Abstract
Objective
To determine the expression profile of small non-coding RNAs (sncRNAs) which have not been investigated to date in leiomyoma.
Design
Laboratory based investigation.
Setting
Academic center.
Patients
Women undergoing hysterectomy for benign indications.
Intervention
Next generation sequencing and screening of sncRNA database with confirmatory analysis by QRT-PCR.
Main Outcome Measure
Expression profile of sncRNAs in leiomyoma and matched myometrium.
Results
Screening our previously determined RNA sequencing data with sncRNAs database resulted in identification of 15 snRNAs, 284 snoRNAs, 98 piRNAs, 152 tRNAs and 45 rRNAs of which 15 snoRNAs, 24 piRNAs, 7 tRNAs and 6 rRNAs were differentially expressed at 1.5 fold change cutoff in leiomyoma as compared to myometrium. We selected 5 snoRNAs, 4 piRNAs, 1 tRNA, and 1 rRNA which were differentially expressed and confirmed their expression in paired tissues (N=20) from both phases of the menstrual cycle using QRT-PCR. The results indicated upregulation of snoRNAs (SNORD30, SNORD27, SNORA16A, SNORD46 and SNORD56) and down-regulation of piRNAs (piR-1311, piR-16677, piR-20365, piR-4153), tRNA (TRG-GCC5-1), and rRNA (RNA5SP202) expression in leiomyoma as compared to myometrium (P<0.05). The pattern of expression of these sncRNAs was similar to RNA sequencing analysis with no menstrual cycle-dependent differences detected except for SNORD30. Since Argonaute 2 (AGO2) is required for sncRNAs mediated gene silencing, we determined its expression and found greater abundance in leiomyoma.
Conclusion
Our results provide the first evidence for the differential expression of additional classes of sncRNAs and AGO2 in leiomyoma, and implicate their roles as a gene regulatory mechanism.
Keywords: sncRNA, piRNA, snoRNA, AGO2, leiomyoma
Introduction
Leiomyomas are benign uterine tumors with an unknown etiology affecting 40-70% of women during their reproductive years (1). Symptomatic tumors are a major cause of chronic pelvic pain, abnormal uterine bleeding and associated infertility, and account for over 30% of all hysterectomies performed in the United States annually (1). Although ovarian steroids are known to be key regulators of leiomyoma growth, altered expression of many protein-coding genes (1–3) as well as genetic heterogeneity associated with chromosomal re-arrangements and mutation in a number of genes have also been associated with their development and growth progression (1). In addition to protein-coding genes, conventional, microarray and recent high-throughput sequencing have provided further evidence for the expression of a large number of non-protein-coding RNAs (ncRNAs) in leiomyoma (4–6).
As of to date tens of thousands of non-protein coding RNAs (ncRNAs) derived from different genomic loci have been identified. These RNAs are classified as small (17–120 nucleotides long) and long (>200 nucleotides long) non-coding RNAs (sncRNAs and lncRNAs) (7). SncRNAs are transcribed as precursors of 60- to 300 nucleotides long and in their mature form are further classified as miRNAs (17–22 nucleotides), small nuclear/nucleolar RNAs (snRNA and snoRNAs)[70–120 nucleotides], PIWI-interacting RNAs (piRNAs)[26–33 nucleotides]. SnoRNAs are highly conserved and classified as C/D box (SNORDs) or H/ACA box (SNORAs) based on their sequence and structure (8). MiRNAs have been most intensively investigated in the past few years, and accumulated evidence supports their regulatory function on a vast number of protein-coding genes involved in various normal cellular activities. Aberrant expression of miRNAs is associated with a wide range of disorders, including tumorigenesis and tissue fibrosis (9, 10). In previous studies including our own high throughput sequencing identified the expression profile of a large number of miRNAs in leiomyoma and myometrium and provided support for altered expression and regulatory function of a number of them, including let7, miR-21, miR-29, miR-200 and miR-25/93/106 cluster in leiomyoma and leiomyoma smooth muscle cells (6, 11–15).
Far less is known about the function and expression profile of other members of sncRNAs. Functionally snoRNAs play a central role in modifying and processing sncRNAs, ribosomal (rRNA) and transfer RNA (tRNA) (16). PiRNAs are the largest class of sncRNA family and functionally regulate epigenetic, post-transcriptional gene expression and degradation (17). Transfer RNAs (tRNAs) are derived from pre-tRNA by endonuclease RNase Z and in their mature form play a well-defined role in protein translation (18). However, shorter tRNA fragments (tRFs) of 30–35 nucleotides long are also generated through cleavage of mature tRNAs at both 5′ and 3′ end at the anticodon loop by angiogenin (19). In addition, snRNAs, snoRNAs, tRNAs and rRNAs can be further processed into miRNAs or siRNAs, and incorporated into Argonaute proteins to form RNA-induced silencing complex (RISC) for cleavage, degradation or inhibition of translation of downstream target mRNA (20).
Argonaute proteins are the active part of RISC and play a central role in RNA silencing processes, resulting in RNA degradation or translation inhibition (21). In humans, there are eight Argonaute family members and are separated into two subfamilies: 1). four members of Argonaute-like subfamily interact with miRNAs and siRNAs, and 2). four members of PIWI-like subfamily bind to piRNAs (20). The Argonaute family members are composed of four characteristic domains: the N- terminal domain, the PIWI-Argonaute-Zwille (PAZ) domain, the middle (MID) domain and the C-terminal PIWI domain (20). Among them, the PIWI domain, structurally resembles RNase H, is essential for target RNA cleavage through an active catalytic motif “Asp-Asp-Asp/His/Glu/Lys” that harbors a divalent metal ion, and is necessary for catalysis. The PIWI domain also mediates protein-protein interaction with Dicer at one of its RNase III domain (20). Although four Argonaute-like proteins are capable of loading miRNAs or siRNAs, only Argonaute 2 (AGO2) has endonuclease activity in human, thus playing a key role in gene silencing (22).
The identity and pattern of expression of snRNAs, snoRNAs, piRNAs, tRNAs and rRNAs as well as AGO2 in leiomyoma and myometrium has not been evaluated to date. As such in the present study using our existing high-throughput sequencing data set generated from leiomyoma and paired myometrium we carried out an in-depth data analysis to identify the expression profile of snRNAs, snoRNAs, piRNAs, tRNAs and rRNAs. Using QRT-PCR we selected and confirmed the expression of a number of differentially expressed sncRNAs along with detection of AGO2 protein in twenty pairs of leiomyoma and matched myometrium tissues from both phases of the menstrual cycle.
Materials and Methods
Myometrium and Leiomyoma Tissues Collection
Leiomyomas (3–5 cm in diameters) and paired myometrium (N=20) were collected at Harbor-UCLA Medical Center with prior approval obtained from Institutional Review Board at LA BioMed Research Institute at Harbor-UCLA Medical Center. The paired tissues were from white Hispanic (N=15) and African-American (N=5) women ages ranging from 30 to 49 years (mean=42±5.6 years). This sample set included 3 paired tissues previously used for high throughput sequencing (4). Based on endometrial histology the paired leiomyoma and myometrium were from follicular (N=10) and luteal (N=10) phase of the menstrual cycle. The tissues were snap-frozen and stored in liquid nitrogen for further analysis.
Total RNA isolation and RNA sequencing
Total RNA was isolated from leiomyoma and matched myometrium using miRNeasy Mini Kit (Qiagen, Germantown, MD) and RNA concentration and integrity were determined as previously described (4). Samples with RNA integrity numbers (RIN) greater than or equal to 9 were used for library preparation. The RNA sequencing was carried out at UCLA Clinical Microarray Core Facility (http://pathology.ucla.edu/tcgb) as previously described (4). Briefly, for the production of small RNA sequencing libraries 500 ng of total RNA from each tissue was used and libraries were prepared according to manufacturer’s instructions of the Truseq small-RNA kit (Illumina, San Diego, CA). Each library was then pooled and sequenced on a MiSeq single-ended 35-bp run (Illumina) to 10 million reads with 80-90% alignment per library. Our sequencing GEO accession number is GSE100338.
Assembly of snRNA, snoRNA, piRNA, tRNA and rRNA annotations
For data quality control, FASTQC was used to check the raw fastq data quality and Trimmomatic was used to remove adaptors and to trim quality bases. After adapter clipping, we removed leading and trailing ambiguous or low quality bases (below Phred quality scores of 33). Trimmomatic works with a user-defined window spanning the read from 5′ to 3′ and removes bases only at the 3′-end; we set up a window length of 4 and a quality threshold Q of 20. When the average quality droped below 20, the 3′-end was clipped. The low-quality reads and reads shorter than 15nt were trimmed by Cutadapt (23). The filtered reads were mapped to known human sncRNA reference index (24), including mature miRNA (miRBase, release 21), precursor miRNA (miRBase, release 21), tRNA (Genomic tRNA Database), piRNA (piRBase), rRNA (ENSEMBL, release 76), snRNA, snoRNA and mitochondrial RNA (ENSEMBL, release 76). Read count outputs from AASRA pipeline (An Anchor Alignment-Based Small RNA Annotation Pipeline) (25) were statistically normalized by DESeq2 (26). Analyzed read counts for snRNA, snoRNA, piRNA, tRNA and rRNA were further sorted based on 1.5 fold change cutoff and p<0.05.
Quantitative RT-PCR Analysis
Total RNA was extracted from leiomyoma and matched myometrium (N=20) using TRIzol (Thermo Fisher Scientific, Carlsbad, CA) and their quantity and quality was determined (ND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington, DE) as previously described (27–29). One μg of RNA was reverse transcribed using random primers for snoRNAs detection. Quantitative RT-PCR was carried out using SYBR gene expression master mix (Applied Biosystems). Reactions were incubated for 10 min at 95°C followed by 40 cycles for 15 seconds at 95°C and 1 min at 60°C. For piRNAs, tRNA and rRNA detection, primer design and PCR conditions were followed as described previously (30, 31). The level of sncRNAs was determined using Invitrogen StepOne System with RNU6B used for normalization. All reactions were run in triplicate and relative expression was analyzed with the comparative cycle threshold method (2−ΔΔCT) according to the manufacturer (Applied Biosystems). Values were expressed as fold change compared to the control group. The primer sequences used are listed in supplementary Table 1.
Immunoblotting
Total protein was isolated from leiomyoma and paired myometrium (N=20) and subjected to immunoblotting as previously described (32, 33). AGO2 antibody (Cell Signaling Technology, Inc., Danvers, MA) was used for AGO2 detection. The band densities were determined using image J program (http://imagej.nih.gov/ij/), normalized to a band obtained from staining the membrane with Ponceau S. Results were expressed as a ratio relative to the control group designated as 1.
Statistical analysis
Throughout the text, results are expressed as mean ± SEM and analyzed by PRISM software (Graph-Pad, San Diego, CA). Comparisons involving two groups were analyzed using Student’s t-test. Statistical significance was established at P<0.05.
Results
Analysis of snRNAs sequencing in Leiomyoma and Matched Myometrium
Using high throughput RNA sequencing we have recently reported the expression profile of lncRNAs, miRNAs (a member of sncRNAs) and mRNAs in leiomyoma and paired myometrium (4). Using the existing sequencing data set we remapped and aligned our data with sncRNA sequenced database (miRbase, piRBase, Genomic tRNA database, and ENSEMBL annotations) which contains the curated sncRNAs, with specific emphasis placed on snoRNAs, snRNAs, piRNAs, tRNAs and rRNAs. Screening of 437 snoRNAs, 1828 snRNAs, 23439 piRNAs, 610 tRNAs and 522 rRNAs in the database resulted in identification of 31 snRNAs, 284 snoRNAs, 180 piRNAs, 152 tRNAs and 63 rRNAs in leiomyoma and paired myometrium (Supplementary Table 2). Despite considerable variation, further analysis after normalization by DESeq2 (26) revealed that 15 snoRNAs, 24 piRNAs, 7 tRNAs, and 6 rRNAs were differentially expressed at 1.5 fold change cutoff with no significant difference in snRNA expression in leiomyoma as compared to myometrium (Table 1).
Table 1.
Summarized Results of RNA Sequencing Data in leiomyoma as compared to matched myometrium (N=3; ≥ 1.5 or ≤ 0.75 fold change) and the background information of the differential expressed snoRNAs, piRNAs, tRNAs and rRNAs.
| Transcripts Total | Expressed in all Paired tissues |
Differentially Expressed |
Gene symbol | Gene description | Aliases | Regulation | Fold Change | p Value | Chromosome Location | |
|---|---|---|---|---|---|---|---|---|---|---|
| piRNA | 180 | 85 | 24 | hsa_piR_000753 | Piwi-interacting RNA 753 | piR-31052 | Down | 0.5263 | 0.0177 | chr17 |
| hsa_piR_000794 | Piwi-interacting RNA 794 | piR-31104 | Down | 0.5347 | 0.0136 | chr6 | ||||
| hsa_piR_001311 | Piwi-interacting RNA 1311 | piR-31924 | Down | 0.6059 | 0.0194 | chr1 and 3 | ||||
| hsa_piR_004153 | Piwi-interacting RNA 4153 | piR-43772 | Down | 0.5468 | 0.0009 | chr3 | ||||
| hsa_piR_004307 | Piwi-interacting RNA 4307 | piR-43993 | Down | 0.6105 | 0.1194 | chr3, 5 and 6 | ||||
| hsa_piR_006426 | Piwi-interacting RNA 6426 | piR-46895 | Down | 0.6222 | 0.0626 | chr17 | ||||
| hsa_piR_009228 | Piwi-interacting RNA 9228 | piR-32608 | Down | 0.6283 | 0.1112 | chr17 | ||||
| hsa_piR_009294 | Piwi-interacting RNA 9294 | piR-32678 | Down | 0.6953 | 0.1063 | chrY | ||||
| hsa_piR_009295 | Piwi-interacting RNA 9295 | piR-32679 | Down | 0.6867 | 0.2222 | chr17 | ||||
| hsa_piR_015026 | Piwi-interacting RNA 15026 | piR-57660 | Down | 0.5742 | 0.0610 | chr1, 3 and 4 | ||||
| hsa_piR_016677 | Piwi-interacting RNA 16677 | piR-33065 | Down | 0.6695 | 0.0412 | chr2 | ||||
| hsa_piR_016742 | Piwi-interacting RNA 16742 | piR-33161 | Down | 0.4766 | 0.0123 | chr1, 5 and 16 | ||||
| hsa_piR_016945 | Piwi-interacting RNA 16945 | piR-33468 | Down | 0.5214 | 0.0031 | chr1, 5, 6 and 16 | ||||
| hsa_piR_017723 | Piwi-interacting RNA 17723 | piR-60576 | Down | 0.6963 | 0.2539 | chr2, 3, 5 and 6 | ||||
| hsa_piR_017724 | Piwi-interacting RNA 17724 | piR-60577 | Down | 0.6487 | 0.1722 | chr1, 5, 6 and 11 | ||||
| hsa_piR_018569 | Piwi-interacting RNA 18569 | piR-61645 | Down | 0.7030 | 0.2534 | chr1, 3 and 4 | ||||
| hsa_piR_018570 | Piwi-interacting RNA 18570 | piR-61648 | Down | 0.6259 | 0.0271 | chr1, 2, 5, 6, 16 and 17 | ||||
| hsa_piR_019825 | Piwi-interacting RNA 19825 | piR-35284 | Down | 0.4448 | 0.0023 | chr1 | ||||
| hsa_piR_019914 | Piwi-interacting RNA 19914 | piR-35413 | Down | 0.5331 | 0.0102 | chr1, 5, 6 and 16 | ||||
| hsa_piR_020326 | Piwi-interacting RNA 20326 | piR-35982 | Down | 0.5240 | 0.0319 | chr1, 3, 6 and 16 | ||||
| hsa_piR_020365 | Piwi-interacting RNA 20365 | piR-36041 | Down | 0.6139 | 0.0467 | chr11 | ||||
| hsa_piR_000765 | Piwi-interacting RNA 765 | piR-31068 | Down | 0.7164 | 0.2774 | chr1, 2, 5 and 16 | ||||
| hsa_piR_001312 | Piwi-interacting RNA 1312 | piR-31925 | Down | 0.7116 | 0.1113 | chr1, 2, 5, 6, 16 and 17 | ||||
| hsa_piR_017716 | Piwi-interacting RNA 17716 | piR-60565 | Down | 0.7487 | 0.3280 | chr1, 3 and 6 | ||||
| tRNA | 152 | 72 | 7 | tRNA-Glu-CTC-1-3 | Transfer RNA-Glu (CTC) 1-3 | TRNAE22 | Down | 0.6755 | 0.2088 | chr1 |
| tRNA-Glu-CTC-1-5 | Transfer RNA-Glu (CTC) 1-5 | TRNAE5 | Down | 0.6395 | 0.1482 | chr1 | ||||
| tRNA-Gly-GCC-5-1 | Transfer RNA-Gly (GCC) 5-1 | TRNAG20 | Down | 0.5275 | 0.0059 | chr16 | ||||
| tRNA-Lys-CTT-4-1 | Transfer RNA-Lys (CTT) 4-1 | TRNAK9 | Down | 0.6500 | 0.1105 | chr16 | ||||
| tRNA-Glu-CTC-1-2 | Transfer RNA-Glu (CTC) 1-2 | TRNAE13 | Down | 0.7134 | 0.2638 | chr1 | ||||
| tRNA-Glu-CTC-1-7 | Transfer RNA-Glu (CTC) 1-7 | TRNAE19 | Down | 0.7554 | 0.3511 | chr6 | ||||
| tRNA-His-GTG-1-9 | Transfer RNA-His (GTG) 1-6 | TRNAH5; TRH-GTG1-9 | Down | 0.7128 | 0.2502 | chr15 | ||||
| rRNA | 63 | 29 | 6 | RNA5SP202 | RNA, 5S ribosomal pseudogene 202 | RN5S202 | Down | 0.5882 | 0.0259 | chr6 |
| RNA5SP150 | RNA, 5S ribosomal pseudogene 150 | RN5S150 | Down | 0.6798 | 0.1125 | chr3 | ||||
| RNA5SP355 | RNA, 5S ribosomal pseudogene 355 | RN5S355 | Down | 0.6894 | 0.2412 | chr12 | ||||
| RNA5SP199 | RNA, 5S ribosomal pseudogene 199 | RN5S199 | Down | 0.7243 | 0.2294 | chr5 | ||||
| RNA5SP103 | RNA, 5S ribosomal pseudogene 103 | RN5S103 | Down | 0.7558 | 0.2727 | chr2 | ||||
| RNA5SP450 | RNA, 5S ribosomal pseudogene 450 | RN5S450 | Down | 0.7270 | 0.3132 | chr18 | ||||
| snoRNA | 284 | 243 | 15 | SNORD36A | Small nucleolar RNA, C/D box 36A | RNU36A; U36a | Up | 1.5998 | 0.0228 | chr9 |
| SNORA68 | Small nucleolar RNA, H/ACA box 68 | RNU68A; U68 | Up | 1.5299 | 0.0928 | chr19 | ||||
| SNORD113 | Small nucleolar RNA, C/D box 113 | 14q(I) | Up | 1.7936 | 0.0201 | chr14 | ||||
| SNORD56 | Small nucleolar RNA, C/D box 56 | RNU56; U54 | Up | 1.5767 | 0.0272 | chr20 | ||||
| SNORD54 | Small nucleolar RNA, C/D box 54 | RNU54; U54 | Up | 1.6456 | 0.0626 | chr8 | ||||
| SNORD52 | Small nucleolar RNA, C/D box 52 | RNU52; U52 | Up | 1.5259 | 0.0202 | chr6 | ||||
| SNORA77 | Small nucleolar RNA, H/ACA box 77 | ACA63; SNORA77A | Up | 1.5513 | 0.1650 | chr1 | ||||
| SNORD114-17 | Small nucleolar RNA, C/D box 114-17 | 14q(II-17) | Up | 1.9226 | 0.0101 | chr14 | ||||
| SNORA16A | Small nucleolar RNA, H/ACA box 16A | ACA16 | Up | 1.6404 | 0.0310 | chr1 | ||||
| SNORD96A | Small nucleolar RNA, C/D box 96A | U96A | Up | 1.5495 | 0.0210 | chr5 | ||||
| SNORD73A | Small nucleolar RNA, C/D box 73A | RNU73A; U73a | Up | 1.5996 | 0.0377 | chr4 | ||||
| SNORD94 | Small nucleolar RNA, C/D box 94 | U94 | Up | 1.4956 | 0.0495 | chr2 | ||||
| SNORD49B | Small nucleolar RNA, C/D box 49B | U49B | Up | 1.6227 | 0.0227 | chr17 | ||||
| SNORD16 | Small nucleolar RNA, C/D box 16 | U16 | Up | 1.6850 | 0.0095 | chr15 | ||||
| SNORD113-5 | Small nucleolar RNA, C/D box 113-5 | 14q(I-5) | Up | 1.8938 | 0.0057 | chr14 | ||||
| snRNA | 31 | 15 | 0 |
QRT-PCR Expression of sncRNAs in Leiomyoma and Matched Myometrium
To provide support and validate RNA sequencing data analysis we selected a number of differentially expressed snoRNAs (SNORD30, SNORD27, SNORA16A, SNORD46 and SNORD56), piRNAs (piR-1311, piR-16677, piR-20365, piR-4153), tRNA (TRG-GCC5-1) and rRNA (RNA5SP202) and confirmed their expression by QRT-PCR in leiomyoma and paired myometrium (N=20) including the same tissues used in RNA sequencing. The selection of these sncRNAs was based on their differential expression obtained from RNA sequencing in leiomyoma as compared to myometrium (N=3) as well as prior reports demonstrating their altered expression in various disorders, including tumorigenesis and tumor progression (34–40). QRT-PCR analysis demonstrated that all the above snoRNAs, piRNAs, tRNA, and rRNA are expressed in leiomyomas and paired myometrium with up-regulated expression of snoRNAs and down-regulated expression of piRNAs, tRNA, rRNA in leiomyomas as compared to matched myometrium (P<0.05; Fig. 1A–C). Expression of these sncRNAs as determined by QRT-PCR displayed a similar pattern as RNA sequencing analysis. Further analysis of QRT-PCR results was also carried out in paired tissues based on the phase of the menstrual cycle. This analysis indicated that there were no statistically significant differences in expression of selected piRNAs, tRNA, rRNA and snoRNAs between follicular (N=10) and luteal (N=10) phase, with the exception of SNORD30 which was down-regulated in myometrium from luteal phase as compared to follicular phase (P<0.05; Fig. 2A–C).
Figure 1.
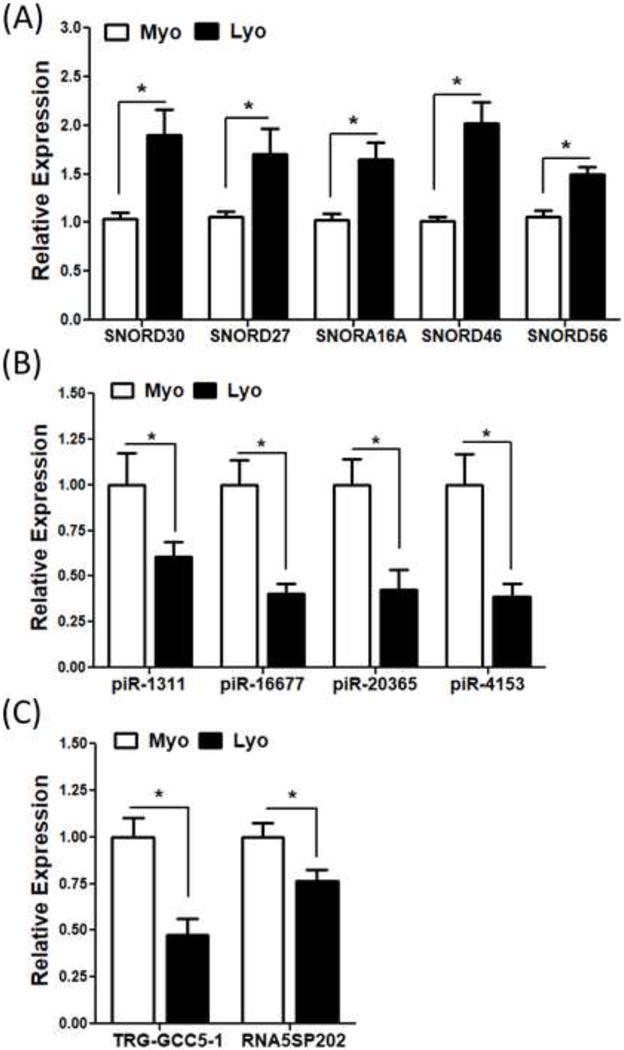
The expression of selected (A) snoRNAs (SNORD30, SNORD27, SNORA16A, SNORD46 and SNORD56), (B) piRNA (piR-1311, piR-16677, piR-20365 and piR-4153) and (C) tRNA (TRG-GCC5-1) and rRNA (RNA5SP202) in twenty paired leiomyoma tissues by QRT-PCR. The results are presented as mean ± SEM with P values (*P<.05) indicated by corresponding lines.
Figure 2.
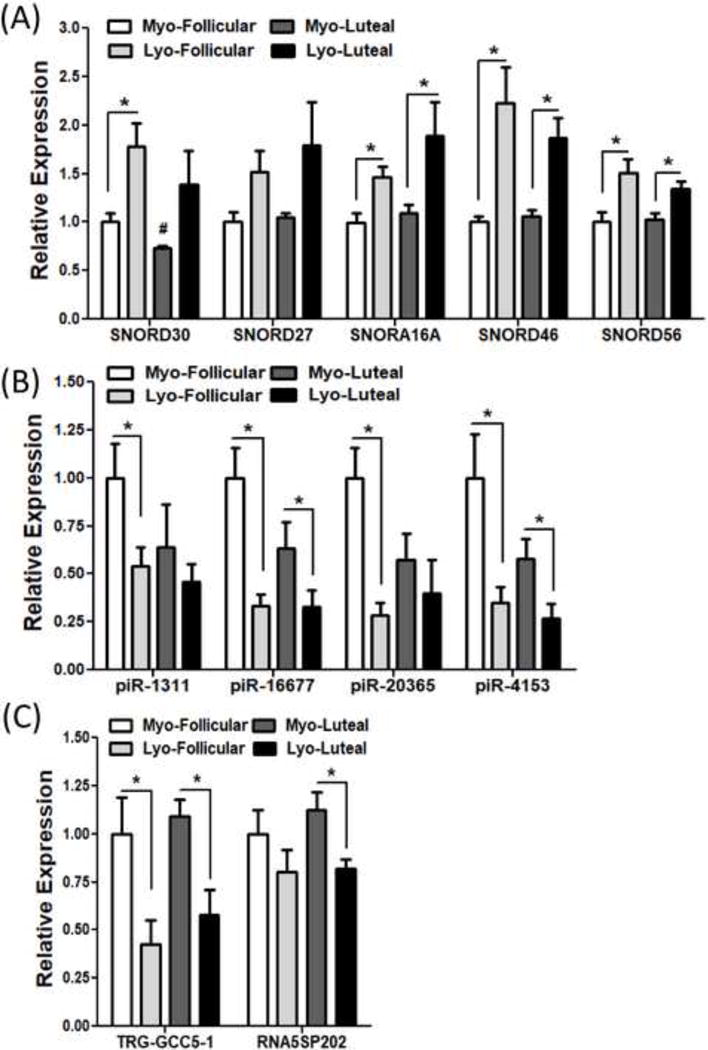
The expression of selected (A) snoRNAs (SNORD30, SNORD27, SNORA16A, SNORD46 and SNORD56), (B) piRNA (piR-1311, piR-16677, piR-20365 and piR-4153) and (C) tRNA (TRG-GCC5-1) and rRNA (RNA5SP202) in twenty paired leiomyoma tissues separated by follicular (N=10) or luteal (N=10) phase of menstrual cycle by QRT-PCR. The results are presented as mean ± SEM with P values (*P<.05) indicated by corresponding lines. #: P<.05 as compared to Myo-Follicular group.
AGO2 expression in Leiomyoma and Matched Myometrium
Since AGO2 plays an essential role in sncRNAs mediated gene silencing (41), we assessed the expression of AGO2 in paired leiomyoma and myometrium (N=20) using western blot analysis. The results indicated that AGO2 was expressed in greater abundance in leiomyomas as compared to myometrium (P<0.05; Fig. 3A–B) with no significant difference in AGO2 expression between follicular as compared to luteal phase of the menstrual cycle (Fig. 3C).
Figure 3.
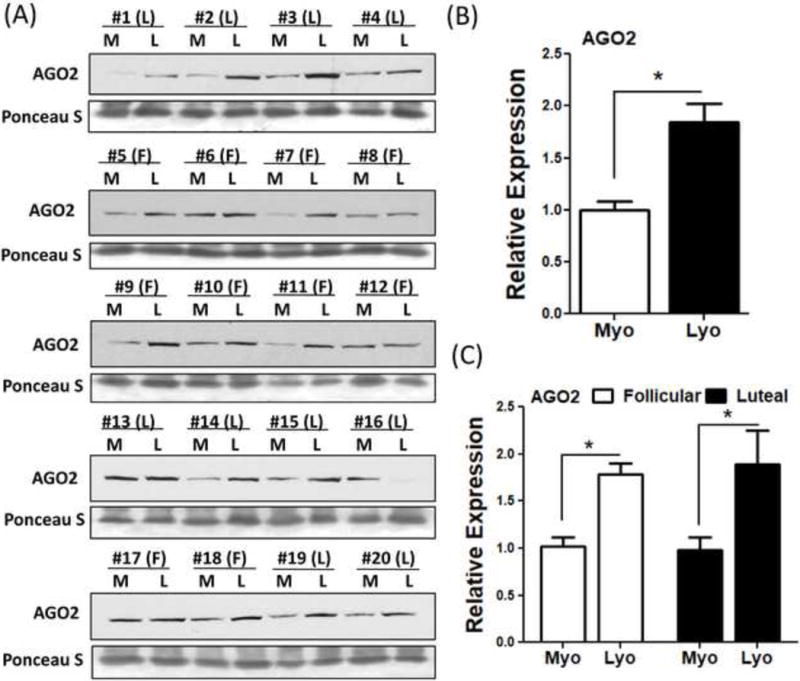
Representative protein levels of AGO2 (A) in twenty paired leiomyoma tissues. The mean expression of AGO2 is shown in (B) (N=20) and separated according to menstrual cycle phase: Follicular (F, N=10) and Luteal (L, N=10) phase is shown in (C). The results are presented as mean ± SEM. *P<.05.
Discussion
Through high-throughput RNA sequencing we have recently reported the expression profile of a large number of lncRNAs with concurrent expression of miRNAs and protein-coding RNAs profiles in leiomyoma and paired myometrium (4). Using the existing RNA sequencing data in the present study through annotation with AASRA pipeline and miRbase, piRBase, Genomic tRNA database, and ENSEMBL annotations, which provides comprehensive up-to-date database information on human sncRNAs (437 snoRNAs, 1828 snRNAs, 23439 piRNAs, 610 tRNAs and 522 rRNAs), enabled us to further characterize the expression profile of other classes of sncRNAs (snRNAs, snoRNAs, piRNAs, tRNAs and rRNAs) in leiomyoma and paired myometrium. The annotation analysis resulted in identification of 284 snoRNAs, 31 snRNAs, 180 piRNAs, 152 tRNAs and 63 rRNAs transcripts in leiomyoma and myometrium of which 15 snoRNAs, 24 piRNAs, 7 tRNAs, and 6 rRNAs were differentially expressed in leiomyomas, and confirmed by QRT-PCR. Although we expected to detect a substantially larger number of sncRNAs transcripts in these paired tissues, we consider possible optimal library preparation utilized in our initial study and/or degradation or chemical modification of transcripts may have accounted for detection of fewer numbers of sncRNAs. Despite this limitation to the best of our knowledge these results are the first to identify differential expression of these sncRNAs in leiomyoma.
Leiomyomas used in our RNA sequencing study were from the luteal phase while the QRT-PCR analysis included paired tissues from both phases of the menstrual cycle in leiomyomas that were 3–5 cm in diameters in size. Under these conditions, QRT-PCR analysis displayed similar pattern of expression as RNA sequencing data. Several studies have previously reported the influence of menstrual cycle phase, tumor size and race on expression of mRNAs, lncRNAs as well as miRNAs, a member of sncRNA family (5, 42). Additional studies are warranted to determine the influence of race, tumor size and menstrual cycle phase on expression profile of sncRNAs in leiomyomas and the influence of ovarian steroids in isolated leiomyoma smooth muscle cells.
Currently with the exception of a number of studies that addressed the regulatory function of few miRNAs (6, 11–15), the regulatory function of other classes of sncRNAs in leiomyoma remains unknown. Accumulated evidence suggests that snoRNAs play key regulatory functions in various normal cellular processes and their altered expression has been associated with a wide range of disorders, including chronic lymphocytic leukemia, hepatocellular carcinoma and colorectal, prostate, endometrial and lung cancers. As such snoRNAs might be useful as diagnostic and prognostic biomarkers in colorectal and lung cancers (43–48). In addition, snoRNAs have a housekeeping role and are involved in biogenesis and modification of rRNAs, tRNAs and snRNAs (49). Other functions of snoRNAs include alternative splicing of the trans gene transcript as exemplified by SNORD115 (50), and may function as miRNAs after further processing into 20–24 nucleotides long small RNAs (51). Interestingly, there is overexpression of SNORD30 and SNORD27 in smoldering myeloma and this increased expression correlates with a higher incidence of tumor progression (34). SNORD27 modifies rRNA methylation by targeting fibrillarin, a methyltransferase, and through direct RNA-RNA interaction regulates alternative pre-mRNA splicing of several genes, including E2F7, MAP4K3, ZBTB37, FER, and ABCA8 (35). Additionally, SNORD27 has been shown to associate with ribosomes from the early pre-ribosomes to the later maturation stage, whereas SNORD56 is only recruited at the stage of later pre-ribosomal complexes, mediated by DDX21 RNA helicase (36). Moreover, SNORD46 which is embedded within the intronic regions of RPS8 was down-regulated in breast tumor (37). Furthermore, elevated expression of SNORA16A correlated with a higher risk of relapse in squamous cell carcinomas (38). Our results demonstrated that the expression levels of SNORD30, SNORD27 and SNORA16A were all increased in leiomyomas as in other tumors (34, 38); however, leiomyomas expressed elevated levels of SNORD46 in contrast to repressed SNORD46 expression in breast tumors (37), indicating the tumor-specificity of this snoRNA expression. The function and the regulatory mechanisms of differentially expressed snoRNAs in leiomyoma progression remain to be elucidated.
piRNAs are derived from long RNA precursors through a dicer-independent mechanism, and are also transcribed from the 3′ untranslated regions of intergenic non-protein coding and protein-coding genes (52). Although piRNAs transcripts were initially identified in gonadal tissues they are also expressed in somatic tissues (53), circulating or cancer stem cells (54). Functionally piRNAs through interaction with PIWI proteins regulate expression of genes through translational control, silencing transposons or epigenetically through control of telomeres via particular methyltransferases or histone modifications (55–58). Aberrant PIWI protein expression has been reported in various cancers including breast, esophageal, pancreas, gastric and endometrial carcinoma (59–67). Several studies reported elevated expression of piR-20365 in breast tumors and lymph node metastasis (39), while no differences in piR-4153 expression was found in synovial fibroblasts from rheumatoid arthritis and osteoarthritis (40). Despite the existence of a large number of piRNAs in the database our analysis only detected 180 piRNAs of which the expression of 24 piRNAs including piR-1311, piR-16677, piR-20365 and piR-4153 were significantly decreased in leiomyoma as compared to myometrium. The role of these piRNAs as potential silencers of protein coding genes in leiomyomas remains to be determined.
Transfer RNAs (tRNAs) are derived from pre-tRNA by endonuclease RNase Z and in their mature form play a well-defined role in protein translation (18). However, tRNAs derived small RNAs (tsRNAs) or tRNA fragments (tRFs) of 30–35 nucleotides long are also generated through cleavage of mature tRNAs at both 5′ and 3′ end at the anticodon loop during the maturation process of tRNAs (18). tRFs similar to piRNAs have been shown to associate with PIWIL2, a protein involved in the silencing of transposons (68). Altered expression of tRFs has been reported in several tumors including colon, breast and ovarian cancers upon oncogene activation and during cancer staging (68). Under in vitro condition, knock-out of tRFs-101 and tRFs-46 in HEK-293 cells resulted in significant alteration of gene-expression patterns, with activation of genes involved in cell survival with concurrent down-regulation of genes involved in apoptosis and chromatin structure. Overexpression of tRFs-46 and tRFs-47 in lung cancer cell lines resulted in inhibition of colony formation and cell proliferation, implying tsRNAs play a key role in cell growth and survival (68). Although our sequencing resulted in identification of 152 tRFs transcripts only 7 tRFs were differentially expressed in leiomyoma. It remains to be determined if these tRFs have a role in leiomyoma cell proliferation and or apoptosis. Our results also demonstrated that leiomyoma expressed significantly more AGO2 protein as compared to matched myometrium, possibly suggesting greater levels of post-transcriptional RNA processing in leiomyomas. However, the impact of altered AGO2 expression in leiomyoma tumorigenesis and progression remain further elucidation.
A dynamic regulatory interaction among the noncoding (sncRNA and lncRNAs) and protein-coding genes has emerged to influence the outcome of various normal cellular activities while their aberrant expression has been associated with a wide range of disorders. In the present study and our previous report (4) through next generation RNA sequencing we provided evidence that leiomyoma also display an altered expression of a number of sncRNAs and lncRNAs as compared to myometrium. Although the result presented here provided the necessary initial step in sncRNAs identification in leiomyoma, further studies are required to address their expression, regulation and molecular mechanism of actions in leiomyoma pathogenesis and progression.
Supplementary Material
Supplementary Table 1: Primers sequences used for QRT-PCR.
Supplementary Table 2: Excel spread sheets showing the raw data of expression profile of all sncRNAs (snoRNAs, snRNAs, piRNAs, tRNAs and rRNAs) in leiomyoma and paired myometrium.
Capsule.
The expression profile of sncRNAs was determined by next generation sequencing and confirmed by QRT-PCR, indicating differential expression of selected snoRNAs, piRNAs, tRNA and rRNA in leiomyoma.
Acknowledgments
This study was supported by LA BioMed Research Committee Seed Grant Program (530592-01-00), LA BioMed Research Committee Bridge Grant Program (5311720100) and NIH (HD088868). The work performed by the Yan lab was supported by NIH (HD071736 and HD085506) and the Templeton Foundation (50183). Bioinformatics were conducted in the Single Cell Genomics Core of the University of Nevada, Reno School of Medicine, which was supported, in part, by the NIH COBRE Grant 1P30GM110767.
Footnotes
The authors have nothing to declare and no competing financial interests
Reference List
- 1.Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Human reproduction update. 2014;20:309–33. doi: 10.1093/humupd/dmt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Journal of the Society for Gynecologic Investigation. 2003;10:161–71. doi: 10.1016/s1071-5576(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 3.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Seminars in reproductive medicine. 2010;28:180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang TD, Khorram O. Expression Profiling of lncRNAs, miRNAs, and mRNAs and Their Differential Expression in Leiomyoma Using Next-Generation RNA Sequencing. Reproductive sciences (Thousand Oaks, Calif) 2018;25:246–55. doi: 10.1177/1933719117711265. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Zhang X, Dong R, Liu X, Li Y, Lu S, et al. Integrated analysis of long noncoding RNAs and mRNAs reveals their potential roles in the pathogenesis of uterine leiomyomas. Oncotarget. 2014;5:8625–36. doi: 10.18632/oncotarget.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgieva B, Milev I, Minkov I, Dimitrova I, Bradford AP, Baev V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics. 2012;99:275–81. doi: 10.1016/j.ygeno.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB. Annotating non-coding regions of the genome. Nature reviews Genetics. 2010;11:559–71. doi: 10.1038/nrg2814. [DOI] [PubMed] [Google Scholar]
- 8.Kiss T, Fayet-Lebaron E, Jady BE. Box H/ACA small ribonucleoproteins. Molecular cell. 2010;37:597–606. doi: 10.1016/j.molcel.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–74. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. The FEBS journal. 2010;277:2015–21. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X, Chegini N. The expression and potential regulatory function of microRNAs in the pathogenesis of leiomyoma. Seminars in reproductive medicine. 2008;26:500–14. doi: 10.1055/s-0028-1096130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang TD, Khorram O. miR-200c Regulates IL8 Expression by Targeting IKBKB: A Potential Mediator of Inflammation in Leiomyoma Pathogenesis. PloS one. 2014;9:e95370. doi: 10.1371/journal.pone.0095370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang TD, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertility and sterility. 2016;105:236–45.e1. doi: 10.1016/j.fertnstert.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Chuang TD, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Molecular endocrinology (Baltimore, Md) 2012;26:1028–42. doi: 10.1210/me.2012-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocrine-related cancer. 2012;19:541–56. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falaleeva M, Stamm S. Processing of snoRNAs as a new source of regulatory non-coding RNAs: snoRNA fragments form a new class of functional RNAs. BioEssays: news and reviews in molecular, cellular and developmental biology. 2013;35:46–54. doi: 10.1002/bies.201200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng KW, Anderson C, Marshall EA, Minatel BC, Enfield KS, Saprunoff HL, et al. Piwi-interacting RNAs in cancer: emerging functions and clinical utility. Molecular cancer. 2016;15:5. doi: 10.1186/s12943-016-0491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Lee I, Lee YS, Bao X. Small Non-coding Transfer RNA-Derived RNA Fragments (tRFs): Their Biogenesis, Function and Implication in Human Diseases. Genomics & informatics. 2015;13:94–101. doi: 10.5808/GI.2015.13.4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson P, Ivanov P. tRNA fragments in human health and disease. FEBS letters. 2014;588:4297–304. doi: 10.1016/j.febslet.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nature reviews Molecular cell biology. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 21.Kupferschmidt K. A lethal dose of RNA. Science (New York, NY) 2013;341:732–3. doi: 10.1126/science.341.6147.732. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Li S, Qi HH, Chowdhury D, Shi Y, Novina CD. Distinct passenger strand and mRNA cleavage activities of human Argonaute proteins. Nature structural & molecular biology. 2009;16:1259–66. doi: 10.1038/nsmb.1712. [DOI] [PubMed] [Google Scholar]
- 23.Beauchef G, Bigot N, Kypriotou M, Renard E, Poree B, Widom R, et al. The p65 subunit of NF-kappaB inhibits COL1A1 gene transcription in human dermal and scleroderma fibroblasts through its recruitment on promoter by protein interaction with transcriptional activators (c-Krox, Sp1, and Sp3) The Journal of biological chemistry. 2012;287:3462–78. doi: 10.1074/jbc.M111.286443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hon CC, Ramilowski JA, Harshbarger J, Bertin N, Rackham OJ, Gough J, et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang C, Xie Y, Yan W. AASRA: An Anchor Alignment-Based Small RNA Annotation Pipeline. bioRxiv. 2017 doi: 10.1093/biolre/ioab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang TD, Khorram O. Tranilast Inhibits Genes Functionally Involved in Cell Proliferation, Fibrosis, and Epigenetic Regulation and Epigenetically Induces miR-29c Expression in Leiomyoma Cells. Reproductive sciences (Thousand Oaks, Calif) 2017;24:1253–63. doi: 10.1177/1933719116682878. [DOI] [PubMed] [Google Scholar]
- 28.Chuang TD, Ho M, Khorram O. The Regulatory Function of miR-200c on Inflammatory and Cell-Cycle Associated Genes in SK-LMS-1, A Leiomyosarcoma Cell Line. Reproductive sciences (Thousand Oaks, Calif) 2015;22:563–71. doi: 10.1177/1933719114553450. [DOI] [PubMed] [Google Scholar]
- 29.Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. American journal of physiology Cell physiology. 2015;309:C117–25. doi: 10.1152/ajpcell.00254.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sai Lakshmi S, Agrawal S. piRNABank: a web resource on classified and clustered Piwi-interacting RNAs. Nucleic acids research. 2008;36:D173–7. doi: 10.1093/nar/gkm696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC bioinformatics. 2014;15:29. doi: 10.1186/1471-2105-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khorram O, Chuang TD, Pearce WJ. Long-term effects of maternal undernutrition on offspring carotid artery remodeling: role of miR-29c. J Dev Orig Health Dis. 2015:1–8. doi: 10.1017/S2040174415001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang TD, Khorram O. Glucocorticoids regulate MiR-29c levels in vascular smooth muscle cells through transcriptional and epigenetic mechanisms. Life sciences. 2017;186:87–91. doi: 10.1016/j.lfs.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Corral L, Mateos MV, Corchete LA, Sarasquete ME, de la Rubia J, de Arriba F, et al. Genomic analysis of high-risk smoldering multiple myeloma. Haematologica. 2012;97:1439–43. doi: 10.3324/haematol.2011.060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falaleeva M, Pages A, Matuszek Z, Hidmi S, Agranat-Tamir L, Korotkov K, et al. Dual function of C/D box small nucleolar RNAs in rRNA modification and alternative pre-mRNA splicing. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1625–34. doi: 10.1073/pnas.1519292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sloan KE, Leisegang MS, Doebele C, Ramirez AS, Simm S, Safferthal C, et al. The association of late-acting snoRNPs with human pre-ribosomal complexes requires the RNA helicase DDX21. Nucleic acids research. 2015;43:553–64. doi: 10.1093/nar/gku1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan P, Ghosh S, Wang B, Heyns M, Graham K, Mackey JR, et al. Profiling of Small Nucleolar RNAs by Next Generation Sequencing: Potential New Players for Breast Cancer Prognosis. PloS one. 2016;11:e0162622. doi: 10.1371/journal.pone.0162622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirisola V, Mora R, Esposito AI, Guastini L, Tabacchiera F, Paleari L, et al. A prognostic multigene classifier for squamous cell carcinomas of the larynx. Cancer letters. 2011;307:37–46. doi: 10.1016/j.canlet.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Huang G, Hu H, Xue X, Shen S, Gao E, Guo G, et al. Altered expression of piRNAs and their relation with clinicopathologic features of breast cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2013;15:563–8. doi: 10.1007/s12094-012-0966-0. [DOI] [PubMed] [Google Scholar]
- 40.Plestilova L, Neidhart M, Russo G, Frank-Bertoncelj M, Ospelt C, Ciurea A, et al. Expression and Regulation of PIWIL-Proteins and PIWI-Interacting RNAs in Rheumatoid Arthritis. PloS one. 2016;11:e0166920. doi: 10.1371/journal.pone.0166920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Molecular cell. 2007;26:611–23. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes, chromosomes & cancer. 2007;46:336–47. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 43.Ronchetti D, Mosca L, Cutrona G, Tuana G, Gentile M, Fabris S, et al. Small nucleolar RNAs as new biomarkers in chronic lymphocytic leukemia. BMC medical genomics. 2013;6:27. doi: 10.1186/1755-8794-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu G, Yang F, Ding CL, Zhao LJ, Ren H, Zhao P, et al. Small nucleolar RNA 113-1 suppresses tumorigenesis in hepatocellular carcinoma. Molecular cancer. 2014;13:216. doi: 10.1186/1476-4598-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravo M, Cordella A, Rinaldi A, Bruno G, Alexandrova E, Saggese P, et al. Small non-coding RNA deregulation in endometrial carcinogenesis. Oncotarget. 2015;6:4677–91. doi: 10.18632/oncotarget.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao J, Yu L, Mei Y, Guarnera M, Shen J, Li R, et al. Small nucleolar RNA signatures as biomarkers for non-small-cell lung cancer. Molecular cancer. 2010;9:198. doi: 10.1186/1476-4598-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao L, Ma J, Mannoor K, Guarnera MA, Shetty A, Zhan M, et al. Genome-wide small nucleolar RNA expression analysis of lung cancer by next-generation deep sequencing. International journal of cancer Journal international du cancer. 2015;136:E623–9. doi: 10.1002/ijc.29169. [DOI] [PubMed] [Google Scholar]
- 48.Okugawa Y, Toiyama Y, Toden S, Mitoma H, Nagasaka T, Tanaka K, et al. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut. 2017;66:107–17. doi: 10.1136/gutjnl-2015-309359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filipowicz W, Pogacic V. Biogenesis of small nucleolar ribonucleoproteins. Current opinion in cell biology. 2002;14:319–27. doi: 10.1016/s0955-0674(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 50.Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science (New York, NY) 2006;311:230–2. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- 51.Taft RJ, Glazov EA, Lassmann T, Hayashizaki Y, Carninci P, Mattick JS. Small RNAs derived from snoRNAs. RNA (New York, NY) 2009;15:1233–40. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robine N, Lau NC, Balla S, Jin Z, Okamura K, Kuramochi-Miyagawa S, et al. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Current biology: CB. 2009;19:2066–76. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan Z, Hu HY, Jiang X, Maierhofer V, Neb E, He L, et al. Widespread expression of piRNA-like molecules in somatic tissues. Nucleic acids research. 2011;39:6596–607. doi: 10.1093/nar/gkr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez VD, Vucic EA, Thu KL, Hubaux R, Enfield KS, Pikor LA, et al. Unique somatic and malignant expression patterns implicate PIWI-interacting RNAs in cancer-type specific biology. Scientific reports. 2015;5:10423. doi: 10.1038/srep10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, et al. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell. 2012;149:693–707. doi: 10.1016/j.cell.2012.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Molecular cell. 2008;31:785–99. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development (Cambridge, England) 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 58.Le Thomas A, Toth KF, Aravin AA. To be or not to be a piRNA: genomic origin and processing of piRNAs. Genome biology. 2014;15:204. doi: 10.1186/gb4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan Y, Liu L, Liao M, Zhang C, Hu S, Zou M, et al. Emerging roles for PIWI proteins in cancer. Acta biochimica et biophysica Sinica. 2015;47:315–24. doi: 10.1093/abbs/gmv018. [DOI] [PubMed] [Google Scholar]
- 60.Wang DW, Wang ZH, Wang LL, Song Y, Zhang GZ. Overexpression of hiwi promotes growth of human breast cancer cells. Asian Pacific journal of cancer prevention: APJCP. 2014;15:7553–8. doi: 10.7314/apjcp.2014.15.18.7553. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Xu G, Lan J, Huang Q, Tang Z, Tian L. High expression of piwi-like RNA-mediated gene silencing 1 is associated with poor prognosis via regulating transforming growth factor-beta receptors and cyclin-dependent kinases in breast cancer. Molecular medicine reports. 2016;13:2829–35. doi: 10.3892/mmr.2016.4842. [DOI] [PubMed] [Google Scholar]
- 62.He W, Wang Z, Wang Q, Fan Q, Shou C, Wang J, et al. Expression of HIWI in human esophageal squamous cell carcinoma is significantly associated with poorer prognosis. BMC cancer. 2009;9:426. doi: 10.1186/1471-2407-9-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grochola LF, Greither T, Taubert H, Moller P, Knippschild U, Udelnow A, et al. The stem cell-associated Hiwi gene in human adenocarcinoma of the pancreas: expression and risk of tumour-related death. British journal of cancer. 2008;99:1083–8. doi: 10.1038/sj.bjc.6604653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Liu Y, Shen X, Zhang X, Chen X, Yang C, et al. The PIWI protein acts as a predictive marker for human gastric cancer. International journal of clinical and experimental pathology. 2012;5:315–25. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W, Gao Q, Chen K, Xue X, Li M, Chen Q, et al. Hiwi facilitates chemoresistance as a cancer stem cell marker in cervical cancer. Oncology reports. 2014;32:1853–60. doi: 10.3892/or.2014.3401. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Liu J, Wu G, Yang F. Manipulations in HIWI level exerts influence on the proliferation of human non-small cell lung cancer cells. Experimental and therapeutic medicine. 2016;11:1971–6. doi: 10.3892/etm.2016.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L, Bi L, Liu Q, Zhao M, Cao B, Li D, et al. Hiwi Promotes the Proliferation of Colorectal Cancer Cells via Upregulating Global DNA Methylation. Disease markers. 2015;2015:383056. doi: 10.1155/2015/383056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, et al. tsRNA signatures in cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:8071–6. doi: 10.1073/pnas.1706908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Primers sequences used for QRT-PCR.
Supplementary Table 2: Excel spread sheets showing the raw data of expression profile of all sncRNAs (snoRNAs, snRNAs, piRNAs, tRNAs and rRNAs) in leiomyoma and paired myometrium.


