Abstract
Human-induced global change is expected to amplify the disease risk for marine biota. However, the role of disease in the rapid global decline of seagrass is largely unknown. Global change may enhance seagrass susceptibility to disease through enhanced physiological stress, while simultaneously promoting pathogen development. This review outlines the characteristics of disease-forming organisms and potential impacts of global change on three groups of known seagrass pathogens: labyrinthulids, oomycetes and Phytomyxea. We propose that hypersalinity, climate warming and eutrophication pose the greatest risk for increasing frequency of disease outbreaks in seagrasses by increasing seagrass stress and lowering seagrass resilience. In some instances, global change may also promote pathogen development. However, there is currently a paucity of information on these seagrass pathosystems. We emphasise the need to expand current research to better understand the seagrass-pathogen relationships, serving to inform predicative modelling and management of seagrass disease under future global change scenarios.
Keywords: Global change, Halophytophthora, Labyrinthula, Marine infectious disease, Phytophthora, Phytomyxea
1. Introduction
Human-induced global change facilitates disease outbreaks on a global scale (Harvell et al., 2002). Mass-mortalities due to disease in keystone species marine species have been reported in corals (Hoegh-Guldberg et al., 2007), marine mammals (Cosby et al., 1988), oysters (Van Banning, 1991), sea-stars (Hewson et al., 2014) and seagrasses (Sullivan et al., 2013). Through the negative impacts on these keystone species, the effects of disease may rapidly cascade through ecosystems, with potentially catastrophic consequences through local species extinction, community regime shifts and disruptions in economically-linked ecosystem services, such as fisheries production (Groner et al., 2015; Sullivan et al., 2013). Despite emerging threats to several ecologically important species, epidemiological studies in marine systems are sparse compared to terrestrial systems (Harvell et al., 1999).
Seagrasses are marine flowering plants that form large swaths of grass-like intertidal and subtidal meadows in coastal areas. Seagrass ecosystems provide vital coastal habitats that are increasingly recognised for their ecological function and the provision of human services, including reduction in human and wildlife pathogens (Lamb et al., 2017) coastal protection (Christianen et al., 2013), fisheries habitat (Jackson et al., 2001) and carbon sequestration (Fourqurean et al., 2012). Seagrasses also provide positive feedback mechanisms for themselves that drive continued provision of suitable habitat for their own survival (De Boer, 2007; Maxwell et al., 2016). Despite increasing attention to seagrasses as a keystone species, researchers estimate nearly 30% of the global seagrass area world-wide was lost between 1879 and 2006 (Waycott et al., 2009).
Under the threat of ongoing global change, ecologists look to understand how marine ecosystems will function, and how community and population dynamics may be altered in the near and distant future. The majority of publications available on the effects of global change in coastal ecosystems have focused on the role of abiotic environmental conditions, namely temperature, on the health of marine systems, and most often at the level of an individual species or population (Harley et al., 2006). Still, research on the role of biologically-mediated environmental conditions (Alsterberg et al., 2013; Eklöf et al., 2012; Sunday et al., 2017) suggests that organism-to-organism interactions are important in understanding both direct and indirect effects of global change on the functional capacity of coastal and marine ecosystems.
1.1. Known global change impacts to seagrass
Abiotic drivers potentially affecting seagrass resilience under future scenarios include increasing ocean temperatures, sea level rise, increased frequency of storms, ocean acidification (increased CO2 and decreased pH) and changes to salinity (Duarte, 2002; Harley et al., 2006; Koch et al., 2013; Short et al., 2016). Increasingly, studies are emerging to quantify the effects of global change on seagrasses. These include theoretical investigations of seagrass physiology, communities and populations (Duarte, 2002; Edwards, 1995; Harley et al., 2006; Occhipinti-Ambrogi and Savini, 2003; Orth et al., 2006; Short and Neckles, 1999) and modelling and experimentation (Jordà et al., 2012; Koch et al., 2013; Palacios and Zimmerman, 2007; Rasheed and Unsworth, 2011).
Firstly, elevated seawater temperatures will affect plant physiology, reproduction, growth and geographic distribution (Short and Neckles, 1999). For some seagrasses, warmer waters may be beneficial, resulting in increased growth rates (Short and Coles, 2001) and reproduction (Diaz-Almela et al., 2007). However, plants growing at the edges of their geographical range are found to experience increased mortality due to drought stress (de Fouw et al., 2016) and heat waves (Collier and Waycott, 2014; Fraser et al., 2014).
Secondly, increasing atmospheric CO2 levels are increasing the amount of dissolved CO2 in coastal waters, resulting in a lower pH (Orr et al., 2005). Although the effects of ocean acidification are unfavourable to calcifying marine organisms, they are known to enhance resilience of seagrasses (Alsterberg et al., 2013). Seagrasses themselves are expected to benefit from high CO2 levels that result in increased plant production (Garrard and Beaumont, 2014; Takahashi et al., 2016) and carbon storage (Russell et al., 2013).
Global change may also affect salinity of coastal waters in two ways: either warming will raise coastal salinities by higher evaporation levels and reduced freshwater input, or salinity in coastal areas may decrease for prolonged periods due to increased precipitation. Effects of altered salinities vary among seagrass species (Salo et al., 2014), but extremes of salinity < 10 ppt or > 45 ppt negatively affect seed germination (Pan et al., 2011) and plant performance (Kahn and Durako, 2006; Koch et al., 2007a).
Additionally, there may be important shifts to environmental conditions that may further threaten seagrasses under global change scenarios. One such threat to seagrasses is human-induced eutrophication (Burkholder et al., 2007), which causes an increase in algal cover. Increased nutrient loads in coastal waters may occur as a result of agricultural run-offs and wastewater discharge. Nutrient loading favours fast growing plankton and macroalgal species at the expense of seagrasses (Hauxwell et al., 2003; Kemp et al., 2005). Thus, substantial inputs of additional nutrients to water bodies is known to result in increased turbidity and lower light availability for seagrasses. These conditions undermine general seagrass health and growth (Burkholder et al., 2007) and may impose direct ammonium (van Katwijk et al., 1997) and sulfide stress and toxicity (Lamers et al., 2013). The ability of known seagrass diseases to complete their lifecycles in eutrophic conditions is unknown. Global change thus seems to play a pivotal role in the acute decline of seagrasses worldwide (Orth et al., 2006; Waycott et al., 2009), though those effects are not always simply positive or negative.
1.2. Potential increased risk of seagrass disease in the future
The impact and ecological roles of co-occurring biological interactions with seagrasses, such as pathogen presence, are largely unknown. In fact, while the importance of the microbiome in seagrass is gaining momentum (Fahimipour et al., 2017), the role of pathogenic disease is often left out of seagrass research entirely, and is largely missing or overlooked in reviews and analyses of global change impacts in seagrass ecosystems (Bjork et al., 2008; Brown et al., 2013; Short et al., 2016). Still, there is recognition of the vulnerability of seagrass to global change scenarios (Short et al., 2016), which is alarming, given multiple evidence of accelerating risk of emerging infectious diseases in terrestrial and marine systems, largely through range expansion and human introduction (Anderson et al., 2004; Hoegh-Guldberg and Bruno, 2010).
We theorise that since human-induced changes to global systems pose major threats to seagrasses in the future (Orth et al., 2006; Short et al., 2016), they may decrease seagrass resilience and fitness (De Boer, 2007; Ehlers et al., 2008; Fraser et al., 2014; Ruesink, 2016) and thus, increase the risk of susceptibility to disease (Harvell et al., 2002). Multiple and repeated disturbances, such as those that may occur as global change progresses, are known to further reduce seagrass resilience (Eklöf et al., 2010; Fraser et al., 2014) and may create hotspots of seagrass disease. In addition to enhanced susceptibility of the host, global change may also promote infection and transmission of diseases by promoting pathogen development, survival and reproduction (Burge et al., 2014; Harvell et al., 1999). Combined effects of global change on host, pathogen and host-pathogen relationships in seagrass diseases are understudied. Still, available research points to significant effects for seagrasses from temperature, salinity, eutrophication and biological interactions, such as disease. This research reveals the urgency for continued and inclusive investigations into the potential roles of disease in seagrass ecosystems, especially those under direct stress from global change phenomena.
2. History of seagrass disease research
Disease events in seagrass ecosystems have been observed for nearly a century (Fischer-Piette et al., 1932). Initial work in this field was driven by early observations of rapid decline and acute losses to large areas of Zostera marina L. on both sides of the Atlantic Ocean in the 1930's (Den Hartog, 1987; Giesen et al., 1990; Rasmussen, 1977). It was estimated that up to 90% of all Z. marina of the North Atlantic was lost in a wasting disease epidemic (Muehlstein, 1989). Since that time several wasting disease events have been observed and have resulted in ecologically significant losses of Z. marina meadows in Maine, New Hampshire and Massachusetts in the United States (Short et al., 1987) and Thalassia testudinum ex Koenig in Florida Bay, USA (Hall et al., 2016; Robblee et al., 1991). There have been other localised disease events around the globe that may be attributed to wasting disease, including observations of the pathogen causing disease symptoms in Japan (Ralph and Short, 2002), Canada, Europe and the United States (Groner et al., 2016a; Short et al., 1988) and New Zealand (Armiger, 1964).
Presumably due to the large scale of losses in the 1930's, seagrass pathology has progressed with a focus on Labyrinthula, the etiological agent of seagrass wasting disease. Much of this work has been done in Z. marina and other North American and European species. However, more recently, research has emerged on the biology and ecology of Labyrinthula at the global level where researchers have investigated phylogeny and global trends in pathogenicity of Labyrinthula (Martin et al., 2016), including the most recent work describing isolation, phylogeny (Sullivan et al., 2016) and pathogenicity (Trevathan-Tackett et al., unpublished) of Labyrinthula spp. in the southern hemisphere.
Although reports of others seagrass pathogens are rare, Plasmodiophora bicaudata and Plasmodiophora diplantherae were discovered to cause shoot galls in Zostera and Halodule, respectively (Den Hartog, 1965, 1989; Walker and Campbell, 2009). Those parasites were described as new species, but there was no comment on their potential to cause diseases or dieback in the populations other than Walker and Campbell (2009) and Den Hartog (1989), who noted increased uprooting of the plants. Very recently, two additional pathogens of seagrasses were discovered: Phytophthora gemini and Halophytophthora zostera (Govers et al., 2016; Govers et al., 2017; Man in 't Veld et al., 2011). These oomycetes were shown to affect the viability of seeds and restricts seedling development. In light of these novel discoveries, the relevance of additional pathogens in seagrass disease events and resilience of seagrass ecosystems is gaining momentum. A summary of seagrass diseases and their hosts is provided in Table 1.
Table 1.
Overview of known seagrass pathogens and hosts.a
| Agent of disease | Seagrass host species | Type | Symptoms | References |
|---|---|---|---|---|
| Halophytophthora sp. Zostera | Zostera marina | Oomycetes, Heterokonta | Reduced seed germination, impaired seedling development | (Govers et al., 2016, Govers et al., 2017) |
| Labyrinthula sp. including L. zosterae |
Zostera marina Zostera caulescens Zostera japonica Zostera noltii Zostera pacifica Cymodocea nodosa Posidonia oceanica Ruppia cirrhosa Ruppia maritima Syringodium isoetifolium Thalassia testudinum |
Labyrinthulomycetes, Heterokonta | Leaf lesions, mortality if lesion advancement out-grows new leaf growth | (Garcias-Bonet et al., 2011), (Martin et al., 2016), Muehlstein et al. (1991) |
| Phytophthora gemini | Zostera marina | Oomycetes, Heterokonta | Reduced seed germination (seed pathogen) | (Govers et al., 2016, Govers et al., 2017) |
| Plasmodiophora diplantherae |
Halodule wrightii Halodule uninervis |
Phytomyxea, Rhizaria | Shoot galls | (Den Hartog, 1965, Walker and Campbell, 2009) |
| Plasmodiophora bicaudata |
Zostera noltii Zostera capensis Zostera muelleri Zostera japonica |
Phytomyxea, Rhizaria | Shoot galls | (Den Hartog, 1989) |
| Plasmodiophora halophila | Halophila ovalis | Phytomyxea, Rhizaria | Shoot galls | (Karling, 1968) |
| Tetramyxa parasitica |
Ruppia brachypus Ruppia rostellata Ruppia spiralis Ruppia maritimus |
Phytomyxea, Rhizaria | Shoot galls | (Karling, 1968) |
Labyrinthula sp. have been isolated from the following seagrass species but pathogenicity was not tested, or could not be confirmed: Halodule uninervis, Halophila ovalis, Thalassodendron ciliatum (Vergeer and Den Hartog, 1994), Phyllospadix scouleri, Phyllospadix torreyi (Martin et al., 2016).
3. The role of environmental parameters in seagrass disease
There is a long history of trying to decouple the influence of environmental factors on prevalence and occurrence of periodic seagrass disease events (Cottam and Munro, 1954; Stevens, 1936; Tutin, 1938). For instance, following the 1930's epidemic, evidence was presented showing correlations of Zostera marina declines at the time with extreme drought and flood cycles (Martin, 1954), moon phases, and warm water transgressions (Stevens, 1936). A correlation was later discovered between solar insolation and wasting disease events during that time (Giesen et al., 1990), suggesting reduction in light by increased storm events or cloud cover may contribute to disease severity. More recent assessments of independent environmental variables, temperature and salinity, have found variable effects of these factors on seagrass disease progression (Burdick et al., 1993; Olsen et al., 2015; Trevathan et al., 2011). Given no studies have positively demonstrated specific environmental factors contribute to presence or severity of disease, it is clear more theory and experimentation in this field is needed.
Our review is the first paper to summarise the roles of global change and diseases in seagrass ecosystems, including 1) detailed descriptions of three known pathogenic groups, 2) investigations into known and potential effects of disease on seagrass in a changing climate, 3) discussions of the need for advancing disease related research and monitoring and 4) providing recommendations for future research considering disease in seagrass resilience and recovery models into the future.
4. Known seagrass pathogens
4.1. Labyrinthula
4.1.1. Description
Labyrinthula spp. are unicelluar protists (Fig. 1a) in the family Labyrinthulomycetes (at the base of the Stramenopila), which grow in distinctly branching colonies formed by secretion of extracellular mucus, which forms intricate networks the cells utilise for transport (Fig. 1b). Along with thraustochytrids and aplanochytrids, Labyrinthula species typically decompose plant material in marine and coastal ecosystems (Tsui et al., 2009). When penetration of Labyrinthula into green, live seagrass leaf tissue, does occur it is resulting in chloroplast and cell necrosis. As a result of the necrosis, affected areas of the leaf become blackened while surrounded by green leaf tissue, which is the primary way to identify possible wasting disease infection (Fig. 1c). In the 1930s, the presence of Labyrinthula and blackened seagrass leaves closely coupled with a massive die-off of Z. marina was affecting > 90% of the seagrass populations in the northern Atlantic Ocean. It wasn't until decades later that the causative agent, Labyrinthula zosterae was identified by fulfilling Koch's Postulates (Muehlstein et al., 1988; Muehlstein et al., 1991). The mechanisms behind the spread was shown to be from blade-to-blade contact (Muehlstein, 1992) as well as floating leaf detritus and waterborne introductions (Martin et al., 2016), with lesions bisecting the width of the leaf being detrimental to plant oxygen and nutrient transport. Since then, Labyrinthula has been considered primarily an opportunistic pathogen under the hypothesis that unhealthy seagrasses would more likely be susceptible to wasting disease and thus larger-scale die-offs.
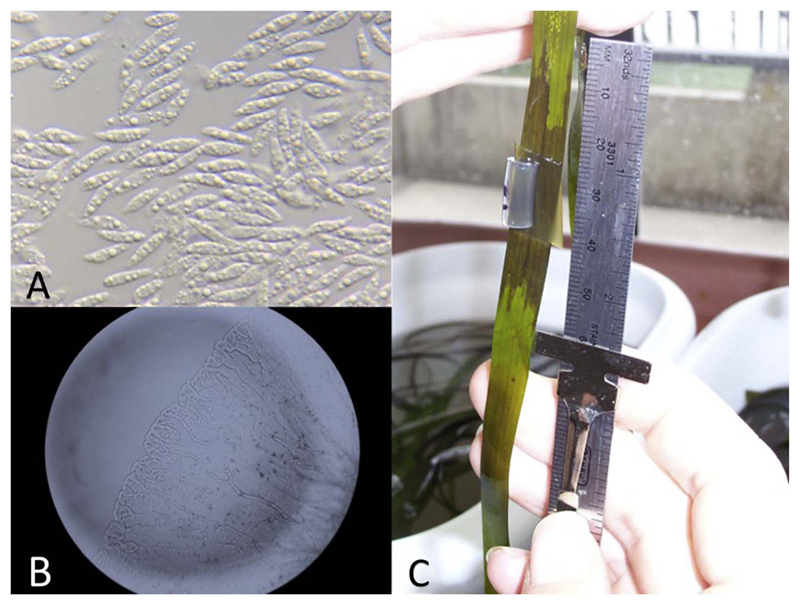
Fig. 1.
A) Spindle-shaped cells of Labyrinthula spp., B) Unique ectoplasmodic network forms distinctly branching Labyrinthula colony, and C) Infected Zostera marina leaf with an expanding wasting disease lesion after 4 days.
4.1.2. Host-pathogen relationship
The Labyrinthula-seagrass-wasting disease relationship is not entirely well defined. For one, it is unclear why some isolates of Labyrinthula are able to infect living seagrass tissues, yet other times, the same isolate may only act as a saprobe of seagrass litter. Martin et al. (2016) made advances in identifying ITS rDNA clades showing pathogenic or non-pathogenic characteristics. The second facet of this relationship is host susceptibility, which can be tested via environmental stressors (decreased health) and availability of a host defence mechanism (secondary metabolites, oxidative burst, hypersensitive response) (Loucks et al., 2013; Trevathan-Tackett, 2011). As previously stated, the majority research thus far has focused on wasting disease in temperate Z. marina and (sub) tropical T. testudinum (Table 1). However, recently there has been a push to understand the host-pathogen-environment interactions for other Zostera species which can infect Cymodocea nodosa Asch. and Posidonia oceanica Delile (Martin et al., 2016; Olsen et al., 2015; Olsen and Duarte, 2015).
4.1.3. Global change dynamics
Previously, the hypothesised effects of global change and anthropogenic stressors on seagrass wasting disease were primarily based on seagrass stress studies, absent of infection treatments (e.g. Koch et al., 2007b; Short et al., 2016). More recently, there has been an effort in understanding how the environment influences wasting disease dynamics. These studies have shown that elevated temperatures, particularly above 28 °C, had no effect on wasting disease proliferation for Mediterranean Cymodocea nodosa (Olsen and Duarte, 2015) and Posidonia oceanica (Olsen et al., 2015). However, testing the effect of warming on disease severity in temperate species, such as Zostera, is needed, as these species at the edges of climate tolerance may not be adapted to warmer waters. Labyrinthula growth and incidence of wasting disease are generally inhibited by low salinities (e.g. oligo- and mesohaline < 15) and thrive at polyhaline salinities (up to ~ 30–35). Elevated salinities above 35–40, however, do not increase susceptibility to infection, due to acclimation by the seagrass, as well as detrimental effects of hypersalinity on Labyrinthula itself (Bishop, 2013; Burdick et al., 1993; Martin et al., 2009; McKone and Tanner, 2009; Sykes and Porter, 1973; Trevathan et al., 2011). Given that seagrass ecosystems will potentially be subject to several sub-optimal environmental conditions simultaneously, it will be important to understand the combinations of health ‘thresholds’ within and beyond current climatic conditions (e.g. temperature, salinity). It has been hypothesised that multiple stressors have provided conditions for enhanced seagrass die- off in the past (Koch and Erskine, 2001; Koch et al., 2007b; Koch et al., 2007c), but as of yet the relationship between seagrass and Labyrinthula and a changing environment is complex and necessitates further investigation (Bishop, 2013; Groner et al., 2014; Martin et al., 2016).
4.1.4. Current topics
In comparison with other well-studied terrestrial and marine pathogens, there are still many questions to answer about Labyrinthula speciation, ecology and seagrass wasting disease (Martin et al., 2016). Firstly, while there are a handful of studies that have tested pathogenicity of Labyrinthula of seagrass species other than Z. marina, it is critical to understand the wasting disease dynamics of the ~ 60 other seagrass species world-wide, particularly those that are threatened and/or endemic. It is possible that certain Labyrinthula isolates demonstrate host-specific or effective climatic-, species- or genera-specific infection and subsequent responses of seagrass hosts to infection. This could in turn limit wide-spread incidences of wasting disease (Martin et al., 2016; Vergeer and Den Hartog, 1994). There are also large gaps in our knowledge about Labyrinthula ecology, particularly, its infection mechanisms from the perspective of enzymatic degradation of live tissue and its own defence against seagrass metabolites. While Labyrinthula growth is effectively reduced in vitro by phenolic acids common in seagrass tissues (Jensen et al., 1998; Steele et al., 2005; Trevathan-Tackett et al., 2015), the role of such compounds as an active, induced response to infection is still to be evidenced in vivo. There could also be a biological control of infection through interactions with other epibiotic microbes as seen with marine macroalgal and animal biofilms (Wahl et al., 2012). Lastly, one of the largest research needs surrounding seagrass wasting disease is the detection and monitoring of infectious Labyrinthula. Most recent monitoring efforts have been performed with visual lesion identification through a wasting index (Burdick et al., 1993). However, this method can be quite time-consuming and costly as well as unreliable for identifying Labyrinthula-based infections, if not followed up with culturing and Koch's Postulates tests. With the growing affordability and accessibility of broader genetic typing techniques such as eDNA profiling of communities on all taxonomic levels (i.e. species, genera, phyla) together with improved detection methods (PCR, qPCR, single cell genomics) large scale screening of the pathogen load and the pathogen community will be viable in the near future (Bockelmann et al., 2013).
4.2. Phytophthora
4.2.1. Description
Phytophthora gemini was first discovered on rotting leaves of Zostera marina together with another Phytophthora species, Phytophthora inundata (Man in 't Veld et al., 2011). This was the first report on Phytophthora species associated with a seagrass species, but up to that point, nothing was yet known about potential pathogenicity of these Phytophthora species (Govers et al., 2016). This knowledge gap is surprising as Phytophthora spp. are considered the most vigorous group of terrestrial plant pathogens but virtually nothing is known about freshwater and marine Phytophthora species.
4.2.2. Host-pathogen relationship
Phytophthora species are fungi-like oomycetes that can reproduce both sexually and asexually by means of motile zoospores. Infection by Phytophthora species can cause a range of disease symptoms in terrestrial plants including stem rot, root rot and leaf blight (Lamour, 2013). The most infamous Phytophthora species is Phytophthora infestans, the causative agent of potato blight (Bourke, 1964; Yoshida et al., 2013). Next to causing economic damage through the loss of agricultural crops, several Phytophthora species are also known to harm natural ecosystems. Phytophthora ramorum is currently threatening forest ecosystems in California by causing mortality in oaks (Rizzo and Garbelotto, 2003; Rizzo et al., 2002), and Phytophthora cinnamomi infects and kills many endemic tree species in eucalyptus forests in Southwest Australia (Weste and Marks, 1987). Phytophthora alni is a widespread lethal disease of alder that represents a threat to riparian ecosystems in Europe (Brasier et al., 2004). In contrast to this extensive list of examples from terrestrial systems, virtually nothing is known about marine Phytophthora species, with the exception of two reports of Phytophthora species associated with mangroves (Pegg et al., 1980; Zeng et al., 2009).
Recent studies on seagrasses have shown that Phytophthora gemini infection of Zostera marina seeds is widespread across the North Atlantic with infection rates as high as 74%. Infection strongly reduces seed germination (by 6 times) and impairs seedling development (Fig. 2a). (Govers et al., 2016; Govers et al., 2017) of Z. marina. Although Phytophthora inundata has also been found on Zostera marina leaf and seed material (Man in 't Veld et al., 2011), pathogenicity trials with this Phytophthora species have yet to be performed. Another, newly described Phytophthora species, Phytophthora chesapeakensis was recently found on Zostera marina seeds from Chesapeake Bay, U.S. (Man in 't Veld et al., in review). Based on the pathogenic character of the Phytophthora genus, it is very likely that both P. inundata and P. chesapeakensis may cause disease in Zostera marina, but this needs further investigation. So far, Phytophthora species have only been found associated to Zostera marina (Govers et al., 2016; Man in 't Veld et al., 2011) and very recently with Zostera noltii Hornem. growing intermingled with infected Zostera marina (Man in 't Veld et al., in review). This may indicate that other seagrass species, especially from the Zostera genus may also be susceptible to infection by Phytophthora species.
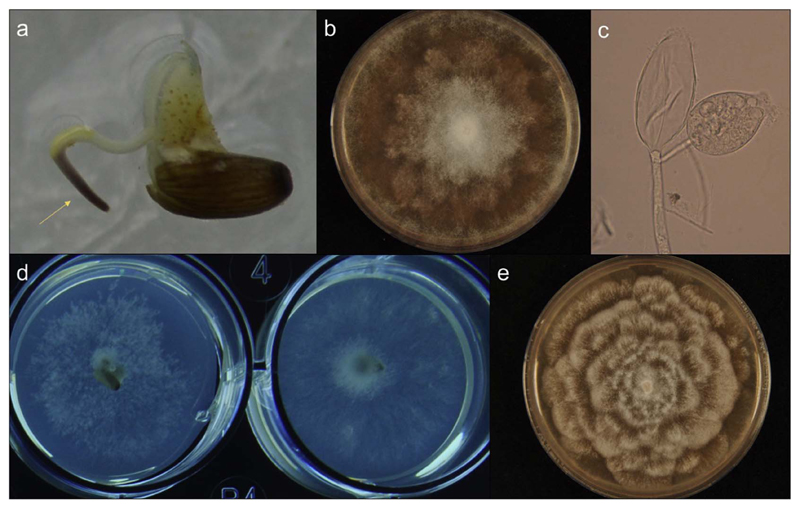
Fig. 2.
a) Impaired seedling development of Zostera marina due to Phytophthora gemini infection, b) Colony morphology of Phytophthora gemini grown on cherry decoction agar (CHA), c) Microscopic detail of Phytophthora gemini sporangia characterized by the occasional formation of two neighbouring sporangia on the zoospore, d) Phytophthora gemini (left) and Halophytophthora sp. Zostera and Phytophthora gemini mycelium (right) growing from infected Zostera marina seeds on a ParpH medium, d) colony morphology of Halophytophthora sp. Zostera grown on CHA. Photos are reproduced from Govers et al. (2016) and Govers et al. (2017).
Although Phytophthora spp. have been isolated from both Zostera marina leaves and seeds, no symptoms of infection have been found on leaves so far (Govers et al., 2016). Instead, Phytophthora spp. seem to be seed pathogens, killing dormant seeds and newly emerging pre-seedlings (Govers et al., 2017).
Infection numbers may be extremely high in dense seagrass meadows (Govers et al., 2016), but transmission pathways have not yet been studied. However, presence of oomycetes such as Phytophthora spp. in aquatic systems are often baited for by using pollen grains (Goldstein, 1960). Therefore, it may be expected that infection of Zostera marina by Phytophthora spp. zoospores takes places in the young sporophyte and gametophyte stages (Gleason et al., 2013).
4.2.3. Global change dynamics
Global change is known to negatively affect seagrass performance (see above), thereby likely enhancing susceptibility of seagrasses to disease. In addition, global change may very likely also affect Phytophthora survival, motility and infection potential. Climate warming may affect plant diseases by altering stages and rates of pathogen development, leading to range shifts and altered infection patterns (Coakley et al., 1999). In many high-temperature optimum Phytophthora species, winter is generally considered the period of major mortality. Increasing winter sea temperatures due to global warming is therefore expected to lessen the effects of this bottleneck period of the Phytophthora growth cycle, thereby expanding the range of these pathogens. In addition, warming is predicted to increase pathogen activity at existing disease locations (Bergot et al., 2004; Brasier, 1996; Sturrock et al., 2011; Thompson et al., 2014). Although no research has yet been done on Phytophthora species that infect seagrasses, growth curves of Phytophthora gemini and Phytophthora inundata show optimum mycelium growth rates around 27 °C and steeply increasing growth rates between 6 and 27 °C (Man in 't Veld et al., 2011). In contrast, Phytophthora gemini infection of Zostera marina seeds seems to be higher in seeds wintering in low temperature sediments (5 °C) than high temperature sediments (12 °C) (Govers et al., 2016). This ambiguity indicates that temperature effects on seagrass-Phytophthora interactions may be hard to predict and will depend on both plant and pathogen life stage and seasons.
Although nothing is known about marine Phytophthora species in relation to seawater pH, many terrestrial Phytophthora species can disperse via waterways and are affected by water quality (Granke et al., 2009). These common, water dispersing Phytophthora species such as P. alni, P. kernoviae, P. ramorum, generally have a broad pH tolerance, which is species specific (Kong et al., 2012). pH levels outside the species-specific tolerance zones negatively affected motility, encystment and germination of Phytophthora zoospores (Kong et al., 2012). However, ocean acidification is only expected to change seawater pH by 0.1–0.2 pH points (Pachauri et al. IPCC). Such a change will very likely fall within the pH tolerance of marine Phytophthora species. We, therefore, expect no effects of ocean acidification on Phytophthora infection in seagrasses.
In contrast, high salinities generally seem to facilitate Phytophthora as a result of increased sporangium production under high saline conditions (Bouchibi et al., 1990). Salinity extremes due to global change may negatively affect seagrass performance (Kahn and Durako, 2006), thereby enhancing susceptibility to disease, including Phytophthora as is seen in many terrestrial plant species (Blaker and MacDonald, 1986; Sanogo, 2004; Snapp et al., 1991). However, during periods of extremely low salinity (e.g. high precipitation or flooding events) may have opposite effects on Phytophthora spp. Infection at salinities as low as 0.5 ppt have been observed to completely eliminate infection of Zostera marina seeds, possibly because Phytophthora are weak competitors in these conditions (Govers et al., 2017).
4.2.4. Current topics
As Phytophthora species have only recently been discovered as seagrass pathogens (Govers et al., 2016; Govers et al., 2017), current topics on Phytophthora-seagrass interactions cover every aspect of this host-pathogen interaction, from a plant physiological to ecosystem level, including the use of novel techniques for pathogen detection (Menning et al. unpublished data). Research on seagrass-Phytophthora interactions is still in a pioneering phase and additional research is urgently needed as Labyrinthula infection of eelgrass has shown that massive, disease-driven die-offs may occur in seagrass beds, affecting both seagrasses and associated fauna (Den Hartog, 1987; Rasmussen, 1977). Many stringent questions have arisen. 1) Ecology: What other seagrass species may be vulnerable to Phytophthora infection? What conditions determine infection and pathogen transmission? How widespread are Phytophthora spp. associated with seagrasses? 2) Phytopathology: How do seagrass plants get infected by Phytophthora species? How does Phytophthora kill seagrass seeds? What kind of defence mechanisms do seagrasses have against infection? Finally, 3) Global change: How do temperature, ocean acidification and extreme salinities affect seagrass-Phytophthora interactions.
4.3. Halophytophthora
4.3.1. Description
Just like Phytophthora species, Halophytophthora species are oomycetes, with similar morphology (Fig. 2c–d) and life cycles. The genus of Halophytophthora is not monophyletic and so far, only 14 species have been named (Robideau et al., 2011). Some Halophytophthora species even group with Pythium, another genus of plant parasites (Cooke et al., 2000). Halophytophthora are commonly found inhabiting brackish and saltwater and were only very recently positioned in a genus separate from Phytophthora based on rDNA-ITS sequences (Cooke et al., 2000).
4.3.2. Host-pathogen relationship
Halophytophthora species were previously unknown as pathogens and were only described as saprophytes, decomposing dead mangrove and saltmarsh plant leaves (Ho and Jong, 1990; Nakagiri, 2000; Newell, 1996). Some saprophytes may however become pathogenic under favourable conditions (Freitag et al., 2009; Marois and Mitchell, 1981), and Halophytophthora species have very recently indeed been found to be pathogenic to seagrasses (Govers et al., 2016). In total, three Halophytophthora species (sp. 1, 3, 4) have been isolated from Zostera marina, which have not yet been named officially (Man in 't Veld et al., in review). Potential pathogenic effects of Halophytophthora species on other higher marine plant species (mangroves, salt marsh plants) have not yet been discovered.
As indicated above, Halophytophthora species have traits very similar to Phytophthora species and infection rates in the field can be as high as 67% (Govers et al., 2016). In addition, Halophytophthora spp. may be equally pathogenic, causing seed mortality of infected seeds and impaired seedling development (Govers et al., 2016; Govers et al., 2017). Halophytophthora infection of Zostera is as widespread as Phytophthora infection (Govers et al., 2016; Govers et al., 2017; Man in 't Veld et al., in review) and Halophytophthora spp. have also been isolated from both leaves and seeds of Zostera marina (Govers et al., 2016; Man in 't Veld et al., in review). So far, Halophytophthora species have only been found to infect Zostera marina, but since this group of oomycetes is very widespread across ecosystems and climatic zones (Nakagiri, 2000; Newell, 1996; Nigrelli and Thines, 2013), it is very likely that other seagrasses may also be at risk of infection.
4.3.3. Global change dynamics
Most reports of Halophytophthora species come from tropical to subtropical coastal ecosystems (Fell and Master, 1975; Nakagiri and Newell, 1994; Pegg and Alcorn, 1982), until their recent discovery in temperate coastal waters (Govers et al., 2016; Man in 't Veld et al., in review; Nigrelli and Thines, 2013). These recent discoveries are probably not a consequence of biogeographic expansion due to global change, but rather a result of increased sampling efforts for eukaryotic microbes. This is because growth rates of temperate Halophytophthora spp. are similar (between 5 and 25 °C), whereas tropical Halophytophthora spp. showed optimum growth rates around 30 °C (Nigrelli and Thines, 2013). Thus, climate warming may not necessarily benefit temperate Halophytophthora species. However, the range of common tropical and sub-tropical Halophytophthora species may expand because of increased temperatures.
Potential effects of ocean acidification on Halophytophthora have not yet been studied, but there is one study reporting on the effects of pH on Halophytophthora growth (Leano et al., 1998). The best-studied species in this group is Halophytophthora vesicula, commonly isolated from mangrove leaves. It has a narrower pH range for growth than most Phytophthora species (Kong et al., 2012), growing between pH 6 and 8.5, with an optimum of pH 7 (Leano et al., 1998). For this particular species, ocean acidification would thus provide more suitable vegetative growth conditions. The Halophytophthora species infecting seagrasses however may have very different pH tolerances and hence generic effects of ocean acidification on Halophytophthora are hard to predict.
Salinity extremes are likely to occur in shallow coastal zones were seagrasses generally grow and Halophytophthora pathogens (and decomposers) may be affected by such conditions. Although “Halo”, coming from the Greek word “Háls” – meaning salt, would indicate that Halophytophthora are salt-loving Phytophthora relatives, many Halophytophthora have a broad salinity tolerance ranging from 0 to 50 ppt (Leano et al., 1998; Nakagiri and Newell, 1994). Optimum levels for vegetative growth are however somewhat more specified (between 15 and 30 ppt) and requirements for sporulation (production of zoospores for dispersal) are even more specific and depend on species (Leano et al., 1998; Nakagiri and Newell, 1994). Thus, Halophytophthora will very likely survive both high and low salinity extremes, although growth and dispersal of these pseudo-fungi may be limited during such events.
4.3.4. Current topics
Halophytophthora species in temperate waters have long been overlooked (Nigrelli and Thines, 2013) and only very recently, Halophytophthora species were discovered to cause disease in seagrasses (Zostera marina). Current themes in seagrass-Halophytophthora research therefore comprise the same basic questions as for seagrass-Phytophthora research. In addition, stringent questions that needs further investigation is if the same Halophytophthora species that act as decomposers may also act as pathogens and under what conditions these saprophytes turn pathogenic.
4.4. Phytomyxea
4.4.1. Description
Phytomyxea are obligate biotrophic parasites known to infect vascular plants, brown algae, diatoms and oomycetes. Phytomyxea are currently classified in the Phytorhiza, a sub-group of the Rhizaria, a protistan supergroup (Sierra et al., 2016). The best known species is Plasmodiophora brassicae, the causal agent in the development of clubroot in cabbage (Dixon, 2014) (Fig. 3a–b). They are able to infect and reinfect host species in multiple kingdoms, but there seems to be a separation between marine and terrestrial species (Neuhauser et al., 2014). However, the role of Phytomyxea beyond their phytopathological potential is less well-known and data about their abundance and occurrence in natural ecosystems are largely missing (Neuhauser et al., 2011). In seagrass environments, four phytomyxid species have been described which all have been identified as the causal agents in the formation of shoot galls (Fig. 3c–d), which are hypertrophic enlargements of the cortical tissues in seagrass rhizomes (Den Hartog, 1965). Enlargements in host root tissues can appear as a ‘string of beads’ forming from the roots tissues while appearing to advance up the stem (Fig. 3d). They greatly alter the morphology of affected tissues, however, the uninfected part of the plant may appear normal. Morphologically phytomyxids are characterized by their distinct resting spores. In their vegetative form, they can be identified as large, amoeba like, multinucleate single-celled plasmodia that fill the entire host cell before cleaving into spores, which then release flagellated motile zoospores and are free to infect new hosts.
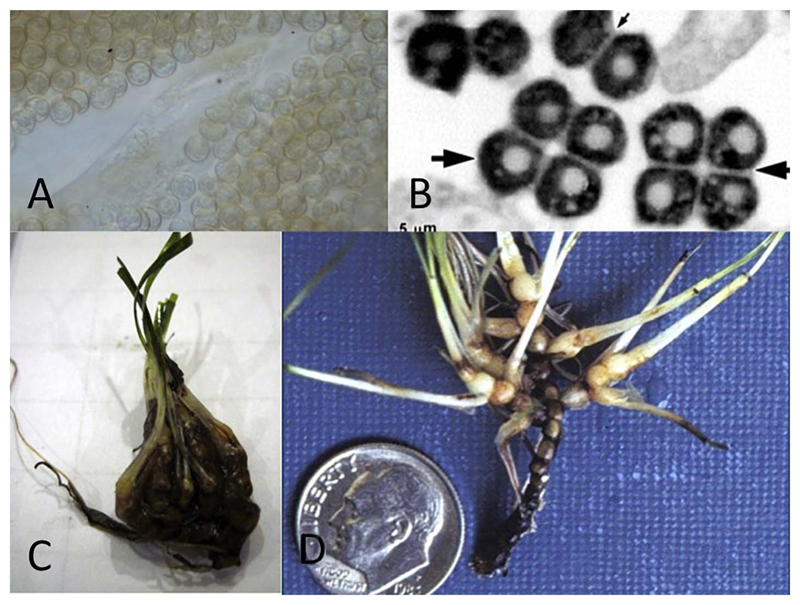
Fig. 3.
a) Resting spores of Plasmodiophora diplanthera a parasite of Halodule wrightii, light microscopy. b) Resting spores of Tetramyxa parasitica, parasite of Ruppia spp. TEM picture (picture from James Braselton), c) Plasmodiophora bicaudata infected Zostera noltii. d) Plasmodiophora diplantherae infected Halodule (picture from James Braselton).
4.4.2. Host-pathogen relationship
All described phytomyxid pathogens of seagrasses cause enlargements, or hypertrophies, of the rhizomes, internodes or shoots of the seagrasses. From genetic studies of the clubroot pathogen, it is known that these hypertrophies are linked to a pathogen-induced alteration of the auxin and cytokinin metabolism of the host (Ludwig-Müller, 2014), so it is likely that similar strategies will be employed in seagrasses. Two species of Plasmodiophora have been well documented and have been repeatedly linked to shoot gall disease in seagrasses of both temperate and tropical regions of both northern and southern hemispheres (Den Hartog, 1965, 1989; Walker and Campbell, 2009). Infection of seagrasses by Plasmodiophora bicaudata J. Feldman was confirmed across a wide range of Zostera species in temperate regions of the globe, including Z. muelleri ‘complex’ in Australia, Z. capensis Setch. in South Africa, Z. noltii in Europe and Z. japonica in Asia and the United States (Den Hartog, 1989). Plasmodiophora diplantherae (F & W) Iv. Cook affects Halodule species, including Halodule wrightii Asch., Halodule uninervis forssk., Halodule tridentata Steinh., Halodule pinifolia Hartog, and Halodule beaudettei Hartog in tropical regions of the world (Karling, 1968). Plasmodiophora halophila was described in 1913 from herbarium material of Halophila ovalis (r.Br) Hook.f., and the species is now considered to be doubtful (Karling, 1968). The fourth known species, Tetramyxa parasitica, forms galls on Ruppia spp. and seems to be widespread in northern Europe (Karling, 1968). Like in all phytomyxids, no direct antagonistic effect between the parasite and the host can be observed, although infected plants often show dwarfed growth as a result of the enlarged internodes which divert the energy otherwise directed into lateral growth into the formation of gall tissue and the parasite. Additionally, Den Hartog (1989) reported that he could not find any inflorescences on Halodule spp. infected with P. diplantherae.
4.4.3. Global change dynamics
Gall formation from Phytomyxea infection is accompanied by alterations of the seagrass rooting tissues, and in some species largely eliminates rhizome elongation at the internodes. This effect has been discussed to reduce resilience of individual plants to existing hydrodynamic forces (Walker and Campbell, 2009). This suggests even further risk of losses to seagrasses, including Zostera and Halodule, under global change scenarios that predict increased storm events, including larger and stronger waves and currents in seagrass habitats (Short and Neckles, 1999). As seagrasses are championed for their ability to reduce coastal erosion and alleviate storm surges (De Boer, 2007), impacts from phytomyxid infection on seagrasses could have cascading effects on ecosystems and existing conservation and management plans. Acidification and changes in chemical properties can potentially impact the disease prevalence and abundance: the clubroot pathogen P. brassicae causes the largest damage in agricultural ecosystems when the soil pH is 6.5 or lower and is often kept below the detection limit when the pH is higher (Dixon, 2014; Gossen et al., 2014). Whether or not a similar effect of increased disease severity and abundance will be observable in seagrass ecosystems needs to be tested, as not all phytomyxid show such a marked pH dependence than P. brassicae (Dixon, 2014).
4.4.4. Current topics
There is a paucity of information surrounding seagrass-infecting phytomyxids. There are large gaps in our understanding of the life cycle of these parasites, as well as of the host-parasite interactions, including infection mechanisms and the impact of infection on plant physiology in the marine environment. While infection does not seem to affect the development of leaf tissues (Den Hartog, 1989) it is known that phytomyxids impact on the photosynthesis of their host (Ludwig-Müller, 2014). Also potential alterations of inflorescence were described in an anecdotal report and a greater understanding of physiological effects on root tissues are also needed (Den Hartog, 1989). In land plants the seed quality of clubroot infected plants is inferior to plants free of infection (Gossen et al., 2014), an effect which has not been studied to date but which would be dramatic in the context of restoration efforts. As species in this genus are capable of host shifts across kingdoms, and have very resistant and long-lived resting spores, the persistence of spores and potential reservoirs in natural systems must be explored.
5. Potential risk of increasing disease for seagrasses in future
Through an analysis of known relationships between host, pathogen, and host-pathogen effects under given climate thresholds (Table 2), we may begin to predict key drivers of increasing disease events under future global change scenarios. Generally, with current and predicted alterations to marine environments globally, the likelihood of infection due to decreased defences and likewise increased susceptibility of the host, is hypothesised to increase (Harvell et al., 1999; Harvell et al., 2002).
Table 2.
Summary of predicted host and pathogen responses to anthropogenic global change. Potential responses of seagrass and known pathogens to anticipated future global change scenarios and identification of research gaps. (“ ↑ ” = positive relationship, “ ↓ ” = negative relationship, “ ± ” = neutral relationship, and “?” = relationship unknown). Global change stressors derived from Short and Neckles (1999), Duarte (2002), Harley et al. (2006), and Koch et al. (2013).
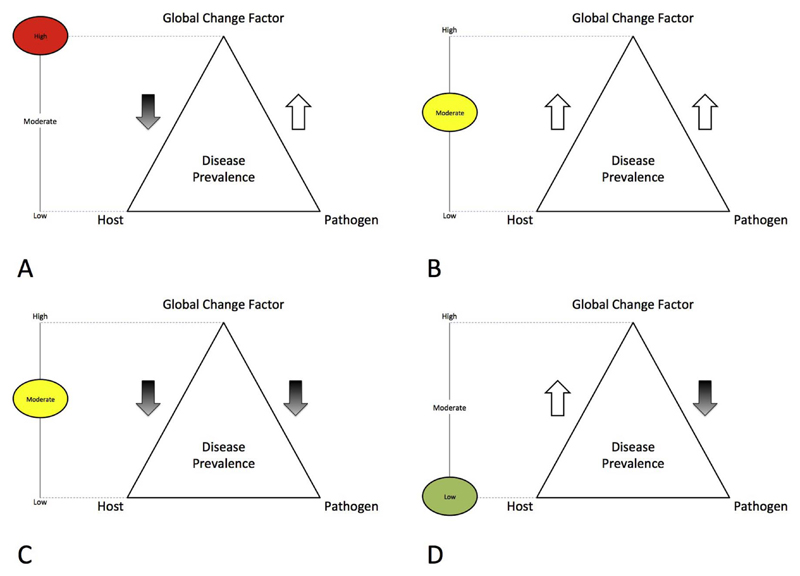
We developed a novel framework for using known responses of hosts and pathogens to described global change parameters to determine a risk factor for global change stressors contributing to disease in seagrasses (Fig. 4). Here, depending on positive or negative responses of hosts and pathogens under a given parameter level (Table 2), we were able to deduce one of three potential levels of disease risk associated with that parameter. These risk levels are 1) High, when host immunity is suppressed and pathogen is enhanced (e.g. warming temperatures and ocean acidification), 2) Moderate, when both or neither host or pathogen are enhanced (e.g. freshwater inputs) and 3) Low, when the host is enhanced and the pathogen is suppressed (e.g. increased atmospheric CO2, potentially). The results of our investigation suggest the highest overall risk of infectious disease outbreaks in seagrass ecosystems may be related to ocean acidification, hypersalinity and warming temperatures. Still, Table 2 demonstrates the lack of basic research into the roles of several environmental parameters on host, pathogen and host pathogen relationships. Thus, further research in these areas is urgently needed to develop and test these theories, and to ultimately better understand the risks and mechanisms of disease and severity related to these parameters.
Fig. 4.
Conceptual framework demonstrating three potential levels of disease risk. These include, 1) high (when the host is supressed and pathogen is enhanced), 2) moderate (when both or neither host or pathogen are enhanced) and 3) low (when the host is enhanced and the pathogen is supressed. Here we evaluate the results of potential co-effects of described global change stressors on known seagrass diseases (summarised in Table 2).
6. Expanding capacity and resilience in seagrass disease research
Similar to human diseases, the onset of disease in plants and animals can be sudden and widespread (Sullivan et al., 2013; Woodroffe, 1999), causing long-term loss or alteration of function (Cottam and Munro, 1954; Dexter, 1944), and thus losses of associated ecosystems services that are beneficial to humankind (Fisher et al., 2012; Harvell et al., 2002). Given the lack of knowledge about seagrass diseases, it is critical to develop a strategy for managers and researchers to use when prioritising resources for monitor existing populations and preparing for future outbreaks.
When strategising responses to emerging threats to human health from infectious disease, researchers have identified key focus areas to promote preparedness for outbreaks of infectious disease (Gostin et al., 2002). Other researchers have reviewed critical predictors of infectious disease outbreaks under global change regimes (Altizer et al., 2013). We have used these considerations as a building block for prioritising activities that increase our capacity to respond to global outbreaks of seagrass diseases. Thus, we offer five key recommendations, or action items, summarising priorities for building our capacity to respond to outbreaks and advance resilience of seagrasses in the future.
Continue support for basic seagrass disease research. The lack of basic research, management and monitoring for marine disease escalation under global change scenarios are presumably due to a lack of specific evidence that disease events in seagrass may be exacerbated under future global change scenarios. In fact, as noted above, some studies even point to some contrary evidence in results. Still, evidence in multiple host-pathogen fields indicate that the role of climate is exacerbating disease in marine and coastal ecosystems around the world and warrants support of research, monitoring and management programs for these ecologically important seagrass ecosystems. Successful development of pathogenesis is a function of properties of both the host and pathogen. The immune response of the host and virulence of the pathogen both play a part in the resulting severity of disease (Casadevall and Pirofski, 2003). We also know that seagrasses are expected to be impacted by global change conditions (Short and Neckles, 1999). Impacts from global change are increasing instances of disease and these increases are expected to continue in ecosystems worldwide (Altizer et al., 2013). In fact, attention to climate-related increases of disease in other marine ecosystems, such as corals and fisheries aquaculture, are gaining momentum. We find the role of global change phenomenon in exacerbating the threat of disease in seagrass ecosystems around the world has not been sufficiently investigated to warrant the current omissions in research and management schemes. Further, we suggest that continued research will help build models to identify potential disease hotspots and areas of vulnerability or resilience around the world. We offer a framework for advancing future seagrass disease research that responds to host, pathogen and environmental conditions (Fig. 4).
Establish emergency funding sources. Traditional models of funding, such as those that rely on lengthy annual applications or specific collaborations with identified institutions, may not be successful in relieving emerging infectious disease events (Groner et al., 2016b; Woodroffe, 1999). When access to immediate funding may be required to investigate an emerging disease event, we suggest an international organization could be tasked to collect and quickly release funds, as is the case for diseases that directly affect humans through the oversight of the World Health Organization. Funding opportunities generally must include both long-term and short-term solutions, including availability of emergency funding, alongside traditional grant funding schemes that support long-term basic research objectives. Provision of emergency funds will allow researchers to engage with active epidemics and derive real-world data to compliment the seagrass disease research framework and fill current research gaps.
Enhancing global expert collaborations and perspectives. We suggest international collaborations are key to getting ahead of recognised marine infectious diseases, especially those known to have global reach. We suggest the creation of a seagrass task force aimed at advancing global understanding of issues in marine plant diseases. This task force should specifically oversee the coordination of conference sessions and workshops at the International Seagrass Biology Workshop on current topics in seagrass disease. In addition, this task force could connect to similar groups, such as the Coastal and Estuarine Research Federation and individual researchers studying disease in other marine plants such as mangroves or salt marsh plants. By encouraging an exchange of knowledge and greater awareness of disease issues, conference sessions may help in generating evidence of global disease occurrences while also helping form expert research collaborations. Through the exchange of ideas, we believe greater progress in basic research and modelling of global trends is more likely, and is warranted for this critical ecosystem.
Develop an adaptive framework for responding to emerging seagrass disease events. When a monitoring event reveals an epidemic disease event is underway or is at risk of developing, a response framework for effective action would aid managers and researchers in attempts to harness emerging infectious disease events in seagrass ecosystems and thus potentially prevent or reduce acute losses to ecosystem function and human services. For instance, we know transmission rates and severity of disease may be reduced by mitigation measures such as optimising host resilience, managing environmental conditions known to facilitate disease and reducing pathogen abundance (Groner et al., 2016b; Lamb et al., 2017). In addition, transmission rates globally may also be reduced through enhanced international biosecurity measures. For example, a newly described species Phytophthora gemini is very likely an invasive species in the Wadden Sea as genotypic variation in this species is very low. In Alaska, the same species was isolated displaying a great deal more genotypic diversity, and as such, it may have been the source of introduction to the Wadden Sea (Man in 't Veld et al., in review). The potential transmission of seagrass disease agents through transference of ballast water or distribution of plant materials is unknown.
Development of an on-going global seagrass disease database. We propose the development of a world-wide GIS-database of seagrass monitoring and disease events, similar to those developed to understand the spread of human diseases (Hightower and Klein, 1995). A centralised data source could assist researchers and managers in regional areas and facilitate further international collaborations. The potential to link to existing seagrass citizen science mapping programs like Seagrass Spotter (https://seagrassspotter.org/) may also be effective at encouraging engagement and collecting large amounts of monitoring data.
In summary, there are three known groups of pathogenic organisms currently known to cause disease symptoms in seagrass, including labyrinthulids, oomycetes and phytomyxids. Global change is likely to affect seagrass-pathogen relationships, for instance through climate warming, hypersalinity and eutrophication. Ocean acidification, low salinity events and increased sulfide production may also to have some effects on seagrass-pathogen dynamics. However, research into the specific impacts and mechanisms of these effects, and co-effects, are largely unknown. We summarise three key areas of research needed to advance our understanding of disease phenomenon, especially as we face a future of global changes.
Biological Research. Many of the research gaps revealed in our assessment can be described as simple lack of basic research into key biological features and interactions of hosts, pathogens or host-pathogen interactions and environmental parameters. These gaps include unknown mechanisms of infection and transmission, genetic precursors to disease, and host defence or immune systems. With regards to Labyrinthula, even after decades of research, we are still unclear about the basic mechanisms by which the host is able to enter and infect the host leaf tissues. Several theories have been presented (Muehlstein, 1992), but no conclusive evidence has been obtained. It will be vital in the future to establish model seagrass systems needed to study disease ex-situ. We suggest analysing basic biological interactions in a targeted and systematic way to understand the processes that form the basis of the pathogenesis. Additionally, the role of biota outside of the immediate host-pathogen relationship may play a role in disease presence, transmission and severity. However, there is no research into the potential role of parasitism in reducing pathogen loads (Gleason et al., 2014), or for mesograzers to facilitate disease in seagrasses, as has been shown in several terrestrial and saltmarsh plants through host tissue damage (Daleo et al., 2009). Additionally, pathogenic microbes can have direct predators, which could further reduce pathogen loading (Schmeller et al., 2014). However, food web dynamics at smaller scales and identification of key players in the microbiome of seagrass communities are only just beginning to be explored (Fahimipour et al., 2017). Biological control and biological causes of disease transmission and severity in seagrasses, has not been studied.
Pathogen discovery. There are roughly 60 recognised species of seagrass globally. So far, only pathogens belonging to three different eukaryotic groups have been identified, and these mostly affect the most extensively studied seagrass species (e.g. Zostera marina). The recent discovery of new and widespread seagrass pathogens, such as Phytophthora spp. and Halophytophthora species, are indications that diseases may be present that have been previously overlooked. Additionally, research suggests there may be a pathogenic role of the Ascomycete, Lindra thalassiae, described as causing dieback in the seagrass Thalassia testudinum (Porter, 1986). More recently, researchers have found the oomycetes Salisapilia sp. on Zostera noltii (Man in 't Veld et al., in review) and the fungus Phialophora malorum on Zostera marina (Govers et al., 2017) may generate disease symptoms. Bacteria and viruses also threaten marine sessile organisms like algae, corals and sponges (Harvell et al., 1999; Suttle et al., 1990). However, other research demonstrates bacteria and viruses can moderate eukaryotic diseases (Gil-Turnes et al., 1989; Rohwer and Thurber, 2009). Thus, it is worthwhile to investigate the effects bacterial and viral pathogens and symbionts may have on seagrass ecosystems. These discoveries indicate that there are potentially many more seagrass pathogens to be identified. Since global change will very likely also affect these unknown pathogens and their relationship with seagrass hosts, it is very important to increase efforts to discover, characterise and understand symbiotic relationships of eukaryotic microorganisms and seagrasses.
Modelling. Using results from basic biological and ecological research, we can build mathematical models to assist our understanding of the complex environmental and biological conditions that lead to disease. Additionally, disease is shaped by, and may in turn shape, host demography with population level consequences (Metcalf et al. 2015). Modelling may assist in the identification of populations that are more or less vulnerable to disease emergence, or to predict disease transmission and severity in an emerging epidemic. We know the ability to model the emergence and transmission of disease are key to preparing for, and potentially mitigating, disease events in human and animal systems (Groner et al., 2016a; Heesterbeek et al., 2015). We believe the time is right to begin modelling efforts in seagrass disease as well.
Monitoring. In order to respond to an emerging disease event, consistent monitoring of hosts and environmental conditions are required (Groner et al., 2016b). Observations should include improving existing monitoring of ambient environmental conditions known to promote disease, to include rapid diagnosis methods for targeted diseases, such as rt-qPCR and analysis of eDNA (Thomsen and Willerslev, 2015). Consistent observations of vulnerable populations are needed. In order to appropriately identify monitoring targets, continued support of basic research into host, pathogen and host-pathogen biology is essential. This basic research aids in the development of disease transmission and expression models that may help identify environmental thresholds and key hotspots or seasonal factors where infection may lead to disease emergence.
In the face of future global change phenomenon, such as climate disruptions, marine ecosystems are believed to face several challenges. We predict seagrasses are likely to be increasingly vulnerable to disease under projected global change scenarios. Thus, there is a need to investigate a variety of potential environmental conditions, including disturbance thresholds and co-occurring effects, as effects may be accumulative and non-linear. In addition, climate related disease effects are likely to be species and region specific, therefore international and cross disciplinary collaborations on seagrass disease issues, such as this one, are key to ensure adequate use of existing and novel techniques for understanding, monitoring, and hopefully in the future, managing the role of diseases in seagrass ecosystems.
Acknowledgements
The authors would like to acknowledge T. van der Heide, J. Heusinkveld, J. Meffert, P. van Rijswick, W. Man in ‘t Veld, E. van der Zee, H. Olff, R. Orth, D. Menning, T. Bouma, M. Van Katwijk for the fruitful discussions on Phytophthora/Halophytophthora-seagrass interactions, and to F. Gleason for his unrelenting interest and support for research on labyrinthulid, oomycete and Phytomyxea disease ecology.
Funding
Laura Govers was funded by Natuurmonumenten (Waddenfonds) and Rijkswaterstaat and NWO-VENI grant 016.Veni.181.087. Sigrid Neuhauser was funded by the Austrian Science Fund: grant Y0810-B16.
References
- Alsterberg C, Eklöf JS, Gamfeldt L, Havenhand JN, Sundbäck K. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc Natl Acad Sci. 2013;110:8603–8608. doi: 10.1073/pnas.1303797110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Armiger LC. An occurrence of Labyrinthula in New Zealand Zostera. N Z J Bot. 1964;2:3–9. [Google Scholar]
- Arnold T, Mealey C, Leahey H, Miller AW, Hall-Spencer JM, Milazzo M, Maers K. Ocean acidification and the loss of phenolic substances in marine plants. PLoS one. 2012;7:e35107. doi: 10.1371/journal.pone.0035107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Rogerson A, Leftley JW. Utilisation of seaweed carbon by three surface-associated heterotrophic protists, Stereomyxa ramosa, Nitzschia alba and Labyrinthula sp. Aquat Microb Ecol. 2000;21:49–57. [Google Scholar]
- Beer S, Axelson L, Björk M. Modes of photosynthetic bicarbonate utilisation in seagrasses, and their possible roles in adaptations to specific habitats. Biol Mar Mediterr. 2006;13:3–7. [Google Scholar]
- Bergot M, Cloppet E, Perarnaud V, Deque M, Marcais B, Desprez-Loustau ML. Simulation of potential range expansion of oak disease caused by Phytophthora cinnamomi under climate change. Glob Chang Biol. 2004;10:1539–1552. [Google Scholar]
- Bishop ND. The Effects of Multiple Abiotic Stressors on the Susceptibility of the Seagrass Thalassia testudinum to Labyrinthula sp., the Causative Agent of Wasting Disease. University of North Florida; 2013. p. 108. [Google Scholar]
- Bjork M, Short F, McLeod E, Beer S. Managing Seagrasses for Resilience to Climate Change. IUCN; 2008. [Google Scholar]
- Blaker NS, MacDonald JD. The role of salinity in the development of Phytophthora root rot of citrus. Phytopathology. 1986;76:970–975. [Google Scholar]
- Bockelmann A-C, Tams V, Ploog J, Schubert PR, Reusch TB. Quantitative PCR reveals strong spatial and temporal variation of the wasting disease pathogen, Labyrinthula zosterae in northern European eelgrass (Zostera marina) beds. PLoS One. 2013;8:e62169. doi: 10.1371/journal.pone.0062169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchibi N, Van Bruggen AHC, MacDonald JC. Effects of ion concentration and sodium:calcium ratio of a nutrient solution on Phytophthora root rot of zoospore motility and viability of Phytophthora parasitica. Phytopathology. 1990;80:1323–1336. [Google Scholar]
- Bourke PM. Emergence of potato blight. Nature. 1964;203:805–808. [Google Scholar]
- Brasier CM. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann Sci For. 1996;63:347–358. [Google Scholar]
- Brasier CM, Kirk SA, Delcan J, Cooke DE, Jung T, In't Veld WAM. Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycol Res. 2004;108:1172–1184. doi: 10.1017/s0953756204001005. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Saunders MI, Possingham HP, Richardson AJ. Managing for interactions between local and global stressors of ecosystems. PLoS One. 2013;8:e65765. doi: 10.1371/journal.pone.0065765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick DM, Short FT, Wolf J. An index to assess and monitor the progression of wasting disease in eelgrass Zostera marina. Mar Ecol Prog Ser. 1993;94:83. [Google Scholar]
- Burge CA, Mark Eakin C, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, Petes LE, Prager KC, Weil E, Willis BL, Ford SE, et al. Climate change influences on marine infectious diseases: implications for management and society. Annu Rev Mar Sci. 2014;6:249–277. doi: 10.1146/annurev-marine-010213-135029. [DOI] [PubMed] [Google Scholar]
- Burkholder J, Tomasko D, Touchette B. Seagrasses and eutrophication. J Exp Mar Biol Ecol. 2007;350:46–72. [Google Scholar]
- Casadevall A, Pirofski L-a. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianen MJA, Van Belzen J, Herman PMJ, van Katwijk MM, Lamers LPM, Van Leent PJM, Bouma TJ. Low-canopy seagrass beds still provide important coastal protection services. PLoS One. 2013;8:e62413. doi: 10.1371/journal.pone.0062413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley SM, Scherm H, Chakraborty S. Climate change and plant disease management. Annu Rev Phytopathol. 1999;37:399–426. doi: 10.1146/annurev.phyto.37.1.399. [DOI] [PubMed] [Google Scholar]
- Collier CJ, Waycott M. Temperature extremes reduce seagrass growth and induce mortality. Mar Pollut Bull. 2014;83:483–490. doi: 10.1016/j.marpolbul.2014.03.050. [DOI] [PubMed] [Google Scholar]
- Cooke DE, Drenth A, Duncan JM, Wagels G, Brasier CM. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet Biol. 2000;30:17–32. doi: 10.1006/fgbi.2000.1202. [DOI] [PubMed] [Google Scholar]
- Cosby SL, MCQuaid S, Duffy N, Lyons C, Rima BK, Allan GM, McCullough SJ, Kennedy S, Smyth JA, McNeilly F, Craig C, et al. Characterization of a seal morbillivirus. Nature. 1988;336:115–116. [Google Scholar]
- Cottam C, Munro DA. Eelgrass status and environmental relations. J Wildl Manag. 1954;18:449–460. [Google Scholar]
- Daleo P, Silliman B, Alberti J, Escapa M, Canepuccia A, Peña N, Iribarne O. Grazer facilitation of fungal infection and the control of plant growth in south-western Atlantic salt marshes. J Ecol. 2009;97:781–787. [Google Scholar]
- De Boer WF. Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia. 2007;591:5–24. [Google Scholar]
- de Fouw J, Govers LL, van de Koppel J, Van Belzen J, Dorigo W, Cheikh MS, Christianen MJA, Van der Reijden K, van der Geest M, Piersma T, Smolders AJP, et al. Drought, mutualism breakdown and landscape-scale degradation of seagrass beds. Curr Biol. 2016;26:1–6. doi: 10.1016/j.cub.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Den Hartog C. Some Notes on the Distribution of Plasmodiophora diplantherae, a Parasitic Fungus on Species of Halodule. Persoonia-Molecular Phylogeny and Evolution of Fungi. 1965;4:15–18. [Google Scholar]
- Den Hartog C. “Wasting disease” and other dynamic phenomena in Zostera beds. Aquat Bot. 1987;27:3–14. [Google Scholar]
- Den Hartog C. Distribution of Plasmodiophora bicaudata, a parasitic fungus on small Zostera species. Dis Aquat Org. 1989;6:227–229. [Google Scholar]
- Dexter RW. Ecological significance of the disappearance of eel-grass at Cape Ann, Massachusetts. J Wildl Manag. 1944;8:173–176. [Google Scholar]
- Diaz-Almela E, Marbà N, Duarte CM. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob Chang Biol. 2007;13:224–235. [Google Scholar]
- Dixon GR. Clubroot (Plasmodiophora brassicae Woronin)–an agricultural and biological challenge worldwide. Can J Plant Pathol. 2014;36:5–18. [Google Scholar]
- Duarte CM. The future of seagrass meadows. Environ Conserv. 2002;29:192–206. [Google Scholar]
- Edwards AJ. Impact of Climate Change on Coral Reefs, Mangroves and Tropical Seagrass Ecosystems. Lewis Publishers; Boca Raton, FL, USA: 1995. [Google Scholar]
- Ehlers A, Worm B, Reusch TB. Importance of genetic diversity in eelgrass Zostera marina for its resilience to global warming. Mar Ecol Prog Ser. 2008;355:1–7. [Google Scholar]
- Eklöf JS, McMahon K, Lavery PS. Effects of multiple disturbances in seagrass meadows: shading decreases resilience to grazing. Mar Freshw Res. 2010;60:1317–1327. [Google Scholar]
- Eklöf JS, Alsterberg C, Havenhand JN, Sundbäck K, Wood HL, Gamfeldt L. Experimental climate change weakens the insurance effect of biodiversity. Ecol Lett. 2012;15:864–872. doi: 10.1111/j.1461-0248.2012.01810.x. [DOI] [PubMed] [Google Scholar]
- Fahimipour AK, Kardish MR, Lang JM, Green JL, Eisen JA, Stachowicz JJ. Global-scale structure of the eelgrass microbiome. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.03391-16. e03391–03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell JW, Master IM. Phycomycetes (Phytophthora spp. nov and Pythium sp. nov) associated with degrading mangrove (Rhizophora mangle) leaves. Can J Bot. 1975;53:2908–2922. [Google Scholar]
- Fischer-Piette E, Heim R, Lami R. Note préliminaire sur une maladie bactérienne des Zostéres. CR Acad Sci Paris. 1932;195:1420–1422. [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery KJ. Seagrass ecosystems as a globally significant carbon stock. Nat Geosci. 2012;5:505–509. [Google Scholar]
- Fraser MW, Kendrick GA, Statton J, Hovey RK, Zavala-Perez A, Walker DI. Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. J Ecol. 2014;102:1528–1536. [Google Scholar]
- Freitag NE, Port GC, Miner MD. Listeria monocytogenes - from saprophyte to intracellular pathogen. Nat Rev Microbiol. 2009;7:623–628. doi: 10.1038/nrmicro2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcias-Bonet N, Sherman TD, Duarte CM, Marbà N. Distribution and pathogenicity of the protist Labyrinthula sp. in western Mediterranean seagrass meadows. Estuar Coasts. 2011;34:1161–1168. [Google Scholar]
- Garrard SL, Beaumont NJ. The effect of ocean acidification on carbon storage and sequestration in seagrass beds. Mar Pollut Bull. 2014;86:138–146. doi: 10.1016/j.marpolbul.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Giesen W, Van Katwijk M, Den Hartog C. Temperature, salinity, insolation and wasting disease of eelgrass (Zostera marina L.) in the Dutch Wadden Sea in the 1930's. Neth J Sea Res. 1990;25:395–404. [Google Scholar]
- Gil-Turnes MS, Hay ME, Fenical W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science. 1989;246:116. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- Gleason FH, van Ogtrop F, Lilje O, Larkum AWD. Ecological roles of zoosporic parasites in blue carbon ecosystems. Fungal Ecol. 2013;6:319–327. [Google Scholar]
- Gleason FH, Lilje O, Marano AV, Sime-Ngando T, Sullivan BK, Kirchmair M, Neuhauser S. Ecological functions of zoosporic hyperparasites. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. Degradation of pollen by phycomycetes. Ecology. 1960;41:543–545. [Google Scholar]
- Gossen BD, Deora A, Peng G, Hwang S-F, McDonald MR. Effect of environmental parameters on clubroot development and the risk of pathogen spread. Can J Plant Pathol. 2014;36:37–48. [Google Scholar]
- Gostin LO, Sapsin JW, Teret SP, Burris S, Mair JS, Hodge JG, Jr, Vernick JS. The Model State Emergency Health Powers Act: planning for and response to bioterrorism and naturally occurring infectious diseases. JAMA. 2002;288:622–628. doi: 10.1001/jama.288.5.622. [DOI] [PubMed] [Google Scholar]
- Govers LL, Man in 't Veld WA, Meffert JP, Bouma TJ, Van Rijswick PCJ, Heusinkveld JHT, Orth RJ, van Katwijk MM, van der Heide T. Marine Phytophthora species can hamper conservation and restoration of vegetated coastal ecosystems. Proc R Soc B. 2016;283 doi: 10.1098/rspb.2016.0812. 20160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers LL, Van der Zee EM, Meffert JP, Van Rijswick PCJ, Man in 't Veld WA, Heusinkveld JHT, van der Heide T. Copper treatment during storage reduces Phytophthora and Halophytophthora infection of Zostera marina seeds used for restoration. Sci Rep. 2017;7 doi: 10.1038/srep43172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granke LL, Windstam ST, Hoch HC, Smart CD, Hausbeck MK. Dispersal and movement mechanisms of Phytophthora capsici sporangia. Phytopathology. 2009;99:1258–1264. doi: 10.1094/PHYTO-99-11-1258. [DOI] [PubMed] [Google Scholar]
- Groner M, Burge C, Yang S, Van Alstyne K, Wyllie-Echeverria S, Harvell D. Identification of Demographic and Environmental Risk Factors Associated With Eelgrass Wasting Disease in the Salish Sea. 2014 [Google Scholar]
- Groner M, Breyta R, Dobson AP, Friedman CS, Froelich B, Garren M, Gulland F, Maynard J, Weil E, Wyllie-Echeverria S, Harvell D. Emergency response for marine diseases. Science. 2015;347:1210–1211. doi: 10.1126/science.347.6227.1210-a. [DOI] [PubMed] [Google Scholar]
- Groner ML, Burge CA, Kim CJ, Rees E, Van Alstyne KL, Yang S, Wyllie-Echeverria S, Harvell CD. Plant characteristics associated with widespread variation in eelgrass wasting disease. Dis Aquat Org. 2016a;118:159–168. doi: 10.3354/dao02962. [DOI] [PubMed] [Google Scholar]
- Groner ML, Maynard J, Breyta R, Carnegie RB, Dobson A, Friedman CS, Froelich B, Garren M, Gulland FM, Heron SF. Managing marine disease emergencies in an era of rapid change. Philos Trans R Soc B. 2016b;371 doi: 10.1098/rstb.2015.0364. 20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MO, Furman BT, Merello M, Durako MJ. Recurrence of Thalassia testudinum seagrass die-off in Florida Bay, USA: initial observations. Mar Ecol Prog Ser. 2016;560:243–249. [Google Scholar]
- Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. The impacts of climate change in coastal marine systems. Ecol Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Harvell C, Kim K, Burkholder J, Colwell R, Epstein PR, Grimes D, Hofmann E, Lipp E, Osterhaus A, Overstreet RM. Emerging marine diseases—climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hauxwell J, Cébrian J, Valiela I. Eelgrass (Zostera marina L.) loss in temperate estuaries: relationship to land-derived nitrogen loads and effects of light limitation imposed by algae. Mar Ecol Prog Ser. 2003;247:59–73. [Google Scholar]
- Heesterbeek H, Anderson RM, Andreasen V, Bansal S, De Angelis D, Dye C, Eames KT, Edmunds WJ, Frost SD, Funk S. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347 doi: 10.1126/science.aaa4339. aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson I, Button JB, Gudenkauf BM, Miner B, Newton AL, Gaydos JK, Wynne J, Groves CL, Hendler G, Murray M, Fradkin S, et al. Densovirus associated with sea-star wasting disease and mass mortality. Proc Natl Acad Sci U S A. 2014;111:17278–17283. doi: 10.1073/pnas.1416625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower A, Klein R. Building a geographic information system (GIS) public health infrastructure for research and control of tropical diseases. Emerg Infect Dis. 1995;1:156. doi: 10.3201/eid0104.950414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HH, Jong SC. Halophytophthora gen. nov., a new member of the family Pythiaceae. Mycotaxon. 1990;36:377–382. [Google Scholar]
- Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world's marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez C, Harvell D, Sale PF, Edwards AJ, Caldeira K, Knowlton N, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Holmer M, Bondgaard EJ. Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquat Bot. 2001;70(1):29–38. [Google Scholar]
- Jackson EL, Rowden AA, Attrill MJ, Bossey SJ, Jones MB. The importance of seagrass beds as a habitat for fishery species. Oceanogr Mar Biol. 2001;39:269–304. [Google Scholar]
- Jensen PR, Jenkins KM, Porter D, Fenical W. Evidence that a new antibiotic flavone glycoside chemically defends the sea grass Thalassia testudinum against zoosporic fungi. Appl Environ Microbiol. 1998;64:1490–1496. doi: 10.1128/aem.64.4.1490-1496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps MW. Note on a marine Labyrinthula. J Mar Biol Assoc U K (New Series) 1931;17:833–838. [Google Scholar]
- Jordà G, Marbà N, Duarte CM. Mediterranean seagrass vulnerable to regional climate warming. Nat Clim Chang. 2012;2:821–824. [Google Scholar]
- Kahn AE, Durako MJ. Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. J Exp Mar Biol Ecol. 2006;335:1–12. [Google Scholar]
- Karling JS. The Plasmodiophorales. Hafner Publishing Company; New York: 1968. [Google Scholar]
- Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, et al. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser. 2005;303:1–29. [Google Scholar]
- Koch MS, Erskine JM. Sulfide as a phytotoxin to the tropical seagrass Thalassia testudinum: interactions with light, salinity and temperature. J Exp Mar Biol Ecol. 2001;266:81–95. [Google Scholar]
- Koch MS, Schopmeyer SA, Holmer M, Madden CJ, Kyhn-Hansen C. Thalassia testudinum response to the interactive stressors hypersalinity, sulfide and hypoxia. Aquat Bot. 2007a;87:104–110. [Google Scholar]
- Koch M, Schopmeyer S, Kyhn-Hansen C, Madden C, Peters J. Tropical seagrass species tolerance to hypersalinity stress. Aquat Bot. 2007b;86:14–24. [Google Scholar]
- Koch MS, Schopmeyer SA, Nielsen OI, Kyhn-Hansen C, Madden CJ. Conceptual model of seagrass die-off in Florida Bay: links to biogeochemical processes. J Exp Mar Biol Ecol. 2007c;350:73–88. [Google Scholar]
- Koch M, Bowes G, Ross C, Zhang XH. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol. 2013;19:103–132. doi: 10.1111/j.1365-2486.2012.02791.x. [DOI] [PubMed] [Google Scholar]
- Kong P, Lea-Cox JD, Moorman GW, Hong C. Survival of Phytophthora alni, Phytophthora kernoviae, and Phytophthora ramorum in a simulated aquatic environment at different levels of pH. FEMS Microbiol Lett. 2012;332:54–60. doi: 10.1111/j.1574-6968.2012.02574.x. [DOI] [PubMed] [Google Scholar]
- Lamb JB, van de Water JA, Bourne DG, Altier C, Hein MY, Fiorenza EA, Abu N, Jompa J, Harvell CD. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science. 2017;355:731–733. doi: 10.1126/science.aal1956. [DOI] [PubMed] [Google Scholar]
- Lamers LP, Govers LL, Janssen IC, Geurts JJ, Van der Welle ME, Van Katwijk MM, Van der Heide T, Roelofs JG, Smolders AJ. Sulfide as a soil phytotoxin - a review. Front Plant Sci. 2013;4:268. doi: 10.3389/fpls.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour K. Phytophthora: A Global Perspective. CABI; Oxfordshire: 2013. [Google Scholar]
- Larkum A, Wood W. The effect of UV-B radiation on photosynthesis and respiration of phytoplankton, benthic macroalgae and seagrasses. Photosynth Res. 1993;36:17–23. doi: 10.1007/BF00018071. [DOI] [PubMed] [Google Scholar]
- Leano EM, Vrijmoed LLP, Jones EBG. Physiological studies on Halophytophthora vesicula (Straminipilous fungi) isolated from fallen mangrove leaves from Mai Po, Hong Kong. Bot Mar. 1998;41:411–419. [Google Scholar]
- Lirman D, Cropper WP. The influence of salinity on seagrass growth, survivorship, and distribution within Biscayne Bay, Florida: field, experimental, and modeling studies. Estuar Coasts. 2003;26(1):131–141. [Google Scholar]
- Loucks K, Waddell D, Ross C. Lipopolysaccharides elicit an oxidative burst as a component of the innate immune system in the seagrass Thalassia testudinum. Plant Physiol Biochem. 2013;70:295–303. doi: 10.1016/j.plaphy.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J. Auxin homeostasis, signaling, and interaction with other growth hormones during the clubroot disease of Brassicaceae. Plant Signal Behav. 2014;9:e28593. doi: 10.4161/psb.28593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man in 't Veld WA, Rosendahl KC, Brouwer H, de Cock AW. Phytophthora gemini sp. nov., a new species isolated from the halophilic plant Zostera marina in the Netherlands. Fungal Biol. 2011;115:724–732. doi: 10.1016/j.funbio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Man in 't Veld WA, Rosendahl KC, Van Rijswick PCJ, Meffert JP, Govers LL, van der Heide T, Menning D, Talbot S, Boer E, Westenberg M. Multiple Halophytophthora spp. and Phytophthora spp. including P. gemini, P. indundata and P. chesapeakensis sp. nov. isolated from the seagrass Zostera marina in the Northern Hemisphere. Eur J Plant Pathol. 2017 (in review) [Google Scholar]
- Marois JJ, Mitchell DJ. Effects of fungal communities on the pathogenic and saprophytic activities of Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology. 1981;71:1251–1256. [Google Scholar]
- Martin AC. A clue to the eelgrass mystery. Transactions of the North American Wildlife Conference; 1954. pp. 441–449. [Google Scholar]
- Martin DL, Boone E, Caldwell MM, Major KM, Boettcher AA. Liquid culture and growth quantification of the seagrass pathogen, Labyrinthula spp. Mycologia. 2009;101:632–635. doi: 10.3852/08-171. [DOI] [PubMed] [Google Scholar]
- Martin DL, Chiari Y, Boone E, Sherman TD, Ross C, Wyllie-Echeverria S, Gaydos JK, Boettcher AA. Functional, phylogenetic and host-geographic signatures of Labyrinthula spp. provide for putative species delimitation and a global-scale view of seagrass wasting disease. Estuar Coasts. 2016:1–19. [Google Scholar]
- Maxwell PS, Eklof JS, van Katwijk MM, O'Brien KR, de la Torre-Castro M, Bostrom C, Bouma TJ, Krause-Jensen D, Unsworth RK, van Tussenbroek BI, van der Heide T. The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems - a review. Biol Rev Camb Philos Soc. 2016 doi: 10.1111/brv.12294. [DOI] [PubMed] [Google Scholar]
- McKone KL, Tanner CE. Role of salinity in the susceptibility of eelgrass Zostera marina to the wasting disease pathogen Labyrinthula zosterae. Mar Ecol Prog Ser. 2009;377:123–130. [Google Scholar]
- Metcalf CJE, Graham AL, Martinez-Bakker M, Childs DZ. Opportunities and challenges of Integral Projection Models for modelling host–parasite dynamics. J Anim Ecol. 2015 doi: 10.1111/1365-2656.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlstein LK. Perspectives on the wasting disease of eelgrass Zostera marina. Dis Aquat Org. 1989;7:211–221. [Google Scholar]
- Muehlstein LK. The host–pathogen interaction in the wasting disease of eelgrass, Zostera marina. Can J Bot. 1992;70:2081–2088. [Google Scholar]
- Muehlstein L, Porter D, Short F. Labyrinthula sp., a marine slime mold producing the symptoms of wasting disease in eelgrass, Zostera marina. Mar Biol. 1988;99:465–472. [Google Scholar]
- Muehlstein LK, Porter D, Short FT. Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass, Zostera marina. Mycologia. 1991;83:180–191. [Google Scholar]
- Nakagiri A. Ecology and diversity of Halophytophthora species. In: Hyde KD, Ho WH, Pointing SB, editors. Aquatic Mycology Across the Millennium. 2000. [Google Scholar]
- Nakagiri A, Newell SY. Two new Halophytophthora species, H. tartarea and H. masteri, from intertidal decomposing leaves in saltmarsh and mangrove regions. Mycoscience. 1994;38:223–232. [Google Scholar]
- Neuhauser S, Kirchmair M, Gleason FH. The ecological potentials of Phytomyxea (“plasmodiophorids”) in aquatic food webs. Hydrobiologia. 2011;659:23–35. doi: 10.1007/s10750-010-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauser S, Kirchmair M, Bulman S, Bass D. Cross-kingdom host shifts of phytomyxid parasites. BMC Evol Biol. 2014;14:33. doi: 10.1186/1471-2148-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell SY. Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Biol Ecol. 1996;200:187–206. [Google Scholar]
- Nigrelli L, Thines M. Tropical oomycetes in the German Bight – climate warming or overlooked diversity? Fungal Ecol. 2013;6:152–160. [Google Scholar]
- Occhipinti-Ambrogi A, Savini D. Biological invasions as a component of global change in stressed marine ecosystems. Mar Pollut Bull. 2003;46:542–551. doi: 10.1016/S0025-326X(02)00363-6. [DOI] [PubMed] [Google Scholar]
- Olsen YS, Duarte CM. Combined effect of warming and infection by Labyrinthula sp. on the Mediterranean seagrass Cymodocea nodosa. Mar Ecol Prog Ser. 2015;532:101–109. [Google Scholar]
- Olsen Y, Potouroglou M, Garcias-Bonet N, Duarte C. Warming reduces pathogen pressure on a climate-vulnerable seagrass species. Estuar Coasts. 2015;38:659–667. [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- Orth RJ, Carruthers TJ, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S. A global crisis for seagrass ecosystems. Bioscience. 2006;56:987–996. [Google Scholar]
- Palacios SL, Zimmerman RC. Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar Ecol Prog Ser. 2007;344 [Google Scholar]
- Pan J, Jiang X, Li X, Cong Y, Zhang Z, Li Z, Zhou W, Han H, Luo S, Yang G. Influence of temperature and salinity on germination of eelgrass (Zostera marina) seeds. J Ocean Univ China. 2011;10:147–152. [Google Scholar]
- Pegg KG, Alcorn JL. Phytophthora operculata sp. nov., a new marine fungus. Mycotaxon. 1982;16:99–102. [Google Scholar]
- Pegg KG, Gillespie NC, Forsberg LL. Phytophthora sp. associated with mangrove death in central coastal Queensland, Australia. Plant Pathol. 1980;9:6–7. [Google Scholar]
- Pokorny KS. Labyrinthula. The Journal of protozoology. 1967;14:697–708. [Google Scholar]
- Porter D. Mycoses of Marine Organisms: An Overview of Pathogenic Fungi. Cambridge University Press; Cambridge, Great Britain: 1986. [Google Scholar]
- Ralph PJ, Short FT. Impact of the wasting disease pathogen, Labyrinthula zosterae, on the photobiology of eelgrass Zostera marina. Mar Ecol Prog Ser. 2002;226:e271. [Google Scholar]
- Rasheed MA, Unsworth RK. Long-term climate-associated dynamics of a tropical seagrass meadow: implications for the future. Mar Ecol Prog Ser. 2011;422:93–103. [Google Scholar]
- Rasmussen E. The wasting disease of eelgrass (Zostera marina) and its effects on environmental factors and fauna. In: McRoy CP, Helferrich C, editors. Seagrass Ecosystems: A Scientific Perspective. Marcel Dekker, New York: 1977. [Google Scholar]
- Rizzo DM, Garbelotto M. Sudden Oak Death: endangering California and Oregon forest ecosystems. Front Ecol Environ. 2003;1:197–204. [Google Scholar]
- Rizzo DM, Garbelotto M, Davidson JM, Slaughter GW, Koike ST. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002;86:205–214. doi: 10.1094/PDIS.2002.86.3.205. [DOI] [PubMed] [Google Scholar]
- Robblee M, Barber T, Carlson P, Jr, Durako M, Fourqurean J, Muehlstein L, Porter D, Yarbro L, Zieman R, Zieman J. Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA) Mar Ecol Prog Ser. 1991;71:297–299. [Google Scholar]
- Robideau GD, de Cock AW, Coffey MD, Voglmayr H, Brouwer H, Bala K, Chitty DW, Désaulniers N, Eggertson QA, Gachon CMM, Hu CH, et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol Ecol. 2011;11:1002–1011. doi: 10.1111/j.1755-0998.2011.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer F, Thurber RV. Viruses manipulate the marine environment. Nature. 2009;459:207. doi: 10.1038/nature08060. [DOI] [PubMed] [Google Scholar]
- Ruesink JL. Epiphyte load and seagrass performance are decoupled in an estuary with low eutrophication risk. J Exp Mar Biol Ecol. 2016;481:1–8. [Google Scholar]
- Russell BD, Connell SD, Uthicke S, Muehllehner N, Fabricius KE, Hall-Spencer JM. Future seagrass beds: can increased productivity lead to increased carbon storage? Mar Pollut Bull. 2013;73:463–469. doi: 10.1016/j.marpolbul.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Salo T, Pedersen MF, Bostrom C. Population specific salinity tolerance in eelgrass (Zostera marina) J Exp Mar Biol Ecol. 2014;461:425–429. [Google Scholar]
- Sanogo S. Response of Chile pepper to Phytophthora capsici in relation to soil salinity. Plant Dis. 2004;88:205–209. doi: 10.1094/PDIS.2004.88.2.205. [DOI] [PubMed] [Google Scholar]
- Schmeller DS, Blooi M, Martel A, Garner TW, Fisher MC, Azemar F, Clare FC, Leclerc C, Jäger L, Guevara-Nieto M. Microscopic aquatic predators strongly affect infection dynamics of a globally emerged pathogen. Curr Biol. 2014;24:176–180. doi: 10.1016/j.cub.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Short FT, Coles RG. Global Seagrass Research Methods. Elsevier; Amsterdam: 2001. [Google Scholar]
- Short FT, Neckles HA. The effects of global climate change on seagrasses. Aquat Bot. 1999;63:169–196. [Google Scholar]
- Short FT, Muehlstein LK, Porter D. Eelgrass wasting disease: cause and recurrence of a marine epidemic. Biol Bull. 1987;173:557–562. doi: 10.2307/1541701. [DOI] [PubMed] [Google Scholar]
- Short F, Ibelings BW, Den Hartog C. Comparison of a current eelgrass disease to the wasting disease in the 1930s. Aquat Bot. 1988;30:295–304. [Google Scholar]
- Short FT, Kosten S, Morgan PA, Malone S, Moore GE. Impacts of climate change on submerged and emergent wetland plants. Aquat Bot. 2016 [Google Scholar]
- Sierra R, Cañas-Duarte SJ, Burki F, Schwelm A, Fogelqvist J, Dixelius C, González-García LN, Gile GH, Slamovits CH, Klopp C, Restrepo S, et al. Evolutionary origins of Rhizarian parasites. Mol Biol Evol. 2016;33:980–983. doi: 10.1093/molbev/msv340. [DOI] [PubMed] [Google Scholar]
- Snapp SS, Shennan C, Van Bruggen AHC. Effects of salinity on severity of infection by Phytophthora parasitica Dast., ion concentrations and growth of tomato Lycopersicon esculentum Mill. New Phytol. 1991;119:275–284. doi: 10.1111/j.1469-8137.1991.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Steele LT, Caldwell M, Boettcher A, Arnold T. Seagrass-pathogen interactions: ‘pseudo-induction’ of turtlegrass phenolics near wasting disease lesions. Mar Ecol Prog Ser. 2005;303:123–131. [Google Scholar]
- Stevens NE. Environmental conditions and the wasting disease of eel-grass. Science. 1936;84:87–89. doi: 10.1126/science.84.2169.87. [DOI] [PubMed] [Google Scholar]
- Sturrock R, Frankel S, Brown A, Hennon P, Kliejunas J, Lewis K, Worrall J, Woods A. Climate change and forest diseases. Plant Pathol. 2011;60:133–149. [Google Scholar]
- Sullivan BK, Sherman TD, Damare VS, Lilje O, Gleason FH. Potential roles of Labyrinthula spp. in global seagrass population declines. Fungal Ecol. 2013;6:328–338. [Google Scholar]
- Sullivan BK, Robinson KL, Trevathan-Tackett SM, Lilje E, Gleason FH, Lilje O. The First Isolation and characterisation of the protist Labyrinthula sp. in Southeastern Australia. J Eukaryot Microbiol. 2016 doi: 10.1111/jeu.12387. [DOI] [PubMed] [Google Scholar]
- Sunday JM, Fabricius KE, Kroeker KJ, Anderson KM, Brown NE, Barry JP, Connell SD, Dupont S, Gaylord B, Hall-Spencer JM. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat Clim Chang. 2017;7:81–85. [Google Scholar]
- Suttle CA, Chan AM, Cottrell MT. Infection of phytoplankton by viruses and reduction of primary productivity. Nature. 1990;347:467–469. [Google Scholar]
- Sykes EE, Porter D. Nutritional studies of Labyrinthula sp. Mycologia. 1973:1302–1311. [Google Scholar]
- Takahashi M, Noonan S, Fabricius K, Collier C. The effects of long-term in situ CO2 enrichment on tropical seagrass communities at volcanic vents. ICES J Mar Sci. 2016;73:876–886. [Google Scholar]
- Thompson SE, Levin S, Rodriguez-Iturbe I. Rainfall and temperatures changes have confounding impacts on Phytophthora cinnamomi occurrence risk in the southwestern USA under climate change scenarios. Glob Chang Biol. 2014;20:1299–1312. doi: 10.1111/gcb.12463. [DOI] [PubMed] [Google Scholar]
- Thomsen PF, Willerslev E. Environmental DNA – An Emerging Tool in Conservation for Monitoring Past and Present Biodiversity. Special Issue: Environmental DNA: A Powerful New Tool for Biological Conservation. 2015;183:4–18. [Google Scholar]
- Touchette BW. Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. J Exp Mar Biol Ecol. 2007;350:194–215. [Google Scholar]
- Trevathan SM, Kahn A, Ross C. Effects of short-term hypersalinity exposure on the susceptibility to wasting disease in the subtropical seagrass Thalassia testudinum. Plant Physiol Biochem. 2011;49:1051–1058. doi: 10.1016/j.plaphy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Trevathan-Tackett SM. Physiology and Biochemistry of the Tropical Seagrass Thalassia testudinum in Response to Hypersalinity Stress and Labyrinthula sp. Infection. Department of Biology. University of North Florida; 2011. p. 99. [Google Scholar]
- Trevathan-Tackett SM, Lane AL, Bishop N, Ross C. Metabolites derived from the tropical seagrass Thalassia testudinum are bioactive against pathogenic Labyrinthula sp. Aquat Bot. 2015;122:1–8. [Google Scholar]
- Tsui CK, Marshall W, Yokoyama R, Honda D, Lippmeier JC, Craven KD, Peterson PD, Berbee ML. Labyrinthulomycetes phylogeny and its implications for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding. Mol Phylogenet Evol. 2009;50:129–140. doi: 10.1016/j.ympev.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Tutin TG. The autecology of Zostera marina in relation to its wasting disease. New Phytol. 1938;37:50–71. [Google Scholar]
- Van Banning P. Observations on bonamiasis in the stock of the European flat oyster, Ostrea edulis, in the Netherlands, with special reference to the recent developments in Lake Grevelingen. Aquaculture. 1991;93:205–211. [Google Scholar]
- van Katwijk MM, Vergeer LHT, Schmitz GHW, Roelofs JGM. Ammonium toxicity in eelgrass Zostera marina. Mar Ecol Prog Ser. 1997;157:159–173. [Google Scholar]
- van Katwijk MM, Bos AR, De Jonge VN, Hanssen L, Hermus DCR, De Jong DJ. Guidelines for seagrass restoration: importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Mar Pollut Bull. 2009;58:179–188. doi: 10.1016/j.marpolbul.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Vergeer LHT, Den Hartog C. Omnipresence of Labyrinthulaceae in seagrasses. Aquat Bot. 1994;48:1–20. [Google Scholar]
- Vishniac HS. The nutritional requirements of isolates of Labyrinthula spp. J Gen Microbiol. 1955;12:455–463. doi: 10.1099/00221287-12-3-455. [DOI] [PubMed] [Google Scholar]
- Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F. The second skin: ecological role of epibiotic biofilms on marine organisms. Front Microbiol. 2012;3:292. doi: 10.3389/fmicb.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Campbell J. First records of the seagrass parasite Plasmodiophora diplantherae from the northcentral Gulf of Mexico. Gulf Caribbean Res. 2009;21:63–65. [Google Scholar]
- Watson SW, Ordal EJ. Studies on Labyrinthula. University of Washington Oceanographic Laboratories; Seattle and Friday Harbor, Washington: 1951. https://digital. lib.washington.edu/researchworks/bitstream/handle/1773/15933/51-1.pdf? sequence=1. [Google Scholar]
- Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Jr, Hughes AR, Kendrick GA, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A. 2009;106:12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weste G, Marks GC. The Biology of Phytophthora cinnamomi in Australasian forests. Annu Rev Phytopathol. 1987;25:207–229. [Google Scholar]
- Woodroffe R. Managing disease threats to wild mammals. Anim Conserv. 1999;2:185–193. [Google Scholar]
- Yoshida K, Schuenemann VJ, Cano LM, Pais M, Mishra B, Sharma R, Lanz C, Martin FN, Kamoun S, Krause J. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. elife. 2013;2:e00731. doi: 10.7554/eLife.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EL. Studies on Labyrinthula. The Etiologic Agent of the Wasting Disease of Eel-Grass. Am J Bot. 1943;30:586–593. [Google Scholar]
- Zeng HC, Ho HH, Zheng FC. A survey of Phytophthora species on Hainan Island of South China. J Phytopathol. 2009;157:33–39. [Google Scholar]
- Zimmerman RC, Hill VJ, Jinuntuya M, Celebi B, Ruble D, Smith M, Cedeno T, Swingle WM. Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina. Mar Ecol Prog Ser. 2017;566:1–15. [Google Scholar]