Abstract
Isoproterenol is used widely for inducing heart failure in mice. Isoproterenol is a nonselective beta-adrenergic agonist. The acute model mimics stress-induced cardiomyopathy. The chronic model mimics advanced heart failure in humans. In this chapter, we describe a protocol that we used to induce heart failure in 100+ strains of inbred mice. Techniques on surgical pump implantation and echocardiography are described in detail. We also discuss the impact of drug dosage, duration, mortality, age, gender, and strain on cardiac remodeling responses. The success of model creation may be assessed by echocardiogram or molecular markers. This chapter may be relevant to those who are interested in using this heart failure model.
Keywords: Heart failure, Isoproterenol, Mouse model, Cardiac remodeling, Mouse strains, Echocardiography
1. Introduction
Heart failure is a condition caused by inadequate pumping function of the heart. Several mouse models are available for basic investigations and preclinical testing. Left anterior descending artery ligation, transaortic constriction, and isoproterenol (ISO) aim at different cardiac pathologies [1]. Investigators should choose a model that best recapitulates their pathology of interest.
Both acute and chronic models of ISO have been used to study cardiac injury (Fig. 1). The acute model mimics stress-induced cardiomyopathy (SIC). SIC is characterized by apical segment ventricular dysfunction with basal segment sparing. It occurs in the setting of stressful life events. Adrenergic overstimulation has been postulated to play important roles in its pathogenesis [2, 3]. Protocols using one or few bolus injections have been described to mimic SIC. In contrast, the chronic model uses an implanted minipump to release ISO continuously. This model mimics advanced heart failure where there is chronic adrenergic stimulation. A few representative protocols in literature are listed under Table 1.
Fig. 1.
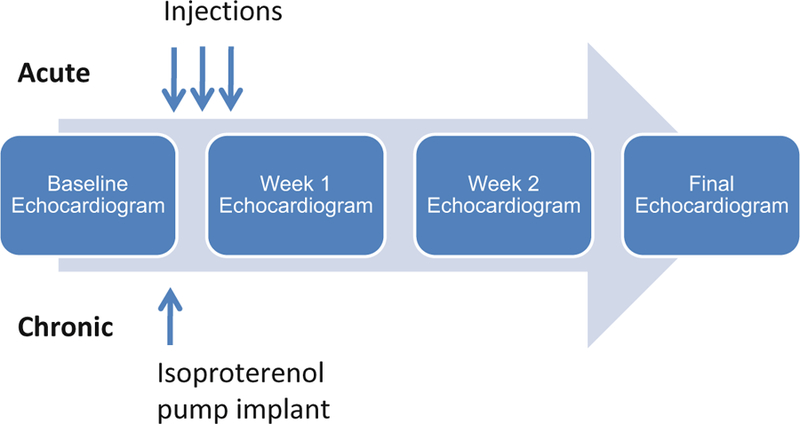
Acute and chronic isoproterenol models
Table 1.
Representative dosages, route of administration, and duration for chronic isoproterenol models of heart failure in mice
| Strain | Age | Gender (see Note 6) | Dosage (mg/kg/d) | Route | Duration |
|---|---|---|---|---|---|
| C57BL/6 [12] | 6–8 weeks | Male | 3 | s.c. daily | 2 weeks |
| 23 inbred mouse strains [13] | 10–12 weeks | Male | 10 | s.c. osmotic pump | 2 weeks |
| p110γ (unknown genetic background) [14] | 10–12 weeks | Male | 15 | Osmotic pump | 7 days |
| 100+ Hybrid Mouse Diversity Panel strains [8, 9] | 10–12 weeks | Female | 30 | i.p. osmotic pump | 3 weeks |
| C57BL/6 [15] | 8 weeks | Male | 40 | s.c. osmotic pump | 10 days |
| C57BL/6 [16] | 6–8 weeks | Female | 50 | s.c. daily | 7 days |
| BALB/c [17] | 8–10 weeks | Male | 60 | s.c. daily | 7 days |
| A/J C57BL/6J [18] | 12–15 weeks | Male | 100 | s.c. daily | 5 days |
| C57BL/6 [19] | 8 weeks | Male | 150 | s.c. daily | 7 days |
| AS-Ren mice [20] | 3–5 months | NA | 600 | s.c. BID | Two Consecutive days |
Success for ISO model creation can be assessed in vivo by serial echocardiography demonstrating hypertrophy, dilation, and ventricular dysfunction. Histological findings include intramyocardial lipid accumulation and mild fibrosis [4]. Molecular signature from the collected heart tissue samples can be performed ex vivo. ISO causes cardiomyocyte ER stress, apoptosis, and a downregulation of beta-adrenergic receptors and adenyl cyclase activities [5–7].
In our previously published study performed across 105 Hybrid Mouse Diversity Panel (HMDP) strains, we used a dosage of 30 mg/kg/d over 21 days via the osmotic pump implanted intraperitoneally [8, 9]. We observed striking interstrain variation in terms of response to interventricular wall and left ventricular mass hypertrophy, dilation, and ejection fraction (see Note 1). After 3 weeks of treatment, cardiac gene expression demonstrated significant changes between treatment and control groups across the HMDP [9]. The differentially expressed genes spanned cellular processes from extracellular matrix to inflammatory responses. These processes gave a glimpse of heart failure mechanisms due to beta-adrenergic stimulation. Here we describe the protocol we used for the preparation and surgical implantation of osmotic pumps and echocardiography.
2. Materials
Osmotic Pumps. Osmotic pumps of different sizes and drug duration are available for purchase from Alzet. We chose the ALZET Model 1004 minipumps with a 100 μL reservoir to administer isoproterenol over 3 weeks.
Isoproterenol (Concentration varies depending on the body weight of the animal due to constant flow rate.)
Depilatory cream.
Buprenorphine.
Ketamine.
Xylazine.
Ophthalmic ointment.
Surgical tools for mouse: scissors, forceps.
5.0 absorbable suture.
6.0 nonabsorbable suture.
Carprofen.
Amoxicillin.
Small animal anesthesia system: A tabletop anesthesia system that includes an isoflurane vaporizer/regulator, tubing, induction chamber, face mask/nose cone, and passive scavenging system is required for echocardiography.
Echocardiography imaging system. An ultrahigh frequency ultrasound imaging system with cardiovascular analysis software, such as Visulsonics Vevo systems, is required for assessment of cardiac structure and function. We used a 30 MHz transducer for all image studies.
3. Methods
House animals in a vivarium with easy access to a surgical suite and an echocardiogram imaging system.
3.1. Anesthesia for Echocardiography
The use of inhaled isoflurane enables lesser trained operators to achieve physiologic images (see Note 2). Minimize measurement errors by using a uniform dosage of isoflurane throughout the experiment. Different mouse strains have varying susceptibility to isoflurane. Isoflurane dosage must be tailored to each mouse strain. Physiological heart rate for mice is around 600 bpm. Heart rate <475 bpm may be due to deep or prolonged sedation and should be avoided.
Fill the isoflurane regulator reservoir with isoflurane.
Turn on the oxygen to 2 L.
Turn on the isoflurane regulator to 1.25% or 1.5% to anesthetize each mouse in the induction chamber.
Once the mouse is appropriately sedated and secured on to the warmed echocardiography platform with tape, decrease isoflurane to a maintenance dosage of around 1%.
Take frequent notes of respiratory rate and heart rate.
Fine adjust isoflurane dosage as needed to maintain sedation without affecting heart rate and respiratory rate throughout the study.
3.2. Echocardiography
Echocardiography enables in vivo assessment of cardiac structures and function. Relevant measurements include left ventricular wall thickness, internal dimension, mass, and ejection fraction. Baseline assessment is helpful in controlling for biological variation. After isoproterenol treatment, reevaluation by echocardiogram can be performed as frequently as desired. In our study we monitored the mice weekly. The following steps are recommended to ensure accurate comparisons between serial studies.
Image the left ventricle in B-mode in the parasternal long-axis view.
Carefully adjust the mouse handling table to position the long-axis of the left ventricle in the same plane as the ultrasound beam. In practice, we place the aortic valve and the apex of the left ventricle in the same plane as the ultrasound beam. The aortic valve and the apex serve as fix points that remain invariant between serial studies on the same animal.
Adjust the tilt of the mouse handling table so that the long axis of the left ventricle is at 90 to the ultrasound beam.
Turn the ultrasound probe 90 to reveal the LV in short axis. Fine adjustments are made so that the maximum diameter of the LV is seen.
Perform M-mode through the middle of the left ventricle to image the walls and internal dimensions (see Note 3).
3.3. Preparation of Isoproterenol Osmotic Pump
Prepare osmotic pumps in a sterile environment such as a biosafety laboratory cabinet. The dosage of isoproterenol should be adjusted based on mortality and susceptibility to cardiac remodeling for each strain [8]. We chose 30 mg/kg/d to induce cardiac remodeling in most of the mouse strains. The osmotic pumps have a fixed delivery rate. The #1004 pump we chose has a pumping rate of 0.11 μL/h, duration of 28 days and reservoir volume of 100 μL. Prepare enough isoproterenol solution (~120 μL) as some volume will be lost in the filling tubing.
Weigh and record body weight for each mouse to calculate appropriate amounts of isoproterenol drug needed. See Table 2.
Use an analytical balance to weigh out the appropriate amount of isoproterenol for each mouse.
Dissolve isoproterenol in sterile 0.9% NaCl solution. Appropriate negative control for the experimental treatment is a solution identical to that used for solubilizing the isoproterenol drug, for example the 0.9% saline solution.
Weigh the empty pump together with its flow modulator.
Draw the isoproterenol solution into a 1.0 mL small syringe. Attach the provided blunt-tipped, 27 gauge filling tube. Make sure that the syringe and filling tube are free of air bubbles.
Remove the flow moderator. Hold the pump in the upright position. Insert the filling tube through the opening at the top until it can go no further. This places the tip of the tube near the bottom of the pump reservoir.
Push the plunger of the syringe slowly to load the osmotic pump. When the solution appears at the outlet, stop filling and carefully remove the tube.
Wipe off excess solution and insert the flow moderator until the white flange is flushed with the top of the pump. Wipe off any overflow.
Weigh the filled pump to ensure that the fill volume is over 90% of the reservoir volume of 100 μL.
Table 2.
Required dose of isoproterenol for commonly used animal weights
| Isoproterenol 30 μg/g/d | ||||
|---|---|---|---|---|
| Body weight (g) | Daily dose (μg/d) | Flow rate (μL/d) | Concentration (μg/μL) | Isoproterenol (mg/120 μL) |
| 20 | 600 | 2.64 | 227.3 | 27.3 |
| 21 | 630 | 2.64 | 238.6 | 28.6 |
| 22 | 660 | 2.64 | 250.0 | 30.0 |
| 23 | 690 | 2.64 | 261.4 | 31.4 |
3.4. Isoproterenol Osmotic Pump Surgical Implantation
Potential complications from isoproterenol pump surgery include infection and death. Therefore, survival surgery should be performed using sterile instruments, sutures, and septic procedures to minimize microbial infection. Surgeons should put on a surgical mask, bonnet and clean lab coat, and then wash and dry their hands before aseptically donning sterile gloves. Please see Notes 3–5 regarding special considerations for age, gender, body size, and mortality rate with regards to surgical morbidity and mortality.
Prepare the animal by removing hair using a depilatory, such as Nair, at planned site of incision to minimize infections or other complications from incident hair. Perform this procedure in an area separate from where the surgery is to be conducted.
Administer buprenorphine 0.1 mg/kg s.c. to the back of the neck between the shoulder blades. Wait 10 min for the medication to work.
Anesthetize with 100 μg/kg ketamine i.p. and 10 μg/kg xyla-zine i.p.
Put ophthalmic ointment in the eyes to prevent the corneas from drying out.
Prepare the surgical site with an appropriate skin disinfectant, such as diluted Betadine or chlorhexidine. Alcohol is not an adequate disinfectant and its evaporation may lead to hypothermia in small animals.
Make a 1 cm long midline skin incision in the lower abdomen with surgical scissors. Carefully separate the skin from underlying connective tissues using blunt-ended scissors.
Use forceps to pick up the peritoneal wall. Cut a small (0.8 cm) hole in the musculoperitoneal layer and the peritoneal wall using fine surgical scissors while avoiding damage to the underlying bowel.
Insert the osmotic pump, flow moderator side first, into the peritoneal cavity.
Close the musculoperitoneal layer and the peritoneal wall with 5.0 absorbable sutures in an interrupted fashion.
Close the skin incision with 6.0 nonabsorbable sutures.
Move the animal to a warm, dry area, such as a dedicated incubator, and monitor it during recovery or overnight. Return the animal to its routine housing only after it has fully recovered from anesthesia.
To minimize pain and discomfort in the postoperative period, we recommend carprofen 5 mg/kg s.c., which may be repeated every 48 h as needed.
Administer antibiotic solution orally to prevent surgical site infections. For example, amoxicillin (0.25 mg/mL) in drinking water for 5 days.
Monitor every few days to check for complications (see Notes 4 and 5).
Remove nonabsorbable sutures after 7–10 days.
3.5. Tissue Collection
At the end of the protocol, endpoint assessment of cardiac structure and function using echocardiography may be performed. In our study, the minipump was capable of delivering constant isoproterenol dosage for 28 days. We made our end measurements at 21 days. After sublethal dosage of inhaled isoflurane followed by cervical dislocation, heart weight can be confirmed and heart and other tissues can be collected.
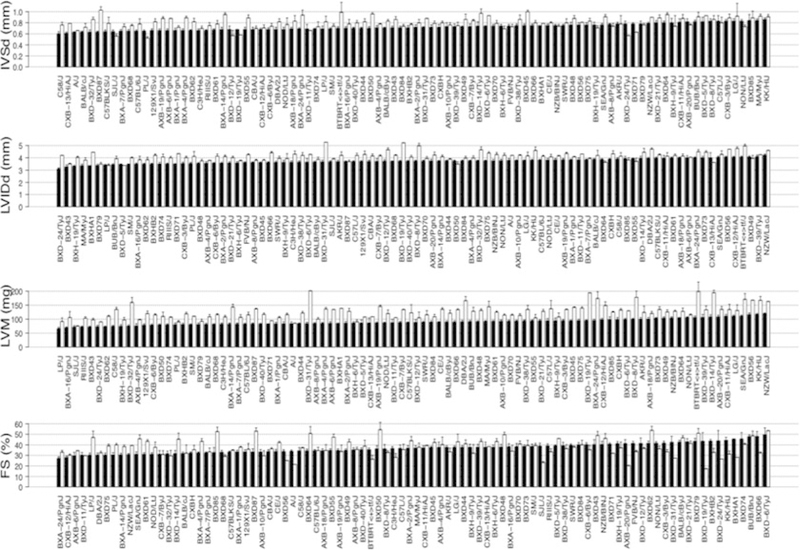
Fig. 2.
Variation in echocardiographic measures of cardiac structure and function among HMDP mouse strains. Black bars represent measurements under the baseline condition in ranked order. White bars represent measurements after 3 weeks of continuous ISO infusion. IVSd Interventricular septal wall thickness, LVIDd Left ventricular diastolic diameter, LVM Left ventricular mass, FS Fractional shortening. Error bars represent the standard errors of the means
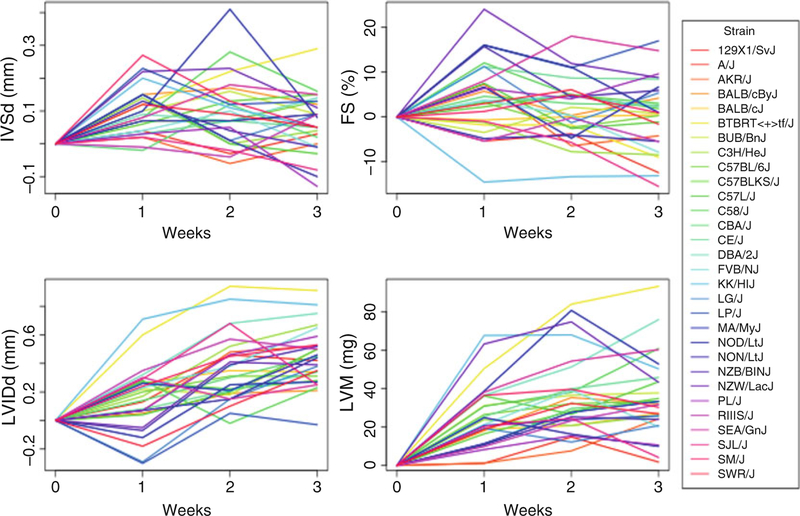
Fig. 3.
The changes in echocardiographic measures compared to baseline at each ISO time point for individual classical inbred strains
Fig. 4.
Distribution of cardiac fibrosis before and after isoproterenol treatment in the HMDP. Data is organized based on the amount of fibrosis observed after isoproterenol treatment and displays mean +/ standard deviation
Table 3.
Mouse strains and deaths in the isoproterenol group
| Strain | Control | Isoproterenol | Deaths (<48 h) |
|---|---|---|---|
| 129X1/SvJ | 3 | 8 | 1 |
| A/J | 3 | 8 | 3 |
| AKR/J | 2 | 6 | 0 |
| AXB-10/PgnJ | 2 | 3 | 0 |
| AXB-12/PgnJ | 1 | 2 | 2 |
| AXB-13/PgnJ | 0 | 1 | 1 |
| AXB-18/PgnJ | 4 | 4 | 1 |
| AXB-19/PgnJ | 5 | 5 | 2 |
| AXB-20/PgnJ | 1 | 4 | 2 |
| AXB-4/PgnJ | 1 | 2 | 0 |
| AXB-6/PgnJ | 1 | 2 | 1 |
| AXB-8/PgnJ | 3 | 5 | 0 |
| BALB/cByJ | 2 | 4 | 1 |
| BALB/cJ | 6 | 12 | 7 |
| BTBRT<+>tf/J | 5 | 14 | 12 |
| BUB/BnJ | 3 | 8 | 3 |
| BXA-1/PgnJ | 2 | 3 | 1 |
| BXA-11/PgnJ | 1 | 1 | 0 |
| BXA-12/PgnJ | 1 | 3 | 3 |
| BXA-14/PgnJ | 4 | 5 | 0 |
| BXA-16/PgnJ | 2 | 4 | 0 |
| BXA-2/PgnJ | 3 | 4 | 1 |
| BXA-24/PgnJ | 5 | 8 | 4 |
| BXA-4/PgnJ | 4 | 4 | 0 |
| BXA-7/PgnJ | 5 | 5 | 0 |
| BXA-8/PgnJ | 1 | 2 | 1 |
| BXD-1/TyJ | 1 | 0 | 0 |
| BXD-11/TyJ | 2 | 2 | 1 |
| BXD-12/TyJ | 2 | 2 | 0 |
| BXD-14/TyJ | 1 | 3 | 0 |
| BXD-15/TyJ | 1 | 2 | 2 |
| BXD-19/TyJ | 0 | 2 | 1 |
| BXD-20/TyJ | 0 | 1 | 1 |
| BXD-21/TyJ | 6 | 9 | 2 |
| BXD-22/TyJ | 0 | 1 | 1 |
| BXD-24/TyJ | 2 | 1 | 0 |
| BXD-27/TyJ | 1 | 3 | 3 |
| BXD-31/TyJ | 1 | 1 | 0 |
| BXD-32/TyJ | 4 | 5 | 2 |
| BXD-33/TyJ | 1 | 1 | 1 |
| BXD-34/TyJ | 2 | 5 | 5 |
| BXD-38/TyJ | 4 | 6 | 4 |
| BXD-39/TyJ | 2 | 3 | 0 |
| BXD-40/TyJ | 7 | 14 | 9 |
| BXD43 | 2 | 5 | 0 |
| BXD44 | 2 | 2 | 0 |
| BXD45 | 3 | 4 | 2 |
| BXD48 | 2 | 4 | 0 |
| BXD49 | 3 | 3 | 0 |
| BXD-5/TyJ | 1 | 1 | 0 |
| BXD50 | 4 | 5 | 1 |
| BXD55 | 2 | 5 | 1 |
| BXD56 | 3 | 4 | 3 |
| BXD-6/TyJ | 1 | 1 | 0 |
| BXD61 | 4 | 7 | 2 |
| BXD62 | 3 | 4 | 1 |
| BXD64 | 3 | 3 | 0 |
| BXD66 | 3 | 3 | 0 |
| BXD68 | 3 | 5 | 0 |
| BXD69 | 1 | 0 | 0 |
| BXD70 | 3 | 4 | 1 |
| BXD71 | 1 | 1 | 0 |
| BXD73 | 3 | 5 | 1 |
| BXD74 | 2 | 2 | 0 |
| BXD75 | 3 | 6 | 1 |
| BXD79 | 3 | 4 | 0 |
| BXD-8/TyJ | 1 | 2 | 0 |
| BXD84 | 3 | 6 | 1 |
| BXD85 | 1 | 2 | 0 |
| BXD86 | 2 | 2 | 2 |
| BXD87 | 3 | 3 | 0 |
| BXH-19/TyJ | 1 | 3 | 0 |
| BXH-6/TyJ | 4 | 5 | 0 |
| BXH-9/TyJ | 1 | 2 | 0 |
| BXHA1 | 0 | 1 | 0 |
| BXHB2 | 4 | 5 | 0 |
| C3H/HeJ | 4 | 8 | 1 |
| C57BL/6J | 6 | 11 | 4 |
| C57BLKS/J | 3 | 5 | 3 |
| C57L/J | 2 | 3 | 0 |
| C58/J | 2 | 4 | 1 |
| CBA/J | 3 | 7 | 0 |
| CE/J | 2 | 3 | 0 |
| CXB-11/HiAJ | 2 | 3 | 1 |
| CXB-12/HiAJ | 4 | 6 | 2 |
| CXB-13/HiAJ | 2 | 5 | 1 |
| CXB-3/ByJ | 4 | 3 | 0 |
| CXB-6/ByJ | 3 | 7 | 4 |
| CXB-7/ByJ | 2 | 3 | 1 |
| CXBH | 2 | 5 | 1 |
| DBA/2J | 6 | 11 | 3 |
| FVB/NJ | 6 | 10 | 1 |
| KK/HlJ | 2 | 4 | 0 |
| LG/J | 4 | 4 | 1 |
| LP/J | 3 | 3 | 0 |
| MA/MyJ | 3 | 4 | 0 |
| NOD/LtJ | 3 | 7 | 0 |
| NON/LtJ | 4 | 6 | 1 |
| NZB/BlNJ | 2 | 4 | 1 |
| NZW/LacJ | 3 | 6 | 5 |
| PL/J | 2 | 7 | 2 |
| RIIIS/J | 4 | 9 | 3 |
| SEA/GnJ | 3 | 9 | 5 |
| SJL/J | 2 | 7 | 2 |
| SM/J | 3 | 3 | 0 |
| SWR/J | 4 | 9 | 5 |
Acknowledgments
The discussed protocol has been developed in the laboratory of Dr. Yibin Wang.
Footnotes
Notes
In our study, we included females from >100 inbred mouse strains. Isoproterenol caused a wide spectrum of cardiac remodeling phenotypes (Fig. 2 reproduced from PLoS Genet 2016 with permission from the Public Library of Science (PLOS) [9]). Expected time for the model development and weekly changes in echocardiography parameters for each strain are shown in Fig. 3 (reproduced from PLoS Genet 2016 with permission from PLOS) [9]. Overall, LVIDd and LVM increased over a period of 3 weeks. IVSd and FS increased in the first week but decreased by later time points. Of note, during the final assessment, most strains showed normal systolic heart function. With ongoing isoproterenol infusion, normal systolic function represents impaired contractile reserve.
Isoflurane can have a negative effect on cardiac chronotropy and inotropy. Echocardiogram may be performed without sedation by experienced operators.
The sternum may cast a bony shadow over the image. The probe should be repositioned to image from a different rib interspace. The right ventricular wall may make it difficult to measure the interventricular wall borders. Measure cardiac dimensions in real time so that poorly delineated borders can be immediately reinterrogated by taking additional modified views. Accurate measurements are critical to the downstream comparisons.
Age and body size. Mice weighing <20 g have a higher early surgical mortality associated with surgical procedures (death within 48 h). Allowing female mice to mature to at least 9–10 weeks of age will lower early surgical mortality.
Mortality rate and cardiac fibrosis. It is important to consider strain-specific rates of operative mortality and susceptibility to isoproterenol when planning an experiment (Table 3) [8]. In the HMDP study, 139 (29.6%) mice out of 470 mice in the isoproterenol treatment group died before the end of the protocol. Most (127) died within the first 48 h of treatment. Once mice survived the initial surgery, few died during the course of the study from heart failure complications. Specifically, 100% mortality occurred in BXA-12/PgnJ and BXD-34/TyJ; 86% in BTBRT<+>tf/J; 83% in NZW/LacJ; 64% in BXD40/TyJ; and 58% in BALB/cJ. Finally, ISO caused more cardiac fibrosis in some strains than in others. For example, the strain KK/HlJ had markedly increased fibrosis after ISO stimulation (Fig. 4 reproduced from Circ Cardiovasc Genet 2015 with permission from Lippincott Williams & Wilkins) [8].
Gender. Sex differences in cardiovascular disease are well-known in the literature. Considerations regarding gender must be made when planning an experiment. In an ischemia reperfusion injury model, female mouse demonstrated higher postischemic contractile function and lesser ATP-depletion [10]. In a chronic isoproterenol model, Klingman et al. found elevated heart to body weight ratio in males but not in females [11]. Finally, total norepinephrine levels in parotid and sub-maxillary glands were reduced in males but not in females.
References
- 1.Balakumar P, Singh AP, Singh M (2007) Rodent models of heart failure. J Pharmacol Toxicol Methods 56(1):1–10 [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa T (2015) Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol 182:297–303 [DOI] [PubMed] [Google Scholar]
- 3.Kono T, Sabbah HN (2014) Takotsubo cardiomyopathy. Heart Fail Rev 19(5):585–593 [DOI] [PubMed] [Google Scholar]
- 4.Shao Y, Redfors B, Stahlman M, Tang MS, Miljanovic A, Mollmann H, Troidl C, Szardien S, Hamm C, Nef H, Boren J, Omerovic E (2013) A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. Eur J Heart Fail 15 (1):9–22 [DOI] [PubMed] [Google Scholar]
- 5.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF (1997) Effects of chronic beta-adrenergic receptor stimulation in mice. J Mol Cell Cardiol 29(10):2735–2746 [DOI] [PubMed] [Google Scholar]
- 6.Zhuo XZ, Wu Y, Ni YJ, Liu JH, Gong M, Wang XH, Wei F, Wang TZ, Yuan Z, Ma AQ, Song P (2013) Isoproterenol instigates cardiomyocyte apoptosis and heart failure via ampk inactivation-mediated endoplasmic reticulum stress. Apoptosis 18(7):800–810 [DOI] [PubMed] [Google Scholar]
- 7.El-Demerdash E, Awad AS, Taha RM, El-Hady AM, Sayed-Ahmed MM (2005) Probucol attenuates oxidative stress and energy decline in isoproterenol-induced heart failure in rat. Pharmacol Res 51(4):311–318 [DOI] [PubMed] [Google Scholar]
- 8.Rau CD, Wang J, Avetisyan R, Romay MC, Martin L, Ren S, Wang Y, Lusis AJ (2015) Mapping genetic contributions to cardiac pathology induced by beta-adrenergic stimulation in mice. Circ Cardiovasc Genet 8 (1):40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JJ, Rau C, Avetisyan R, Ren S, Romay MC, Stolin G, Gong KW, Wang Y, Lusis AJ (2016) Genetic dissection of cardiac remodeling in an isoproterenol-induced heart failure mouse model. PLoS Genet 12(7):e1006038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross HR, Murphy E, Koch WJ, Steenbergen C (2002) Male and female mice overexpressing the beta(2)-adrenergic receptor exhibit differences in ischemia/reperfusion injury: role of nitric oxide. Cardiovasc Res 53(3):662–671 [DOI] [PubMed] [Google Scholar]
- 11.Klingman GI, McKay G, Ward A, Morse L (1973) Chronic isoproterenol treatment of mice: effects on catecholamines and rectal temperature. J Pharm Sci 62(5):798–801 [DOI] [PubMed] [Google Scholar]
- 12.Ma S, Yang D, Wang K, Tang B, Li D, Yang Y (2012)Cryptotanshinoneattenuates isoprenaline-induced cardiac fibrosis in mice associated with upregulation and activation of matrix metalloproteinase-2. Mol Med Rep 6 (1):145–150 [DOI] [PubMed] [Google Scholar]
- 13.Berthonneche C, Peter B, Schupfer F, Hayoz P, Kutalik Z, Abriel H, Pedrazzini T, Beckmann JS, Bergmann S, Maurer F (2009) Cardiovascular response to beta-adrenergic blockade or activation in 23 inbred mouse strains. PLoS One 4(8):e6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM (2003) Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation 108(17):2147–2152 [DOI] [PubMed] [Google Scholar]
- 15.Galindo CL, Skinner MA, Errami M, Olson LD, Watson DA, Li J, McCormick JF, McIver LJ, Kumar NM, Pham TQ, Garner HR (2009) Transcriptional profile of isoproterenol-induced cardiomyopathy and comparison to exercise-induced cardiac hypertrophy and human cardiac failure. BMC Physiol 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J, Yang L, Tian W, Zhu M, Liu J, Lu P, Li J, Yang L, Qi Z (2015) Nitric oxide synthase inhibition abolishes exercise-mediated protection against isoproterenol-induced cardiac hypertrophy in female mice. Cardiology 130 (3):175–184 [DOI] [PubMed] [Google Scholar]
- 17.Yang YH, Fang HL, Zhao M, Wei XL, Zhang N, Wang S, Lu Y, Yu XJ, Sun L, He X, Li DL, Liu JJ, Zang WJ (2017) Specific alpha7 nicotinic acetylcholine receptor agonist ameliorates isoproterenol-induced cardiac remodelling in mice through tgf-beta1/smad3 pathway. Clin Exp Pharmacol Physiol 44:1192–1200 [DOI] [PubMed] [Google Scholar]
- 18.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD (2005) Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. Am J Physiol Heart Circ Physiol 289(1):H30–H36 [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang ZL, Wang HF (2017) Fusaric acid (fa) protects heart failure induced by isoproterenol (isp) in mice through fibrosis prevention via tgf-beta1/smads and pi3k/akt signaling pathways. Biomed Pharmacother 93:130–145 [DOI] [PubMed] [Google Scholar]
- 20.Vergaro G, Prud’homme M, Fazal L, Merval R, Passino C, Emdin M, Samuel JL, Cohen Solal A, Delcayre C (2016) Inhibition of galectin-3 pathway prevents isoproterenol-induced left ventricular dysfunction and fibrosis in mice. Hypertension 67(3):606–612 [DOI] [PubMed] [Google Scholar]