there is increasing evidence regarding the biological effects of NAC (see discussion of Das et al. 2013).
Clinical Significance
This trial failed to demonstrate a benefit of NAC for the treatment of tic disorders in children with TS. However, it also provides further evidence of the safety and tolerability of NAC in the pediatric population. The trial's negative results highlight the need for larger, better-powered, multisite studies evaluating the efficacy of NAC for several OC spectrum disorders (and other psychiatric conditions) using ideal placebo comparator formulations. The increasing evidence of biological effects of NAC across psychiatric conditions provides additional rationale for these larger trials.
Supplementary Material
Acknowledgments
We thank Alisa Yaffa for assistance with recruiting participants and Dr. Haya Rubin for assistance with study oversight.
Disclosures
Dr. Leckman receives royalties from John Wiley & Sons, McGraw Hill, and Oxford University. He also serves on the scientific advisory boards of National Organization for Rare Disorders (NORD) and the Brain and Behavior Research Foundation (formerly NARSAD). Other authors have no conflcits of interest to declare.
References
- Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, Wang T: Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatr Genet 21:90–97, 2011 [DOI] [PubMed] [Google Scholar]
- Adler LA, Spencer T, Faraone SV, Kessler RC, Howes MJ, Biederman J, Secnik K: Validity of pilot Adult ADHD Self-Report Scale (ASRS) to rate adult ADHD symptoms. Ann Clin Psychiatry 18:145–148, 2006 [DOI] [PubMed] [Google Scholar]
- Afshar H, Roohafza H, Mohammad–Beigi H, Haghighi M, Jahangard L, Shokouh P, Sadeghi M, Hafezian H: N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol 32:797–803, 2012 [DOI] [PubMed] [Google Scholar]
- Alvarenga P, Mastrorosa R, Rosário M: Obsessive-compulsive disorder in children and adolescents. In: The IACAPAP Textbook of Child and Adolescent Mental Health. Edited by Rey JM. Paris: IACAPAP; 2012 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Anderson GM, Pollak ES, Chatterjee D, Leckman JF, Riddle MA, Cohen DJ: Postmortem analysis of subcortical monoamines and amino acids in Tourette syndrome. Adv Neurol 58:123–133, 1992 [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson–Hunt M, Bush AI: N-acetyl cysteine for depressive symptoms in bipolar disorder––a double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475, 2008a [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson–Hunt M, Judd F, Katz F, Katz P, Ording–Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI: N-acetyl cysteine as a glutathione precursor for schizophrenia––a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64:361–368, 2008b [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, Cullen B, Valle D, Hoehn-Saric R, Greenberg BD, Pinto A, Knowles JA, Piacentini J, Pauls DL, Liang KY, Willour VL, Riddle M, Samuels JF, Feng G, Nestadt G: Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet 150B:710–720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Landeros–Weisenberger A, Dombrowski P, Kelmendi B, Wegner R, Nudel J, Pittenger C, Leckman JF, Coric V: Systematic review: Pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry 62:839–846, 2007 [DOI] [PubMed] [Google Scholar]
- Bloch MH, Panza KE, Grant JE, Pittenger C, Leckman JF: N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry 52:231–240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Panza KE, Landeros–Weisenberger A, Leckman JF: Meta-analysis: Treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry 48:884–893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, Leckman JF: Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med 160:65–69, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Boulougouris V, Fineberg NA, Grant JE. Trichotillomania: Neurobiology and treatment. Neurosci Biobehav Rev 33:831–842, 2009 [DOI] [PubMed] [Google Scholar]
- Cotgreave IA: N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol 38:205–227, 1997 [PubMed] [Google Scholar]
- Crane J, Fagerness J, Osiecki L, Gunnell B, Stewart SE, Pauls DL, Scharf JM, Tourette Syndrome International Consortium for Genetics (TSAICG): Family-based genetic association study of DLGAP3 in Tourette Syndrome. Am J Med Genet B Neuropsychiatr Genet 156B:108–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Tanious M, Fritz K, Dodd S, Dean OM, Berk M, Malhi GS: Metabolite profiles in the anterior cingulate cortex of depressed patients differentiate those taking N-acetyl-cysteine versus placebo. Aust N Z J Psychiatry 47:347–354, 2013 [DOI] [PubMed] [Google Scholar]
- De Rosa SC, Zaretsky MD, Dubs JG, Roederer M, Anderson M, Green A, Mitra D, Watanabe N, Nakamura H, Tjioe I, Deresinski SC, Moore WA, Ela SW, Parks D, Herzenberg LA, Herzenberg LA: N-acetylcysteine replenishes glutathione in HIV infection. Eur J Clinical Invest 30:915–929, 2000 [DOI] [PubMed] [Google Scholar]
- DeVito TJ, Drost DJ, Pavlosky W, Neufeld RW, Rajakumar N, McKinlay BD, Williamson PC, Nicolson R: Brain magnetic resonance spectroscopy in Tourette's disorder. J Am Acad Child Adolesc Psychiatry 44:1301–1308, 2005 [DOI] [PubMed] [Google Scholar]
- Dion Y, Annable L, Sandor P, Chouinard G: Risperidone in the treatment of Tourette syndrome: A double-blind, placebo-controlled trial. J Clin Psychopharmacol 22:31–39, 2002 [DOI] [PubMed] [Google Scholar]
- Ferrao YA, Miguel E, Stein DJ: Tourette's syndrome, trichotillomania, and obsessive-compulsive disorder: How closely are they related? Psychiatry Res 170:32–42, 2009 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011, 1989 [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW: N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 66:756–763, 2009 [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT: A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry 169:805–812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, Frazier TW, Tirouvanziam R: A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry 71:956–961, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression, Inventory (CDI). Psychopharmacol Bull 21:995–998, 1985 [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW: The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71:978–986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman J, Bloch M, Sukhodolsky D, Scahill L, King R: Phenomenology of tics and sensory urges: the self under siege. In: Tourette Syndrome. Edited by Martino D. New York: Oxford Unity Press; 2013; pp. 3–25 [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ: The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28:566–573, 1989 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK: The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36:554–565, 1997 [DOI] [PubMed] [Google Scholar]
- McElroy SL, Phillips KA, Keck PE, Jr: Obsessive compulsive spectrum disorder. J Clin Psychiatry 55 Suppl:33–51, 1994 [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, Storch EA: A meta-analysis of behavior therapy for Tourette Syndrome. J Psychiatr Research 50:106–112, 2014 [DOI] [PubMed] [Google Scholar]
- Minns AB, Clark RF, Schneir A: Guanfacine overdose resulting in initial hypertension and subsequent delayed, persistent orthostatic hypotension. Clin Toxicol 48:146–148, 2010 [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK: Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25:6389–6393, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW: N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT: Behavior therapy for children with Tourette disorder: A randomized controlled trial. JAMA 303:1929–1937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel–Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, Leckman JF, Bansal R, Peterson BS: Altered interhemispheric connectivity in individuals with Tourette's disorder. Am J Psychiatry 161:2028–2037, 2004 [DOI] [PubMed] [Google Scholar]
- Robertson MM, Stern JS: Gilles de la Tourette syndrome: Symptomatic treatment based on evidence. Eur Child Adolesc Psychiatry 9 Suppl 1:I60–75, 2000 [DOI] [PubMed] [Google Scholar]
- Roessner V, Rothenberger A: Pharmacological treatment of tics. In: eds. Tourette Syndrome. Edited by Martino D. New York: Oxford Unity Press; 2013; pp. 454–452 [Google Scholar]
- Roessner V, Rothenberger A, Rickards H, Hoekstra PJ: European clinical guidelines for Tourette syndrome and other tic disorders. Eur Child Adolesc Psychiatry 20:153–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, Cohen DJ, Leckman JF: A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 158:1067–1074, 2001 [DOI] [PubMed] [Google Scholar]
- Scahill L, Leckman JF, Schultz RT, Katsovich L, Peterson BS: A placebo-controlled trial of risperidone in Tourette syndrome. Neurology 60:1130–1135, 2003 [DOI] [PubMed] [Google Scholar]
- Shapiro M, Silva SG, Compton S, Chrisman A, DeVeaugh-Geiss J, Breland-Noble A, Kondo D, Kirchner J, March JS: The child and adolescent psychiatry trials network (CAPTN): Infrastructure development and lessons learned. Child Adolesc Psychiatry Ment Health 3:12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS: Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40:181–191, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA: Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci 8:419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH: Systematic review: Pharmacological treatment of tic disorders––efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev 37:1162–1171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterneck CT, Teng EJ, Stanley MA: Current issues in the treatment of OC-spectrum conditions. Bull Menninger Clin 74:141–166, 2010 [DOI] [PubMed] [Google Scholar]
- Woods DW, Piacentini J, Himle MB, Chang S: Premonitory urge for tics scale (PUTS): Initial psychometric results and examination of the premonitory urge phenomenon in youths with tic disorders. J Dev Behav Pediatr 26:397–403, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.