Abstract
Background
Neuropathic pain is a consequence of damage to the central nervous system (CNS), for example, cerebrovascular accident, multiple sclerosis or spinal cord injury, or peripheral nervous system (PNS), for example, painful diabetic neuropathy (PDN), postherpetic neuralgia (PHN), or surgery. Evidence suggests that people suffering from neuropathic pain are likely to seek alternative modes of pain relief such as herbal medicinal products due to adverse events brought about by current pharmacological agents used to treat neuropathic pain. This review includes studies in which participants were treated with herbal medicinal products (topically or ingested) who had experienced neuropathic pain for at least three months.
Objectives
To assess the analgesic efficacy and effectiveness of herbal medicinal products or preparations for neuropathic pain, and the adverse events associated with their use.
Search methods
We searched CENTRAL and the Cochrane Database of Systematic Reviews, MEDLINE, Embase, CINAHL and AMED to March 2018. We identified additional studies from the reference lists of the retrieved papers. We also searched trials registries for ongoing trials and we contacted experts in the field for relevant data in terms of published, unpublished or ongoing studies.
Selection criteria
We included randomised controlled trials (including cross‐over designs) of double‐blind design, assessing efficacy of herbal treatments for neuropathic pain compared to placebo, no intervention or any other active comparator. Participants were 18 years and above and had been suffering from one or more neuropathic pain conditions, for three months or more.
We applied no restrictions to language or gender. We excluded studies monitoring effects of isolated, single chemicals derived from the plant or synthetic chemicals based on constituents of the plant, if they were not administered at a concentration naturally present within the plant.
We excluded studies monitoring the effects of traditional Asian medicine and Cannabinoids as well as studies looking at headache or migraine as these treatments and conditions are addressed in distinct reviews.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently considered trials for inclusion, assessed risk of bias, and extracted data. We calculated the risk ratio (RR) and number needed to treat for an additional beneficial outcome (NNTB). The primary outcomes were participant‐reported pain relief of 30%, or 50%, or greater, and participant‐reported global impression of clinical change (PGIC). We also collected information on adverse events. We assessed evidence using GRADE and created a 'Summary of findings' table.
Main results
We included two studies (128 participants). Both diabetic neuropathy and non‐diabetic neuropathic pain conditions were investigated across these two studies.
Two herbal medicinal products, namely nutmeg (applied topically as a 125 mL spray for four weeks, containing mace oil 2%, nutmeg oil 14%, methyl salicylate 6%, menthol 6%, coconut oil and alcohol) and St John's wort (taken in capsule form containing 900 μg total hypericin each, taken three times daily, giving a total concentration of 2700 mg for five weeks). Both studies allowed the use of concurrent analgesia.
Both reported at least one pain‐related outcome but we could not carry out meta‐analysis of effectiveness due to heterogeneity between the primary outcomes and could not draw any conclusions of effect. Other outcomes included PGIC, adverse events and withdrawals. There were no data for participant‐reported pain relief of 50% or greater or PGIC (moderate and substantial) outcomes.
When looking at participant‐reported pain relief of 30% or greater over baseline, we observed no evidence of a difference (P = 0.64) in response to nutmeg versus placebo (RR 1.12, 95% confidence interval (CI) 0.69 to 1.85; 48.6% vs 43.2%). We downgraded the evidence for this outcome to very low quality.
We observed no change between placebo and nutmeg treatment when looking at secondary pain outcomes. Visual analogue scale (VAS) scores for pain reduction (0 to 100, where 0 = no pain reduction), were 44 for both nutmeg and placebo with standard deviations of 21.5 and 26.5 respectively. There was no evidence of a difference (P = 0.09 to 0.33) in total pain score in response to St John’s wort compared to placebo, as there was only a reduction of 1 point when looking at median differences in change from baseline on a 0 to 10‐point numeric rating scale.
There was a total of five withdrawals out of 91 participants (5%) in the treatment groups compared to six of 91 (6.5%) in the placebo groups, whilst adverse events were the same for both the treatment and placebo groups.
We judged neither study as having a low risk of bias. We attributed risk of bias to small study size and incomplete outcome data leading to attrition bias. We downgraded the evidence to very low quality for all primary and secondary outcomes reported in this review. We downgraded the quality of the evidence twice due to very serious limitations in study quality (due to small study size and attrition bias) and downgraded a further level due to indirectness as the included studies only measured outcomes at short‐term time points. The results from this review should be treated with scepticism as we have very little confidence in the effect estimate.
Authors' conclusions
There was insufficient evidence to determine whether nutmeg or St John's wort has any meaningful efficacy in neuropathic pain conditions.
The quality of the current evidence raises serious uncertainties about the estimates of effect observed, therefore, we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Plain language summary
Herbal products for neuropathic pain
Background
Neuropathic pain is a complex and often disabling condition and many people suffer moderate or severe pain for many years, affecting quality of life. This condition is difficult to treat and typically only 40% to 60% of people with this condition achieve partial relief.
Neuropathic pain is pain coming from damaged nerves. It is different from pain messages that are carried along healthy nerves from damaged tissue (for example, a fall or cut, or arthritic knee). Neuropathic pain is often treated by different medicines to those used for pain from damaged tissue. Medicines that are sometimes used to treat neuropathic pain can have damaging side effects and therefore people are now trying herbal products to help relieve pain instead.
We conducted a search for relevant clinical trials in March 2018. We looked for studies in adults suffering from moderate neuropathic pain who took some form of herbal product, either by consuming it in their diet, in tablet form, or by applying it to the skin to relieve pain. We also collected information on side effects these herbal products might have.
Study characteristics
We included two studies with 128 participants. Study size ranged from 54 to 74 participants with an age range of 21 to 85 years. Both studies included men and women. Both studies compared herbal medicines (nutmeg or St John’s wort) to placebo and allowed continued use of painkillers. Both studies reported side effects.
Key results
There were no reports from participants of any reduction in pain intensity of 30% or above and there was no observable reduction in the total pain score in response to either nutmeg or St John’s wort. There were also no reductions in dropout rates or number of side effects between the treatment and placebo.
Quality of the evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident.
Only two small studies met this review’s search criteria. Neither provided any high‐quality evidence for either possible benefits or harms. We judged the evidence to be of very low quality. Thus, results from the studies contained in this review are very uncertain and prevent any meaningful conclusions. Larger, high‐quality studies are needed to assess accurately if herbal products are of any benefit or have the potential to harm when used to treat adults with neuropathic pain.
Summary of findings
Summary of findings 1. Herbal treatment compared with placebo for adults with neuropathic pain.
| Herbal treatment compared with placebo for adults with neuropathic pain | ||||||
|
Patient or population: adults with neuropathic pain Settings: primary care centre, hospital research unit Intervention: herbal treatment Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Herbal product | |||||
|
Participant‐reported pain relief of 30% or greater Immediately post‐intervention |
432 per 1000 | 486 per 1000 | RR 1.12 (0.69 to 1.85) | 74 participants (1 study) |
⊝⊝⊝⊝ Very low a,b,c,d,e |
Downgraded −2 due to very serious limitations in study quality in addition to −1 due to indirectness |
|
Participant‐reported pain relief of 50% or greater Immediately post‐intervention |
No data | No data | No data | No data | No data | Neither study reported this outcome |
|
Participant‐reported global impression of clinical change (PGIC) much or very much improved (moderate) Immediately post‐intervention |
No data | No data | No data | No data | No data | Neither study reported this outcome |
|
Participant‐reported global impression of clinical change (PGIC) very much improved (substantial) Immediately post‐intervention |
No data | No data | No data | No data | No data | Neither study reported this outcome |
|
Any pain‐related outcome indicating some improvement VAS 1‐100 Immediately post‐intervention (high score indicates more pain relief) |
44 + 21.5 | 44 + 26.5 | No data | 74 participants (1 study) |
⊝⊝⊝⊝ Very low a,b,c,d,e |
Downgraded −2 due to very serious limitations in study quality in addition to −1 due to indirectness |
|
Study withdrawals Post‐intervention |
66 per 1000 | 55 per 1000 (22 to 33) | RR 0.83 (0.26 to 2.64) | 128 participants (2 studies) | ⊝⊝⊝⊝
Very low a,b,c,e,f |
Downgraded −2 due to very serious limitations in study quality in addition to −1 due to indirectness |
|
Adverse events Post‐intervention |
187 per 1000 | 187 per 1000 (44 to 143) | RR 1.0 (0.55 to 1.81) | 128 participants (2 studies) | ⊝⊝⊝⊝
Very low a,b,c,e,f |
Downgraded −2 due to very serious limitations in study quality in addition to −1 due to indirectness |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for very serious study limitations due to risk of bias: small population (< 50 per treatment arm) and incomplete outcome data (> 10%). bDowngraded once for indirectness, outcomes only reported at short‐term time points. cNot downgraded for imprecision. dNot downgraded for publication bias, only 1 study identified but thorough search strategy carried out. eNot downgraded for inconsistency, I2 = 0%, P = 0.37, confidence intervals overlap fNot downgraded for publication bias, only 2 studies identified but thorough search strategy carried out.
Background
Description of the condition
The International Association for the Study of Pain (IASP) classifies neuropathic pain according to three features: the underlying disease; the site of the lesion (i.e. a peripheral nerve lesion or spinal cord); and the underlying mechanism (IASP 2006). It is defined as, "Pain arising as a direct consequence of a lesion or disease affecting the somatosensory system" (IASP 2006). Unlike nociceptive pain, such as gout and other forms of arthritis, neuropathic pain is caused by nerve damage, often accompanied by anatomical and physiological changes in the central nervous system (CNS) or peripheral nervous system (PNS). The pain can be described as burning, tingling, shooting, stabbing or shocking. Injury to the brain, brain tumours, diabetic neuropathy and herpes zoster are all examples of conditions that may cause this type of pain.
Neuropathic pain can be very difficult to treat, with only 40% to 60% of patients achieving partial relief (Dworkin 2007), fewer than those experiencing nociceptive pain. Determining the best treatment for individual patients remains challenging, with favoured treatments including certain antidepressants, such as tricyclics and selective serotonin‐norepinephrine reuptake inhibitors (SNRIs), anticonvulsants, especially pregabalin (Lyrica) and gabapentin (Neurontin), and topical lidocaine.
A study carried out in 1998 in the USA reported that approximately four million people suffered from neuropathic pain (Dickson 2010). The highest prevalence rates were observed for peripheral diabetic neuropathy (600,000 cases) and postherpetic neuralgia (500,000 cases), based on a population of 270 million (Bennett 1998). In Europe, neuropathic pain is estimated to affect between 3% and 8% of individuals, with 5% of these people reporting moderate to severe pain leading to significant reductions in quality of life (Bouhassira 2008; Gustorff 2008; Torrance 2006). In the UK, the prevalence of neuropathic pain is as high as 8% (Torrance 2006), with incidence rate estimates for specific conditions of 34 to 40 cases per 100,000 person‐years observation for postherpetic neuralgia and 27 to 400 cases for trigeminal neuralgia, one for phantom limb pain and 15 to 400 cases of painful diabetic neuropathy. While rates for phantom limb pain and postherpetic neuralgia appear to have declined in recent years, painful diabetic neuropathy has increased (Hall 2006; McQuay 2007).
Anatomical and physiological changes in the CNS include age‐dependent total grey matter volume decrease, reduced presynaptic dopamine activity, disruption of dopaminergic neurotransmission resulting in increased pain and discomfort, hippocampus dysfunction, and metabolite and cerebral metabolite ratio abnormalities, all of which demonstrate CNS dysfunction (Emad 2008; Kuchinad 2007; Petrou 2008; Wood 2007a; Wood 2007b; Wood 2009). People with chronic neuropathic display features of the central hypersensitivity responsible for enhanced neuronal excitability and increased pain (Curatolo 2006).
For the purpose of this review, the definition of 'neuropathic pain' will be restricted to those disorders with a primary aetiology clearly related to the PNS or CNS.
Pharmacological interventions include unconventional analgesics such as antidepressants and anticonvulsants, in addition to conventional medications such as strong opioids. Most of these agents have significant side effects and as one of the first‐line treatment options there are concerns about the associated costs to the health service (NICE 2010). Population‐based surveys suggest that people with chronic neurological pain are likely to try complementary and alternative (CAM) therapies such as herbal treatments (Kanodia 2010; Metcalfe 2010; Thomas 2004). For this reason, it is important for policy makers to become aware of the impact these products may have.
Description of the intervention
Oral herbal remedies include standardised extracts (encapsulated or tablet form), tinctures (e.g. alcohol, glycerine), dried herbs (encapsulated or tablet form), raw whole herb infusions (e.g. tea) and decoctions (e.g. boiled down tea). Topical herbal applications include ointments, essential oils, creams (petroleum or glycerine based), powders, plasters and poultices. Constituents of a single plant or of herbal mixtures are claimed to work synergistically to produce a greater effect than a single constituent. It is also claimed that the combined actions of the various constituents reduce the toxicity of the extract compared with single, isolated constituents (Ernst 2001). Both these synergistic and buffering effects extend to the use of different plant extracts in combination preparations.
Three definitions of herbal medicines have been identified to inform this review. Ernst 2001 has previously defined herbal medicine as "The medical use of preparations that contain exclusively plant material". Gagnier 2011 defined herbal treatments as all or part of a plant used for medicinal purposes, administered orally (ingestion) or applied topically. This definition does not include plant substances that are smoked (e.g. Cannabis sativa), individual chemicals that are derived from plants or synthetic chemicals that are based on constituents of plants. The European Medicines Agency Directive (2004/24/EC) defines a herbal medicinal product as "Any medicinal product, exclusively containing as active ingredients, one or more herbal substances or one or more herbal preparations, or one or more such herbal substances in combination with one or more such herbal preparations". Herbal preparations are defined as preparations obtained by subjecting herbal substances to treatments such as extraction, distillation, expression, fractionation, purification, concentration or fermentation.
In the current review, we included herbal preparations that contained whole plants, parts of plants, or comminuted or powdered herbal substances, tinctures, extracts, essential oils, expressed juices, processed exudates, infusions or decoctions. To clarify, we included preparations exclusively containing plant material that were ingested or applied topically, at any dose and that contained active ingredients of one or more herbal substance or preparation. We defined herbal preparations as outlined by the EMA Directive above.
Current guidelines on the treatment and management of neuropathic pain do not report on the use of herbal products for pain intensity reduction, possibly due to a lack of research studies. However, there is a body of literature suggesting a pain‐reducing effect in response to cannabis that is being investigated in a separate Cochrane Review (Mücke 2016). There is also some preliminary evidence that capsaicin is beneficial for reduction of pain intensity in people with some neuropathic pain conditions, as demonstrated in two recent Cochrane Reviews (Derry 2012; Derry 2013). This was based on studies of adequate methodological quality and involved pooling of the neuropathic conditions (postherpetic neuralgia, diabetic neuropathy, HIV neuropathy, postmastectomy pain and postsurgical cancer pain). Whole essential oils have also been reported to have analgesic effects in neuropathic pain in a randomised, double‐blind, placebo‐controlled trial of 60 participants (Li 2010). These preliminary results appear promising for the use of herbal products/preparations in the treatment of neuropathic pain, however more robust evidence is required before definitive guidance on their use can be recommended.
Why it is important to do this review
Neuropathic pain is a complex and often disabling condition. Many people suffer moderate or severe pain for many years, and in the UK 7% to 8% of adults currently have chronic pain with neuropathic characteristics (EFIC 2015), which leads to significant reductions in quality of life. In a UK study, 17% of people who had neuropathic pain characteristics had health‐related quality of life (QOL) scores equivalent to 'worse than death' (Torrance 2014). Conventional analgesics are usually not effective in alleviating the symptoms, although opioids may be effective in some individuals. Treatment is therefore usually by unconventional analgesics such as antidepressants or antiepileptics. However, there has been negative publicity surrounding the side effects associated with current pharmacological treatments for specific types of neuropathic pain (BNF 2006; Glassman 1998; Peretti 2000), and evidence from population‐based surveys has shown that people with chronic pain are likely to try herbal treatments. It is therefore important to determine the efficacy and safety of herbal medicines in the treatment of such conditions.
New standards have evolved for assessing efficacy in neuropathic pain. More strict criteria for the inclusion of trials and assessment of outcomes are now applied, and researchers are more aware of problems that may affect overall assessment. For this reason, a review applying these new standards to an assessment of the efficacy of herbal medicinal products or preparations in neuropathic pain is necessary.
Objectives
To assess the analgesic efficacy and effectiveness of herbal medicinal products or preparations for neuropathic pain, and the adverse events associated with their use.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including cross‐over designs, of double‐blind design, which assess the efficacy and effectiveness of herbal medicinal products or preparations for neuropathic pain.
We applied no restriction with regard to language.
Types of participants
We included adult participants aged 18 years and above. Participants had been suffering from one or more neuropathic pain conditions, for three months or more. Neuropathic pain conditions included (but were not limited to) the following.
Painful diabetic neuropathy (PDN)
Post‐herpetic neuralgia (PHN)
Trigeminal neuralgia
Phantom limb pain
Postoperative or traumatic neuropathic pain
Complex regional pain syndrome (CRPS)
Cancer‐related neuropathy
HIV neuropathy
Spinal cord injury
We included studies of participants with more than one type of neuropathic pain with the intention of analysing these results according to the primary condition.
We did not make restrictions based on gender.
We excluded studies of headache or migraine.
Types of interventions
For the purpose of this review, we included studies that investigated the effects of herbal medicinal products or preparations administered in the form of whole plants, parts of plants or extracts for the relief of neuropathic pain compared to placebo, no intervention or any other active comparator. These preparations were either administered topically or orally. In the case of single, isolated substances, we only included studies using a treatment dose of the herbal product/preparation that was directly proportionate to the concentration that would be present in the whole plant.
We also extracted data from dose‐comparison studies.
Co‐interventions
We included studies monitoring other analgesic consumption, alongside herbal medicinal products.
Exclusions
Studies monitoring the effects of isolated, single chemicals derived from the plant or synthetic chemicals based on constituents of the plant if they were not being administered at a concentration that would be naturally present within the plant.
Studies monitoring the effects of traditional Asian medicine as this involves complex mixtures of plant products individualised for the patient.
Studies monitoring the effects of capsaicin or cannabis as these have been dealt with in separate Cochrane Reviews.
Types of outcome measures
We required studies to report pain assessment as either the primary or secondary outcome. The majority of studies used standard subjective scales for pain intensity or pain reduction, or both.
We considered the IMMPACT definitions of moderate and substantial benefit in chronic pain studies (Dworkin 2008).
Primary outcomes
Participant‐reported pain relief of 30% or greater, over baseline (moderate)
Participant‐reported pain relief of 50% or greater, over baseline (substantial)
Participant‐reported global impression of clinical change (PGIC) much or very much improved (moderate)
Participant‐reported global impression of clinical change (PGIC) very much improved (substantial)
Secondary outcomes
Any pain‐related outcome indicating some improvement
Withdrawals: for any reason, due to lack of efficacy, due to adverse events
Adverse events: participant reporting of any adverse event; participant reporting of any serious adverse event; death
We collected outcome assessment data for all treatment durations and reported the extracted data.
Search methods for identification of studies
Electronic searches
To identify studies for inclusion in this review, we developed detailed search strategies for each electronic database to be searched. These were based on the search strategy developed for MEDLINE but revised appropriately for each database. The search strategy combined the subject search with phase one and two of the Cochrane highly sensitive search strategy for RCTs (Lefebvre 2011), and was developed with the assistance of Cochrane Pain, Palliative and Supportive Care's (PaPaS) Information Specialist. We undertook the latest search in March 2018. The subject search used a combination of controlled vocabulary and free‐text terms. The search strategies used can be found in Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5.
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) in the Cochrane Library;
the Cochrane Database of Systematic Reviews (CDSR; 2018, Issue 3) in the Cochrane Library;
MEDLINE ‐ OVID (1946 to 13 March 2018);
Embase ‐ OVID (1974 to 13 March 2018);
CINAHL ‐ EBSCO (1982 to 13 March 2018);
AMED ‐ OVID (1985 to 13 March 2018).
Searching other resources
We screened any systematic reviews on the effectiveness or efficacy (or both) of herbal medicinal products or preparations for neuropathic pain for additional references and identified additional studies from the reference lists of the retrieved papers. We also supplemented the electronic search strategy by using the Science Citation Index to perform citation tracking of the RCTs identified.
We also searched the metaRegister of controlled trials (mRCT) (http://www.controlled-trials.com/mrct (at March 2019, this website is under review)), Clinicaltrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials. We carried out the last search in March 2018.
We contacted experts in the field (identified by personal contacts, lead authors in published studies, world wide web searching) for relevant data in terms of published, or ongoing studies, to identify other relevant articles that may have been missed by the electronic search.
We also intended to identify herbal medicinal products or preparations being used without sufficient evidence of effectiveness (unpublished data) by contacting experts in the field of complementary and alternative medicine but it decided it was not productive to do this for the purposes of the review at this stage as most experts in the field appeared to be investigating those preparations that we had chosen to exclude from this review, namely cannabis and capsaicin at higher levels not present naturally in chili peppers. We plan to revisit this decision in the future.
Our searches identified all relevant studies irrespective of language. We assessed non‐English papers and translated them with the assistance of a native speaker.
Data collection and analysis
Selection of studies
Two review authors (AB, CB) independently selected trials for inclusion and screened the titles and abstracts of publications obtained by the search strategy. If no abstract was available we obtained and assessed the full paper. We retrieved all trials classified as relevant by either of the review authors for further assessment. We resolved disagreement between review authors by consensus, or third party adjudication (SMcD). We included a PRISMA flow chart in this review, which shows the status of identified studies (Moher 2009), as recommended in the Cochrane Handbook of Systematic Reviews of Interventions (Schünemann 2011). We included studies in this review irrespective of whether measured outcome data were reported in a 'usable' way. Where necessary, we attempted to contact primary authors for clarification of study characteristics.
Data extraction and management
Two review authors (AB, DH) extracted data independently using a customised form, tested prior to use. We used this to extract relevant data on methodological issues, eligibility criteria, interventions (including the pain condition, number of participants treated, herbal medicinal product/preparation, dosing regimen, study design, study duration and follow‐up, comparisons, outcome measures and results, withdrawals and adverse events). Again, we resolved any disagreement by consensus, or third party adjudication (SMcD). We attempted to contact the primary study authors to clarify any omitted data or study characteristics. With the intention‐to‐treat analysis in mind, we extracted data according to the original allocation groups, and noted losses to follow‐up where possible.
Where data seemed to be missing from a study we attempted to obtain these data through correspondence with the study authors.
There was no blinding to study author, institution or journal at this stage.
We collected characteristics of the included studies in sufficient detail to populate a table of 'Characteristics of included studies' in this review.
Assessment of risk of bias in included studies
Two authors (AB and CB) independently assessed the risk of bias for each study, using the 'Risk of bias' tool available in the Review Manager 5 (RevMan 5) software (Review Manager 2014), outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and adapted from those used by Cochrane Pregnancy and Childbirth. We resolved any disagreements by discussion, with SMcD acting as third party adjudicator. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated); we excluded any studies at high risk of bias (studies using a non‐random process such as odd or even date of birth).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated); we excluded any studies at high risk of bias (studies that do not conceal allocation).
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies that were not double‐blind are considered to have high risk of bias.
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study has a clear statement that outcome assessors were unaware of treatment allocation, and ideally describes how this was achieved); unclear risk of bias (study states that outcome assessors were blind to treatment allocation but lacks a clear statement on how it was achieved). We judged studies where outcome assessment was not blinded as having a high risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We will assess the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); we excluded studies that were high risk of bias (used 'completer' analysis).
Selective reporting (reporting bias). We assessed the risk of reporting bias as: low risk of bias (all intended outcomes reported); unclear risk of bias (any anomaly in reporting, such as participants contributing more than one set of data, or some outcomes not participant‐reported); we excluded studies that were high risk of bias (pre‐specified outcome of interest not reported).
Size of study (Moore 1998; Nuesch 2010), (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
We regarded differences in treatment intervention detail (e.g. type of herbal product/preparation, dosage of herbal product/preparation or different pain condition) as a potential source of bias as there was previous evidence of different effects in different neuropathic pain conditions for some interventions (Moore 2009). We planned to address these in the subgroup analysis, however, the type of painful condition could not be subjected to a subgroup analysis due to heterogeneity among the included studies.
We also intended to consider additional risks of bias including issues of withdrawal (Moore 2010a), and duration (Moore 2010b), in addition to standard risks of bias.
Measures of treatment effect
For each study, we calculated risk ratio (RR) and 95% confidence intervals (CI) for dichotomous outcomes, and mean differences (MD) and 95% CI for continuous outcomes. Only one of the two included studies provided continuous outcome data, so it was not necessary to pool different scales to use standardised mean differences. We used changes from baseline (mean change scores) in preference to follow‐up scores.
Unit of analysis issues
We split the control treatment arm between active treatment arms in the single study of Motilal 2013, where the active treatment arms were not combined for analysis, in order to determine individual treatment effects.
Dealing with missing data
We used intention‐to‐treat (ITT) analysis wherever possible. The ITT population consisted of participants who were randomised, took the assigned herbal product/preparation and provided at least one post‐baseline assessment. We contacted the original investigators to request missing data by email, with reminder emails sent when no response was given. For both included studies it was necessary to contact the original authors, however, only one author provided the requested information despite email and phone call attempts to the author of the other study.
We did not need to consider missing data during sensitivity analyses.
Standard deviations were available in both studies.
Assessment of heterogeneity
Initially, we qualitatively assessed clinical diversity between the two studies. We considered whether the studies were similar for intervention (dosage and duration), type of participant, outcomes assessed and follow‐up time. As we deemed the studies to be clinically homogeneous according to the above terms, we assessed the data for statistical heterogeneity using RevMan 5 (Review Manager 2014). We used the I² statistic (Higgins 2003), to assess this and considered values of I² greater than 50% to represent substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
We contacted study authors when data were not clearly presented in the papers included in this review. We requested clarification around what the data were demonstrating as well as the scales used. When we felt it necessary, we requested raw data from the authors of the study.
Should it have become apparent that a large enough body of hidden data (participants or trials) existed, we would have followed guidance from the Pain, Palliative and Supportive Care Review Group and the Cochrane Handbook.
Data synthesis
We considered individual herbal medicinal products/preparations separately. In order to assess the effectiveness of the intervention we extracted the dichotomous data from the included studies. We used these data to calculate risk ratio (RR) or benefit using Review Manager 2014 with 95% CIs together with numbers needed to treat for an additional beneficial outcome (NNTBs) (Cook 1995), using a fixed‐effect model, as there was no evidence of heterogeneity of effect. We did not calculate the NNTB for pain or the number needed to treat for an additional harmful outcome (NNTH), as too few data were available to carry out a meta‐analysis. For unwanted effects, the NNTB becomes the NNTH and we calculated this in the same way. We calculated the NNTH for both minor and major adverse events. Major adverse events are those that lead to withdrawal from the study. We reported the number and type of adverse events.
Continuous data were not used as it is inappropriate when there is an underlying skewed distribution. When continuous data were used, we used RevMan 5 to report on summary continuous data where available and appropriate. We carried out a meta‐analysis using a fixed‐effect model when there was no evident heterogeneity of effect.
Meta‐analysis was not possible for the primary outcome due to study heterogeneity and the availability of too few data, therefore we provided a narrative review.
We attempted to collect outcome assessment data for participants for all treatment durations and report extracted data as close to eight weeks as possible but not less than four weeks. Where longer‐duration outcomes were available we also extracted these data. Where multiple observations of the same outcome occurred, we extracted data at clinically relevant time points. This reflected short‐term (immediately after the intervention), medium‐term (closest to 12 weeks) and long‐term (24 weeks or more) outcomes.
Subgroup analysis and investigation of heterogeneity
Due to the limited number of studies identified fitting the inclusion criteria, there were too few data to carry out subgroup analyses as planned, for:
type of herbal product/preparation;
dose of herbal product/preparation;
concurrent analgesia;
different painful conditions.
Sensitivity analysis
We did not carry out any sensitivity analysis due to a small evidence base and difficulty in determining the potency of the herbal products or preparations. We pooled results for different neuropathic pain conditions. We did not carry out any sensitivity analysis due to a high or unclear risk of bias in the studies.
Summary of findings and assessment of the certainty of the evidence
Two review authors (AB, SMcD) independently rated the quality of the outcomes. We used the GRADE system to rank the quality of the evidence using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased the GRADE rating by one (−1) or two (−2) if we identified:
serious (−1) or very serious (−2) limitations to study quality
important inconsistency (−1)
some (−1) or major (−2) uncertainty about directness
some (‐1) or serious (‐2) imprecise or sparse data
high probability of reporting bias (‐ 1)
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings for herbal products/preparations and neuropathic pain relief in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes: participant‐reported pain relief of 30% or greater, participant‐reported pain relief of 50% or greater, PGIC much or very much improved, PGIC very much improved, any pain‐related outcome indicating some improvement, withdrawals and adverse events.
Results
Description of studies
Results of the search
The searches of the five databases retrieved 11,559 records (see Electronic searches). Our searches of the trials registers identified 35 further studies. Our screening of the reference lists of the included publications did not reveal additional RCTs. Our searches of other resources (e.g. hand searches) identified no additional studies that appeared to meet the inclusion criteria. We therefore had a total of 11,594 records.
Once duplicates had been removed, we had a total of 9560 records. We excluded 8533 records based on titles and a further 1008 based on abstracts. We obtained the full text of 19 records. We included two studies (see Characteristics of included studies). We excluded 15 studies (see Characteristics of excluded studies). We added one record to Characteristics of studies awaiting classification. We identified one ongoing study (see Characteristics of ongoing studies).
For a further description of our screening process, see the study flow diagram (Figure 1).
1.
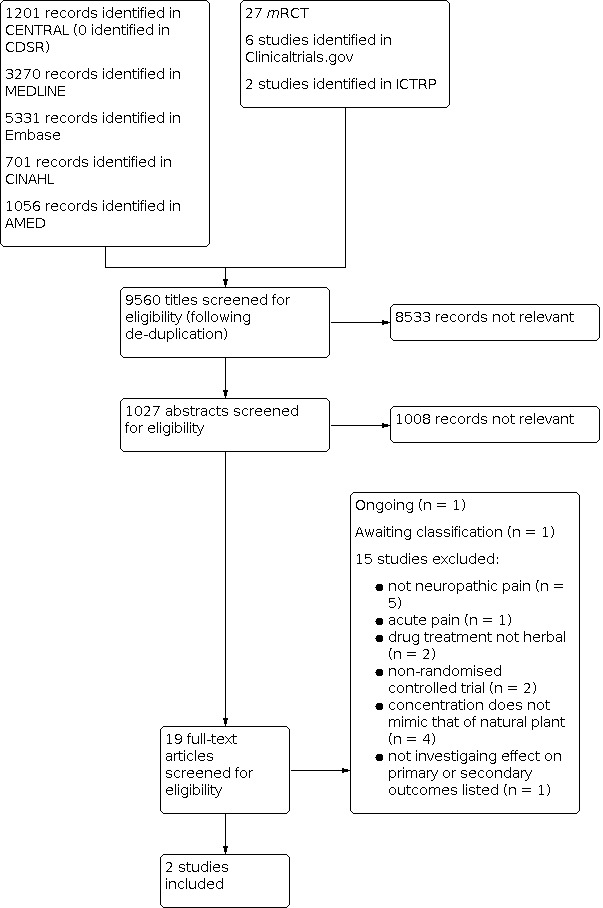
Study flow diagram
Included studies
We included two studies, with 128 participants in total, 91 of whom were treated with the herbal treatment, in comparison with placebo (Motilal 2013; Sindrup 2000). These two studies investigated both diabetic neuropathy (Motilal 2013; Sindrup 2000), and non‐diabetic neuropathic pain conditions (Sindrup 2000). One study enrolled participants with idiopathic peripheral neuropathy (Sindrup 2000). Whilst both studies enrolled participants with diabetic neuropathy, only Sindrup 2000 enrolled other non‐diabetic polyneuropathy patients. Study size ranged from 54 to 74 participants with an age range of 21 to 85 years. Both studies included both men and women.
The studies investigated two herbal medicinal products, namely nutmeg and St John's wort. We planned to include studies looking only at whole plant products or preparations, however, we later decided that we would also include preparations containing the active ingredient at a concentration range that would naturally be present in the plant.
Nutmeg was applied topically as a 125 mL spray for four weeks, which contained nutmeg oil 14%, methyl salicylate 6%, menthol 6%, mace oil 2%, coconut oil and alcohol (Motilal 2013). St John's wort was taken in capsule form containing 900 μg total hypericin each, which were taken three times daily, giving a total concentration of 2700 mg (Sindrup 2000); this study lasted for five weeks.
The exclusion criterion of the identified studies varied slightly depending on the herbal product/preparation being investigated. Examples of exclusion criteria applied to these studies were allergies to the treatment, severe terminal illness, soft tissue infections or injuries, treatment with monoamine oxidase (MAO) inhibitors, use of HIV antiretroviral drugs, elderly people or individuals who may not understand the treatment, or individuals who cannot read or understand English.
Both studies reported participants to have at least moderate pain (pain rated as 4 or above on a 10‐point numerical rating scale) at baseline, regardless of the type of neuropathic pain condition. Pain was reported as having been present for at least three months in Sindrup 2000, however, Motilal 2013 did not report the actual duration in included participants. Based on the information given in this study, we deemed it likely that the majority of participants in these studies had experienced pain for at least three months (i.e. chronic pain), and therefore we decided to include it.
Both studies were placebo‐controlled without active ingredients. Placebos took the form of a topical spray of 6% salicylate, 6% menthol coconut oil and alcohol (Motilal 2013), and tablets dosed in the same manner as the total hypericin in Sindrup 2000. Sindrup 2000 used a cross‐over design, with a washout period of at least one week between treatment phases. Motilal 2013 did not specify any washout period as it was a parallel study.
Both studies allowed continued use of stable oral analgesics, but all other use of the treatment substance was prohibited.
Excluded studies
We excluded studies if they were non‐randomised, case reports or clinical observations. We excluded 15 studies from this review. We excluded two studies due to non‐randomisation (Mankowski 2017; Staiger 2012). Five studies assessed pain outcomes in non‐neuropathic painful conditions (ISRCTN29199098; Salazar Sanchez 2010; Wade 2004; Willich 2010; Woolridge 2005). We excluded two studies based on the fact that the intervention was a pharmacological agent (Khodari 2017), the second of which used a treatment of three drugs in the preparation (Barton 2011). We excluded one study as it did not look at neuropathic pain of a chronic nature; it investigated the effects of cannabis against heat‐induced acute pain (Abrams 2007). We excluded four studies based on the rationale that the active ingredient was not present at a concentration that was naturally present in the plant (Hambardzumyan 2017; Moon 2017; Paice 2000; Torre‐Mollinedo 2001). We excluded one study as it did not investigate any of the primary or secondary outcomes being investigated in this review (Cruccu 2018). See Characteristics of excluded studies.
Studies awaiting classification
We identified one study that is awaiting classification as the trial has been completed but it has not yet been fully published (NCT02107469 see Characteristics of studies awaiting classification).
Ongoing studies
We identified one study that is ongoing (IRCT201201248815N1; see Characteristics of ongoing studies).
Risk of bias in included studies
Comments on potential biases in individual studies are reported in the 'Risk of bias' section of the Characteristics of included studies tables. The findings are displayed in Figure 2 and Figure 3. We undertook no sensitivity analysis as we judged no studies as having a low risk of bias. Risk of bias was attributed to small study size and incomplete outcome data leading to attrition bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Both studies adequately described the method used to generate the random sequence.
Both studies were randomised and adequately described the method used to conceal allocation.
Blinding
Blinding of participants and personnel
We judged that both studies were double blind and both reported the method used.
Blinding of outcome assessment
Both studies clearly identified the participants and outcome assessors remained blind.
Incomplete outcome data
We judged Motilal 2013 as having an unclear risk as they did not report the amount of missing data and used the last observation carried forward (LOCF) imputation method for missing data. This study also recorded a 7% dropout rate.
We judged Sindrup 2000 to be at a high risk of bias as they reported greater than 10% dropout, with LOCF imputation of data also being reported.
Selective reporting
Both of the included studies in this review had a low risk of selective reporting bias. Both reported on secondary outcomes including any pain‐related outcome indicating some improvement, withdrawals and adverse events. When we contacted primary study authors, Motilal 2013 provided raw data for pain scores, thereby allowing us to calculate the number of individuals with a participant‐reported pain intensity reduction of 30% or greater over baseline. No anomalies in the reporting of data were evident.
Other potential sources of bias
We considered issues of withdrawal as part of 'incomplete outcome data'. We could not investigate duration as a source of bias since both studies only assessed pain immediately post‐intervention. Neither study made any longer‐term follow‐up assessments.
Size of study
Sindrup 2000 had treatment groups with slightly over 50 participants randomised per treatment arm. We judged this study as having an unclear risk for this item as only 47 participants completed each arm of the study. Motilal 2013 had more than 50 participants in total (74) but as it was a parallel study there were only 37 per treatment arm. We therefore judged this as being at a high risk of bias.
Effects of interventions
See: Table 1
See 'Summary of findings' table 1 for the comparison herbal treatment versus placebo for neuropathic pain (Table 1).
See also Table 2 for the summary of effect in each study.
1. Data extraction: summary of effect in individual studies.
| Reference | Participants | Treatment | Washout | Duration (weeks) | Size n | Imputation | Pain outcome | Withdrawals | AEs |
| Motilal 2013 | Adults aged 21‐85 years, with PDN of:
Symptoms limited to the extremities of limbs, and an average neuropathic pain > 4 as determined by the DN4 Questionnaire. |
Commercially available topical preparation of nutmeg extracts (NEMM). Colourless with same odour as MM in 125 mL spray bottle. Participants instructed to apply 4 sprays to affected area 3 times/day, followed by gentle massage for 4 weeks. Placebo (MM). Colourless with same odour as NEMM, in 125 mL spray bottle. |
No washout period as not a cross‐over trial | 4 sprays to affected area 3 times/day, followed by gentle massage for 4 weeks | 74 | LOCF used for missing data | No statistically significant difference between groups for worst (P = 0.594) or average pain (P = 0.970) as measured by BPI for PDN and total NPSI score (P = 0.620). No change scores given. No difference in % achieving at least 33% reduction in worst pain from baseline at 4 weeks: NEMM (48.6%) v MM (43.2%) (P = 0.64, RR 1.12, 95% CI 0.69 to1.85) |
Treatment NEMM n = 3 1 could not be contacted from week 1 visit 1 had adverse event and withdrew after week 2 1 could not be contacted at week 4 Control MM (placebo) n = 2 1 had adverse event and withdrew after 2 days 1 could not be contacted from week 2 visit |
Treatment NEMM n = 4 1 eye pain and headache, withdrew after 2 weeks 2 burning, transient and tolerable, continued therapy 1 stiffness, transient and tolerable, continued therapy Control MM (placebo) n = 2 1 blisters on heels, withdrew after 2 days 1 heaviness, transient, continued therapy |
| Sindrup 2000 | Adults > 20 years, with painful polyneuropathy:
Confirmed by electrophysiological tests for > 6 months n = 54 entered and 47 completed study |
St John’s wort 3 tablets (900 μg total hypericin each) total daily dose 2700 mg total hypericin given in the evening x 5 weeks or placebo (3 tablets identical in appearance were dosed similarly in the placebo phase) x 5 weeks |
1 week washout | 5 weeks | 54 | LOCF | Marginally lower total pain score for St Johns wort (median 14, 25‐75 percentile 7‐21) v placebo (15, 9‐19; P = 0.05) Diabetic participants (n = 18) trend towards lower total pain score during St Johns wort (P = 0.08) and reduction in lancinating pain (P = 0.02) Non‐diabetic participants (n = 29) no significant difference in total or individual pain scores between groups |
Treatment St John’s wort n = 2 1 adverse event 1 lost to follow‐up Control Placebo n = 4 1 adverse event 3 needed pain treatment |
Comparable number and type of AEs for St Johns wort (n = 13) and placebo (n = 15) groups:
|
AE: adverse event; BPI: Brief Pain Inventory; CI: confidence interval; DN: Douleur Neuropathique; LOCF: last outcome carried forward; mL: millilitres; MM: methyl salicylate (6%), menthol (6%), coconut oil, alcohol; n: number of participants; NEMM: nutmeg oil (14%), methyl salicylate (6%), menthol (6%), mace oil (2%), coconut oil, alcohol; NPSI: Neuropathic Pain Symptom Inventory; PDN: painful diabetic neuropathy; RR: risk ratio; μg: microgram
Primary outcomes
Both included studies reported at least one pain‐related outcome and reported some improvement compared with placebo, as seen in the data extraction table (Table 2), however, we could not carry out any meta‐analysis due to there only being two studies with heterogeneity existing between their primary outcomes. We downgraded the evidence derived from this review to very low quality due to limitations in study quality and imprecision. Low study quality was attributed to various factors such as study size, attrition bias, short duration of intervention and follow‐up. For this reason, we deemed it unnecessary to carry out a subgroup analysis.
Participant‐reported pain relief of 30% or greater over baseline (moderate)
One study reported a participant‐reported pain relief of 30% or above over baseline, in response to nutmeg versus placebo (RR 1.12, 95% CI 0.69 to 1.85; 48.6% vs 43.2%; Motilal 2013). participant‐reported pain relief of 30% or greater over baseline is a moderate effect as described by the IMMPACT definitions of moderate and substantial benefit in chronic pain studies (Dworkin 2008), however, this finding was not demonstrative of an effect (P = 0.64). We downgraded the quality of the evidence by three levels (using GRADE criteria) to very low due to very serious limitations in study quality (small participant numbers and attrition bias) and indirectness (short‐term outcomes only). These limitations caused serious uncertainties about the estimates observed (see Characteristics of included studies ‐ 'Risk of bias' tables, Table 1, and additional Table 2).
Participant‐reported pain relief of 50% or greater, over baseline (substantial)
Neither study reported substantial pain relief of 50% or greater.
Participant‐reported global impression of clinical change (PGIC) much or very much improved (moderate)
Neither study reported PGIC much or very much improved.
Participant‐reported global impression of clinical change (PGIC) very much improved (substantial)
Neither study reported PGIC to be very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement
We attempted to obtain raw data from study authors in order to calculate percentage change in pain as assessed by VAS, however only Motilal 2013 provided this information, reporting the mean values in pain reduction (0 to 100, where 0 = no pain reduction) and standard deviation (SD) for baseline and post‐intervention, revealing no change between placebo (44 ± 21.5) and nutmeg (44 ± 26.5) treatments.
Whilst Sindrup 2000 did not provide raw data, they did report a lower total pain score in response to St John’s wort compared to placebo, with a reduction of 1 point from baseline at weeks two to five on a 0 to 10‐point numeric rating scale. This small change demonstrated no evidence of change between the two groups.
We did not include Sindrup 2000 in Table 1 with regards to this secondary outcome as the author did not provide us with any raw data but reported only the median pain scores with percentiles as opposed to mean and standard deviations. Should the author have presented the data to us, they would have been of limited value due to the downgrading of the evidence by three levels to very low quality as a result of very serious limitations to study quality, and indirectness.
Withdrawals
Motilal 2013 observed three withdrawals in response to nutmeg (3/37; 8%) compared to placebo (2/37; 5%). Reasons were similar for both groups. In the treatment group, two of the participants could not be contacted (one after week one and one after week four), and one had an adverse event, whilst in the placebo group one could not be contacted after week two and one had an adverse event. In Sindrup 2000, St John’s wort resulted in 2/54 (4%) withdrawals (due to loss to follow‐up and adverse events) compared to 4/54 (7%) in the placebo group (three were due to lack of efficacy and one was due to adverse events).
This gave a total of five withdrawals out of 91 participants (5%) in the treatment groups compared to six withdrawals out of 91 participants (6.5%) in the placebo groups, giving an increased RR for withdrawal with active treatment (RR 0.83, 95% CI 0.26 to 2.64; NNTH = 1.7; Analysis 1.1). See Table 1.
1.1. Analysis.

Comparison 1: Herbal treatment versus placebo, Outcome 1: Study withdrawals
Again it should be noted that we downgraded the quality of this evidence by three levels to very low as a result of very serious limitations in study quality and also indirectness.
Adverse events
Motilal 2013 documented four adverse events recorded in those who were treated with nutmeg (37 participants), whilst two adverse events were reported in the placebo group (37 participants). Sindrup 2000 reported that St John’s wort resulted in 13 adverse events in the treatment group (54 participants) and 15 in the placebo group (54 participants). When we combined these studies, we observed a RR of 1.00 (95% CI 0.55 to 1.81; NNTH = 10; Analysis 2.1), for adverse events in response to these herbal treatments, and an odds ratio of 1.00 (95% CI 0.47 to 2.15). See Table 1.
2.1. Analysis.

Comparison 2: Herbal treatment versus placebo, Outcome 1: Adverse events
Additional adverse events noted with nutmeg treatment were mild, transient and tolerable, and there were no major systemic adverse events (Motilal 2013). Adverse events were also few with the dose of St John's wort and were not different in spectrum and severity from adverse events reported with placebo (Sindrup 2000). This is in line with previous observations with St John's wort (Ernst 2001). However, we downgraded the quality of the evidence for this outcome to very low as a result of very serious limitations in study quality and also indirectness.
Neither study documented any deaths or serious adverse events.
Discussion
Summary of main results
Table 1 outlines the main results of this review by highlighting the effects of herbal medicinal products or preparations on each primary and secondary outcome. The main findings demonstrate a RR of 1.12 (95% CI 0.69 to 1.85), for the primary outcome of 'number of participants obtaining 30% pain relief over baseline' in response to treatment (nutmeg) compared to placebo. The secondary outcome of 'any pain‐related outcome indicating some improvement' highlighted no difference between treatment (nutmeg) and placebo when it was assessed on a VAS. We observed a RR of 0.83 (95% CI 0.26 to 2.64) for the secondary outcome 'study withdrawals' between treatment and control. Finally, adverse events were no different between treatment and placebo (RR 1.00, 95% CI 0.55 to 1.81).
All of the main findings reported in this review are limited in their meaningfulness as we downgraded all primary and secondary outcomes to very low quality (Table 1). We have little confidence in the findings as the quality of the evidence is too low to draw any definitive conclusions.
Overall completeness and applicability of evidence
Based on the evidence collated in this review, it is not possible to draw any meaningful conclusions. Whilst the evidence presented in this review is relevant to the research question in that it examines the effects of herbal medicinal products towards neuropathic pain, overall, the evidence presented is of very low quality and therefore does not permit the research question or indeed the objectives, to be answered. The studies were carried out with low participant numbers and with only one condition. This prevented the pooling of studies, resulting in little confidence about effects or size of effect observed. In addition, both studies were of short duration (maximum of five weeks), so it was not possible to assess whether any early response would be maintained in the longer term. This is important in chronic conditions. The outcomes investigated in the studies were also limited in that they mainly reported secondary outcomes.
In summary, the evidence presented in this review was trivial in amount and therefore is not applicable to clinical practice at this stage. Further studies of higher quality, in larger numbers of participants, across a number of neuropathic pain conditions and looking at primary pain outcomes as specified by IMMPACT, are required (Dworkin 2008). These should also be carried out over longer follow‐up time points in order to answer the research question looking at the effect of herbal medicinal products or preparations on neuropathic pain and to assess the analgesic efficacy and effectiveness of herbal medicinal products or preparations for neuropathic pain, and also the adverse events they may cause. We anticipate that the two studies listed as ongoing (IRCT201201248815N1), and awaiting classification (NCT02107469), will provide limited evidence to answer the research question due to the low quality of the evidence their methodology will allow.
Quality of the evidence
Both studies were randomised and double‐blind, with one of the two studies providing primary outcome data, the other only providing secondary outcome data. We could carry out meta‐analysis only for withdrawals and adverse events (secondary outcomes).
We downgraded the quality of the evidence three times to very low using the GRADE approach. This prevented us from drawing any conclusions about the effects of the herbal treatments investigated in the studies. We have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
Small study size, a large number of dropouts and missing data (attrition bias), as well as short study duration, caused us to downgrade evidence twice for very serious study limitations. The studies assessed outcomes only at baseline and immediately after treatment. There were no follow‐up outcomes after this time point and the actual interventions themselves were of short duration (four and five weeks) with a lack of follow‐up time points to assess longer‐term effects of the intervention after the treatment phase. For this reason we downgraded the quality of the evidence a further level due to indirectness.
Potential biases in the review process
We carried out a broad search for studies, and think it is unlikely that significant numbers of studies remain unknown to us regarding the efficacy of herbal medicinal products or preparations in neuropathic pain conditions. We attempted to identify medicinal herbal products or preparations being used without sufficient evidence of effectiveness (unpublished data) by contacting experts in the field of complementary and alternative medicine.
We contacted study authors to request information surrounding the results presented in the papers in addition to the raw data if we deemed it essential. Whilst only one out of the two study authors responded to this request, we do not feel it would have changed the outcome of this review as we classed both studies as very low quality regardless of this information.
Agreements and disagreements with other studies or reviews
The evidence collated in this review is of very low quality and also very limited, and therefore making comparisons of agreement or disagreement with other studies is difficult. The results of a Cochrane Review investigating the effects of capsaicin on neuropathic pain relief suggested that capsaicin applied repeatedly at a low dose (0.075% cream), or as a single application of a high dose (8% patch), may provide a degree of pain relief to some individuals (Derry 2009). However, similar to the current review, estimates of benefit and harm were not robust due to limited amounts of data for different neuropathic conditions in addition to having inconsistent outcome definitions. By way of adverse events and withdrawals, local skin irritation resulting from capsaicin led to some withdrawals, which were common but were often mild and transient, which again is similar to our observations for nutmeg and St John's wort in this review. Systemic adverse events were also rare for capsaicin.
Authors' conclusions
Implications for practice.
For people with neuropathic pain
There was insufficient evidence to suggest that nutmeg or St John's wort has any efficacy in any neuropathic pain conditions. The current evidence is of very low quality resulting in serious uncertainties about the estimates of effect observed. The evidence on adverse events is very low quality and therefore caution should be applied to its usage until more research has been done in this area.
For clinicians
There was insufficient evidence to suggest that nutmeg or St John's wort has any efficacy in any neuropathic pain conditions. The current evidence is of very low quality resulting in serious uncertainties about the estimates of effect observed.
For policy makers
There was insufficient evidence to suggest that nutmeg or St John's wort has any efficacy in any neuropathic pain conditions and therefore should not be recommended by policy makers at present. Further clinical trials are necessary.
For funders
There was insufficient evidence to suggest that nutmeg or St John's wort has any efficacy in any neuropathic pain conditions. The body of the evidence from the two included studies is of too low quality, resulting in serious uncertainties about the estimates of effect observed. Establishing whether these particular herbal products/preparations, or indeed any other herbal product or preparation, have any efficacy would require large clinical trials in several types of neuropathic pain. The evidence surrounding the adverse events associated with current pharmacological treatments for specific types of neuropathic pain and the knowledge that people with this type of pain are likely to try herbal treatments are both justification for further clinical trials investigating the safety and efficacy of herbal medicines in the treatment of such conditions. To ascertain whether pain relief is brought about as a result of nutmeg and St John's wort requires development of the evidence base. This would permit a better assessment of their efficacy and safety.
Implications for research.
General
Nutmeg and St John's wort have only been investigated in one study each and therefore more studies are required to draw any conclusions on these types of herbal products or preparations. Randomised controlled trials (RCTs) of adequate sample size (i.e. more than 200 participants per treatment arm), duration (longer than 12 weeks), with analysis that does not use imputation methods are required to establish whether herbal medicinal products are effective in reducing neuropathic pain. The two studies that are listed as ongoing (IRCT201201248815N1), or awaiting classification (NCT02107469), will not address this review question any more clearly than those published studies that are reported within this review. The reasons for this are outlined below. We recognise, however, that although further studies would be desirable, it is unlikely that there will be interest to fund these.
Design
Studies of cross‐over design with comparison to placebo, no intervention or active comparator and assessing a large study population are required. In addition, studies should be carried out in participants suffering from various types of neuropathic pain and should include long‐term follow‐up assessment of efficacy. Outcome measures should be collected at baseline, at regular meaningful time‐points and at the end of the study. Longer duration studies are required to assess the meaningfulness of any efficacy that might be observed in response to herbal medicinal products. The two studies in this review do not include follow‐up assessment past two months and therefore this highlights the need for further longer‐term studies. Those studies that are ongoing (IRCT201201248815N1), or awaiting classification (NCT02107469), in this area investigate the effects of ajwain cream and Phyllanthus niruri and Sida cordifolia towards neuropathic pain via double‐blind randomised placebo‐controlled trials in participants with neuropathic pain diagnosis as a result of diabetic peripheral polyneuropathy and also postsurgical/post‐traumatic neuropathic pain. These studies did not record outcomes past eight weeks.
Measurement (endpoints)
The measurements or outcomes assessed by the studies included in the current review were mostly secondary outcomes that are recommended by IMMPACT, with no data being extracted for primary outcomes aside from 30% pain relief or greater. Future research is needed to investigate these primary outcomes of neuropathic pain management, namely the number of participants obtaining 50% pain relief or greater over baseline, the number of participants obtaining 30% pain relief or greater over baseline, participant‐reported global impression of clinical change (PGIC) much or very much improved (moderate) and participant‐reported global impression of clinical change (PGIC) very much improved (substantial). The ongoing study (IRCT201201248815N1), and study awaiting classification (NCT02107469), also used secondary measures of pain assessment as opposed to those listed as primary outcomes by IMMPACT.
Other
Due to the limited number of trials, with few participants, investigating whole plant herbal products/preparations, there is a clear need for large, good‐quality, long‐duration, RCTs in participants suffering from various types of neuropathic pain. These have been done in other chronic conditions (Mills 1996; Oltean 2014), but not of a neuropathic nature. The number of participants investigated in the ongoing study IRCT201201248815N1 and the study awaiting classification, NCT02107469 does not exceed 200 and this, therefore, still poses a high risk of bias, lowering the methodological quality of both studies.
Motilal 2013 was the first clinical trial to be carried out on nutmeg, and therefore further human studies are required on the evidence base, however, the cost of these trials would be at least several million GBP, USD, or EUR. To date, all evidence supporting the analgesic effects of nutmeg has been demonstrated in animal models only (Hayfaa 2013; Sonavane 2001; Zhang 2016).
This review found no high‐quality evidence from good‐quality RCTs to support the use of herbal medicinal products and preparations for neuropathic pain. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
What's new
| Date | Event | Description |
|---|---|---|
| 28 April 2021 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 5, 2013 Review first published: Issue 3, 2019
| Date | Event | Description |
|---|---|---|
| 6 January 2020 | Amended | Corrected errors in Summary of findings table. |
| 3 April 2019 | Amended | 'Next stage expected' date amended. |
| 11 July 2017 | Amended | This protocol has been reinstated following withdrawal and we have made the following amendments:
|
Notes
Assessed for updating in 2021
In March 2021 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The review authors would like to acknowledge the Health and Social Care Research and Development Division of the Public Health Agency (Northern Ireland) for their funding of a Cochrane Fellowship.
We would like to thank the following peer reviewers who commented on the review: Dr Grace Lai; Theresa Wrangham.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pain, Palliative and Supportive Care (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
1. MeSH descriptor: [Herbal Medicine] this term only
2. MeSH descriptor: [Medicine, Traditional] this term only
3. MeSH descriptor: [Plant Extracts] this term only
4. MeSH descriptor: [Plant Preparations] explode all trees
5. MeSH descriptor: [Complementary Therapies] this term only
6. MeSH descriptor: [Phytotherapy] this term only
7. (herb or herbs or herbal):ti,ab,kw (Word variations have been searched)
8. (herbal near/5 medicine*):ti,ab,kw (Word variations have been searched)
9. (traditional near/5 medicine*):ti,ab,kw (Word variations have been searched)
10. (plant* near/5 extract*):ti,ab,kw (Word variations have been searched)
11. (plant* near/5 preparation*):ti,ab,kw (Word variations have been searched)
12. (herb* near/5 tea*):ti,ab,kw (Word variations have been searched)
13. (plant* near/5 oil*):ti,ab,kw (Word variations have been searched)
14. (complementary near/5 therap*):ti,ab,kw (Word variations have been searched)
15. (alternative near/5 therap*):ti,ab,kw (Word variations have been searched)
16. (phytotherap* or homeopath*):ti,ab,kw (Word variations have been searched)
17. (herbal near/5 drug*):ti,ab,kw (Word variations have been searched)
18. (medicinal near/5 herb*):ti,ab,kw (Word variations have been searched)
19. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. MeSH descriptor: [Pain] explode all trees
21. MeSH descriptor: [Peripheral Nervous System Diseases] explode all trees
22. MeSH descriptor: [Somatosensory Disorders] explode all trees
23. MeSH descriptor: [Myofascial Pain Syndromes] explode all trees
24. MeSH descriptor: [Polymyalgia Rheumatica] this term only
25. ((pain* or discomfort*) near/10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):ti,ab,kw (Word variations have been searched)
26. ((neur* or nerv*) near/6 (compress* or damag*)):ti,ab,kw (Word variations have been searched)
27. 20 or 21 or 22 or 23 or 24 or 25 or 26
28. 27 and 19
Appendix 2. MEDLINE search strategy
MEDLINE (OVID)
1 Herbal Medicine/ (1793)
2 Medicine, Traditional/ (10088)
3 Plant Extracts/ (95457)
4 exp Plant Preparations/ (192252)
5 Complementary Therapies/ (15775)
6 Phytotherapy/ (35713)
7 (herb or herbs or herbal).ab,kw,ti. (37463)
8 (herbal adj5 medicine$).ab,kw,ti. (9904)
9 (traditional adj5 medicine$).ab,kw,ti. (24774)
10 (plant$ adj5 extract$).ab,kw,ti. (14675)
11 (plant$ adj5 preparation$).ab,kw,ti. (1451)
12 (herb$ adj5 tea$).ab,kw,ti. (946)
13 (plant$ adj5 oil$).ab,kw,ti. (3737)
14 (complementary adj5 therap$).ab,kw,ti. (4529)
15 (alternative adj5 therap$).ab,kw,ti. (22804)
16 (phytotherap$ or homeopath$).ab,kw,ti. (5773)
17 (herbal adj5 drug$).ab,kw,ti. (2278)
18 (medicinal adj5 herb$).ab,kw,ti. (4167)
19 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (277813)
20 exp PAIN/ (354243)
21 exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (134931)
22 exp SOMATOSENSORY DISORDERS/ or exp MYOFASCIAL PAIN SYNDROMES/ or POLYMYALGIA RHEUMATICA/ (28056)
23 ((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (78160)
24 ((neur* or nerv*) adj6 (compress* or damag*)).mp. (56235)
25 20 or 21 or 22 or 23 or 24 (538660)
26 randomized controlled trial.pt. (454574)
27 controlled clinical trial.pt. (92184)
28 randomized.ab. (353744)
29 placebo.ab. (170695)
30 drug therapy.fs. (1997167)
31 randomly.ab. (245826)
32 trial.ab. (366625)
33 or/26‐32 (2707765)
34 exp animals/ not humans.sh. (4430952)
35 33 not 34 (2411193)
36 19 and 25 and 35 (2863)
37 (201612* or 2017* or 2018*).ed. (1108476)
38 36 and 37 (229)
Appendix 3. Embase search strategy
1 *Herbal Medicine/ (8118)
2 *Medicine, Traditional/ (8640)
3 *Plant Extracts/ (81005)
4 exp *Plant Medicinal Product/ (682022)
5 *Complementary Therapies/ (18404)
6 *Phytotherapy/ (9512)
7 (herb or herbs or herbal).ab,kw,ti. (70708)
8 (herbal adj5 medicine$).ab,kw,ti. (19383)
9 (traditional adj5 medicine$).ab,kw,ti. (47620)
10 (plant$ adj5 extract$).ab,kw,ti. (30237)
11 (plant$ adj5 preparation$).ab,kw,ti. (2241)
12 (herb$ adj5 tea$).ab,kw,ti. (1626)
13 (plant$ adj5 oil$).ab,kw,ti. (6555)
14 (complementary adj5 therap$).ab,kw,ti. (8208)
15 (alternative adj5 therap$).ab,kw,ti. (37968)
16 (phytotherap$ or homeopath$).ab,kw,ti. (10435)
17 (herbal adj5 drug$).ab,kw,ti. (5717)
18 (medicinal adj5 herb$).ab,kw,ti. (8032)
19 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (816908)
20 exp PAIN/ (1125569)
21 exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (63250)
22 exp SOMATOSENSORY DISORDERS/ (85464)
23 exp MYOFASCIAL PAIN SYNDROMES/ or POLYMYALGIA RHEUMATICA/ (10793)
24 ((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (155761)
25 ((neur* or nerv*) adj6 (compress* or damag*)).mp. (85034)
26 20 or 21 or 22 or 23 or 24 or 25 (1299091)
27 random$.tw. (1276123)
28 factorial$.tw. (32197)
29 crossover$.tw. (65088)
30 cross over$.tw. (28907)
31 cross‐over$.tw. (28907)
32 placebo$.tw. (269611)
33 (doubl$ adj blind$).tw. (186897)
34 (singl$ adj blind$).tw. (20712)
35 assign$.tw. (331653)
36 allocat$.tw. (124760)
37 volunteer$.tw. (229600)
38 Crossover Procedure/ (54565)
39 double‐blind procedure.tw. (239)
40 Randomized Controlled Trial/ (490541)
41 Single Blind Procedure/ (30578)
42 or/27‐41 (1964186)
43 (animal/ or nonhuman/) not human/ (5476035)
44 42 not 43 (1744410)
45 19 and 26 and 44 (8107)
46 (201612* or 2017* or 2018*).dd. (1640185)
47 45 and 46 (519)
Appendix 4. CINAHL search strategy
S38 S36 AND S37
S37 20161201‐20180314
S36 S29 AND S38
S35 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34
S34 (allocat* random*)
S33 (MH "Quantitative Studies")
S32 (MH "Placebos")
S31 placebo*
S30 (random* allocat*)
S29 (MH "Random Assignment")
S28 (Randomi?ed control* trial*)
S27 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) S29 S17 AND S28
S26 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25
S25 FM or FMS
S24 ((neur* or nerv*) n6 (compress* or damag*))
S23 ((pain* or discomfort*) N10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*))
S22 (MH "Polymyalgia Rheumatica")
S21 (MH "Myofascial Pain Syndromes+")
S20 (MH "Somatosensory Disorders+")
S19 (MH "Peripheral Nervous System Diseases+")
S18 (MH "Pain+")
S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16
S16 (medicinal n5 herb*)
S15 (herbal n5 drug*)
S14 (phytotherap* or homeopath*)
S13 (alternative n5 therap*)
S12 (complementary n5 therap*)
S11 (plant* n5 oil*)
S10 (herb* n5 tea*)
S9 (plant* n5 preparation*)
S8 (plant* n5 extract*)
S7 (traditional n5 medicine*)
S6 (herbal n5 medicine*)
S5 (herb or herbs or herbal)
S4 (MH "Alternative Therapies")
S3 (MH "Plant Extracts")
S2 (MH "Medicine, Traditional")
S1 (MH "Medicine, Herbal")
Appendix 5. AMED search strategy
1 Plant Extracts/ (17424)
2 Complementary Therapies/ (3906)
3 Phytotherapy/ (4745)
4 (herb or herbs or herbal).ab,kw,ti. (4989)
5 (herbal adj5 medicine$).ab,kw,ti. (1550)
6 (traditional adj5 medicine$).ab,kw,ti. (3153)
7 (plant$ adj5 extract$).ab,kw,ti. (1632)
8 (plant$ adj5 preparation$).ab,kw,ti. (154)
9 (herb$ adj5 tea$).ab,kw,ti. (87)
10 (plant$ adj5 oil$).ab,kw,ti. (220)
11 (complementary adj5 therap$).ab,kw,ti. (1268)
12 (alternative adj5 therap$).ab,kw,ti. (1076)
13 (phytotherap$ or homeopath$).ab,kw,ti. (4068)
14 (herbal adj5 drug$).ab,kw,ti. (338)
15 (medicinal adj5 herb$).ab,kw,ti. (504)
16 exp PAIN/ (20678)
17 exp MYOFASCIAL PAIN SYNDROMES/ or POLYMYALGIA RHEUMATICA/ (430)
18 ((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (6478)
19 ((neur* or nerv*) adj6 (compress* or damag*)).mp. (910)
20 16 or 17 or 18 or 19 (23759)
21 or/1‐15 (32073)
22 20 and 21 (1072)
23 limit 22 to yr="2016 ‐Current" (41)
Data and analyses
Comparison 1. Herbal treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Study withdrawals | 2 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.64] |
Comparison 2. Herbal treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adverse events | 2 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.55, 1.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Motilal 2013.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Adults aged 21‐85 years, with PDN of: hands (5.4% NEMM), feet (51.4% NEMM; 67.6% MM), both (43.2% NEMM; 32.4% MM) Symptoms limited to the extremities of limbs, and an average neuropathic pain > 4 as determined by the DN4 questionnaire n = 74 (37/arm) M 24 (32.4%): F 50 (67.6%) Mean (SD) age: NEMM 60.7 (11.5) years, MM 59.7 (8.1) years |
|
| Interventions |
Treatment Commercially available topical preparation of nutmeg extracts (NEMM). Colourless with same odour as MM in 125 mL spray bottle Participants instructed to apply 4 sprays to affected area 3 times/day, followed by gentle massage for 4 weeks Control Placebo (MM). Colourless with same odour as NEMM, in 125 mL spray bottle Participants instructed to apply 4 sprays to affected area 3 times/day, followed by gentle massage for 4 weeks |
|
| Outcomes | Worst or average pain as measured by BPI for PDN and total NPSI score Percentage achieving at least 30% reduction in worst pain from baseline at 4 weeks Withdrawals AEs |
|
| Follow‐up | Post‐intervention | |
| Method of delivery | Topical via spray | |
| Exclusion criteria | Soft‐tissue infections and injuries, radiating cervical or lumbosacral pain, tendinitis, spurs, broken skin or rash at pain sites and salicylate allergy | |
| Notes | Noelville Ltd, Grenada agreed to manufacture and supply both the treatments and placebos used in this trial. University of West Indies (Trinidad) ‐ St Augustine Campus financially supported the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number‐generating software used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes each containing 1‐80 chosen at random by participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All treatments were in similar 125 mL spray bottles with contents colourless and same viscosity. Odours same |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All scoring of the primary outcome, NPSI, were measured by a blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Doesn’t state how much data is missing. LOCF used for missing data. 7% dropout |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome data were reported |
| Size | High risk | n = 74 participants in total but n = 37 per treatment arm |
Sindrup 2000.
| Study characteristics | ||
| Methods | Randomized, double‐blind, placebo‐controlled and cross‐over | |
| Participants | Adults > 20 years, mean of 58 years (30‐82), with painful polyneuropathy (idiopathic n = 17, diabetic n = 18, alcohol n = 1, drug‐induced n = 5, others n = 6) confirmed by electrophysiological tests for > 6 months n = 54 entered and 47 completed trial |
|
| Interventions |
Treatment St John’s wort: 3 tablets (900 μg total hypericin each); total daily dose 2700 mg total hypericin given in the evening x 5 weeks Control Placebo (3 tablets identical in appearance were dosed similarly in the placebo phase) x 5 weeks At least 1 week washout ≤ 6 tablets of 500 mg paracetamol could be used daily as escape medication during all study phases |
|
| Outcomes | Total pain score and lancinating pain for St Johns wort vs placebo, total and individual pain scores between groups Withdrawals AEs |
|
| Follow‐up |
|
|
| Method of delivery | Oral | |
| Exclusion criteria | Causes of pain other than polyneuropathy, previous allergic reactions to St John’s wort, treatment with MAO inhibitors, pregnancy, severe terminal illness | |
| Notes | SanoPharm A/S, Denmark provided study drugs. The Foundation of 1870 and the Danish National Research Council (NASTRA grant no. 42820) financially supported the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block size of 4 |
| Allocation concealment (selection bias) | Low risk | Study drugs were packed in boxes marked with participant number and treatment period |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment and placebo were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded to treatment allocation of participant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF for 13% dropout |
| Selective reporting (reporting bias) | Low risk | Secondary pain‐related outcome indicating some improvement and other secondary outcomes reported |
| Size | Unclear risk | n = 54 participants |
AE: adverse event; BPI: Brief Pain Inventory; CI: confidence interval; DN: Douleur Neuropathique; F: female; LOCF: last outcome carried forward; M: male; MAO: monoamine oxidase mL: millilitres; MM: methyl salicylate (6%), menthol (6%), coconut oil, alcohol; n: number of participants; NEMM: nutmeg oil (14%), methyl salicylate (6%), menthol (6%), mace oil (2%), coconut oil, alcohol; NPSI: Neuropathic Pain Symptom Inventory; PDN: painful diabetic neuropathy; RR: risk ratio; SD: standard deviation; μg: microgram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 2007 | Acute pain not chronic |
| Barton 2011 | Combination of 3 drugs, not a whole plant medicinal product |
| Cruccu 2018 | Did not look at primary or secondary neuropathic pain outcomes |
| Hambardzumyan 2017 | Active ingredient not present in the concentration naturally present in the plant |
| ISRCTN29199098 | Not neuropathic pain |
| Khodari 2017 | Pharmacological topical agent not plant |
| Mankowski 2017 | Not an RCT |
| Moon 2017 | Active ingredient not present in the concentration naturally present in the plant |
| Paice 2000 | Active ingredient not present in the concentration naturally present in the plant |
| Salazar Sanchez 2010 | Not neuropathic pain |
| Staiger 2012 | Not an RCT |
| Torre‐Mollinedo 2001 | Active ingredient not present in the concentration naturally present in the whole plant |
| Wade 2004 | Not neuropathic pain |
| Willich 2010 | Not neuropathic pain |
| Woolridge 2005 | Not neuropathic pain |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
NCT02107469.
| Methods | Randomised, double‐blind, parallel |
| Participants | 98 men and women aged 20‐80 years |
| Interventions | Phyllanthus niruri 3 g fine dry powder 3 times/day and Sida cordifolia 7 g coarse dry powder 2 times/day for 8 weeks |
| Outcomes | Improvement of NTSS‐6 in % from baseline, validated symptom score containing 6 questions investigation severity |
| Notes | NCT02107469 |
g: gram; NTSS: Neuropathy Total Symptom Score
Characteristics of ongoing studies [ordered by study ID]
IRCT201201248815N1.
| Study name | Evaluation of ajwain cream in participants with neuropathic foot, a double blind randomized controlled clinical trial |
| Methods | Randomised, double‐blind, parallel |
| Participants | 92 men and women |
| Interventions | Ajwain cream (5 cm of cream on the affected area of feet twice/day for 30 days) |
| Outcomes | Change or any decline in foot burn in neuropathic foot |
| Starting date | 21 April 2012 |
| Contact information | mrmoein@sums.ac.ir |
| Notes | IRCT201201248815N1 |
cm: centimetre
Differences between protocol and review
The protocol for this review was reinstated following withdrawal, and we made the following amendments.
Removed fibromyalgia in line with PaPaS policy to separate the two conditions.
We excluded studies monitoring the effects of cannabinoids/cannabis or capsaicin as these have now been dealt with in separate Cochrane Reviews (Mücke 2016 (cannabis); Derry 2012; Derry 2013 (capsaicin)). As we have now excluded cannabis studies from this review, we included only orally or topically applied herbal products or preparations.
Updated background text and references.
Added GRADE methods wording and removed tiers of evidence.
Added selective outcome reporting to risk of bias methods, and also assessed both performance and detection bias.
We decided to include preparations containing the active ingredient at a concentration range that would naturally be present in the plant.
Contributions of authors
AB, SMcD and CB wrote the protocol. AB and CB carried out searches and assessed studies for inclusion. AB and DH extracted data. SMcD acted as arbitrator. All authors reviewed the protocol and were involved in writing the review. AB drafted the final write‐up. AB will be responsible for updating the review. PB acted as a content expert.
Sources of support
Internal sources
No sources of support provided
External sources
Health and Social Care Research and Development Division of the Public Health Agency (Northern Ireland) ‐ Cochrane Fellowship, UK
Declarations of interest
AB: none known
CB: none known
DH: none known
CG: none known
MHF: none known
PB is a retired consultant in pain medicine who has treated patients with neuropathic pain in the past. She received funding from Grunenthal pharmaceutical company in 2017.
SMcD: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Motilal 2013 {published data only}
- Motilal S, Maharaj R. Nutmeg extracts for painful diabetic neuropathy: a randomized, double blind, controlled study. Journal of Alternative and Complementary Medicine 2013;19(4):337-2. [DOI] [PubMed] [Google Scholar]
Sindrup 2000 {published data only}
- Sindrup S, Masden C, Bach F, Gram L, Jensen T. St John's wort has no effect on pain in polyneuropathy. Pain 2000;91:361-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 2007 {published data only}
- Abrams D, Jay C, Shade S, Vizso H, Reda H, Press S. Cannabis in painful HIV associated sensory neuropathy. Neurology 2007;68:515-1. [DOI] [PubMed] [Google Scholar]
Barton 2011 {published data only}
- Barton D, Wos E, Bassam-Mattar R, Green N, Lanier K, Bearden J. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Supportive Care in Cancer 2011;19:833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruccu 2018 {published data only}
- Cruccu G, Nurmikko TJ, Ernault E, Riaz FK, McBride WT, Haanpaa M. Superiority of capsaicin 8% patch versus oral pregabalin on dynamic mechanical allodynia in patients with peripheral neuropathic pain. European Journal of Pain 2018;22(4):700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hambardzumyan 2017 {published data only}
- Hambardzumyan H, Manvelyan H. The treatment of painful diabetic neuropathy with capsaicin gel. Journal of the Neurological Sciences 2017;381 (supplement):976. [Google Scholar]
ISRCTN29199098 {unpublished data only}29199098
- ISRCTN29199098. Efficacy of a topical cannabinoid preparation in decreasing symptoms of rheumatoid arthritis. www.isrctn.com/ISRCTN29199098 (accessed 18 December 2016).
Khodari 2017 {published data only}
- Khodari SN, Noordin MI, Chan L, Chik Z. In vitro and in vivo evaluation of new topical anaesthetic cream formulated with palm oil base. Current Drug Delivery 2017;14(5):690-5. [DOI] [PubMed] [Google Scholar]
Mankowski 2017 {published data only}
- Mankowski C, Poole CD, Ernault E, Thomas R, Berni E, Currie CJ, et al. Effectiveness of the capsaicin 8% patch in the management of peripheral neuropathic pain in European clinical practice: the ASCEND study. BMC Neurology 2017;17(80):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moon 2017 {published data only}
- Moon JY, Lee PB, Kim YC, Lee SC, Nahm FS, Choi E. Efficacy and safety of 0.625% and 1.25% capsaicin patch in peripheral neuropathic pain: multi-center, randomized, and semi-double blind controlled study. Pain Physician 2017;20(2):27-35. [PubMed] [Google Scholar]
Paice 2000 {published data only}
- Paice J, Ferrans C, Lashley F, Shott S, Vizgirda V, Pitrak D. Topical capsaicin in the management of HIV-associated peripheral neuropathy. Journal of Pain and Symptom Management 2000;19(1):45-52. [DOI] [PubMed] [Google Scholar]
Salazar Sanchez 2010 {published data only}
- Salazar-Sanchez N, Lopez-Jornet P, Camacho-Alonso F, Sanchez-Siles M. Efficacy of topical Aloe vera in patients with oral lichenplanus: a randomised double-blind study. Journal of Oral Pathology and Medicine 2010;39:735-40. [DOI] [PubMed] [Google Scholar]
Staiger 2012 {published data only}
- Staiger C. Comfrey: a clinical overview. Phytotherapy Research 2012;26:1441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torre‐Mollinedo 2001 {published data only}
- Torre-Mollinedo F, Barez E, Fernandez-Landaluce A, Barreira R, Raposo F. 0.025% topical capsaicin in the treatment of postherpetical neuralgia [Capsaicina 0,025% tipica en el tratamiento de la neuralgia postherpetica]. Journal of the Spanish Society of Pain 2001;8:468-75. [Google Scholar]
Wade 2004 {published data only}
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomised, placebo-controlled study on 160 patients. Multiple Sclerosis 2004;10:434-41. [DOI] [PubMed] [Google Scholar]
Willich 2010 {published data only}
- Willich S, Rossnagel K, Roll S, Wagner A, Mune O, Erlendson J. Rose hip herbal remedy in patients with rheumatoid arthritis – a randomised controlled trial. Phytomedicine 2010;17:87-93. [DOI] [PubMed] [Google Scholar]
Woolridge 2005 {published data only}
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. Journal of Pain and Symptom Management 2005;29(4):358-67. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02107469 {published and unpublished data}
- NCT02107469. A study of Phyllanthus niruri and Sida cordifolia in diabetic peripheral polyneuropathy (VEDICINE). ClinicalTrials.gov (accessed 13 December 2016). [NCT02107469]
References to ongoing studies
IRCT201201248815N1 {published and unpublished data}
- IRCT201201248815N1. Evaluation of ajwain cream in patients with neuropathic foot, a double blind randomized controlled clinical trial. WHO International Clinical Trials Registry Platform (ICTRP) (accessed 18 December 2016). [IRCT201201248815N1]
Additional references
Bennett 1998
- Bennett G. Neuropathic pain: new insights, new interventions. Hospital Practice 1998;33(10):95-110. [DOI] [PubMed] [Google Scholar]
BNF 2006
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary Number 51. www.bnf.org (accessed 14 November 2016).
Bouhassira 2008
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380-7. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook R, Sackett D. The number needed to treat: a clinically useful measure of treatment effect. BMJ (Clinical Research Ed.) 1995;310:452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curatolo 2006
- Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Physical Medicine and Rehabilitation Clinics of North America 2006;17:287-302. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Derry 2009
- Derry S, Lloyd R, Moore R, McQuay H. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD007393. [DOI: 10.1002/14651858.CD007393.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Moore R. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD010111. [DOI: 10.1002/14651858.CD010111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Sven-Rice A, Cole P, Tan T, Moore R. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD007393. [DOI: 10.1002/14651858.CD007393.pub4] [DOI] [PubMed] [Google Scholar]
Dickson 2010
- Dickson B, Head C, Gitlow S, Osbahr A 3rd. Maldynia: pathophysiology and management of neuropathic and maladaptive pain - a report of the AMA Council on Science and Public Health. Pain Medicine 2010;11:1635-53. [DOI] [PubMed] [Google Scholar]
Dworkin 2007
- Dworkin R, O'Connor A, Backonja M, Farrar J, Finnerup N, Jensen T et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132(3):237-51. [DOI: 10.1016/j.pain.2007.08.033] [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin R, Turk D, Wyrwich K, Beaton D, Cleeland C, Farrar J, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI: 10.1016/jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
EFIC 2015
- EFIC. European Pain Federation and International Association for the Study of Pain. Global Year Against Neuropathic Pain 2014-2015. www.efic.org/ (accessed 20 May 2015).
Emad 2008
- Emad Y, Ragab Y, Zeinhom F, El-Khouly G, Abou-Zeid A, Rasker JJ. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy. Journal of Rheumatology 2008;35(7):1371-7. [PubMed] [Google Scholar]
Ernst 2001
- Ernst E, Pittler M, Stevinson C, White A. Therapies - herbalism. In: Ernst E, Pittler MH, Stevinson C, White AR, editors(s). The Desktop Guide to Complementary and Alternative Medicine: an Evidence-based Approach. London: Harcourt Publishers Limited, 2001:50-2. [ISBN: 0723432074] [Google Scholar]
European Medicines Agency Directive (2004/24/EC)
- European Medicines Agency. Herbal medicinal products. www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000208.jsp&mid=WC0b01ac05800240cf (date accessed 13 December 2016).
Gagnier 2011
- Gagnier J, Moher D, Boon H, Beyene J, Bombardier C. Randomized controlled trials of herbal interventions underreport important details of the intervention. Journal of Clinical Epidemiology 2011;64(7):760-9. [DOI] [PubMed] [Google Scholar]
Glassman 1998
- Glassman A. Cardiovascular effects of antidepressant drugs: updated. Journal of Clinical Psychiatry 1998;59 (Suppl):13-8. [PubMed] [Google Scholar]
Gustorff 2008
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52(1):132-6. [DOI] [PubMed] [Google Scholar]
Hall 2006
- Hall G, Carroll D, Parry D, McQuay H. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain 2006;122(1-2):156-62. [DOI] [PubMed] [Google Scholar]
Hayfaa 2013
- Hayfaa A, Sahar A, Awatif M. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. Journal of Pain Research 2013;6:611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
IASP 2006
- International Association for the Study of Pain. Guidelines on the Management of Neuropathic Pain. www.iasp-pain.org (accessed 1 September 2013).
Kanodia 2010
- Kanodia A, Legedza A, Davis R, Eisenberg D, Phillips R. Perceived benefit of complementary and alternative medicine (CAM) for back pain: a national survey. Journal of the American Board of Family Medicine 2010;23(3):354-62. [DOI] [PubMed] [Google Scholar]
Kuchinad 2007
- Kuchinad A, Schweinhardt P, Seminowicz D, Wood P, Chizh B, Bushnell M. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? Journal of Neuroscience 2007;27(15):4004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Li 2010
- Li L. The effect of Neuragen PN on neuropathic pain: a randomized, double blind, placebo controlled clinical trial. BMC Complementary and Alternative Medicine 2010;10:22. [DOI: 10.1186/1472-6882-10-22] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay H, Smith L, Moore R. Chronic pain. In: Stevens A, Rafferty J, Mont J, Simpson S, editors(s). Health Care Needs Assessment. Oxford: Radcliffe Publishing Limited, 2007:517-600. [Google Scholar]
Metcalfe 2010
- Metcalfe A, Williams J, McChesney J, Patten S, Jetté N. Use of complementary and alternative medicine by those with a chronic disease and the general population - results of a national population based survey. BMC Complementary and Alternative Medicine 2010;10:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 1996
- Mills S, Jacoby R, Chacksfteld M, Willoughby M. Effect of a proprietary herbal medicine on the relief of chronic arthritic pain: a double-blind study. British Journal of Rheumatology 1996;35:874-8. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore R, Gavaghan D, Tramèr M, Collins S, McQuay H. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI] [PubMed] [Google Scholar]
Moore 2009
- Moore R, Straube S, Wiffen P, Derry S, McQuay H. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007076. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore R, Straube S, Derry S, McQuay H. Topical review: chronic low back pain analgesic studies - a methodological minefield. Pain 2010;149(3):431-4. [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore R, Moore O, Derry S, Peloso P, Gammaitoni A, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mücke 2016
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabinoids for chronic neuropathic pain. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD012182. [DOI: 10.1002/14651858.CD012182] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Clinical Excellence. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. www.nice.org.uk/cg96 (accessed 15 June 2017). [PubMed]
Nuesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes A, Tschannen B, Altman D, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oltean 2014
- Oltean H, Robbins C, Van Tulder M, Berman B, Bombardier C, Gagnier J. Herbal medicine for low-back pain. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD004504. [DOI: 10.1002/14651858.CD004504.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peretti 2000
- Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants versus selective serotonin reuptake inhibitors. Acta Psychiatrica Scandanavica 2000;403 (Suppl):17-25. [DOI] [PubMed] [Google Scholar]
Petrou 2008
- Petrou M, Harris R, Foerster B, McLean S, Sen A, Clauw D, et al. Proton MR spectroscopy in the evaluation of cerebral metabolism in patients with fibromyalgia: comparison with healthy controls and correlation with symptom severity. American Journal of Neuroradiology 2008;29:913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing 'Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sonavane 2001
- Sonavane G, Sarveiya V, Kasture V, Kasture S. Behavioural actions of Myristica fragrans seeds. Indian Journal of Pharmacology 2001;33:417-24. [Google Scholar]
Thomas 2004
- Thomas K, Colmen P. Use of complementary or alternative medicine in general population in Great Britain, results of the National Omnibus Survey. Journal of Public Health 2004;26:152-7. [DOI] [PubMed] [Google Scholar]
Torrance 2006
- Torrance N, Smith B, Bennett M, Lee A. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281-9. [DOI] [PubMed] [Google Scholar]
Torrance 2014
- Torrance N, Lawson K, Afolabi E, Bennett M, Serpell M, Dunn K et al. Estimating the burden of disease in chronic pain with and without neuropathic characteristics: does the choice between the EQ-5D and SF-6D matter? Pain 2014;155(10):1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2007a
- Wood P, Patterson J 2nd, Sunderland J, Tainter K, Glabus M, Lilien D. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. Journal of Pain 2007;8(1):51-8. [DOI] [PubMed] [Google Scholar]
Wood 2007b
- Wood P, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. European Journal of Neuroscience 2007;25(12):3576-82. [DOI] [PubMed] [Google Scholar]
Wood 2009
- Wood P, Ledbetter C, Glabus M, Broadwell L, Patterson J 2nd. Hippocampal metabolite abnormalities in fibromyalgia: correlation with clinical features. Journal of Pain 2009;10(1):47-52. [DOI] [PubMed] [Google Scholar]
Zhang 2016
- Zhang W, Tao S, Li T, Li Y, Li X, Tang H, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food and Nutrition Research 2016;60(10):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


