Abstract
IMPORTANCE
Extubation failure is common in extremely preterm infants. The current paucity of data on the adverse long-term respiratory outcomes associated with reinitiation of mechanical ventilation prevents assessment of the risks and benefits of a trial of extubation in this population.
OBJECTIVE
To evaluate whether exposure to multiple courses of mechanical ventilation increases the risk of adverse respiratory outcomes before and after adjustment for the cumulative duration of mechanical ventilation.
DESIGN, SETTING, AND PARTICIPANTS
We performed a retrospective cohort study of extremely low-birth-weight (ELBW; birth weight <1000 g) infants born from January 1, 2006, through December 31, 2012, who were receiving mechanical ventilation. Analysis was conducted between November 2014 and February 2015. Data were obtained from the Alere Neonatal Database.
EXPOSURES
The primary study exposures were the cumulative duration of mechanical ventilation and the number of ventilation courses.
MAIN OUTCOMES AND MEASURES
The primary outcome was bronchopulmonary dysplasia (BPD) among survivors. Secondary outcomes were death, use of supplemental oxygen at discharge, and tracheostomy.
RESULTS
We identified 3343 ELBW infants, of whom 2867 (85.8%) survived to discharge. Among the survivors, 1695 (59.1%) were diagnosed as having BPD, 856 (29.9%) received supplemental oxygen at discharge, and 31 (1.1%) underwent tracheostomy. Exposure to a greater number of mechanical ventilation courses was associated with a progressive increase in the risk of BPD and use of supplemental oxygen at discharge. Compared with a single ventilation course, the adjusted odds ratios for BPD ranged from 1.88 (95% CI, 1.54–2.31) among infants with 2 ventilation courses to 3.81 (95% CI, 2.88–5.04) among those with 4 or more courses. After adjustment for the cumulative duration of mechanical ventilation, the odds of BPD were only increased among infants exposed to 4 or more ventilation courses (adjusted odds ratio, 1.44; 95% CI, 1.04–2.01). The number of ventilation courses was not associated with increased risk of supplemental oxygen use at discharge after adjustment for the length of ventilation. A greater number of ventilation courses did not increase the risk of tracheostomy.
CONCLUSIONS AND RELEVANCE
Among ELBW infants, a longer cumulative duration of mechanical ventilation largely accounts for the increased risk of chronic respiratory morbidity associated with reinitiation of mechanical ventilation. These results support attempts of extubation in ELBW infants receiving mechanical ventilation on low ventilator settings, even when success is not guaranteed.
Severe respiratory insufficiency is common among extremely preterm infants. More than two-thirds of those born at less than 29 weeks’ gestation require mechanical ventilation for some duration during the newborn period.1 Although this therapy can be lifesaving, prolonged exposure is associated with multiple adverse effects, including upper airway injury, nosocomial infection, bronchopulmonary dysplasia (BPD), and neurocognitive impairment.2–4 Early weaning from mechanical ventilation with successful extubation may reduce the risk of these complications.2,5 However, 30% to 40% of extremely preterm infants require replacement of their endotracheal tube within 1 week of extubation.6,7 Moreover, 15% to 20% of infants born weighing less than 1000 g are exposed to 3 or more courses of mechanical ventilation before discharge from the neonatal intensive care unit (NICU).8 In older children and adults, the association between extubation failure and adverse outcomes is well established.9–12 In preterm infants, the association is less clear.13,14 After reintubation, pre-term infants may initially require higher ventilator pressures and greater supplemental oxygen compared with immediately before extubation.15,16 Whether the possible postextubation atelectrauma and temporary increase in respiratory support after reintubation increase the risk of adverse long-term respiratory outcomes, particularly when compared with continued ventilation without a trial of extubation, is unknown.
The objectives of the present study were to evaluate whether exposure to additional courses of invasive mechanical ventilation compared with successful extubation after a single course independently increases the risk of BPD, use of supplemental oxygen at discharge, tracheostomy, or death among extremely low-birth-weight (ELBW; birth weight <1000 g) infants. We hypothesized that each additional course of mechanical ventilation would increase the risk of adverse respiratory outcomes even after accounting for the cumulative duration of mechanical ventilation.
Methods
Population and Data Source
We performed a retrospective cohort study of infants with birth weights less than 1000 g and gestational ages of 32 weeks or less born from January 1,2006, through December 31,2012. Analysis was conducted between November 2014 and February 2015. Because our goal was to evaluate the effect of reintubation, all included infants were intubated and received mechanical ventilation at least once. We excluded infants who died in the delivery room and infants who received surfactant but never received mechanical ventilation. Data were obtained from the Alere Neonatal Database. The Alere Neonatal Database provides neonatal care management services for multiple private, government, and self-insured employer health plans. The infants included in this analysis were cared for in 554 level II or higher academic and community-hospital based NICUs. Daily clinical, sociodemo-graphic, and cost-related information is abstracted concurrent with each hospitalization 3to4 times per week by experienced neonatal nurses. The institutional review board at Thomas Jefferson University approved this study. Informed consent was not required because this was an analysis of deidentified data.
Study Outcomes and Exposure Definitions
The primary study outcome was BPD, defined as the use of supplemental oxygen at 36 weeks’ postmenstrual age, among infants who survived to NICU discharge. Prespecified secondary outcomes were death, continued supplemental oxygenuse at the time of discharge, and tracheostomy among survivors. We evaluated the rates and risk-adjusted odds of these outcomes based on 2 primary study exposures: the cumulative duration of mechanical ventilation before death or NICU discharge and the number of individual courses of mechanical ventilation. The database used to define these exposures contains the highest level of respiratory support administered each day. Therefore, distinct ventilation courses were identified if separated by 1 or more days without mechanical ventilation. We evaluated the primary study exposures as continuous and multilevel categorical variables. The categorical variables were created suchthat the median duration of mechanical ventilation was similar between the corresponding levels of each exposure.
We assessed the following potential confounding variables in our statistical modeling: gestational age, birth weight, sex, small for gestational age, 5-minute Apgar score, treatment with surfactant, surgical or medical treatment of a patent ductus arteriosus (PDA), necrotizing enterocolitis, bacterial sepsis, and postnatal dexamethasone treatment. Small for gestational age was defined as a birth weight less than the 10th percentile for gestational age based on the Olsen infant growth curves.17 Necrotizing enterocolitis was defined as modified Bell stage 2 or higher. The diagnosis of bacterial sepsis required blood culture confirmation.
Statistical Analysis
Demographic data were summarized with standard descriptive statistics and compared with Wilcoxon rank sum and χ2 tests as appropriate. To assess the independent association between the primary study exposures and each study outcome, we first developed separate multivariable logistic regression models for the duration of mechanical ventilation and the number of ventilation courses. To compare the differential effects of these 2 exposures, we then constructed multivariable models that included categorical variables for both the duration of mechanical ventilation and the number of ventilation courses.
To build the regression models, all variables associated with a study outcome at P ≤ .20 in the bivariate testing were evaluated in a stepwise manner for inclusion in the final regression model. P ≤ .05 in the multivariable model, evidence of confounding of the association between a primary study exposure and outcome, or agreement among the investigators of the covariate’s importance resulted in inclusion in the final model. Year of birth was included in each model to account for potential change in outcome incidence over time. We used a robust variance estimator for cluster-correlated data to account for possible within-hospital outcome correlation.18,19 To maintain consistency, we included the same covariates in the final model for each study outcome. An interaction term between the 2 primary exposures was included in the combined regression model if significant at P ≤ .05.20 The Hosmer-Lemeshow test and receiver operating characteristic analysis were used to assess model fit. Risk-adjusted probabilities were determined from the logistic regression model using marginal standardization.21 All analyses were performed with STATA software, version 13.1 (StataCorp).
Results
Characteristics of the Study Participants
Among the 3343 ELBW infants with a history of at least one course of mechanical ventilation, 2867 (85.8%) survived to discharge and were assessed for the primary outcome. Demographic characteristics and comorbidity rates among survivors for each level of the 2 study exposures are given in Table 1 and Table 2 (the eTable in the Supplement gives the characteristics of all infants). Lower gestational age, lower birth weight, and male sex were associated with a longer cumulative duration of mechanical ventilation and greater number of ventilation courses. Treatment of a PDA, necrotizing enterocolitis, bacterial sepsis, and postnatal use of dexamethasone were more common among infants with greater exposure to mechanical ventilation. Most infants received mechanical ventilation for less than 36 days (59.4%; n = 1703) and for 2 or fewer distinct courses (54.3%; n = 1557) (Table 1 and Table 2). When the duration of ventilation was treated as a continuous variable, each individual ventilation course was associated with a mean of 10.7 (95% CI, 10.3–11.2) additional days of mechanical ventilation.
Table 1.
Characteristics of the Infants Who Survived to Discharge Based on the Cumulative Duration of Mechanical Ventilationa
| Cumulative Duration of Mechanical Ventilation, d | ||||
|---|---|---|---|---|
| Characteristic | ≤7 (n = 759) | 8–21 (n = 562) | 22–35 (n = 382) | ≥36 (n = 1164) |
| Demographics | ||||
| Gestational age, median (IQR), wk | 27.3 (26.1–28.6) | 26.4 (25.7–27.6) | 25.9 (25.0–26.7) | 25.0 (24.1–26.0) |
| Birth weight, median (IQR), g | 870 (780–940) | 820 (724–910) | 790 (690–883) | 698 (681–800) |
| Male | 315 (41.5) | 275 (48.9) | 191 (50.0) | 608 (52.2) |
| SGA | 263 (34.7) | 134 (23.8) | 84 (22.0) | 276 (23.7) |
| 5-Minute Apgar score, median (IQR) | 8 (6–8) | 7 (6–8) | 7 (6–8) | 7 (6–8) |
| Treatments and morbidities | ||||
| Surfactant | 530 (69.8) | 399 (71.0) | 293 (76.7) | 917 (78.8) |
| PDA treatment | 274 (36.1) | 289 (51.4) | 238 (62.3) | 811 (69.7) |
| NEC | 15 (2.0) | 39 (6.9) | 17 (4.5) | 139 (11.9) |
| Bacterial sepsis | 160 (21.1) | 204 (36.3) | 163 (42.7) | 684 (58.8) |
| Postnatal dexamethasone treatment | 40 (5.3) | 75 (13.4) | 75 (19.6) | 528 (45.4) |
| Mechanical ventilation duration, median (IQR), d | 3 (2–5) | 14 (10–18) | 29 (25–32) | 59 (46–80) |
| Mechanical ventilation courses, median (IQR), No. | 1 (1–1) | 2 (1–3) | 2 (2–3) | 4 (3–4) |
Abbreviations: IQR, interquartile range; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; SGA, small for gestational age.
Data are presented as number (percentage) of patients unless otherwise indicated. Differences between categories for all patient characteristics are statistically significant at P < .001.
Table 2.
Characteristics of the Infants Who Survived to Discharge Based on the Number of Mechanical Ventilation Coursesa
| No. of Mechanical Ventilation Courses | ||||
|---|---|---|---|---|
| Characteristic | 1 (n = 921) | 2 (n = 636) | 3 (n = 502) | ≥4 (n = 808) |
| Demographics | ||||
| Gestational age, median (IQR), wk | 27.0 (25.9–28.1) | 26.0 (25.0–27.1) | 25.7 (24.9–26.9) | 25.1 (24.3–26.3) |
| Birth weight, median (IQR), g | 848 (737–930) | 800 (685–890) | 750 (650–890) | 710 (614–815) |
| Male | 393 (42.7) | 309 (48.6) | 246 (49.0) | 441 (54.6) |
| SGA | 297 (32.3) | 148 (23.3) | 114 (22.7) | 198 (24.5) |
| 5-Minute Apgar score, median (IQR) | 8 (6–8) | 7 (6–8) | 7 (6–8) | 7 (6–8) |
| Treatments and morbidities | ||||
| Surfactant | 624 (67.8) | 471 (74.1) | 406 (80.9) | 638 (79.0) |
| PDA treatment | 376 (40.8) | 346 (54.4) | 313 (62.4) | 577 (71.4) |
| NEC | 23 (2.5) | 33 (5.2) | 39 (7.8) | 115 (14.2) |
| Bacterial sepsis | 217 (23.6) | 242 (38.1) | 245 (48.8) | 507 (62.8) |
| Postnatal dexamethasone treatment | 74 (8.0) | 122 (19.2) | 142 (28.3) | 380 (47.0) |
| Mechanical ventilation duration, median (IQR), d | 4 (2–13) | 19 (9–37) | 36 (19–55) | 59 (41–87) |
| Mechanical ventilation courses, median (IQR), No. | KD | 2 (2) | 3 (3) | 5 (4–6) |
Abbreviations: BPD, bronchopulmonary dysplasia; IQR, interquartile range; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; SGA, small for gestational age.
Data are presented as number (percentage) of patients unless otherwise indicated. Differences between categories for all patient characteristics are statistically significant at P < .001.
Primary Outcome
A total of 1695 infants (59.1%) who survived to discharge developed BPD. The rates of BPD progressively increased as both the duration of mechanical ventilation and the number of ventilation courses increased (Table 3). After adjustment for potential confounding variables, a greater number of ventilation courses and a longer cumulative duration of mechanical ventilation were associated with increased odds of BPD (Table 4). A longer duration of mechanical ventilation was associated with a greater incremental increase in BPD risk. Compared with infants who received mechanical ventilation for 7 days or less, the adjusted odds of BPD ranged from 2.73 (95% CI, 2.16–3.45) among infants who received mechanical ventilation for 8 to 21 days to 8.69 (95% CI, 6.58–11.48) among infants who received mechanical ventilation for 36 days or longer. In contrast, the adjusted odds of BPD ranged from 1.88 (95% CI, 1.54–2.31) among infants exposed to 2 ventilation courses to 3.81 (95% CI, 2.88–5.04) among those with 4 or more ventilation courses.
Table 3.
Rates of Death and the Study Outcomes Determined for Survivors Based on the Cumulative Duration of Mechanical Ventilation and the Number of Mechanical Ventilation Courses
| No. (%) of Patients | ||||
|---|---|---|---|---|
| Variable | BPD | Discharged on Oxygen | Tracheostomy | Deatha |
| Duration of mechanical ventilation, d | ||||
| ≤7 (n = 759) | 202 (26.6) | 102 (13.4) | 1 (0.1) | 177 (18.9) |
| 8–21 (n = 562) | 287 (51.1) | 109 (19.4) | 1 (0.2) | 116 (17.1) |
| 22–35 (n = 382) | 248 (64.9) | 115 (30.1) | 2 (0.5) | 60 (13.6) |
| ≥36 (n = 1164) | 957 (82.2) | 530 (45.5) | 27 (2.3) | 123 (9.6) |
| P value | <.001 | <.001 | <.001 | <.001 |
| No. of mechanical ventilation courses | ||||
| 1 (n = 921) | 335 (36.4) | 165 (17.9) | 2 (0.2) | 162 (15.0) |
| 2 (n = 636) | 360 (56.5) | 183 (28.8) | 7 (1.1) | 93 (12.8) |
| 3 (n = 502) | 345 (68.7) | 178 (35.5) | 3 (0.6) | 71 (12.4) |
| ≥4 (n = 808) | 654 (80.9) | 330 (40.8) | 19 (2.4) | 150 (15.7) |
| P value | <.001 | <.001 | <.001 | .18 |
Numbers of deaths determined for the full cohort are as follows: 936, 678, 442, and 1287 for the 7 days or less, 8 through 21 days, 22 through 35 days, and 36 days or more of mechanical ventilation, respectively, and 1083, 729, 573, and 958 for 1, 2, 3, and 4 or more courses of mechanical ventilation, respectively.
Table 4.
Adjusted ORs for Study Outcomes Based on the Cumulative Duration of Mechanical Ventilation and the Number of Ventilation Courses
| Adjusted 0Ra (95% Cl) | ||||
|---|---|---|---|---|
| Exposure | BPD | Discharged on Oxygen | Tracheostomy | Death |
| Duration of mechanical ventilation, d | ||||
| ≤7 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 8–21 | 2.73 (2.16–3.45) | 1.35 (1.00–1.81) | 0.97 (0.06–17.01) | 0.60 (0.46–0.77) |
| 22–35 | 4.62 (3.37–6.35) | 2.13 (1.54–2.95) | 3.02 (0.25–36.88) | 0.33 (0.21–0.51) |
| ≥36 | 8.69 (6.58–11.48) | 3.28 (2.40–4.47) | 6.30 (0.52–76.41) | 0.11 (0.08–0.17) |
| No. of ventilation courses | ||||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 1.88 (1.54–2.31) | 1.41 (1.08–1.85) | 2.73 (0.45–16.73) | 0.75 (0.56–0.99) |
| 3 | 2.56 (1.99–3.28) | 1.62 (1.26–2.10) | 1.10 (0.14–8.82) | 0.68 (0.48–0.96) |
| ≥4 | 3.81 (2.88–5.04) | 1.58 (1.14–2.19) | 2.27 (0.34–14.94) | 0.66 (0.49–0.91) |
Abbreviations: BPD, bronchopulmonary dysplasia; OR, odds ratio.
The ORs for death were determined for 3433 infants. The ORs for nondeath outcomes were determined for 2867 infants who survived to discharge. The ORs were adjusted for birth weight, gestational age, small for gestation age, sex, birth year, surfactant exposure, postnatal dexamethasone treatment, patent ductus arteriosus treatment, bacterial sepsis, and diagnosis of necrotizing enterocolitis.
Secondary Outcomes
Among the surviving infants, 856 (29.9%) required supplemental oxygen at discharge and 31 (1.1%) underwent tracheostomy (Table 3). A longer duration of mechanical ventilation and a greater number of ventilation courses were associated with significantly higher adjusted odds of oxygen use at discharge (Table 4). The adjusted odds ratios for tracheostomy suggested a similar increase in the risk associated with both study exposures, but the CIs were wide and included the point of equivalence (Table 4). The adjusted odds of death were inversely proportional to the length of mechanical ventilation and the number of ventilation courses.
Differential Effects of the Study Exposures
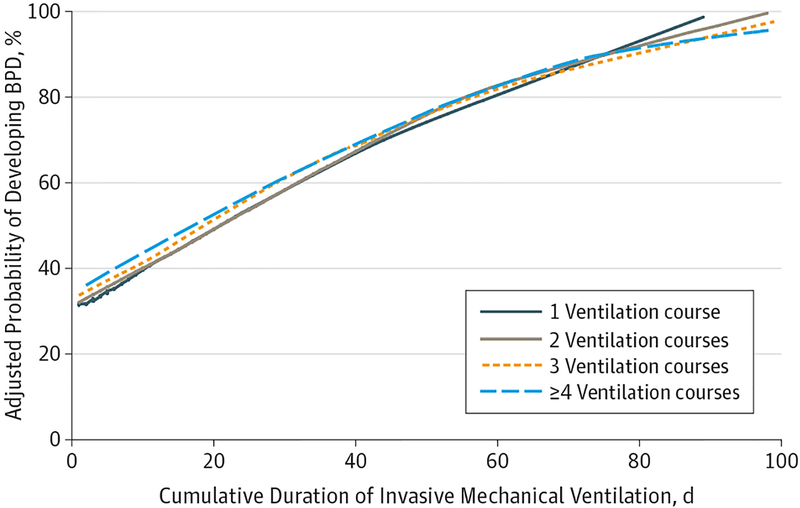
To explore the differential effects of the primary study exposures on the evaluated respiratory outcomes, we constructed multivariable logistic regression models that included categorical definitions of both study exposures. This approach allowed us to determine the relative effect of each study exposure after adjustment for the effect of the other. With this approach, we found that the duration of mechanical ventilation was the stronger predictor of BPD and the use of supplemental oxygen at NICU discharge (Table 5). After adjustment for the cumulative duration of mechanical ventilation, a greater number of ventilation courses was not associated with an increase in the risk-adjusted odds of supplemental oxygen use at discharge. The number of ventilation courses remained associated with increased risk of BPD only among infants exposed to 4 or more courses. When we considered the length of mechanical ventilation as a continuous variable, a longer cumulative duration of ventilation resulted in a nearly identical baseline and incremental increase in the risk-adjusted probability of developing BPD, irrespective of the number of ventilation courses (Figure). Neither of the primary study exposures were associated with increased risk of tracheostomy (Table 5).
Table 5.
Adjusted ORs for Adverse Respiratory Outcomes Among Survivors Obtained From the Logistic Regression Model Inclusive of the Duration of Mechanical Ventilation and the Number of Ventilation Courses
| Adjusted 0Ra (95% Cl) | |||
|---|---|---|---|
| Exposure | BPD | Discharged on Oxygen | Tracheostomy |
| Duration of mechanical ventilation, d | |||
| ≤7 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 8–21 | 2.44 (1.90–3.13) | 1.32 (0.96–1.83) | 0.86 (0.04–20.74) |
| 22–35 | 4.04 (2.90–5.64) | 2.14 (1.46–3.13) | 2.91 (0.17–48.89) |
| ≥36 | 7.10 (5.18–9.74) | 3.50 (2.30–5.31) | 7.80 (0.41–147.23) |
| No. of ventilation courses | |||
| 1 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2 | 1.22 (0.97–1.53) | 1.10 (0.83–1.50) | 2.13 (0.28–16.04) |
| 3 | 1.23 (0.92–1.63) | 1.03 (0.76–1.41) | 0.57 (0.05–6.00) |
| ≥4 | 1.44 (1.04–2.01) | 0.85 (0.56–1.31) | 0.96 (0.12–7.67) |
| Interaction term P valueb | .15 | .14 | .07c |
Abbreviations: BPD, bronchopulmonary dysplasia; OR, odds ratio.
The ORs were determined for 2867 infants who survived to discharge. The ORs were adjusted for birth weight, gestational age, small for gestation age, sex, birth year, surfactant exposure, postnatal dexamethasone treatment, patent ductus arteriosus treatment, bacterial sepsis, diagnosis of necrotizing enterocolitis, cumulative duration of mechanical ventilation, and number of ventilation courses.
P values for the interaction terms provided for reference only. Interaction terms not included in the regression models secondary to P > .05.
The multivariable tracheostomy model did not converge when categorical definitions for the duration of mechanical ventilation and the number of ventilation courses were used. When the duration of mechanical ventilation was used as a continuous variable, the interaction P value was .07.
Figure.
Adjusted Probability of Developing Bronchopulmonary Dysplasia (BPD)
Probabilities are based on the cumulative duration of mechanical ventilation for infants exposed to 1, 2, 3, or 4 or more separate courses of mechanical ventilation and are adjusted for birth weight, gestational age, small for gestation age, sex, birth year, surfactant exposure, postnatal dexamethasone treatment, patent ductus arteriosus treatment, bacterial sepsis, and diagnosis of necrotizing enterocolitis. Plots were generated using locally weighted scatterplot smoothing (LOWESS).
Discussion
Extubation failure and reinitiation of invasive mechanical ventilation are common in extremely preterm infants.7,8 Whether this exposure increases the risk of chronic respiratory morbidity above that attributable to mechanical ventilation is unclear. To address this question, we compared the independent effects of the number of ventilation courses and the cumulative duration of mechanical ventilation on the risk of BPD, death, supplemental oxygen use at NICU discharge, and tracheostomy in a large cohort of ELBW infants. We found that each successive course of mechanical ventilation was associated with a progressive increase in the risk of BPD among survivors and the use of supplemental oxygen at NICU discharge. Contrary to our hypothesis, however, the duration of mechanical ventilation was the stronger predictor of both outcomes. After adjustment for the total length of mechanical ventilation, the number of ventilation courses did not affect the risk of supplemental oxygen use at NICU discharge. The risk of BPD remained increased only amonginfantsexposedto4ormoreventilationcoursesbutwith substantial attenuation of the odds ratio. These findings suggest that reinitiation of invasive mechanical ventilation may not result in additional risk of chronic respiratory morbidity in extremely preterm infants.
Similar to other investigators, we found that each successive course of ventilation was associated with a longer cumulative duration of mechanical ventilation.15,16 However, whether extubation failure in preterm infants directly prolongs mechanical ventilation is unknown. In our cohort, re-intubation rates were higher among infants with lower gestational ages and birth weights. This suggests that extubation failure may be a marker of respiratory and developmental immaturity rather than a cause of prolonged mechanical ventilation. Moreover, Danan et al6 found in a small randomized clinical trial that delay of extubation, once minimal ventilator settings are reached, may not improve extubation success or decrease the total duration of mechanical ventilation.
Unlike the findings of reports in older children and adults,10,11,22 our findings did not reveal an association between multiple intubations and increased risk of tracheostomy or death. In fact, longer durations of ventilation and greater number of ventilation courses were associated with lower odds of death. Because nearly three-quarters of deaths in extremely premature infants occur within the first 2 weeks of life, this observation is not unexpected.23 The low tracheostomy rate (approximately 1%) among the infants who survived to discharge in our cohort limited our ability to draw conclusions about this study end point. Although the odds ratios for tracheostomy increased as the duration of ventilation increased, the CIs were wide and the differences not statistically significant. Analysis of data pooled from large academic centers where the overall tracheostomy rate among ELBW infants is approximately 4% may produce different results.24
Multiple studies15,16,25–31 have explored methods to predict extubation success in preterm infants. Although several techniques show promise, none are clearly better than physician judgment. Given the well-established sequelae associated with prolonged mechanical ventilation in preterm infants, our results suggest that a practice of trialing extubation when low ventilator settings are reached, even if extubation success is not guaranteed, may reduce the risk of chronic respiratory morbidity.
This study has several strengths. To our knowledge, this is the largest evaluation of the association between multiple intubations and clinically relevant respiratory outcomes in ELBW infants. Our findings should be broadly generalizable because the infants included in the present cohort were cared for in both academic- and community-based NICUs located throughout the United States. Although this analysis was conducted retrospectively, our data set includes concurrently collected data abstracted by trained neonatal health care professionals.
We also acknowledge several limitations. Our data source only permitted identification of ventilation courses separated by a day or more. We did not have information on the criteria used to determine extubation timing or the reasons for reintubation. In particular, it is possible that ventilation courses initiated with an elective compared with an emergency reintubation may have a different effect on the risk of adverse respiratory outcomes. We also did not assess the potential effects of the postmenstrual age at ventilation, the duration of time between the periods of mechanical ventilation, or the characteristics of the provided respiratory support. Finally, we evaluated respiratory outcomes among survivors instead of morbidity-mortality composite outcomes. Although this approach may produce selection bias due to the competing risk of death with the study outcomes, morbidity-mortality composite outcomes are often uninformative when the effect size of the individual components is large and in the opposite direction, as was the case in this study.32,33
Conclusions
Our findings suggest that reinitiation of invasive mechanical ventilation does not increase the risk of chronic respiratory morbidity above that attributable to the cumulative duration of mechanical ventilation. A practice of routinely trialing extubation when low ventilator settings are reached, even if extubation success is not guaranteed, may reduce the risk of lung injury and chronic respiratory impairment in extremely preterm infants.
Supplementary Material
At a Glance.
There is a paucity of data on the adverse long-term respiratory outcomes associated with reinitiation of mechanical ventilation in extremely preterm infants.
The present study evaluated whether exposure to multiple courses of mechanical ventilation increased the risk of bronchopulmonary dysplasia (BPD), tracheostomy, and supplemental oxygen use at discharge among extremely low-birth-weight (ELBW) infants.
Compared with a single ventilation course, the adjusted odds ratios for BPD ranged from 1.88 (95% CI, 1.54–2.31) among ELBW infants with 2 ventilation courses to 3.81 (95% CI, 2.88–5.04) among those with 4 or more courses.
After adjustment for the cumulative duration of mechanical ventilation, the risk-adjusted odds of BPD were only increased among infants exposed to 4 or more ventilation courses (adjusted odds ratio, 1.44; 95% CI, 1.04–2.01). A greater number of ventilation courses did not increase the risk of tracheostomy or supplemental oxygen use at discharge after adjustment for the length of ventilation.
These findings support a practice of routinely trialing extubation when low ventilator settings are reached, even if extubation success is not guaranteed.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sant’Anna GM, Keszler M. Weaning infants from mechanical ventilation. Clin Perinatol 2012;39(3): 543–562. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Walsh MC, Vohr BR, et al. ; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. [DOI] [PubMed] [Google Scholar]
- 4.Walsh MC, Morris BH, Wrage LA, et al. ; National Institutes of Child Health and Human Development Neonatal Research Network. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr 2005;146(6):798–804. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007;357(19):1893–1902. [DOI] [PubMed] [Google Scholar]
- 6.Danan C, Durrmeyer X, Brochard L, Decobert F, Benani M, Dassieu G. A randomized trial of delayed extubation for the reduction of reintubation in extremely preterm infants. Pediatr Pulmonol 2008;43(2):117–124. [DOI] [PubMed] [Google Scholar]
- 7.Giaccone A, Jensen E, Davis P, Schmidt B. Definitions of extubation success in very premature infants: a systematic review. Arch Dis Child Fetal Neonatal Ed 2014;99(2):F124–F127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS; NIPPV Study Group. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med 2013;369(7):611–620. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Alía I, Gordo F, et al. ; Spanish Lung Failure Collaborative Group. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. Am J Respir Crit Care Med 1997;156(2, pt 1):459–465. [DOI] [PubMed] [Google Scholar]
- 10.Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med 2005;6(3):312–318. [DOI] [PubMed] [Google Scholar]
- 11.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192. [DOI] [PubMed] [Google Scholar]
- 12.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011;39(12):2612–2618. [DOI] [PubMed] [Google Scholar]
- 13.Manley B, Owen L, Doyle L, Davis P. Extubating extremely preterm infants: predictors of success and outcomes of failure In: Proceedings from the Pediatric Academic Societies Annual Meeting; April 25–28, 2015; San Diego, CA: Abstract 1675.4. [Google Scholar]
- 14.Berger J, Mehta P, Bucholz E, Dziura J, Bhandari V. Impact of early extubation and reintubation on the incidence of bronchopulmonary dysplasia in neonates. Am J Perinatol 2014;31(12):1063–1072. [DOI] [PubMed] [Google Scholar]
- 15.Chawla S, Natarajan G, Gelmini M, Kazzi SNJ. Role of spontaneous breathing trial in predicting successful extubation in premature infants. Pediatr Pulmonol 2013;48(5):443–448. [DOI] [PubMed] [Google Scholar]
- 16.Kavvadia V, Greenough A, Dimitriou G. Prediction of extubation failure in preterm neonates. Eur J Pediatr 2000;159(4):227–231. [DOI] [PubMed] [Google Scholar]
- 17.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. [DOI] [PubMed] [Google Scholar]
- 18.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. [DOI] [PubMed] [Google Scholar]
- 19.Rogers W Regression standard errors in clustered samples. Stata Tech Bull. 1993;13:19–23. http://www.stata.com/support/faqs/statistics/stb13_rogers.pdf. Accessed June, 28, 2015. [Google Scholar]
- 20.Marshall SW. Power for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014;43(3):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein SK. Extubation failure: an outcome to be avoided. Crit Care. 2004;8(5):310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel RM, Kandefer S, Walsh MC, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015;372(4):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMauro SB, D’Agostino JA, Bann C, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Developmental outcomes of very preterm infants with tracheostomies. J Pediatr 2014;164(6):1303–1310.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitriou G, Fouzas S, Vervenioti A, Tzifas S, Mantagos S. Prediction of extubation outcome in preterm infants by composite extubation indices. Pediatr Crit Care Med 2011;12(6):e242–e249. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie LM, White SD, Sinha SK, Donn SM. Usefulness of the minute ventilation test in predicting successful extubation in newborn infants: a randomized controlled trial. J Perinatol 2003;23(3):205–207. [DOI] [PubMed] [Google Scholar]
- 27.Hiremath GM, Mukhopadhyay K, Narang A. Clinical risk factors associated with extubation failure in ventilated neonates. Indian Pediatr 2009; 46(10):887–890. [PubMed] [Google Scholar]
- 28.Kamlin COF, Davis PG, Argus B, Mills B, Morley CJ. A trial of spontaneous breathing to determine the readiness for extubation in very low birth weight infants: a prospective evaluation. Arch Dis Child Fetal Neonatal Ed 2008;93(4):F305–F306. [DOI] [PubMed] [Google Scholar]
- 29.Kamlin COF, Davis PG, Morley CJ. Predicting successful extubation of very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2006;91(3):F180–F183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller M, Almeida JS, Stanislaus R, Wagner CL. Can machine learning methods predict extubation outcome in premature infants as well as clinicians? J Neonatal Biol 2013;2(2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vento G, Tortorolo L, Zecca E, et al. Spontaneous minute ventilation is a predictor of extubation failure in extremely-low-birth-weight infants. J Matern Fetal Neonatal Med 2004;15(3): 147–154. [DOI] [PubMed] [Google Scholar]
- 32.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–2559. [DOI] [PubMed] [Google Scholar]
- 33.Montori VM, Permanyer-Miralda G, Ferreira-González I, et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.