Abstract
Intestinal transplantation in children has evolved with more isolated small intestine transplants being performed compared to combined liver-intestine transplants. Consequently, surgical techniques have changed, frequently requiring the use of vascular homografts of small caliber to revascularize the isolated small intestine, the impact of which on outcomes is unknown. Among 106 pediatric intestine and multivisceral transplants performed at our center since 2003, 33 recipients of an isolated small intestine graft were included in this study. Outcome parameters were thrombotic complications, graft, and patient survival. A total of 29 of 33 (87.9%) patients required arterial and/or venous homografts from the same donor, mainly iliac or carotid artery and iliac or innominate vein, respectively (donor’s median age 1.1 years [2 months to 23 years], median weight 10 kg [14.7–48.5]). Post-transplant, there were three acute arterial homograft thromboses and one venous thrombosis resulting in two peri-operative graft salvages and two graft losses. Three of four thromboses occurred in patients with primary hypercoagulable state, including the two graft losses. Overall, at a median of 4.1 years (1–10.2) from transplant, 29 of 33 (88%) patients are alive with 26 of 33 (79%) functioning grafts. The procurement of intact, size-matched donor vessels and the management of effective post-transplant anticoagulation are critical.
Keywords: complications, conduit, pediatric intestine transplantation, thrombosis
1 |. INTRODUCTION
Current worldwide registry data show that at least 2887 intestinal transplants have been performed since 1985,1 and approximately half of intestinal transplant recipients were performed in children, where pediatric (age <18 years) intestinal transplant contributed 63.6% in 2002 and 52.8% in 2012.2 Traditionally, children with intestinal failure, especially small children, were transplanted using a combined liver-intestine graft due to the commonly associated liver failure as a consequence of late referral,3 and the surgical technique for the implantation of the combined liver-intestine graft has been described elsewhere.4 Over the last decade, the incidence and severity of liver failure in children with intestinal failure have changed since the introduction of omega-3 fatty acid-based parenteral nutrition (Omegaven, Fresenius Kabi, Bad Homburg vdh, Germany).5,6 As a result, an increasing number of children maintain the function of the native liver while on TPN, therefore are being transplanted using an isolated small intestinal graft instead of a combined liver-intestine graft.7 This switch of grafts requires technical modifications in the procurement and in the implantation of the graft. Specifically, the isolated small intestine graft is procured with a pedicle containing the SMA and vein, unlike the combined liver-intestine, which is procured en bloc with the aorta. The mesenteric vessels are of small caliber in children (approximately 3–5 mm in typical 2-year-old donor), increasing the risk of thrombosis.
The key surgical aspect of isolated intestinal transplantation is the revascularization of the two graft great vessels: SMA and SMV. The technique of revascularization of the isolated small intestine graft depends, in part, on the status of the native mesenteric vessels. Many patients lack native SMA and SMV due to previous thrombosis or trauma or other reasons. In this case, the graft is revascularized via aortic inflow and vena cava outflow also known as via central drainage (Figure 1). In cases of patent native mesenteric vessels, mesenteric revascularization via the native SMA and SMV can be performed also known as via mesenteric drainage (Figure 2). In both instances, use of vascular conduits is often required to allow enough length of the vascular pedicle of the graft thus avoiding twisting and torsion and therefore reducing the risk of vascular complications. The most feared vascular complication is thrombosis because of the risk of graft loss. Usually, vascular conduits are procured from the same donor and consist of iliac or supra-aortic vessels. In children, the size and length of these vascular conduits, as well as the size of the native mesenteric vessels, are small, increasing the risk of complications. In addition, several recipients have a procoagulant state that further increases the risk of thrombosis. The impact of this change in types of grafts on complication rates and graft survival is unknown.
FIGURE 1.
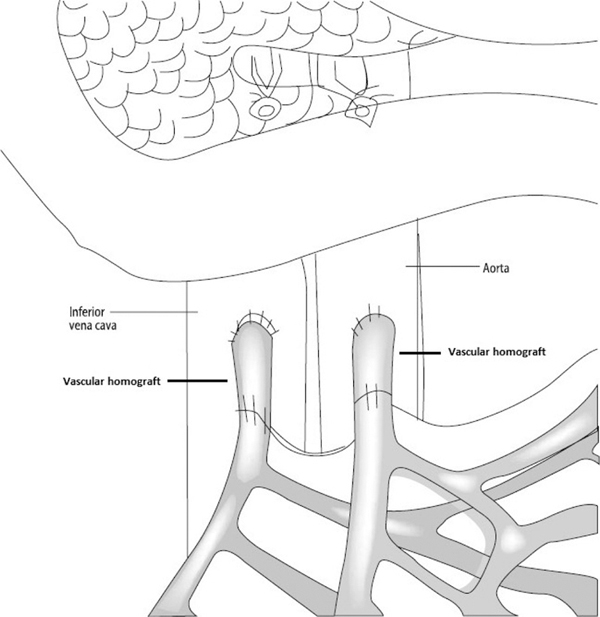
Central drainage
FIGURE 2.
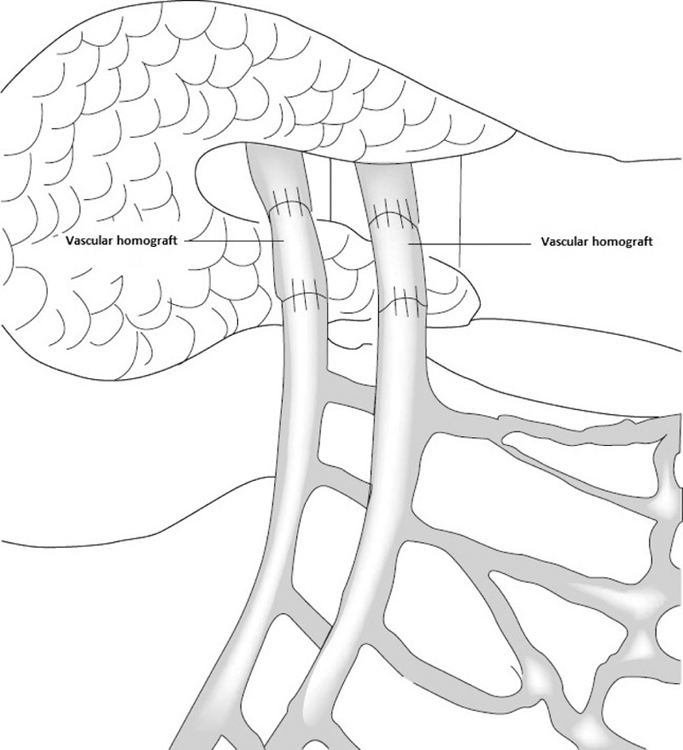
Mesenteric drainage
To determine thrombotic complications and graft survival in isolated small intestinal transplants in children, we reviewed our single-center experience. We evaluated the types of interposition homografts used and its effect on the outcome. In addition, we compared the two methods of revascularization (central vs mesenteric) to determine whether there were any differences on outcome between the two types of revascularization.
2 |. PATIENTS AND METHODS
This was a single-center retrospective review study. Our institution’s ethical committee approved the study, and informed consent was obtained from the patients, parents, or legal guardians of the participants of the study.
Among 106 pediatric intestine and multivisceral transplants performed at our center since 2003, 39 recipients underwent isolated small intestine transplant. Four patients transplanted between 2003 and 2005 with insufficient medical records, and two patients recently transplanted with follow-up <1 year were excluded. The remaining 33 patients (16 M, 17 F, median age 3.3 years [range: 1.2–17.2], median weight 14.2 kg [8.1–56]), which included four retransplants, were included in this study (Table 1).
TABLE 1.
Recipient demographics
| N | Age (years old) | Gender (M/F) | Weight (kg) | Donor age (years old) | Donor weight (kg) | Indications for transplant | Types of transplant (SB/SB + C) |
|---|---|---|---|---|---|---|---|
| 1 | 4.4 | F | 8.3 | 1.1 | 11.8 | Chronic rejection | SB |
| 2 | 9.4 | F | 30.0 | 9.0 | 41.0 | Hirschsprung’s disease | SB + C |
| 3 | 17.2 | M | 45.0 | 8.0 | 33.3 | Cystic fibrosis | SB + C |
| 4 | 3.0 | F | 11.6 | 0.6 | 8.1 | Gastroschisis | SB |
| 5 | 4.8 | M | 20.0 | 5.0 | 17.1 | Pseudo-obstruction | SB |
| 6 | 5.3 | M | 12.0 | 1.3 | 10.0 | Atresia | SB + C |
| 7 | 6.2 | F | 20.0 | 6.0 | 19.1 | Pseudo-obstruction | SB + C |
| 8 | 1.2 | F | 8.1 | 0.5 | 8.2 | Sclerosing mesenteritis | SB |
| 9 | 3.7 | M | 15.0 | 2.0 | 13.0 | Volvulus | SB |
| 10 | 1.2 | F | 11.0 | 3.0 | 12.0 | Hirschsprung’s disease | SB + C |
| 11 | 16.4 | F | 56.0 | 23.0 | 48.5 | Neurofibromatosis | SB |
| 12 | 3.2 | F | 15.1 | 0.8 | 11.0 | Microvillus inclusion disease | SB + C |
| 13 | 10.5 | F | 24.0 | 0.8 | 10.0 | Tufting enteropathy | SB + C |
| 14 | 15.2 | F | 3.0 | 6.0 | 24.8 | Volvulus | SB + C |
| 15 | 1.6 | F | 11.6 | 1.0 | 6.8 | Necrotizing enterocolitis | SB + C |
| 16 | 2.7 | M | 14.0 | 0.8 | 6.1 | Necrotizing enterocolitis | SB + C |
| 17 | 2.3 | F | 11 | 0.3 | 8.9 | Tufting enteropathy | SB + C |
| 18 | 12.1 | F | 31.9 | 6.0 | 29.0 | Chronic rejection | SB |
| 19 | 3.3 | M | 17.8 | 0.5 | 7.9 | Volvulus | SB |
| 20 | 1.5 | M | 10.1 | 0.2 | 5.0 | Gastroschisis | SB |
| 21 | 2.4 | M | 10.5 | 0.2 | 5.1 | Hirschsprung’s disease | SB + C |
| 22 | 1.7 | M | 13.2 | 2.0 | 9.3 | Atresia | SB |
| 23 | 3.4 | M | 12.1 | 2.0 | 9.5 | Gastroschisis | SB |
| 24 | 4.7 | M | 17 | 1.0 | 10.0 | Atresia | SB |
| 25 | 1.3 | M | 10.4 | 0.3 | 7.6 | Volvulus | SB |
| 26 | 1.2 | M | 8.9 | 0.5 | 6.3 | Volvulus | SB |
| 27 | 1.7 | F | 8.1 | 0.5 | 8.5 | Gastroschisis | SB |
| 28 | 6.9 | M | 19.9 | 3.0 | 16.6 | Necrotizing enterocolitis | SB + C |
| 29 | 2.1 | M | 15.1 | 1.0 | 8.7 | Necrotizing enterocolitis | SB + C |
| 30 | 3.3 | M | 14.4 | 3.0 | 14.0 | Necrotizing enterocolitis | SB |
| 31 | 4.8 | F | 14.2 | 1.5 | 10.7 | Tufting enteropathy | SB + C |
| 32 | 2.4 | F | 10.8 | 0.8 | 9.5 | Atresia | SB + C |
| 33 | 13.8 | F | 44.9 | 9.0 | 39.0 | Chronic rejection | SB + C |
SB, small bowel; CB + C, small bowel and colon.
After completion of the pretransplant diagnostic work-up,8 recipient candidacy was determined in a multidisciplinary approach and discussed at the patient selection committee before being added to the waitlist. Donors were selected according to blood-type compatibility and size match. The procurement technique and the implantation techniques have been described elsewhere.4 The vascular anastomoses were constructed using non-absorbable 6–0 or 7–0 Prolene running suture (Ethicon, Somerville, NJ, USA). Our decision to use vascular conduits and the proper length of these conduits was made at the time of the organ implantation at the discretion of the performing transplant surgeon with the aim to achieve tension-free anastomosis and at the same time to avoid redundancy to prevent potential kinking. Upon completion of the anastomosis, the graft’s root of the mesentery was tacked down to the recipient’s retroperitoneum to ensure no twisting and tension at the anastomosis. The post-transplant management was conducted according to our protocol.9 Briefly, at the time of transplant, all patients received induction immunosuppression with high-dose methylprednisolone bolus and either basiliximab or thymoglobulin, depending on the degree of sensitization. Maintenance immunosuppression consisted of tacrolimus (target through level of 20–25 ng/mL) and steroids. Sirolimus was added within the first month post-transplant, unless contraindicated. Patients with hypercoagulable state received systemic anticoagulation with intravenous heparin from the time of graft reperfusion, subsequently transitioned to subcutaneous low molecular weight heparin or warfarin. Grafts were monitored by clinical examination of the stoma, including bedside Doppler of the stoma and angiography when necessary. Outcome parameters were thrombotic complications, and graft and patient survival. Patients living in the region were discharged home, whereas patients from out-of-state were housed locally for the first 3 months or more until deemed stable to return home. After returning home, patients were followed up locally with periodic blood tests and monthly in our clinic for the first year. All patients were followed in our outpatient transplant clinic with surveillance endoscopy and mucosal biopsy thru the ileostomy constructed at the time of transplant (either end-ileostomy or loop ileostomy) twice a week during the first 6 weeks, weekly for the following 6 weeks, and then monthly thereafter.
3 |. RESULTS
As previously mentioned, the cohort of this study included 33 patients (16 M, 17 F, median age 3.3 years [range: 1.2–17.2], median weight 14.2 kg [8.1–56]), which included four retransplants (Table 1). The median age of the corresponding donors was 1.1 years old (range: 2 months to 23 years old), and the median weight was 10 kg (range: 14.7–48.5 kg; Table 1).
The grafts of 25 patients of 33 patients (76%) were reperfused “centrally” to recipient aorta and vena cava, and the remaining eight patients were reperfused via recipient’s native SMA and SMV (Table 2).
TABLE 2.
Revascularization methods and types of conduits used
| N | Types of revascularization (central/mesenteric) | Arterial interposition graft | Venous interposition graft |
|---|---|---|---|
| 1 | Mesenteric | - | - |
| 2 | Mesenteric | - | - |
| 3 | Central | Iliac artery | Iliac vein |
| 4 | Central | Iliac artery | Iliac vein |
| 5 | Mesenteric | Iliac artery | Iliac vein |
| 6 | Central | Iliac artery | Iliac vein |
| 7 | Mesenteric | Iliac artery | Iliac vein |
| 8 | Central | Carotid artery | Iliac vein |
| 9 | Central | Iliac artery | Iliac vein |
| 10 | Central | Iliac artery | Iliac vein |
| 11 | Mesenteric | Iliac artery | Iliac vein |
| 12 | Mesenteric | Iliac artery | - |
| 13 | Central | Iliac artery | IVC |
| 14 | Central | Iliac artery | Iliac vein |
| 15 | Central | Carotid artery | Jugular vein |
| 16 | Central | Carotid artery | Jugular vein |
| 17 | Central | Carotid artery | Innominate vein |
| 18 | Central | Iliac artery | Iliac vein |
| 19 | Central | Carotid artery | Innominate vein |
| 20 | Central | Composite grafta | Innominate vein |
| 21 | Central | Carotid artery | Iliac vein |
| 22 | Central | Iliac artery | IVC |
| 23 | Central | Carotid artery | IVC |
| 24 | Central | Carotid artery | Innominate vein |
| 25 | Central | Carotid artery | Innominate vein |
| 26 | Central | - | - |
| 27 | Central | Carotid artery | Jugular vein |
| 28 | Central | Iliac artery | Iliac vein |
| 29 | Central | Carotid artery | Jugular vein |
| 30 | Central | Carotid artery | Innominate vein |
| 31 | Mesenteric | Carotid artery | Iliac vein |
| 32 | Central | Carotid artery | Innominate vein |
| 33 | Mesenteric | - | - |
Combined carotid and iliac artery.
Twenty-nine of 33 patients (87.9%) required homografts from the same donor to perform the arterial anastomosis, and 28 of 33 patients (84.8%) required the homograft to perform venous anastomosis. For the arterial anastomosis, 14 patients used carotid arteries, 14 patients used iliac arteries, and one patient used combined carotid and iliac artery (due to lack of length). For the venous anastomosis, 14 patients used iliac vein, seven patients used innominate vein, four patients used jugular vein, and three patients used IVC.
Among the 25 patients who were reperfused centrally, almost all except one patient (96%) used arterial and venous homografts. Among the eight patients who were drained via recipient’s native mesenteric vessels, five patients (62.5%) required homograft for the arterial anastomosis, and only four patients required homograft for the venous anastomosis (50.0%).
Among the 33 patients, we had one intra-op graft loss (n21, Tables 1 and 2). After reperfusion, graft did not appear be adequately reperfused, and despite multiple anastomosis revisions and ruling out vessel thrombosis, the graft could not be salvaged likely due to microvascular circulatory events or ischemic reperfusion event and therefore discarded intra-operatively.
Three of the 33 patients were found to have arterial thrombosis on POD 0, 2, and 13 (n5, n17, and n28, respectively, on Tables 1 and 2). Two of these patients were drained centrally (n17 and n28), and one of these patients was drained via recipient’s mesenteric vessels (n5). The two centrally drained patients (n17 and n28) lose the graft due to the arterial thrombotic events, and they both had hypercoagulable disease.
One of the 33 patients was found to have venous thrombosis on POD 7 (n3 on Tables 1 and 2). This patient was drained centrally and also found to have also hypercoagulable disease. The graft was salvaged after venous thrombectomy.
In summary, we had one patient with intra-op graft loss and 4 patients with post-op thrombotic events where two of them lost the grafts. The two patients who lost the graft had hypercoagulable disease. Overall, at a median of 4.1 years (1–10.2) from transplant, 29 of 33 (88%) patients are alive with 26 of 33 (79%) functioning grafts.
4 |. DISCUSSION
Since 2007, the total number of intestinal transplants began to decline in children and as well as in adults.1 This trend is likely to continue for several reasons. With adaptation of omega-3 fatty acid-based parenteral nutrition (Omegaven; Fresenius Kabi, Bad Homburg vdh, Germany), more children are being managed on TPN without liver failure. With more aggressive use of bowel rehabilitation surgeries like STEP procedure, more patients are being weaned from TPN. In fact, the most recent 2015 OPTN/SRTR data report showed dramatic decline in number of pediatric intestinal transplant: 73 pediatric patients (<18) underwent intestinal transplant in 2003, and only 36 pediatric patients required intestinal transplant in 2013.10
As expected, the proportion of intestine transplants without liver has also increased over the past decade as more isolated intestinal transplants are being performed before irreversible liver failure occurs.11 Also, this is due to early referral to intestinal transplant center.12 According to the Intestinal Transplant Registry data, isolated intestinal transplant constitutes 37.5% between 1985 and 1995 and went up to 46.6% between 2001 and 2011.1 However, these data do not separate into pediatric vs adults patients. 2015 OPTN/SRTR data, which separated the data based on adults vs pediatric, showed that 45.2% (33/73) was isolated pediatric intestine transplants in 2003 vs only 27.8% (10/36) in 2013. These data are surprising as we expected that the isolated intestinal transplant proportion will be increased in 2013.
Our study analyzes the impact of using vascular homografts to revascularize the small intestine graft in children. The important finding of our study is that isolated intestinal transplant so far has been successful without compromising the graft survival compared to combined liver-intestine.
For isolated intestinal transplant procurements, SMA and SMV are often dissected and cut at the level of pancreas (Figures 1 and 2), and the key of successful isolated intestinal transplantation is sound vascular reanastomosis of these two great vessels. As mentioned previously, successful transplantation often times requires homograft conduits to avoid tension and/or torsion of the vascular pedicle and therefore reduce the risk of thrombosis. As our data showed, the central drainage type is more likely to require homografts to make up for the extra length to reach aorta and IVC, whereas in the case of the mesenteric drainage, it is often possible to do anastomosis without vascular conduit. Often times, the SMA and SMV and vascular conduits are of small caliber (3–5 mm), therefore increasing the technical complexity and the risk of complications.
In our series, only three of 33 (6%) pediatric isolated intestine grafts were lost due to thrombotic/intra-op complications of the vascular homograft and/or intestinal graft in patients with hypercoagulable state. Most of these isolated intestinal transplant required interposition grafts to prevent tension on the anastomosis. Although number of patients was small, our data showed that there is no significant difference in outcome between the mesenteric drainage and central drainage. Overall, at a median of 4.1 years (1–10.2) from transplant, 29 of 33 (88%) patients are alive with 26 of 33 (79%) functioning grafts.
As for the type of homograft that was used as interposition graft, frequently carotid artery, jugular and innominate veins were used rather than iliac vessels. The procurement of intact, size-matched donor vessels is critical. Our data showed that unusually procured vessels including carotid arteries, jugular, and innominate veins can be used as interposition grafts and do not affect outcome as long as there was good size match. The number of isolated pediatric intestinal transplant is likely to continue to decrease with advancement of TPN formula and bowel rehabilitation program and also early referral to intestinal transplant center. Our data showed that although number of patients was small, there is no significant difference in outcome between the mesenteric drainage and central drainage. Procurement of intact, size-matched donor vessels is very critical for successful isolated intestinal transplant.
Abbreviations:
- IVC
inferior vena cava
- OPTN
Organ Procurement and Transplantation Network
- POD
postoperative day
- SMA
superior mesenteric artery
- SMV
superior mesenteric vein
- SRTR
Scientific Registry of Transplant Recipients
- STEP
serial transverse enteroplasty
- TPN
total parenteral nutrition
REFERENCES
- 1.Grant D, Abu-Elmagd K, Mazariegos G, et al. Intestinal transplant registry report: global activity and trends. Am J Transplant 2015;15:210–219. [DOI] [PubMed] [Google Scholar]
- 2.Smith JM, Skeans MA, Horslen SP, et al. OPTN/SRTR 2012 annual data report: intestine. Am J Transplant 2014;14(Suppl 1):97–111. [DOI] [PubMed] [Google Scholar]
- 3.Vianna RM, Mangus RS, Tector AJ. Current status of small bowel and multivisceral transplantation. Adv Surg 2008;42:129–150. [DOI] [PubMed] [Google Scholar]
- 4.Fishbein T, Matsmoto C. Intestinal and multivisceral transplantation. In: Humar A, Sturdevant M, eds. Atlas of Organ Transplantation, 2nd edn. London, UK: Springer; 2015:339–348. [Google Scholar]
- 5.Fallon EM, Le HD, Puder M. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant 2010;15:334–340. [DOI] [PubMed] [Google Scholar]
- 6.Venick RS, Calkins K. The impact of intravenous fish oil emulsions on pediatric intestinal failure-associated liver disease. Curr Opin Organ Transplant 2011;16:306–311. [DOI] [PubMed] [Google Scholar]
- 7.Khan KM, Desai CS, Mete M, et al. Developing trends in the intestinal transplant waitlist. Am J Transplant 2014;14:2830–2837. [DOI] [PubMed] [Google Scholar]
- 8.Swerdlow DR, Trotter A, Girlanda R, Matsumoto C, Fenelly E. Computed tomography (CT) colonography with CT arteriography and venography for the workup of intestinal transplant candidates. Clin Transplant 2013;27:126–131. [DOI] [PubMed] [Google Scholar]
- 9.Hauser GJ, Kaufman SS, Matsumoto CS, Fishbein TM. Pediatric intestinal and multivisceral transplantation: a new challenge for the pediatric intensivist. Intensive Care Med 2008;34:1570–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JM, Skeans MA, Horslen SP, et al. OPTN/SRTR 2013 annual data report: intestine. Am J Transplant 2015;15(Suppl 2):1–16. [DOI] [PubMed] [Google Scholar]
- 11.Lopushinsky SR, Fowler RA, Kulkarni GS, Fecteau AH, Grant DR, Wales PW. The optimal timing of intestinal transplantation for children with intestinal failure: a markov analysis. Ann Surg 2007;246:1092–1099. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein TM, Matsumoto CS. Intestinal replacement therapy: timing and indications for referral of patients to an intestinal rehabilitation and transplant program. Gastroenterology 2006;130(2 Suppl 1):S147–S151. [DOI] [PubMed] [Google Scholar]


