Abstract
Background:
Inflammation initiated by damage-associated molecular patterns has been implicated for the cognitive decline associated with surgical trauma and serious illness. We determined whether resolution of inflammation mediates dexmedetomidine-induced reduction of damage-associated molecular pattern-induced cognitive decline.
Methods:
Cognitive decline (assessed by trace fear-conditioning) was induced with high molecular group box 1 protein, a damage-associated molecular pattern, in mice that also received blockers of neural (vagal) and humoral inflammation-resolving pathways. Systemic and neuroinflammation was assessed by pro-inflammatory cytokines.
Results:
Damage-associated molecular pattern-induced cognitive decline and inflammation (mean ± SD) was reversed by dexmedetomidine (trace fear-conditioning: 58.77 ± 8.69% vs 41.45 ± 7.64%, p<0.0001; plasma IL-1β: 7.0 ± 2.2 pg/ml vs 49.8 ± 6.0pg/ml, p < 0.0001; plasma IL-6: 3.2 ± 1.6pg/ml vs 19.5 ± 1.7pg/ml, p<0.0001; hippocampal IL-1β: 4.1 ± 3.0pg/mg vs 41.6 ± 8.0pg/mg, p<0.0001; hippocampal IL-6: 3.4 ± 1.3pg/mg vs 16.2 ± 2.7pg/mg, p < 0.0001). Reversal by dexmedetomidine was prevented by blockade of vagomimetic imidazoline and α7 nicotinic acetylcholine receptors but not by α2 adrenoceptor-blockade. Netrin-1, the orchestrator of inflammation-resolution, was upregulated (fold-change) by dexmedetomidine (Lung: 1.5 ± 0.1 vs 0.7 ± 0.1, p<0.0001; Spleen: 1.5 ± 0.2 vs 0.6 ± 0.2, p<0.0001) resulting in upregulation of pro-resolving (lipoxin-A4: 1.7 ± 0.2 vs 0.9 ± 0.2, p<0.0001) and down-regulation of pro-inflammatory (leukotriene-B4: 1.0 ± 0.2 vs 3.0 ± 0.3, p<0.0001) humoral mediators that was prevented by α7 nicotinic acetylcholine receptor-blockade.
Conclusions:
Dexmedetomidine resolves inflammation through vagomimetic (neural) and humoral pathways thereby preventing damage-associated molecular pattern-mediated cognitive decline.
Introduction:
Over the last decade we have generated several lines of evidence that implicate surgery-initiated systemic- and neuro-inflammation in the development of postoperative cognitive decline both in preclinical1–3 as well as in clinical settings.4 The engagement of the innate immune system, that triggers the inflammatory response which results in postoperative cognitive decline, is due to the damage-associated molecular pattern, high molecular group box 1 (HMGB1) protein, that is passively released from traumatized tissue.5–6
While postoperative cognitive decline was first reported in the setting of general anesthesia7 no difference has been reported in the incidence of postoperative cognitive decline in patients randomized to receive general vs regional anesthetic techniques.8 These earlier studies may have been underpowered and larger trials are progressing to understand whether regional or general anesthesia is less likely to result in postoperative cognitive decline (REGAIN Trial;NCT02507505).9 Notwithstanding the outcome of the REGAIN trial, many patients will require either sedatives and/or general anesthesia combined with a regional technique. While some comparative studies have suggested that the frequency and/or severity of postoperative cognitive decline may be affected by the choice and dose of anesthetic/sedative agent that is used for the surgical procedure,10 and that exposure to deeper anesthetic stages results in a higher incidence of delirium11, larger, appropriately powered studies are needed to avoid a type 2 statistical error as was suspected in the HIPELD study (NCT01199276).12
It is notable that animal cohorts receiving anesthesia and analgesia alone did not differ from the control group (no anesthesia/analgesia, no surgery).1–2 Ethical considerations prevented us from using a “surgery only” (i.e., without anesthesia/analgesia) cohort to which we could compare the neuroinflammatory and cognitive effects provoked by the addition of anesthetic and analgesic drugs. Now that we have demonstrated that systemically-administered HMGB1 reproduces the surgical phenotype with high fidelity5, we are ethically able to study a surrogate of the “surgery only” cohort without use of anesthesia/hypnotics to which we can compare the effects of adding these drugs.
In order to determine whether anesthetic/hypnotics have a modulating effect on the surgical phenotype, we have chosen to first study dexmedetomidine because it reduces cognitive decline13–14 and inflammation15, including that associated with acute neurologic injury16 by an as yet undetermined mechanism. Because of the challenge that there is “questionable biological plausibility” for the beneficial effects that were noted postoperatively with dexmedetomidine14 we addressed the hypothesis that the ameliorative effect of dexmedetomidine on cognitive decline is due to resolution of inflammation through neural and humoral processes.
Methods and Materials
Animals (Figure 1)
Figure 1: Study design.
(A) Mice were randomly allocated to 10 groups (n=15/group) and were pre-treated intraperitoneally (ip) with antagonists (yohimbine/atipamezole/methyllycaconitine). Thirty minutes later mice were trained in the trace-fear conditioning paradigm. After the training session, high mobility group box 1 protein (HMGB1) or vehicle (phosphate-buffered saline) was administered ip dexmedetomidine was administered every 2 hours × 3 times. 72 hours after HMGB1, testing was performed in the trace-fear conditioning.
(B) Mice were randomly allocated to 10 groups (n=8/group) and were pre-treated ip with antagonists (yohimbine/atipamezole/methyllycaconitine) and 30 minutes later HMGB1 was administered. Dexmedetomidine was administered every 2 hours × 3 times. Blood and tissue were collected 24 hours later.
(C) Mice were randomly allocated to three groups (n=15/group): control (vehicle only); surgery/anesthesia and surgery/anesthesia + dexmedetomidine. Mice were trained in the trace fear-conditioning paradigm. After the training session, animals were anesthetized with isoflurane and subjected to aseptic trauma. Dexmedetomidine was administered and the mice were tested in the trace-fear conditioning 3 days later.
(D) Mice were randomly allocated to 3 groups (n=5–6/group): control (vehicle only); surgery/anesthesia and surgery/anesthesia + dexmedetomidine. Mice were anesthetized with isoflurane and subjected to aseptic trauma. Dexmedetomidine was administered and blood and tissue were collected 24 hours later.
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, (AN 167062) and conformed to the National Institutes of Health Guidelines. 12–14 week-old wild-type male C57BL/6J mice (Jackson Laboratory Bar Harbor, ME) were used for this study. All animals were fed standard rodent food and water and were housed (five mice per cage) in a controlled environment with 12-hour light/dark cycles. Mice were tagged and randomly allocated to each group before any treatment or procedure. Researchers were blinded to the group assignment, which was revealed only after completing analysis. Mice did not experience unexpected lethality in the study and animals were euthanized according to our institutional animal care and use committee guidelines.
Drug Administration (Figure 1)
Recombinant HMGB1 (R&D System, Minneapolis, MN) was dissolved in phosphate-buffered saline and administered ip 50 μg/kg, a dose that we had earlier reported to produce a similar inflammatory and cognitive response as that seen after surgery.5
Dexmedetomidine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline and administered 50 μg/kg ip every 2 hours for three doses immediately following HMGB1 (Figure 1 A, B) or at 20 μg/kg ip for four doses in the surgical model (Figure 1 C, D). These doses were selected to simulate perioperative sedation either with (Figure 1 C, D) or without isoflurane anesthesia (Figure 1 A, B).
Yohimbine (Sigma-Aldrich) was dissolved in 0.9% sterile saline and 1.5 mg/kg was administered ip, a dose that effectively blocks α2 adrenoceptor-mediated responses.17
Atipamezole (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 5% dimethyl sulfoxide in saline and 3 mg/kg was administered ip, a dose that effectively blocks both imidazole receptor and α2 adrenoceptor-mediated responses.
Methyllycaconitine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline and 4mg/kg ip was administered, a dose that blocks α7 nicotinic acetylcholine receptor-mediated response.2
Aseptic surgical trauma
Under aseptic conditions, groups of mice were subjected to an open tibia fracture of the left hind paw with an intramedullary fixation as previously described.5 Briefly, mice received general anesthesia with 2% isoflurane and analgesia was achieved with buprenorphine 0.1 mg/kg administered subcutaneously, immediately after anesthetic induction. Warming pads and temperature-controlled lights were used to maintain body temperature. The entire procedure from induction of anesthesia to end of surgery lasted 12 ± 5 minutes.
Cognitive Testing (Figure 1A, C)
Trace fear-conditioning was used to assess learning and memory in rodents as previously described.2–3 Briefly, mice are trained to associate a conditional stimulus, such as a tone, with an aversive, unconditional stimulus, such as a foot-shock. Aversive memory is associated with freezing behavior when the rodent is re-exposed to the same context. The behavioral study was conducted using a conditioning chamber (Med. Associates Inc., St. Albans, VT) and an unconditional stimulus (two periods of 2-seconds foot shock of 0.75 mA). Behavior was captured with an infrared video camera (Video Freeze; Med. Associates Inc.). Mice underwent a context test 72 hours after training, during which no tones or foot-shocks were delivered. Lack of movement, indicating freezing behavior, was analyzed by software of video-recordings. With this model, perturbations of the hippocampus that are associated with memory impairment result in disruption of recall of the fear responses to the presentation of the same context, resulting in a reduction in freezing behavior.5
Blood and Tissue Sample harvesting (Figure 1B, D)
Twenty-four hours after the specific intervention, blood was collected transcardially after thoracotomy under isoflurane anesthesia and placed in heparin-coated syringes. After collection of blood, mice were immediately perfused with saline, and the hippocampus, brain, lung and spleen were then rapidly extracted and stored at −80°C for further testing. After centrifugation of the blood sample at 3,400 rotations per minute for 10 minutes at 4°C, plasma was collected and stored at −80°C until these were assayed.
Circulating Cytokines (Figure 1B, D)
Plasma interleukin IL-6 and IL-1β were quantified using commercially available enzyme-linked immunosorbent kits, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Hippocampal Inflammatory Markers (Figure 1B, D)
Two different techniques were used to assess hippocampal inflammation. For experiments described in Figure 1B, the hippocampus was homogenized and sonicated in cell lysis buffer (Cell Signaling Technology), plus protease inhibitor (Halt Protease Inhibitor Single-Use Cocktail, Thermo Fisher Scientific) and phenylmethanesulfonyl fluoride (PMSF, Cell Signaling Technology). Protein concentration was assayed with Pierce BCA Protein Assay kit (Thermo Prod). Interleukin IL-6 and IL-1β were measured using commercially available enzyme-linked immunosorbent kits, according to the manufacturer’s instructions (R&D Systems). For experiments described in Figure 1D, after mice were perfused with saline the hippocampus was rapidly extracted, placed in RNAlater™ solution (Qiagen) and stored at 4°C overnight. Total RNA was extracted using RNeasy Lipid tissue Kit (Qiagen). Extracted RNA was treated with recombinant DNase I by using a RNase-Free Dnase set™ (Qiagen). Messenger RNA (mRNA) concentrations were determined with a ND-1000 Spectrophotometer (NanoDrop®; Thermo Fisher Scientific) and mRNA was reverse transcribed to complementary DNA with a High Capacity RNA to-cDNA Kit (Applied Biosystems). TaqMan Fast Advanced Master Mix (Applied Biosystems) and specific gene-expression assays were used for quantitative polymerase chain reaction, actin beta (NM_007393.1) and IL-6 (Mm00446190_m1). Quantitative polymerase chain reaction was performed using StepOnePlus™ (Applied Biosystems). Each sample was run in triplicate, and relative gene expression was calculated using the comparative threshold cycle ΔΔCt and normalized to β-actin. Results are expressed as fold increases relative to controls.
Measurement of circulating leukotriene B4 (LTB4) and lipoxin A4 (LXA4)
Plasma LTB4 and LXA4 were quantified using commercially available enzyme-linked immunosorbent kits, according to the manufacturer’s instructions (Biomatik USA, LLC). Results are expressed as fold-change compared with that measured in control mice that did not receive any intervention.
Measurement of Netrin-1 (in the Lung and Spleen) and Albumin (in the Brain)
Tissues were homogenized with RIPA Lysis Buffer (Cell Signaling Technology) plus protease inhibitor (Halt Protease Inhibitor Single-Use Cocktail, Thermo Fisher Scientific) and phenylmethanesulfonyl fluoride (PMSF, Cell Signaling Technology) and sonicated. Protein concentration was assayed with Pierce BCA Protein Assay kit (Thermo Prod). For immunoblotting the buffer for the samples was prepared by adding 950μl of 2× Laemmli Sample Buffer to 50μl of 2-mercaptoethanol (Bio-Rad). The protein samples were mixed in a 1:1 ratio with the sample buffer. After boiling for 5 minutes, 20 μg of protein was loaded onto 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis the analytes were transferred onto nitrocellulose transfer membranes. After the membranes were incubated with blocking buffer (LICOR® Biosciences) for 1 hour at room temperature, these were incubated with primary antibodies at 1: 1,000 dilution overnight at 4°C. The primary antibodies that were used for immunoblotting were rabbit monoclonal antibodies directed against murine netrin-1 (ab126729, Abcam) and murine albumin (ab207327, Abcam). For the loading control, rabbit monoclonal directed at murine GAPDH (ab181602, Abcam) was used. After washing 4 times with TBS containing 0.1% tween (TBST), membranes were incubated with 1: 10,000 dilution of IRDye 680RD or 800RD labeled goat anti-rabbit antibody (LICOR® Biosciences) for 1 hour at room temperature. Membranes were washed 3 times with TBST and once with TBS, and images were captured and quantified using a LI-COR Imager (LICOR® Biosciences).
Statistical Analysis
All data in this study were analyzed using Prism 6.0 (GraphPad Software, San Diego, CA, USA) and were expressed as mean ± SD. Statistical comparison was performed by a one-way ANOVA followed by Tukey test for post hoc analysis. Significance was set at P < 0.05. No statistical power calculation was conducted before our study, and the sample size selected was based on our previous experience using this design.3,18
Results
Dexmedetomidine prevents cognitive decline induced by HMGB1 in an imidazoline- and α7 nicotinic acetylcholine receptor-dependent mechanism (Figure 2)
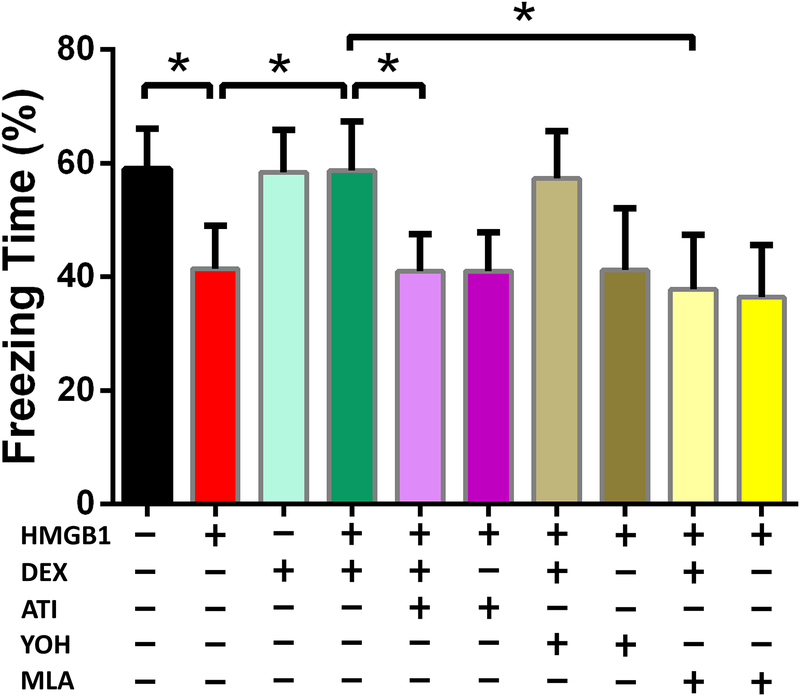
Figure 2. Dexmedetomidine prevents HMGB1-induced decrement in freezing behavior in an Atipamezole and Methyllycaconitine sensitive manner.
Ten groups of randomly-assigned mice (n=15/group) were administered antagonists (methyllycaconitine, atipamezole, yohimbine) prior to HMGB1 and subjected to trace-fear conditioning training with and without dexmedetomidine exposure. Testing for freezing behavior in the trace-fear conditioning context was undertaken 72 hours later. Freezing time data are expressed as means ± SD and were analyzed by one-way ANOVA and Tukey post hoc test, * = P<0.0001 for comparisons shown.
Consistent with our previous report5, HMGB1 significantly decreased freezing time (%) compared to the control group (41.45 ± 7.64% vs 58.94 ± 7.22%, p < 0.0001). Administration of dexmedetomidine prevented HMGB1-induced cognitive decline (58.77 ± 8.69% vs 41.45 ± 7.64%; p < 0.0001). In contrast to the lack of effect by an α2 adrenoceptor-blocking dose of yohimbine, atipamezole, which has blocking activity at both the α2 adrenergic receptor and the imidazoline receptor, prevented dexmedetomidine-induced reversal of HMGB1-mediated cognitive decline (40.87 ± 6.60% vs 58.77 ± 8.69%; p < 0.0001). The reversal effect by dexmedetomidine of HMGB1-induced cognitive decline was prevented by the α7 nicotinic acetylcholine receptor antagonist, methyllycaconitine (37.79 ± 9.51% vs 58.77 ± 8.69%; p < 0.0001).
Dexmedetomidine prevents HMGB1-induced systemic inflammation through an imidazoline and α7 nicotinic acetylcholine receptor-dependent mechanism (Figure 3)
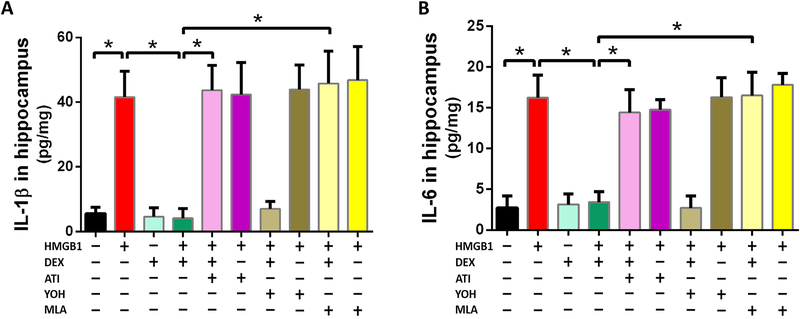
Figure 3: Dexmedetomidine prevents HMGB1-induced peripheral inflammation in an Atipamezole and Methyllycaconitine sensitive manner.
Ten groups of randomly-assigned mice (n=8/group) were administered antagonists (methyllycaconitine, atipamezole, yohimbine) prior to HMGB1 in the presence or absence of dexmedetomidine. Twenty-four hours after HMGB1, mice were sacrificed and the blood was harvested and assayed by ELISA for circulating IL-1β (A) and IL-6 (B). Data are expressed as means ± SD and analyzed by one-way ANOVA and Tukey post hoc test. * = P<0.0001, # = P<0.01 for comparisons shown.
Twenty-four hours after HMGB1 administration, plasma IL-1β (A) and IL-6 (B) were significantly increased 9-(IL-1β: 49.8 ± 6.0 pg/ml vs 5.2 ± 2.5 pg/ml, p < 0.0001) and 6-fold (IL-6: 19.5 ± 1.7 pg/ml vs 3.1 ± 1.7 pg/ml, p < 0.0001), respectively. Exposure to dexmedetomidine reduced the plasma concentration of both pro-inflammatory cytokines to normal levels (IL-1β: 7.0 ± 2.2 pg/ml vs, 49.8 ± 6.0 pg/ml, p < 0.0001; IL-6: 3.2 ± 1.6 pg/ml vs 19.5 ± 1.7 pg/ml, p < 0.0001). Among the α2 adrenoceptor antagonists, only atipamezole, which also has activity at the imidazole receptor, abolished the anti-inflammatory response of dexmedetomidine (IL-1β: 47.8 ± 7.2 pg/ml vs 7.0 ± 2.2 pg/ml, p < 0.0001). While methyllycaconitine, the α7 nicotinic acetylcholine receptor antagonist, also prevented inhibition by dexmedetomidine of the peripheral inflammatory response to HMGB1 (IL-1β: 65.0 ± 9.0 pg/ml vs 7.0 ± 2.2 pg/ml, p < 0.0001) it is notable that methyllycaconitine, alone, significantly enhanced the inflammatory response to HMGB1 (IL-1β: 68.4 ± 5.8 pg/ml vs 49.8 ± 6.0 pg/ml, p = 0.006).
Dexmedetomidine prevents HMGB1-induced hippocampal inflammation through an imidazoline and α7 nicotinic acetylcholine receptor-dependent mechanism (Figure 4)
Figure 4: Dexmedetomidine prevents HMGB1-induced hippocampal inflammation in an Atipamezole and Methyllycaconitine sensitive manner.
Ten groups of randomly-assigned mice (n=8/group) were administered antagonists (methyllycaconitine, atipamezole, yohimbine) prior to HMGB1 in the presence or absence of dexmedetomidine. Twenty-four hours after HMGB1, mice were sacrificed and the hippocampus was harvested and assayed by ELISA for IL-1β (A) and IL-6 (B). Data are expressed as means ± SD and were analyzed by one-way ANOVA and Tukey post hoc test, * = P<0.0001 for comparisons shown.
At 24 hours after HMGB1, hippocampal IL-1β (A) and IL-6 (B) were significantly increased 8-(41.6± 8.0 pg/mg vs 5.5 ± 2.0 pg/mg, p < 0.0001) and 6-fold (16.2 ± 2.7 pg/mg vs 2.7 ± 1.5 pg/mg, p < 0.0001), respectively. Exposure to dexmedetomidine reduced the hippocampal concentration of both pro-inflammatory cytokines to normal levels for IL-1β (4.1 ± 3.0 pg/mg vs 41.6± 8.0 pg/mg, p < 0.0001) and for IL-6 (3.4 ± 1.3 pg/mg vs 16.2 ± 2.7 pg/mg, p < 0.0001). Among the α2 adrenoceptor antagonists, only atipamezole abolished dexmedetomidine’s anti-inflammatory response of IL-1β (43.7 ± 7.7 pg/mg vs 4.1 ± 3.0pg/mg, p < 0.0001) and IL-6 (14.4 ± 2.8 pg/mg vs 3.4 ± 1.3 pg/mg, p < 0.0001). Methyllycaconitine, the α7 nicotinic acetylcholine receptor antagonist, also prevented inhibition by dexmedetomidine of HMGB1-induced peripheral inflammatory response as reflected by IL-1β (45.8 ± 10.1 pg/mg vs 4.1 ± 3.0 pg/mg, p < 0.0001) and IL-6 (16.5 ± 2.9 pg/mg vs 3.4 ± 1.3 pg/mg, p < 0.0001) Unlike systemic inflammation (Figure 3) methyllycaconitine, did not enhance the hippocampal inflammatory response to HMGB1 (Figure 4).
Dexmedetomidine reverses HMGB1-induced down-regulation of Netrin-1 expression in an α7 nicotinic acetylcholine receptor sensitive manner (Figure 5).
Figure 5. Dexmedetomidine prevents HMGB1-induced downregulation of Netrin-1 expression in the lung (A) and spleen (B) in an α7nicotinic acetylcholine receptor dependent manner.
Four groups of randomly-assigned mice (n=5/group) were administered saline vehicle (control), HMGB1 alone, HMGB1+ dexmedetomidine, or HMGB1 + dexmedetomidine + methyllycaconitine. Twenty-four hours later, mice were sacrificed and lung (A) and spleen (B) were harvested for expression of netrin-1 by immunoblotting. Data are expressed as means ± SD fold-change relative to control and were analyzed by one-way ANOVA and Tukey post hoc test. # = P<0.05 and * = P<0.0001 for comparisons shown
Accompanying the sterile inflammation induced by HMGB1 (Figures 3, 4) netrin-1 expression is significantly decreased in the lung (0.7 ± 0.1 vs 1.0 ± 0.2, p = 0.0173, Figure 5A,) and spleen (0.6 ± 0.2 vs 1.0 ± 0.2, p = 0.0220, Figure 5B), organs that are vagally-innervated. Exposure to dexmedetomidine reverses HMGB1-induced netrin-1 downregulation in both organs (1.5 ± 0.1 vs 0.7 ± 0.1, p < 0.0001, Figure 5A; 1.4 ± 0.2 vs 0.7 ± 0.2 p < 0.0001, Figure 5B) and pretreatment with methyllycaconitine, the α7 nicotinic acetylcholine receptor antagonist, prevented reversal of netrin expression by dexmedetomidine (0.7 ± 0.1 vs 1.5 ± 0.1, p < 0.0001 Figure 5A; 0.6 ± 0.2 vs 1.4 ± 0.2, p < 0.0001, Figure 5B).
Dexmedetomidine reverses HMGB1-induced changes in the expression of circulating leukotriene B4 (A) and lipoxin A4 (B) in an α7 nicotinic acetylcholine receptor sensitive mechanism (Figure 6)
Figure 6. Dexmedetomidine downregulates the circulating pro-inflammatory mediator leukotriene B4, (LTB4; A) and upregulates the circulating pro-resolving mediator lipoxin A4 (LXA4; B).
Four groups of randomly-assigned mice (n=8/group) were administered saline vehicle (control), HMGB1 alone, HMGB1+ dexmedetomidine, or HMGB1 + dexmedetomidine + methyllycaconitine. Twenty-four hours later, mice were sacrificed and the blood was harvested and assayed by ELISA for plasma LTB4 (A) and LXA4 (B). Data are expressed as means ± SD fold-change relative to control and were analyzed by one-way ANOVA and Tukey post hoc test. * = P<0.0001 for comparisons shown.
Accompanying the sterile inflammation induced by HMGB1 (Figures 3, 4) and the down-regulation of netrin-1 expression (Figure 5), there is a 3-fold upregulation in the relative expression of leukotriene B4 (LTB4), the pro-inflammatory lipid mediator (2.7 ± 0.4 vs 1.0 ± 0.2, p < 0.0001, Figure 6A). Dexmedetomidine reverses HMGB1-induced upregulation of LTB4 (1.0 ± 0.2 vs 2.7 ± 0.4, p < 0.0001, Figure 6A), and this reversal is prevented by methyllycaconitine (3.0 ± 0.3 vs 1.0 ± 0.2, p < 0.0001, Figure 6A). Conversely, dexmedetomidine upregulated the expression of LXA4 (1.7 ± 0.2 vs 1.2 ± 0.2, p < 0.0001, Figure 6B), the specific pro-resolving mediator, an effect that was reversed by methyllycaconitine (0.9 ± 0.2 vs 1.7 ± 0.2, p < 0.0001, Figure 6B).
Dexmedetomidine reverses HMGB1-induced leakage of the blood brain barrier (Figure 7)
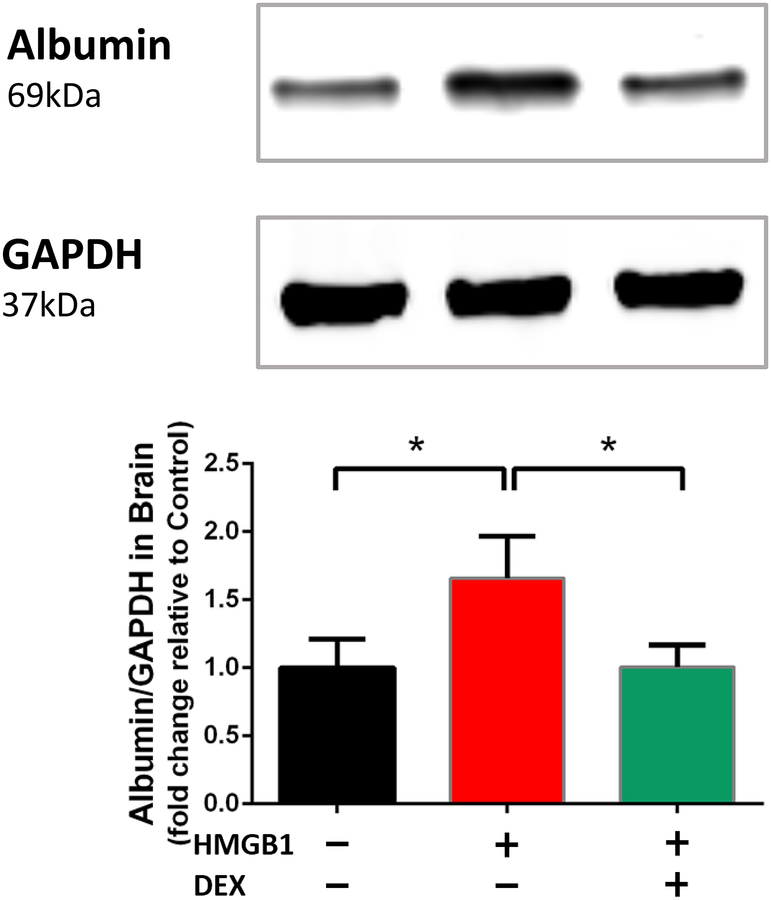
Figure 7. Dexmedetomidine reverses HMGB1-induced leakage of blood brain barrier.
Three groups of randomly-assigned mice (n=5/group) were treated with vehicle (control), HMGB1, or HMGB1 + dexmedetomidine. 24 hours after treatment, mice were sacrificed and the brains were harvested for immunoblotting of albumin expression. Data are expressed as means ± SD relative to control and were analyzed by one-way ANOVA and Tukey post hoc test. * = P<0.01 for comparisons shown.
Accompanying the HMGB1-induced inflammation (Figures 3, 4), the blood brain barrier is disrupted as evidenced by a significant upregulation of albumin expression in the brain assessed by immunoblotting. The upregulation in brain albumin expression (1.0 ± 0.2 vs 1.7 ± 0.3, p = 0.0019) is suppressed by dexmedetomidine (1.7 ± 0.3 vs 1.0 ± 0.2, p = 0.002).
Dexmedetomidine-reverses Surgery-induced Cognitive Decline (A) and Inflammation (B, C) (Figure 8)
Figure 8. Dexmedetomidine reverses Surgery-induced Cognitive Decline (A) and Peripheral (B), and Neuro-Inflammation (C).
Three groups of randomly-assigned mice (n=15/group) were treated with (i) vehicle, (ii) tibia fracture under anesthesia (Sx/Anesth) + vehicle and (iii) Sx/Anesth + dexmedetomidine prior to training in the trace fear-conditioning paradigm (A). Testing for freezing behavior in the trace-fear conditioning context was undertaken 72 hours later. Freezing time data are expressed as means ± SD and were analyzed by one-way ANOVA and Tukey post hoc test. Three groups of randomly-assigned mice (n=6/group) were treated with (i) vehicle, (ii) tibia fracture under anesthesia (Sx/Anesth) + vehicle and (iii) Sx/Anesth + dexmedetomidine and mice were sacrificed at 24 hours and blood and brain were harvested. Plasma IL-6 was assayed by ELISA (B) and hippocampal IL-6 was assayed by quantitative PCR (C). The means ± SD of the mean for expression of IL-6 protein (B) and mRNA (C) and were analyzed by one-way ANOVA and Tukey post hoc test. # = P < 0.05; * = P < 0.01; **P<0.0001
Because isoflurane had no effect on HMGB1-induced cognitive decline (data not shown), the effect of dexmedetomidine on surgery + isoflurane-induced cognitive decline was investigated. Surgery (Sx)-induced cognitive decline was reversed by dexmedetomidine (35.91 ± 5.03% vs 54.62 ± 8.82%, p<0.0001; Figure 8A). Twenty-four hours after surgery, we observed a significant decrease in circulating IL-6 (83.2 ± 60.2 pg/mL vs 30.1 ± 13.7 pg/mL, p = 0.013; Figure 8B). The change of hippocampal mRNA expression of IL-6 after surgery was reversed by dexmedetomidine (8.6 ± 1.3 vs 10.7 ± 1.0, p = 0.012 Figure 8C).
Discussion
Recapitulation of Main Findings
Using HMGB1 to generate a surrogate of the surgical phenotype without the confounding influence of general anesthesia, dexmedetomidine reversed HMGB1-induced cognitive decline (Figure 2), as well as systemic (Figure 3) and hippocampal (Figure 4) inflammation. In each case, reversal by dexmedetomidine of the HMGB1-induced surgical phenotype was blocked by atipamezole (Figures 2, 3, 4), an α2 adrenoceptor antagonist that also has activity at the imidazole receptor19, but not by yohimbine an α2 adrenoceptor antagonist which has no activity at the imidazole receptor. Reversal by dexmedetomidine of the surgical phenotype was also blocked by methyllycaconitine (Figures 2, 3, 4) the α7nAChR antagonist. Dexmedetomidine reversed the HMGB1-induced downregulation of netrin-1 in the vagally-innervated lung (Figure 5A) and spleen (Figure 5B); reversal by dexmedetomidine was attenuated by antagonism of signaling at the vagal termini by α7 nicotinic acetylcholine receptor blockade with methyllycaconitine. Upregulation of LTB4, the pro-inflammatory humoral response to HMGB1 was prevented by dexmedetomidine (Figure 6A) while the pro-resolving mediator, LXA4, was upregulated by dexmedetomidine (Figure 6B). In each case, the effects of dexmedetomidine were negated by pretreatment with methyllycaconitine, the α7 nicotinic acetylcholine receptor blocker (Figure 6). Disruption of the blood brain barrier by HMGB1 was reversed by dexmedetomidine (Figure 7). Finally, perioperative exposure to dexmedetomidine prevented surgery-induced cognitive decline and peripheral and hippocampal inflammation (Figure 8).
Justification for the use of HMGB1 to produce the Surgical Phenotype
HMGB1 regulates transcription of NF-κB20 establishing it as an extracellular orchestrator of the systemic inflammatory response.21 Previously, we showed the causal role of HMGB1 in mediating postoperative cognitive decline following passive release from traumatized tissues.5 Following binding to pattern recognition receptors on circulating immunocytes, HMGB1 initiates the innate immune response to aseptic surgical trauma. Exogenously administered HMGB1 reproduces the surgical phenotype if myeloid-derived circulating monocytes are present,5 a feature also noted following surgery. Other cardinal features of the surgical phenotype, including peripheral (Figure 3) and neuro-inflammation (Figure 4) and disruption of the blood brain barrier (Figure 7), are reproduced following HMGB1 administration.2
Use of Antagonists with which to probe the mechanism for Dexmedetomidine’s Effects in reversing the Surgical Phenotype
α2 adrenoceptor agonists such as dexmedetomidine and clonidine have an imidazole ring structure facilitating binding to and activation of the imidazoline receptor.22 Because atipamezole, which has antagonist activity at the imidazoline receptor20, was able to block dexmedetomidine’s reversal of the surgical phenotype while yohimbine, which has no activity at the imidazoline receptor, was ineffective, we invoke an action mediated by the imidazoline receptor for the reversal of dexmedetomidine’s effect. An important property of imidazoline receptor agonists is its negative chronotropic property23 that is prevented by vagotomy.24 As enhancement of vagal activity by α2 agonists with an imidazole ring has been well-documented25 and as the bradycardic effect of α2 agonists with an imidazole ring structure is unrelated to its binding affinity to α2 adrenoceptors19 we propose that this imidazoline receptor-mediated vagomimetic action may be the mechanism whereby dexmedetomidine reverses the surgical phenotype.
Increase in vagal activity has been shown to be an important mechanism for resolving inflammation.26 The α7 nicotinic acetylcholine receptor transduces the inflammation-resolving vagomimetic effect.27 A supporting finding that the enhanced vagal activity is the likely explanation for dexmedetomidine’s reversal of the surgical phenotype is provided by the fact that α7 nicotinic acetylcholine receptor blockade by methyllycaconitine eliminated the ameliorative effect produced by dexmedetomidine (Figures 2, 3, 4).
The Role of Vagal Stimulation on Neural and Humoral mediated Resolution of Inflammation
Neural28 and humoral23 pathways have been implicated in the resolution of acute inflammation. Neural mechanisms involve efferent vagal fibers in which release of acetylcholine activates α7 nicotinic acetylcholine receptor on immunocompetent cells29 thereby inhibiting NF-κB and downregulating synthesis of pro-inflammatory cytokines.30 For the humoral pathway biotransformation of free fatty acids (arachidonic acid, eicosopentaenoic acid, and docosahexaenoic acid) elaborates specific pro-resolving lipid mediators.31 During synthesis of eicosanoid hormones there are juncture points at which a precursor, such as leukotriene A4, can elaborate two different products (LTB4 and LXA4) with diametrically opposite effects, namely, pro-inflammation and pro-resolution, respectively.32 Dexmedetomidine appears to shunt the biotransformation pathway to the synthesis of LXA4 at the expense of LTB4 (Figure 6).
Humoral and neural pathways have been recently linked to vagally-mediated expression of netrin-1.33 Netrin-1, originally identified as a neuronal guidance protein, also limits inflammation.34 Interestingly, netrin-1 expression is downregulated in vagally-innervated lung and spleen following HMGB1 administration, an effect that is reversed by dexmedetomidine (Figure 5). A similar downregulation of netrin-1 expression has been noted in other inflammation-inducing experimental settings.33
Integrating results into existing molecular mechanism model for postoperative cognitive decline
Earlier, we had shown that trauma-induced release of HMGB1 engages circulating monocytes5 to stimulate the synthesis of pro-inflammatory cytokines that are capable of disrupting the blood brain barrier2 enabling the passage of bone marrow-derived monocytes to enter into the brain, attracted by upregulation of the chemokine MCP-1 by microglia in the hippocampus.3 Within the hippocampus the bone marrow-derived monocytes activate microglia resulting in release of pro-inflammatory cytokines that disrupt long-term potentiation35, the neurobiologic correlate of learning and memory. Within 7 days the inflammation usually resolves, as does postoperative cognitive decline, except in vulnerable animals in which inflammation and cognitive decline can both be exaggerated3 and more persistent.18
Dexmedetomidine appears to reverse postoperative cognitive decline by enhancing the inflammation-resolving pathways. Dexmedetomidine stimulates the vagus through imidazoline receptor activation resulting in inhibition of the NF-κB-dependent synthesis of pro-inflammatory cytokines (Figures 3, 4) through activation of α7 nicotinic acetylcholine receptor (Figures 3, 4). Netrin-1 is downregulated by HMGB1 through a vagal pathway and this is reversed by dexmedetomidine (Figure 5) resulting in a change in the elaboration of specific pro-resolving mediators (increase in LXA4 and decrease in LTB4) that resolves inflammation (Figure 6).
Caveats
Methyllycaconitine-induced upregulation of circulating pro-inflammatory Cytokines:
Methyllycaconitine was used in a dose that blocks the α7 nicotinic acetylcholine receptor-mediated inhibition of synthesis of proinflammatory cytokines.2 However, at this dose there was an increase in HMGB1-induced pro-inflammatory cytokines (Figure 3) and it may be argued that the reversal of the anti-inflammatory effect of dexmedetomidine could be due to the non-specific enhancement of pro-inflammatory cytokines independent of any specific action that dexmedetomidine exerts on α7 nicotinic acetylcholine receptor through vagal stimulation. It is notable that in the hippocampus, where cognitive decline is produced, there was not a similar methyllycaconitine-induced enhancement of pro-inflammatory cytokines.
Doses of Dexmedetomidine used in HMGB1- and surgery-induced cognitive decline:
In the HMGB1-induced surrogate of the surgical phenotype we used a dexmedetomidine dose of 50 μg/kg while in the trauma-induced model a dexmedetomidine dose of 20 μg/kg was used. The reason that these doses differ was to prevent a significant increase in the sedative effect of dexmedetomidine in the presence of isoflurane36 that may increase postoperative cognitive decline.11 The lower dexmedetomidine dose in the surgical model (Figure 8) was as effective as the higher dose in the HMGB1 model (Figures 2, 3, 4) at reversing cognitive decline and inflammation.
Relevance of findings in young mice to vulnerable animal models:
In the current study we only tested the efficacy and putative mechanisms whereby dexmedetomidine prevents inflammation and cognitive decline in young mice. Whether or not these observed cognitive decline-reducing properties of dexmetomidine occurs in vulnerable models (including aging, obesity3, and metabolic syndrome18), remains to be determined.
Relevance of Preclinical Mechanistic Findings in the light of Dexmedetomidine’s abilityto reverse the Surgical Phenotype in Clinical Studies
Sedation with dexmedetomidine reduces the likelihood of delirium in the ICU when compared to benzodiazepines13,37 and when dexmedetomidine was administered during the first postoperative night to elderly surgical patients.14 Because dexmedetomidine changes the activity in neuronal pathways in asimilar manner to those altered during natural sleep38 and produces similar EEG changes to that seen during natural sleep39 and because both sleep deprivation40 and sleep fragmentation41 induce neuroinflammation and cognitive decline, we had conjectured that the cognition-enhancing effects of dexmedetomidine were due to its unique sedative profile. However, as dexmedetomidine-induced sedation is mediated by the α2A adrenoceptors42 that are antagonized by yohimbine43, our finding that reversal by dexmedetomidine of the surgical phenotype is insensitive to yohimbine challenges that explanation.
We recognize that the reversal of postoperative cognitive decline by dexmedetomidine is not universal. Recently, Deiner and colleagues reported that dexmedetomidine lacked efficacy in preventing the onset of postoperative delirium.44 Further studies are needed to define the patient subgroups that may be resistant to the cognitive decline-reducing properties of dexmedetomidine.
Future applications
These preclinical studies were performed in mice that do not exhibit exaggerated and persistent cognitive decline. The effectiveness of dexmedetomidine will need to be established in reagents that have abnormalities in their inflammation-resolving pathways including in advanced age with its “inflammaging” processes45 and the metabolic syndrome with its precocious aging phenotype.46
While we have already shown that dexmedetomidine is effective at decreasing delirium in mechanically-ventilated medical/surgical intensive care patients13 as well as in non-ventilated surgical patients14 it will be important to demonstrate that wound healing and the ability to combat infection are not jeopardized by a dexmedetomidine intervention that perturbs the innate immune system. Regarding infection it is notable that survival is enhanced by dexmedetomidine in a preclinical model of sepsis47 a finding that was also noted in a post hoc analysis of the MENDS trial.48 However, dexmedetomidine was not shown to improve outcome in a recently-reported trial of septic patients; this question is being further addressed in the MENDS II trial (NCT01739933) comparing outcomes in septic patients sedated with dexmedetomidine vs propofol. Whether the existing role of dexmedetomidine as a perioperative and procedural sedative agent can be supplemented by indications in which resolution of inflammation requires bolstering will need to be studied further. Furthermore, if the activation of the imidazoline receptor and subsequent vagal outflow is confirmed to be the reason for dexmedetomidine’s anti-inflammatory properties, it may be possible to reproduce these effects with a selective imidazoline receptor agonist and avoid α2 adrenoceptor properties such as sedation.
Acknowledgments:
The authors wish to thank Dr. Jinbao Chen (Department of Anesthesia, Tongling People’s Hospital, Tongling Anhui 244000, PRC) for providing postdoctoral support to JH and to Mr. Christopher Chang for help with the preparation of the manuscript.
Funding Statement:
Work was supported by a grant from the NIH to M.M. (R01GM104194)
Footnotes
Conflicts of Interest
MM is a co-inventor on a patent for the use of dexmedetomidine for sedation. Between 1987–1991 MM’s laboratory at Stanford University received $250,000 for the assignment of the patent to Farmos, the company that synthesized dexmedetomidine. Between 1995–2008, MM was intermittently paid as a consultant by Orion-Farmos, Abbott Labs and Hospira for advising on the pivotal Phase III clinical trials, approval of the New Drug Application, and for subsequent marketing of the product. MM has not received any payments for at least the last 5 years. MM has not and will not receive royalty payments for sales of dexmedetomidine.
References
- 1.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M: Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010; 107: 20518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M: Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011; 70: 986–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng X, Valdearcos M, Uchida Y, Lutrin D, Koliwad SK, Maze M: Microglia mediate postoperative hippocampal neuroinflammation and cognitive decline in mice. JCI Insight 2017; 2: e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsberg A, Cervenka S, Fagerlun MJ, Rasmussen L, Zetterberg H, Harris HE, Stridh P, Christensson E, Granstrom A, Schening A, Dymmel K, Knave N, Terrando N, Maze M, Borg J, A. V, Varrone A, Halldin C, Blennow K, Farde L, Eriksson L: The immune response of the human brain to abdominal surgery. Ann Neurol 2017; 84: 572–82 [DOI] [PubMed] [Google Scholar]
- 5.Vacas S, Degos V, Tracey KJ, Maze M: High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology 2014; 120: 1160–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrando N, Yang T, Wang X, Fang J, Cao M, Andersson U, Erlandsson HH, Ouyang W, Tong J: Systemic HMGB1 Neutralization Prevents Postoperative Neurocognitive Dysfunction in Aged Rats. Front Immunol 2016; 7: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedford PD: Adverse cerebral effects of anaesthesia on old people. Lancet 1955; 269: 259–63 [DOI] [PubMed] [Google Scholar]
- 8.Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME: Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA 1995; 274: 44–50 [PubMed] [Google Scholar]
- 9.Neuman MD, Ellenberg SS, Sieber FE, Magaziner JS, Feng R, Carson JL, and the REGAIN Investigators: Regional versus General Anesthesia for Promoting Independence after Hip Fracture (REGAIN): protocol for a pragmatic, international multicentre trial. BMJ Open 2016; 6: e013473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD: Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110 Suppl 1: i98–105 [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, Simpkins SA: Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016; 3: CD005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coburn M, Sanders RD, Maze M, Rossaint R: The Hip Fracture Surgery in Elderly Patients (HIPELD) study: protocol for a randomized, multicenter controlled trial evaluating the effect of xenon on postoperative delirium in older patients undergoing hip fracture surgery. Trials 2012; 13: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW: Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298: 2644–53 [DOI] [PubMed] [Google Scholar]
- 14.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D: Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016; 388: 1893–1902 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Miao L, Yao Y, Wu W, Wu X, Gong C, Qiu L, Chen J: Dexmedetomidine Ameliorate CLP-Induced Rat Intestinal Injury via Inhibition of Inflammation. Mediators Inflamm 2015; 2015: 918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rong H, Zhao Z, Feng J, Lei Y, Wu H, Sun R, Zhang Z, Hou B, Zhang W, Sun Y, Gu X, Ma Z, Liu Y: The effects of dexmedetomidine pretreatment on the pro- and anti-inflammation systems after spinal cord injury in rats. Brain Behav Immun 2017; 64: 195–207 [DOI] [PubMed] [Google Scholar]
- 17.Hsing CH, Lin CF, So E, Sun DP, Chen TC, Li CF, Yeh CH: alpha2-Adrenoceptor agonist dexmedetomidine protects septic acute kidney injury through increasing BMP-7 and inhibiting HDAC2 and HDAC5. Am J Physiol Renal Physiol 2012; 303: F1443–53 [DOI] [PubMed] [Google Scholar]
- 18.Feng X, Degos V, Koch LG, Britton SL, Zhu Y, Vacas S, Terrando N, Nelson J, Su X, Maze M: Surgery results in exaggerated and persistent cognitive decline in a rat model of the Metabolic Syndrome. Anesthesiology 2013; 118: 1098–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernsberger P, Giuliano R, Willette RN, Reis DJ: Role of imidazole receptors in the vasodepressor response to clonidine analogs in the rostral ventrolateral medulla. J Pharmacol Exp Ther 1990; 253: 408–18 [PubMed] [Google Scholar]
- 20.Tang D, Kang R, Zeh HJ 3rd, Lotze MT: High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 2011; 14: 1315–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L: Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004; 10: 1216–21 [DOI] [PubMed] [Google Scholar]
- 22.Khan ZP, Ferguson CN, Jones RM: alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999; 54: 146–65 [DOI] [PubMed] [Google Scholar]
- 23.Serhan CN: Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 2017; 31: 1273–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boblewski K, Lehmann A, Saczewski F, Kornicka A, Rybczynska A: Vagotomy reveals the importance of the imidazoline receptors in the cardiovascular effects of marsanidine and 7-ME-marsanidine in rats. Pharmacol Rep 2014; 66: 874–9 [DOI] [PubMed] [Google Scholar]
- 25.Philbin KE, Bateman RJ, Mendelowitz D: Clonidine, an alpha2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res 2010; 1347: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavan SS, Tracey KJ: Essential Neuroscience in Immunology. J Immunol 2017; 198: 3389–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallowitsch-Puerta M, Tracey KJ: Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann N Y Acad Sci 2005; 1062: 209–19 [DOI] [PubMed] [Google Scholar]
- 28.Pavlov VA, Tracey KJ: Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 2017; 20: 156–166 [DOI] [PubMed] [Google Scholar]
- 29.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ: Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334: 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP: Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 2009; 60: 114–22 [DOI] [PubMed] [Google Scholar]
- 31.Dalli J, Serhan CN: Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012; 120: e60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN: Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001; 2: 612–9 [DOI] [PubMed] [Google Scholar]
- 33.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN: Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 2014; 211: 1037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, Parnes JA, Furuta GT, Rivera-Nieves J, Eltzschig HK: Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut 2012; 61: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, Harding RE, Lindskog M, Eriksson LI: Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J 2013; 27: 3564–71 [DOI] [PubMed] [Google Scholar]
- 36.Savola MK, MacIver MB, Doze VA, Kendig JJ, Maze M: The alpha 2-adrenoceptor agonist dexmedetomidine increases the apparent potency of the volatile anesthetic isoflurane in rats in vivo and in hippocampal slice in vitro. Brain Res 1991; 548: 23–8 [DOI] [PubMed] [Google Scholar]
- 37.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG, Group SS: Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301: 489–99 [DOI] [PubMed] [Google Scholar]
- 38.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M: The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003; 98: 428–36 [DOI] [PubMed] [Google Scholar]
- 39.Akeju O, Kim SE, Vazquez R, Rhee J, Pavone KJ, Hobbs LE, Purdon PL, Brown EN: Spatiotemporal Dynamics of Dexmedetomidine-Induced Electroencephalogram Oscillations. PLoS One 2016; 11: e0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z: Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis 2012; 48: 348–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vacas S, Degos V, Maze M: Fragmented Sleep Enhances Postoperative Neuroinflammation but Not Cognitive Dysfunction. Anesth Analg 2017; 124: 270–276 [DOI] [PubMed] [Google Scholar]
- 42.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE: Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A 1997; 94: 9950–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y, Axelsson AS, Spegel P, Andersson LE, Mulder H, Groop LC, Renstrom E, Rosengren AH: Genotype-based treatment of type 2 diabetes with an alpha2A-adrenergic receptor antagonist. Sci Transl Med 2014; 6: 257ra139. [DOI] [PubMed] [Google Scholar]
- 44.Deiner S, Luo X, Lin HM, Sessler DI, Saager L, Sieber FE, Lee HB, Sano M; and the Dexlirium Writing Group, Jankowski C, Bergese SD, Candiotti K, Flaherty JH, Arora H, Shander A, Rock P: Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA 2017; 152:e171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S: Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab 2017; 28: 199–212 [DOI] [PubMed] [Google Scholar]
- 46.Su X, Feng X, Terrando N, Yan Y, Chawla A, Koch LG, Britton SL, Matthay MA, Maze M: Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of the metabolic syndrome. Mol Med 2013; 18: 1481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao H, Sanders RD, Ma D, Wu X, Maze M: Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care 2009; 13: R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, Herr DL, Maze M, Ely EW, investigators M: Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 2010; 14: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]