Abstract
Background:
A high degree of co-morbidity exists between methamphetamine (MA) addiction and alcohol use disorders and both sequential and simultaneous MA-alcohol mixing increases risk for co-abuse. As little preclinical work has focused on the biobehavioral interactions between MA and alcohol within the context of drug-taking behavior, we employed simple murine models of voluntary oral drug consumption to examine how prior histories of either MA- or alcohol-taking influence the intake of the other drug.
Methods:
In one study, mice with a 10-day history of binge alcohol-drinking [5,10, 20 and 40% (v/v); 2 h/day] were trained to self-administer oral MA in an operant-conditioning paradigm (10–40 mg/L). In a second study, mice with a 10-day history of limited-access oral MA-drinking (5, 10, 20 and 40 mg/L; 2 h/day) were presented with alcohol (5–40% v/v; 2 h/day) and then a choice between solutions of 20% alcohol, 10 mg/L MA or their mix.
Results:
Under operant-conditioning procedures, alcohol-drinking mice exhibited less MA reinforcement overall, than water controls. However, when drug availability was not behaviorally-contingent, alcohol-drinking mice consumed more MA and exhibited greater preference for the 10 mg/L MA solution than drug-naïve and combination drug-experienced mice. Conversely, prior MA-drinking history increased alcohol intake across a range of alcohol concentrations.
Discussion:
These exploratory studies indicate the feasibility of employing procedurally simple murine models of sequential and simultaneous oral MA-alcohol mixing of relevance to advancing our biobehavioral understanding of MA-alcohol co-abuse.
Keywords: Co-abuse, Self-administration, Oral methamphetamine, Animal models, Binge drinking, Reinforcement
1. Introduction
The prevalence of methamphetamine (MA) and alcohol co-abuse is high, with MA ranking 3rd as the illicit drug most co-abused in individuals with alcohol-use disorders (AUDs) (e.g., UN Office on Drugs and Crime, 2015). Conversely, the percentage of current MA users that report alcohol co-abuse (a.k.a. mixing) ranges from 34 to 99% (e.g., Brecht et al., 2007; Celentano et al., 2008; Furr et al., 2000; O’Grady et al., 2008; Sattah et al., 2002). Prior AUD history is a major predisposing factor for MA abuse, with recent excessive alcohol consumption associated with a 4–5-fold greater incidence of co-abuse (e.g., Brecht et al., 2007; Bujarski et al., 2014; Chen et al., 2014; Furr et al., 2000; Herbeck et al., 2013; O’Grady et al., 2008; Sattah et al., 2002) and coabuse is a risk factor for treatment discontinuation and non-compliance in MA-dependent individuals (Brecht et al., 2005). This latter fact is particularly serious as primary MA use accounts for ~30% of all addiction treatment admissions in the U.S. (SAMHSA, 2009, 2012), the world-wide treatment admission rate for MA use is rising annually (UN Office on Drugs and Crime, 2015) and currently, there exists no effective treatment for MA addiction, let alone addiction co-morbidity.
In humans, the increased MA abuse risk observed in problem drinkers reflects, in part, alcohol’s ability to potentiate MA’s stimulant-related subjective effects (Bershad et al., 2015; Kirkpatrick et al., 2012a; Mendelson et al., 1995). Of direct relevance here, prescription MA (Desoxyn) has high abuse liability (NIDA, 2013) and oral MA administration at doses of 20 or 40 mg (i.e., 0.33 or 0.66 mg/kg) elicit positive subjective effects in current stimulant abusers (e.g., Kirkpatrick et al., 2012b) and MA-alcohol co-abusers (c.f., Bershad et al., 2015; Kirkpatrick et al., 2012a, 2012b). In these populations, an alcoholic beverage increases ratings of “good drug effect”, “drug liking” and “desire to take drug”, over that produced by oral MA alone (Bershad et al., 2015; Kirkpatrick et al., 2012a). Thus, both sequential and simultaneous MA-alcohol mixing increases risk for co-abuse in humans. Yet, there is little biobehavioral research into the sequelae of MA-alcohol interactions to inform abuse-related outcomes (Gutierrez-Lopez et al., 2010; Winkler et al., 2016). Thus, we conducted two exploratory studies designed to probe MA-alcohol interactions in voluntary drug-taking, with the intention of developing procedurally facile murine models of sequential and simultaneous MA-alcohol mixing suitable for the high-throughput biobehavioral study of MA-alcohol co-abuse.
2. Materials and methods
2.1. Subjects
Experiment 1 (Fig. 1A) employed adult (8–10 weeks old) male and female mice on a mixed C57BL/6J and 129 × 1/SvJ genetic background (B6.129) that were generated in house at the University of California Santa Barbara. These mice were selected for this study primarily because they commonly serve as the background strain for transgenic mice, with the parental strains exhibiting differences in response to drugs of abuse, including cocaine (Schlussman et al., 1998, 2003), heroin (Szumlinski et al., 2005), morphine (e.g., Belknap et al., 1993b; Dockstader and van der Kooy, 2001; Metten et al., 2009) and alcohol (e.g., Belknap et al., 1993a; Homanics et al., 1999), but there is extremely limited information regarding their response to methamphetamine (Szumlinski et al., 2017) or alcohol-methamphetamine mixing. Experiment 2 (Fig. 2A) employed adult (8 weeks old), male C57BL/6J (B6) mice, obtained from Jackson Laboratories (Sacramento, CA). This exploratory study was a small part of a larger research effort in our laboratory to study the neurobiology of MA addiction in this common mouse strain (see Lominac et al., 2014, 2016; Szumlinski et al., 2017). B6 mice were allowed to acclimatize to the housing conditions for 10 days prior to experimentation.
Fig. 1.
Summary of the effects of a prior history of binge alcohol-drinking upon MA reinforcement. (A) Procedural time-line for Experimental 1. During the alcohol-drinking phase of this experiment, females exhibited: (B) a shift upwards in the dose-response function for alcohol intake; (C) consistently higher levels of daily total alcohol intake; and (D) greater average total alcohol intake, than their male counterparts. For panels B-D,]* indicates a main Sex effect (ANOVA, p < 0.05). (E) When compared to water-drinking controls (Prior Water), male and female mice with a prior history of binge ls. (G) The dose-response function for MA intake was shifted downwards, but to the left, of water controls. (H) No effect of prior alcohol history was observed upon the percentage of total responses directed at the active hole (% Active Hole Responding) during the initial 5 days of operant-conditioning. (I) However, alcohol-experienced mice exhibited lower response allocation with increasing response demand and (J) the dose-response function for response allocation was shifted downwards in alcohol-experienced mice, relative to water controls. The data represent the mean ± SEMs of the number of mice indicated in parentheses. For panels E-J,]* indicates a main Binge History effect (p < 0.005); *p < 0.05 vs. Prior Water (tests for simple main effects).’.
Fig. 2.
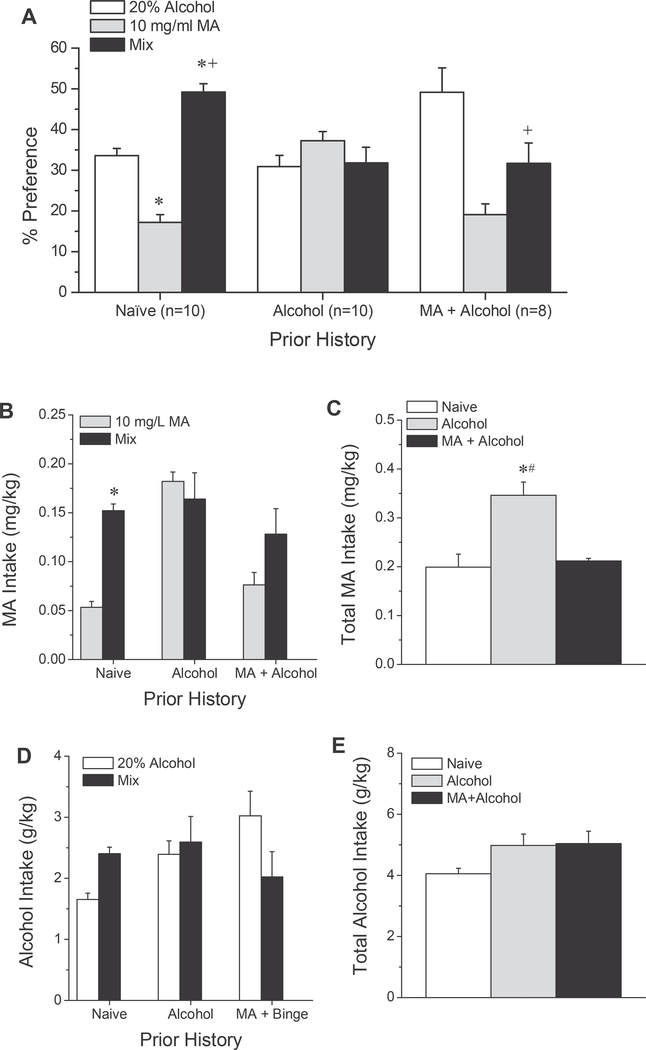
Summary of oral MA by B6 mice. (A) Procedural time-line for Experimental 2. (B) Summary of the dose-response function for the average MA intake in the home cage, indicating that the majority of daily MA intake by adult, male, B6 mice was derived from the 40 mg/L solution. (C) The average total daily MA intake fluctuated over the course of the 10-day drinking period, with mice exhibiting an escalation of total MA intake from the first to the last day of drinking. (D) The average total daily MA intake from each of the different MA concentrations over the course of the 10-day drinking period indicated that the intake of both the 20 and 40 mg/L concentrations fluctuated over the 10-day drinking period. The data represent the means ± SEMs of 8 mice.
In both experiments, mice were housed individually in standard mouse cages on a ventilated rack, in a temperature and humidity-controlled colony room, under a 12-h reverse light cycle (lights off: 10 am). All procedures were approved by the Institutional Animal Care and Use Committee of the University of California Santa Barbara and were conducted according to the guidelines outlined in the Guide to the Care and Use of Laboratory Animals (6th edition, revised 2014). Note that one female B6.129 mouse developed ulcerative dermatitis during testing for oral MA reinforcement that did not respond to nursing care and was dropped from the study.
2.2. Experimental designs
Two independent experiments were conducted in order to probe the potential interactions between methamphetamine and alcohol with respect to measures of drug-taking. As outlined in Fig. 1A, the mice in Experiment 1 were first allowed to binge-drink alcohol in the home cage and then MA reinforcement and intake were determined under operant-conditioning procedures, as detailed below. As access to the operant-conditioning equipment was limited at the time of study, the mice in Experiment 2 were first trained to drink MA in the home cage and the effects of this prior MA experience upon alcohol intake in the home cage was assessed (Fig. 2A). As the aforementioned experimental designs assayed for the effects of sequential drug experience, mice in Experiment 2 were also presented with a choice between an alcoholonly solution, a MA-only solution and a solution of a mix of alcohol and MA (see Fig. 2A, right). This was done to determine whether or not (1) drug-naïve mice preferred a simultaneous MA-alcohol mix over single-drug solutions alone and (2) a prior history of either alcohol-drinking alone or sequential MA and alcohol drinking influences mix preference. The details of the experimental procedures employed in these two experiments are provided in the subsections below.
2.2. Binge-alcohol drinking procedures
Both experiments employed a modified version of the Drinking-in-the-Dark (DID) binge alcohol-drinking paradigm (see Rhodes et al., 2005), in which mice are presented simultaneously with 4 sipper tubes containing 5, 10, 20 and 40% alcohol (v/v) for a total of 2 h, beginning at 3 h into the dark phase of the circadian cycle (Cozzoli et al., 2014). In Experiment 1, mice were presented with alcohol 5 days/week (Mon-Fri) for 2 weeks for a total of 10 days (Fig. 1A). In Experiment 2, mice were presented with alcohol for 7 days, following the end of the MA drinking period (Fig. 2A). Animals were weighed weekly. The amount of alcohol consumed at each concentration were calculated as function of the animals’ body weight and corrected for spillage induced by bottle handling. Water-drinking animals served as controls.
2.3. Home-cage MA drinking procedures
We also employed a multi-bottle-choice procedure to entice the consumption of unsweetened MA solutions in Experiment 2 (Fig. 2A). The MA bottle-presentation procedures were identical to those described in Section 2.2, with the exception that solutions of 5, 10, 20 and 40 mg/L MA were presented. These MA concentrations are voluntarily consumed by B6 mice (e.g., Shabani et al., 2012; Wheeler et al., 2009) and are reinforcing in both B6 and B6.129 hybrid mice (Szumlinski et al., 2017). Again, water-drinking animals served as controls.
2.4. Drug-choice drinking procedures
Upon completion of testing for binge alcohol-drinking, the mice in Experiment 2 were then offered a choice between a 20% (v/v) alcohol solution, a 10 mg/L MA solution or a solution of 10 mg/L MA in 20% alcohol (i.e., a simultaneous mix) to determine how simultaneous MA-alcohol mixing alters drug preference/intake (Fig. 2A). The 20% alcohol and 10 mg/L MA solutions were selected to be employed as the simultaneous mix to mimimize the contribution of a ceiling effect upon intake and the mice were presented with the 3 solutions over the course of 3 consecutive days. As controls, a group of drug-naïve mice and mice with a 7-day history of binge alcohol-drinking only were included.
2.5. MA reinforcement
The procedures for training the B6.129 hybrid mice in Expeirment 1 to respond for oral MA reinforcement and the apparatus used for operant-conditioning were similar to those described recently by our group (see Szumlinski et al., 2017). Behavioral training and testing for MA reinforcement was conducted Mon-Fri, over the course of several months, as outlined in Fig. 1A. First, mice were trained to nose-poke for delivery of a 10 mg/L MA solution under an FR1 reinforcement schedule (i.e., one poke/reinforcer) with 20 s time-out for a total of 10 training session. As illustrated in Fig. 1A, following this 10-day training period, the response requirement was increased to 2 pokes/reinforcer (FR2) for 5 days and then to 5 pokes/reinforcer (FR5) for 5 days. As we observed an inverse relationship between MA intake and response requirement (see Fig. 1F), the dose-response phase of testing (2.5, 5, 10, 20 and 40 mg/L) was conducted under the original FR1 schedule. For dose-response testing, each concentration was presented, in ascending order, until responding on the active hole stabilized (less than 25% variability across 3 consecutive days) or for a maximum of 5 days and the average of the last 3 days of responding was employed in the statistical analyses of the results. To determine the volume of the MA solution consumed during each 1-h session, the volume of MA remaining in the well was determined by pipetting and subtracted from the total volume delivered during the session. The amount of MA consumed each day was expressed as a function of the animal’s body weight, which was determined weekly.
2.6. Statistical analyses
The data were analyzed with SPSS v.23 software using mixed ANOVAs, and significant interactions were deconstructed and analyzed using tests for simple main effects (corrected for multiple comparisons) and/or LSD post-hoc tests, when appropriate. For comparisons involving only two means, t-tests for dependent or independent conditions were used. Alpha was set to 0.05 and all analyses were conducted two-tailed.
3. Results
3.1. Study 1: binge-drinking blunts MA reinforcement
3.1.1. Alcohol intake prior to operant-conditioning
B6.129 mice consumed the greatest amount of alcohol from the 40% solution (Fig. 1B) [Alcohol Concentration effect: F(3,42) = 60.57, p < 0.0001]. While the total alcohol consumption of the mice fluctuated somewhat over the 10 days of limited alcohol-access (Fig. 1C), the shape of the alcohol concentration-response function remained stable (not shown) [Day effect: F(9,126) = 4.19, p < 0.0001; no Alcohol Concentration X Day interaction, p > 0.20]. As expected based on the literature (e.g., Melón et al., 2013), female mice consumed more alcohol than males, with a shift upwards in the alcohol concentration-intake function (Fig. 1B) and greater total alcohol intake across days [Sex effect: F(1,14) = 5.63, p = 0.03; no Sex x Alcohol Concentration or Sex X Day interactions, p’s > 0.15]. Despite sex differences in the average alcohol intake (Fig. 1D), no sex difference was apparent in the BACs attained immediately following the 4th day of alcohol-drinking [males: 144.90 ± 11.90 vs. females: 155.44 ± 13.43 mg/dL; t-test: p = 0.57]. As these BACs were well above the 80 mg/dL criterion for binge-drinking put forth by NIAAA (NIAAA, 2007), both the male and female mice were binge alcohol-drinking prior to testing for MA reinforcement.
3.1.2. Acquisition of oral MA reinforcement
Prior alcohol-water differences in MA intake were not observed for either sex during the first 5 days of FR1 training (Fig. 1E) [Day effect: F (4,96) = 15.40, p < 0.0001; no Binge History effects or interactions, p’s > 0.25]. The average MA intake during this initial training period was equivalent between alcohol-bingers and water controls [Water = 0.28 ± 0.04 mg/kg vs. Alcohol = 0.24 ± 0.52 mg/kg] and there was no sex difference in response allocation or MA intake during initial training (for both variables, Sex effect and interactions, p’s > 0.25). Likewise, alcohol-water differences in response-allocation were not apparent during the first 5 days of nose-poke training (Fig. 1H) [Day effect: F(4,96) = 4.07, p = 0.004; no Binge History effect or interactions, p’s > 0.25].
However, over the subsequent weeks, MA intake dropped precipitously in all animals as task demand increased (Fig. 1F) [Schedule effect: F(2,48) = 30.90, p < 0.0001]. Importantly, mice with a prior alcohol history exhibited lower MA intake than controls during this phase of training (Fig. 1F) [Binge History effect: F(1,24) = 5.70, p = 0.03; Binge History X Schedule: F(2,48) = 3.19, p = 0.05; other effects and interactions, p’s > 0.80], with group differences detected during the 2nd week of training under the FR1 reinforcement schedule [test for simple main effects: F(1,26) = 4.44, p = 0.03], which is suggestive, but not significant when a Bonferroni correction is applied. However, a significant group difference was observed under the FR2 reinforcement schedule [test for simple main effects: F(1,26) = 8.24, p = 0.008], but not under the FR5 schedule, when MA intake was very low (test for simple main effects, p = 0.13). In contrast to MA intake, response-allocation was maintained in both groups as task demand increased; however, alcohol-bingers allocated a lower proportion of total nose-pokes towards the MA-reinforced hole, overall, than water controls (Fig. 1I) [Binge History effect: F(1,23) = 4.70, p = 0.04; other main effects and interactions, p’s > 0.06]. No sex differences in either MA intake or response allocation were apparent during this phase of testing (for both variables, Sex effect and interactions: all p’s > 0.30).
3.1.3. MA concentration-response testing
The MA concentration-intake function was shifted downwards in alcohol-experienced mice versus water controls (Fig. 1G) [Binge History effect: F(1,24) = 5.15, p = 0.03; Dose effect: F(4,96) = 37.54, p < 0.0001; Binge History X MA Concentration: F(4,96) = 5.32, p = 0.001 and this alcohol effect was observed irrespective of sex (no Sex effect or interactions, p’s > 0.20). Tests for simple main effects indicated that alcohol-bingers exhibited lower MA intake at the 5 mg/L [F(1,26) = 5.81, p = 0.02] and 10 mg/L [F(1,26) = 4.93, p = 0.04], but these effects were not significant when a Bonferroni correction was applied. However, a significant reduction nin MA intake was observed in alcohol-bingers at the highest MA concentration tested (40 mg/L) [F (1,26) = 7.13, p = 0.01] concentrations.
The concentration-response function for MA reinforcement was also shifted downwards in alcohol-experienced mice vs. water controls (Fig. 1J) [Binge History effect: F(1,24) = 4.97, p = 0.04; Dose effect: F (4,96) = 4.15, p = 0.004; no Binge History interactions, p’s > 0.40]. For this variable, a sex difference in the shape of the dose-response-allocation function was detected by ANOVA [Sex X Dose: F(4,96) = 2.71, p = 0.04], however, tests for simple main effects post-hoc comparisons between male and female subjects failed to indicate significant sex differences at any MA dose (see Table 1).
Table 1.
Comparison of the MA dose-response allocation function for male and female B6.129 mice. Although a significant Sex X Dose interaction was detected by ANOVA, post-hoc comparisons failed to indicate significant group differences at any of the MA doses. Data represent the means ± SEMs of the number of mice indicated.
| MA dose (mg/L) | Females (n = 14) | Males (n = 14) | Post-hoc result |
|---|---|---|---|
| 2.5 | 76.89 ± 3.84 | 77.03 ± 4.08 | F(1,26) = 0.009, p = 0.98 |
| 5 | 77.49 ± 2.92 | 74.74 ± 5.39 | F(1,26) = 0.20, p = 0.66 |
| 10 | 73.10 ± 5.10 | 64.11 ± 6.27 | F(1,26) = 1.23, p = 0.28 |
| 20 | 78.29 ± 3.99 | 80.27 ± 3.88 | F(1,26) = 0.13, p = 0.73 |
| 40 | 72.88 ± 4.33 | 82.79 ± 2.90 | F(1,26) = 3.61, p = 0.07 |
3.2. Experiment 2: a prior history of MA-taking augments subsequent binge alcohol-drinking
3.2.1. MA intake in the home cage
Male B6 mice consumed the majority of their daily MA intake from the 40 mg/L MA solution, with a test of within-subjects contrasts indicating linearity within the dose-range tested (Fig. 2B) [F(1,7) = 66.94, p < 0.0001]. The total daily oral MA intake varied across the 10 days of MA availability (Fig. 2C), as did the MA concentration-intake function (Fig. 2D) [Day effect: F(9,63) = 26.83, p < 0.0001; MA Concentration X Day: F(27,189) = 2.25, p = 0.001]. The MA Concentration X Day interaction reflected different experience-dependent changes in the daily intake of all four MA solutions over the 10-day MA drinking period [one-way ANOVAs, 5 mg/L: F(9,63) = 4.08, p < 0.0001; 10 mg/L: F(9,63) = 5.98, p < 0.0001; 20 mg/L: F(9,63) = 3.21, p = 0.003; 40 mg/L: F(9,63) = 3.24, p = 0.003], with tests of within-subjects contrasts indicating linear increases in the intake of both 5 mg/L MA (Fig. 4C) and 10 mg/L MA (Fig. 2C) [5 mg/L: F(1,7) = 22.08, p = 0.002; 10 mg/L: F(1,7) = 26.71, p = 0.001]. In contrast, the intake of 20 and 40 mg/L MA exhibited more complex patterns of change (Fig. 2C) [for 20 mg/L, 8th order: F(1,7) = 15.30, p = 0.006; for 40 mg/L, 5th order: F(1,7) = 27.24, p = 0.001]. Thus, when MA-access is limited, male B6 mice escalate their intake of lower MA concentrations, but exhibit higher, undulating, patterns of intake at higher MA concentrations.
Fig. 4.
Effects of prior drug/alcohol history upon the preference for a simultaneous MA-alcohol mix. Summary of the results from the drug-choice study. (A) Naïve mice preferred the mix solution over both single-drug solutions, while Alcohol mice did not discriminate. MA + Alcohol mice preferred equally the alcohol-only and mix solutions and did so above that of MA alone. *p < 0.05 vs. 20% EtOH, +p < 0.05 vs. 10 mg/L MA (LSD post-hoc tests). (B) The average amount of MA consumed from each of the MA-containing solutions indicated greater MA intake from the mix in both Naïve and MA + Alcohol mice. *p < 0.05 vs. 10 mg/L MA (LSD post-hoc tests). (C) The average total MA intake from the two MA-containing solutions was greatest in Alcohol mice. *p < 0.05 vs. Naïve; #p < 0.05 vs. MA + Binge (LSD post-hoc tests). In contrast, a prior history of neither binge-drinking nor sequential MA-taking and alcohol binge-drinking influenced the relative (D) or total (E) alcohol intake from the two alcohol-containing solutions. The data represent the means ± SEMs of the number of mice indicated in panel A.
3.2.2. Binge alcohol-Drinking following MA-taking experience
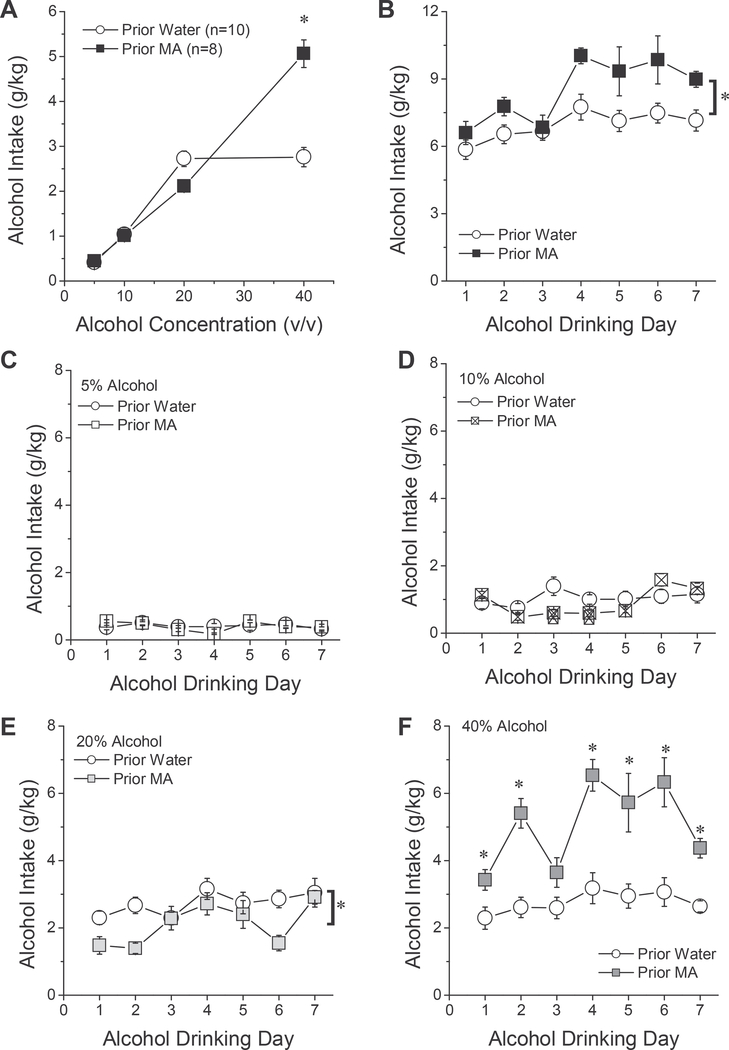
A prior history of MA-drinking augmented total daily alcohol intake and shifted the alcohol concentration-intake function (Fig. 3A,B) [MA History effect: F(1,16) = 13.53, p = 0.002; Alcohol Dose effect: F (3,48) = 174.69, p < 0.0001; Dose X MA History: F(3,48) = 30.14, p < 0.0001], but in a manner that varied as a function of alcohol-experience [Alcohol Dose X MA History X Day: F(18,288) = 3.44, p < 0.0001]. The significant 3-way interaction did not reflect MA-water differences in the intake-pattern for 5% alcohol (Fig. 3C) [Day effect: F(6,96) = 2.37, p = 0.04; MA History effect: F(1,16) = 0.006, p = 0.94; MA History X Day: p = 0.13]. In contrast, the daily intake of 10% alcohol varied as a function of MA-taking [MA History X Day: F (6,96) = 5.23, p < 0.0001], which reflected undulations in 10% alcohol intake over the course of testing in MA-experienced animals [F (6,42) = 12.62, p < 0.0001], but stable intake in controls (Fig. 3D) [F (6,54) = 1.97, p = 0.09]. Overall, MA-experienced mice exhibited lower intake of 20% alcohol versus water controls (Fig. 3E) [Day effect: F(6,96) = 4.72, p < 0.0001; MA History effect: F(1,16) = 8.46, p = 0.01; MA History X Day: p = 0.11]. Finally, group differences in alcohol intake were also apparent at the 40% concentration, but at this higher dose, MA-experienced mice consumed more alcohol than water controls, in a manner that fluctuated across drinking days [Day effect: F (6,96) = 8.01, p < 0.0001; MA History effect: F(1,16) = 39.93, p < 0.0001; MA History X Day: F(6,96) = 3.22, p = 0.006]. While the intake of 40% alcohol was stable in water controls [Day effect: F(6,54) = 1.11, p = 0.37], the higher alcohol intake exhibited by MA-experienced mice waxed and waned over the 7-day drinking period (Fig. 3F) [Day effect: F(6,42) = 6.73, p < 0.0001]. Despite group differences in the dose-dependent patterns of alcohol intake over the course of the 7day drinking period, the average total daily alcohol intake exhibited by both MA-experienced mice (8.45 ± 0.36 g/kg) and water controls (6.80 ± 0.24 g/kg) were well above that predicted to elicit BACs ≥ 80 mg/dL (Rhodes et al., 2005; see Experiment 1 above). Thus, a prior history of limited-access MA-drinking potentiates binge alcohol-drinking in B6 mice, particularly at high alcohol concentrations.
Fig. 3.
Effects of a prior history of oral MA intake upon binge alcohol-drinking. Relative to a prior history of water-drinking (Prior Water), (A) a prior history of MA-taking (Prior MA) increased the intake of 40% alcohol. (B) MA-experienced mice exhibited greater total daily alcohol intake, compared to water controls and this group difference was more or less consistent over the 7-day alcohol-drinking period. When comparing the intake of the different alcohol concentrations across the 7-day drinking period, a prior history of oral MA intake did not alter the intake of the 5% (C) or 10% (D) alcohol solutions. (E) Prior MA history reduced the intake of 20% alcohol on certain drinking days, while (F) prior MA history augmented the intake of 40% alcohol. The data represent the means ± SEMs of the number of mice indicated in parentheses in Panel A.]* indicates main prior MA History effect (p < 0.05); *p < 0.05 vs. Prior Water (tests for simple main effects).
3.2.3. Choice drinking
Both groups with a prior drug and/or alcohol history consumed a greater amount of total fluid, relative to drug-naïve controls [Naive: 0.80 ± 0.02; Alcohol: 1.23 ± 0.07; MA + Alcohol: 1.04 ± 0.09 ml; F(2,27) = 12.24, p < 0.0001; LSD post-hoc tests: Naïve vs. Alcohol: p < 0.0001; Naïve vs. MA + Alcohol: p = 0.012]. Importantly, the relative preference for the different solutions also varied as a function of prior drug/alcohol history (Fig. 4A, left) [Drug History X Solution: F (4,50) = 10.50, p < 0.0001]. This interaction reflected a greater preference for the mix over either of the single-drug solutions in drug-naïve controls [F(2,18) = 47.92, p < 0.0001; LSD post-hoc tests]. In contrast, alcohol-binging mice did not discriminate between the three solutions (Fig. 4A, middle) [F(2,18) = 0.87, p = 0.44]. As observed in drug-naïve animals, mice with a combination drug-history discriminated between the solutions [F(2,14) = 6.76, p = 0.009]. Although inspection of Fig. 4A suggested that MA/alcohol-experienced mice preferred the alcohol-only solution over the mix, this difference was not statistically significant (LSD post-hoc tests). However, these mice exhibited a significantly higher preference for the mix over the MA-only solution (Fig. 4A, right) (LSD post-hoc tests). Thus, in both drug-naïve and “mix”-experienced mice, MA preference is augmented in the presence of alcohol.
As observed for MA preference, MA intake varied with both prior drug history and simultaneous mixing, and these factors interacted to influence MA intake (Fig. 4B) [Drug History X Solution: F(2,25) = 7.05, p = 0.004; Drug History effect: F(2,25) = 12.06, p < 0.0001; Solution effect: F(1,25) = 11.05, p = 0.003]. This interaction reflected greater MA intake from the mixed versus MA-only solution in both drug-naïve mice [test for simple main effects: F(1,9) = 78.69, p < 0.0001]. and mice with a combination drug-history [F(1,7) = 9.43, p = 0.02], although the latter difference was not statistically significant upon Bonferroni correction. In contrast, MA intake from the two MA-containing solutions was comparable in alcohol-binging mice [F(1,9) = 0.37, p = 0.56]. However, when MA intake from both solution was totaled, MA intake was greatest in alcohol-binging mice (Fig. 4C) [F(2,27) = 9.46, p = 0.001; LSD post-hoc tests, Binge vs. Naïve: p < 0.0001; Binge vs. MA + Binge: p = 0.005]. These data contrast with those from Experiment 1 and suggest that the behavioral-contingency of MA availability may be a major factor influencing drug intake in alcohol-experienced mice.
A comparable analysis of alcohol intake from the alcohol-only and mixed solution did not support effects of either prior drug-taking history or simultaneous drug-mixing (Fig. 4D) [Drug History effect: F (2,25) = 2.72, p = 0.09; Solution effect: F(1,25) = 0.003, p = 0.96; Drug History X Solution: F(2,25) = 3.16, p = 0.06]. Further, prior drug-taking history did not influence the total alcohol consumption during our choice-procedures (Fig. 4E) [F(2,27) = 1.31, p = 0.29]. Importantly, the total daily alcohol intakes exhibited by the mice during choice procedures were approximately 1.5–2 g/kg less than that exhibited by the mice under our 4-bottle procedures (Fig. 2F vs. 4 E). Thus, a ceiling effect did not likely limit alcohol intake during this last phase of testing.
4. Discussion
Consistent with the human literature (e.g., Brecht et al., 2005, 2007; Chen et al., 2014; Burjarski et al., 2014; Chen et al., 2014; Furr et al., 2000; Herbeck et al., 2013; Sattah et al., 2002; O’Grady et al., 2008; Sattah et al., 2002), our exploratory study demonstrates that both sequential and simultaneous MA-alcohol mixing engenders greater drug intake in mice, at least when drug availability is behaviorally-non-contingent. Under our home-cage, limited-access procedures, the effects of sequential drug-mixing were bi-directional; a prior history of oral MA-taking potentiated binge alcohol-drinking (Fig. 3B) and vice versa (Fig. 4C). In line with evidence that an alcoholic beverage increases MA-craving over that produced by oral MA alone (Bershad et al., 2015; Kirkpatrick et al., 2012a), drug-naïve mice preferred, and consumed more MA from, a simultaneous MA-alcohol mix, relative to MA alone (Fig. 4B). In fact, the high relative preference for, and MA consumption from, the simultaneously mixed solution was similar between drug-naïve mice and mice with a prior history of sequential consumption of both drugs (Fig. 4B). Thus, factors associated with prior drug-taking history (e.g., expectancies/anticipation, conditioning, changes in drug pharmacokinetics, changes in pharmacodynamics/neuroplasticity) are not necessary to observe an alcohol-induced facilitation of oral MA-preference/taking in mice, at least when MA availability is behaviorally-noncontingent.
The facilitation of alcohol-drinking by B6 mice with a prior history of oral MA-taking observed herein (Fig. 3B) contrasts with the results of a recent study by Winkler et al. (2016), in which P rats, trained to self-administer intravenous (IV) MA during daily, 2-h, operant-conditioning sessions, exhibited reduced intake of, and preference for, 10% alcohol under several experimental variations. Importantly, a 5-day prior history of IV MA markedly reduced the initiation of alcohol- drinking by P rats and although tolerance developed to this MA effect with subsequent MA/alcohol-taking, at no point did concurrent IV MA self-administration augment the intake of 10% alcohol, above that of saline self-administering controls (Winkler et al., 2016). It is noteworthy that while prior MA history augmented total alcohol intake by B6 mice under our 4-bottle-choice, binge-drinking, procedure (Fig. 3B), this effect was driven entirely by a MA-induced potentiation of the intake of 40% alcohol (Fig. 3A,F). In fact, B6 mice with prior MA experience consumed less alcohol from the 10% and 20% solutions, relative to MA-naïve controls (although MA-induced reduction in 10% alcohol intake was inconsistent across days; Fig. 3D,E). Furthermore, the MA-induced potentiation of the intake of 40% alcohol also reflected, in part, the relatively low intake of 40% alcohol by the Prior Water controls [ < 3 g/kg (Fig. 3A) vs. ~4.5 g/kg observed in B6.129 hybrids (Fig. 1B). While other procedural differences such as the route of MA self-administration (and associated differences in dose), and the duration of alcohol-access (i.e., limited vs. continuous) very likely influence the effect of MA upon subsequent alcohol intake, the selective effect of prior MA history upon the intake of 40% alcohol by B6 mice (Fig. 3A) argues that alcohol concentration and/or choice of concentrations is another key variable to consider when designing studies of drug-alcohol mixing or co-use.
Oral MA is reinforcing in several mouse strains (e.g., Shabani et al., 2012; Szumlinski et al., 2017) and thus, we determined also the effects of a recent history of alcohol binge-drinking upon oral MA reinforcement. However, despite their excessive alcohol consumption (BA-Cs > 100 mg/dL), mice with a prior alcohol-drinking history exhibited lower levels of oral MA intake and MA-appropriate responding when MA availability was behaviorally-contingent (Fig. 1). The results in Fig. 1 also contrast with the results of Winkler et al. (2016), in which a 2-week prior history of continuous alcohol-access, in addition to concurrent alcohol-access in the home-cage, enhanced IV MA intake by P rats during the first week of operant-conditioning. However, in contrast to the present study in which the alcohol-induced reduction in MA reinforcement/intake persisted once responding had stabilized (Fig. 1E vs. 1F), the alcohol-enhancement of IV MA intake in P rats was not apparent beyond the first week of training, nor did it extend to MA reinforcement at any time during testing (Winkler et al., 2016). At the present time, too many procedural differences exist between the present study and that of Winkler et al. (2016) to delineate the relevant factors driving the conflicting results with respect to alcohol’s effect upon MA self-administration/reinforcement. However, based on the results from our choice study (Fig. 4), alcohol-availability and the opportunity to self-administer MA while under the influence of alcohol is likely a critical factor determining MA consumption in rodent models and this issue will be addressed in future studies.
MA-taking elicits a range of positive and negative drug effects in the individual user (Cruickshank and Dyer, 2009; Sheridan et al., 2009) and the perception of drug effects as appetitive versus aversive influences risk of continued drug use (Chait, 1993; de Wit et al., 1986). One major drawback of drug self-administration studies (either operant or non-operant) is their inability to inform as to whether or not changes in drug-taking reflect alterations in the motivational valence of the drug. For instance, the blunted MA intake/reinforcement exhibited by the alcohol-experienced mice in the present study could reflect decreased sensitivity to MA’s positive effects (i.e., reduced MA-reinforcer efficacy). Indeed, the concentration-response functions for both MA intake and response-allocation are shifted downwards in alcohol-experienced mice versus naïve controls. However, this interpretation is difficult to reconcile with the results from Experiment 2 in which alcohol-experienced mice: (a) exhibited a higher preference for the MA-only solution; (b) consumed more MA from the MA-only solution; and (c) consumed more MA overall, relative to both drug-naïve mice and mice with a combination drug-history. These data from the drug-choice study are in line with the alcohol-enhancement of MA-taking by P rats (Winkler et al., 2016) demonstrate that a prior alcohol-drinking history promotes, rather than prevents, MA-taking. As different strains of mice were employed in the study of MA reinforcement (B6–129 hybrids) versus limited-access intake (inbred B6), the possibility exists that the discrepancies in findings could merely reflect strain differences in MA intake or alcohol-MA interactions. Although the results of the present study (Fig. 1C vs. Fig. 3B), as well as prior work (Cozzoli et al., 2014, 2016; Lominac et al., 2016; Szumlinski et al., 2017), indicate that alcohol binge-drinking and MA reinforcement are relatively comparable between B6 and B6–129 hybrid mice, B6 mice exhibit blunted MA intake, relative to inbred DBA2/J mice – an effect attributable to genetic variance in trace amine-associated receptor 1 function (c.f., Shi et al., 2016). Thus, further work is required in order to delineate the relative roles played by species, strain within a species and experimental paradigm in the apparently conflicting effects of a prior history of alcohol-drinking intake upon MA intake under behaviorally-contingent vs. non-contingent drug availability. Furthermore, it would be important to understand how a prior history of alcohol-drinking impacts the subjective (rewarding and aversive) effects of MA and MA-mixes as measured by place-conditioning procedures. For instance, the high oral MA intake exhibited by selectively bred MAHDR mice reflect both diminished place-aversion at high MA doses, in addition to greater place-preferences induced by low-moderate MA doses (Shabani et al., 2011, 2012; Wheeler et al., 2009).
Given the outcomes from the drug-choice study, a more parsimonious explanation for the blunted MA intake/reinforcement in alcohol-binging mice may reflect facilitated sensitization of the psychomotor-activating and/or negative subjective effects of MA. Sensitization is a progressive increase in a particular drug effect that occurs upon repeated treatment. The fact that blunted reinforcement manifested during the second week of operant-training is consistent with a “sensitization-like”, process. Sensitization is also reflected by a leftward shift in the dose-response function for a particular drug effect. Indeed, estimation of the EC50 for MA intake in Experiment 1 (Fig. 1G) suggests a lower EC50 in alcohol-drinking mice (~10 mg/L), relative to alcohol-naïve controls (∼22 mg/L). High MA doses induce repetitive, stereotyped, behaviors in rodents (notably focused sniffing) and the prevalence/intensity of stereotypic behaviors sensitizes with repeated MA experience (c.f., Grant et al., 2012; Hadamitzky et al., 2012; Randrup et al., 1988). Given the nature of the nose-poke operandae employed in Experiment 1, it is entirely possible that alcohol-drinking mice were engaged in a higher degree of MA-induced stereotypy, than naïve controls, which limited their ability to respond for drug. While MA-induced stereotypy per se has not been measured in the drug cross-sensitization literature, a chronic (3-week) history of alcohol consumption is reported to augment locomotor sensitivity to d-amphetamine (Manley and Little, 1997). As we had no a priori rationale for studying motor behavior, our mice were not assayed for psychomotor activity upon completion of their operant-conditioning sessions. Thus, the potential for a prior history of binge-drinking to facilitate MA-induced stereotypy will be an important consideration for future study of MA reinforcement, particularly when considering employing nose-poke apertures as the operandae during operant-conditioning.
Higher MA doses are also anxiogenic in both humans and laboratory animals (e.g., Cruickshank and Dyer, 2009; Miladi-Gorji et al., 2015; Sheridan et al., 2009; Šlamberová et al., 2015), raising the possibility that the blunted intake/reinforcement exhibited by alcohol-drinking mice reflects a heightening of MA’s anxiogenic effects. Indeed, evidence exists for greater behavioral and neuroendocrine signs of hyper-anxiety in mice co-administered alcohol and methamphetamine, compared to mice treated with either drug alone (Chuang et al., 2011) and stress-amphetamine cross-sensitization of hyper-anxiety was recently demonstrated in humans (Booij et al., 2016). While the design of the experiments in this report cannot discern between the above possibilities, the present results argue that MA and alcohol interact in complex ways to influence drug-taking behavior, even in relatively simple murine models, and further psychopharmacological research is required in order to understand the bases of these interactions.
Acknowledgments
Role of funding source
Nothing declared.
Funding
This work was supported, in small part, by the National Institutes of Health [grant numbers AA024044 and DA039168] and largely, by a UCSB Academic Senate Individual Faculty Research Award to KKS.
Footnotes
Conflict of interest
No conflict declared
References
- Belknap JK, Crabbe JC, Riggan J, O’Toole LA, 1993a. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl.) 112, 352–358. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER, 1993b. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl.) 112, 503–510. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Kirkpatrick MG, Seiden JA, de Wit H, 2015. Effects of acute doses of prosocial drugs methamphetamine and alcohol on plasma oxytocin levels. J. Clin. Psychopharmacol 35, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Welfeld K, Leyton M, Dagher A, Boileau I, Sibon I, Baker GB, Diksic M, Soucy JP, Pruessner JC, Cawley-Fiset E, Casey KF, Benkelfat C, 2016. Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl. Psychiatry 6, e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD, 2005. Methamphetamine treatment: trends and predictors of retention and completion in a large state treatment system (1992–2002). J. Subst. Abuse Treat 29, 295–306. [DOI] [PubMed] [Google Scholar]
- Brecht ML, Greenwell L, Anglin MD, 2007. Substance use pathways to methamphetamine use among treated users. Addict. Behav 32, 24–38. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, Allen V, Hartwell E, Leventhal A, Rohrbaugh T, Ray LA, 2014. The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug Alcohol Depend 142C, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano DD, Aramrattana A, Sutcliffe CG, Sirirojn B, Quan VM, Taechareonkul S, Sherman S, Sintupat K, Thomson N, Latkin C, 2008. Associations of substance abuse and sexual risks with self-reported depressive symptoms in young adults in northern Thailand. J. Addict. Med 2, 66–73. [DOI] [PubMed] [Google Scholar]
- Chait LD, 1993. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav. Pharmacol 4, 191–199. [PubMed] [Google Scholar]
- Chen LY, Strain EC, Alexandre PK, Alexander GC, Mojtabai R, Martins SS, 2014. Correlates of nonmedical use of stimulants and methamphetamine use in a national sample. Addict. Behav 39, 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JY, Chang WT, Cherng CG, Kao GS, Yu L, 2011. Repeated co-administrations of alcohol- and methamphetamine-produced anxiogenic effect could be associated with the neurotoxicity in the dentate gyrus. J. Neural Transm 118, 1559–1569. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK, 2014. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology 39, 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR, 2009. A review of the clinical pharmacology of methamphetamine. Addiction 104, 1085–1099. [DOI] [PubMed] [Google Scholar]
- Dockstader CL, van der Kooy D, 2001. Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J. Neurosci 21, 9077–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr CD, Delva J, Anthony JC, 2000. The suspected association between methamphetamine (‘ice’) smoking and frequent episodes of alcohol intoxication: data from the 1993 National Household Survey on Drug Abuse. Drug Alcohol Depend. 59, 89–93. [DOI] [PubMed] [Google Scholar]
- Grant KM, LeVan TD, Wells SM, Li M, Stoltenberg SF, Gendelman HE, Carlo G, Bevins RA, 2012. Methamphetamine-associated psychosis. J. Neuroimmune Pharmacol. 7, 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Lopez MD, Llopis N, Feng S, Barrett DA, O’Shea E, Colado MI, 2010. Involvement of 2-arachidonoyl glycerol in the increased consumption of and preference for ethanol of mice treated with neurotoxic doses of methamphetamine. Br. J. Pharmacol 160, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, McCunney S, Markou A, Kuczenski R, 2012. Development of stereotyped behaviors during prolonged escalation of methamphetamine self-administration in rats. Psychopharmacology (Berl.) 223, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Brecht ML, Lovinger K, Raihan A, Christou D, Sheaff P, 2013. Polydrug and marijuana use among adults who primarily used methamphetamine. J. Psychoactive Drugs 45, 132–140. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL, 1999. Pharmacologic and behavioral responses of inbred C57BL/6: J and strain 129/SvJ mouse lines. Pharmacol. Biochem. Behav 63, 21–26. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL, 2012a. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology 219, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL, 2012b. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl.) 219, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Phillips TJ, Szumlinski KK, 2014. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front. Syst. Neurosci 8, 70 10.3389/fnsys.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK, 2016. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur. J. Neurosci 43, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley SJ, Little HJ, 1997. Enhancement of amphetamine- and cocaine-induced locomotor activity after chronic ethanol administration. J. Pharmacol. Exp. Ther 281, 1330–1339. [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Upton R, Jacob P 3rd, 1995. Methamphetamine and ethanol interactions in humans. Clin. Pharmacol. Ther 57, 559–568. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC, Belknap JK, 2009. Genetic correlates of morphine withdrawal in 14 inbred mouse strains. Drug Alcohol Depend. 99, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miladi-Gorji H, Fadaei A, Bigdeli I, 2015. Anxiety assessment in methamphetamine − sensitized and withdrawn rats: immediate and delayed effects. Iran. J. Psychiatry 10, 150–157. [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA), 2007. FAQs for the General Public. NIH, Bethesda, MD: http://www.niaaa.nih.gov/FAQs/General-English/default.htm. [Google Scholar]
- National Instituteon Drug Abuse (NIDA), 2013. Research Report Series: Methamphetamine. NIH, Bethesda, MD: NIH Publication Number 13–4210. [Google Scholar]
- O’Grady KE, Arria AM, Fitzelle DM, Wish ED, 2008. Heavy drinking and polydrug use among college students. J. Drug Issues 38, 445–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A, Sørensen G, Kobayashi M, 1988. Stereotyped behaviour in animals induced by stimulant drugs or by a restricted cage environment: relation to disintegrated behaviour, brain dopamine and psychiatric disease. Yakubutsu Seishin Kodo 8, 313–327. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav 84, 53–63. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. NIH, Rockville, MD: Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA; 09–4434. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2012. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. NIH, Rockville, MD: Office of Applied Studies, NSDUH Series H-44, HHS Publication No.(SMA) 12–4713. [Google Scholar]
- Sattah MV, Supawitkul S, Dondero TJ, Kilmarx PH, Young NL, Mastro TD, Chaikummao S, Manopaiboon C, Griensven Fv., 2002. Prevalence of and risk factors for methamphetamine use in northern Thai youth: results of an audio-computer-assisted self-interviewing survey with urine testing. Addiction 97, 801–818. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Ho A, Zhou Y, Curtis AE, Kreek MJ, 1998. Effects of binge pattern cocaine on stereotypy and locomotor activity in C57BL/6: J and 129/J mice. Pharmacol. Biochem. Behav 60, 593–599. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ, 2003. Locomotion, stereotypy, and dopamine D1 receptors after chronic binge cocaine in C57BL/6: J and 129/J mice. Pharmacol. Biochem. Behav 75, 123–131. [DOI] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ, 2011. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav 10, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ, 2012. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology 62, 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J, Butler R, Wheeler A, 2009. Initiation into methamphetamine use: qualitative findings from an exploration of first time use among a group of New Zealand users. J. Psychoactive Drugs 41, 11–17. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Frys KA, Middaugh LD, 2005. Genetic variation in heroin-induced changes in behaviour: effects of B6 strain dose on conditioned reward and locomotor sensitization in 129-B6 hybrid mice. Genes Brain Behav 4, 324–336. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugmann M, Phillips TJ, Kippin TE, 2017. Methamphetamine addiction vulnerability: the glutamate, the bad and the ugly. Biol. Psychiatry 81, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2015. World Drug Report 2015. United Nations publication Sales No; E.15. XI.6. [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ, 2009. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 8, 758–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MC, Greager EM, Stafford J, Bachtell RK, 2016. Methamphetamine self-administration reduces alcohol consumption and preference in alcohol-preferring P rats. Addict. Biol 10.1111/adb.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE, 1986. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 16, 341–360. [DOI] [PubMed] [Google Scholar]