Abstract
Urban areas are often perceived to have lower biodiversity than the wider countryside, but a few small-scale studies suggest that some urban land uses can support substantial pollinator populations. We present a large-scale, well-replicated study of floral resources and pollinators in 360 sites incorporating all major land uses in four British cities. Using a systems approach, we developed Bayesian network models integrating pollinator dispersal and resource switching to estimate city-scale effects of management interventions on plant-pollinator community robustness to species loss. We show that residential gardens and allotments (community gardens) are pollinator ‘hotspots’: gardens due to their extensive area, and allotments due to their high pollinator diversity and leverage on city-scale plant-pollinator community robustness. Household income was positively associated with pollinator abundance in gardens, highlighting the influence of socio-economic factors. Our results underpin urban planning recommendations to enhance pollinator conservation, using increasing city-scale community robustness as our measure of success.
Introduction
Pollinators are currently the focus of international concern as numerous studies document their declines and the multiple threats they face1–5. Land use change is a major driver of pollinator declines, and urbanisation is regarded as one of the main threats to biodiversity6. However, cities can contain high levels of biodiversity for some taxa7; pollinator abundance and diversity in urban areas often compare favourably with those in agricultural and even conservation areas8–11. Urban areas are complex mosaics of different land uses and habitats12 that are likely to differ in their value for pollinators. However, studies have yet to describe urban pollinator communities fully, for three main reasons. Firstly, most studies focus on just one or a small subset of urban land uses, e.g. allotments (urban food-growing areas, also known as community gardens)13–15, cemeteries and churchyards16,17, gardens15, or parks17–19. Secondly, many studies consider only subsets of potential pollinators, typically bees, hoverflies or butterflies, rather than entire pollinator communities (e.g.13–17,20–22). Finally, most studies have limited replication, collecting data from a small number of sites13,14,18–20, often in a single city13,14,16,19–22. A more complete understanding of urban plant-pollinator biology is required for effective pollinator conservation. To achieve this, data need to be collected at a much larger scale using a well-replicated experimental design, and include all urban land uses and pollinator groups. Such ecological data are essential to identify conservation opportunities in existing urban environments and to inform actions that promote sustainable urban development.
Data on plant-pollinator interactions are also needed to estimate key parameters associated with community composition and structure. A high level of community robustness to species loss is increasingly recognised as an important goal in restoration ecology, since robust communities are better able to withstand perturbations23–25. Robustness measures a community’s vulnerability to cascading secondary extinctions following an initial loss of species26–28 and is determined by the pattern of interactions between species26. Here we use a systems approach to analyse plant-pollinator community robustness throughout the entire matrix of urban land uses in replicate cities. This allows us to make evidence-based recommendations for pollinator conservation at the scale of entire cities.
We present a multi-city assessment of all major urban land uses for all pollinator groups. We identify the most important land uses for pollinator communities in UK cities, compare floral availability between land uses, and consider the effect of a key socio-economic factor (household income) on pollinators. We also develop mathematical models that can be used to assess the contribution of different urban land uses to city-scale plant-pollinator community robustness, an approach that could be applied in the future to any landscape consisting of multiple habitats. To do this we mapped the distribution of nine major land uses in four UK cities (Bristol, Reading, Leeds and Edinburgh; Supplementary Fig. 1) and sampled ten replicate areas of each land use per city (360 sites in total) during 2012 and 2013 (sampling months April-September; see Methods section for details). Together the nine land uses - allotments, cemeteries, gardens, manmade surfaces (e.g. car parks and industrial estates), nature reserves, other greenspaces, parks, pavements (sidewalks) and road verges - comprised 72-76% of the total area per city (Supplementary Table 1), or 99% of each city once buildings, roads and water were excluded. For full descriptions of the nine land uses see Supplementary Fig. 2 and Supplementary Table 2. We collected data on plant-pollinator interactions by catching and identifying all flower-visiting insect taxa along fixed transects (2 m x 100 m transect per site), sampling 4,996 insects in the four cities during 2,160 transect walks and documenting interactions between 347 flower-visiting insect taxa (hereafter ‘pollinators’) and 326 plant taxa. The data were used to construct a quantitative plant-pollinator network for each site (360 networks in total; 90 per city). Quantitative plant-pollinator networks describe the relative frequency of observed interactions, rather than simply whether an interaction was observed between a particular plant-pollinator pair. We also quantified the floral abundance along each transect to explore the extent to which variation in floral resources explains variation in pollinator communities between urban land uses, and to identify the important floral resources for pollinators in urban areas. We developed Bayesian network models of community robustness to test the effects of management methods that could be applied to improve pollinator habitats at a city scale. These models are computationally efficient, and our application incorporates two key aspects of pollinator behaviour: dispersal and resource switching. We also examined how a socio-economic factor relates to pollinator abundance, given that socio-economic status can act as a filter for species composition within cities29. To do this we compared our data between residential neighbourhoods with different levels of household income to assess whether income correlates with pollinator abundances in residential gardens. The majority of previous studies have shown positive associations between socio-economic status and plant diversity (e.g.30), and given pollinators’ reliance on floral resources we expected pollinators to be more abundant in wealthier neighbourhoods.
Results
Abundance, occurrence and richness of pollinating insects and plants
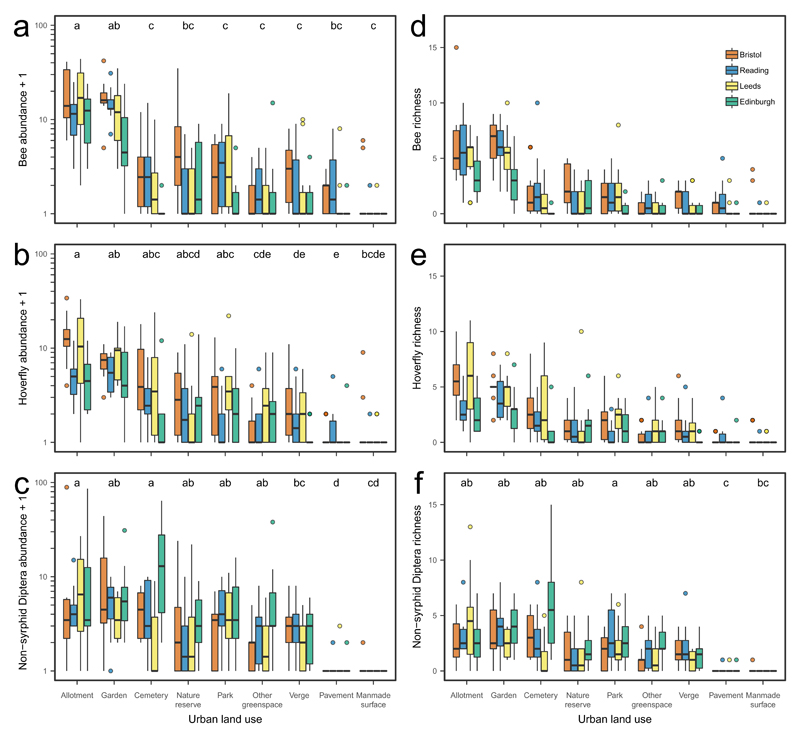
The abundance of key pollinator groups (bees, hoverflies and non-syrphid Diptera, together comprising 90% of flower-visitors) varied significantly among land uses in group-specific ways (Fig. 1; for full results for all pollinator taxa see Supplementary Tables 3 & 4). Allotments and gardens supported the highest bee and hoverfly abundances, while manmade surfaces (e.g. car parks and industrial estates) supported the lowest abundances (Fig. 1). Bees (honeybees, bumble bees and solitary bees) were significantly more abundant in allotments than in all other land uses except gardens, and more abundant in gardens than in most other land uses (Fig. 1a). Mean bee abundances were between 4 and 52 times higher in allotments and gardens than in other land uses (Supplementary Table 3). Overall, bumble bees, honey bees and solitary bees respectively comprised 62%, 24% and 14% of bees, and 20%, 8% and 4% of all pollinators collected. Bumble bees were significantly more likely to be found in allotments than in cemeteries and verges, and significantly more likely to be found in gardens than in cemeteries (Supplementary Table 4). Honey bees were more likely to be found in allotments and gardens than in cemeteries, other greenspaces and verges. Solitary bees were more likely to be found in allotments and gardens than in other greenspaces and verges (Supplementary Table 4).
Figure 1. Pollinator abundance and richness for the nine urban land uses in four cities.
Box and whisker plots of the raw data for a-c log10 (x+1) pollinator abundance, d-f pollinator richness for (a, d) bees, (b, e) hoverflies and (c, f) non-syrphid Diptera. Significantly different land uses are indicated by different letters (Tukey multiple comparisons tests). See Supplementary Tables 3-5 for GLMM results and Tukey post hoc pairwise comparisons for all pollinator groups. Plots show the median, 25th and 75th percentiles (lower and upper hinges), trimmed ranges that extend from the hinges to the lowest and highest values within 1.5× inter-quartile range of the hinge (lower and upper whiskers) plus outliers (filled circles).
For hoverfly abundance, allotments did not differ significantly from gardens, cemeteries, nature reserves or parks, although hoverfly abundance was significantly higher (4-30 times higher) in allotments and gardens than in other greenspaces, verges and pavements (Fig. 1b; Supplementary Table 3). Non-syrphid Diptera were significantly less abundant on pavements and manmade surfaces than in any other land use, and more abundant in allotments and cemeteries than on road verges (Fig. 1c).
Having controlled for variation in sample size, we found no significant differences in species richness among land uses for bees, hoverflies or any of the bee groups (bumble bees, honey bees and solitary bees), although non-syrphid Diptera showed significantly lower species richness for pavements than for most other land uses (Fig. 1d-f, Supplementary Table 5).
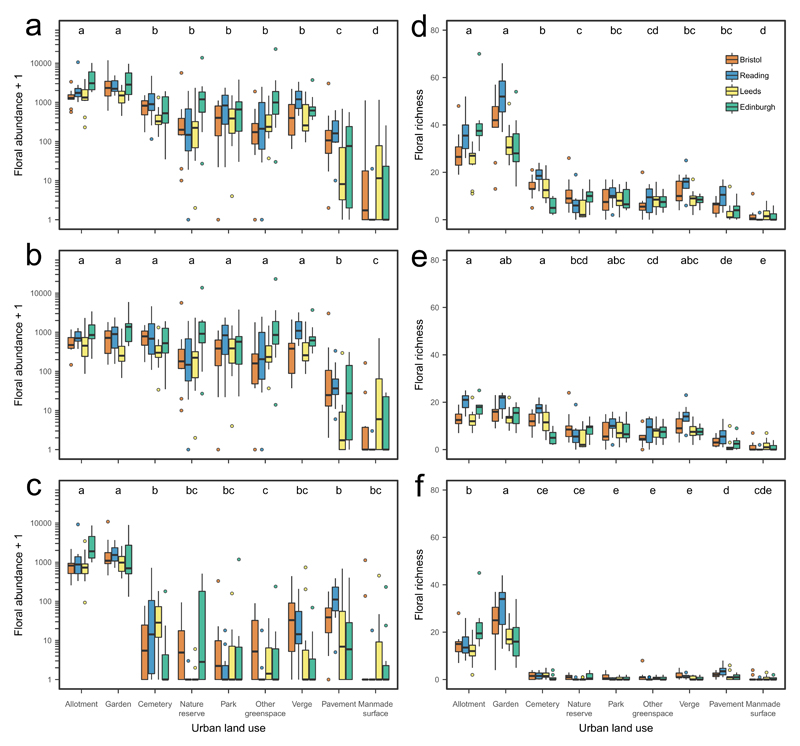
We found a significant positive effect of floral abundance on pollinator abundance and richness in all models (Fig. 2, Supplementary Tables 3-5). Floral abundance was significantly higher in allotments and gardens than in all other land uses (Fig. 2a); mean abundance was 6 to 30 times that in the poorest land uses (pavements and manmade surfaces; Supplementary Table 6). This pattern is driven by the significantly higher floral abundance of non-native plant taxa in allotments and gardens (Fig. 2c); native floral abundance did not differ significantly among most land uses (Fig. 2b). Similarly, the richness of flowering plant taxa was significantly higher in allotments and gardens than in all other land uses (Fig. 2d), a pattern caused by the higher richness of non-native taxa in allotments and gardens than in all other land uses (Fig. 2f).
Figure 2. Floral abundance and richness for the nine urban land uses in four cities.
Box and whisker plots of the raw data for a-c log10 (x+1) floral abundance, d-f floral richness for all plant taxa (a, d), native plant taxa (b, e) and non-native plant taxa (c, f). Significantly different land uses are indicated by different letters (Tukey multiple comparisons tests). See Supplementary Table 6 for GLMM results and Tukey post hoc pairwise comparisons for all analyses. Plots show the median, 25th and 75th percentiles (lower and upper hinges), trimmed ranges that extend from the hinges to the lowest and highest values within 1.5× inter-quartile range of the hinge (lower and upper whiskers) plus outliers (filled circles).
Household income level
When controlling for floral abundance, we found significantly higher pollinator abundance in gardens located in neighbourhoods with higher median household income (GLM: z= 2.170, p= 0.0299). This is consistent with the so-called ‘luxury effect’ whereby socio-economic status is often positively correlated with urban biodiversity30,31. In our case, the effect is driven by the greater quality of floral resources for pollinators in wealthier neighbourhoods. Additional models that examined the effect of household income directly on the floral data showed that both floral abundance (GLM: z=1.962, p=0.0498) and especially flowering plant species richness (GLM: z=3.118, p=0.0018) were significantly higher in gardens with higher median household income.
Plant selection by pollinating insects
Insects were recorded visiting a wide diversity of native and non-native plant taxa in all four cities. We used null models (following32) to assess which plant taxa were visited more often than expected according to their floral abundance, in order to identify which plants are disproportionately important to pollinators in urban areas (see Methods section). Fourteen plant taxa, comprising nine native and five non-native taxa, were visited significantly more often than expected in three or more cities (Table 1); a further 17 species were visited significantly more often than expected in two cities (Supplementary Tables 7 & 8). Four native species (Cirsium arvense, Heracleum sphondylium, Ranunculus repens, Taraxacum agg.) and one non-native species (Borago officinalis) were visited significantly more often than expected in all four cities. Two of the native species, Cirsium arvense and Taraxacum agg., are common urban weeds that rank highly in provision of both nectar and pollen resources to flower-visitors33,34. Three taxa (Bellis perennis, Hydrangea macrophylla, Myosotis spp.) had significantly fewer visits than expected in all four cities (Supplementary Table 8), and of these, Bellis perennis and Myosotis spp. offer low or very low pollen and nectar resources to flower visitors33,34.
Table 1. Plant species with significantly more insect visits than expected in three or more cities.
Native (n=9) and non-native (n=5) plant species which have significantly more visitors than expected based on their floral abundance according to null models. Number of observed visits is shown, followed by 95% confidence intervals from the null models in brackets. * indicates species with significantly more visits than expected, † indicates species with significantly fewer visits than expected and NR indicates the species was not included in the model for that city (due to no recorded visits or no floral abundance data). For null model results for all plant taxa in all cities see Supplementary Tables 7 and 8.
| Plant species/taxon | Common name | Bristol | Reading | Leeds | Edinburgh |
|---|---|---|---|---|---|
| Native taxa | |||||
| Cirsium arvense | Creeping thistle | 40 (0-3) * | 3 (0-2) * | 32 (0-5) * | 166 (0-2) * |
| Geum urbanum | Wood avens | 7 (0-5) * | 12 (0-5) * | 1 (1-8) | 6 (0-3) * |
| Heracleum sphondylium | Common hogweed | 18 (0-5) * | 20 (0-5) * | 9 (1-8) * | 66 (1-9) * |
| Hypochaeris radicata | Cat’s ear | 12 (0-5) * | 37 (2-11) * | 2 (0-1) * | NR |
| Leucanthemum vulgare | Ox-eye daisy | 2 (0-1) * | 11 (0-3) * | NR | 50 (0-4) * |
| Ranunculus repens | Creeping buttercup | 44 (3-14) * | 41 (2-12) * | 31 (8-22) * | 25 (5-18) * |
| Rubus fruticosus.agg. | Bramble/blackberry | 53 (2-11) * | 37 (9-23) * | 50 (29-47) * | 10 (0-6) * |
| Scorzoneroides autumnalis | Autumn hawkbit | 34 (16-32) | 13 (2-12) * | 41 (2-13) * | 1 (0-1) * |
| Taraxacum agg. | Dandelion | 56 (3-14) * | 87 (3-13) * | 92 (16-33) * | 404 (1-10) * |
| Non-native taxa | |||||
| Borago officinalis | Borage | 5 (0-3) * | 6 (0-3) * | 11 (1-9) * | 3 (0-3) * |
| Buddleja davidii | Butterfly bush | 17 (0-6) * | 8 (0-2) * | 4 (0-1) * | 1 (0-5) |
| Calendula officinalis | Common marigold | 12 (0-3) * | 12 (0-5) * | 6 (0-2) * | NR |
| Lavandula angustifolia, L. latifolia & hybrids | Lavender | 71 (11-29) * | 37 (1-10) * | 18 (2-12) * | 10 (28-47) † |
| Symphytum spp. | Comfrey | 26 (4-17) * | 17 (1-8) * | 3 (0-4) | 37 (4-15) * |
Scaling to the city level
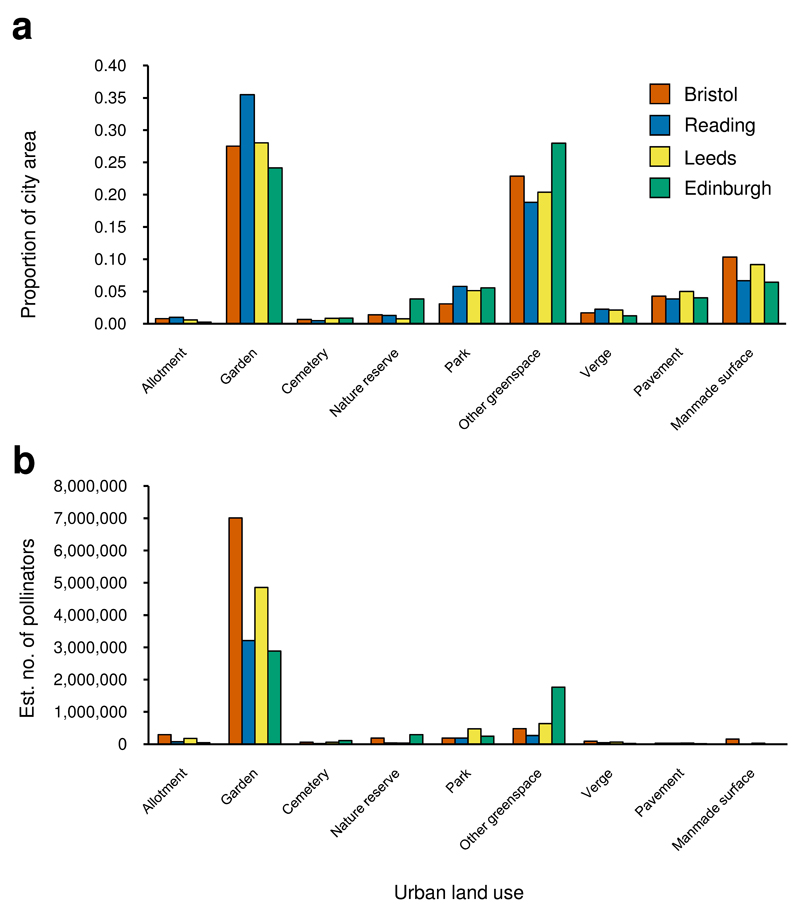
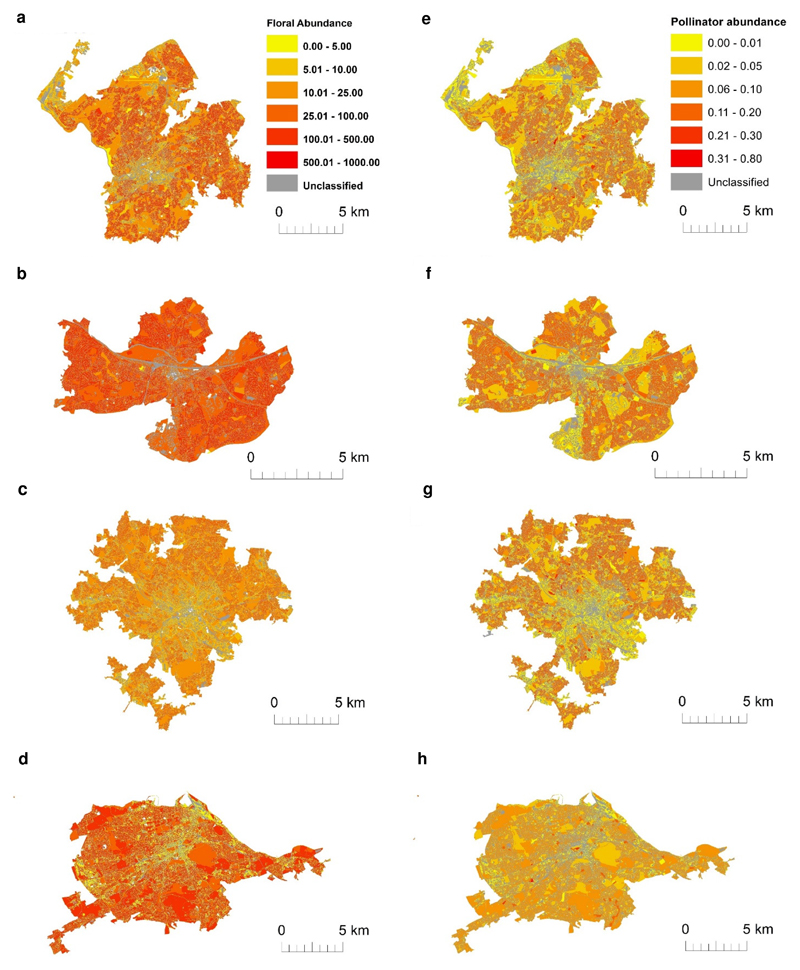
The nine land uses varied markedly in area within each city. For example, allotments comprise <1% of the four cities whereas residential gardens make up 24-36% of each city (Fig. 3a, Supplementary Table 1). However, the proportions of each land use are remarkably consistent among the four cities (Fig. 3a). Heat maps based on the data from the 90 sampling sites show substantial spatial variation in the estimated abundance of both flowers and pollinators in each city, reflecting patterns of land use composition (Fig. 4; Supplementary Figs. 3 & 4). We estimated the numbers of pollinators foraging on plants at the level of entire cities by combining abundance values per unit area for all pollinators, and specifically for bees, hoverflies and non-syrphid Diptera, with land use areas (Fig. 3b, Supplementary Fig. 5). Our estimates show that gardens contain 54-83% of pollinators in the four cities (Fig. 3b). By contrast, allotments are predicted to contain relatively few pollinators at a city scale (1-3%), as, although they host high pollinator numbers per unit area, they represent a very small component of the overall area (<1% of cities). Publicly managed greenspaces (parks, road verges and other greenspaces) comprise 27-35% of the total area across cities, but are predicted to support far fewer pollinators than gardens (which comprise 24-36% of cities), despite covering a similar area. Managing public greenspaces to benefit pollinators thus provides a clear opportunity for city-level improvement of urban areas for pollinators.
Figure 3. Land use proportions and estimated numbers of pollinators per land use at a city scale for four cities.
a, Proportions of sampled land uses and b, estimated numbers of pollinators per land use at a city scale. See Supplementary Fig. 5 for equivalent graphs for bees, hoverflies and non-syrphid Diptera. Note that in a proportions for each city do not sum to 1.00 as other non-sampled land uses (buildings, roads, railways, water) were also present; for proportions of all sampled and non-sampled land uses in each city see Supplementary Table 1.
Figure 4. Heat maps of estimated city-scale floral and pollinator abundances.
Estimated a-d floral abundances (measured as floral units per m2) and e-h pollinator abundances (individuals per m2) across the four cities. ‘Unclassified’ denotes land uses that were not sampled and comprises roads, buildings, railways and water. High resolution versions of these maps are available for download as Supplementary files (Supplementary Figs. 3 & 4). Crown copyright and database rights 2018 Ordnance Survey (100025252).
Network models and management strategies
There are two main opportunities to improve conditions for pollinators in urban areas: (i) increase the quantity of land favourable to pollinators by converting currently unfavourable land to better quality land uses (e.g. converting parks into allotments); and (ii) improve the quality of existing land through better management of current land uses for pollinators (e.g., increasing the number and quality of floral resources available in publicly managed greenspaces). We developed a modelling approach to test the impact of both strategies on the robustness of plant-pollinator communities to species loss at a city scale, with the aim of identifying management interventions which have a positive effect on plant-pollinator communities. Species loss was modelled using a method based on Bayesian networks35 that we extended to include pollinator dispersal and switching between forage plants.
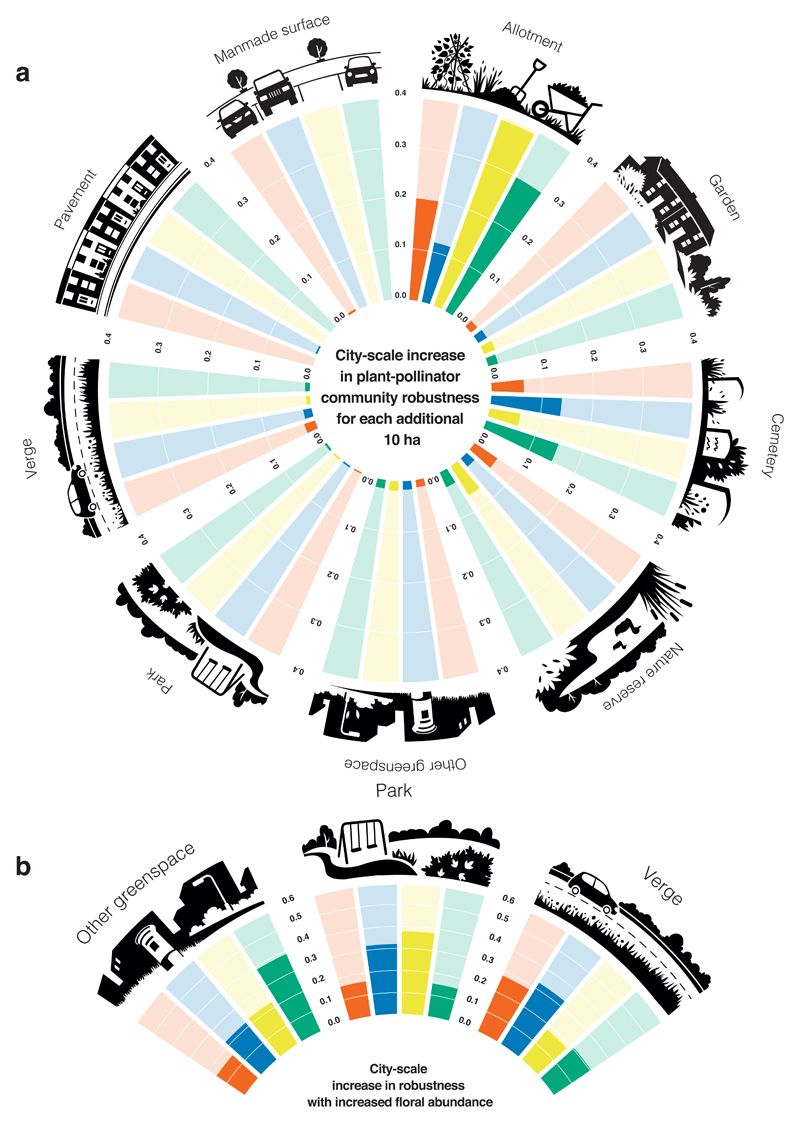
We simulated the loss of plant and pollinator species from the 90 quantitative plant-pollinator networks sampled in each city (nine land uses sampled ten times per city) and measured the robustness of the plant-pollinator communities at a city scale. We predicted the effect of increasing the area of each land use by 25%, 50% or 75% of their current totals. For ease of comparison across land uses, we express the results as changes in robustness per 10 ha increase in each land use (Fig. 5a, Supplementary Table 9). Increasing the area of allotments resulted in the greatest increase per 10 ha in city-scale robustness in three cities, and the second greatest increase after cemeteries in the remaining city (Reading; Fig. 5a). Increasing cemetery area also enhanced robustness compared to the remaining land uses in Bristol and Edinburgh (Fig. 5a). These findings are consistent across area increases of 25%, 50% and 75% (Supplementary Table 9). While adding new cemeteries to cities is rarely practical as a conservation measure, enlarging the area of allotments could be, due to their small area (1-2% of cities) and the benefits they provide for both pollinators and people36.
Figure 5. Predicted increase in city-scale plant-pollinator network robustness for two management strategies.
a, City-scale network robustness increase per 10 ha of additional land area when each land use is increased by 25% of its original area. See Supplementary Table 9 for equivalent robustness values for land use area increases of 50% and 75%. b, Maximum increase in city-scale network robustness following simulated increases in floral abundances of Bellis perennis, Taraxacum spp. and Trifolium repens for parks, other greenspaces and road verges. Bristol: red, Reading: blue, Leeds: yellow, Edinburgh: green
Given that our empirical data suggest improved management of public greenspaces holds the greatest potential for increasing pollinator habitat quality (Figs. 1 and 3), we modelled the effect of increasing three abundant and commonly visited plant species found in parks, other greenspaces and road verges in all four cities: Bellis perennis (common daisy), Taraxacum agg. (dandelion) and Trifolium repens (white clover). These plants have the added benefit of being species whose floral abundances can easily be increased by reduced mowing18, providing an easy way to implement this treatment, with the potential for reduced management costs. In simulations, we added flowers of all three plant species to each land use in turn and recorded the network robustness at saturation (i.e. when adding further flowers had no additional effect on robustness). Our model predicts that adding flowers, whether of species that were visited more often (Taraxacum agg.) or less often (Bellis perennis) than expected for their abundance in our surveys, will increase city-scale robustness for all three land uses in all cities (Fig. 5b).
Discussion
Our study demonstrates that urban land uses differ substantially in the floral resources they offer for pollinating insects, which can help inform how urban areas could be planned and managed more effectively to benefit pollinators. Urban areas are highly heterogeneous, and pollinators will move between sites based on the availability of floral and nesting resources. Therefore, conservation strategies for pollinators in urban areas need to be holistic in scope and consider the extent and diversity of urban land uses.
Allotments and gardens were visited by large numbers of pollinators (particularly bees) per unit area, although other land uses, including nature reserves, public parks and cemeteries, contained similar numbers of some taxa. Species richness did not differ between land uses for bees or hoverflies, perhaps because there is such small-scale heterogeneity of land uses in urban areas (multiple land uses can be found within a small area) and many pollinating insects can easily move between flowers in different adjacent land uses. Our findings suggest that both native and non-native plants are important for foraging pollinators in urban areas. Native plants were important food sources in all the urban land uses we sampled, while non-native plants were particularly important in areas of cultivation (allotments and gardens). The higher floral abundance and richness observed in gardens and allotments is likely to be one of the drivers of higher pollinator abundance in these land uses. Our findings highlight opportunities for pollinator conservation, such as ensuring that new housing developments contain gardens, and that new and existing gardens are managed to provide better floral resources for pollinators33,37. While city densification is considered to be beneficial for biodiversity at a large scale, in that the spread of cities may be limited (i.e. “land sharing” sensu 38), it could lead to a loss of gardens in urban areas. Our results support the concept of a “land sharing” approach to pollinator conservation in towns and cities, with gardens and urban food growing areas providing essential habitat and resources for pollinators, although this concept would need to be examined more closely as different taxa have been found to respond differently to urban densification and local context can be important39. Public greenspaces, including parks and road verges, also offer key conservation opportunities for pollinators in urban areas: they comprise large areas of cities and changing management approaches to promote increased floral resources is predicted to increase plant-pollinator community robustness at a city scale. We also show that pollinator abundance in gardens is positively associated with socio-economic status. This finding suggests that initiatives to support pollinators in lower-income neighbourhoods could help to reduce inequities in the distribution of pollinators and the delivery of pollination services within cities. These initiatives could include preferential investment of councils in greenspace enrichment in poorer areas, free seed schemes or demonstration plantings in public spaces.
If conservation organisations, land managers and policy makers are to manage biodiversity in the long term, then they need to understand the ways in which species interact across complex landscapes, since these interactions can have a profound impact on community responses to species loss, stress and ecological restoration. Robustness to species loss is rarely assessed for decision-making purposes, and wider adoption of this community-focused measure opens new evidence-based opportunities for conservation research and practice40. We extended a computationally efficient method for calculating community robustness to plant-pollinator communities by including the important context-specific mechanisms of pollinator dispersal and resource switching. Our models allow identification of key land uses that contribute most to community robustness at the level of entire systems, in this case for cities, but they could be used for any landscape consisting of multiple habitats. Our findings indicate that allotments, while small in area, are disproportionately important for plant-pollinator community robustness. Allotments have a high floral abundance and diversity as they host many weeds, in addition to flowers grown for cutting, and flowering fruit and vegetables. Allotments are also recognised as beneficial for human health and wellbeing36, while urban agriculture more generally is considered important for food security and poverty alleviation41. Thus, expanding areas cultivated for urban food growing confers multiple benefits and should be incorporated into city-level planning strategies for pollinators.
With the intention of managing for robustness more generally, adding allotments (particularly in Leeds and Edinburgh), cemeteries (Reading and Edinburgh), and nature reserves (particularly in Bristol and Leeds) would all be effective options for increasing community robustness. Land-use enhancement for pollinators through addition of floral resources achieves similar benefits in parks, other greenspaces and verges, though our modelling identified some city-specific effects that reflect variation in the make-up and quality of green spaces in different cities. For example, enhancement of parks has an especially strong impact in Leeds, while similar strong effects were revealed for enhancement of other greenspaces in Leeds and Edinburgh, and for verges in Bristol and Reading. In practice, decisions on what to manage will be constrained by how much of each land use currently exists within each city, what local development plans are in place, and what is practical. For example, adding allotments is probably simpler (and faster) than adding nature reserves, and while adding parks is expensive, improving floral resources in parks could be a cost-effective option (as mowing less can reduce costs, and all three species in our models are expected to increase in floral abundance with reduced mowing) and one which could also be popular with the human users of the park.
Results from the four cities were remarkably similar despite the four cities being geographically distant. So even though our study took place in UK cities, we expect our results to hold for other urban areas with similar land uses and management. However, we recognise that other factors (e.g. land use spatial arrangement, surrounding landscape, presence of larval host plants, availability of nesting sites) will also affect pollinator communities found in cities42, and that cities vary in their layout. That said, urbanisation is increasing globally43, and it is thus crucial to promote management strategies that support key ecosystem services, such as pollination, provided by urban biodiversity44. Furthermore, given the threats to pollinators present in farmland4, urban areas provide an increasingly important opportunity for pollinator conservation.
Methods
PART 1. Field site selection
1.1. City selection
We selected four urban areas in the UK with populations of >100,000 people, three cities (Bristol, Leeds and Edinburgh) and one large town (Reading), which are hereafter collectively referred to as cities. These cities were selected to provide good geographical coverage of the UK (Scotland, northern England, south-west England and south-east England) and for logistical reasons (they are where the four main research groups involved in the study are located).
1.2. Mapping and identification of land uses
We mapped the land uses in all four cities using ArcGIS (see Supplementary Fig. 1, Supplementary Methods). Sampling categories based on land use rather than habitat were used as these provide the basis for most management practices in urban environments. For example, urban land managers are responsible for parks, nature reserves or cemeteries, rather than grassland, heathland or woodland. Nine land use categories were selected for sampling: (1) allotments, (2) cemeteries (including churchyards and other burial grounds), (3) residential gardens (referred to as gardens), (4) manmade surfaces (impermeable surfaces not categorised as pavement or road; including car parks and industrial estates), (5) urban nature reserves (sites designated as Local Nature Reserves or Sites of Special Scientific Interest), (6) other greenspaces (including school playing fields and amenity grassland), (7) public parks (referred to as parks), (8) pavements and (9) road verges (including roundabouts). For descriptions of each land use see Supplementary Table 2. Together the nine land uses sampled comprised 72-76 % of the total area of each city and 99% of each city area excluding roads, railways, buildings and water, which could not be sampled and which (with the exception of railway verges) are very unlikely to contain flowers (Supplementary Table 1).
1.3. Site selection
Ten sampling sites were selected per land use in each city, giving 90 sites per city and 360 sites in total. Sampling sites were geographically stratified by dividing the urban area of each city into ten approximately equally sized regions, each region comprising adjacent electoral wards. One site per land use was selected in each region to provide geographical replication across each city. Sites that were too small for a 100 m transect or for which permission to sample could not be obtained were excluded. In each region, one allotment, one park, one cemetery and one nature reserve site was selected at random from all possible options. If a region did not contain a suitable site, the nearest suitable site in an adjacent region was used (5% of sites). There were only two nature reserves within the Leeds urban boundary, so multiple sampling sites were located in these two: eight sites in Middleton Woods LNR and two in Meanwood LNR. Sampling sites for verges, pavements, other greenspaces and manmade surfaces were each selected at random by choosing a random point (‘create random points’ function in ArcGIS) in each region and sampling the closest suitable site (see Supplementary Table 10 for further details on selecting sampling sites).
Since very few gardens were large enough for a 100 m transect, ten gardens in each region in each city were sampled collectively as a single unit, with each garden containing a 10 m transect. One neighbourhood was selected at random in each region using stratified random sampling to capture variation in garden size and management across a gradient of median household incomes (based on census data with five income bands per city; for more details see Supplementary Methods). All households within randomly selected neighbourhoods (89–252 households per neighbourhood) were asked for permission to sample their back garden and ten gardens for which access permission was granted were selected at random for sampling. In case a garden could not be accessed in a given sampling round, we had alternative gardens available in each neighbourhood to ensure that ten gardens could be sampled each time.
PART 2. Sampling pollinators, flowers and interactions
2.1. Transect sampling
Each site was sampled three times: twice between 14th May and 26th September 2012 and once between 15th April and 5th September 2013. Regions within cities were sampled in turn. The order in which regions were visited in each sampling round was randomly chosen subject to the following rules: (1) adjacent regions were not sampled consecutively, (2) the first five regions sampled included all five income bands, (3) regions with the same income band were not sampled consecutively.
Plants and pollinators were sampled at each site along a 100 m transect, 2 m in width. Transect locations were fixed and the same transects were sampled on all three sampling visits. Transects in gardens were split between ten individual gardens, with a 10 m transect located in each one. Sampling in gardens was stratified so that both garden edges (typically flower beds) and centres (typically lawns) were sampled: a 5 m transect was located at random along the garden edge and a second 5 m transect was located at random in the centre of the garden. Sampling in nature reserves, parks and other greenspaces was stratified to ensure that the main habitats at the site were sampled. To do this, the habitats present (broad-leaved woodland, mixed woodland, rough grassland, other grassland and heathland) were mapped, their area at the site quantified and the 100 m transect split proportionally among all habitats comprising more than 5% of a site (excluding water). Thus nature reserve, park and other greenspace sites with more than one habitat contained multiple transect locations, with a combined length of 100 m. Transect locations within a site were selected at random (see Supplementary Table 11 for details of how transect locations were selected in all land uses).
2.2. Sampling flowers
Flowers were sampled at 4 m intervals along each transect. All flowering plant species in a 1 m x 1 m quadrat were identified and the number of floral units was counted for each species. A floral unit, defined as an individual flower or collection of flowers following Baldock et al. (2015)9, comprised a single capitulum for Asteraceae, a secondary umbel for Apiaceae and a single flower for most other taxa (see Supplementary Table 12 for definitions for all plant taxa). All forbs were sampled irrespective of whether they might be wind or insect pollinated (e.g. Plantago species were included in sampling); grasses, rushes and sedges were not sampled.
2.3. Sampling pollinators
All flower-visitors (hereafter referred to as pollinators) and their interactions with flowers were quantified by walking along each transect and collecting all insects (except thrips, order Thysanoptera) visiting flowers. Collections were made up to 1 m either side of the transect line and to a height of 2 m, this including flowers in trees and bushes overhanging the transect width. Each transect was walked twice on each visit with a 10 minute gap between the two samples to allow disturbed pollinators to return. Each transect was sampled on three occasions, so that in total 2,160 transect walks, each of 100 m, were carried out in the four cities over two years (90 sites x 4 cities x 6 transect walks per site). When pollinators were highly numerous and morphologically similar and could not all be captured, a subsample was collected for identification and the remainder simply counted rather than collected (17% of insects, predominantly Coleoptera and small Diptera). Sampling for pollinators and their interactions took place between 09.00 and 17.00h on dry, warm, non-windy days spanning the activity periods of diurnally active UK pollinators45.
2.4. Plant and insect identification
All insects were identified by taxonomists (see Acknowledgements), 90% to species or morphospecies groups and the remainder to morphologically distinct genera (6%) or families (4%). The majority (90%) of plant taxa visited by insects and sampled in floral counts were identified to species. The remainder (10%; mostly apomicts and hybrids) were identified to genus level.
PART 3. Data analysis
3.1. Comparing pollinator and floral abundance and species richness among land uses
Analyses were performed using R version 3.2.046. Generalized linear mixed models (GLMM) were fitted using the R package lme447 and plots of the residuals were inspected to check the fits of all models. Post hoc Tukey tests were conducted using the multcomp package48. The effect of land use on the response variable was tested using a log-likelihood ratio test49 comparing models with and without land use included (n=360 sampling sites for all models; data for all transect walks were pooled for the three sampling visits at each site). The majority of pollinators belonged to one of three main taxonomic groups: bees (35% of recorded visits), hoverflies (Diptera; Syrphidae; 24% of recorded visits) and non-syrphid Diptera (all true flies other than hoverflies; 31% of visits). The remaining 10% of pollinators were wasps, beetles (Coleoptera) and butterflies and moths (Lepidoptera). Analyses were carried out: (i) for the whole dataset; (ii) separately for the two dominant insect orders, Diptera and Hymenoptera, (iii) separately for the subset of Hymenoptera comprising the bees (Apoidea: bumblebees, honeybees and solitary bees), and for two types of Diptera: hoverflies (Syrphidae) and non-syrphid Diptera and (iv) separately for each of the main bee groups: bumble bees, honey bees and solitary bees. Recent studies demonstrate the importance of Dipteran flower visitors and they formed a large part of our dataset50,51. Separate analyses were not carried out for wasps, Coleoptera and Lepidoptera because of small sample sizes. Pollen beetles (Nitidulidae: Brassicogethes, Kateretes or Brachypterus) were excluded from analyses as they were not observed to move between flowers; ants (Hymenoptera: Formicidae) and true bugs (Hemiptera) were excluded because they are considered unimportant as pollinators in the UK52.
(i). Pollinator abundance
We tested for effects of land use on pollinator abundance using GLMMs fitted using a negative binomial error distribution, as residuals for models fitted using a Poisson error distribution were overdispersed. Models included the fixed effects City (Bristol, Reading, Leeds, Edinburgh) and Land use (allotment, cemetery, garden, manmade surface, nature reserve, park, pavement, other greenspace and road verge), and the random effect term of Region (n=40 regions, 10 per city). Floral abundance was included to account for the variation in numbers of flowers between sites and log-transformed to meet model assumptions. Models for the whole dataset, Diptera and non-syrphid Diptera were run twice, with and without high abundance values attributed to large numbers of a scatopsid fly (Reichertellia geniculata) recorded at two Edinburgh sites. The results from models with and without the outlier values are both shown in Supplementary Table 3 and results excluding the outlier values presented in the main text.
The probability of bumblebee, solitary bee and honeybee occurrence was compared among land uses using a GLMM fitted using a binomial error distribution as we were unable to model differences in abundance with GLMMs due to high numbers of zero values in these datasets. The findings are presented in Supplementary Table 4.
(ii). Pollinator species richness
We tested for effects of land use on pollinator species richness using GLMMs fitted using a Poisson error distribution. Models were checked for overdispersion. We compared species richness for the same pollinator groups as for abundance. Models included the same fixed and random effects as for the pollinator abundance models above. Pollinator abundance (log transformed) was included as a covariate in models comparing species richness to control for sample size effects, as there is an increased chance of larger sample sizes containing higher richness. The findings are presented in Supplementary Table 5.
(iii). Floral abundance and species richness
We tested for effects of land use on floral abundance and species richness using GLMMs fitted using a negative binomial distribution. Models included the fixed effects City and Land use and the random effect term of Region. Models testing for differences in floral richness between land uses included floral abundance as a covariate to account for the variation in floral abundance. Models were run separately to test for the effect of land use on the following plant groups: (i) all plant taxa, (ii) native plant taxa and (iii) non-native plant taxa. Non-native plant taxa were defined as those categorised as ‘archeophyte’ or ‘neophyte’ according to PLANTATT53. The findings are presented in Supplementary Table 6.
3.2. Relationships between household income on pollinator abundance, floral abundance and floral richness in gardens
We tested for the effect of median household income (combined incomes of all people sharing a household; see Supplementary Methods) on pollinator abundance, floral abundance and floral richness in gardens using Generalized Linear Models (GLMs) fitted using a negative binomial distribution using the MASS package in R54. Data were pooled across the ten gardens sampled in each region, removing the need for a region-level random effect, so GLMs were used rather than GLMMs. Models included City as a factor and median household income (log transformed) as a covariate. Floral abundance (log transformed) was included in models that compared pollinator abundances to account for the variation in floral abundance among gardens. Model fit was checked using plots of the residuals.
3.3. Identifying plants that are visited disproportionately more frequently than expected
We used the resource selection null model of Vaughan et al. (2018)32 to identify flower taxa that were visited more frequently than expected based on their abundance, suggesting that they were preferred by pollinators. The model randomly reallocated the flower visits made by pollinators, with the probability of a plant taxon being visited proportional to its floral abundance. The analysis was run separately for the four cities using all of the observed pollinators (860–1352 per city) and plant species that were visited at least once (101–131 taxa): pollinators visiting plants not recorded in the accompanying floral abundance data were removed. Across all four cities, the analyses incorporated 246 of the 326 plant taxa; most taxa that were not included in analyses due to absence of floral data received very few visits (<5). Floral data were pooled within land uses separately for each sampling occasion, and pollinator visits were reallocated within each of these before combining them to produce city-level results. After 10,000 iterations of the model, 95% confidence limits for the visitation frequency to each flower taxon were estimated from the respective 2.5 and 97.5 percentiles of the frequency distributions. Using a 5% significance level, extensive tests of the null model have shown that the Type I error rate is typically < 2%32, so should have minimal impact on the results.
3.4. Scaling pollinator abundance to city level
For each city, we first combined the pollinator abundance data for the ten sites sampled for each land use. The transects sampled across the ten sites for each land use represent an area of 2,000 m2 (10 transects of 100 m x 2 m). We divided the pollinator abundance data for each land use in each city by 2,000 to give a value for the number of pollinators per m2. This was multiplied by the total area (m2) of the land use present in the city to estimate the number of pollinators present per land use per city. We repeated this calculation for (i) all pollinator taxa, (ii) bees, (iii) hoverflies and (iv) non-syrphid Diptera.
Heat maps were created from the land use maps of each city (see Supplementary Methods and Supplementary Fig. 1). Mean floral and pollinator abundances per m2 (calculated across the ten sampled sites for each land use in each city) are shown in the heat maps for all locations in each city that were not sampled directly. For each of the 90 sampled sites in each city, the floral abundance and pollinator abundance data per m2 sampled at the site are shown in the heat maps. Land uses that were not sampled for pollinators (buildings, roads, railways and water) are shown as unclassified areas in the heat maps.
PART 4. Network models of plant-pollinator community robustness
We developed a modelling approach to test the effect of different management strategies on the robustness of plant-pollinator communities at a city scale. Our models were based on quantitative networks built from the plant-pollinator interaction data collected from the 90 sites in each city. We first obtained robustness values for each site - defined as the expected proportion of pollinator species lost due to primary and secondary extinctions, averaged over all possible extinction outcomes - then summed the 90 values to give a city-scale measure of community robustness. With this definition, our value of robustness provides a measure of how a community will react to future species loss: primary extinctions represent future losses of plant and pollinator species due to both natural reasons and anthropogenic pressure, while secondary extinctions26–28 represent additional pollinator losses resulting from primary extinctions of plants that leave pollinators without any resource species. When considering the effect of management strategies on robustness, an increase in community robustness following an intervention would correspond to a decrease in expected pollinator loss due to the intervention. This logic forms the basis for our predictions of the impact of two management strategies. We computed robustness values using the Bayesian network method for secondary extinctions in food webs proposed by Eklöf et al. (2013)35, which we extended to include two important ecological mechanisms displayed by pollinators: dispersal between sites and switching between forage plants. For dispersal, we modelled the potential for pollinators in neighbouring sites to move into focal sites and mitigate the loss of pollinators caused by primary extinctions. For switching, we modelled the potential for pollinators to visit new plant species following the loss of preferred plant species caused by primary extinctions (also known as “re-wiring”27,28). Both mechanisms served to increase nominal robustness, but increases varied between sites owing to differences in plant species composition and in the surrounding land uses (in addition to inter-site variability in robustness due to different underlying quantitative network structures). See Supplementary Methods for full details of how both mechanisms were incorporated into models.
After establishing a reference value of community robustness for each city, we simulated two management strategies: (i) increasing the quantity of particular land uses and (ii) improving the quality of particular land uses. For the first strategy, we simulated the effect of changing, in turn, the city-wide coverage of the nine sampled land uses by ±25%, ±50% and ±75% of their current areas. We focus on the effects of adding, rather than removing, each land use in our models, as our aim was to assess the effect of increasing particular land uses on community robustness. The effects of removal are symmetrical though, i.e., of the same magnitude but in the opposite direction, so they are straightforward to envisage. As the total area of the different land uses varies widely, the relative increases in area are equivalent to very different increases in absolute area (in m2). To facilitate comparisons between land uses, we divided the city-scale change in robustness by the change in absolute area for each land use in turn, presenting the changes in robustness expected for an additional 10 hectares (100,000 m2) of each land use (see Supplementary Methods). For the second management strategy (increasing land use quality), we simulated the effect of increasing the floral abundances of three common and frequently visited plant species (Bellis perennis, Trifolium repens and Taraxacum agg.) in three land uses for which this would be practical (parks, other greenspaces and road verges).
For each city, we modelled 27 scenarios for the first strategy (increasing the quantity of all sampled land uses - 9 land uses x 3 area changes) and three scenarios for the second strategy (increasing the quality of three land uses - 3 land uses x 1 intervention of adding flowers). Each scenario produced a new community robustness value that was compared to the reference value for the city to determine each scenario’s relative effectiveness. Results for strategy (i) are presented in Fig. 5a and Supplementary Table 9, and those for strategy (ii) in Fig. 5b. For a complete description of the models used see Supplementary Methods.
Supplementary Material
Acknowledgements
This research was supported by the UK Insect Pollinators Initiative (IPI), funded by BBSRC, Defra, NERC, the Scottish Government and the Wellcome Trust under the auspices of the Living with Environmental Change partnership: grant BB/I00047X/1 (www.urbanpollinators.org). We thank M. Pavett, J. Deeming, B. Levey, M. Wilson, R. Morris & R. Barnett for taxonomic expertise and land owners and managers for access to sites. We thank S. Bettoni, P. Cannard, S. Cartwright, R. Comont, E. Elliot, C. Grey, P. Harris, R. Harris, B. Jarrett, K. Mikolajczak, V. Miravent, H. Morse, E. Moss, P. Ouvrard, L. Riggi, V. Radhakrishnan, D. Roumpeka, F. Sinclair, M. Stone and V. Williams for assistance with field data collection. This work is based on data provided through Ordnance Survey, Office for National Statistics, UK Data Service (EDINA UKBORDERS, and Casweb MIMAS), Natural England, Countryside Council for Wales and Scottish Natural Heritage, and uses boundary material which is copyright © of the Crown. Census output is Crown copyright and is reproduced with the permission of the Controller of HMSO and the Queen's Printer for Scotland.
Footnotes
Data availability
The data that support the findings of this study are available within the article and Supplementary Information (see Supplementary Tables 1-9 and Supplementary Data 1-5). Supplementary Data 1 contains pollinator and floral abundance and richness data that support Figures 1 and 2. Supplementary Data 2 contains data used in the socio-economic analyses. The data used in the floral null model analyses are presented in Supplementary Data 3 and the model outputs are summarised in Supplementary Tables 7 & 8. Supplementary Data 4 contains data used in Figures 3 & 4 and Supplementary Figures 3-5. Supplementary Data 5 contains data used in the robustness models.
Code availability
The modelling code used in the robustness models is available upon request from the corresponding author.
Author contributions
The study was conceived by JM and designed with input from all authors. Fieldwork was carried out by KCRB, MAG, DMH, NM, HM, LMO and KMR, with local teams supervised by JM, GNS, SGP and WEK. KCRB, IPV and PPAS carried out the analyses. KCRB and JM led the writing of the manuscript, all authors contributed to drafts of the manuscript and gave final approval for publication.
Competing interests
The authors declare no competing financial interests.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Dicks LV, et al. Ten policies for pollinators. Science. 2016;354:975–976. doi: 10.1126/science.aai9226. [DOI] [PubMed] [Google Scholar]
- 2.Potts SG, et al. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–229. doi: 10.1038/nature20588. [DOI] [PubMed] [Google Scholar]
- 3.Potts SG, Imperatriz-Fonseca VL, Ngo HT, editors. IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; 2016. [Google Scholar]
- 4.Ollerton J, Erenler H, Edwards M, Crockett R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science. 2014;346:1360–1362. doi: 10.1126/science.1257259. [DOI] [PubMed] [Google Scholar]
- 5.Knop E, et al. Artificial light as a new threat to pollination. Nature. 2017;548:206. doi: 10.1038/nature23288. [DOI] [PubMed] [Google Scholar]
- 6.Seto KC, Guneralp B, Hutyra LR. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. P Natl Acad Sci USA. 2012;109:16083–16088. doi: 10.1073/pnas.1211658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronson MFJ, et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc Lond B Biol Sci. 2014;281 doi: 10.1098/rspb.2013.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortel L, et al. Decreasing abundance, increasing diversity and changing structure of the wild bee community (Hymenoptera: Anthophila) along an urbanization gradient. PLoS ONE. 2014;9(8):e104679. doi: 10.1371/journal.pone.0104679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldock KCR, et al. Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc R Soc Lond B Biol Sci. 2015;282 doi: 10.1098/rspb.2014.2849. 20142849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall DM, et al. The city as a refuge for insect pollinators. Conserv Biol. 2017;31:24–29. doi: 10.1111/cobi.12840. [DOI] [PubMed] [Google Scholar]
- 11.Turrini T, Knop E. A landscape ecology approach identifies important drivers of urban biodiversity. Global Change Biology. 2015;21:1652–1667. doi: 10.1111/gcb.12825. [DOI] [PubMed] [Google Scholar]
- 12.Grimm NB, et al. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- 13.Matteson KC, Ascher JS, Langellotto GA. Bee richness and abundance in New York city urban gardens. Ann Entomol Soc Am. 2008;101:140–150. [Google Scholar]
- 14.Ahrne K, Bengtsson J, Elmqvist T. Bumble bees (Bombus spp) along a gradient of increasing urbanization. PLoS ONE. 2009;4(5):e5574. doi: 10.1371/journal.pone.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster G, Bennett J, Sparks T. An assessment of bumblebee (Bombus spp) land use and floral preference in UK gardens and allotments cultivated for food. Urban Ecosyst. 2017;20:425–434. [Google Scholar]
- 16.Bates AJ, et al. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE. 2011;6(8):e23459. doi: 10.1371/journal.pone.0023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Normandin E, Vereecken NJ, Buddle CM, Fournier V. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ. 2017;5:e3051. doi: 10.7717/peerj.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbuzov M, Fensome KA, Ratnieks FLW. Public approval plus more wildlife: twin benefits of reduced mowing of amenity grass in a suburban public park in Saltdean, UK. Insect Conserv Divers. 2015;8:107–119. [Google Scholar]
- 19.Geslin B, Le Féon V, Kuhlmann M, Vaissière BE, Dajoz I. The bee fauna of large parks in downtown Paris, France. Ann Soc Entomol Fr. 2016;51:487–493. [Google Scholar]
- 20.Banaszak-Cibicka W, Ratyńska H, Dylewski Ł. Features of urban green space favourable for large and diverse bee populations (Hymenoptera: Apoidea: Apiformes) Urban For Urban Gree. 2016;20:448–452. [Google Scholar]
- 21.Pauw A, Louw K. Urbanization drives a reduction in functional diversity in a guild of nectar-feeding birds. Ecol Soc. 2012;17 [Google Scholar]
- 22.Chong KY, et al. Not all green is as good: Different effects of the natural and cultivated components of urban vegetation on bird and butterfly diversity. Biol Conserv. 2014;171:299–309. [Google Scholar]
- 23.Mace GM. Whose conservation? Science. 2014;345:1558–1560. doi: 10.1126/science.1254704. [DOI] [PubMed] [Google Scholar]
- 24.Oliver TH, et al. Biodiversity and resilience of ecosystem functions. Trends Ecol Evol. 2015;30:673–684. doi: 10.1016/j.tree.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 25.de Visser SN, Freymann BP, Olff H. The Serengeti food web: empirical quantification and analysis of topological changes under increasing human impact. J Anim Ecol. 2011;80:484–494. doi: 10.1111/j.1365-2656.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunne JA, Williams RJ, Martinez ND. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol Lett. 2002;5:558–567. [Google Scholar]
- 27.Kaiser-Bunbury CN, Muff S, Memmott J, Muller CB, Caflisch A. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol Lett. 2010;13:442–452. doi: 10.1111/j.1461-0248.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 28.Staniczenko PPA, Lewis OT, Jones NS, Reed-Tsochas F. Structural dynamics and robustness of food webs. Ecol Lett. 2010;13:891–899. doi: 10.1111/j.1461-0248.2010.01485.x. [DOI] [PubMed] [Google Scholar]
- 29.Aronson MFJ, et al. Hierarchical filters determine community assembly of urban species pools. Ecology. 2016;97:2952–2963. doi: 10.1002/ecy.1535. [DOI] [PubMed] [Google Scholar]
- 30.Hope D, et al. Socioeconomics drive urban plant diversity. P Natl Acad Sci USA. 2003;100:8788–8792. doi: 10.1073/pnas.1537557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong M, Dunn RR, Trautwein MD. Biodiversity and socioeconomics in the city: a review of the luxury effect. Biol Lett. 2018;14 doi: 10.1098/rsbl.2018.0082. 20180082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan IP, et al. econullnetr: an R package using null models to analyse the structure of ecological networks and identify resource selection. Methods Ecol Evol. 2018;9:728–733. [Google Scholar]
- 33.Baude M, et al. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature. 2016;530:85–88. doi: 10.1038/nature16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks DM, et al. Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE. 2016;11:e0158117. doi: 10.1371/journal.pone.0158117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eklöf A, Tang S, Allesina S. Secondary extinctions in food webs: a Bayesian network approach. Methods Ecol Evol. 2013;4:760–770. [Google Scholar]
- 36.Wood CJ, Pretty J, Griffin M. A case–control study of the health and well-being benefits of allotment gardening. J Public Health. 2016;38:e336–e344. doi: 10.1093/pubmed/fdv146. [DOI] [PubMed] [Google Scholar]
- 37.Salisbury A, et al. Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? J Appl Ecol. 2015;52:1156–1164. [Google Scholar]
- 38.Stott I, Soga M, Inger I, Gaston KJ. Land sparing is crucial for urban ecosystem services. Front Ecol Environ. 2015;13:387–393. [Google Scholar]
- 39.Soga M, Yamaura Y, Koike S, Gaston KJ. Land sharing vs. land sparing: does the compact city reconcile urban development and biodiversity conservation? J App Ecol. 2014;51:1378–1386. [Google Scholar]
- 40.Pocock MJO, Evans DM, Memmott J. The robustness and restoration of a network of ecological networks. Science. 2012;335:973–977. doi: 10.1126/science.1214915. [DOI] [PubMed] [Google Scholar]
- 41.Orsini F, Kahane R, Nono-Womdim R, Gianquinto G. Urban agriculture in the developing world: a review. Agron Sustain Dev. 2013;33:695–720. [Google Scholar]
- 42.Lepczek CA, et al. Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience. 2017;67:799–807. [Google Scholar]
- 43.United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352) United Nations; 2014. [Google Scholar]
- 44.Aronson MFJ, et al. Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ. 2017;15:189–196. [Google Scholar]
- 45.Willmer PG, Stone GN. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv Stud Behav. 2004;34:347–466. [Google Scholar]
- 46.R Core Team. 2014. R: a language and environment for statistical computing. [Google Scholar]
- 47.Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999–2. 2013 See http://CRAN.R-project.org/package=lme4.
- 48.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 49.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith G. Mixed effects models and extensions in ecology with R. Springer: 2009. [Google Scholar]
- 50.Orford KA, Vaughan IP, Memmott J. The forgotten flies: the importance of non-syrphid Diptera as pollinators. Proc R Soc Lond B Biol Sci. 2015;282 doi: 10.1098/rspb.2014.2934. 20142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rader R, et al. Non-bee insects are important contributors to global crop pollination. P Natl Acad Sci USA. 2016;113:146–151. doi: 10.1073/pnas.1517092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willmer PG. Pollination and floral ecology. Princeton University Press; 2011. [Google Scholar]
- 53.Hill MO, Preston CD, Roy DB. PLANTATT- attributes of British and Irish plants: status, size, life history, geography and habitats. Centre for Ecology and Hydrology; 2004. [Google Scholar]
- 54.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth edn. Springer: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.