Abstract
A new series of quinazolinone compounds 16–34 incorporating isatin moieties was synthesized. The antitumor efficacy of the compounds against MDA-MB-231, a breast cancer cell line, and LOVO, a colon cancer cell line, was assessed. Compounds 20, 21, 22, 23, 25, 27, 28, 29, 30, 31, 32, 33, and 34 displayed potent antitumor activity against MDA-MB-231 and LOVO cells (IC50: 10.38–38.67 μM and 9.91–15.77 μM, respectively); the comparative IC50 values for 5-fluorouracil and erlotinib in these cells lines were 70.28 μM, 22.24 μM and 15.23 μM, 25.31 μM respectively. The EGFR-TK assay and induction of apoptosis for compound 31 were investigated to assess its potential cytotoxic activity as a representative example of the novel synthesized compounds. At a concentration of 10 μM, compound 31 exhibited efficient inhibitory effect against EGFR-TK and induced apoptosis in MDA-MB-231 cells. Furthermore, a molecular docking study for compound 31 and erlotinib was performed to verify the binding mode toward the EGFR kinase enzyme, and showed a similar interaction as that with erlotinib alone.
Graphical Abstract: Compound 31 showed potent antitumor activity and efficient inhibitory effect against EGFR-TK and induced apoptosis of MDA-MB-231 cells at a concentration of 10 μM.
Keywords: Synthesis, isatin, quinazolinone, antitumor, EGFR-TK, molecular docking
Graphical Abstract
Introduction
Cancer is one of the most worldwide dangerous health problems and is one of the leading causes of death1. Many of the current anticancer agents are highly toxic and nonspecific, so the production of innovative, safe, and selective anticancer molecules is an important goal for the medicinal chemistry researchers. The quinazolinone scaffold is a vital structure in medicinal chemistry2–22.
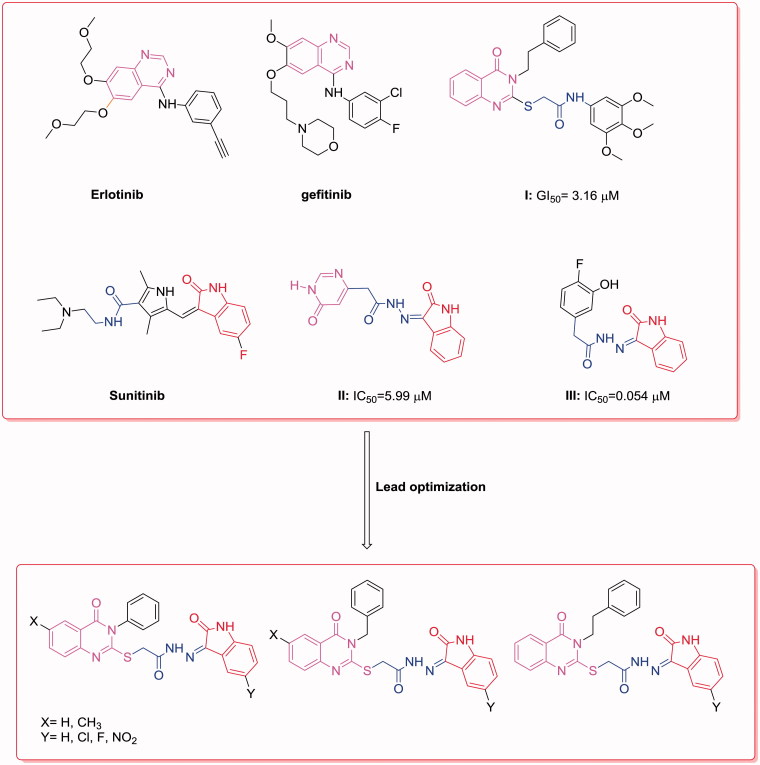
Anilinoquinazolines, such as gefitinib23,24 and erlotinib25, have been established as EGFR kinase inhibitors for the treatment of breast cancer (Figure 1). The 3-phenethylquinazoline derivative (I) has broad spectrum antitumor activity with a mean GI50 value of 3.16 μM, in addition to EGFR-TK inhibitory activity11 (Figure 1).
Figure 1.
Reported and proposed quinazoline–isatin conjugates with antitumor and tyrosine kinase inhibitory activity.
Additionally, isatin derivatives exhibit broad spectrum biological effects such as anticancer activity26. A 5-fluoro-3-substituted isatin analog (Sunitinib) was approved by the FDA for the treatment of renal carcinoma and gastrointestinal stromal tumors27,28 (Figure 1).
Methyl 3-(1-(4-bromobenzyl)-2,3-dioxoindolin-5-yl)acrylate showed broad spectrum anticancer activity and a weak cytotoxic effect in normal human cells29. A series of indolinone hydrazides, including 2-(6-oxo-1,6-dihydropyrimidin-4-yl)-N′-(2-oxoindolin-3-ylidene)acetohydrazide (II) and 2-(4-fluoro-3-hydroxyphenyl)-N′-(2-oxoindolin-3-ylidene)acetohydrazide (III), were reported as potent anticancer agents with IC50 values of 5.99 and 0.054 μM, respectively30 (Figure 1). As an attempt to develop effective cytotoxic agents, we synthesized hybrids of quinazoline conjugated to 5-substituted isatin that contained an acylhydrazone moiety and evaluated their cytotoxic activity. Additionally, the EGFR-TK assay and apoptosis induction were investigated for the most active compound, as a representative example of the novel synthesized compounds, to identify their potential cytotoxic activity. A molecular docking study was conducted to verify the structural requirements of the antitumor activity of the target molecules and to support the results of binding of the active compounds to EGFR31.
Materials and methods
Chemistry
Melting points were recorded on Barnstead 9100 Electrothermal melting point apparatus (UK). IR spectra (KBr) were recorded on a FT-IR Perkin-Elmer spectrometer (Perkin Elmer Inc., MA). Nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on Bruker 500 or 700 MHz spectrometers (Zurich, Switzerland) using DMSO-d6 as the solvent. Microanalytical data (C, H, and N) were performed on a Perkin-Elmer 240 analyzer (Perkin Elmer Inc., MA) and agreed with the proposed structures within ±0.4% of the theoretical values. Mass spectra were recorded on a Varian TQ 320 GC/MS/MS mass spectrometer (Varian, Palo Alto, CA). 2-[(3-Substituted-4(3H)-quinazolinon-2-yl)thio]acetohydrazides (11–15) were prepared according to previously reported methods11,19,22.
Synthesis of 2-((3-substituted-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazides (16–34)
An equimolar amount of the appropriate 2-[(3-substituted-4(3H)-quinazolinon-2-yl)thio]acetohydrazide (11–15) and substituted isatin was added to methanol (15 ml) containing glacial acetic acid (0.2 ml) and refluxed for 4–6 h. The reaction mixture was filtered while hot; the solid obtained was washed with methanol and dried.
2-((3-Benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (16)
Yield: 83%; mp: 250–251 oC; IR (KBr, cm−1) ν: 3421, 3160 (2NH), 1744, 1725, 1693 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 29.5, 47.5, 111.6, 115.6, 119.1, 122.1, 123.1, 126.3, 126.6, 127.1, 127.2, 127.9, 129.0, 135.3, 135.4, 136.0, 142.9, 147.1, 156.9, 161.2, 163.0; 1H-NMR (700 MHz, DMSO-d6): δ 11.54 (s, 0.5H), 11.33 (s, 0.5H), 10.86 (s, 0.5H), 8.14 (s, 0.5H), 8.11 (d, 1H, J = 5.5 Hz), 7.75 (s, 1H), 7.57–7.46 (m, 2H), 7.41–7.28 (m, 6H), 7.08–7.03 (m, 1H), 6.93 (d, 1H, J = 5.5 Hz), 5.38 (s, 2H), 4.69 (s, 1H), 4.24 (s, 1H); MS: [m/z, 469].
2-((3-Benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-chloro-2-oxoindolin-3-ylidene)acetohydrazide (17)
Yield: 83%; mp: 275–276 oC; IR (KBr, cm−1) ν: 3448, 3178 (2NH), 1723, 1718, 1695 (3C=O); 13C NMR (176 MHz, DMSO-d6): δ 29.4, 47.5, 113.1, 116.8, 119.1, 120.8, 121.9, 126.3, 126.6, 127.1, 127.2, 127.9, 129.0, 135.3, 135.4, 136.0, 141.6, 147.0, 147.1, 156.8, 161.2; 1H-NMR (700 MHz, DMSO-d6): δ 11.77 (s, 0.5 H), 11.44 (s, 0.5 H), 10.98 (s, 0.5 H), 8.36 (s, 0.5 H), 8.10 (d, 1H, J = 6.5 Hz), 7.74 (t, 1H, J = 5.5 Hz), 7.65–7.29 (m, 9H), 6.99–6.93 (m, 1H), 5.38 (s, 2H), 4.68 (s, 1H), 4.25 (s, 1H); MS: [m/z, 503; M + 2, 505].
2-((3-Benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-fluoro-2-oxoindolin-3-ylidene)acetohydrazide (18)
Yield: 83%; mp: 244–245 oC; IR (KBr, cm−1) ν: 3410, 3169 (2NH), 1717, 1702, 1692 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 29.3, 47.5, 108.4, 112.7, 118.5, 119.1, 121.5, 126.4, 126.7, 127.1, 127.2, 127.9, 129.0, 134.5, 135.4, 135.9, 139.2, 147.0, 156.2, 158.1, 159.4, 161.2, 163.0; 1H-NMR (700 MHz, DMSO-d6): δ 13.48 (s, 0.4H), 12.74 (s, 0.6H), 11.38 (s, 1H), 8.13 7.36 (m, 12H), 5.40 (s, 2H), 4.70 (s, 1H), 4.28 (s, 1H); MS: [m/z, 487].
2-((3-Benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-nitro-2-oxoindolin-3-ylidene)acetohydrazide (19)
Yield: 83%; mp: 313–315 oC; IR (KBr, cm−1) ν: 3467, 3279 (2NH), 1741, 1701, 1655 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 47.5, 111.2, 111.8, 115.5, 119.1, 121.0, 122.1, 126.3, 126.6, 127.1, 127.2, 127.9, 128.0, 129.0, 135.3, 135.4, 136.0, 142.5, 143.2, 147.0, 148.1, 156.8, 161.2; 1H-NMR (700 MHz, DMSO-d6): δ 12.27 (s, 0.5H), 11.93 (s, 0.5H), 11.56 (s, 0.5H), 9.12 (s, 0.5H), 8.34 (dd, 1H, J = 8.5 Hz), 8.10 (d, 1H, J = 8.0 Hz), 7.72 (t, 1H, J = 7.5 and 8.0 Hz), 7.45 (t, 1H, J = 7.5 Hz), 7.37–7.27 (m, 6H), 7.11 (d, 1H, J = 9.0 Hz), 5.38 (s, 2H), 4.66 (s, 1H), 4.30 (s, 1H); MS: [m/z, 514].
2-((3-Benzyl-6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (20)
Yield: 83%; mp: 270–271 oC; IR (KBr, cm−1) ν: 3412, 3273 (2NH), 1793, 1724, 1686 (3C=O); 13C-NMR (125 MHz, DMSO-d6): δ 20.7, 39.9, 46.9, 110.6, 115.2, 118.4, 121.6, 125.9, 126.7, 127.4, 128.5, 135.6, 136.0, 144.7, 155.2, 160.7; 1H-NMR (500 MHz, DMSO-d6): δ 11.51–11.32 (m, 1H), 10.84 (d, 1H, J = 7.0 Hz), 8.13 (s, 1H), 7.88 (d, 1H, J = 4.5 Hz), 7.55–7.32 (m, 8H), 7.06–6.92 (m, 2H), 5.37 (d, 2H, J = 10.0 Hz), 4.65 (s, 1H), 4.41 (s, 1H), 2.41 (d, 3H, J = 11.0 Hz); MS: [m/z, 483].
2-((3-Benzyl-6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-chloro-2-oxoindolin-3-ylidene)acetohydrazide (21)
Yield: 83%; mp: 246–247 oC; IR (KBr, cm−1) ν: 3456, 3163 (2NH), 1741, 1713, 1685 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 21.2, 39.6, 47.4, 113.2, 116.8, 126.1, 126.4, 127.2, 127.9, 129.0, 136.1, 136.6, 141.6, 145.2, 155.7, 161.2; 1H-NMR (700 MHz, DMSO-d6): δ 11.74 (s, 0.5H), 11.42 (s, 0.5H), 10.96 (s, 0.5H), 8.36 (s, 0.5H), 7.90 (s, 1H), 7.68–7.56 (m, 2H), 7.50–7.28 (m, 7H), 6.94 (d, 1H, J = 5.5 Hz), 5.37 (s, 2H), 4.67 (s, 1.5H), 4.23–4.12 (m, 0.5H), 2.41 (s, 3H); MS: [m/z, 517; M + 2, 519].
2-((3-Benzyl-6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-fluoro-2-oxoindolin-3-ylidene)acetohydrazide (22)
Yield: 83%; mp: 272–274 oC; IR (KBr, cm−1) ν: 3448, 3182 (2NH), 1762, 1717, 1686 (3C=O); 13C-NMR (125 MHz, DMSO-d6): δ 20.6, 34.6, 46.9, 111.3, 112.2, 113.2, 113.4, 115.5, 115.6, 118.4, 125.6, 125.8, 126.7, 127.4, 128.5, 135.6, 135.8, 136.0, 138.7, 140.1, 144.7, 155.2, 156.5, 158.4, 160.7, 164.6; 1H-NMR (500 MHz, DMSO-d6): δ 11.58 (s, 0.5H), 11.31 (s, 0.5H), 10.84 (s, 1H), 8.17 (d, 1H, J = 8.0 Hz), 7.88 (s, 1H), 7.54 (dd, 1H, J = 1.5 and 7.0 Hz), 7.36–7.25 (m, 7H), 6.93–6.90 (m, 1H), 5.37 (s, 2H), 4.65 (s, 1H), 4.59 (s, 1H), 2.39 (s, 3H); MS: [m/z, 501].
2-((3-Benzyl-6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-nitro-2-oxoindolin-3-ylidene)acetohydrazide (23)
Yield: 83%; mp: 292–294 oC; IR (KBr, cm−1) ν: 3467, 3167 (2NH), 1741, 1702, 1687 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 21.1, 40.4, 47.48, 111.9, 115.5, 116.3, 118.9, 121.0, 122.0, 126.3, 126.4, 127.2, 127.9, 129.0, 136.1, 136.3, 136.6, 136.6, 142.5, 143.2, 145.1, 148.0, 155.7, 161.2, 165.4; 1H-NMR (700 MHz, DMSO-d6): δ 12.56 (0.5H), 11.92 (0.5H), 11.53 (0.5H), 9.12 (0.5H), 8.29 (dd, 1H, J = 5.5 and 15.0 Hz), 7.88 (s, 1H), 7.54 (d, 1H, J = 5.5 Hz), 7.36–7.27 (m, 7H), 7.10 (d, 1H, J = 6.0 Hz), 5.37 (s 2H), 4.60 (s, 1H), 4.27 (s, 1H), 2.40 (s, 3H); MS: [m/z, 528].
2-((4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (24)
Yield: 83%; mp: 304–305 oC; IR (KBr, cm−1) ν: 3449, 3223 (2NH), 1726, 1712, 1698 (3C=O); 13C-NMR (125 MHz, CDCl3-DMSO-d6): δ 34.6, 111.0, 119.4, 119.6, 120.538, 120.8, 122.4, 126.0, 126.4, 129.3, 129.4, 129.9, 131.5, 134.6, 135.4, 137.6, 142.4, 146.9, 155.7, 160.6, 162.4, 164.9; 1H NMR (500 MHz, DMSO-d6): δ 13.49 (s, 0.56H), 12.72 (s, 0.46H), 11.26 (s, 1H), 8.07 (dd, 1H, J = 1.0 and 8.0 Hz), 7.74 (s, 1H), 7.60–7.42 (m, 8H), 7.33 (t, 1H, J = 8.0 Hz), 7.0526 (d, 1H, J = 6.0 Hz), 6.95–6.87 (m, 1H), 4.55 (s, 1H), 4.08 (s, 1H); MS: [m/z, 455].
N'-(5-chloro-2-oxoindolin-3-ylidene)-2-((4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (25)
Yield: 83%; mp: 328–329 oC; IR (KBr, cm−1) ν: 3447, 3259 (2NH), 1730, 1702, 1659 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 21.2, 47.4, 47.4, 113.2, 116.8, 126.1, 126.4, 127.2, 127.9, 129.0, 136.1, 136.6, 141.6, 145.2, 155.7, 161.2; 1H-NMR (700 MHz, DMSO-d6): δ 13.42 (s, 0.5H), 12.63 (s, 0.5H), 11.43 (s, 1H), 8.07 (d, 1H, J = 5.5 Hz), 7.79 (s, 1H), 7.72–7.61 (m, 4H), 7.51–7.42 (m, 5H), 6.99–6.93 (m, 1H), 4.57 (s, 1H), 4.12 (s, 1H); MS: [m/z, 489; M + 2, 491].
N'-(5-fluoro-2-oxoindolin-3-ylidene)-2-((4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (26)
Yield: 83%; mp: 310–312 oC; IR (KBr, cm−1) ν: 3429, 3256 (2NH), 1733, 1709, 1686 (3C=O); 13C-NMR (125 MHz, DMSO-d6): δ 34.7, 108.0, 112.2, 118.1, 119.4, 120.9, 126.0, 126.5, 129.4, 129.5, 130.0, 134.8, 135.6, 138.69 146.9, 157.3, 159.2, 160.5, 162.6; 1H-NMR (500 MHz, DMSO-d6): δ 13.47 (s, 0.5H), 12.69 (s, 0.5 H), 11.34 (s, 1H), 8.06 (d, 1H, J = 8.0 Hz), 7.77 (s, 1H), 7.61–7.34 (m, 8H), 7.20 (s, 1H) 6.95– 6.90 (m, 1H), 4.56 (s, 1H), 4.11 (s, 1H); MS: [m/z, 473].
N'-(5-nitro-2-oxoindolin-3-ylidene)-2-((4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (27)
Yield: 83%; mp: 337–338 oC; IR (KBr, cm−1) ν: 3431, 3188 (2NH), 1730, 1712, 1691 (3C=O); 1H-NMR (500 MHz, DMSO-d6): δ 13.31 (s, 0.5 H), 12.53 (s, 0.5), 11.94 (s, 1H), 8.29 (d, 2H, J = 6.5 Hz), 8.00 (d, 1H, J = 7.5 Hz), 7.78 (s, 1H), 7.61–7.47 (m, 8H), 7.13 (s, 1H), 4.61 (s, 1H), 4.16 (s, 1H); MS: [m/z, 500].
2-((6-Methyl-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (28)
Yield: 83%; mp: 305–306 oC; IR (KBr, cm−1) ν: 3421, 3298 (2NH), 1725, 1695, 1652 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 21.1, 35.1, 111.6, 115.7, 119.7, 120.1, 121.3, 122.1, 123.1, 126.2, 126.3, 129.9, 130.0, 130.4, 136.3, 136.4, 136.5, 136.6, 142.9, 144.3, 145.6, 161.1, 163.0, 165.0; 1H-NMR (700 MHz, DMSO-d6): δ 11.46 (s, 0.5H), 11.31 (s, 0.5H), 10.85 (s, 0.5H), 8.15 (s, 0.5H), 7.86 (s, 1H), 7.63–7.48 (m, 7H), 7.40–7.35 (m, 2H), 7.05 (t, 1H, J = 5.0 and 5.5 Hz), 6.97–6.90 (m, 1H), 4.55 (s, 1H), 4.28 (s, 0.5H), 4.08 (s, 0.5 H), 2.40 (s, 3H); MS: [m/z, 469].
N'-(5-chloro-2-oxoindolin-3-ylidene)-2-((6-methyl-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (29)
Yield: 83%; mp: 328–330 oC; IR (KBr, cm−1) ν: 3419, 3149 (2NH), 1721, 1689, 1646 (3C=O); 1H-NMR (500 MHz, DMSO-d6): δ 11.70 (s, 0.5H), 11.44 (s, 0.5H), 10.95 (s, 1H), 8.35 (s, 1H), 7.87 (s, 1H), 7.60–7.49 (m, 7H), 7.36 (s, 1H), 6.93 (d, 1H, J = 8.0 Hz), 4.51 (s, 1H), 4.35 (s, 0.75H), 4.10 (s, 0.25H), 2.42 (s, 3H); MS: [m/z, 503; M + 2, 505].
N'-(5-fluoro-2-oxoindolin-3-ylidene)-2-((6-methyl-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (30)
Yield: 83%; mp: 281–282 oC; IR (KBr, cm−1) ν: 3448, 3283 (2NH), 1725, 1699, 1662 (3C=O); 13C-NMR (125 MHz, DMSO-d6): δ 20.6, 34.6, 111.3, 112.2, 119.2, 121.0, 125.7, 125.8, 129.4, 129.5, 129.9, 135.6, 135.8, 136.0, 136.1, 138.6, 145.0, 145.1, 155.6, 156.5, 157.3, 159.2, 160.5, 162.6, 164.6; 1H-NMR (500 MHz, DMSO-d6): δ 11.57 (s, 0.4H), 11.33 (s, 0.6H), 10.83 (s, 0.4H), 8.14 (s, 0.6H), 7.86 (s, 1H), 7.61–7.49 (m, 7H), 7.35 (s, 1H), 7.26–7.21 (m, 1H), 6.91 (t, 1H, J = 4.0 Hz), 4.55 (s, 1.4H), 4.09 (s, 0.6H), 2.41 (s, 3H); MS: [m/z, 487].
2-((6-Methyl-4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)thio)-N'-(5-nitro-2-oxoindolin-3-ylidene)acetohydrazide (31)
Yield: 83%; mp: 344–345 oC; IR (KBr, cm−1) ν: 3446, 3196 (2NH), 1744, 1707, 1648 (3C=O); 1H-NMR (700 MHz, DMSO-d6): δ 13.29 (s, 0.7H), 12.52 (s, 0.3H), 11.92 (s, 0.7H), 11.52 (s, 0.3H), 8.30 (s, 1H), 7.87 (s, 1H), 7.60–7.11 (m, 9H), 4.59 (s, 1H), 4.35 (s, 0.3H), 4.14 (s, 0.7H), 2.41 (s, 1H); MS: [m/z, 514].
2-((4-Oxo-3-phenethyl-3,4-dihydroquinazolin-2-yl)thio)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (32)
Yield: 83%; mp: 273–274 oC; IR (KBr, cm−1) ν: 3448, 3133 (2NH), 1715, 1686, 1636 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 33.9, 40.4, 46.0, 111.1, 111.5, 119.2, 120.2, 122.1, 123.1, 126.2, 126.5, 126.9, 127.2, 129.1, 135.1, 135.2, 138.1, 142.9, 147.0, 147.0, 160.8, 160.8; 1H-NMR (700 MHz, DMSO-d6): δ 11.55 (s, 0.5H), 11.28, (s, 0.5H), 10.83 (s, 0.5H), 8.16 (s, 0.5H), 8.15 (d, 1H, J = 2.0 Hz), 8.07 (d, 1H, J = 5.5 Hz), 7.80 (d, 1H, J = 6.0 Hz), 7.68–7.26 (m, 8H), 7.10–7.00 (m, 1H), 6.93–6.90 (m, 1H), 4.74–4.49 (m, 1.5H), 4.41–4.36 (m, 2.5H, J = 5.0 and 7.5 Hz), 3.07–3.00 (m, 2H); MS: [m/z, 483].
N'-(5-chloro-2-oxoindolin-3-ylidene)-2-((4-oxo-3-phenethyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (33)
Yield: 83%; mp: 233–235 oC; IR (KBr, cm−1) ν: 3469, 3167 (2NH), 1710, 1676, 1646 (3C=O); 13C-NMR (176 MHz, DMSO-d6): δ 33.9, 40.4, 46.0, 112.4, 116.8, 119.2, 121.9, 126.1, 126.2, 126.5, 126.9, 127.2, 129.1, 135.1, 135.2, 138.1, 143.0, 147.0, 156.2, 160.8, 164.8, 172.4; 1H-NMR (700 MHz, DMSO-d6): δ 11.67 (s, 0.5H), 11.58 (s, 0.5H), 10.97 (s, 1H), 8.37 (s, 1H), 8.06 (d, 1H, J = 5.5 Hz), 7.70 (t, 1H, J = 5.0 Hz), 7.44–7.21 (m, 8H), 6.93 (d, 1H, J = 6.0 Hz), 4.70 (s, 1H), 4.52 (s, 1H), 4.29 (t, 2H, J = 5.5 Hz), 3.05 (t, 2H, J = 5.5 and 5.5 Hz); MS: [m/z, 517; M + 2, 519].
N'-(5-fluoro-2-oxoindolin-3-ylidene)-2-((4-oxo-3-phenethyl-3,4-dihydroquinazolin-2-yl)thio)acetohydrazide (34)
Yield: 83%; mp: 257–258 oC; IR (KBr, cm−1) ν: 3442, 3267 (2NH), 1719, 1683, 1639 (3C=O); 1H-NMR (500 MHz, DMSO-d6): δ 11.61 (s, 0.5H), 11.30 (s, 0.5H), 10.85 (s, 0.5H), 8.19 (d, 0.5H, J = 8.5 Hz), 8.05 (d, 1H, J = 8.0 Hz), 7.70 (t, 1H, J = 7.0 and 7.5 Hz), 7.50–7.21 (m, 9H), 6.91 (dd, 1H, J = 4.0 and 4.5 Hz), 4.73 (s, 1H), 4.65 (s, 1H), 4.28 (dd, 2H, J = 4.0 Hz), 3.05 (t, 2H, J = 7.5 Hz); MS: [m/z, 501].
Biology
WST-1 cell proliferation assay
The cell proliferation assay was conducted according to a previously reported method32.
Immunofluorescence microscopy
The EGFR immunofluorescence assay was conducted according to a previously reported method33.
Apoptosis assay
Vybrant apoptosis assay kit (Annexin-V, APC conjugate; Molecular Probes™) was used to evaluate cell viability in accordance with the manufacturer’s recommendation33.
Docking methodology
All modeling experiments were conducted with MOE programs running on a PC34. Hydrogen bonds with a bond length of up to 3.5 Å were considered. The starting coordinates of the X-ray crystal structure of the EGFR enzyme in complex with erlotinib (PDB code: 1M17) were obtained from the RCSB Protein Data Bank of Brookhaven National Laboratory35. All hydrogens were added and the enzyme structure was subjected to a refinement protocol in which the constraints on the enzyme were gradually removed and minimized until the RMS gradient was 0.01 kcal/mol Å. The energy minimization was conducted using the AMBER molecular mechanics force field. The lowest energy conformer, the “global-minima,” was pre-positioned using the crystal structure ligand “erlotinib” as a template at the enzyme-binding pocket.
Results and discussion
Chemistry
2-Mercapto-3-substituted-4(3H)-quinazolinones (1–5) were prepared by heating anthranilic acid derivatives with an appropriate isothiocyanate in ethanol containing a catalytic amount of triethylamine. Accordingly, 2-[(3-substituted-4(3H)-quinazolinon-2-yl)thio]acetohydrazides (11–15) were obtained by stirring compounds 1–5 with ethyl 2-bromoacetate in acetone to yield the corresponding ethyl 2-[(3-substituted-4(3H)-quinazolinon-2-yl)thio]acetates (6–10), which were then stirred with hydrazine hydrate in ethanol11,19,22 (Scheme 1).
Scheme 1.
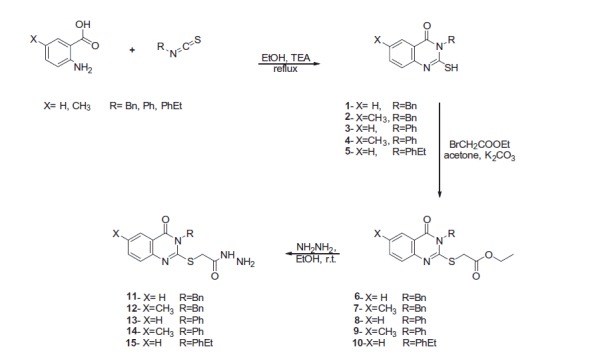
Synthesis of 2-[(3-substituted-4(3H)-quinazolinon-2-yl)thio]acetohydrazides 11–15.
The 2-[(3-substituted-4-quinazolinon-2-yl)thio]-N'-(2-oxoindolin-3-ylidene)acetohydrazides (16–34) were produced at 80–85% yield by heating an appropriate 2-[(3-substituted-4(3H)-quinazolinon-2-yl)thio]acetohydrazide (11–15) and isatin derivative in methanol containing a catalytic amount of acetic acid26 (Scheme 2).
Scheme 2.

Synthesis of quinazoline-isatin conjugates 16–34.
1H-NMR of compounds 16–34 revealed singlet signals corresponding to the two NH groups at 13.48–10.85 and 11.94–8.13 ppm, in addition to presence of signals for SCH2CO at 4.79–4.05 ppm as a mixture of the E/Z isomers. Additionally, the IR spectra of compounds 16–34 showed new bands at 3467–3410 cm–1 and 3298–3133 cm–1, which corresponded to the NH group of amides, and 1793–1713 cm–1 and 1676–1725 cm–1, owing to the presence of two C=O groups in addition to the C=O of the 4-quinazolinone nucleus at 1698–1636 cm–1.
Biological activity
Cell proliferation inhibition assay
The in vitro antitumor activity of compounds 16–34 against the human breast cancer cell line, MDA-MB-231, and the colon cancer cell line, LOVO, was determined by WST-1 assay32 using 5-FU and erlotinib as a reference drugs, and IC50 was calculated for each cell line (Table 1). In the present study, the active compounds exhibited a characteristic selectivity potential in addition to broad-spectrum antitumor activity.
Table 1.
In vitro antitumor activity of the newly synthesized compounds 16–34.
| Compounds | MDA-MB-231a IC50 (μM)c | LOVOb IC50 (μM)c |
|---|---|---|
| 16 | 16.23 ± 0.32 | 33.97 ± 0.26 |
| 17 | 14.97 ± 0.37 | 23.98 ± 0.06 |
| 18 | 12.38 ± 0.3 | 21.46 ± 0.13 |
| 19 | 12.31 ± 0.11 | 17.53 ± 0.04 |
| 20 | 16.82 ± 0.13 | 14.80 ± 0.1 |
| 21 | 14.48 ± 0.03 | 14.21 ± 0.06 |
| 22 | 18.33 ± 0.01 | 14.14 ± 0.06 |
| 23 | 17.14 ± 0.01 | 13.39 ± 0.23 |
| 24 | 11.50 ± 0.36 | 20.39 ± 0.02 |
| 25 | 11.41 ± 0.07 | 12.00 ± 0.05 |
| 26 | 11.80 ± 0.02 | 23.62 ± 0.01 |
| 27 | 18.05 ± 0.04 | 12.80 ± 0.03 |
| 28 | 37.41 ± 0.06 | 14.20 ± 0.09 |
| 29 | 38.67 ± 0.04 | 14.00 ± 1.02 |
| 30 | 13.77 ± 0.4 | 14.12 ± 0.06 |
| 31 | 10.38 ± 0.22 | 9.91 ± 0.12 |
| 32 | 18.35 ± 0.14 | 16.51 ± 0.15 |
| 33 | 20.21 ± 0.05 | 14.37 ± 0.46 |
| 34 | 20.06 ± 0.11 | 15.77 ± 0.16 |
| 5-FU | 70.28 ± 0.2 | 15.23 ± 0.09 |
| Erlotinib | 22.24 ± 0.22 | 25.31 ± 0.12 |
Aggressive human MDA-MB-231 (representative triple negative breast cancer cells with high metastasis potential).
Aggressive human LOVO colon cell line (type IV metastasized colon cancer).
IC50: concentration of the compound (μM) that produced 50% inhibition of cell growth inhibition after 48 h of treatment.
For the selectivity against the MDA-MB-231 cell line, compounds 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 30, 31, 32, 33, and 34 showed high activity (IC50: 10.38–20.21 μM); the comparative IC50 values for 5-FU and erlotinib were 70.28 and 22.24 μM respectively. On the other hand, compounds 28 and 29 (IC50: 37.41 and 38.67 μM); were less active than erlotinib but more active than 5-FU.
Moreover, the LOVO cell line was sensitive toward compounds 19, 20, 21, 22, 23, 25, 27, 28, 29, 30, 31, 32, 33, and 34 (IC50: 9.91–17.53 μM); the comparative IC50 value for 5-FU and erlotinib were 15.23 and 25.31 μM respectively. Compounds 17, 18, 24, and 26 were less active than 5-FU with IC50 values of 20.39–23.98 μM but more active than erlotinib.
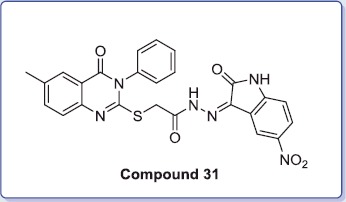
With regards to broad-spectrum antitumor activity, compounds 20, 21, 22, 23, 25, 27, 30, 31, 32, 33, and 34 showed strong antitumor activities against MDA-MB-231 cells and LOVO cells, which was supported by the IC50 values (10.38–20.21 μM and 9.91–15.77 μM, respectively). Moreover, compound 31 showed the highest potency toward MDA-MB-231 cells and LOVO cells with IC50 values of 10.38 and 9.91 μM, respectively.
GFR tyrosine kinase enzyme inhibition assay
The enzyme activity assay of the most active compound 31 toward the MDA-MB-231 breast cancer cell line was selected as representative example of the compounds and administered at a single concentration (10 μM) against EGFR-TK to investigate the mechanism of action of the newly synthesized compounds33. The immunofluorescence staining of EGFR in MDA-MB-231 cells treated with compound 31 at 10 μM indicated a good selectivity of compound 31 to EGFR-TK, as shown by inhibition of the level of EGFR on the cell membrane as well as in the nucleus (Figure 2).
Figure 2.
EGFR (left panel; green color) of MDA-MB-231 breast cell line and (right panel) MDA-MB-231 breast cell line after treatment with compound 31.
Apoptosis detection by flow cytometry
The effect of compound 31 on the apoptosis was investigated using DAPI (4,6-diamidino-2-phenylindole) and annexin V-FITC biparametric cytofluorimetric analysis32. After treatment with compound 31 (10 μM for 24 h), the MDA-MB-231 breast cancer cells were stained with DAPI and annexin V, and analyzed by flow cytometry (Figure 3). Compound 31 was able to induce apoptosis in MDA-MB-231 cells. Compound 31 induced apoptosis by a 30-fold increase in the percentage of fluorescein isothiocyanate annexin V (Annexin V-FITC)-positive apoptotic cells (right panel) in comparison with untreated cells (left panel). Compound 31 increased the percentage of apoptotic cells by 5.6% and late apoptotic cells by 61.4% compared with 1.3% and 2.6% in untreated control cells, respectively. Moreover, the tested compound induced necrosis in treated cells by 8.3% compared with 0.2% in untreated control cells.
Figure 3.
MDA-MB-231 breast cancer cell line was treated with compound 31 (right panel), which displayed an increased percentage of fluorescein isothiocyanate annexin V (Annexin V–FITC), and untreated control cells (left panel).
Structure–activity relationships
The structure–activity relationships of the tested compounds revealed that 5-methyl-3-benzyl derivatives 20–23 (IC50: 14.48–18.33 μM and 13.39–14.80 μM) and 5-methyl-3-phenyl derivatives 28–31 (IC50: 10.38–38.67 μM and 9.91–14.20 μM) showed significant inhibition of MDA-MB-231 cells and LOVO cells, compared with 5-FU (IC50: 70.28 μM and 15.23 μM), respectively (Table 1).
Moreover, unsubstituted 3-benzyl derivatives 16–19 (IC50: 12.31–16.23 μM and 17.53–33.97 μM), 3-unsubstituted phenyl derivatives 24–27 (IC50: 11.41–18.05 and 12.0–23.62 μM) and unsubstituted 3-phenethyl derivatives 32–34 (IC50: 18.35–20.21 and 14.37–17.87 μM) were more selective for MDA-MB-231 cells than LOVO colon cells, compared with 5-FU (IC50: 70.28 μM and 15.23 μM), respectively (Table 1).
In MDA-MB-231 cells, the unsubstituted 3-benzyl derivatives 16–19 (IC50: 12.31–16.23 μM) and unsubstituted 3-phenyl derivatives 24–27 (IC50: 11.41–18.05 μM) were more active than the 5-methyl-3-benzyl derivatives 20–23 (IC50: 14.48–18.33 μM) and 5-methyl-3-phenyl derivatives 28–31 (IC50: 10.38–38.67 μM) respectively. In the LOVO cells, the 5-methyl-3-benzyl derivatives 20–23 (IC50: 13.39–14.80 μM) and 5-methyl-3-phenyl derivatives 28–31 (IC50: 9.91–14.2 μM) were more active than the unsubstituted 3-benzyl derivatives 16–19 (IC50: 17.53–33.97 μM) and unsubstituted 3-phenyl derivatives 24–27 (IC50: 12.0–23.62 μM), respectively (Table 1).
Molecular docking results
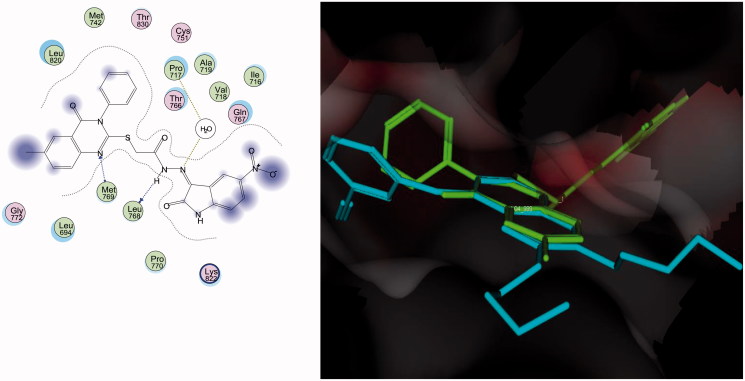
The antitumor activities of the weakly active compound 28 and the highly active compound 31 in MDA-MB-23 cells, which highly express epidermal growth factor receptor (EGFR)7,10,11,15,19,22 and the binding activity of compound 31 with EGFR, encouraged us to conduct molecular docking simulations of the binding site of the EGFR kinase.
Compounds 28 and 31 were docked into the receptor active site of EGFR along with their inhibitor erlotinib (Tarceva™) (PDB code: 1M17)35. All calculations were performed using MOE 2008.10 software34. The docking study of the most active compound 31 revealed that the quinazoline ring typically overlaid the corresponding ring of erlotinib without clashing with the surrounding amino acids. The substituted linkage at the C-2 hybrid of the binding of compound 31 in both the activation and catalytic loops where N1 was uniquely bound with the distinctive residue Met769. A semicarbazide nitrogen atoms was recognized via hydrogen bonding with Leu768, while the second semicarbazide nitrogen atom performed hydrophilic interaction by cross interaction with Pro717 through the water molecule in the pocket. The two adjacent conserved amino acids Leu768 and Met769 firmly held the backbone of compound 31, which augmented the recognition and the overall inhibition activity (Figure 4).
Figure 4.
Docking of compound 31 (left panel) and superposition with erlotinib (right panel) in the receptor pocket of EGFR kinase. Compound 31 and erlotinib are shown in green and cyan, respectively.
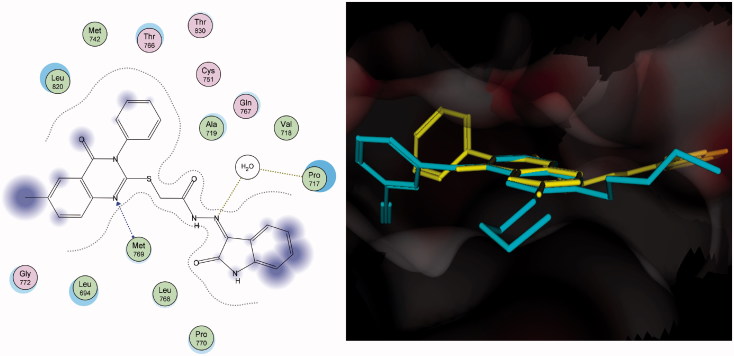
In contrast, compound 28 was bound in different manner, which dramatically lowered the overall complementarity. Although N1 was clearly recognized with hydrogen bonding to the distinctive residue Met769, N3 was buried away from the surrounding amino acids owing to the rigidity of the connected phenyl group. However, the semicarbazide linkage enriched the hydrophilic interaction by cross interaction with Pro717 through the water molecule in the pocket (Figure 5).
Figure 5.
Docking of compound 28 (left panel) and superposition with erlotinib (right panel) in the receptor pocket of EGFR kinase. Compound 28 and erlotinib are shown in yellow and cyan, respectively.
Conclusions
A new series of quinazolinone-isatin conjugates 16–34, which strongly inhibited growth in the MDA-MB-231 breast cancer cell line and LOVO colon cancer cell line, was synthesized. Compounds 16–34 showed high activity against the human MDA-MB-231 breast cell line (IC50: 10.38–38.67 μM) in comparison with 5-FU and erlotinib (IC50: 70.28 μM and 22.24 μM, respectively). Similarly, compounds 19–23, 25, and 27–34 possessed strong activity against the LOVO colon cancer cell line (IC50: 9.91–17.87 μM) in comparison with 5-FU and erlotinib (IC50: 15.23 μM and 25.31 μM, respectively). Compounds 20–23, 25, and 27–34 showed potent antitumor activity against the MDA-MB-231 and LOVO cell lines (IC50: 10.38–38.67 μM and 9.91–15.77 μM, respectively. Compound 31 inhibited the level of EGFR-TK in the cell membrane, as well as in the nucleus, of MDA-MB-231 cells as a representative example of quinazolinone–isatin conjugates at a single concentration (10 μM). Compound 31 increased the number of apoptotic cells by 5.6% and late apoptotic cells by 61.4% compared with 1.3 and 2.6%, respectively, in untreated control cells. Additionally, compound 31 induced necrosis in treated cells by 8.3% compared with 0.2% in untreated control cells. A molecular docking simulation was performed for compounds 31 and 28 into the binding site of EGFR kinase, which showed a similar binding mode to erlotinib. The results of molecular docking can help in the design of new molecules with potential antitumor activity and good binding to the enzyme receptor site.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-1435-046.
References
- 1.Senwar KR, Reddy TS, Thummuri D, et al. Design, synthesis and apoptosis inducing effect of novel (Z)-3-(3′-methoxy-4′-(2-amino-2-oxoethoxy)-benzylidene) indolin-2-ones as potential antitumour agents. Eur J Med Chem 2016;118:34–46. [DOI] [PubMed] [Google Scholar]
- 2.Alaa A-M, Abou-Zeid LA, ElTahir KEH, et al. Design, synthesis of 2, 3-disubstitued 4 (3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents: COX-1/2 inhibitory activities and molecular docking studies. Bioorg Med Chem 2016;24:3818–28. [DOI] [PubMed] [Google Scholar]
- 3.Alaa A-M, El-Azab AS, Abou-Zeid LA, et al. Synthesis, anti-inflammatory, analgesic and COX-1/2 inhibition activities of anilides based on 5, 5-diphenylimidazolidine-2, 4-dione scaffold: molecular docking studies. Eur J Med Chem 2016;115:121–31. [DOI] [PubMed] [Google Scholar]
- 4.Alafeefy AM, El-Azab AS, Mohamed MA, et al. Synthesis of some new substituted iodoquinazoline derivatives and their antimicrobial screening. J Saudi Chem Soc 2011;15:319–25. [Google Scholar]
- 5.Alanazi AM, Alaa A-M, Al-Suwaidan IA, et al. Design, synthesis and biological evaluation of some novel substituted quinazolines as antitumor agents. Eur J Med Chem 2014;79:446–54. [DOI] [PubMed] [Google Scholar]
- 6.Alanazi AM, Al-Suwaidan IA, Alaa A-M, et al. Design, synthesis and biological evaluation of some novel substituted 2-mercapto-3-phenethylquinazolines as antitumor agents. Med Chem Res 2013;22:5566–77. [Google Scholar]
- 7.Al-Obaid A, Abdel-Hamide S, El-Kashef H, et al. Synthesis, in vitro antitumor activity and molecular modeling study of certain 2-thieno-4 (3H)-quinazolinone analogs. Eur J Med Chem 2009;44:2379–91. [DOI] [PubMed] [Google Scholar]
- 8.Al-Omar M, El-Azab A, El-Obeid S, Hamide HA.. Sythesis of some new 4-(3H)-quinazoline analogs as potential antioxidant agents. Saudi J Chem Soc 2006;10:113–28. [Google Scholar]
- 9.Al-Omary FA, Abou-Zeid LA, Nagi MN, et al. Non-classical antifolates. Part 2: synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg Med Chem 2010;18:2849–63. [DOI] [PubMed] [Google Scholar]
- 10.Al-Suwaidan IA, Abdel-Aziz AA-M, Shawer TZ, et al. Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4 (3H) quinazolinone analogues. J Enzyme Inhib Med Chem 2016;31:78–89. [DOI] [PubMed] [Google Scholar]
- 11.Al-Suwaidan IA, Alanazi AM, Alaa A-M, et al. Design, synthesis and biological evaluation of 2-mercapto-3-phenethylquinazoline bearing anilide fragments as potential antitumor agents: molecular docking study. Bioorg Med Chem Lett 2013;23:3935–41. [DOI] [PubMed] [Google Scholar]
- 12.AzizaNassar M, AbdelHamide M, ElHakim S, El-Azab AA.. Synthesis and antimicrobial activities of some new 3-heteroaryl-quinazolin-4-ones. Indian J Heterocycl Chem 1996;6:25–30. [Google Scholar]
- 13.El-Azab AS.Synthesis of some new substituted 2-mercaptoquinazoline analogs as potential antimicrobial agents. Phosphorus Sulfur Silicon Relat Elem 2007;182:333–48. [Google Scholar]
- 14.El-Azab AS, Abdel-Hamide SG, Sayed-Ahmed MM, et al. Novel 4 (3H)-quinazolinone analogs: synthesis and anticonvulsant activity. Med Chem Res 2013;22:2815–27. [Google Scholar]
- 15.El-Azab AS, Al-Omar MA, Alaa A-M, et al. Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur J Med Chem 2010;45:4188–98. [DOI] [PubMed] [Google Scholar]
- 16.El-Azab AS, ElTahir KE.. Synthesis and anticonvulsant evaluation of some new 2,3,8-trisubstituted-4 (3H)-quinazoline derivatives. Bioorg Med Chem Lett 2012;22:327–33. [DOI] [PubMed] [Google Scholar]
- 17.El-Azab AS, ElTahir KE.. Design and synthesis of novel 7-aminoquinazoline derivatives: antitumor and anticonvulsant activities. Bioorg Med Chem Lett 2012;22:1879–85. [DOI] [PubMed] [Google Scholar]
- 18.El-Azab AS, ElTahir KE, Attia SM.. Synthesis and anticonvulsant evaluation of some novel 4 (3H)-quinazolinones. Chem Monthly 2011;142:837–48. [Google Scholar]
- 19.Mohamed MA, Ayyad RR, Shawer TZ, et al. Synthesis and antitumor evaluation of trimethoxyanilides based on 4 (3H)-quinazolinone scaffolds. Eur J Med Chem 2016;112:106–13. [DOI] [PubMed] [Google Scholar]
- 20.Alafeefy AM, Kadi AA, El‐Azab AS, et al. Synthesis, analgesic and anti‐inflammatory evaluation of some new 3H‐quinazolin‐4‐one derivatives. Archiv Der Pharmazie 2008;341:377–85. [DOI] [PubMed] [Google Scholar]
- 21.Alaa A-M, Abou-Zeid LA, ElTahir KEH, et al. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4 (3H)-quinazolinones. Eur J Med Chem 2016;121:410–21. [DOI] [PubMed] [Google Scholar]
- 22.Alanazi AM, Abdel-Aziz AA, Shawer TZ, et al. Synthesis, antitumor and antimicrobial activity of some new 6-methyl-3-phenyl-4(3H)-quinazolinone analogues: in silico studies. J Enzyme Inhib Med Chem 2016;31:721–35. [DOI] [PubMed] [Google Scholar]
- 23.Barlési F, Tchouhadjian C, Doddoli C, et al. Gefitinib (ZD1839, Iressa®) in non‐small‐cell lung cancer: a review of clinical trials from a daily practice perspective. Fundam Clin Pharmacol 2005;19:385–93. [DOI] [PubMed] [Google Scholar]
- 24.Barker AJ, Gibson K, Grundy HW, et al. Studies leading to the identification of ZD1839 (Iressa™): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett 2001;11:1911–14. [DOI] [PubMed] [Google Scholar]
- 25.Ganjoo KN, Wakelee H.. Review of erlotinib in the treatment of advanced non-small cell lung cancer. Biologics 2007;1:335–46. [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim HS, Abou-Seri SM, Tanc M, et al. Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumor-associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 2015;103:583–93. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:16–24. [DOI] [PubMed] [Google Scholar]
- 28.Prenen H, Cools J, Mentens N, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res 2006;12:2622–7. [DOI] [PubMed] [Google Scholar]
- 29.Teng Y-O, Zhao H-Y, Wang J, et al. Synthesis and anti-cancer activity evaluation of 5-(2-carboxyethenyl)-isatin derivatives. Eur J Med Chem 2016;112:145–56. [DOI] [PubMed] [Google Scholar]
- 30.Koenig M, Cui J, Wei C, et al. Indolinone hydrazides as c-Met inhibitors. Google Patents, 2006. [Google Scholar]
- 31.Al-Suwaidan IA, Alanazi AM, El-Azab AS, et al. Molecular design, synthesis and biological evaluation of cyclic imides bearing benzenesulfonamide fragment as potential COX-2 inhibitors. Part 2. Bioorg Med Chem Lett 2013;23:2601–5. [DOI] [PubMed] [Google Scholar]
- 32.Radwan AA, Al-Mohanna F, Alanazi FK, et al. Target β-catenin/CD44/Nanog axis in colon cancer cells by certain N′-(2-oxoindolin-3-ylidene)-2-(benzyloxy) benzohydrazides. Bioorg Med Chem Lett 2016;26:1664–70. [DOI] [PubMed] [Google Scholar]
- 33.Naglah AM, Shinwari Z, Bhat MA, et al. Targeting leukemic side population cells by isatin derivatives of nicotinic acid amide. J Biol Regul Homeost Agents 2016;30:353. [PubMed] [Google Scholar]
- 34.Chemical Computing Group. MOE: molecular operating environment. 2008. 10 of Chemical Computing Group. Inc. Available from: http://www.chemcomp.com/press_releases/2008-11-04.htm [Google Scholar]
- 35.Stamos J, Sliwkowski MX, Eigenbrot C.. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002;277:46265–72. [DOI] [PubMed] [Google Scholar]