Abstract
Extracellular vesicles (EVs) are released by cells and contain a complex mixture of proteins, genetic information and lipids. EVs mediate cell:cell communication by transferring their molecular cargo between cells. EVs, initially discovered in mammalian systems, have been demonstrated to play critical role in immunology and cancer biology. More recently, EVs have been identified in a broad range of both unicellular and multicellular parasites. In this review we focus on the emerging roles for EVs in parasitic infections. Parasite-derived EVs can transfer virulence factors and drug-resistance markers, modify host cell gene expression and promote parasite adherence and host cell proliferation. EVs can also suppress or stimulate host immune responses. Thus, EVs are likely important in determining the outcome of parasitic infections.
Introduction
Parasitic diseases continue to have an enormous public health impact worldwide, despite global efforts for control, elimination and eradication of many of the major human parasites. Drug resistance and lack of efficacious vaccines pose formidable challenges to successful intervention. To surmount these challenges we must better understand the intrinsic capability of parasites to manipulate and evade host responses. Parasites are highly sensitized to environmental changes and capable of modulating host responses and synchronizing behavior within the population in response to such changes. Until recently, such regulated parasite-host and parasite-parasite communication was attributed to soluble parasite factors. However, recent evidence suggests that extracellular vesicles (EVs), ranging in size from 30–100 nanometers, are key players in these processes. Initially identified during red blood cell maturation, EVs are now considered major mediators of communication across all domains of life. EVs produced and secreted by parasites can fuse with both host cells and other parasites, thereby delivering protein and RNA cargo that may modulate recipient cells (Fig. 1). In this review we discuss recent progress, challenges and major questions regarding the role of EVs in human parasitic diseases.
Figure 1. Modes of EV-mediated communication.
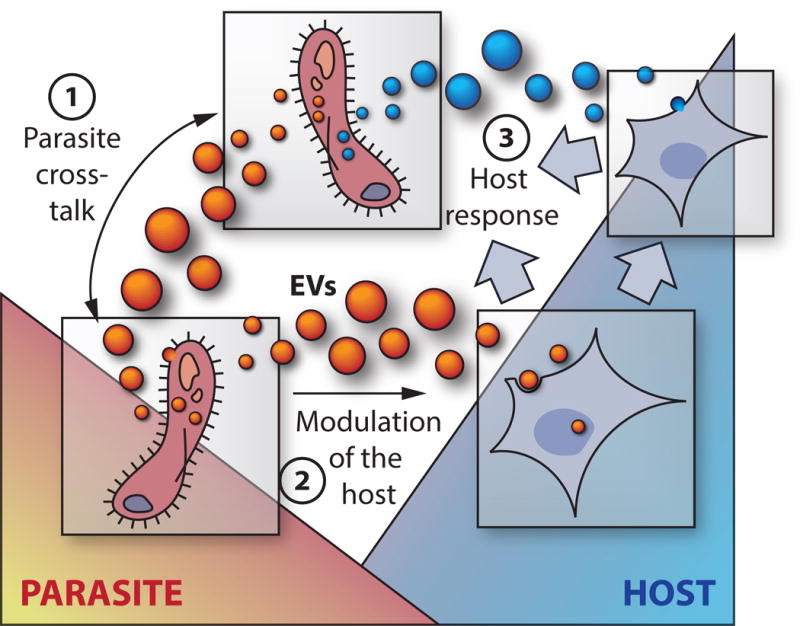
1. Parasite cross-talk. Parasite-parasite communication mediated by EVs can regulate population level behavior and the exchange of traits such as drug resistance markers (P. falciparum) or virulence factors (T. brucei). 2. Parasite-host communication. Modulation of host responses via EVs includes the activation and/or suppression of inflammatory responses, as observed in the majority of systems where parasite-derived EVs have been described. EVs can also induce host responses that facilitate specific parasite phenotypes such as increased host cell adherence (T. vaginalis) and induction of host cell proliferation, supporting growth and replication of parasitic worms. 3. Host response. Parasites can trigger the release of EVs from host cells that either promote or suppress infection. Activation of host EVs upon parasite infection include the induction of endothelial and platelet-derived EVs during malaria infection, as well as release of epithelial EVs with anti-microbial properties upon C. parvum infection.
EVs in Apicomplexa: Cellular communication and modulation of immune responses
Apicomplexa are obligate intracellular parasites that cause a variety of veterinary and human diseases, including malaria (caused by Plasmodium spp.) and cryptosporidiosis (caused by Cryptosporidium spp.). Their intracellular lifestyle poses unique challenges for signaling and communication in the parasite population and within the host.
Infection of animal and human hosts by Plasmodium parasites triggers the release of EVs from various cell types, including endothelial cells, platelets and RBCs. Such elevated EV levels are associated with severe disease both in the rodent malaria model and in malaria patients. A causal link, however, remains to be established. EVs derived from parasite-infected RBCs activate the innate immune response via pro- and anti-inflammatory cytokines in P. falciparum and P. berghei [1,2] and they may therefore contribute to the “cytokine storm” at the onset of malaria infection. In combination with the observed neutrophil activation, these EVs may also contribute to vascular activation and dysfunction, thereby promoting parasite sequestration and related pathology. Notably, recent work by El-Assad and colleagues in the rodent malaria parasite, P. berghei, also demonstrated that EVs derived from endothelial cells can cause cerebral lesions when transferred to naïve mice [3], suggesting a role for host EVs in malaria pathology.
Two studies have demonstrated the transfer of EVs between parasite-infected RBCs, suggesting existence of a cellular communication pathway within the parasite population [1,4]. In both cases, the rate of EV transfer between infected RBCs appeared to be correlated with the rate of parasite differentiation into the transmission stage, or gametocyte, suggesting quorum sensing-like mechanisms. Regev-Rudzki and colleagues [4] demonstrated the transfer of DNA encoding a drug resistance marker, suggesting that parasites may be able to exchange traits within the population in the absence of sexual recombination in the mosquito. Mantel and colleagues [1] directly demonstrated EV release and uptake and identified parasite antigens in EVs by proteomics, suggesting involvement of parasite-induced pathways in cellular communication. Recent lipidomics analysis has revealed specific lipid content of P. falciparum EVs [5]. Martin-Jaular and colleagues demonstrated in a proof-of-concept study, that the unique properties of EVs, including the presence of parasite antigens and enrichment of specific lipid species, can elicit protective immune responses in a series of EV vaccination and challenge studies in the rodent model [6].
Similar to Plasmodium, the coccidian parasite Cryptosporidium parvum triggers EV release from its epithelial host cell, and Hu and colleagues demonstrated a role in inducing inflammation against Cryptosporidium for these EVs [7]. Similarly, the coccidian parasite Toxoplasma gondii triggers release of EVs from infected cells that show a specific host mRNA and miRNA signature. However, the physiological role of coccidian-induced EVs remains to be determined [8].
Kinetoplastid EVs: Cellular communication and combating immune response
Kinetoplastids are a diverse group of free-living and parasitic flagellated protozoa that share as the common feature the kinetoplast, a network of DNA within the single mitochondrion of these cells. Kinetoplastids include several important human and veterinary parasite lineages including Leishmania spp, Trypanosoma cruzi and Trypanosoma brucei. A series of studies have identified EVs in kinetoplastids, both in the vertebrate host and the arthropod vector, and unraveled a role for kinetoplastid EVs in modulating the host immune system. The first description of EVs in the context of parasitic infection was published by Silverman and colleagues in 2008 in Leishmania donovani [9]. Here and in subsequent studies, the authors described the composition and immunomodulatory effects of L. donovani EVs [10,11], setting the stage for similar analyses in other parasites. A recent study by Atayde and colleagues has demonstrated production of Leishmania infantum EVs in the sand fly midgut, which repress the immune response and exacerbate disease outcome upon transmission to the vertebrate host [12]. Similarly, EVs were identified to be produced by Trypanosoma cruzi both in trypomastigote forms infecting humans,and in epimastigote forms in the Triatoma vector [13].
Most recently, Szempruch and colleagues identified an EV-mediated cellular communication pathway in Trypanosoma brucei, the etiological agent of human sleeping sickness [14]. Remarkably, these EVs are released from nanotubes that extend from the flagellar pocket, the major site of protein secretion in kinetoplastids. Importantly, T. brucei EVs are transferred within the parasite population via the flagellar pocket and are capable of spreading a major virulence trait: serum resistance-associated protein (SRA), allowing evasion of a host innate immune factor. Moreover, T. brucei EVs also fuse with RBC membranes and induce anemia in a mouse model, suggesting that they contribute to the pathology of animal and human trypanosomiasis.
Worm EVs: Functional miRNAs dampen host gene expression and drive cellular proliferation
Parasitic worms are the most common human infectious agents found in developing countries and are a major global health problem. The first evidence of the secretion of EVs from parasitic worms was reported by Marcilla and colleagues in 2012 [15]. Focusing on the trematodes Fasciola hepatica and Echinostoma caproni, they reported the release of EVs from liver flukes and subsequent EV incorporation into host cells in vitro. Proteomic analyses revealed that the EVs are enriched in homologs of proteins that are typically found in mammalian EVs but also contain species-specific protein cargo. A significant overlap in the protein content of the EVs with the parasites’ secretomes indicated that EVs are major contributors to trematode protein export, as also observed for Leishmania EVs [9,10]. While these studies on trematode EVs suggested a role for the vesicles in mediating host-parasite communication, no molecular or cellular modulation of host cell function was reported.
Loukas and colleagues recently demonstrated host cell internalization of EVs produced by another trematode, the carcinogenic liver fluke Opisthorchis viverrini [16]. EVs taken up by cholangiocyte epithelial cells of the bile duct were shown to drive cell proliferation and IL-6 secretion. Furthermore, internalization promoted cholangiocytes to adopt a tumorigenic phenotype and induced changes in protein expression associated with wound repair, endocytosis and cancer. These analyses indicated a role for O. viverrini EVs in promoting liver cancer and chronic periductal fibrosis. Notably, these researchers also identified the presence of EVs in the bile of infected hamsters and humans, providing the first demonstration of EV release from a multicellular parasite in infected host tissue.
Interestingly, antibodies against tetraspanin, a conserved EV membrane protein that is a candidate antigen for Schistosoma vaccine development [17], were shown to block both O. viverrini EV uptake and EV-induced IL-6 secretion [16]. These observations demonstrate a role for trematode EVs in delivering antigens to the host, suggesting utility for EVs in vaccine strategies. This is further supported by the presence of several other vaccine candidates in EVs secreted by S. mansoni [18]. If EV internalization by host cells plays a critical role in establishing parasitemia, the disruption of this process via neutralizing antibodies may explain why vaccines directed against EV membrane proteins show efficacy. Notably, this study also found a high abundance of hemolytic and heme-storage proteins involved in trematode feeding pathways, suggesting a role for EVs in nutrient acquisition [18].
The presence of small RNAs in EVs from unicellular parasites [8,10,19] led to the prediction that RNA cargo in EVs may modulate host cell gene expression by acting as miRNAs. The discovery of miRNAs with homology to mammalian miRNAs in the EVs from the trematode Dicrocoelium dendriticum [20] and the nematodes Heligmosomoides polygyrus and Litomosoides sigmodontis) [21] reinforced these predictions. H. polygyrus EVs were also shown to contain Argonaute, a protein known to be essential for the down-regulation of gene expression via miRNA pathways [22]. A direct role for EV-derived miRNA modulation of host cell pathways was demonstrated by microarray analyses, which revealed the down-regulation of mouse genes predicted to be targets of the worm miRNAs upon incubation of mouse cells with H. polygyrus EVs in vitro. L. sigmodontis miRNAs were also found in sera of infected mice pointing to the secretion of these miRNAs during infection. In addition to providing a possible mechanism for RNA transfer between worms and their mammalian hosts, this study demonstrated a role for H. polygyrus EVs in the suppression of Type 2 innate immune response and eosinophilia in mice [21]. These data indicate a role for H. polygyrus EVs in down-regulating host cell immune responses that would otherwise lead to parasite clearance. Modulation of host cell immune responses by S. japonicum EVs was also demonstrated recently [23].
Trichomonas vaginalis EVs: Host parasite interactions and immune modulation
The sexually transmitted parasite Trichomonas vaginalis secretes EVs containing proteins and small RNAs typically found in mammalian and other parasitic EVs. T. vaginalis EVs were the first parasite-derived EVs directly shown to deliver protein cargo into host cells in vitro [19]. T. vaginalis EVs were also demonstrated to increase parasite adherence to host cells in vitro, indicating a role for EVs in promoting host colonization by this extracellular parasite. Interestingly, EVs from T. vaginalis strains that are highly adherent to host epithelial cells could confer enhanced adherence properties, when incubated with either host cells or a poorly adherent parasite strain. Similarly, EVs from T. vaginalis strains with a preference for binding prostate (relative to vaginal) epithelial cells can transfer this phenotype to a T. vaginalis strain not previously exhibiting preferential binding. Thus, T. vaginalis EVs have the potential to mediate both parasite-parasite and parasite-host interactions, via mechanisms yet to be deciphered. Similar to Leishmania [11] and helminth [16] EVs, T. vaginalis EVs also modulate host immune responses, specifically by inducing an IL-6 response and dampening the IL-8 response. Reducing secretion of IL-8, a key cytokine for neutrophil recruitment, by EVs may be critical in establishing infection, as neutrophils are the front line of defense against this parasite. T. vaginalis EVs are thus likely to enhance colonization of the host by increasing both parasite adherence to epithelial cells of the urogenital tract and reducing parasite clearance by remodeling the immune response.
Summary/Conclusion
EVs mediate host-parasite and parasite-parasite communication (Fig. 1) via an impressive array of strategies that result in wide-ranging outcomes. Specific interactions are influenced by physiological context and vary depending on the type of parasite and host cells involved in the dialogue. EVs mediate the transfer of parasite cargo, including proteins, nucleic acids and lipids, to host cells. The transfer of parasite (or host) miRNAs can modulate host cell gene expression, and protein transfer of virulence traits may lead to evasion of host innate immunity.
In many cases studied so far, parasite-derived EVs have been shown to suppress host immune responses, which in turn, may promote parasite survival. In different environments EVs can activate pro-inflammatory cytokines to prime immune cells. EVs can also increase adherence of parasites to host cells, thereby enhancing colonization and sequestration of parasite-infected host cells and contributing to pathological outcomes. EVs affect parasite population dynamics by influencing population dissemination and can drive the developmental switch from one life stage to another.
Studies of parasitic EVs are in their infancy and have primarily been descriptive. The molecular mechanisms underlying the formation and the packaging of selective cargo, as well as the budding and fusion of EVs are unknown. Are specific host receptors required for EV docking and fusion? If not, how are EVs targeted to specific cell types? The full array of EV function in parasite infections also remains uncharacterized. Although several physiological roles influencing pathogenesis have been revealed, these are likely the tip of the iceberg. Uncovering the full extend of EVs on infective processes is predicted to expose additional physiological roles and may provide insight for the use of EVs in therapeutic and vaccination strategies.
Highlights.
Parasites secrete extracellular vesicles (EVs) that mediate cellular communication
EVs can transfer virulence factors, drug-resistance markers & modify host response
microRNAs contained in EVs have been shown to down-regulate host cell proteins
EVs can support parasite growth as well as host cell proliferation
Most described parasite EVs either suppress or stimulate the host immune response
Acknowledgments
We thank Drs. Frances Mercer and Nicolas Brancucci for helpful comments on the manuscript and preparation of the figure. This work was conducted with the support of the National Institutes of Health grants AI103182 and AI105779 (to PJJ) and an Investigator Award in Pathogenesis of Infectious Diseases from the Burroughs Wellcome Fund (to MM).”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
of special interest *
of outstanding interest **
- 1.Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell host & microbe. 2013;13:521–534. doi: 10.1016/j.chom.2013.04.009. A systematic analysis of EVs released from infected red blood cells during Plasmodium infection identifies a link between EV concentration and stage conversion, at least under controlled in vitro conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS pathogens. 2010;6:e1000744. doi: 10.1371/journal.ppat.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Assaad F, Wheway J, Hunt NH, Grau GE, Combes V. Production, fate and pathogenicity of plasma microparticles in murine cerebral malaria. PLoS Pathog. 2014;10:e1003839. doi: 10.1371/journal.ppat.1003839. This comprehensive EV investigation in the mouse malaria model demonstrates that adoptive transfer of endothelial cell-derived EVs from infected mice induces pathology in naïve mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. The companion paper to Ref 1 provides independent evidence for EV-mediated stage-conversion and DNA transfer in P. falciparum. Together these 2 papers provide a rationale for future studies of quorum sensing-like mechanisms and transfer of genetic traits in P. falciparum. [DOI] [PubMed] [Google Scholar]
- 5.Gulati S, Ekland EH, Ruggles KV, Chan RB, Jayabalasingham B, Zhou B, Mantel PY, Lee MC, Spottiswoode N, Coburn-Flynn O, et al. Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe. 2015;18:371–381. doi: 10.1016/j.chom.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PloS one. 2011;6:e26588. doi: 10.1371/journal.pone.0026588. This is a first proof-of-concept study demonstrating the protective effect of EVs in the mouse malaria model, supporting the idea of EV-based vaccination strategies in Plasmodium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013;9:e1003261. doi: 10.1371/journal.ppat.1003261. This study identified a set of host EVs released during Cryptosporidium parvum infection with anti-parasitic activity. The findings demonstrate that TLR signaling regulates EV release and suggest existence of a new arm of mucosal immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope SM, Lasser C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, Reiner NE. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008;9:R35. doi: 10.1186/gb-2008-9-2-r35. This analysis of the secretome of Leishmania donovani found the majority of known eukaryotic exosomal (EV) proteins. These proteins were also shown to lack a classic eukaryotic amino-terminal signal peptide. Together, this study provided the first molecular data indicating the production and secretion of EVs in a parasite system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 11.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. 2010;185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 12.Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell Rep. 2015;13:957–967. doi: 10.1016/j.celrep.2015.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res. 2013;12:883–897. doi: 10.1021/pr300947g. [DOI] [PubMed] [Google Scholar]
- 14.Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell. 2016;164:246–257. doi: 10.1016/j.cell.2015.11.051. In this elegant study the authors provide in vitro and in vivo evidence that EVs mediate virulence factor transfer within African trypanosome parasite populations and induce anemia in mice by altering red blood cell properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sanchez del Pino MM, Munoz-Antoli C, Toledo R, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7:e45974. doi: 10.1371/journal.pone.0045974. These authors provided the first evidence of secretion of EVs by parasitic worms in studies on the trematodes Faciola and Echinostoma. Microscopic analyses suggesting the EVs were incorporation into host cells was also reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS, Turnbull L, Whitchurch CB, Potriquet J, Laohaviroj M, et al. Carcinogenic Liver Fluke Secretes Extracellular Vesicles That Promote Cholangiocytes to Adopt a Tumorigenic Phenotype. J Infect Dis. 2015;212:1636–1645. doi: 10.1093/infdis/jiv291. Using the carcinogenic liver fluke Opisthorchis viverrini, the authors published the first demonstration of roles for parasitic EVs in wound repair and cancer. These studies were also the first to demonstrate the presence of EVs from a multicellular parasite in infected host tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, Loukas A. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. 2016;46:1–5. doi: 10.1016/j.ijpara.2015.09.002. This study on the proteome of EVs secreted by the most deadly human pathogenic worm, Schistosoma mansoni, found an abundance of proteins involved in trematode feeding pathways, providing the first evidence for a role for EVs in nutrient acquisition. Data implying a role for S. mansoni EVs in presenting candidate vaccine antigens to the host were also reported. [DOI] [PubMed] [Google Scholar]
- 19.Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. 2013;9:e1003482. doi: 10.1371/journal.ppat.1003482. This work on the extracellular parasite Trichomonas vaginalis was the first molecular to demonstrate the delivery of a parasite protein into a host cell in vitro. The ability of EVs to modulate parasite adherence and host cell preference thus possibly aiding in host colonization was also shown. The dampening of IL8 responses, the key cytokine that recruits neutrophils, the front line of defense against T. vaginalis, also suggested a role for EVs in combatting parasite clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernal D, Trelis M, Montaner S, Cantalapiedra F, Galiano A, Hackenberg M, Marcilla A. Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. J Proteomics. 2014;105:232–241. doi: 10.1016/j.jprot.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. In a series of elegant analyses, the authors demonstrate for the first time the modulation of host cell pathways via EV miRNAs derived from the nematodes Heligmosomoides and Litomosoides. EV miRNA-mediated down regulation of host immune response which might otherwise lead to parasite clearance was also shown, indicating a role for EVs in parasite survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Li Z, Shen J, Liu Z, Liang J, Wu X, Sun X, Wu Z. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune-activity of macrophage. Parasitol Res. 2015;114:1865–1873. doi: 10.1007/s00436-015-4373-7. [DOI] [PubMed] [Google Scholar]


