Abstract
Scaling evolutionary trees to time is essential for understanding the origins of clades. Recently developed methods allow including the entire fossil record known for the group of interest and eliminated the need for specifying prior distributions for node ages. Here we apply the fossilized birth-death (FBD) approach to reconstruct the diversification timeline of the viperines (subfamily Viperinae). Viperinae are an Old World snake subfamily comprising 102 species from 13 genera. The fossil record of vipers is fairly rich and well assignable to clades due to the unique vertebral and fang morphology. We use an unprecedented sampling of 83 modern species and 13 genetic markers in combination with 197 fossils representing 28 extinct taxa to reconstruct a time-calibrated phylogeny of the Viperinae. Our results suggest a late Eocene-early Oligocene origin with several diversification events following soon after the group’s establishment. The age estimates inferred with the FBD model correspond to those from previous studies that were based on node dating but FBD provides notably narrower credible intervals around the node ages. Viperines comprise two African and an Eurasian clade, but the ancestral origin of the subfamily is ambiguous. The most parsimonious scenarios require two transoceanic dispersals over the Tethys Sea during the Oligocene.
Introduction
Scaling phylogenetic trees to time is one of the major challenges in evolutionary biology. Reliable estimates for the age of evolutionary events are essential for addressing a wide array of questions, such as deciphering micro- and macroevolutionary processes, identifying drivers of biodiversity patterns, or understanding the origins of life1.
The most widely used approach for dating trees is calibration at internal nodes (hereafter ‘node dating’)2, in which some nodes are a priori assigned ages based, for example, on the fossil record for that clade. Despite its wide use, there are drawbacks to this method. First of all, the fossil record is extremely fragmentary and the likelihood of recovering a fossil of a common ancestor of two lineages is extremely low3. Also, the earliest fossil representatives of newly divergent taxa might be virtually indistinguishable4. Fossils thus may provide information about the absolute minimum clade ages, but the maximum ages remain obscure5. For this reason, the age of the oldest known fossil is commonly used as the minimum hard bound, while the maximum clade age is given a soft bound with a specified prior distribution. Despite recent progress in prior distribution specification6 this task still remains challenging as the decision regarding optimal prior density is often problematic, especially for groups with a poor fossil record2. Recently, alternative methods that use fossils to calibrate trees have been developed. One is the fossilized birth-death model (FBD)7 that uses the entire fossil record rather than just the oldest fossil for a given node. Similar to node dating, FBD requires some prior knowledge about the phylogenetic affinities of the fossils, but contrary to node dating, no prior calibration densities of the node ages need to be specified as fossils are assigned to branches or clades and not nodes7,8. While node dating requires the user to parameterize a prior density for each calibrated node, the FBD model assumes random sampling of extant and fossil species, constant speciation and extinction rates, and prior information on these FBD model parameters: speciation rate (λ), extinction rate (μ), fossil recovery rate (ψ), and proportion of sampled extant species (ρ).
Here, we apply the FBD approach to reconstruct the timeline of viperine diversification. Viperines (subfamily Viperinae) are an Old World radiation distributed through Africa (excluding Madagascar), Arabia, Europe and Asia9. The subfamily comprises 102 species in 13 genera. The sister relationships within Viperidae (Viperinae to Azemiopinae + Crotalinae) and its monophyly are well supported by available genetic evidence10,11, although this does not reflect relationships based on analyses of morphological data. Instead, morphology shows that the genus Causus possesses features similar to Azemiops or other colubroid snakes12. Latest phylogenies based on hundreds of phenotypic characters reconstructed Causus as sister to the Viperinae + Crotalinae13,14. Furthermore, the incongruence in the phylogenetic position of Causus is also obvious from genetic-based phylogenies. While early studies placed it as sister to the Viperinae15,16, more recent analyses with broader taxon and gene sampling placed it within the radiation of viperines. However, the placement within Viperinae has been inconsistent and the genus has been placed as sister to Echis + Cerastes17, sister to Atheris18, sister to Echis10,19,20, sister to six Eurasian genera21, and sister to Proatheris11,22,23. As a result of these topological inconsistencies, the geographic origin of vipers has not been resolved, although both genetic and fossil evidence suggest a non-European origin17,24.
Vipers are well represented in the fossil record, although their remains are fragmentary and usually consist of isolated vertebrae, cranial segments and fangs24,25. The oldest viperine fossil is a central European Vipera antiqua, which is dated to the early Miocene, ca. 22.5 million years ago (Ma, Fig. 1)26. Osteological features preserved in viperid fossils generally permit identification, although certain characters are rather homogeneous and shared across different species. This conserved morphology has led to the establishment of species groups, which contain taxa that are osteologically similar12,27. For example, the small European vipers of the genus Vipera form two morphological groups (V. berus and V. aspis groups) that differ in vertebral and cranial morphology, but differentiating species within these groups is nearly impossible24. Likewise, fossil remains of large-bodied species have traditionally been clustered in a group of ‘Oriental vipers’28. However, monophyly of the ‘Oriental vipers’ is questionable as it has not been confirmed either by morphological analyses29, immunological comparisons30 or multilocus phylogenetic analyses10,11,22.
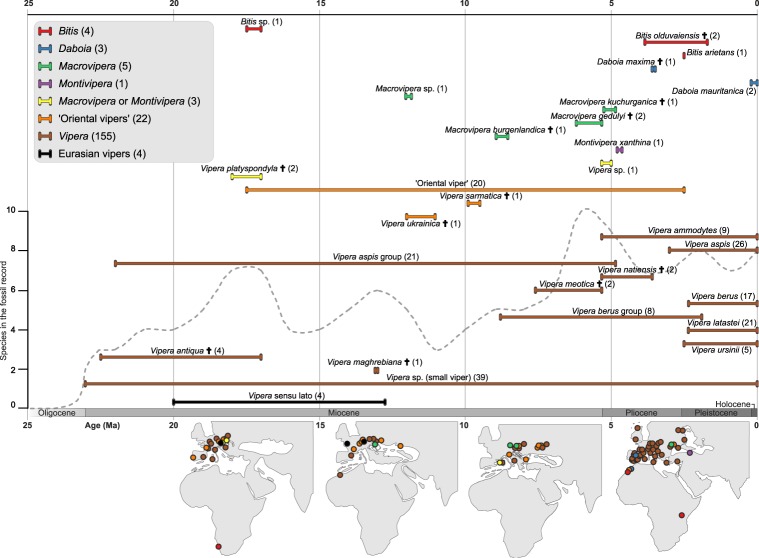
Figure 1.
Temporal and spatial distribution of the viperine fossil record. All fossil specimens that belong to a species or species group are shown as one horizontal bar. Bars are colored by genera or higher clades as denoted in the upper left panel. Black crosses denote extinct species. ‘Oriental vipers’ represents an assemblage of generally large-bodied species belonging to either of the modern genera Daboia, Macrovipera, or Montivipera. Eurasian vipers are a clade of the genera Vipera, Daboia, Montivipera, and Macrovipera. Numbers in parentheses show the number of individual fossils for each species and genus. The dashed grey line shows the number of species in the fossil record through time. The maps show approximate locations of fossils reported from five-million-year timeframes from 20 Ma to the present. Note that not all fossils are plotted for the lack of space and one locality for an ‘Oriental viper’ in eastern Russia is not shown in the map.
Several studies have estimated the time-calibrated phylogeny for viperines, all of which used the node dating approach with calibration points almost exclusively outside the viperines. The sole exception was fossil evidence for the initial radiation of the Eurasian vipers used by some authors17,18, but without referring to an actual fossil. Most agree on the origin of the crown Viperinae to be dated between the middle Eocene and early Miocene (ca. 34–42 Ma with confidence intervals ranging between 32 and 50 Ma; Fig. 2)11,13,17–19,22 (but see31).
Figure 2.
Comparison of crown ages of the viperines and their genera as estimated in this (in red) and previous studies. Circles represent mean age estimates and bars indicate their 95% HPD credible intervals (not available for some studies). Circles marked with an ‘a’ indicate that the oldest species was missing in the analysis. The monotypic genera Eristicophis and Proatheris are not shown as they only have stem ages. Exact age estimates are given in detail in Supplementary Table S4.
In this study, we reconstruct the phylogeny of Viperinae using an unprecedented taxon sampling of 83 modern species (out of 102) and 13 genetic markers in combination with their entire known fossil record. This new assessment allowed for a comparison of the FBD approach to previously published results based on node dating. Additionally, we use the new phylogeny for ancestral area reconstructions to examine the origin and speciation history of viperines.
Materials and Methods
Sampling of Modern Species
Of the 102 species of Viperinae, there were sequence data available for 79 species on GenBank, which was downloaded to collate a dataset of 13 genes, six mitochondrial and seven nuclear (Supplementary Table S1). The number of available sequences ranged from 1 to 12 per species (with the mean of 4.7). Additionally, we generated a total of 46 new DNA sequences of seven markers for 23 species of the genera Bitis and Causus (Supplementary Table S2). The mitochondrial dataset was 59% complete, the nuclear one was 22% complete. The best sampled mitochondrial markers were cytochrome b (available for 77 species) and 16S rRNA (available for 62 species), the nuclear ones were PRLR (available for 27 species) and UBN1 (available for 23 species). Eristicophis, Proatheris, and Pseudocerastes only had mitochondrial markers available. The final data matrix comprised 422 sequences representing 83 modern species.
Sampling of Fossil Species
Data on the viper fossils, their age ranges, and geographic origins were obtained from the regularly updated fosFARbase32 that contained 302 unique fossils of the Viperinae. We pruned the list by removing duplicate, dubious, and undetermined records. As dubious we considered fossils with doubtful phylogenetic placement (e.g. Vipera aegertica, V. kargii)24 or those originally assigned to viperines but later redetermined (e.g. Vipera sp. from Oggenhausen, Germany)24,33,34. Fossils were assigned to species or alternatively to higher taxonomic groups when quality of the material prevented assignment to a particular species. We verified all records with primary and secondary literature for a more accurate taxonomic assignment (details are given in Supplementary Table S3). The final dataset contained 197 unique fossil records (Fig. 1, Supplementary Table S3). The species groups of Vipera and the ‘Oriental vipers’ as used in the paleontological literature must be treated with caution as they might not represent clades. For example, the Vipera aspis group found in the paleontological literature is in fact a paraphyletic assemblage composed of two clades, one of V. aspis, V. latastei, and V. monticola and the other of V. ammodytes and V. transcaucasiana. Under the FBD model, these fossils may be assigned to deeper nodes of the tree, although this increases uncertainty in the placement of the fossil7. There were five recent viper genera represented in the fossil material: Bitis (4 records), Daboia (3), Macrovipera (5), Montivipera (1), Vipera (155). Additionally, 29 records could not be assigned to a genus and were assigned to higher groups: the ‘Oriental vipers’ (22 records), Macrovipera or Montivipera (3), the Eurasian genera Vipera, Daboia, Montivipera, Macrovipera (4) (Fig. 1).
Phylogenetic Analyses and FBD Dating
All sequences were checked and edited in Geneious 11.1.3 (www.geneious.com). We aligned each marker independently using MAFFT 735 and the ‘auto’ settings for all genes. We used Gblocks36 to remove poorly alignable sites of the rRNA mtDNA genes. Fifteen outgroup species were included in the dataset, six from the closest subfamilies Crotalinae (5 species) and Azemiopinae (1 species) and nine from other snake families (Supplementary Table S1). The concatenated alignment had a length of 10418 bp (Supplementary Table S2). The dataset was partitioned by gene, with the best models of nucleotide evolution estimated using PartitionFinder (PF)37. To avoid over-parameterization we used the HKY instead of the GTR model for all partitions, with the among-site rate variation parameter (+Γ) included for the 12S, 16S, cox1, cytb, nd2, nd4, cmos, nt3, and prlr partitions as identified by PF. Invariant sites (+I) were excluded, as this is accounted for by the +Γ parameter38.
All analyses were run with BEAST 2.5.139 at the CIPRES Science Gateway40, using the Sampled Ancestors (SA 2.0) package41. Likelihood ratio tests (LRT) implemented in MEGA642 rejected the clock-like models of evolution for all partitions except the cox1; we therefore used a strict-clock prior for the cox1 and relaxed clock lognormal priors for all other partitions. We used the FBD model as the tree prior. The parameters of the FBD model are reparameterized as follows: diversification rate d = λ – μ; turnover rate r = μ/λ; fossil sampling proportion s = ψ/(μ + ψ)7. We assumed an exponential prior for d with the mean of 2.0 (lineages per million years). This broad prior distribution (5% and 95% quantiles being, respectively, 0.103 and 5.99) reflected the difficulty of predicting the speciation and extinction rates a priori. For the s and r parameters we used beta prior distributions with shape parameters α = 2.0 and β = 2.0. Beta distribution ranges between 0 and 1 and such shape parameters α and β produced a convex-shaped distribution with the highest probability at 0.5. This shape was chosen because extremely high (close to 1.0) or low (close to 0.0) values for these two parameters could not be ruled out, but are not probable. The ρ parameter was given a value of 0.81 as 83 of 102 modern viperines were included in the analysis. We assumed a lognormal prior distribution for the rate of the mtDNA genes (parameter ucld.mean; mean = 0.3 [substitutions per site per million years], SD = 1.0) and an exponential prior distribution for the nDNA genes (mean = 0.05). Rate heterogeneity among lineages (parameter ucld.stdev) was assumed to have an exponential prior distribution for all partitions (mean = 1.0). The clock rate of cox1 was assumed to have a lognormal prior distribution (mean = 1.0 [substitutions per site per million years], SD = 1.25).
The set of 197 fossil records represented 28 different species or species groups, which were added to the dataset with sequences coded as missing data. For fossil species known only from a single record (11 species in total), the stratigraphic age was represented by the minimum and maximum age estimate for the particular record. For fossils with more than one record, the youngest and oldest records were used as the age limits. Means of the stratigraphic ages were used as the initial values for all records. Because all fossils in the dataset were crown fossils we conditioned the FBD model on the root. We specified 21 constraints on the phylogeny to indicate the phylogenetic placement of the fossils (Supplementary Table S3). They may be found in the supplementary nexus alignment file. The Vipera aspis group and ‘Oriental vipers’ were defined without enforcing their monophyly because the two groups may not be monophyletic. We ran three independent runs each for 5 × 108 generations with parameters logged every 25,000 generations. Posterior trace plots, stationarity, convergence and effective sample sizes (ESS) were inspected in Tracer 1.543. We discarded the first 10% of sampled trees from each run as burn-in based on inspection of likelihood trace plots and combined the tree files using LogCombiner. Because the phylogenetic placement of the fossil samples was entirely a result of the prior we removed them from the posterior trees and then summarized the resulting trees with a maximum clade credibility (MCC) tree with mean node heights using TreeAnnotator.
Ancestral Area Reconstruction
To infer geographic origins of viperines, we performed likelihood-based analyses in BioGeoBEARS44. Given the distribution pattern of modern viperines and geological history of the Old World, we defined four discrete biogeographic areas: Africa, Arabia, Asia, and Europe. Tree tips were assigned to one or more areas based on their current distributions9 and the outgroup was removed prior to the analysis. Using the viperine-only tree allowed reconstructing the ancestral range for the subfamily, but without accounting for deeper phylogenetic and biogeographic context. Therefore, to gauge the effect of outgroup on the biogeographic reconstruction we ran an additional analysis on a tree that besides viperines included all sequenced species of their sister clade - the subfamilies Azemiopinae (1 sp.; 50% of species included) and Crotalinae (198 spp.; 82% of species included). Sequence data for the additional subfamilies were obtained from GenBank and the dataset comprised 282 species (Supplementary Table S1). To generate the tree we used the same settings as described above for the FBD analysis except we assumed a birth-death model for the tree prior with a beta prior distribution (α = 2.0, β = 2.0) for the relative death rate and a uniform prior (0, 1000) for the birth rate. We ran the analysis four times for 2 × 108 generations, logging parameters and trees every 25,000 generations, and 10% of trees were discarded as burn-in. The MCC tree was then used as the input tree.
For both trees, we applied all biogeographic analyses available in BioGeoBEARS (dispersal–extinction–cladogenesis – DEC; dispersal–vicariance analysis – DIVA; BayArea), all with and without the parameter + J employed, which allows for founder-event speciation44. The fit of the models was assessed by the sample-size corrected Akaike Information Criterion (AICc).
Results
Phylogenetic Analyses and FBD Dating
The three MCMC runs converged after 5% of the 5 × 108 generations. Most parameters had adequate ESS, except for the age parameter of the most recent common ancestors of Bitis arietans and its fossil sample, and of B. arietans and B. olduvaiensis (ESS = 50 and 64, respectively). These low values were likely caused by the long branch leading to B. arietans (23.5 Ma) and the uncertainty associated with exact placement of the two fossils along this branch. The mean estimate of the net diversification rate was 0.072 lineages per million years with the 95% highest posterior density (HPD) interval ranging between 0.052–0.09. The mean estimate of the sampled ancestors was 22.7 individuals (HPD: 19–25), indicating that of the 28 fossils most were direct ancestors of other fossils or modern taxa. Evolutionary rates estimated for the individual genes are given in Supplementary Table S2.
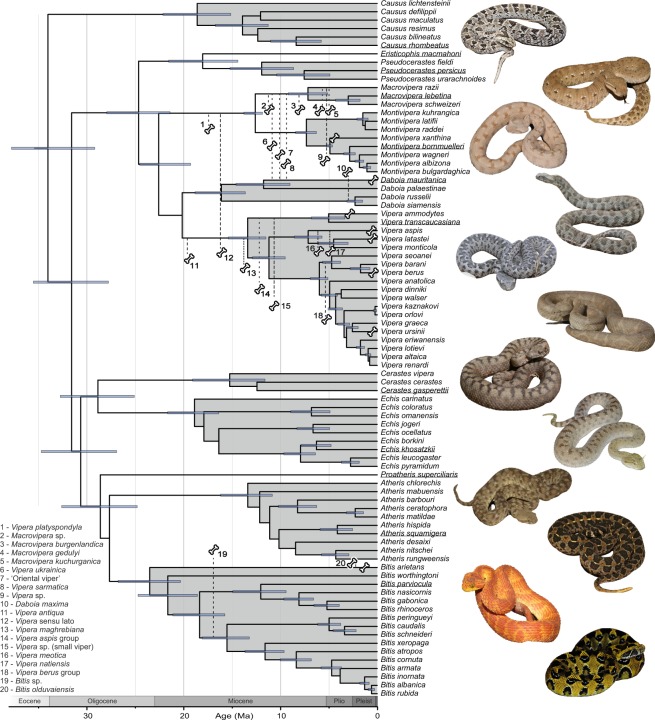
Monophyly of all genera for which monophyly was not enforced was highly supported (posterior probability [pp] = 1.0). The genus Causus was placed as sister to all other viperine genera (pp = 0.98; Fig. 3). The remaining genera formed two well supported clades, one consisting of Cerastes, Echis, Proatheris, Atheris, and Bitis (pp = 0.99), the other of Eristicophis, Pseudocerastes, Daboia, Macrovipera, Montivipera, and Vipera (pp = 0.99). Phylogenetic relationships among the genera were generally well resolved, Cerastes was sister to Echis (pp = 1.0) and these two were sister to Proatheris, Atheris, and Bitis (pp = 0.98). Relationships between these three genera were not resolved. In the other clade, Eristicophis was supported as sister to Pseudocerastes (pp = 1.0), which were together sister to a clade of the Eurasian genera Vipera, Daboia, Montivipera, Macrovipera. Within the latter clade, Montivipera and Macrovipera were supported as sister lineages (pp = 1.0), but otherwise the relationships remained unresolved due to low support (pp = 0.54). The tree with all age estimates and support values is shown in Supplementary Fig. S1 and is supplied as a nexus file in the electronic Supplementary Materials.
Figure 3.
Maximum clade credibility tree for the modern viperines. The tree was calibrated with 197 fossils representing 28 species, whose phylogenetic positions are indicated by bone symbols; the numbered ones were assigned to a branch or a tree portion as indicated by the stippled lines and are of fossils listed in lower left. Unnumbered bone symbols are fossils of species known from the present day. Blue bars indicate 95% HPD intervals of the age estimates and are shown only for supported nodes with pp ≥ 0.95. Genera are highlighted in grey except the monotypic Eristicophis and Proatheris. Outgroup species are not shown. Specimens depicted on the right are of the species underlined and ordered as in the tree. Specimens are not to scale. Photographs are courtesy and are published with permission of T. Mazuch (B. parviocula, D. mauritanica), D. Jandzik (E. macmahoni), S. Carranza (C. gasperettii, P. persicus), D. Hegner (A. squamigera, P. superciliaris); otherwise JŠ. The tree with all age estimate and pp values is available in a nexus format in the supplementary materials.
The mean crown age of the Viperinae was estimated to 34.0 Ma (HPD: 29.2–37.8). Mean crown age estimates for the individual genera were mostly within the Miocene, ranging from 7.2 Ma in Macrovipera to 23.5 Ma in Bitis (Figs 2 and 3). Stem and crown age estimates for all genera and their HPD confidence intervals are given in Supplementary Table S4.
Ancestral Area Reconstruction
In all analyses, models with the + J parameter were favored by LRT. The DEC + J was identified as the best-fitting model for both trees analyzed (Supplementary Table S5). According to the reconstructions (Fig. 4), the genera Causus, Atheris, Bitis, and Proatheris are of African origin, Daboia, Eristicophis, Pseudocerastes, Macrovipera, Montivipera are of Asian origin, Echis is most likely of Arabian origin (marginal probability for an Arabian origin 57%), Vipera is Eurasian (61% for Asia, 22% for Europe, 16% for unresolved Eurasian), and Cerastes is of an unresolved African or Arabian origin (61% for either Africa or Arabia). At deeper phylogenetic levels, the clade of Eristicophis, Pseudocerastes, Macrovipera, Montivipera, Daboia, and Vipera is clearly Asian in origin (96% for an Asian origin). The clade of Proatheris, Atheris, and Bitis is unequivocally African in origin (98%), and the clade of these three genera together with Echis and Cerastes originated either in Afro-Arabia or Africa (45% for Afro-Arabia, 39% for Africa). The geographic origin of all viperines except Causus was not resolved, with Afro-Arabia or Asia being the most supported (49%), followed by the option of either African or Asian origin (21%). Likewise, the biogeographic origin of the entire subfamily Viperinae remained unclear, with 50% support for Afro-Arabia or Asia, 25% for Africa or Asia, and 16% for Africa alone.
Figure 4.
(A) Ancestral area reconstruction of the viperines. Genera are shaded, and species are in the same order as in Fig. 3. The four biogeographic areas defined for the analyses are shown in the map. Probabilities of the regions to be ancestral to a clade are shown as pie charts on the given nodes. (B) Results of the alternative biogeographic analysis that included all members of the Azemiopinae and Crotalinae. A complete tree with all 282 tips is shown in Supplementary Fig. S2.
The alternative uncalibrated phylogenetic analysis that included viperines and all Azemiopinae and Crotalinae species resulted in a topology mostly corresponding to that of the FBD analysis. The difference was in the position of Daboia that was sister to the clade of Macrovipera and Montivipera, as opposed to the FBD analysis in which it was sister to Vipera. However, neither of the topologies was convincingly supported (pp = 0.73 and 0.54, respectively). The biogeographic reconstruction based on this 282-tip tree provided results similar to that of the Viperinae tree alone (Supplementary Fig. S2). While the origin of the individual viperine genera and deeper nodes within the subfamily remained well resolved (the Asian and Afro-Arabian clades were present), the biogeographic origin of the crown Viperinae and the node internal to the Causus clade remained obscure. The basal node of Viperinae was of an unresolved African/Arabian/Asian origin (marginal probability 49%) or African/Asian origin (32%), the node of viperines without Causus was also African/Arabian/Asian in origin (50%) or African/Asian (31%). Of a note is the reconstructed biogeographic origin of the entire family Viperidae, i.e. the clade containing the Viperinae, Azemiopinae and Crotalinae, which originated in Afro-Arabia or Asian (Africa/Arabia/Asia 49%, Africa/Asia 35%). The common ancestor of Azemiopinae and Crotalinae originated in Asia (95%).
Discussion
In this study, we take a novel approach to reconstruct the evolutionary history of viperines. The FBD model used to calibrate the tree allowed integration of the actual fossil record of the group instead of relying on external calibration points, as previous studies using node dating have done11,17–19,22,31,45. We show that diversification within viperines began at the Eocene/Oligocene boundary (ca. 34 Ma). Stem lineages of most genera were established prior to the Miocene, and their crown ages were generally placed in the early to middle Miocene, with Bitis being the oldest genus that began to diversify at the end of the Oligocene (23.5 Ma). The FBD analysis yielded a slightly younger age estimate for the crown Viperinae compared to previous studies, although the credible intervals show considerable overlap (Fig. 2). Our age estimates for the individual genera are essentially congruent with dates from earlier works that used node dating11,17–19,22,45, with the exception of the study by Fenwick, et al.31, who estimated younger ages for most genera in spite of using calibration points similar to some of the other studies.
We show that the age of the genus Macrovipera is older than previously thought. This is, however, not a result of the FBD approach, but due to a recent taxonomic change in the genus46. Macrovipera and Vipera have of all the genera the richest fossil record that spans back before the modern species started diversifying. The earliest record of Macrovipera dates back to the middle Miocene (ca. 12 Ma; Fig. 1)47, while the modern species diverged relatively recently in the late Miocene (7.2 Ma). Vipera started appearing in the fossil record in the early Miocene (ca. 22.5 Ma)24,26, but its crown diversification is nearly ten million years younger; it dates to the middle Miocene (13.4 Ma). While Vipera has radiated into 24 today’s species, the diversity of Macrovipera has not changed significantly since the late Miocene, indicating low net diversification rate of the genus. Therefore, accounting for the fossil record is key not only for estimating the age of modern viperine taxa, but it is also crucial for assessing their past diversity.
The FBD model is being increasingly used for calibrating phylogenies and many studies have shown that node ages estimated under the FBD model rarely match those resulting from node dating analyses7,48–50. The congruence of our age estimates with previous studies that were based on node dating is thus fairly unique. It suggests that the calibration estimates in the squamate phylogeny are relatively stable (at least in the viper clade), and that most previous studies have produced concordant node age estimates, regardless of the uncertainty around the fossil calibration points. Compared to previously published time-trees of viperines, the current analysis is an improvement to the credible intervals around node ages, which are now notably smaller compared to those based on node dating. This may be due to the relatively high number of fossil species in our dataset (25% of taxa in the dataset are fossils) as it has been shown that higher proportion of fossils in the dataset results in shorter credible intervals7,48. Potentially relevant results have been recently provided by Harrington and Reeder51, who utilized FBD to calibrate a squamate-wide phylogeny. However, their taxon sampling of snakes, and vipers in particular, was not dense enough to permit direct comparison with our results.
Despite the relatively rich fossil record of Viperinae with nearly 200 unique fossils, only one of these has been used as a calibration point in node dating analyses17,18. This is most likely due to the difficulty in assigning viper fossils to nodes because of uncertainty regarding their ancestor-descendant relationships24. The FBD model overcomes this problem by allowing the use of the entire fossil record even if some fossils lack accurate taxonomic determination. This eliminates the need for using only the oldest fossil for a given node and the assignment of prior densities placed on the node ages, which is a major step forward in estimating divergence times. Moreover, FBD is robust against disproportional sampling7,48, which makes it applicable even for clades with an unevenly distributed fossil record, as is the case of viperines. Although the tree topology is not required to be fixed41, certain knowledge of the relationships among the taxa studied is still needed to be able to assign the fossils to existing clades. This limitation is overcome by the total evidence approach52,53, which can also use the FBD model for the tree prior. This approach uses the entire fossil record, but requires morphological characters to be scored for both fossil and modern species. Such data matrices may, however, not be readily available or obtainable for many groups of organisms. Hence, of the methods developed to calibrate phylogenetic trees, the FBD has, in our view, the biggest potential to substitute the currently prevailing node dating approach.
Although we have generated the most complete phylogeny of the viperines to date, the ancestral reconstructions of the geographic origin of the group were not conclusive. Our analyses show that the crown Viperinae are of an African, Arabian or Asian origin (or combinations therein). Despite this, we can now make some valuable inferences that have not been possible to date. Firstly, a solely Arabian origin can be ruled out because Arabia was part of the African continent in the early Oligocene when viperines began to diversify54. Likewise, Europe and Asia were a single continent throughout the history of the viper family, although Europe was periodically fragmented into a series of islands by the expanding Paratethys Sea55,56. This narrows down the possible place of origin to two landmasses: Afro-Arabia and Eurasia. Eurasia was part of Laurasia while Afro-Arabia is Gondwanan in origin, and the two landmasses remained widely separated by the Tethys Sea until their collision in the early Miocene57. Thus, the origin of viperines would have to be either one or the other landmass and their current distribution would then necessitate sub-aerial dispersal across the Tethys Sea.
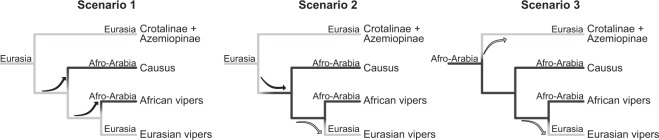
While the origin of the crown Viperinae was not resolved in any of the analysis, the clade of Crotalinae and Azemiopinae is clearly Asian (Supplementary Fig. S2)17,58. Given this, and the viperine topology recovered here, three equally parsimonious but mutually exclusive scenarios could explain the dispersal history of vipers (Fig. 5). The family Viperidae could have originated in Eurasia and dispersed to Afro-Arabia in two consecutive steps ca. 34 and 32 Ma across the Tethys Sea. The second scenario also assumes a Eurasian origin, but with an over-sea dispersal to Afro-Arabia followed by re-colonization of Eurasia between 25–32 Ma. The third scenario is that Viperidae originated in Afro-Arabia and dispersed to Eurasia twice across the Tethys, first as the common ancestor of the Crotalinae and Azemiopinae, then as the ancestor of the Eurasian genera Eristicophis, Pseudocerastes, Daboia, Macrovipera, Montivipera, and Vipera. All three scenarios assume two independent trans-Tethys dispersal events, each followed by a substantial in situ radiation. Previous biogeographic analysis supports the second scenario17, although that study was based on smaller taxon and gene sampling and a tree topology that differed slightly from the present study (Causus sister to Echis + Cerastes). A non-European origin for Viperinae was also suggested based on the fossil evidence24. Given the current evidence available, it is not possible to provide a more definitive answer to this biogeographic conundrum other than the three equally parsimonious scenarios.
Figure 5.
Plausible scenarios of the biogeographic history of the family Viperidae. Black and grey branches correspond to the Afro-Arabian and Eurasian distributions, respectively. Black arrows indicate a trans-Tethys dispersal from Eurasia to Afro-Arabia, grey arrows from Afro-Arabia to Eurasia.
The viperine fossil record shows a clear pattern of increasing diversity towards the middle Miocene and then towards the recent past (Pliocene, Pleistocene; Fig. 1). The Miocene burst corresponds temporarily to the Miocene Climatic Optimum (MCO; ca. 14–18 Ma), an era of increased global temperatures relative to the long-term average59. This warm pulse could have provided suitable conditions for ectothermic vertebrates to thrive and diversify in regions that are currently less favorable to them, such as central and eastern Europe60. MCO was followed by global cooling, which could have been a driver of extinction especially for high-latitude faunas61,62, as evidenced by some viper extinctions at that time (e.g. V. sarmatica, V. ukrainica, M. burgenlandica). Indeed, it appears that the European reptile community was much richer prior to this cooling period, as many clades previously present in Europe shifted toward tropical or subtropical distributions (e.g. Boidae, Chamaeleonidae, Elapidae, Lamprophiidae)63–65.
In conclusion, our genetic dataset of modern viperines is the most comprehensive to date in terms of taxon and gene sampling. The phylogeny was well supported in most relevant nodes including the position of Causus, a genus whose position has proven difficult to infer previously. Our improved dataset and the inclusion of fossils in the dating analyses provide more confident interpretations regarding the evolutionary history of this group. It appears that Viperinae started diversifying at the Eocene/Oligocene boundary and the radiation of most genera took place in the Miocene. The late-Miocene cooling probably resulted in extinctions at high latitudes, with some taxa shifting to lower latitudes. While the biogeographic origin of the subfamily Viperinae remains unclear, we propose scenarios that explain their current distribution pattern, all of which require two transoceanic dispersals.
Supplementary information
Acknowledgements
We are grateful to Axel Barlow and Wolfgang Wüster for use of unpublished Bitis sequences, Eli Greenbaum, Werner Conradie, and Marius Burger for samples, Nick Telford for assistance with preparing figures, and the Molecular Ecology group members at SANBI for their advice, support and lively discussions. We thank anonymous reviewers for providing helpful suggestions that helped to improve the manuscript. J.Š. was funded by the Ministry of Culture of the Czech Republic (DKRVO 2019–2023/6.V.a, 00023272).
Author Contributions
Conceived and designed the experiments: J.Š. Gathered data: J.Š., K.A.T. Analyzed the data: J.Š. Wrote the paper: J.Š., K.A.T.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41290-2.
References
- 1.Hedges SB, Dudley J, Kumar S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- 2.Ho SY, Phillips MJ. Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Syst. Biol. 2009;58:367–380. doi: 10.1093/sysbio/syp035. [DOI] [PubMed] [Google Scholar]
- 3.Springer MS. Molecular clocks and the incompleteness of the fossil record. J. Mol. Evol. 1995;41:531–538. doi: 10.1007/BF00175810. [DOI] [Google Scholar]
- 4.Hedges SB, Kumar S. Precision of molecular time estimates. Trends Genet. 2004;20:242–247. doi: 10.1016/j.tig.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Marshall CR. The fossil record and estimating divergence times between lineages: Maximum divergene times and the importance of reliable phylogenies. J. Mol. Evol. 1990;30:400–408. doi: 10.1007/BF02101112. [DOI] [PubMed] [Google Scholar]
- 6.Nowak MD, Smith AB, Simpson C, Zwickl DJ. A simple method for estimating informative node age priors for the fossil calibration of molecular divergence time analyses. PLoS One. 2013;8:e66245. doi: 10.1371/journal.pone.0066245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath TA, Huelsenbeck JP, Stadler T. The fossilized birth–death process for coherent calibration of divergence-time estimates. PNAS. 2014;111:E2957–E2966. doi: 10.1073/pnas.1319091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didier G, Royer-Carenzi M, Laurin M. The reconstructed evolutionary process with the fossil record. J. Theor. Biol. 2012;315:26–37. doi: 10.1016/j.jtbi.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 9.Phelps, T. Old World Vipers, a natural history of the Azemiopinae and Viperinae. (Edition Chimaira, 2010).
- 10.Pyron RA, Burbrink F, Wiens J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013;13:93. doi: 10.1186/1471-2148-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Wiens JJ. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogen. Evol. 2016;94:537–547. doi: 10.1016/j.ympev.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Groombridge, B. A phyletic analysis of viperine snakes PhD thesis, City of London Polytechnic (1980).
- 13.Hsiang AY, et al. The origin of snakes: revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 2015;15:87. doi: 10.1186/s12862-015-0358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauthier JA, Kearney M, Maisano JA, Rieppel O, Behlke AD. Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History. 2012;53:3–308. doi: 10.3374/014.053.0101. [DOI] [Google Scholar]
- 15.Herrmann H-W, Joger U, Lenk P, Wink M. Morphological and molecular phylogenies of viperines: conflicting evidence? Kaupia. 1999;8:21–30. [Google Scholar]
- 16.Lenk P, Kalyabina S, Wink M, Joger U. Evolutionary relationships among the true vipers (Reptilia: Viperidae) inferred from mitochondrial DNA sequences. Mol. Phylogen. Evol. 2001;19:94–104. doi: 10.1006/mpev.2001.0912. [DOI] [PubMed] [Google Scholar]
- 17.Wüster W, Peppin L, Pook CE, Walker DE. A nesting of vipers: phylogeny and historical biogeography of the Viperidae (Squamata: Serpentes) Mol. Phylogen. Evol. 2008;49:445–459. doi: 10.1016/j.ympev.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Pook CE, Joger U, Stümpel N, Wüster W. When continents collide: phylogeny, historical biogeography and systematics of the medically important viper genus Echis (Squamata: Serpentes: Viperidae) Mol. Phylogen. Evol. 2009;53:792–807. doi: 10.1016/j.ympev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Pyron RA, Burbrink FT. Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol. Lett. 2014;17:13–21. doi: 10.1111/ele.12168. [DOI] [PubMed] [Google Scholar]
- 20.Pyron RA, et al. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol. Phylogen. Evol. 2011;58:329–342. doi: 10.1016/j.ympev.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Tonini JFR, Beard KH, Ferreira RB, Jetz W, Pyron RA. Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 2016;204:23–31. doi: 10.1016/j.biocon.2016.03.039. [DOI] [Google Scholar]
- 22.Alencar LR, et al. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogen. Evol. 2016;105:50–62. doi: 10.1016/j.ympev.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa A, McKelvy AD, Grismer LL, Bell CD, Lailvaux SP. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PloS one. 2016;11:e0161070. doi: 10.1371/journal.pone.0161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szyndlar, Z. & Rage, J.-C. In Biology of the vipers (eds Gordon W. Schuett, Mats Hoggren, & Michael E. Douglas) 419–444 (Eagle Mountain Publishing, 2002).
- 25.Kuch U, Müller J, Mödden C, Mebs D. Snake fangs from the Lower Miocene of Germany: evolutionary stability of perfect weapons. Naturwissenschaften. 2006;93:84–87. doi: 10.1007/s00114-005-0065-y. [DOI] [PubMed] [Google Scholar]
- 26.Szyndlar Z. Snakes from the lower Miocene locality of Dolnice (Czechoslovakia) J. Vert. Paleontol. 1987;7:55–71. doi: 10.1080/02724634.1987.10011637. [DOI] [Google Scholar]
- 27.Groombridge, B. In Studies in Herpetology (ed. Zbyněk Roček) 219–222 (1986).
- 28.Szyndlar, Z. In Proceedings of the 4th Ordinary General Meeting of the Societas Europaea Herpetologica. (eds J. J. Van Gelder, Henk Strijbosch, & P. J. M. Bergers) 387–390 (1987).
- 29.Ashe J, Marx H. Phylogeny of viperine snakes (Viperinae): Part II. Cladistic analysis and major lineages. Fieldiana Zoology. 1988;52:1–23. [Google Scholar]
- 30.Herrmann H-W, Joger U, Nilson G. Phylogeny and systematics of viperine snakes. III: resurrection of the genus Macrovipera (Reuss, 1927) as suggested by biochemical evidence. Amphibia-Reptilia. 1992;13:375–392. doi: 10.1163/156853892X00076. [DOI] [Google Scholar]
- 31.Fenwick AM, Greene HW, Parkinson CL. The serpent and the egg: unidirectional evolution of reproductive mode in vipers? J. Zool. Syst. Evol. Res. 2012;50:59–66. doi: 10.1111/j.1439-0469.2011.00646.x. [DOI] [Google Scholar]
- 32.Böhme, M. & Ilg, A. FosFARbase. http://www.wahre-staerke.com/ Accessed December 2017 (2003).
- 33.Szyndlar Z, Böhme W. Die fossilen Schlangen Deutschlands: geschichte der Faunen und ihrer Erforschung. Mertensiella. 1993;3:381–431. [Google Scholar]
- 34.Szyndlar Z, Rage J-C. Oldest fossil vipers (Serpentes: Viperidae) from the Old. World. Kaupia. 1999;8:9–20. [Google Scholar]
- 35.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 37.Lanfear R, Calcott B, Ho SYW, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 38.Mayrose I, Friedman N, Pupko T. A Gamma mixture model better accounts for among site rate heterogeneity. Bioinformatics. 2005;21:ii151–ii158. doi: 10.1093/bioinformatics/bti1125. [DOI] [PubMed] [Google Scholar]
- 39.Bouckaert R, et al. BEAST 2: a software platform for bayesian evolutionary analysis. PLoS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, M., Pfeiffer, W. & Schwartz, T. In Proceedings of the Gateway Computing Environments Workshop (GCE). 1–8 (IEE) (2010).
- 41.Gavryushkina A, Welch D, Stadler T, Drummond AJ. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comp. Biol. 2014;10:e1003919. doi: 10.1371/journal.pcbi.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rambaut, A. & Drummond, A. Tracer v1.4. Available from, http://beast.bio.ed.ac.uk/Tracer (2007).
- 44.Matzke NJ. BioGeoBEARS: BioGeography with Bayesian (and likelihood) evolutionary analysis in R Scripts. R package, version 0.2. 2013;1:2013. [Google Scholar]
- 45.Lynch VJ. Live-birth in vipers (Viperidae) is a key innovation and adaptation to global cooling during the Cenozoic. Evolution. 2009;63:2457–2465. doi: 10.1111/j.1558-5646.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 46.Oraie H, et al. Molecular and morphological analyses have revealed a new species of blunt-nosed viper of the genus Macrovipera in Iran. Salamandra. 2018;54:233–248. [Google Scholar]
- 47.Venczel M, Stiuca E. Late middle Miocene amphibians and squamate reptiles from Taut. Romania. Geodiversitas. 2008;30:731–763. [Google Scholar]
- 48.Grimm GW, Kapli P, Bomfleur B, McLoughlin S, Renner SS. Using more than the oldest fossils: dating Osmundaceae with three Bayesian clock approaches. Syst. Biol. 2015;64:396–405. doi: 10.1093/sysbio/syu108. [DOI] [PubMed] [Google Scholar]
- 49.Gavryushkina A, et al. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 2017;66:57–73. doi: 10.1093/sysbio/syw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arcila D, Pyron AR, Tyler JC, Ortí G, Betancur-R R. An evaluation of fossil tip-dating versus node-age calibrations in tetraodontiform fishes (Teleostei: Percomorphaceae) Mol. Phylogen. Evol. 2015;82:131–145. doi: 10.1016/j.ympev.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Harrington SM, Reeder TW. Phylogenetic inference and divergence dating of snakes using molecules, morphology and fossils: new insights into convergent evolution of feeding morphology and limb reduction. Biol. J. Linn. Soc. 2017;121:379–394. doi: 10.1093/biolinnean/blw039. [DOI] [Google Scholar]
- 52.Pyron RA. Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Syst. Biol. 2011;60:466–481. doi: 10.1093/sysbio/syr047. [DOI] [PubMed] [Google Scholar]
- 53.Ronquist F, et al. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Syst. Biol. 2012;61:973–999. doi: 10.1093/sysbio/sys058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosworth W, Huchon P, McClay K. The Red Sea and Gulf of Aden Basins. J. Afr. Earth Sci. 2005;43:334–378. doi: 10.1016/j.jafrearsci.2005.07.020. [DOI] [Google Scholar]
- 55.Popov, S. V. et al. Lithological-paleogeographic maps of Paratethys 10 maps Late Eocene to Pliocene. (Courier Forschungsinstitut Senckenberg, 2004).
- 56.Rögl F. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview) Geologica Carpathica. 1999;50:339–349. [Google Scholar]
- 57.Rögl F. Palaeogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene) Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie. 1998;99A:279–310. [Google Scholar]
- 58.Parkinson CL. Molecular systematics and biogeographical history of pitvipers as determined by mitochondrial ribosomal DNA sequences. Copeia. 1999;1999:576–586. doi: 10.2307/1447591. [DOI] [Google Scholar]
- 59.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 60.Böhme M. The Miocene climatic optimum: evidence from ectothermic vertebrates of Central. Europe. Palaeogeogr., Palaeoclimatol., Palaeoecol. 2003;195:389–401. doi: 10.1016/S0031-0182(03)00367-5. [DOI] [Google Scholar]
- 61.Raup DM, Sepkoski JJ. Periodicity of extinctions in the geologic past. PNAS. 1984;81:801–805. doi: 10.1073/pnas.81.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- 63.Szyndlar Z. A review of neogene and quaternary snakes of Central and Eastern Europe. Part 11: natricinae, elapidae, viperidae. Estudios geol. 1991;47:237–266. [Google Scholar]
- 64.Szyndlar Z. A review of Neogene and Quaternary snakes of central and eastern Europe. Part 1: Scolecophidia, Boidae, Colubrinae. Estudios geol. 1991;47:103–126. [Google Scholar]
- 65.Čerňanský A. A revision of chamaeleonids from the Lower Miocene of the Czech Republic with description of a new species of Chamaeleo (Squamata, Chamaeleonidae) Geobios. 2010;43:605–613. doi: 10.1016/j.geobios.2010.04.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.