An intact intestinal epithelial barrier plays a major role in preventing intestinal invasion of luminal pathogens and antigenic molecules and their subsequent migration to the liver and, potentially, the systemic immune system. The clinical relevance of intestinal barrier dysfunction has been recognized by its pathogenic association with several disease states, ranging from inflammatory bowel disease to rheumatoid arthritis to Alzheimer’s disease, and, of direct relevance to this editorial, to alcoholic liver disease (ALD).1,2 Integrity of intestinal barrier function is regulated, in part, by the epithelial tight junction (TJ) complex composed of proteins such as zonula occludens, occludin, and claudins.3 The TJ complex establishes intestinal barrier integrity by connecting the interepithelial cell spaces and inhibiting the paracellular passage of microbes and microbial products and other luminal contents.4 However, intestinal epithelial barrier dysfunction is frequently observed in several acute and chronic enteropathic disorders, such as inflammatory bowel disease, irritable bowel syndrome, and infectious diarrhea.1
Intestinal production of proinflammatory cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)−1β, IL-6, IL-13, and the TNF superfamily member, LIGHT, have been documented to promote intestinal epithelial barrier dysfunction.5–8 Particularly, cytokine-initiated inflammatory signaling plays a major role in breaching TJ integrity.9 Although the role of cytokines and inflammatory signaling in intestinal epithelial barrier dysfunction has been well documented, the underlying mechanisms are still being defined, and potential therapeutic strategies for reestablishing intestinal barrier function are still under investigation.
Work by Chen et al. examined the role of TNF-α-induced receptor 1 (TNFR1) signaling as a critical factor in alcohol-associated loss of intestinal barrier function and the development of liver disease.10 TNF-α-plays a major pathogenic role in many diseases associated with intestinal epithelial barrier dysfunction.11 An interesting aspect of the ALD model of intestinal dysbiosis and barrier dysfunction, in comparison to other enteropathic diseases, such as inflammatory bowel disease, intestinal ischemia, and graft versus host disease, is that the damage to the epithelium is much less extensive, thereby allowing investigation of the pathogenic interaction between proinflammatory signaling and TJ integrity. The data presented in the context of the ALD model showed that chronic alcohol feeding induces intestinal inflammation in the jejunum, as indicated by an increase in TNF-α production by monocytes and macrophages and an increase in the intestinal permeability. The clinical relevance of these findings was supported by similar findings in duodenal biopsies from patients with chronic alcohol abuse. This is especially important because there are multiple experimental models of ALD (usually early stages of ALD), and it is valuable to connect animal findings to human disease.
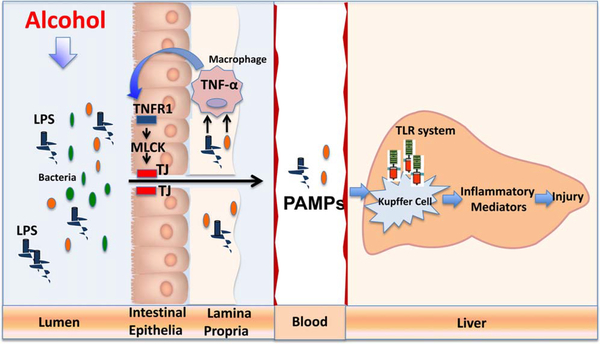
Chen et al.10 were able to specifically address and establish the causal role of TNF/TNFR1 interaction and signaling in the development of alcohol-induced barrier dysfunction and intestinal permeability in this model of ALD. Specifically, the investigators were able to demonstrate that TNFR1 mutant mice are protected from alcohol-induced intestinal barrier dysfunction and liver disease. Importantly, selective reinstatement of TNFR1 expression on intestinal epithelial cells in a TNFR1 mutant mouse carrying a conditional gain-of-function allele for this receptor caused a resumption of intestinal pathology and liver disease similar to wild-type mice. These data certainly support the notion that intestinal TNF/TNFR1 signaling plays an essential pathogenic role in alcohol-induced intestinal barrier dysfunction and subsequent development of liver disease (Fig. 1). These data also suggest that hepatic TNFR1 is not required for development of ALD in this experimental model system.
Fig. 1.
TNFR1 signaling in the intestine is necessary for subsequent development of alcohol-induced liver injury. This demonstrates the critical link between alcohol-induced intestinal inflammation and the subsequent development of alcohol-induced liver injury (gut:liver axis).
In comparison to the research presented by Chen et al., work done by Wang et al.6 investigating intestinal epithelial barrier dysfunction using an IFN-γ-primed human intestinal epithelial cell line, Caco-2 cells, showed that TNFR2, and not TNFR1, is required for TNF-dependent barrier dysfunction. Findings from these two studies indicate that the type of TNFR relevant for mediating TNF-dependent TJ disruption could be contextual and dictated by species and/or disease-type specificity. Importantly, these studies show the need for care in drawing mechanistic conclusions with regard to the development of therapeutic approaches to target inflammatory signaling for reestablishing intestinal epithelial barrier function. Significantly, TNF-mediated intestinal epithelial TNFR1 signaling in the mouse model of alcohol feeding as well as TNFR2 signaling in a human intestinal epithelial cell line both lead to myosin light chain (MLC) phosphorylation by activation of MLC kinase (MLCK) that plays a major role in the proinflammatory cytokine-induced intestinal barrier disruption.9 These data indicate that, regardless of which type of receptor is activated, TNF-dependent disruption of TJ and consequent loss of intestinal barrier function involve MLCK phosphorylation.
Work done by Chen et al.10raises some important questions regarding the mechanisms underlying the pathogenesis of ALD. Particularly, an intriguing aspect of the findings is that in TNFR1 knockout animals, restoration of TNFR1 expression only in the intestinal epithelial cells was sufficient to cause liver pathology, even in the absence of TNFR1-induced inflammatory and cytotoxic signaling in the liver. These data suggest that hepatic TNFR1 activation is not required for the initiation and perpetuation of ALD in this animal model system. Overall, the findings are significant in that they provide an improved understanding of intestinal proinflammatory signaling and malfunction of TJ integrity in the context of chronic alcohol consumption that leads to loss of intestinal epithelial barrier function and development of ALD.
These findings raise other important questions, including those related to the best approach to prevent/ treat ALD. A quarter of a century ago, we reported increased peripheral blood monocyte production of TNF in human alcoholic hepatitis.12 Chen et al. showed that intestinal monocyte TNF production is increased in experimental ALD and that TNF is increased in intestinal biopsies from human alcoholics. Is it only the intestinal TNF that is important, or is the TNF produced by the liver and systemic cells, which recycles back to activate intestinal TNFR1, also important? Chen et al. also showed that intestinal TNFR1 (but not hepatic TNFR1) was critical for the development of experimental ALD. Multiple approaches, such as antibiotics, probiotics, prebiotics, and several dietary factors, have been shown to stabilize gut-barrier function, decrease inflammation, and protect against experimental ALD. Perhaps we should be placing greater emphasis on intestinal inflammation and intestinal barrier disruption as a therapeutic target in human ALD.
Abbreviations
- ALD
alcoholic liver disease
- IFN
nterferon
- IL
nterleukin
- TJ
tight junction
- MLC
myosin light chain
- MLCK
MLC kinase
- TNF
tumor necrosis factor
- TNFR1
TNF-α-induced receptor 1.
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Salim SY,Soderholm JD. Importance of disrupted intestinal barrier ininflammatory bowel diseases. Inflamm Bowel Dis 2011;17:362–381. [DOI] [PubMed] [Google Scholar]
- 2.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013;8:e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 4.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol 2006;248:261–298. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013;70:631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006;131:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 2008;180:5653–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 2007;132:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci 2012; 1258:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates TNFRI and mediates alcoholic liver disease in mice. HEPATOLOGY 2015;61:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 2012;24:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClain CJ,. Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. HEPATOLOGY 1989;9:349–351. [DOI] [PubMed] [Google Scholar]