Abstract
Bacterial contamination of the uterus following calving is ubiquitous in the dairy cow, 40% of cows develop postpartum uterine infection, including metritis. While predisposing factors like twinning and dystocia are associated with metritis, it is unclear why some cows remain healthy following calving and others develop uterine infection, negatively impacting animal health, milk production and economic return. Here, we profiled peripheral blood mononuclear cells of cows before calving and during postpartum metritis. We hypothesized that peripheral blood mononuclear cell function and proportions would be altered during the prepartum period in cows that develop postpartum metritis. Using flow cytometry we observed reduced proportions of peripheral CD3+/CD4+, CD4+/CD62L+, FOXP3+ and CD21+ populations from −10 to 40 days relative to calving associated with metritis, while the proportion of peripheral CD3+/CD4+ lymphocytes were specifically reduced in the prepartum period before the onset of metritis. Peripheral blood mononuclear cells from cows with metritis had a perturbed capacity to secrete IL-1β or IFNγ in response to in vitro stimulus; cells collected during the prepartum period from cows that would go on to develop metritis failed to increase IL-1β secretion in response to stimulation, while IFNγ secretion was altered at calving and postpartum in cows with metritis compared to healthy herd mates. No effect of metritis was observed in the capacity of cows to mount a humoral immune response to antigen administered on the day of calving. The studies discussed here suggest that while minor changes to the prepartum immune system are observed in cows that develop metritis, changes observed in the postpartum period are more prevalent and likely a consequences of disease and not causative. Future studies to modulate the prepartum immune system may help to limit postpartum metritis.
Keywords: immune system, metritis, periparturient, prepartum, uterine disease
INTRODUCTION
Bacterial contamination of the uterus following calving is ubiquitous in the dairy cow (Griffin et al., 1974; Moore et al., 2017; Sheldon et al., 2002). While the majority of cows will be able to clear pathogenic bacteria, approximately 40% of all cows will develop postpartum metritis (Gilbert et al., 2005; Ribeiro et al., 2013; Sheldon et al., 2009). Why some cows in a herd develop metritis and others clear pathogenic bacteria is unclear.
Uterine infection, defined here as metritis or endometritis, is the result of pathogenic bacterial infection of the uterus during the first 3 weeks after calving. Resulting infection has significant negative impacts on the health and productivity of the dairy cow. It is estimated that uterine infection costs the US dairy industry in excess of $650 million per year due to reduced milk production, treatment costs and negative reproductive consequences (Drillich et al., 2001; Sheldon et al., 2009). Cows that resolve uterine infection display persistent reproductive deficiencies with a reduced calving rate, delayed conception and increased days open (Borsberry and Dobson, 1989; Gilbert et al., 2005; LeBlanc et al., 2002; Ribeiro et al., 2013). Negative reproductive consequences of uterine disease force producers to maintain increased replacement animals in the herd, further inflating economic and environmental impacts.
A number of risk factors have been associated with the development of metritis in the dairy cow including, dystocia, retained fetal membranes, twining and ketosis (Bruun et al., 2002; Dohmen et al., 2000; Potter et al., 2010). Many of these risk factors are associated with compromising the normal protective mucosa of the endometrium, leaving the stroma of the endometrium vulnerable to pathogenic bacterial infection. A number of studies have demonstrated the importance of bacterial strain in the development of metritis, with Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes being causative pathogens of disease, while it is interesting to note that non-pathogenic bacterial populations are present in the uterus of healthy cows during gestation and up to 7 weeks postpartum (Gilbert and Santos, 2016; Griffin et al., 1974; Moore et al., 2017; Sheldon et al., 2010). It has been surmised that postpartum negative energy balance plays a role in the predisposition of uterine disease in the dairy cow, potentially by compromising metabolically expensive immune function (Kvidera et al., 2017; Swangchan-Uthai et al., 2013). Little is known about the role of peripheral blood mononuclear cells in the development of uterine disease in the cow. While uterine disease can be treated with systemic antibiotics, treatment does not improve reproductive performance after resolution of disease (Drillich et al., 2001; Haimerl and Heuwieser, 2014). This is also the case with newly developed vaccines targeted to uterine disease causing bacteria (Machado et al., 2014). These observation may suggest that there are inherent difference in immune function of cows that develop uterine disease.
The postpartum innate immune system of the dairy cow has been studied extensively, and data suggests that the functionality of the innate immune response, particularly neutrophil function, during the postpartum period is associated with the development of uterine disease (LeBlanc, 2012; Martinez et al., 2012; Pinedo et al., 2013). Indeed, the innate immune function of the endometrium itself has also been shown to be perturbed during uterine infection and may play a role in uterine disease onset (Herath et al., 2006; Turner et al., 2016). Cows with active uterine infection have alterations in the proportions of peripheral lymphocyte populations postpartum (Hine et al., 2011). Rodent models of systemic immune deficiencies indicate susceptibility to infection, especially in severe scenarios of immune cell depletion, including irradiation, the severe combined immunodeficiency (SCID) mouse and nude mouse(Dickerson et al., 1983; Miller et al., 1960; Teles et al., 1997). Indeed, micronutrient deficiencies reduce immune competence in cows leading to increased disease susceptibility (reviewed in (Spears, 2000)). Here, we asked specifically whether populations and functionality of peripheral blood immune cells are altered in cows before and during metritis in the dairy cow. We hypothesized that functionality and proportional populations of specific peripheral blood immune cells are altered in the dairy cow prior to the onset of metritis. We profiled the periparturient proportions of specific peripheral blood lymphocyte populations by flow cytometry, including T-helper and T-cytotoxic cells, B cells, gamma delta (γδ) cells (which are abundant in cattle) and forkhead box P3 (FOXP3) positive cells thought to have an immune regulatory function (although this is now challenged in cattle (Hoek et al., 2009)) (Mackay and Hein, 1989). We then assessed the functional capacity of peripheral blood mononuclear cells (PBMCs) to secrete immune modulating cytokines interleukin (IL)-1β and interferon gamma (IFNγ), and finally the ability of the cow to mount a humoral immune response to an inert antigen administered at the time of calving (reflecting the normal timing of uterine exposure to disease causing pathogens). We propose that these three factors contribute to the overall immune status of the cow and the capacity for defense from uterine pathogens. Therapeutic modulation of the immune system during the prepartum period may aid in reducing the incidence or severity of uterine disease in the dairy cow and circumvent the negative consequences of pathology.
MATERIALS AND METHODS
All reagents were acquired from Fisher Scientific (Waltham, MA) unless otherwise stated.
Animal Use and Clinical Diagnosis of Uterine Disease
Holstein cows were housed at the University of Florida dairy research unit. All procedures were approved by the University of Florida Institutional Animal Care and Use Committee. The University of Florida herd is free of USDA APHIS notifiable diseases and conditions. A total of 12 cows were used throughout the entire experimental period starting 10 days prior to the expected calving date through 40 days in milk (DIM). Blood was collected every other day prior to calving and then every fifth day during the postpartum period via the coccygeal vein into EDTA vacutainers. Blood was processed according to the desired analysis platform outlined below. Uterine health of all cows was evaluated by in-house veterinary staff after examining vaginal discharge on day 3, 7, 10 and 21 postpartum according to Sheldon et al (Sheldon et al., 2009). Rectal temperature was recorded on days of blood collection. Cows with uterine disease were classified as grade I or II metritis defined by the presence of purulent uterine discharge, without any systemic signs of ill-health, and without fever (> 39.5°C). Cows categorized as grade III or IV metritis were excluded as they routinely receive antibiotic treatment. All cows were monitored for other clinical diseases including mastitis, lameness, ketosis and displaced abomasum. In the absence of other clinical disease during the postpartum period, cows were described as healthy or metritis and all samples retrospectively categorized. All cows were managed as a single group and those with metritis were not treated. Prepartum cows were fed once daily and postpartum cows were fed twice daily. Feed was supplied ad libitum as a total mixed ration. A total of seven healthy and five metritis cows were used throughout the experimental period.
Milk parameters (yield and components) were collected by the AfiLab milk analyzer (Kibbutz Afikim, Isreal) at each milking and compiled using AfiFarm software. Cow weight was collected daily as cows left the milking parlor.
Peripheral Blood Mononuclear Cell Isolation
Whole blood was collected from Holstein cows via the coccygeal vein into EDTA vacutainers (Becton Dickson, Franklin Lakes NJ) and centrifuged at 400 x g for 10 minutes at room temperature. The buffy coat containing PBMCs was aspirated and washed again in PBS by centrifugation at 400 x g for 10 min at room temperature. Cell suspensions were layered above Ficoll 1.078 g/ml Premium (GE Lifesciences, Pittsburgh PA) for density separation centrifugation at 400 x g for 30 min at room temperature. The cell layer was removed and washed in PBS at 400 x g for 10 min at room temperature. Washes were repeated at 300 x g, 200 x g, and 100 x g for 10 min at room temperature to remove platelets. Cells were either cultured for cell stimulation assays or used immediately for flow cytometry analysis (see below for details).
Flow cytometric analysis of peripheral blood lymphocyte populations
Freshly isolated PBMCs were resuspended at 1.5 × 106 cells/ml in cold PBS. Cells were aliquoted into FACS tubes in 200 μl volumes and pelleted by centrifugation at 400 x g for 5 min. For cell surface markers, cells were resuspended in staining buffer containing PBS/1% fetal calf serum and directly conjugated antibody (see Table 1 for antibodies and dilutions). Cells were incubated on ice for 60 min in the dark. Cells were washed three times in cold PBS by centrifugation at 400 x g for 5 min. For intracellular FOXP3, cells were fixed and permeabilized after washing using the commercial Intracellular Fixation & Permeabilization Buffer Set (Thermo Fisher Scientific, Waltham MA). Following permeabilization cells were incubated with directly conjugated antibody for 60 min on ice in the dark. Antibodies were titrated based on the recommended dilutions to determine the minimum concentration required to identify cells with minimal non-specific background. Cells were labelled in antibody cocktails to allow differentiation of various cellular populations, including CD3+/CD4+, CD3+/CD8+ and CD3+/CD4+/CD62L+. Cells were washed by centrifugation as above. Following antibody labeling all cells were resuspended in staining buffer and analyzed for fluorescence intensity immediately using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose CA). A total of 10,000 cells were evaluated and data analyzed by FlowJo V10.0.7 (Treestar, Palo Alto CA). Gating strategies for lymphocytes, viable cells, single cells and double/triple labeling are shown in Supplemental Fig 1.
Table 1.
Antibodies used for flow cytometric analysis of peripheral blood lymphocyte populations.
| Target molecule | Antibody clone | Fluorochrome | Dilution |
|---|---|---|---|
| CD3 | MM1A (WSU) | Alexfluor-488 | 1:200 |
| CD4 | CACT138A (WSU) | Alexfluor-647 | 1:200 |
| CD8α | CACT80C (WSU) | R-phycoerythrin | 1:200 |
| CD21 | GB25A (WSU) | Alexfluor-488 | 1:800 |
| CD62L | IVA94 (ThermoFisher) | Alexfluor-488 | 1:200 |
| FOXP3 | FOX5A (WSU) | R-phycoerythrin | 1:100 |
| γδ TCR (WC+) | ILA29 (WSU) | R-phycoerythrin | 1:50 |
CD, cluster of differentiation. FOXP3, forkhead box P3. TCR, T-cell receptor. WSU, Washington State University, Monoclonal Antibody Center.
In Vitro Culture and Stimulation of Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells were collected from 12 cows at −5, 0 and 5 days relative to calving. Isolated PBMCs were resuspended in complete culture medium (RPMI 1640, 10% FCS, 2mM GlutaMAX, 50 IU/ml penicillin, 50 μg/ml streptomycin) at a concentration of 1.5 × 106 cells/ml and plated in 500 μl aliquots into 24 well culture plates and placed in humidified air incubators at 5% CO2 for 24 h prior to treatment. Following a 24 hour period of equilibration cells were cultured in the presence of a cell stimulation cocktail containing 0.08 μM phorbol 12-myristate 13-acetate (PMA) and 1.3 μM ionomycin for 6 hours, 12 hours, or 24 hours. Cell stimulation with PMA and ionomycin was used to induce robust cytokine production in a non-specific population of cells to evaluate systemic functionality of cells. Following treatment, supernatants were collected and stored at −20°C for analysis of inflammatory mediators by ELISA.
Quantification of In Vivo Humoral Immune Response to Hen Egg Lysozyme
To quantify humoral immune responses in cows, immunizations with an inert antigen were performed on day 0, 14 and 28 relative to calving. Immunizations consisted of 0.5 mg of hen egg lysozyme (Sigma-Aldrich, St Louis MO) suspended in 1 ml of Quil-A adjuvant (Accurate Chemicals, Westbury NY) as previously reported (Hine et al., 2011). Immunizations were performed by intra-muscular injection in the tail-head region.
Using plasma collected above, hen egg lysozyme specific IgG was quantified by ELISA as previously described (Artiaga et al., 2014). Briefly, microtiter plates were coated with 10 μg/ml of hen egg lysozyme overnight at 4°C. Plates were washed three times in PBS containing 0.05% Tween-20 and immediately blocked in 0.05% skim milk for 1 h at 37°C. Blocking solution was aspirated and plasma diluted on 0.2% Tween 20 + 1% BSA DPBS (1:50) was applied and incubated for 1 h at 37°C. Following incubation with samples, plates were washed and a goat anti-bovine IgG conjugated to alkaline phosphatase (Southern Biotechnology Associates, 603004) was applied at a dilution of 1:1000 for 1 hour at 37°C. Plates were washed and incubated with alkaline phosphatase substrate for 40 min before immediately reading optical density (OD) at 405 nm. Data are presented as OD representing relative hen egg lysozyme specific IgG. Blood from D0, D20 and D40 time points was serially diluted prior to quantification with the ELISA to ensure linear detection of hen egg lysozyme-specific IgG. Blood from cows never exposed to hen egg lysozyme was used as a negative control.
Enzyme Linked Immunosorbent Assay
Secretion of inflammatory mediators, IL-1β and IFNγ, into culture supernatants was evaluated by commercial ELISA (ThermoFisher Scientific, Waltham MA). Assays were performed per the manufacturer’s instructions. Supernatants for IFNγ quantification were diluted as appropriate, IL-1β quantification was performed on neat supernatants. All samples were run in duplicate, averaged and multiplied by any dilution factor. Optical density was evaluated at A450 –A550 and concentrations for each sample were extrapolated from a standard curve. The limit of detection for IL-1β or IFNγ was 31 pg/ml and 31.2 pg/ml, respectively.
Data Analysis
SPSS version 20.0 software was used for statistical analysis. All data were analyzed by Generalized Linear Mixed Model procedure with repeat measures. Health, day of collection and partum period were used as fixed effects. Pairwise contrasts were performed for all fixed effects. Data was tested for normality by the Shapiro-Wilk test for normality. Cow weight was used as a weighting factor for milk parameters as it was significantly different between the two groups. Secretion of IL-1β and IFNγ was log transformed for analysis and normality was confirmed by repeating the Shapiro-Wilk test for normality and observing Q-Q plots. Data are presented as means + SEM, and a P value of ≤ 0.05 was assumed to be statistically significant.
RESULTS
Metritis was Associated with Reduced Milk Production During the First 40 DIM
Milk production data was collected for 12 cows enrolled prepartum and retrospectively designated as either healthy or with metritis in the first 40 DIM (Table 2). An average 5.8 kg/d reduction in milk yield was observed in cows with metritis up to 40 DIM (P < 0.001). As expected, an effect of DIM on daily milk yield was observed (P < 0.001). Milk fat, protein or lactose concentrations were not affected by the health status of the cows.
Table 2.
Cow production data 40 DIM.
| Healthy (n = 7) |
Uterine Disease (n = 5) |
P-value |
|||
|---|---|---|---|---|---|
| Health | DIM | Health x DIM | |||
| Parity | 2.1 ± 0.6 | 1.0 ± 0.0 | 0.164 | - | - |
| Weight (kg/d) | 574.4 ± 4.3 | 552.7 ± 4.9 | 0.001 | > 0.0001 | 0.840 |
| Milk yield (kg/d) | 31.09 ± 0.62 | 25.27 ± 0.78 | > 0.0001 | > 0.0001 | 0.436 |
| Fat (g/d) | 985 ± 27 | 1035 ± 32 | 0.230 | 0.152 | 0.254 |
| Protein (g/d) | 779 ± 20 | 802 ± 24 | 0.467 | 0.332 | 0.412 |
| Lactose (g/d) | 1202 ± 34 | 1252 ± 40 | 0.339 | 0.157 | 0.281 |
Milk parameters (yield and components) were collected by the AfiLab milk analyzer. Average daily weights were calculated during the first 40 DIM. Data are presented as mean ± SEM. Data were analyzed by the Generalized Linear Mixed Model procedure after weighting milk parameters to cow weight.
Effect of Uterine Health on Peripheral Blood Lymphocyte Populations
The proportion of CD3+/CD4+ T cells in cows with metritis was reduced by 16.3% during the study period (P < 0.05) compared to cows that remained healthy (Fig 1A). Interestingly, the proportion of CD3+/CD4+ T cells was reduced by 26.7% in the prepartum period of cows that would develop metritis (P < 0.05). Specifically at day −6 relative to calving a proportional reduction in CD3+/CD4+ T cells was observed in cows that would develop metritis compared to those that remained healthy (P < 0.05). The proportion of CD3+/CD4+ T cells in the postpartum period was similar between healthy and metritis cows.
Figure 1. Effect of Metritis on Peripheral Blood Lymphocyte Populations.
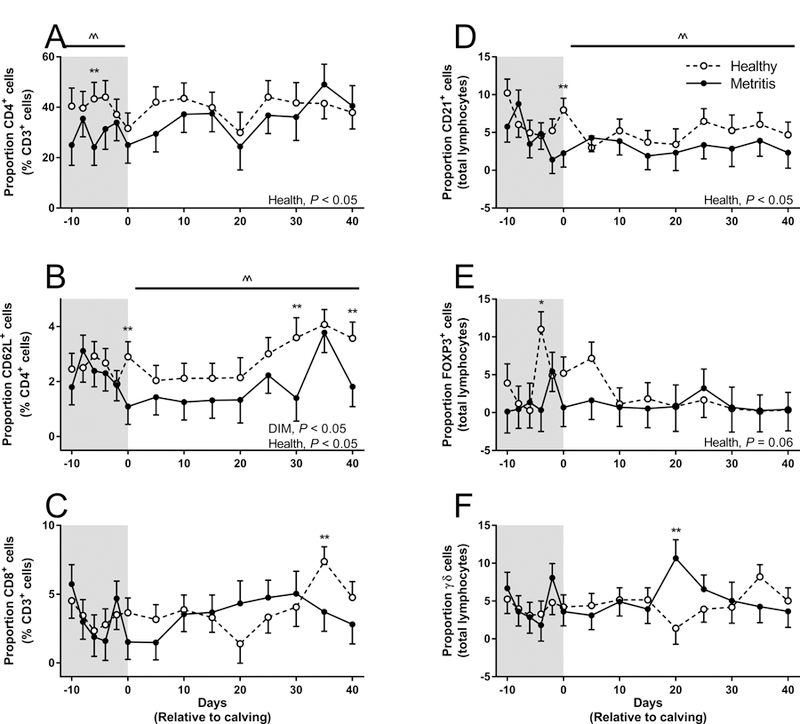
Proportions of peripheral blood lymphocytes was determined in cows from −10 relative to expected calving to 40 DIM. Proportions of CD3+/CD4+ (A), CD4+/CD62L+ (B), CD3+/CD8+ (C), CD21+ (D), FOXP3+ (E) and gamma delta T-lymphocytes (F) were quantified by flow cytometry. Data represent 7 healthy cows (open circles) and 5 metritis cows (closed circles). Data are presented as the mean proportion of total cells analyzed. Data were analyzed using the Generalized Linear Mixed Model procedure and significance was assumed when P ≤ 0.05. Data were first analyzed for the total duration of the study and then analyzed during either the prepartum (shaded area) or postpartum period (including the day of calving). ^^ represents a significant effect of health within the specific pre- or post-partum period.
The proportion of CD4+/CD62L+ (activated CD4+) T cells in cows with metritis was reduced by 28.7% during the study period (P < 0.05) compared to cows that remained healthy (Fig 1B). The proportion of CD4+/CD62L+ T cells was reduced by 38.8% in cows with metritis during the postpartum period (P < 0.05), specifically on day 0, 30 and 40 relative to calving. However the same reduction in CD4+/CD62L+ T cells was not observed in the prepartum period.
The proportion of B cells (CD21+) in cows with metritis was reduced by 33.2% during the study period (P < 0.05) compared to cows that remained healthy (Fig 1D). The proportion of CD21+ cells was reduced by 40.9% in cows with metritis during the postpartum period (P < 0.05), specifically on the day of calving, while the proportion of CD21+ cells was not different between groups in the prepartum period.
The proportion of FOXP3+ cells in cows with metritis tended to be reduced by 58.4% during the study period (P = 0.06) compared to cows that remained healthy (Fig 1E). A specific difference in FOXP3+ cells was observed at day −4 relative to calving in cows that would go on to develop metritis (P < 0.05).
The proportion of CD3+/CD8+ T-cells or γδ T-cells was not effected during the study period by uterine disease (Fig 1C, F). However, a reduction in the proportion of CD3+/CD8+ T-cells was observed in cows with metritis at day 35 relative to calving compared to healthy herd mates (P < 0.05). Conversely, an increase in the proportion of γδ T-cells was observed in cows with metritis at day 20 relative to calving compared to healthy herd mates (P < 0.05).
Impact of Metritis on the Ability to Generate a Humoral Immune Response
Initial immunization on the day of calving was chosen to mimic the timing of uterine exposure to potential disease causing pathogens, allowing us to determine the ability of the cows to generate a humoral response at this specific time. Cows increased hen egg lysozyme specific IgG following initial immunization which became elevated at 10 DIM compared to day of calving (Fig 2; P < 0.05). An effect of DIM was observed in the capacity to generate hen egg lysozyme specific IgG during the study period (P < 0.001). While there was no overall effect of disease on the capacity of cows to generate hen egg lysozyme specific IgG, there was a 27.0% reduction in the relative concertation of hen egg lysozyme specific IgG in metritis cows at 40 DIM.
Figure 2. Effect of Metritis on the Ability to Generate a Humoral Immune Response.
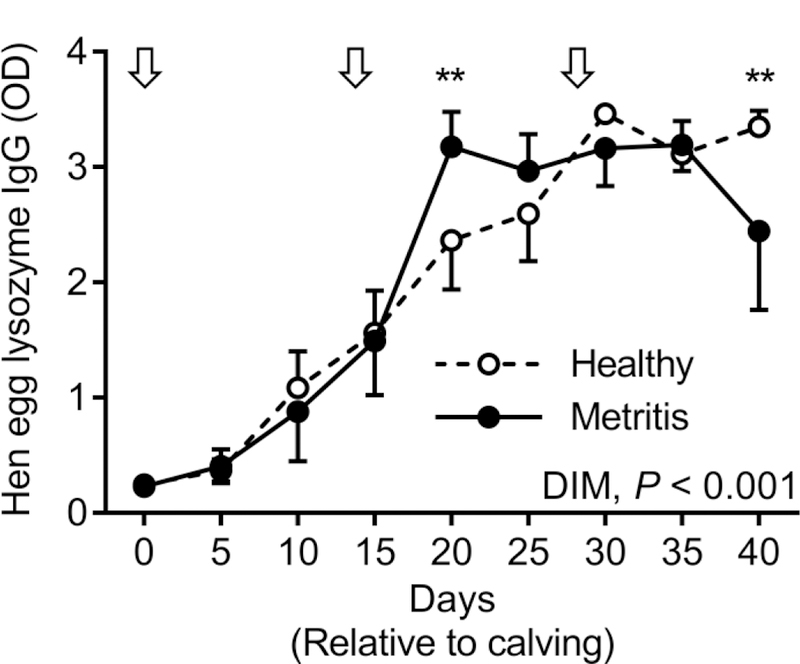
Cows were immunized to hen egg lysozyme at the day of calving and again at days 14 and 28 relative to calving. Blood plasma was collected every fifth day starting at the day of calving and the presence of hen egg lysozyme specific IgG quantified by ELISA. Data represent 7 healthy cows (open circles) and 5 metritis cows (closed circles). Arrows indicate days of immunization. Data were analyzed using the Generalized Linear Mixed Model procedure and significance was assumed when P ≤ 0.05.
The Impact of Uterine Health on the Ability of Peripheral Blood Mononuclear Cells to Respond to Immune Stimulation
There was no effect of the duration of stimulation on cytokine production (P > 0.05); as such, culture times were combined for presentation and analysis.
As expected, the capacity of PBMCs to secrete both IL-1β and IFNγ was increased by stimulation (Fig 3, P < 0.05).
Figure 3. Effect of Metritis on the Ability of Peripheral Blood Mononuclear Cells to Respond to Immune Stimulation.
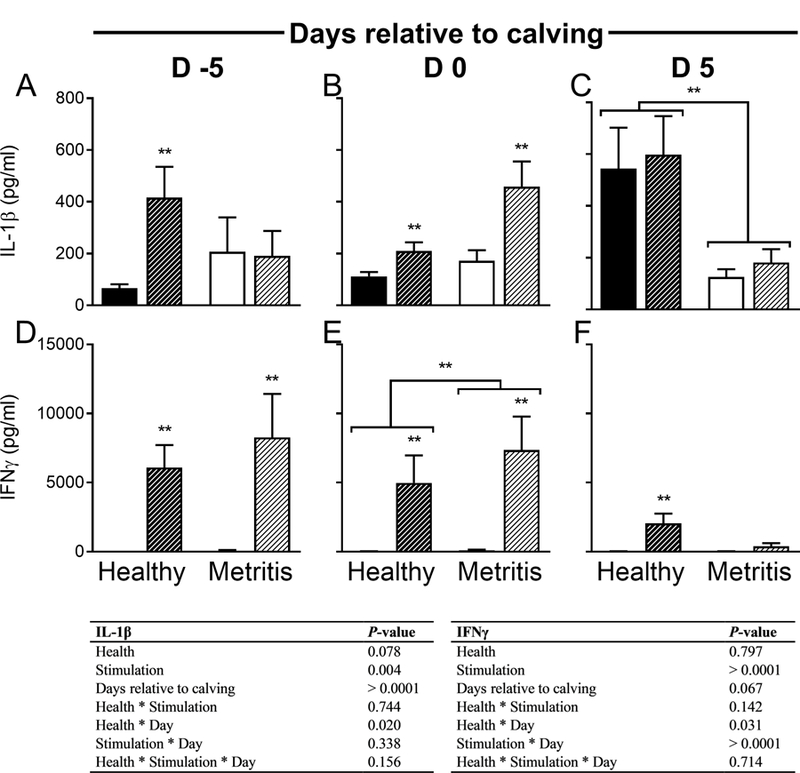
Peripheral blood mononuclear cells were collected at days −5 (A, D), 0 (B, E) and 5 (C, F) relative to calving, cultured and stimulated in vitro with PMA and ionomycin for 6, 12 or 24 h. Following stimulation, cell free supernatants were evaluated for IL-1β (A-C) or IFNγ (D-F) by ELISA. Data are presented as mean + SEM pg/ml from 7 healthy cows and 5 metritis cows. Unstimulated cells are represented by solid bars, stimulated cells are represented by hash bars. Data were analyzed using the Generalized Linear Mixed Model procedure and significance was assumed when P ≤ 0.05. ** represents differences between stimulated and unstimulated cells within group, unless representing differences between groups.
The capacity for PBMCs to secrete IL-1β was affected by the day of collection relative to calving (Fig 3A–C, P < 0.05). There was a tendency for IL-1β secretion to be reduced by 7.4% in metritis PBMC, regardless of in vitro stimulation (P = 0.08). Secretion of IL-1β at day −5 relative to calving was reduced compared to that observed at the day of calving or day 5 relative to calving (P < 0.05). On day −5 relative to calving healthy cows showed an increase in IL-1β secretion after stimulation that was not observed in cows that would go on to develop metritis in the postpartum period (Fig 3C, P < 0.05). On the day of calving, PBMCs from cows in both groups increased IL-1β secretion in response to stimulation (Fig 3B, P < 0.05); however, this stimulation induced secretion was not observed at day 5 relative to calving when IL-1β secretion was higher in healthy cows compared to those with uterine disease, regardless of in vitro stimulation (Fig 3C, P < 0.05).
The capacity for PBMCs to secrete IFNγ tended to be effected by the day of collection relative to calving (Fig 3D–F, P = 0.07). Secretion of IFNγ at day 5 relative to calving was reduced compared to that observed at the day of calving (P < 0.05). On the day of calving, PBMCs from cows with metritis showed a 22.4% increase in IFNγ secretion compared to healthy herd mates, regardless of cell stimulation (Fig 3E, P < 0.05). Secretion of IFNγ was stimulated in PBMCs from healthy cows at all days relative to calving (P < 0.05), but not in cows with metritis at day 5 relative to calving (Fig 3F).
DISCUSSION
These data suggest that proportions of specific lymphocytes are different in dairy cows that develop metritis compared to healthy herd mates. Compared to cows that remain healthy during the postpartum period, cows that go on to develop metritis have reduced proportions of circulating CD3+/CD4+ T cells during the prepartum period, before the onset of disease, while cows with active metritis have reduced proportions of peripheral CD4+/CD62+ T cells, and CD21+ cells. In addition, prepartum PBMCs from cows that would develop metritis had a reduced functional capacity to secrete IL-1β compared to cells from animals that remained healthy, while the capacity to secrete IFNγ at the time of calving was increased in animals that developed metritis. Observations during the postpartum period revealed numerous differences between healthy and metritis cows including reductions in the proportion of peripheral CD3+/CD4+ T cells, CD4+/CD62+ T cells, and CD21+ cell in cows with metritis. Also during the postpartum period, PBMCs had a functional reduction in the capacity to secrete IL-1β and IFNγ. These observations in the postpartum period likely reflect the immune response to metritis, opposed to a causative reason for the development of disease. Interestingly, there was no observed difference in the functional capacity of cows with metritis to mount a humoral immune response to a foreign antigen administered at the time of calving compared to healthy cows. Collectively these data describe differences in the immune system of dairy cows that develop metritis compared to healthy herd mates, both in the prepartum and postpartum period.
Previous work has demonstrated an increase in the number of total lymphocytes during the periparturient period in cows with metritis, specifically two weeks prior to calving (Magata et al., 2016). Interestingly, these studies also demonstrated an increase in the number of CD8+ lymphocytes in the week preceding calving in opposition to our own data here, which suggests there is no difference in the proportion of prepartum CD3+/CD8+ in cows that go on to develop uterine disease. However, it is important to note that data presented here is representative of the proportion of αβ TCR CD8+ cells, not total CD8+ cells as previously reported. While proportional changes to cell populations are suggestive of immune competence, the absolute number of circulating cells is perhaps more indicative of functional changes to the peripheral immune system. Due to the limitations of the studies described here, absolute cell numbers could not be calculated and must be performed in future studies.
Postpartum peripheral lymphocytes are reduced in animals with delayed uterine involution (Levkut et al., 2002). Specifically, cows with delayed uterine involution have reduced CD2+, CD4+, CD8+ and B-cells in peripheral blood. The duration of uterine involution is positively associated with uterine disease, and as such depletion of these cellular subpopulations may be causative of delayed uterine involution, increasing the risk of uterine disease (Heppelmann et al., 2015; Melendez et al., 2004). However, these studies did not quantify changes to lymphocyte populations during the prepartum period which may predispose to delayed uterine involution and subsequent uterine disease. Changes observed here in the proportion of CD3+/CD4+ T cells during the prepartum period of cows that would develop uterine disease may contribute to altered uterine involution and subsequent susceptibility to uterine disease.
While the proportion of circulating lymphocytes may be important to establishing a robust immune response to a pathogen, it is the functional capacity of these cells to respond to a stimulus that is important in a robust immune response. Interleukin-1β is transcribed as a proprotein and activated by caspase in the inflammasome before mediating pleiotropic proinflammatory effects (Ren and Torres, 2009). Our data describe an absence of stimulation induced IL-1β secretion in PBMCs in the prepartum period. A reduction in the capacity of PBMCs to secrete IL-1β during the prepartum period or active disease may be a causative reason behind the establishment of metritis or the duration of disease during the postpartum period. Interleukin-1β regulates expression of secondary inflammatory mediators which coordinate inflammation and activation of the adaptive immune system at the site of infection, as subsequently has a regulatory role in inflammation during disease (Weber et al., 2010). In addition, IL-1β is also involved in immune sensing of tissue damage by the NOD-like receptor (NLR), NALP3 (Weber et al., 2010), similar to what occurs at the time of parturition due to dystocia (and other tissue damage associated risk factors for uterine disease). Indeed, the level of IL-1β secretion by circulating PBMCs from animals with subclinical endometritis is greater than healthy herd mates, suggesting that IL-1β plays a role during active disease (Duvel et al., 2014). There are a number of cytokines responsive to IL-1β, including IL-6 and IL-10 which should be assessed in endometrial tissue at the time of infection onset in cows. This would provide insight into whether the observed reduction in IL-1β secretion alters the functional response of the immune system to infectious pathogens. Additionally, the functional capacity of the cow to mount a robust antibody response to a foreign antigen introduced at the time of calving was not effected in cows with metritis. To some extent this is surprising considering the proportional reduction of B-cells observed during the postpartum period. To our knowledge this is the first report to evaluate humoral immune response or B-cell populations in the metritis dairy cow.
Currently, only risk factors and not causative agents, have been associated with the development of uterine disease which generally occur in the postpartum period, including dystocia, primiparity, twinning, retained placenta and ketosis. It is important to understand the high metabolic demands of immune function in parallel with demands for milk production in the dairy cow (Kvidera et al., 2017). Indeed, high milk production in multiparous cows reduces the functional capacity of innate immune cells potentially compromising their ability to stave off pathogen infection in early lactation (Nonnecke et al., 2003). The metabolic demands of immunity may also be associated with the fact that reduced dry matter intake during the prepartum period is associated with an increased incidence of uterine disease (Huzzey et al., 2007). In conjunction with reduced dry matter intake, cows with reduced prepartum circulating non-esterified fatty acid (NEFA) and insulin-like growth factor 1 (IGF-I) have an increased risk of developing uterine disease (Giuliodori et al., 2013). It may well be that negative energy balance associated with high milk production causes decreases in the functional capacity of the adaptive immune system during the prepartum period.
The role of lymphocyte populations in regard to uterine disease of the dairy cow has been poorly investigated, while the innate immune system has been evaluated more extensively (Hoeben et al., 2000; Kehrli et al., 1989; Rinaldi et al., 2008). The data presented here suggest that functionality and proportions of specific PBMCs are altered in periparturient cows with metritis, and may be involved with the development and duration of metritis. Further investigation into this area may lead to strategies to modulate or improve the immune system and decrease the incidence of uterine disease in the high producing dairy cow.
Supplementary Material
Peripheral blood mononuclear cells were first gated for lymphocytes using forward (FSC) and side (SSC) scatter parameters. Viable lymphocytes were gated as propidium iodine (PI) negative, and singlet cells were then gated by FSC-height and area. Finally cells were gated for antibody specific markers, CD4 and CD62 (A), or CD3 followed by gating for CD4 and CD8 (B). Cells were analyzed with a BD Accuri C6 flow cytometer followed by data analysis by FlowJo V10.0.7.
HIGHLIGHTS.
Uterine infections in the postpartum dairy cow, including metritis, are common and costly to producers.
Data presented here describe changes to proportions of circulating lymphocyte populations during the periparturient period of cows with metritis.
Functional capacity of peripheral blood mononuclear cells to secrete IL-1β and IFNγ is compromised during the periparturient period of cows with metritis.
ACKNOWLEDGMENTS
The authors would like to thank the staff of the University of Florida Dairy Unit and Dr John Driver for assistance with experiments.
FUNDING
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of interest: none.
REFERENCES
- Artiaga BL, Whitener RL, Staples CR, Driver JP, 2014. Adjuvant effects of therapeutic glycolipids administered to a cohort of NKT cell-diverse pigs. Vet. Immunol. Immunopathol 162, 1–13. [DOI] [PubMed] [Google Scholar]
- Borsberry S, Dobson H, 1989. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet. Rec 124, 217–219. [DOI] [PubMed] [Google Scholar]
- Bruun J, Ersboll AK, Alban L, 2002. Risk factors for metritis in Danish dairy cows. Prev. Vet. Med 54, 179–190. [DOI] [PubMed] [Google Scholar]
- Dickerson CL, Taylor RL, Drutz DJ, 1983. Susceptibility of congenitally athymic (nude) mice to sporotrichosis. Infect. Immun 40, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen MJ, Joop K, Sturk A, Bols PE, Lohuis JA, 2000. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 54, 1019–1032. [DOI] [PubMed] [Google Scholar]
- Drillich M, Beetz O, Pfutzner A, Sabin M, Sabin HJ, Kutzer P, Nattermann H, Heuwieser W, 2001. Evaluation of a systemic antibiotic treatment of toxic puerperal metritis in dairy cows. J. Dairy Sci 84, 2010–2017. [DOI] [PubMed] [Google Scholar]
- Duvel A, Maass J, Heppelmann M, Hussen J, Koy M, Piechotta M, Sandra O, Smith DG, Sheldon IM, Dieuzy-Labaye I, Zieger P, Schuberth HJ, 2014. Peripheral blood leukocytes of cows with subclinical endometritis show an altered cellular composition and gene expression. Theriogenology 81, 906–917. [DOI] [PubMed] [Google Scholar]
- Gilbert RO, Santos NR, 2016. Dynamics of postpartum endometrial cytology and bacteriology and their relationship to fertility in dairy cows. Theriogenology 85, 1367–1374. [DOI] [PubMed] [Google Scholar]
- Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M, 2005. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64, 1879–1888. [DOI] [PubMed] [Google Scholar]
- Giuliodori MJ, Magnasco RP, Becu-Villalobos D, Lacau-Mengido IM, Risco CA, de la Sota RL, 2013. Metritis in dairy cows: risk factors and reproductive performance. J. Dairy Sci 96, 3621–3631. [DOI] [PubMed] [Google Scholar]
- Griffin JF, Hartigan PJ, Nunn WR, 1974. Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology 1, 91–106. [DOI] [PubMed] [Google Scholar]
- Haimerl P, Heuwieser W, 2014. Invited review: Antibiotic treatment of metritis in dairy cows: A systematic approach. J. Dairy Sci 97, 6649–6661. [DOI] [PubMed] [Google Scholar]
- Heppelmann M, Krach K, Krueger L, Benz P, Herzog K, Piechotta M, Hoedemaker M, Bollwein H, 2015. The effect of metritis and subclinical hypocalcemia on uterine involution in dairy cows evaluated by sonomicrometry. J Reprod Dev 61, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, Bryant CE, Sheldon IM, 2006. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 147, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine BC, Cartwright SL, Mallard BA, 2011. Effect of age and pregnancy status on adaptive immune responses of Canadian Holstein replacement heifers. J. Dairy Sci 94, 981–991. [DOI] [PubMed] [Google Scholar]
- Hoeben D, Monfardini E, Opsomer G, Burvenich C, Dosogne H, De Kruif A, Beckers JF, 2000. Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J. Dairy Res 67, 249–259. [DOI] [PubMed] [Google Scholar]
- Hoek A, Rutten VP, Kool J, Arkesteijn GJ, Bouwstra RJ, Van Rhijn I, Koets AP, 2009. Subpopulations of bovine WC1(+) gammadelta T cells rather than CD4(+)CD25(high) Foxp3(+) T cells act as immune regulatory cells ex vivo. Vet. Res 40, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzzey JM, Veira DM, Weary DM, von Keyserlingk MA, 2007. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J. Dairy Sci 90, 3220–3233. [DOI] [PubMed] [Google Scholar]
- Kehrli ME Jr., Nonnecke BJ, Roth JA, 1989. Alterations in bovine neutrophil function during the periparturient period. Am. J. Vet. Res 50, 207–214. [PubMed] [Google Scholar]
- Kvidera SK, Horst EA, Abuajamieh M, Mayorga EJ, Fernandez MV, Baumgard LH, 2017. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci 100, 2360–2374. [DOI] [PubMed] [Google Scholar]
- LeBlanc SJ, 2012. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod Domest Anim 47 Suppl 5, 18–30. [DOI] [PubMed] [Google Scholar]
- LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH, 2002. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci 85, 2223–2236. [DOI] [PubMed] [Google Scholar]
- Levkut M, Pisti J, Revajová V, Choma J, Levkutová M, Dávid V, 2002. Comparison of immune parameters in cows with normal and prolonged involution time of uterus. Vet. Med. – Czech 47, 277–282. [Google Scholar]
- Machado VS, Bicalho ML, Meira Junior EB, Rossi R, Ribeiro BL, Lima S, Santos T, Kussler A, Foditsch C, Ganda EK, Oikonomou G, Cheong SH, Gilbert RO, Bicalho RC, 2014. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS One 9, e91734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR, Hein WR, 1989. A large proportion of bovine T cells express the gamma delta T cell receptor and show a distinct tissue distribution and surface phenotype. Int. Immunol 1, 540–545. [DOI] [PubMed] [Google Scholar]
- Magata F, Kitaoka R, Morino I, Teramura M, Kawashima C, Haneda S, Shimizu T, 2016. Long-term impact of puerperal metritis on the profiles of peripheral blood leukocytes in peripartum dairy cows. Anim Sci J 87, 151–155. [DOI] [PubMed] [Google Scholar]
- Martinez N, Risco CA, Lima FS, Bisinotto RS, Greco LF, Ribeiro ES, Maunsell F, Galvao K, Santos JE, 2012. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci 95, 7158–7172. [DOI] [PubMed] [Google Scholar]
- Melendez P, McHale J, Bartolome J, Archbald LF, Donovan GA, 2004. Uterine involution and fertility of holstein cows subsequent to early postpartum PGF2alpha treatment for acute puerperal metritis. J. Dairy Sci 87, 3238–3246. [DOI] [PubMed] [Google Scholar]
- Miller CP, Hammond CW, Anderle SK, 1960. Studies on susceptibility to infection following ionizing radiation. V. Comparison of intraperitoneal and intravenous challenge at intervals following different doses of x-radiation. J. Exp. Med 111, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SG, Ericsson AC, Poock SE, Melendez P, Lucy MC, 2017. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci 100, 4953–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnecke BJ, Kimura K, Goff JP, Kehrli ME Jr., 2003. Effects of the mammary gland on functional capacities of blood mononuclear leukocyte populations from periparturient cows. J. Dairy Sci 86, 2359–2368. [DOI] [PubMed] [Google Scholar]
- Pinedo PJ, Galvao KN, Seabury CM, 2013. Innate immune gene variation and differential susceptibility to uterine diseases in Holstein cows. Theriogenology 80, 384–390. [DOI] [PubMed] [Google Scholar]
- Potter TJ, Guitian J, Fishwick J, Gordon PJ, Sheldon IM, 2010. Risk factors for clinical endometritis in postpartum dairy cattle. Theriogenology 74, 127–134. [DOI] [PubMed] [Google Scholar]
- Ren K, Torres R, 2009. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev 60, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro ES, Lima FS, Greco LF, Bisinotto RS, Monteiro AP, Favoreto M, Ayres H, Marsola RS, Martinez N, Thatcher WW, Santos JE, 2013. Prevalence of periparturient diseases and effects on fertility of seasonally calving grazing dairy cows supplemented with concentrates. J. Dairy Sci 96, 5682–5697. [DOI] [PubMed] [Google Scholar]
- Rinaldi M, Moroni P, Paape MJ, Bannerman DD, 2008. Differential alterations in the ability of bovine neutrophils to generate extracellular and intracellular reactive oxygen species during the periparturient period. Vet. J 178, 208–213. [DOI] [PubMed] [Google Scholar]
- Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ, 2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod 81, 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H, 2002. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 123, 837–845. [PubMed] [Google Scholar]
- Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, Chandler A, Roberts MH, Price SB, Gilbert RO, Simpson KW, 2010. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS One 5, e9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears JW, 2000. Micronutrients and immune function in cattle. Proc Nutr Soc 59, 587–594. [DOI] [PubMed] [Google Scholar]
- Swangchan-Uthai T, Chen Q, Kirton SE, Fenwick MA, Cheng Z, Patton J, Fouladi-Nashta AA, Wathes DC, 2013. Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the post-partum dairy cow. Reproduction 145, 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Wang CY, Stashenko P, 1997. Increased susceptibility of RAG-2 SCID mice to dissemination of endodontic infections. Infect. Immun 65, 3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ML, Cronin JG, Noleto PG, Sheldon IM, 2016. Glucose Availability and AMP-Activated Protein Kinase Link Energy Metabolism and Innate Immunity in the Bovine Endometrium. PLoS One 11, e0151416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M, 2010. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal 3, cm2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peripheral blood mononuclear cells were first gated for lymphocytes using forward (FSC) and side (SSC) scatter parameters. Viable lymphocytes were gated as propidium iodine (PI) negative, and singlet cells were then gated by FSC-height and area. Finally cells were gated for antibody specific markers, CD4 and CD62 (A), or CD3 followed by gating for CD4 and CD8 (B). Cells were analyzed with a BD Accuri C6 flow cytometer followed by data analysis by FlowJo V10.0.7.


