Abstract
Objectives
Radiofrequency interference (RFI) is a known medical device safety issue, but there are no documented cases of interference resulting in erroneous laboratory results.
Methods
We investigated unexpected failure of a hematology analyzer resulting in erroneous WBC counts. Hardware failure was initially suspected, but temporal association with increased power output from a nearby antenna prompted investigation for RFI.
Results
Power output from an antenna located approximately 4 feet from the analyzer was increased to ensure sufficient signal for emergency communications in the building. Interference from the antenna resulted in aberrant side scatter and abnormal WBC counts. Powering down the antenna returned the instrument to normal working conditions.
Conclusions
We have shown RFI as the root cause of erroneous WBC counts in a hematology analyzer. We propose that RFI should be on the list of potential interfering mechanisms when clinical laboratory instruments generate inconsistent or unreliable results.
Keywords: Radiofrequency, Interference, Clinical laboratory, Hematology
Radiofrequency interference (RFI) as a subset of electromagnetic interference (EMI) has been a known safety issue in health care for many years. Subsequent to multiple reports of RFI-associated medical device failure in pacemakers, infusion pumps, and other electronic devices, the Institute of Electrical and Electronics Engineers Committee on Man and Radiation (COMAR) issued a comprehensive report in 1998 with multiple recommendations to ensure the safety of medical devices in the presence of radiofrequency-emitting sources.1 In addition, medical device manufacturers have long followed international standards for design and testing of medical devices that include immunity from RFI.2 Trade associations and the US government provide detailed guidelines to ensure RFI immunity in the health care setting.3,4 Instrument design and testing for RFI immunity, however, do not cover all possible scenarios. Generally, manufacturing guidelines require testing for safe device operation in an electric field strength of 3 V/m for general medical devices and 10 V/m for life safety medical devices.1 To capture postmanufacturing device malfunction due to RFI, the US Food and Drug Administration facilitates reporting and dissemination of RFI events under the Manufacturer and User Facility Device Experience (MAUDE) database. By 2013, the MAUDE database had exceeded approximately 1,000 annual reports related to EMI and/or electromagnetic compatibility (EMC).
Despite engineering safeguards that have proven successful in protecting various traditional electrical equipment from interference with each other, the rapid pace of development and deployment for cellular communication devices have proven to be a challenge in health care. Initial attempts to ban the use of cell phones in hospitals and other health care facilities were abandoned as cell phones became an indispensable part of everyday life, and studies emerged that showed the relative immunity of most medical devices in the presence of cell phones operating under normal conditions of use.5-9 In addition, there has been little to no public support for the ban or restriction of cell phone use in hospitals or other health care facilities, and the general public considers the risk of RFI from cellular devices minimal to nonexistent.10,11 In addition to cellular communication devices, with the emergence of radiofrequency identification (RFID) as a valuable tool in multiple hospital operations, there has been a surge in reports of cases and scenarios in which RFID devices cause malfunction in various clinical equipment.12-14
To our knowledge, there has been no documented case of RFI in the clinical laboratory resulting in erroneous laboratory results. Our laboratory and others are increasingly wired for connectivity, and changes in wiring, antennas, power emissions, and other infrastructural changes that can potentially affect the performance characteristics of clinical instruments are generally viewed as positive, harmless, and/or irrelevant to testing operations. In this report, we present the case of a hematology analyzer malfunction that was directly associated with the operation of a distributed antenna system (DAS) installed for enhancement of wireless communications for emergency responders. In addition to raising awareness about potential RFI in analytical laboratory instruments, this report highlights the importance of buildings and infrastructure as a somewhat neglected issue in determining and investigating performance characteristics of clinical laboratory instruments.
Case Report
The clinical laboratory at Nationwide Children’s Hospital Emergency Department at Lewis Center, Ohio, is part of a new clinical facility designed for pediatric emergency services. The laboratory became operational in February 2017 and is furnished with state-of-the-art equipment. An experienced team of technologists and supervisors oversees the laboratory, and there were no technical or quality issues during construction, validation, and startup of this laboratory.
The laboratory continued clinical operations with no significant technical problems until Thursday, June 29, 2017. On this day, the quality control (QC) samples were successfully run on a Sysmex XN-1000 hematology analyzer (Sysmex America), and patient results were reported with no red flags until mid-afternoon. At 3:21 pm, the instrument reported 4% neutrophils, 15% lymphocytes, and 62% eosinophils in an otherwise routine clinical sample, necessitating a manual differential. The manual differential did not support the instrument findings, and the patient sample was rerun at 4:18 pm. Repeat testing showed a neutrophil count of 70%, with 19% lymphocytes and 1% eosinophils, consistent with the manual differential. The latter results were released into the patient’s medical record.
Because of the above discrepancy, multiple additional QCs were run by the technologist later that afternoon and showed multiple unacceptable results in the WBC differential counts. The technologist attempted to resolve the problem based on various error messages with no success. Faced with these unresolved QC issues, the technologist halted clinical testing, notified the laboratory director, and initiated a service call to the manufacturer.
The following morning, a technical specialist from the manufacturer was on site and concluded that the flow cell laser was malfunctioning. While attempting to place a replacement order for the laser, a second technical specialist suggested the possibility of RFI and recommended additional testing before replacing the laser. Ad hoc attempts were made to physically shield the instrument with commercial aluminum foil with no success. Copper shields were therefore ordered for various internal components of the system, and the instrument remained out of service pending installation of the shields and further testing.
On July 3, 2017, 4 days after the initial instrument failure, a manufacturer specialist returned to the site and, based on the error message “PLT-F scattergram sensitivity error” and various experiments with standard latex beads in the presence and absence of a temporary electrical shield, concluded that the instrument malfunction was indeed due to RFI. Further investigation and discussion with the clinical bioengineering team in charge of the laboratory facility revealed that the building’s new DAS for cellular communications was turned up at 2:30 pm on June 29, 2017, minutes before the first instance of equipment malfunction. Of note, the enhanced DAS was installed to ensure adequate signal and capacity for emergency communications, with the added benefit of improving cellular reception for employees and visitors.
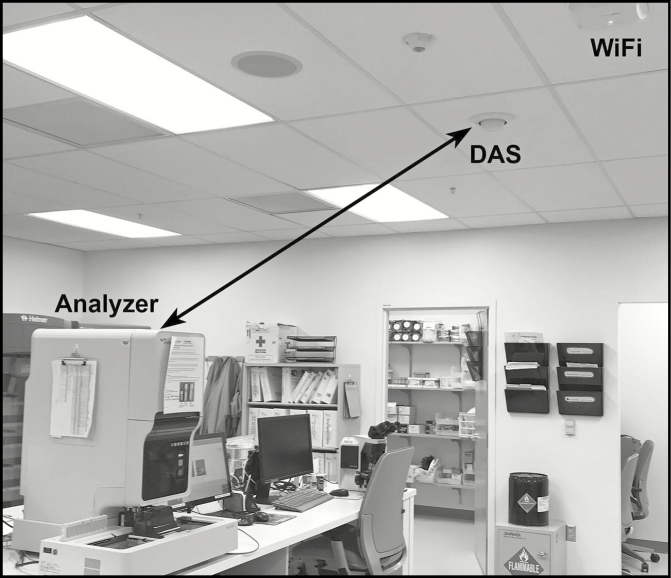
Permanent copper shields were installed on various critical parts of the laser on July 4, 2017 (Figure 1 showing copper shield on some wires around the laser and flow cell) with some improvement, but the instrument continued to remain unreliable. Finally, in the afternoon of July 7, 2017, representatives from the manufacturer, telecommunications, and bioengineering met in the laboratory for a definitive solution. Latex bead experiments were again conducted by the manufacturer and showed repeated failures in the side-scatter window despite the copper shields. The antenna closest to the instrument was disconnected (Figure 2 showing laboratory, instrument, DAS, and WiFi antenna), after which latex bead experiments showed acceptable characteristics and all QCs returned to normal. The instrument was returned to clinical use after multiple additional tests demonstrated acceptable performance. Tests of wireless communication reception in the laboratory and surrounding area showed that first responder radios would not be inhibited by turning off the antenna in the laboratory, and the antenna remained disabled.
Figure 1.
Internal view of the analyzer showing laser and flow cell assembly (inside the ellipse). Also shown are two key input-output electronic bundles (arrows) that were shielded by copper foil by the manufacturer. The addition of the copper shields did not provide sufficient immunity from radiofrequency interference when the distributed antenna system antenna was operating at full power.
Figure 2.
Photograph of the laboratory showing the relationship of the analyzer to two antennas in the room. The two-headed arrow between the distributed antenna system (DAS) antenna and the upper right side of the instrument measures approximately 4 feet. The laser and flow cell assembly is located at the upper right side of the instrument.
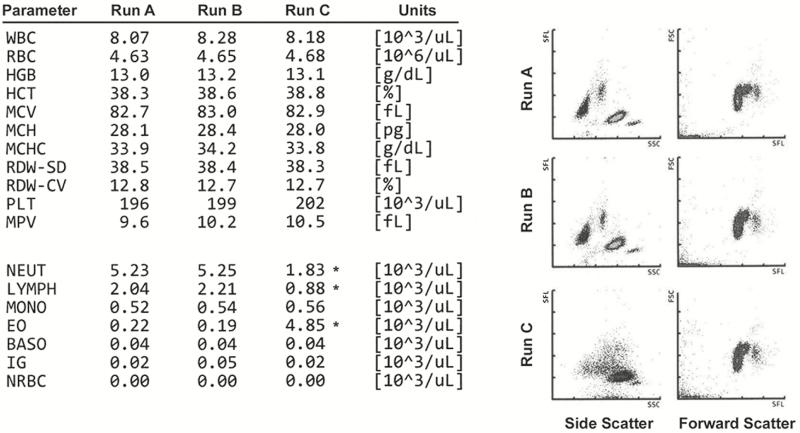
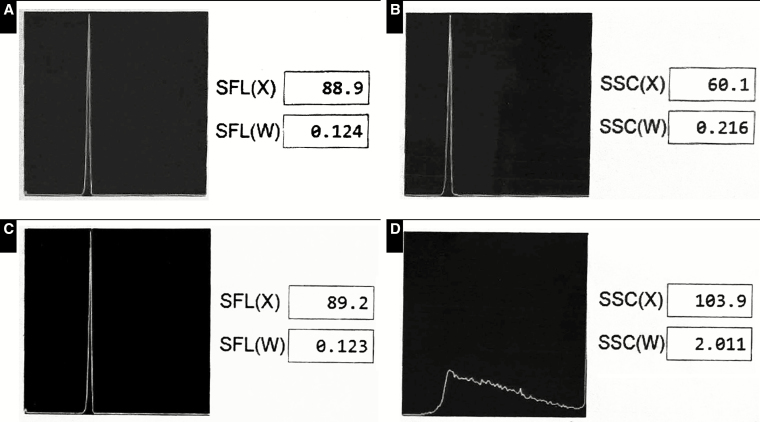
After several months of uneventful operations, the team of laboratory technologists, manufacturer representative, and bioengineering met once again for a formal resolution to the status of the instrument and cellular antenna. With the instrument shielded and the DAS antenna closest to the instrument in the off position, patient specimens were run and repeated successfully (Figure 3, runs A and B). In addition, standard beads used for fine tuning and alignment of the laser and flow cell showed acceptable performance in forward and side scatter Figure 4A and Figure 4B. However, once the antenna was turned on, the same patient showed aberrant results in the automated differential (Figure 3, run C), and standard beads showed unacceptable performance in the side-scatter window Figure 4C and Figure 4D. As such, the team confirmed the DAS antenna as the definitive source of interference and permanently disconnected the antenna.
Figure 3.
Instrument printouts from three separate runs were used to create the composite shown here. Runs A and B are duplicate runs on the same patient sample with the distributed antenna system (DAS) antenna disconnected from power. Numerical value of the results on the left and scatterplots of side and forward scatter are nearly identical between the two runs. A third run of the same sample with the DAS antenna powered up (run C) shows significant aberration in three WBC differential measurements (asterisks) representing neutrophil count (NEUT), lymphocyte count (LYMPH), and eosinophil count (EO). In addition, the side scatterplot shows a significant anomaly compared with runs A and B. Note that the forward scatter is not affected, and neither are any of the parameters in the hemogram portion of the assay. BASO, basophils; HCT, hematocrit; HGB, hemoglobin; IG, immature granulocytes; MCH, mean cell hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MONO, monocytes; MPV, mean platelet volume; NRBC, nucleated red blood cells; PLT, platelets; RDW-CV, red blood cell distribution width coefficient of variation; RDW-SD, red blood cell distribution width standard deviation.
Figure 4.
Standard beads were run on the instrument by the manufacturer representative and the results captured on propriety manufacturer software. Screenshots of the results were captured on a cell phone, cropped and color adjusted to create the composite shown here. Top two panels (A, C) represent forward scatter, including x-axis location of the peak (SFL(X)) and distribution width (SFL(W)). Bottom two panels (B, D) represent side scatter, including x-axis location of the peak (SSC(X)) and distribution width (SSC(W)). The distributed antenna system (DAS) antenna was disconnected in panels A and B and powered up in panels C and D. Panels A, B, and C are the expected results for a properly functioning instrument, while panel D shows a significant anomaly in the side scatter when the DAS antenna was powered up.
Survey of the Environment
To better understand the environment in which the above events took place, a brief survey of the environment was conducted using an RF Explorer 6G Combo spectrum analyzer (Nuts About Nets) running in the 15- to 2,700-MHz frequency range. The analyzer was equipped with a 700- to 2,600-MHz omnidirectional dipole antenna (Techtoo). Measurements were made in three different frequency ranges, covering primary cellular bands used by major US carriers. The three ranges studied included 700 to 900 MHz, 1,700 to 2,000 MHz, and 2,400 to 2,600 MHz.
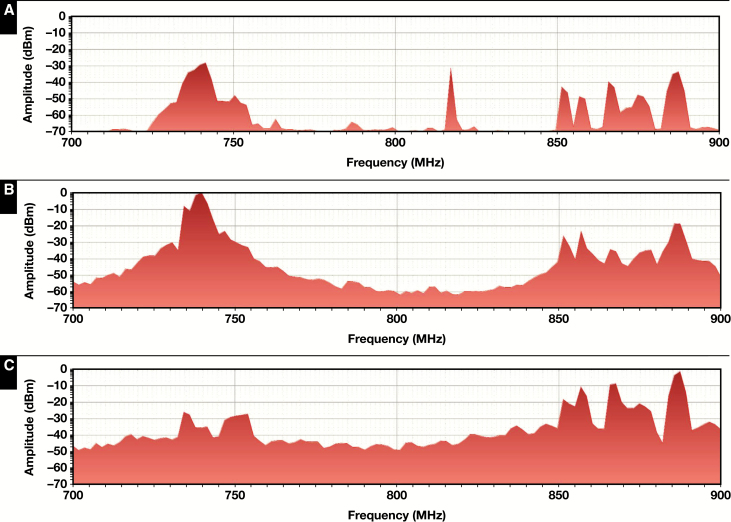
Measurements were conducted at distances of approximately 4, 8, and 32 feet from a DAS antenna adjacent to the laboratory. All three experiments showed essentially the same finding, suggesting significant power output within 4 to 8 feet of the antenna that are orders of magnitude higher than the output at 32 feet. Shown in Figure 5 are the results for the 700- to 900-MHz frequency range covering cellular communications bands in the 700-MHz range, which are the backbone of fixed wireless and broadband communications by major cellular providers in the state of Ohio (based on Spectrum Dashboard information on http://reboot.fcc.gov/reform/systems/spectrum-dashboard accessed on August 23, 2018). Note that these measurements were taken at different times, when different bands of the 700- to 900-MHz spectrum would have been active. Even though the background radiation level rose significantly as the analyzer got closer to the DAS antenna, transient spikes dominated the power spectrum of the DAS antenna. However, the background readings suggest that the DAS antenna is the primary contributor of radiofrequency (RF) noise in the environment and that RFI within 4 feet of the antenna would be approximately 100 times worse than RFI at 32 feet.
Figure 5.
Survey of the environment was conducted to assess distributed antenna system (DAS) antenna power output at distances of 32 feet (A), 8 feet (B), and 4 feet (C) away from the antenna. Shown here is average output in decibel milliwatts (dBm) plotted on a log scale with the spectrum analyzer centered at 800 MHz and a span of 200 MHz. For each plot, a minimum of 250 sweeps were made, with each sweep representing the average of 10 iterations at each of the 112 data points plotted. Note that the peak output in multiple frequencies increases by several orders of magnitude in close proximity to the antenna, and so does the overall power output. At amplitudes greater than –20 dBm, the field strength in most structures acting as antennas will potentially exceed 3 V/m for which non-life-saving instruments are designed.
Power measured by the spectrum analyzer relates to electric field strength through the following equation:
where E is the peak electric field strength of the electromagnetic wave, Pr is the measured average power absorbed, g is the antenna gain, A is the active area of the antenna, and Z0 is the characteristic impedance of air. To guarantee that our measures of E are underestimates, we can assume that the entire area of the receiving antenna is the active receiving surface. In reality, the receiving surface area is smaller than this, since the antenna dimensions are known for the plastic case, not for the metal elements receiving the signal. Therefore, we can reasonably estimate that a power reading of 0 dBm corresponds to an electric field strength greater than 15 V/m. These findings suggest sufficient power output from the DAS antenna during transients to potentially interfere with sensitive or susceptible devices located in close proximity to the antenna.
Also noted is that power output from cellular antennas is not constant and can spike on demand and that clinical laboratory instruments such as the one described here are only susceptible during short periods of activity when a specific measurement is being made. As such, “random” or spot tests can miss the presence of a potential issue or give the false reassurance that the issue has been resolved.
Discussion
Radiofrequencies with the greatest ability to interfere with a device are those with a wavelength comparable to the physical dimension of a wire, circuit, or housing. For frequencies ranging from 700 to 2,600 MHz where most cellular devices and emergency communication systems operate, this corresponds to wavelength ranging from approximately 43 to 12 cm. Antennas as short as one-fourth of the wavelength of a signal can receive a substantial amount of power from a transmitter of a given frequency. Critical components such as circuits, lasers, and detectors in most laboratory analyzers fall in this physical region and are susceptible to RFI. At the same time, medical laboratory equipment often contains extremely sensitive analog components, with signal levels in the region of femtowatts.
Stated differently, many components such as wires, circuits, and various metallic structures used in laboratory analyzers are essentially antennas and, as such, can send and receive electromagnetic radiation in unknown, unplanned, and unintentional ways. Manufacturers of medical and clinical laboratory devices, as well as regulatory agencies such as the US Food and Drug Administration, are keenly aware of this concept and follow strict manufacturing guidelines to ensure EMC.4 Design for EMC ensures that a device is compatible with its electromagnetic environment (ie, not susceptible to interference) and that it does not expose other devices in its vicinity to electromagnetic interference under normal use. Standards for EMC in health care are set by multiple national and international regulatory organizations such as the Federal Communications Commission, American National Standards Institute (ANSI), and the International Organization for Standardization. In particular, detailed protocols for assessment and management of EMC in health care facilities are developed by ANSI Accredited Committee C63. For obvious reasons, most rules, regulations, and testing guidelines focus on wireless medical devices and life safety equipment such as pacemakers. In vitro diagnostic equipment such as laboratory analyzers is specifically covered under International Standard IEC-61326-2-6:2012, which specifies minimum requirements for device immunity and emission. As noted before, these requirements are not designed to foresee or prevent all possible scenarios for potential RFI. Future devices may need to be designed for RF environments up to an order of magnitude worse than those surveyed for the 1998 COMAR report.
Despite the failure of an RF shielding component in this case, RF shields can be an effective solution to EMI issues provided that the shielding covers enough of the surface area around any sensitive components. The frequencies in question are high enough that copper or aluminum shields as thin as 10 µm, approximately the thickness of standard aluminum foil, will attenuate incident RF power by a factor of 10 trillion or more. This suggests that shielding in our case was either ineffective at covering enough surface area or too thin to attenuate enough signal. In practice, components such as flow cells may not be able to be properly shielded from interference because tubes for liquid flow can provide enough surface area for radio waves to enter a sensitive component.
Sandhaus and colleagues15 discussed the issue of electronic noise in various methods of platelet counting but not interference. Similarly, De Smet and colleagues16 discussed the effect of baseline electronic noise in the context of RBC and WBC counts in body fluids without any reference to external interference. To our knowledge, the only published study that refers to interference from electromagnetic fields in the clinical laboratory is an experiment conducted by Vagdatli and colleagues,17 who showed that simultaneous operation of multiple laptops and cell phones in the immediate vicinity of a Mindray BC 3200 hematology analyzer resulted in various effects on multiple parameters, including multiple cells counts and size measurements. Interestingly, the study showed a maximum effect with four cell phones (vs one cell phone, one laptop, or three laptops), but when the analyzer was exposed to the combination of four phones and three laptops, it “gave odd results after 11 measurements and finally stopped working.”17 Vagdatli and colleagues17 did not specifically associate these findings with RFI but instead focused on electronic noise, defined as “electric interference or voltage fluctuations or external noise contamination (e.g., from other electrical equipment).”
The process of instrument validation, which is a well-established laboratory practice, implicitly includes an assessment of the instrument environment, but validation studies are not designed to detect or characterize sporadic events such as peaks in radiofrequency emission. In addition, although the College of American Pathologists and other accrediting bodies specifically require revalidation of an instrument after a physical move, there is generally no concern about changes in the environment other than simple physical parameters such as temperature and humidity. As we experienced in our laboratory, a validated and normally functioning instrument became unstable simply because the power output in a building antenna was turned up for an intangible improvement in wireless communications.
In summary, we have presented a well-documented case of RFI in a hematology analyzer that was discovered when the power output in a nearby antenna was turned up, resulting in erroneous WBC counts. We are unable to determine if previous clinical results were affected in a less obvious manner when the antenna was operating at lower output levels. This report highlights the need for vigilance in clinical laboratories about potential RFI across various test systems. In particular, infrastructure alterations such as new wiring, new antennas, or boosts in wireless signals, which happen routinely in hospitals and other health care facilities, must be closely monitored and scrutinized by the laboratory, especially as facilities strive to maintain compliance with emergency communication requirements.18 Finally, simple environmental surveys may be a valuable tool in ruling out potential RFI in instruments that appear to be “inconsistent” or “unreliable” without a clear explanation.
References
- 1. IEEE Committee on Man and Radiation. Radiofrequency interference with medical devices: a technical information statement. IEEE Eng Med Biol Mag. 1998;17:111-114. [PubMed] [Google Scholar]
- 2. International Electrotechnical Commission (IEC). IEC 60601 (all parts), medical electrical equipment. http://www.iec.ch/. Accessed November 25, 2018. [Google Scholar]
- 3. Association for the Advancement of Medical Instrumentation. Guidance on Electromagnetic Compatibility of Medical Devices in Healthcare Facilities. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2010. [Google Scholar]
- 4. US Food and Drug Administration. Electromagnetic compatibility (EMC). https://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/ElectromagneticCompatibilityEMC/default.htm Accessed July 8, 2018. [Google Scholar]
- 5. Aziz O, Sheikh A, Paraskeva P, et al. Use of mobile phones in hospital: time to lift the ban? Lancet. 2003;361:788. [DOI] [PubMed] [Google Scholar]
- 6. Tri JL, Hayes DL, Smith TT, et al. Cellular phone interference with external cardiopulmonary monitoring devices. Mayo Clin Proc. 2001;76:11-15. [DOI] [PubMed] [Google Scholar]
- 7. Tri JL, Severson RP, Firl AR, et al. Cellular telephone interference with medical equipment. Mayo Clin Proc. 2005;80:1286-1290. [DOI] [PubMed] [Google Scholar]
- 8. Tri JL, Severson RP, Hyberger LK, et al. Use of cellular telephones in the hospital environment. Mayo Clin Proc. 2007;82:282-285. [DOI] [PubMed] [Google Scholar]
- 9. Tang C-K, Chan K-H, Fung L-C, Leung S-W. Electromagnetic interference immunity testing of medical equipment to second- and third-generation mobile phones. IEEE Trans Electromagnetic Compatibility. 2009;51:659-664. [Google Scholar]
- 10. Pogue D. Myth-busting, part XXVII: hospital interference. https://pogue.blogs.nytimes.com/2006/12/19/myth-busting-part-xxvii-hospital-interference/ Accessed September 5, 2018. [Google Scholar]
- 11. Hammond C. Are mobile phones dangerous in hospitals? http://www.bbc.com/future/story/20130513-can-you-use-phones-in-hospitals Accessed September 5, 2018. [Google Scholar]
- 12. Christe B, Cooney E, Maggioli G, et al. Testing potential interference with RFID usage in the patient care environment. Biomed Instrum Technol. 2008;42:479-484. [DOI] [PubMed] [Google Scholar]
- 13. Kapa S, Pierce T, Hayes DL, et al. Electromagnetic interference of magnetic field based auto identification technologies in healthcare settings. Int J Med Inform. 2011;80:239-250. [DOI] [PubMed] [Google Scholar]
- 14. van der Togt R, van Lieshout EJ, Hensbroek R, et al. Electromagnetic interference from radio frequency identification inducing potentially hazardous incidents in critical care medical equipment. JAMA. 2008;299:2884-2890. [DOI] [PubMed] [Google Scholar]
- 15. Sandhaus LM, Osei ES, Agrawal NN, et al. Platelet counting by the Coulter LH 750, Sysmex XE 2100, and Advia 120: a comparative analysis using the RBC/platelet ratio reference method. Am J Clin Pathol. 2002;118:235-241. [DOI] [PubMed] [Google Scholar]
- 16. De Smet D, Van Moer G, Martens GA, et al. Use of the Cell-Dyn Sapphire hematology analyzer for automated counting of blood cells in body fluids. Am J Clin Pathol. 2010;133:291-299. [DOI] [PubMed] [Google Scholar]
- 17. Vagdatli E, Konstandinidou V, Adrianakis N, et al. Effects of electromagnetic fields on automated blood cell measurements. J Lab Autom. 2014;19:362-365. [DOI] [PubMed] [Google Scholar]
- 18. International Code Consortium. Emergency first responder radio coverage. International Fire Code, Section 501; https://archive.org/details/gov.law.icc.ifc.2009/page/n75 Accessed November 12, 2018. [Google Scholar]