Abstract
Purpose
Mutations in the mitochondrial enzyme succinate dehydrogenase (SDH) subunit genes are associated with a wide spectrum of tumours including phaeochromocytoma and paraganglioma (PPGL) 1, 2, gastrointestinal stromal tumours (GIST) 3, renal cell carcinoma (RCC) 4 and pituitary adenomas5. SDH-related tumorigenesis is believed to be secondary to accumulation of the oncometabolite succinate. Our aim was to investigate the potential clinical applications of MRI spectroscopy (1H-MRS) in a range of suspected SDH-related tumours.
Patients and methods
Fifteen patients were recruited to this study. Respiratory-gated single-voxel 1H-MRS was performed at 3T to quantify the content of succinate at 2.4 ppm and choline at 3.22 ppm.
Results
A succinate peak was seen in six patients, all of whom had a germline SDHx mutation or loss of SDHB by immunohistochemistry. A succinate peak was also detected in two patients with a metastatic wild-type GIST (wtGIST) and no detectable germline SDHx mutation but a somatic epimutation in SDHC. Three patients without a tumour succinate peak retained SDHB expression, consistent with SDH functionality. In six cases with a borderline or absent peak, technical difficulties such as motion artefact rendered 1H-MRS difficult to interpret. Sequential imaging in a patient with a metastatic abdominal paraganglioma demonstrated loss of the succinate peak after four cycles of [177Lu]-DOTATATE, with a corresponding biochemical response in normetanephrine.
Conclusions
This study has demonstrated the translation into clinical practice of in vivo metabolomic analysis using 1H-MRS in patients with SDH-deficient tumours. Potential applications include non-invasive diagnosis and disease stratification, as well as monitoring of tumour response to targeted treatments.
Keywords: Translational, metabolic cancer, metabolomics, hereditary, imaging technique
Introduction
The succinate dehydrogenase (SDH) enzyme is composed of four subunits (A-D) and has a key role in the Krebs cycle and oxidative phosphorylation6. In the past two decades germline mutations in the genes encoding the four SDH subunits (SDHA/SDHB/SDHC/SDHD), collectively known as SDHx have emerged as an important cause of human neoplasia and a paradigm for the role of disordered cellular metabolism in oncogenesis 1–5, 7. SDHx mutations were described initially in association with head and neck paragangliomas (derived from parasympathetic ganglia) and in phaeochromocytomas and paragangliomas (PPGL, derived from sympathetic ganglia and often secreting catecholamines) 1, 2. It is now recognised that approximately 40% of PPGL patients harbour a germline mutation in an inherited PPGL gene and SDHx mutations are the most common cause of PPGL predisposition9. In addition, germline SDHB mutations are associated with a high risk of malignancy in PPGL9. Other tumour types associated with SDHx mutations include gastrointestinal stromal tumours (GISTs) and renal cell carcinomas (RCCs)10–13. GISTs are mesenchymal tumours of the gastrointestinal tract and in adults usually associated with somatic activating mutations in the KIT or PDGFRA genes3, 11. However GISTs without KIT and PDGFRA gene mutations3, known as wild-type (wtGIST), account for 15% of adult and 85% of paediatric GIST tumours and recent studies suggest that up to 88% of wtGIST are SDH-deficient11. wtGIST with SDH-deficiency may harbour a germline SDHx mutation (75% of cases) or an SDHC gene epimutation with hypermethylation of the promoter region11. Only about a third of patients with SDH-deficient wtGIST achieve disease stabilisation with imatinib therapy12 and the risk of metastatic disease is higher for SDH-deficient GIST compared to conventional GIST11, 12. SDHx-associated RCC may present in patients with a personal or family history of PPGL or may present with an RCC-only phenotype13. Finally germline SDHx mutations have been described in rare patients with pituitary adenomas10. Despite recent advances in the understanding of the SDHx genes, there are many areas of unmet clinical need including a lack of robust biomarkers to predict aggressive biological behaviour and to inform on clinical surveillance and management14.
Succinate has been shown to be elevated by 100-fold in SDHx-mutated PPGL tumours ex-vivo compared with non-SDHx mutated PPGL tumours15. Recently, in vivo detection of succinate by MR spectroscopy was reported in two patient cohorts with SDH deficient PPGL16, 17. Similarly, the non-invasive detection of 2-hydroxyglutarate with 1H-MRS has been demonstrated in glioma in patients with a gain of function mutation in another citric acid cycle enzyme, isocitrate dehydrogenase 1 (IDH1)18. The ability to measure succinate in vivo has a number of important potential clinical applications including early identification of SDH deficiency, which can enable tailored patient surveillance and management. In vivo detection of succinate accumulation could also serve to verify genetic variant pathogenicity in the era of next generation sequencing. The aim of this study was to investigate the role of 1H-MRS in detecting abnormally elevated succinate in vivo in patients with suspected SDH deficient tumours, expanding the applications of 1H-MRS in SDH deficient tumorigenesis to include GIST and pituitary adenoma for the first time and to explore the technique as a potential non-invasive biomarker of treatment response.
Methods
Patient selection
This study was performed as a prospective case series and subjects were recruited from a dedicated neuroendocrine tumour clinic and a national paediatric and adult wild-type (PAWS) GIST clinic in Cambridge University NHS Foundation Trust. Suitable patients were identified based on SDHx germline status, suspicious clinical phenotype (metastatic PPGL, paraganglioma or wtGIST) and/or immunohistochemistry of tumour tissue showing absent SDHB immunostaining. A minimum tumour size threshold of 1.5cm was applied for inclusion into the study. All participants gave written informed consent and the study was approved by Cambridge South Research Ethics Committee.
MRS Analysis
Both SAGE (GE Healthcare, Waukesha, WI) and LCModel19 spectroscopy analysis programmes were used to reconstruct, analyze and display spectra. For each metabolite, LCModel reports both peak area and the estimated uncertainty in fitting of the peak (%SD). This uncertainty measure was used to stratify the results using the following algorithm: 1) if %SD of choline was >15%, the spectrum was discarded as a technical failure, because it was assumed that choline should be detectable in a metabolically active tumour, such that SD>15% would indicate probable data quality issues; 2) succinate detection was taken as positive if its %SD was <50%, and negative if it was >50%. The succinate to choline ratio was quantified (SCR), the full width at half maximum height (FWHM) of the water peak in Hz was measured in SAGE and recorded as an additional data quality metric, and an expert spectroscopist was asked to rate whether detected succinate peaks were convincing or unconvincing based on data displayed both in LCModel and in SAGE.
Statistical methods, 1H-MRS data acquisition, Germline genetic analysis, SDHB Immunohistochemistry, SDHC hypermethylation analysis and measurement of succinate in ex vivo tissue samples
See supplementary data.
Results
Patients and clinical phenotype
Fifteen subjects (6 females, 9 males; mean age 40 years (range 21-80 years) were studied. Seven wtGIST, three unilateral adrenal phaeochromocytomas, three abdominal PGL’s, a large left glomus PGL and a non-functioning pituitary macroadenoma were examined. Nine patients (60%) had metastatic disease: six with wtGIST, two with an abdominal paraganglioma and one with a unilateral phaeochromocytoma. The liver was the most common site for metastases (7/9, 77.7%). Three patients had multicentric primary tumours, including subject #5 who presented with a metastatic wtGIST and was subsequently diagnosed with a 1.9 cm carotid body PGL (figure 2d, case 5), subject #9 with an abdominal paraganglioma and a small left sided 1.5 cm carotid paraganglioma (figure 3b, case 9), and subject #8 with a large left sided glomus paraganglioma and a 2 cm prolactin secreting pituitary adenoma (figure S1, case 8). Only two patients had a positive family history, (Table 1: case 2 and case 6).
Figure 2.
(A) T1-weighted MR image of a metastatic GIST to the liver (arrow) from case 5. (B) SDHB immunonegativity on SDHB immunohistochemistry performed on the wt GIST tumour from the same patient. (C) Axial fused 18F-FDG PET/CT image demonstrating an FDG-avid carotid body PGL after SDH deficiency was demonstrated on 1H-MRS. (D-E) Spectra acquired at 1H-MRS from the same case before and during treatment with a multi-kinase inhibitor. (F-G) Axial fused 18F-FDG PET/CT images and corresponding coronal PET projections illustrating the increase in disease burden and FDG avidity over time (SUV: 15.1 and 27.1) which correlates with the increase in the succinate peak demonstrated on 1H-MRS.
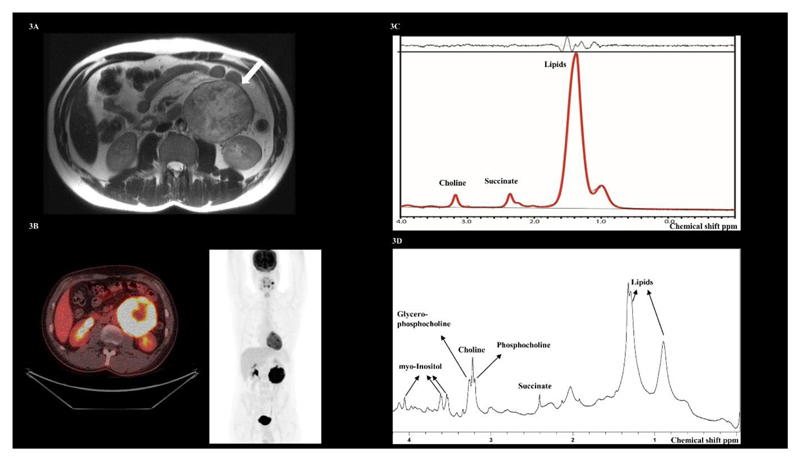
Figure 3.
(A) T2-weighted MRI showing a large non-secretory abdominal paraganglioma from case 9 (arrow). (B) 1H-MR spectra demonstrating a succinate peak at 2.4 ppm. (C) Axial fused 18F-FDG PET/CT image. The corresponding coronal maximum intensity projection (MIP) PET image demonstrates a synchronous left sided carotid paraganglioma. (D) Spectra acquired by High Resolution Magic Angle Spinning (HR-MAS) in vitro on the paraganglioma tumour sample, again confirming a succinate peak at 2.4 ppm.
Table 1. Clinical characteristics of the cohort. PA = pituitary adenoma, PC = phaeochromocytoma.
| Case number | Genetic mutation | Sex | Age | Primary tumour | Metastatic disease | Site of metastatic disease | Family history | Other primary tumour |
|---|---|---|---|---|---|---|---|---|
| 1 | SDHC epimutation | F | 21 | GIST | Yes | Liver, lung | No | No |
| 2 |
SDHB c.268C>T p.(Arg90*) |
F | 53 | Abdominal PGL | Yes | Lymph nodes, bone | Yes-mother (GIST) | No |
| 3 | SDHC epimutation | F | 25 | GIST | Yes | Liver | No | No |
| 4 | No mutation detected | F | 27 | GIST | No | NA | No | No |
| 5 |
SDHB c.137G>A p.(Arg46Gln) |
M | 38 | GIST | Yes | Liver, peritoneum | No | No |
| 6 |
SDHB c.380G>T p.(Ille127Ser) |
M | 80 | PA | No | NA | Yes nephew (PPGL) | No |
| 7 | No mutation detected | M | 70 | PC | Yes | Liver, bone | No | No |
| 8 |
SDHB c.600G>T p.(Trp200Cys) |
M | 41 | Glomus PGL | No | NA | No | Yes, PA |
| 9. |
SDHB c.302G>A p.(Cys101Tyr) |
M | 26 | Abdominal PGL | No | NA | No | Carotid PGL |
| 10. | No mutation detected | M | 23 | PC | No | NA | No | No |
| 11. |
SDHA c.91C>T p.(Arg31Ter) |
F | 21 | GIST | Yes | Liver | No | No |
| 12. |
SDHA c.1765C>T p.(Arg589Trp) |
F | 37 | GIST | Yes | Liver | No | No |
| 13 |
SDHA c.91C>T p.(Arg31Ter) |
M | 46 | PGL | Yes | Bone | No | No |
| 14 |
SDHA c.91C>T p.(Arg31Ter) |
M | 24 | GIST | Yes | Liver | No | No |
| 15 | No mutation | M | 67 | PC | No | NA | No | No |
Genotype
A germline mutation in a SDHx gene was identified in 9/15 (60%) of subjects: 5 in SDHB (4 missense variants and 1 truncating variant) and 4 in SDHA (1 missense and 3 truncating). Two further patients were diagnosed with a somatic SDHC epimutation (Table 1).
1H-MRS succinate analysis
The 1H-MRS characteristics of the 15 patients are shown in Supplementary Table S1. The mean size of the tumour selected for spectra acquisition was 5.5 cm (median: 3.3 cm, range: 1.8-12 cm). The liver was the most common site to be assessed (n = 6), but good quality spectra were also obtained from the pituitary (n = 1), and PPGL tumours (n = 5). The subjects were divided into four groups according to whether a succinate tumour peak was: present, absent, a borderline peak was detected, or technical failure prevented interpretation of the spectra.
Succinate peak detected
Succinate was detected at 2.4 ppm in 6 patients (50 %). The mean SCR in these patients was 1.3 (SD ± 0.71) and the mean tumour size in those six patients with reliable succinate peak detection was 4.8 cm (SD ± 2.94, range 2.3-9 cm). The in vivo detection of succinate on 1H-MRS correlated with tumour SDH deficiency: 4 of the 6 cases had a germline SDHx mutation and loss of SDHB expression on immunohistochemistry and a somatic SDHC epimutation was detected in 2 of the 6 (Figure 1).
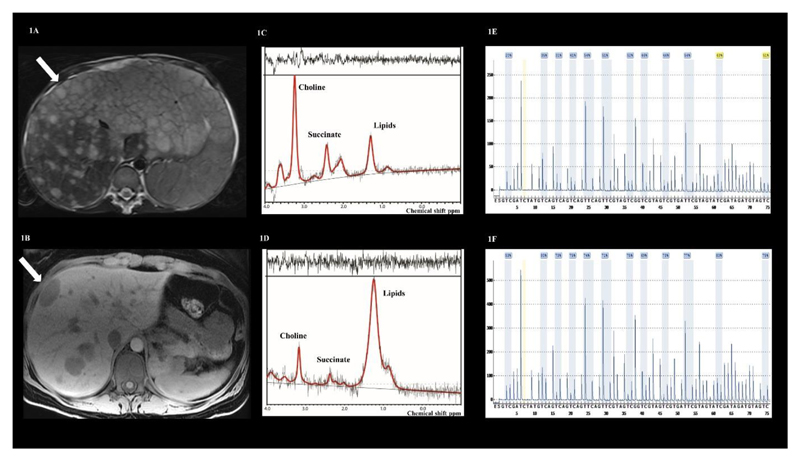
Figure 1.
(A): T2-weighted MR image from case 1 and (B) T1-weighted image from case 3 demonstrating liver metastases from which spectra were acquired in the locations indicated by the white arrows. (C-D) show the spectra from case 1 and case 3 demonstrating a succinate peak at 2.4 ppm. (E-F) demonstrate hypermethylation of the promoter region of the SDHC gene in tumour DNA from cases 1 and 3, confirming a somatic SDHC epimutation: 55% mean methylation in case 1 and 75% mean methylation in case 3.
Borderline succinate peak detected
A borderline succinate peak was detected in two subjects. Patient #8 with a germline SDHB mutation (c.600G>T p.Trp200Cys) and a glomus paraganglioma, demonstrated an SCR of 1.19; however the linewidth (29 Hz) was so broad due to the proximity of metallic dental work that the peak assignments were not reliable (Figure S1). Patient #7 with a metastatic phaeochromocytoma and no detectable germline SDHx mutation demonstrated an SCR of 0.18 but the LCModel detected a very small succinate peak at 2.4 ppm; this patient did not undergo surgery or a diagnostic biopsy and therefore no tissue was available for further analysis and therefore we have classified this case as borderline.
No succinate peak
No succinate peak was detected in three subjects. Patient #4 had a metastatic wtGIST with no detectable germline SDHx mutation and preserved SDHB protein expression in the tumour tissue; choline was confidently fitted on LCModel but no succinate was seen. Patient #6 demonstrated a good quality spectrum from the remnant pituitary adenoma; choline was detected on LCModel and SAGE processing but no succinate was detected and this finding was consistent with the preservation of SDHB protein expression in the pituitary tumour by immunohistochemistry (Figure 4). Patient #10 had no detectable germline SDHx mutation and preserved SDHB protein expression in the tumour tissue; choline was detected in the tumour on 1H-MRS but succinate was not detected.
Figure 4.
(A) Coronal T1-weighted MRI demonstrating a remnant pituitary adenoma in case 6 (white arrow). (B) Spectra acquired from the pituitary tumour at 1H-MRS, with evidence of choline detection but no succinate. (C) SDHB IHC demonstrating preservation of the SDHB protein performed on a section of tumour tissue debulked from the pituitary tumour.
Technical failure
Technical failure occurred in four patients (26%). Patient #12 demonstrated no reliable detection of succinate or choline due to motion artefact and a low signal-to-noise ratio (SNR), which was probably due to inconsistent breathing as the voxel was at the edge of the liver. A small rib metastasis was imaged in patient #13 but only a pure lipid spectrum was obtained from this challenging location. A metastasis on the edge of the liver was imaged in patient #14, where again inconsistent respiration probably led to displacement of the voxel into adjacent adipose tissue. Finally, patient #15 had a unilateral phaeochromocytoma with a large volume of blood, whose paramagnetic properties may have affected acquisition leading to low SNR (Supplementary Table S1).
Sequential 1H-MRS succinate analysis
Subject #2 with a metastatic paraganglioma to the lung, bone and lymph node and a germline SDHB mutation (c.268C>T p.Arg90*) underwent 1H-MRS on a large pelvic nodal metastasis prior to treatment with four cycles of lutetium 177-labelled peptide receptor radionuclide therapy. Succinate and choline peaks were detected with an SCR of 1.32 (Figures 5a, 5b). Following four cycles of treatment, a repeat 1H-MRS examination on the same pelvic nodal metastases revealed a choline peak but no succinate peak (Figure 5c). Though the MRI imaging features of the metastatic lesions were unchanged pre- and post-treatment, the loss of a succinate peak was correlated with a reduction in plasma normetanephrine levels (from 1861 to 1193 pmol/L) and tumour avidity on 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG-PET/CT; standard uptake value of 16.1 pre-treatment and 9.3 post-treatment; Figure 5d-f). The detection of choline on the acquired spectra both before and after treatment indicates that tumour necrosis is unlikely to account for the absent succinate peak post treatment.
Figure 5.
(A) Axial T2-weighted MRI image of a retroperitoneal nodal metastases from case 2 (arrow). (B) Spectra acquired before treatment illustrating succinate accumulation at 2.4 ppm. (C) Spectra acquired following 4 cycles of [177Lu]-DOTATATE with no detectable succinate peak at 2.4 ppm. (D) Plasma metanephrine and methoxytyramine levels before and after treatment with [177Lu]-DOTATATE. (E) Axial fused 18F-FDG PET/CT image and corresponding coronal PET projection showing the FDG-avid nodal metastases (SUV = 16.1, arrowed). (F) The same nodal metastases following treatment with [177Lu]-DOTATATE demonstrating reduced tracer uptake in keeping with the biochemical findings (SUV = 9.3).
A sequential 1H-MRS study was performed on patient #5 due to evidence of progressive disease on surveillance CT, despite treatment with a multi-kinase inhibitor, regorafenib. Serial 1H-MRS demonstrated a larger succinate peak compared to the first study (Figure 2d and 2e) and this correlated with the FDG avidity on PET/CT pre-treatment and ten months post-treatment, which demonstrated an increase in disease burden and avidity (SUV: 15.1 and 27.1 respectively, Figures 2f-g).
Repeatability of 1H-MRS was evaluated in two patients by investigating different tumour deposits during the same study examination (case#5) and the same tumour deposit twice during the same study examination (case#1). The results for succinate: choline were almost identical in these two cases, suggesting good test reproducibility (Supplementary Table 2)
Discussion
This proof-of-principle study has demonstrated that detection of a succinate peak and an increased succinate to choline ratio were specific for a variety of SDH-deficient tumour types. All six tumours with a positive succinate peak and elevated SCR were associated with a germline SDHx mutation (n = 4) or an SDHC epimutation (n = 2). In addition, the three subjects with absent succinate peaks but adequate 1H-MRS, demonstrated preservation of SDHB expression in the tumour analyzed. Our findings are complementary to a previous study in which 1H-MRS was applied to 9 patients with paraganglioma and a succinate peak was detected in all 5 with an SDHx mutation but not in the 4 patients without a mutation16. We have demonstrated for the first time that 1H-MRS can also be used to determine the SDH status of GISTs and pituitary adenomas and that a succinate peak can be detected in SDH-deficient tumours with epigenetic inactivation of SDHC. There are a wide variety of situations in which 1H-MRS might have clinical utility. Potential diagnostic applications of this new approach include: (a) assessing the pathogenicity of patients with a germline SDHx variants of uncertain significance and a potentially SDH-related tumour; (b) investigating possible metastatic lesions e.g. in the liver, in patients with a germline SDHx mutation and a primary SDH-deficient tumour; (c) assessing patients with multiple primary tumours to determine if all are SDH-deficient; (d) identifying patients without a detectable germline SDHx mutation who might benefit from specialist genetic investigations such as SDHC promoter methylation status; and (e) assessing SDH tumour status pre-operatively particularly for patients with possible wtGIST as standard adjuvant treatment with imatinib has proven to be less effective in patients with SDH-deficient disease12.
Notably, here we have used the presence of a choline signal as an internal control for viable tissue to discriminate technical failures from a negative finding. To avoid issues of partial voluming effects within smaller tumours, the voxel for MRS analysis was chosen to fully include tumour where possible. We did not detect a statistically significant correlation between tumour size and succinate/choline ratio although there was a trend towards significance. This trend is the opposite of what would be expected if necrosis was artificially lowering the overall succinate levels in large tumours, and therefore suggests that the method is measuring real differences in succinate, which are independent of tumour size. However, we recommend using a size threshold of greater than 2 cm where possible to improve the sensitivity of the test.
There is increasing interest in understanding the metabolic adaptations that occur during tumorigenesis and how these might be exploited for novel therapeutic interventions. Increased production of lactate during aerobic glycolysis in most cancers, or the Warburg effect, is the best known example of this. SDH-related cancers provides a paradigm for investigating tumour metabolism as succinate is thought to act as an oncometabolite and to drive tumorigenesis6. Succinate inhibits 2-oxoglutarate-dependent dioxygenases including DNA and histone demethylases and hypoxic gene response regulators. As a consequence, SDH-deficient tumours demonstrate epigenetic abnormalities, an activated hypoxic gene response and more recently there is evidence that succinate may have a paracrine effect on stromal tissue20, 21, 22. Understanding the molecular mechanisms of SDH-related tumorigenesis provides a rationale for novel therapeutic interventions such as reversing the epigenetic abnormalities or exploiting metabolic vulnerabilities, similar to the recent discovery that tumoral 2-hydroxyglutarate accumulation may increase responsiveness to olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor23. The availability of sensitive non-invasive biomarkers would greatly facilitate precision medicine-based clinical trials. Imaging with 18F-FDG PET to measure the uptake and phosphorylation of a glucose-analogue to probe the increased glucose utilisation that occurs in many metabolically-active cancers, is a useful form of in vivo metabolic imaging and has been employed for the detection of primary and metastatic disease in many tumour types including PPGL and GIST24, 25 and is in widespread clinical use. However, despite being a very sensitive imaging tool, 18F-FDG PET lacks specificity and cannot differentiate individual metabolites. 1H-MRS is highly specific and allows in vivo detection of individual metabolites without the use of ionising radiation, however, 1H-MRS is significantly less sensitive than PET, which could limit the detection of low levels of succinate and it can be challenging to differentiate intracellular from extracellular metabolites. In the future, 1H-MRS may be complemented by other techniques such as hyperpolarised 13C-MR spectroscopic imaging, which can increase MR signal-to-noise by several orders of magnitude allowing assessment of enzyme flux in vivo26.
We have shown that 1H-MRS could be a valuable tool for the assessment of tumour response in the context of radionuclide and other therapies as alterations in succinate levels were detected despite stable appearances of the tumour diameter. This important application of 1H-MRS could be expanded to include other tumours with specific metabolic defects including fumarate hydratase deficient tumours27, IDH1 mutant tumours28 and the recently identified malate dehydrogenase 2 (MDH2) deficient tumours29. However, important limitations of in vivo metabolomic analysis using 1H-MRS were also revealed by our study: for example, spectral quality was poor in close proximity to metal dental work, adjacent to air spaces including the lung, in bone metastases, and was susceptible to motion artefact. In this study, the technical failure rate was 26%, which is similar to the failure rate reported in previous studies using 1H-MRS16. Importantly, no cases was excluded from this prospective study, with the intention that this would inform on the translation of this imaging modality into clinical practice. Based on the evidence from this exploratory study, we would recommend that tumours were selected for 1H-MRS analysis based on: (i) ideally the largest tumour deposit but at least a size greater than 2 cm, (ii) tumours located close to bone or lung should be avoided, (iii) tumours with significant necrosis or hemorrhage should be avoided, (iv) superficial tumour deposits should be selected preferentially, and (v) respiratory triggered acquisition should be used for tumours in the upper abdomen, such as hepatic metastases. Although the use of 1H-MRS as a diagnostic tool is likely to be limited to specialist centres, the number of scan averages in our study during spectral acquisition was less than half those reported in a previous study16 (200 versus 512), without demonstrating a reduction in sensitivity. Using fewer scan averages reduces the acquisition time, making it more cost effective and convenient for the patient. This is a particularly important consideration if this imaging technique is to be considered for routine clinical practice or for sequential follow-up as part of a clinical trial. Furthermore this imaging modality could be used to investigate other metabolically-driven tumours.
In conclusion, this study is the largest to date to evaluate 1H-MRS in patients with SDH deficiency. It has revealed that 1H-MRS has the potential to be used as a non-invasive biomarker in the precision management of SDH-deficient disease and could have a role as a biomarker of successful treatment response. Lessons learned from this study could be applied to other similar metabolically-driven tumours.
Supplementary Material
Acknowledgements
The authors would like to thank Stephen Provencher for providing the simulated basis set used in spectral fitting, the radiographers and staff of the MRIS Unit at Addenbrooke’s Hospital and the staff of the Tissue Bank at Addenbroke’s hospital for assistance, and all the patients who participated in this study.
Funding:
We thank the following funding organisations; GIST Support UK (RC), Cambridge Experimental Cancer Medicine Centre, Addenbrooke’s Charitable Trust, National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre, Cancer Research UK CRUK (FAG, MM), CRUK Cambridge Centre (MM, FAG, ERM), the University of Cambridge, and Hutchison Whampoa Ltd (MM), NIHR Senior Investigator Award (ERM), European Research Council Advanced Researcher Award (ERM), the British Heart Foundation (ERM), CRUK and Engineering and Physical Sciences Research Council (EPSRC) Imaging Centre in Cambridge and Manchester (FAG). The University of Cambridge has received salary support in respect of EM from the NHS in the East of England through the Clinical Academic Reserve.
Footnotes
The authors have nothing to declare and there are no conflict of interests to report.
Disclosure: The authors have declared no conflicts of interest.
References
- 1.Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000 Feb 4;287(5454):848–51. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 3.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xekouki P, Stratakis CA. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr Relat Cancer. 2012;19:C33–C40. doi: 10.1530/ERC-12-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecchini G. Respiratory complex II: Role in cellular physiology and disease. Biochim Biophys Acta (BBA)-Bioenerg. 2013;1827:541–542. doi: 10.1016/j.bbabio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Morin A, Letouzé E, Gimenez-Roqueplo AP, et al. Oncometabolites-driven tumorigenesis: From genetics to targeted therapy. Int J Cancer. 2014 Nov 15;135(10):2237–48. doi: 10.1002/ijc.29080. [DOI] [PubMed] [Google Scholar]
- 8.Pritchett JW. Familial occurrence of carotid body tumor and pheochromocytoma. Cancer. 1982;49:2578–2579. doi: 10.1002/1097-0142(19820615)49:12<2578::aid-cncr2820491227>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. COMETE Network Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- 10.Evenepoel L, Papathomas TG, Krol N, et al. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet Med. 2015 Aug;17(8):610–20. doi: 10.1038/gim.2014.162. [DOI] [PubMed] [Google Scholar]
- 11.Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016 Jul 1;2(7):922–8. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason EF, Hornick JL. Conventional Risk Stratification Fails to Predict Progression of Succinate Dehydrogenase-deficient Gastrointestinal Stromal Tumors: A Clinicopathologic Study of 76 Cases. Am J Surg Pathol. 2016 Jun 23; doi: 10.1097/PAS.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 13.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008 Sep 3;100(17):1260–2. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 14.Amar L, Fassnacht M, Gimenez-Roqueplo AP, et al. Long-term postoperative follow-up in patients with apparently benign pheochromocytoma and paraganglioma. Hormone and Metabolic Research. 2012;44:385–389. doi: 10.1055/s-0031-1301339. [DOI] [PubMed] [Google Scholar]
- 15.Richter S, Peitzsch M, Rapizzi E, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. 2014;99:3903–11. doi: 10.1210/jc.2014-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varoquaux A, le Fur Y, Imperiale A, et al. Magnetic resonance spectroscopy of paragangliomas: new insights into in vivo metabolomics. Endocr Relat Cancer. 2015 Aug;22(4):M1–8. doi: 10.1530/ERC-15-0246. Epub 2015 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lussey-Lepoutre C, Bellucci A, Morin A, et al. In Vivo Detection of Succinate by Magnetic Resonance Spectroscopy as a Hallmark of SDHx Mutations in Paraganglioma. Clin Cancer Res. 2016 Mar 1;22(5):1120–9. doi: 10.1158/1078-0432.CCR-15-1576. Epub 2015 Oct 21. [DOI] [PubMed] [Google Scholar]
- 18.Andronesi OC, Rapalino O, Gerstner E, et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013 Sep;123(9):3659–63. doi: 10.1172/JCI67229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of a-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letouze E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Garrigue P, Bodin-Hullin A, Balasse L, et al. The evolving role of succinate in tumor metabolism: an 18F-FDG-based study. J Nucl Med. 2017 Jun 15; doi: 10.2967/jnumed.117.192674. pii: jnumed.117.192674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017 Feb 1;9(375) doi: 10.1126/scitranslmed.aal2463. pii: eaal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CA, Pattison DA, Tothill RW, et al. 68Ga-DOTATATE and 18F-FDG PET/CT in Paraganglioma and Pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging. 2016 Aug 17;16(1):22. doi: 10.1186/s40644-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holdsworth CH, Badawi RD, Manola JB, et al. CT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR Am J Roentgenol. 2007;189:W324–30. doi: 10.2214/AJR.07.2496. [DOI] [PubMed] [Google Scholar]
- 26.Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007 Nov;13(11):1382–7. doi: 10.1038/nm1650. Epub 2007 Oct 28. Erratum in: Nat Med. 2007 Dec;13(12):1521. [DOI] [PubMed] [Google Scholar]
- 27.Clark GR, Sciacovelli M, Gaude E, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014 Oct;99(10):E2046–50. doi: 10.1210/jc.2014-1659. [DOI] [PubMed] [Google Scholar]
- 28.Andronesi OC, Rapalino O, Gerstner E, et al. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013 Sep;123(9):3659–63. doi: 10.1172/JCI67229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascón A, Comino-Méndez I, Currás-Freixes M, et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst. 2015 Mar 11;107(5) doi: 10.1093/jnci/djv053. pii: djv053. [DOI] [PubMed] [Google Scholar]
- 30.Madhu B, Shaw GL, Warren AY, et al. Response of Degarelix treatment in human prostate cancer monitored by HR-MAS 1H NMR spectroscopy. Metabolomics. 2016;12:120. doi: 10.1007/s11306-016-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreasson A, Kiss NB, Caramuta S, et al. The VHL gene is epigenetically inactivated in pheochromocytomas and abdominal paragangliomas. Epigenetics. 2013 Dec;8(12):1347–54. doi: 10.4161/epi.26686. Epub 2013 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.