Abstract
Background
Folate and homocysteine are involved in DNA synthesis and methylation processes, which are deregulated during carcinogenesis.
Objectives
The aim of this study was to assess the relationship between folate/homocysteine concentrations, the functional polymorphisms of folate/homocysteine genes and lung cancer risk among cigarette smokers.
Study design
The study included 132 lung cancer patients and 396 controls from northern Poland, matched by sex, age and smoking status. The median cigarette pack-years of smoking among both cases and controls was 30.0. Serum, red blood cell (RBC) folates and serum homocysteine concentrations were measured. The genotypes in selected polymorphic sites of the MTHFR, CBS, SHMT1, MTHFD1, MTRR, MTR, TYMS DHFR, TCN2, and SLC19A1 genes were determined. All study participants underwent scanning with low-dose computed tomography.
Results
Serum folate concentrations above the median (> 17.5 nmol/l among the healthy controls) were associated with an increased lung cancer risk (odds ratio [OR], 1.54, 95% confidence intervals [CI], 1.04–2.29, P = 0.031). An analogous trend was observed when the population was analysed after subdivision according to RBC folate concentrations, that is, above a value of 506.5 nmol/l (OR, 1.53; 95% CI, 0.95–2.47; P = 0.084). Additionally, in a subset of women, an increased risk of lung cancer development was associated with the SLC19A1 c.80AA genotype (c.80AA versus GG OR, 3.14; 95% CI, 1.32–7.46; P = P = 0.010).
Conclusion
These results suggest that, in the population consisting of heavy smokers, high folate levels add to the cancerogenic effect of smoking.
Introduction
Lung cancer accounts for the highest number of cancer deaths globally [1], with cigarette smoking remaining the most prominent risk factor [2].
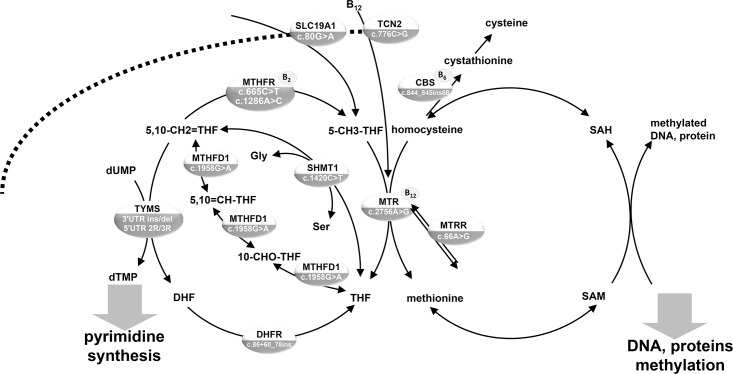
Folate (vitamin B9) plays a pivotal role in cell metabolism by providing metabolites necessary for DNA synthesis and repair, as well as for methylation of DNA, proteins and lipids. While reduced folates are crucial C1 (one-carbon, methyl group) cycle intermediates, several enzymes involved in their metabolism require other cofactors from the B-vitamin group, such as B12 (Fig 1). Moreover, 5-methyl-tetrahydrofolate (5-methyl-THF) acts as a methyl group donor to remethylate homocysteine to methionine, representing an intermediate necessary for methylation processes.
Fig 1. Schematic representation of C1 metabolism. DNA polymorphisms determined in lung cancer case-control study population from northern Poland.
C1, methyl group; CBS, cystathionine-beta-synthase; DHFR, dihydrofolate reductase; HCOOH, formate; MTHFD1, methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase (soluble); MTHFR, 5,10-methylenetetrahydrofolate reductase; MTR, methionine synthase; MTRR, methionine synthase reductase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; SLC19A1, solute carrier family 19 (reduced folate transporter), member 1; SHMT1, serine hydroxymethyltransferase 1 (soluble); TCN2, transcobalamin; THF, tetrahydrofolate; TYMS, thymidylate synthase; 5-CH3-THF, 5-methyltetrahydrofolate; 5-CHO-THF, 5-formyltetrahydrofolate; 5,10-CH2 = THF, 5,10- methylenetetrahydrofolate; 5,10 = CH-THF, 5,10- methenyltetrahydrofolate; 10-CHO-THF, 10-formyltetrahydrofolate.
Folate deficiency is associated with a higher incidence of neural tube defects (NTDs) and it has been proved that periconceptional folic acid supplementation decreases the NTD rate by 70% [3]. This beneficial effect of folic acid supplementation has led to the introduction of mandatory folic acid food fortification in the United States, Canada, Chile, Costa Rica and South Africa [4]. In addition, high homocysteine concentration is a marker of increased stroke risk, and this has been suggested to result from long-term inadequate B-vitamins levels and endothelial dysfunction. There is accumulating evidence that folic acid interventions aimed at lowering homocysteine levels decrease the incidence of stroke [5].
Despite beneficial effects of folic acid supplementation in some malignancies, there are some concerns related to the potential impact of these interventions, or even folate status, on cancer progression. High folate levels may enable the progression of neoplastic cells to cancer via the stimulation of DNA synthesis and cell proliferation [6]. In contrast, inadequate folate levels in neoplastic cells may be associated with the inhibition of DNA synthesis, and thus may lead to the cell growth arrest [6]. However, suboptimal folate levels are also associated with a higher rate of DNA breaks, mutations and alterations in DNA methylation patterns; thus potentially triggering transformation of normal to neoplastic cells.
The combined results of two randomised, double-blind, placebo-controlled clinical trials—the Norwegian Vitamin Trial and the Western Norway B Vitamin Intervention Trial [7]—indicated that folic acid and vitamin B12 supplementation was associated with increased lung cancer incidence. However, in another study from Finland, higher serum B6 levels in men were associated with decreased risk of lung cancer, while serum folate levels were not associated to altered lung cancer risk [8]. Interestingly, a large European study comprising 899 lung cancer cases and 1770 controls showed that high levels of both vitamin B6 and methionine were negatively correlated with lung cancer risk, whereas among ex- and current smokers higher folate levels were associated with a lower lung cancer risk [9]. Further, the mean serum folate level was found to be lower in lung cancer cases as compared to controls [10].
Since several genetic polymorphisms are known to be modulators of folate/homocysteine status, establishing their association with lung cancer risk may elucidate the role of C1 metabolism in lung carcinogenesis. The associations between both well-known, functional polymorphisms: the MTHFR c.665C>T [11–23], MTHFR c.1286A>C [11, 14–20, 22, 23], MTR c.2756A>G (14–18), MTRR c.66A [14, 15, 17, 19], SHMT1 c.1420C>T [14, 24], TYMS 6bp ins/del polymorphism within the 3’UTR (untranslated region) [25], TYMS 5’UTR 2R/3R [15, 25] and other variants within the MTHFR [18, 26], TYMS [18], MTHFD1 [18], CBS [19], SLC19A1 [19], SHMT1 [24] and MTRR gene [26] and lung cancer risk have been evaluated.
The aim of this study was to investigate whether folate metabolism may add to the lung cancer risk in a large, homogenous population of long-term smokers from northern Poland. Specifically, we determined an association between the risk, serum folate, red blood cell (RBC) folate and homocysteine levels or several known functional polymorphisms in the C1 metabolism genes.
Materials and methods
Study design
A total of 1080 blood samples were collected between October 2009 and March 2011 during the Pomeranian Lung Cancer Screening Program performed at the Medical University of Gdańsk, which offered low-dose computed tomography (LDCT) examination for more than 8600 current or former smokers ranging from 50 to 75 years of age. Among the participants of the screening program 16 lung cancer cases were diagnosed. Therefore, between April 2010 and November 2011, blood samples from 116 incidentally detected lung cancer patients before any therapeutic intervention, who did not participate in the screening program, were added to the study, on the condition each case could have been randomly matched to three controls for age, sex and pack-years of smoking. All controls were selected from the participants of the Pomeranian Lung Cancer Screening Program.
The criteria for matching cases and controls were as follows: sex; pack-years with an accuracy of 10 pack-years, and if this criterion could not be fulfilled, an accuracy of 30 pack-years; an age matching with an accuracy of 1 year, and if this criterion could not be fulfilled, an accuracy of 2 years. The matching criteria were fulfilled by 132 cases and 396 controls.
The study was approved by the Ethical Committee of Medical University of Gdańsk, Poland (consent number: NKEBN/42/2009). All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Biochemical analyses
Serum and RBC folate concentrations were determined using chemiluminescent microparticle folate binding protein assay (Abbott, Diagnostics Division, Ireland), while serum homocysteine concentrations were tested by immunonephelometric assay (Siemens, Germany). RBC folate concentrations were determined from the whole blood and were corrected for the hematocrit values. RBC folate concentrations corrected for serum folate concentrations were calculated according to Abbott’s protocol. For 36 cases and 3 controls the measurements of serum folate and serum homocysteine were determined from samples frozen in -80°C prior to analyses. For 33 cases the measurements of RBC folates were determined from whole blood diluted in 1% ascorbic acid at a proportion of 1:10, and frozen in -80°C prior to analyses, using previously validated modified chemiluminescent microparticle folate binding protein assay (Abbott), the mean value from two measurements was taken as the final result. Briefly, 100 μl of Folate RBC Lysis Diluent L2 (Abbott) and 100 μl of whole blood sample diluted 1:10 in 1% ascorbic acid were mixed, next Abbott procedure was followed, samples were read using Folate assay. In order to assess the potential impact of sample pre-treatment, we performed the measurements of metabolites from differently processed aliquots of randomly selected samples and we analysed the correlations between the results (S1 Fig Supplementary Information). These analyses demonstrated that the different pre-treatment conditions were unlikely to have impacted the measurements of metabolites. All the other samples were transferred to the laboratory immediately after collection. The analyses were performed by the Central Laboratory of Clinical Centre of Medical University of Gdańsk, Poland.
Genotyping
DNA for genetic analyses was isolated from buffy coats using QIAamp DNA Blood Midi Kit (Qiagen). The population was genotyped in the MTHFR c.665C>T (rs1801133), MTHFR c.1286A>C (rs1801131), SHMT1 c.1420C>T (rs1979277), MTHFD1 c.1958G>A (rs2236225), MTRR c.66A>G (rs1801394), MTR c.2756A>G (rs1805087), TYMS 3'UTR ins/del (rs202243265, rs11280056, rs16430), TCN2 c.776C>G (rs1801198) and SLC19A1 c.80G>A (rs61510559) polymorphisms using 5’ Nuclease Real-Time PCR assay on a Light Cycler 480 system (Roche). Individual PCR amplification reactions (20 μl) were composed of 2 μl sample DNA, 1xTaqMan Universal PCR Master Mix, No AmpEraseR UNG (Roche), forward and reverse primers and two allele-specific probes. The probes were synthesised by Applied Biosystems. Each plate was set up with a no template control and positive controls representing all genotype classes. Some primers/probes sequences were obtained from published assays [27–33] and the SNP500 Cancer website (http://snp500cancer.nci.nih.gov/cgfseq/pages/snp500.do).
Population was genotyped in the CBS c.844_845ins68, TYMS 5'UTR 2R/3R, DHFR c.86+60_78ins22 polymorphisms using PCR, followed by agarose gel electrophoresis.
Primers, probes sequences and concentrations and genotyping conditions are listed in S1 Table.
Statistical analyses
Descriptive analyses of the study variables included medians and percentiles for continuous variables and proportions for categorical variables. Deviations from the Hardy-Weinberg equilibrium for the genotypes were assessed by χ2 analysis. Correlations between the metabolite values were calculated using Pearson statistics.
To calculate odds ratios (ORs) with 95% confidence intervals (CI) of lung cancer conditional logistic regression (proc logistic in SAS), conditioning on individual case sets, were used. The population was subdivided into groups defined by the upper and the lower half of distribution of biochemical variables. Medians were selected based on the distributions among the healthy controls. Subjects in the lower half of distribution were selected as a reference group. All available biochemical variables were transformed to categorical variables. In analyses involving gene polymorphisms, homozygous carriers of the major allele were selected as a reference group. This was followed by calculations of p-values for the trends by applying score defined by a following natural numbers for each genotype (0- homozygous carriers of the major allele, 1-heterozygotes, 2- homozygous carriers of the minor allele). Next, the OR of lung cancer for each genotype versus the other allele carriers were evaluated. As four test were calculated to evaluate each SNP, the Bonferroni method was used to correct the results for multiple comparisons.
The OR of lung cancer associated with the SLC19A1 c.80G>A genotypes were calculated using unconditional logistic regression controlling for the matching factors, separately for men and women and in either lower or upper serum folate and homocysteine groups (groups were defined as described above).
Differences in distributions of serum folate, RBC folate, RBC folate corrected and homocysteine concentrations between the genotypes in groups defined by disease status and sex were tested using the Kruskal–Wallis test or the Wilcoxon rank-sum tests, when appropriate. P-values ≤0.05 were considered statistically significant. The extreme values: serum folate of 800.5 and 565.4 nmol/l were excluded from those analyses, as indicated in table footnotes when necessary.
Statistical analyses were calculated using SAS 9.3 (NC, USA).
Results
The characteristics of the study group are presented in Table 1. The study included 132 lung cancer patients and 396 controls from northern Poland. Women accounted for 46% of participants. Among both the cases and controls the median cigarette pack-years of smoking was 30.0. The median age of both cases and controls was 62.9. The majority of lung cancer cases were diagnosed with adenocarcinoma (51%) and with squamous cell cancer (36%). The majority of subjects were not supplemented with B-vitamins. Serum folates and homocysteine as well as RBC folate and homocysteine were negatively correlated (r = -0.19; N = 522; P <.0001 and r = -0.19; N = 368; P = 0.0002, respectively) while serum folate and RBC folate levels were positively correlated (r = 0.56, N = 371, P <.0001).
Table 1. Characteristics of participants in lung cancer case-control study population from northern Poland.
| Variable | Lung cancer cases (N = 132) |
Controls (N = 396) |
|
|---|---|---|---|
| Sex | Women | 61 (46%) | 183 (46%) |
| Men | 71 (54%) | 213 (54%) | |
| Age | Mean, SD | 62.8 (6.2) | 62.7 (6,1) |
| Median (Min-Max) | 62.9 (48.6–77.1) | 62.9 (49.2–75.9) | |
| Pack-years of smokinga | Mean, SD | 32.2 (15.0) | 32.3 (11.1) |
| Median (Min-Max) | 30.0 (1–75) | 30.0 (7.5–80) | |
| Source of cases | Screening | 16 (12%) | |
| Incidental | 116 (88%) | ||
| Cancer subtype | Adenocarcinoma | 67 (51%) | |
| Squamous Cell Cancer | 47 (36%) | ||
| Small Cell Lung Cancer | 2 (2%) | ||
| Large Cell Carcinoma | 8 (6%) | ||
| Other | 8 (6%) | ||
| Cancer clinical stage | In situ | 1 (1%) | |
| 1a | 40 (30%) | ||
| 1b | 26 (20%) | ||
| 2a | 30 (23%) | ||
| 2b | 13 (10%) | ||
| 3a | 21 (16%) | ||
| 4 | 1 (1%) | ||
| Serum folates nmol/lb |
Median (25th-75th percentile), N |
19.3 (14.3–24.3), 132 |
17.5 (12.9–23.1), 394 |
| RBC folates nmol/l |
Median (25th-75th percentile), N |
559.7 (401.5–721.9), 93 |
506.5 (383.3–677.4), 279 |
| RBC folates corrected nmol/l |
Median (25th-75th percentile), N |
524.7 (378.4–684.4), 93 |
475.9 (367.0–642.5), 278 |
| Homocysteine μmol/l |
Median (25th-75th percentile), N |
15.7 (11.4–18.6), 129 |
14.7 (12.0–18.6), 395 |
| Supplementation | No | 87 (66%) | 268 (68%) |
| Yes | 12 (9%) | 123 (31%) | |
| No data | 33 (25%) | 5 (1%) | |
| Supplementation with B vitamins | No | 97 (73%) | 362 (91%) |
| Yes | 2 (2%) | 28 (7%) | |
| No data | 33 (25%) | 6 (2%) |
aa pack-year is defined as one pack of cigarettes smoked per day for one year.
bTwo outlying extreme values were excluded from the analyses: serum folate = 800.5; 565.4 nmol/l.
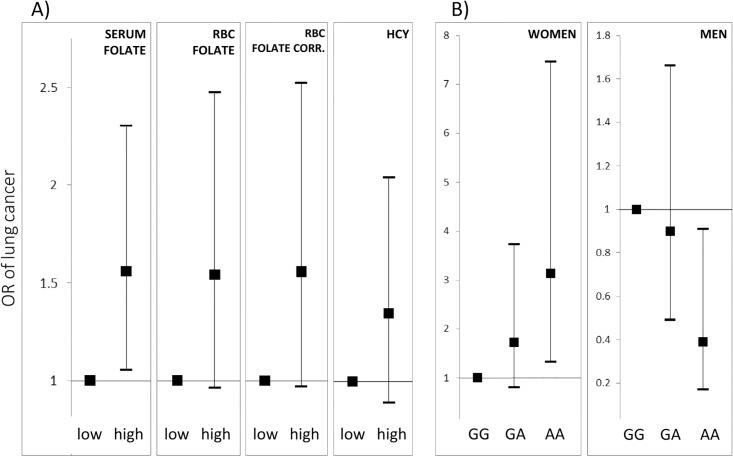
No significant differences between serum folate, RBC folate or homocysteine between the cases and controls (Table 1) were found. Subsequently, the population was subdivided by median values (among healthy subjects) of serum folate, RBC folate, RBC folate corrected, and homocysteine measures (Table 2, Fig 2). The risk of lung cancer incidence correlated with high levels of serum folate (OR = 1.54, 95% CI: 1.04–2.29, P = 0.031). An analogous trend was observed after subdivision according to median RBC folate concentrations (OR = 1.53, 95% CI: 0.95–2.47, P = 0.084) and RBC folate concentrations corrected for serum folates (OR = 1.51, 95% CI: 0.93–2.43, P = 0.093). No differences in lung cancer risk were found when subdivision was applied with regard to homocysteine levels (Table 2).
Table 2. Odds ratios of lung cancer occurrence in relation to serum folate, RBC folate and homocysteine levels, in study population from northern Poland, calculated by conditional logistic regression with adjustment for sex, age and pack-years of smoking.
| Variable | Lung cancer cases (%) | Controls (%) | OR (95% CI), P | |
|---|---|---|---|---|
| Serum folates nmol/l |
≤17.5 | 52 (39.4) | 200 (50.5) | 1 (Ref) |
| >17.5 | 80 (60.6) | 196 (49.5) | 1.54 (1.04–2.29), P = 0.031 | |
| RBC folates nmol/l |
≤506.5 | 37 (39.8) | 140 (50.2) | 1 (Ref) |
| >506.5 | 56 (60.2) | 139 (49.8) | 1.53 (0.95–2.47), P = 0.084 | |
| RBC folates corrected nmol/l |
≤474.8 | 37 (39.8) | 139 (50.0) | 1 (Ref) |
| >474.8 | 56 (60.2) | 139 (50.0) | 1.51 (0.93–2.43), P = 0.0934 | |
| Homocysteine μmol/l |
≤ 14.7 | 56 (43.4) | 203 (51.4) | 1 (Ref) |
| >14.7 | 73 (56.6) | 192 (48.6) | 1.41 (0.93–2.14), P = 0.1021* |
*homocysteine measures for 3 cases were not available, thus 9 matched subjects from the control group were excluded from analyses as uninformative data
Fig 2. Odds ratios of lung cancer occurrence in relation to A) serum folate, RBC folate, RBC folate corrected and homocysteine levels B) the SLC19A1 c.80A>G genotypes among women and men, in case-control study population from northern Poland.
The association between the genotypes in the C1 metabolism-involved genes and the lung cancer risk was assessed for the population as a whole and in the subgroups defined by sex. The more frequent homozygous genotype was selected as the reference for the step-effect of less common allele increase. All analysed genotypes were in accordance with the Hardy-Weinberg equilibrium. The distributions of biochemical variables defined by genotypes under the study are presented in S2 and S3 Tables.
No significant association between the MTHFR c.665C>T polymorphism and lung cancer risk was found, although a trend towards lower risk of lung cancer among T allele carriers as compared to the CC genotype was observed (OR = 0.68, 95% CI: 0.45–1.01, P = 0.057, Table 3). The TYMS 3’UTR del/del genotype was associated with a lower lung cancer risk as compared to the ins/ins genotype (OR = 0.33, 95% CI: 0.11–0.96, P = 0.041, Table 3). This association was not significant, though, after correction for multiple comparisons.
Table 3. Odds ratios of lung cancer occurrence in relation to genotypes in the C1 metabolism genes, in study population from northern Poland.
| Polymorphism | Genotype | Lung cancer cases (% of genotype carriers) |
Controls (% of genotype carriers) |
OR (95% CI), P |
|---|---|---|---|---|
| rs1801133 MTHFR c.665C>T |
CC | 65 (49) | 158 (40) | 1 (Ref) |
| CT | 55 (42) | 199 (50) | 0.66 (0.43–1.01), P = 0.056 | |
| TT | 12 (9) | 39 (10) | 0.75 (0.37–1.51), P = 0.423 | |
| P for trend = 0.120 | ||||
| TvsCC(Ref) | 0.68 (0.45–1.01), P = 0.057 | |||
| TTvsC(Ref) | 0.92 (0.47–1.80), P = 0.801 | |||
| rs1801131 MTHFR c.1286A>C |
AA | 69 (52) | 193 (49) | 1 (Ref) |
| AC | 49 (37) | 169 (43) | 0.80 (0.53–1.23), P = 0.314 | |
| CC | 14 (11) | 34 (9) | 1.16 (0.59–2.29), P = 0.665 | |
| P for trend = 0.818 | ||||
| CvsAA(Ref) | 0.87 (0.59–1.29), P = 0.482 | |||
| CCvsA(Ref) | 1.27 (0.65–2.45), P = 0.484 | |||
| CBS c.844_845ins68 | DD | 110 (83) | 343 (87) | 1 (Ref) |
| I allele | 22 (17) | 53 (13) | 1.32 (0.75–2.31), P = 0.338 | |
| rs1979277 SHMT1 c.1420C>T |
CC | 53 (40) | 164 (41) | 1 (Ref) |
| CT | 61 (46) | 186 (47) | 1.02 (0.66–1.57), P = 0.935 | |
| TT | 18 (14) | 46 (12) | 1.22 (0.64–2.31), P = 0.546 | |
| P for trend = 0.618 | ||||
| TvsCC(Ref) | 1.06 (0.70–1.59), P = 0.794 | |||
| TTvsC(Ref) | 1.21 (0.67–2.18), P = 0.535 | |||
| rs2236225 MTHFD1 c.1958G>A |
GG | 39 (30) | 131 (33) | 1 (Ref) |
| GA | 63 (48) | 182 (46) | 1.15 (0.74–1.80), P = 0.530 | |
| AA | 30 (23) | 83 (21) | 1.21 (0.70–2.09), P = 0.496 | |
| P for trend = 0.470 | ||||
| AvsGG(Ref) | 1.17 (0.77–1.78), P = 0.463 | |||
| AAvsG(Ref) | 1.11 (0.69–1.78), P = 0.669 | |||
| rs1801394 MTRR c.66A>G |
GG | 50 (38) | 119 (30) | 1 (Ref) |
| GA | 58 (44) | 190 (48) | 1.09 (0.63–1.87), P = 0.768 | |
| AA | 24 (18) | 87 (22) | 1.50 (0.87–2.60), P = 0.148 | |
| P for trend = 0.115 | ||||
| AvsGG(Ref) | 0.70 (0.47–1.06), P = 0.095 | |||
| AAvsG(Ref) | 0.79 (0.48–1.30), P = 0.359 | |||
| rs1805087 MTR c.2756A>G |
AA | 91 (69) | 245 (62) | 1 (Ref) |
| AG | 36 (27) | 133 (34) | 0.73 (0.47–1.13), P = 0.162 | |
| GG | 5 (4) | 18 (5) | 0.75 (0.27–2.10), P = 0.587 | |
| P for trend = 0.178 | ||||
| GvsAA(Ref) | 0.73 (0.48–1.12), P = 0.148 | |||
| GGvsA(Ref) | 0.83 (0.30–2.28), P = 0.710 | |||
| rs202243265 rs11280056 rs16430 TYMS 3'UTR ins/del |
II | 67 (51) | 179 (45) | 1 (Ref) |
| ID | 61 (46) | 184 (46) | 0.88 (0.59–1.33), P = 0.547 | |
| DD | 4 (3) | 33 (8) | 0.33 (0.11–0.96), P = 0.041 | |
| P for trend = 0.079 | ||||
| 3*DvsII(Ref) | 0.80 (0.53–1.19), P = 0.261 | |||
| 3*DDvsI(Ref) | 0.35 (0.12–1.00), P = 0.050 | |||
| TYMS 5'UTR 2R/3R | 2R/2R | 32 (24) | 72 (18) | 1 (Ref) |
| 2R/3R | 66 (50) | 205 (52) | 0.716 (0.43–1.18), P = 0.189 | |
| 3R/3R | 34 (26) | 119 (30) | 0.62 (0.34–1.12), P = 0.112 | |
| P for trend = 0.120 | ||||
| 5' 3Rvs2R2R(Ref) | 0.69 (0.42–1.11), P = 0.125 | |||
| 5' 3R3R vs2R(Ref) | 0.80 (0.51–1.26), P = 0.333 | |||
| DHFR c.86+60_78ins22 | II | 36 (27) | 119 (30) | 1 (Ref) |
| ID | 72 (55) | 198 (50) | 1.22 (0.76–1.95), P = 0.414 | |
| DD | 24 (18) | 79 (20) | 1.01 (0.56–1.80), P = 0.985 | |
| P for trend = 0.886 | ||||
| DvsII(Ref) | 1.15 (0.74–1.79), P = 0.541 | |||
| DDvsI(Ref) | 0.89 (0.54–1.48), P = 0.659 | |||
| rs1801198 TCN2 c.776C>G |
CC | 39 (30) | 125 (32) | 1 (Ref) |
| CG | 64 (48) | 183 (46) | 1.12 (0.71–1.75), P = 0.634 | |
| GG | 29 (22) | 88 (22) | 1.05 (0.61–1.82), P = 0.850 | |
| P for trend = 0.810 | ||||
| GvsCC(Ref) | 1.10 (0.72–1.67), P = 0.670 | |||
| GGvsC(Ref) | 0.99 (0.62–1.58), P = 0.952 | |||
| rs61510559 SLC19A1 c.80G>A |
GG | 35 (27) | 115 (29) | 1 (Ref) |
| GA | 68 (52) | 191 (48) | 1.17 (0.73–1.87), P = 0.514 | |
| AA | 29 (22) | 90 (23) | 1.06 (0.60–1.88), P = 0.834 | |
| P for trend = 0.803 | ||||
| AvsGG(Ref) | 1.14 (0.72–1.78), P = 0.574 | |||
| AAvsG(Ref) | 0.96 (0.60–1.54), P = 0.857 | |||
| rs61510559 SLC19A1 c.80G>A Women |
GG | 11 (18) | 57 (31) | 1 (Ref) |
| GA | 31 (51) | 95 (52) | 1.72 (0.80–3.73), P = 0.168 | |
| AA | 19 (31) | 31 (17) | 3.14 (1.32–7.46), P = 0.010 | |
| P for trend = 0.009 | ||||
| AvsGG (Ref) | 2.08 (1.00–4.31), P = 0.050 | |||
| AAvsG (Ref) | 2.16 (1.12–4.17), P = 0.022 | |||
| rs61510559 SLC19A1 c.80G>A Men |
GG | 24 (34) | 58 (27) | 1 (Ref) |
| GA | 37 (52) | 96 (45) | 0.90 (0.49–1.66), P = 0.740 | |
| AA | 10 (14) | 59 (28) | 0.39 (0.17–0.91), P = 0.030 | |
| P for trend = 0.040 | ||||
| AvsGG (Ref) | 0.73 (0.40–1.31), P = 0.284 | |||
| AAvsG (Ref) | 0.42 (0.20–0.88), P = 0.022 |
Sex-stratified analyses revealed that the SLC19A1 c.80G>A polymorphism had different associations with lung cancer risk among men and women (Table 3, Fig 2). In women, the SLC19A1 c.80AA genotype was associated with a higher risk of lung cancer as compared to the GG genotype (OR = 3.14, 95% CI: 1.32–7.46, P = 0.01). In men, inversely, the SLC19A1 c.80AA genotype was associated with a protection against lung cancer as compared to the GG genotype (OR = 0.39, 95% CI: 0.17–0.91, P = 0.03), but this association was no longer significant after correction for multiple comparisons. In women, the risk of lung cancer positively correlated with increasing number of A allele (P for trend = 0.009), while in men, it correlated negatively with increasing number of A allele (P for trend = 0.040).
In order to determine whether the above association might be influenced by serum folate or homocysteine levels, the study population was further divided into groups defined by sex and by either low/high halves of folate or homocysteine distributions, the association between the SLC19A1 c.80G>A genotypes and the lung cancer risk was assessed within each group. The SLC19A1 c.80AA genotype presented higher risk in women and lower risk in men, as compared to the GG genotype, regardless of the metabolite halves within which analyses were conducted (data not presented). The prevailing number of P-values did not reach significance, which may be due to low numbers of subjects within subgroups.
The conditional logistic regression analyses to calculate odds of lung cancer development in relation to the SLC19A1 c.80G>A genotypes with correction for serum folate levels, in subgroups defined by sex, were performed. Crude and adjusted ORs for the association between lung cancer occurrence and the SLC19A1 c.80G>A genotypes were not substantially different. The significance of results did not change after adjustment for folate levels.
Discussion
Our data suggest that serum folate status influence the lung cancer risk among smokers in the northern Polish population. Specifically, more lung cancer cases were found in subjects with serum folate levels above a value of 17.5 nmol/l. This observation corresponds to the report from the Norwegian Vitamin Trial and Western Norway B Vitamin Intervention Trial [7], which found a higher hazard ratio of lung cancer incidence in subjects who were administered folic acid as compared to those who were not. On the contrary, however, Johansson et al. [9] found that higher folate levels were associated with lower lung cancer risk, while Hartman et al. [8] reported no association. This discrepancy may be due to differences in the structures of populations, folate levels distributions in each population, ethnicity or tobacco exposure. Interestingly, heavy smokers were predominant in our study while the association reported by Johansson et al. [9] included the significant fraction of former smokers. Our and Hartman et al. [8] cohorts markedly differ in average folate levels. Specifically, subjects with serum folate levels below 6 ng/ml constituted 90% of the Finnish population but only 26% of that we report herein. Consequently, the majority of the Finnish cohort had folate levels lower than those we associated with increased lung cancer risk. Next, the contribution of smokers in two intervention trials: WAFACS [34] and HOPE-2 [35], which reported no effect of supplementation with folic acid, vitamin B12 and B6 on lung cancer incidence, was low (11.9%; 11.5%, respectively) and subjects mainly came from the countries with mandatory folate fortification.
Folates have been postulated to have a potentially complex impact on carcinogenesis. Folate prevents mutagenesis in normal cells, but after initial DNA lesions occur, increased folate levels may shift the balance towards progression of neoplastic cells to cancer due to an increased synthesis of nucleic acids [6]. Moreover, C1 metabolism, via its impact on SAM levels (Fig 1), has been postulated to effect both DNA and histone methylation [36].
We found that females who in the reduced folate carrier gene, SLC19A1, had A allele variant at the nucleotide position c.80 showed increased lung cancer risk. To our knowledge, this is the first study to report this association. Sex-specific concordance in relation to lung cancer risk might be explained by differences in the activity of oestrogen receptors which were reported in lung adenocarcinoma cells derived from subjects of different gender [37], while the results from the studies of non-small cell lung cancer suggest that these tumours may produce oestradiol which can stimulate the growth of oestrogen receptor-positive malignant cells [38]. Moreover, sex-specific determinants of folate/homocysteine pathway have already been indicated [39]. Interestingly, it has been reported previously that young women, but not men, who carried the A allele, had higher RBC folate concentrations [40]. In this study we report a trend towards an increased lung cancer risk among subjects in the upper half of RBC folate distribution and an increased risk for those in the upper half of serum folate distribution. By linking the published results and our findings we could suspect that female carriers of the SLC19A1 c80AA genotype might have had higher levels of folates during their lifetime as compared to others. This, in combination with smoking habit might result in increased lung cancer risk.
The most widely studied is the MTHFR c.665C>T polymorphism, yet its role in the aetiology of cancer remains controversial. The MTHFR c.665TT genotype, encoding the thermolabile enzyme with a lower affinity for its cofactor, flavin adenine dinucleotide (FAD), has been associated with an increased risk of lung cancer in populations analysed as a whole [18–20, 22] and in the subgroups: of patients with earlier onset or low folate intake [16], in women [17] and in squamous and small cell lung carcinomas [15]. However, the TT genotype has also been associated with a decreased risk of lung cancer [21] and no association between the MTHFR c.665C>T genotypes and lung cancer risk has been found [11–14]. Our results present a trend towards a protective effect of the MTHFR c.665T allele on lung cancer risk.
Similarly, the MTHFR c.1286CC genotype has been associated with an increased lung cancer risk in the general population [17, 20] and among non-smokers [23], with a reduced risk [16] or no risk [11, 14, 15, 18, 19, 22]. In our population, no association between this polymorphism and lung cancer risk was found.
The TYMS 6 bp ins allele within the 3’UTR has been reported to contribute to a higher risk of lung cancer [25] and we also observed a similar trend. As in the previous studies, we found no association between the MTR c.2756A>G [14–16, 18], MTRR c.66A [14, 19], SHMT1 c.1420C>T [14, 24] and TYMS: 5’UTR 2R/3R [15] polymorphisms and lung cancer risk in a general population. However, in a study comprising 2183 subjects, the combined MTR c.2756G and MTRR c.66A alleles have been linked to a higher risk [17].
Several polymorphisms in the C1 metabolism genes were associated with altered folate/homocysteine levels. Importantly, the association between the polymorphisms and folate/homocysteine status may be suppressed by high folate levels. The impact of the C1 polymorphisms on lung cancer risk, if observed, is most likely due to altering either folate levels or distribution of folate forms. Therefore, it is worth to interpret the effect the polymorphisms in the C1 metabolism genes might have on lung cancer outcome in the context of folate profiles for evaluated populations, as reported in this study.
Food fortification with folic acid has been introduced in several countries. However, this practice has been criticised due to its potentially dangerous effects. Our results suggest that high folate levels may be associated with an increased risk of lung cancer in cigarette smokers, a finding that may support concerns of food fortification with folic acid.
While designing the study, several steps were taken to reduce the risk of false discovery. The strength of the study is the rigorous matching of cases and controls, and that study subjects inhabit a relatively confined area in northern Poland. However, several potential confounders should be listed that might have biased our findings. Even though all subjects were diagnosed with LDCT, preclinical disease in control subjects cannot be excluded. The important limitation of the study is the lack of cotinine measurements (smoking status and pack-years of smoking were self-reported). There are several factors that are known to correlate with B-vitamins status on which information was not available such as educational level or lifestyle e.g. physical activity or dietary habits. Further, the disease might have been induced by undisclosed occupational exposures, which might have been incidentally correlated to higher folate measures in lung cancer cases. In addition, the majority of blood draws were from non-fasting subjects.
Conclusions
Our results suggest that serum folate levels in the upper half of the distribution are associated with a moderately increased lung cancer risk among smokers from northern Poland. If so, relatively small differences in folate concentrations may support growth and progression of smoking induced lung cancer. We believe it would be interesting to repeat this study in other populations of smokers, with a strict matching of cases and controls for pack-years of cigarettes smoked, to test if the observed effect is general. Further, the female carriers of the SLC19A1 c.80A allele belong to the group exhibiting increased lung cancer incidence pointing that certain associations between the folate/homocysteine pathway and the development of smoking-related lung cancer are sex-specific.
Supporting information
A) folate concentration determined from serum samples within a day of blood collection vs. serum samples frozen for a few days; B) folate concentrations determined from serum samples frozen for 34–48 days vs. serum samples frozen for 10 months; C) homocysteine concentrations determined from serum samples frozen for 34–48 days vs. serum samples frozen for 10 months; D) RBC folate concentrations determined from whole blood vs. whole blood diluted 1:10 in 1% ascorbic acid, frozen for 2 years. The measures were corrected for the hematocrit values. Correlations were calculated using Pearson statistics.
(PDF)
(PDF)
(PDF)
(PDF)
(XLS)
Acknowledgments
We would like to acknowledge Renata Barańska from the Central Laboratory of Clinical Centre of Medical University of Gdańsk for performing biochemical analyses, Magdalena Szczepanowska and Agata Zakrzewska for samples collection and the Radiologists from the Clinical Centre of Medical University of Gdańsk for performing the computed tomography screenings.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Centre for Research and Development, Poland [grant number: POIG.01.01.02-22-080/09]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. Epub 2011/02/08. 10.3322/caac.20107 . [DOI] [PubMed] [Google Scholar]
- 2.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45 Suppl 2:S3–9. Epub 2004/11/24. 10.1016/j.lungcan.2004.07.998 . [DOI] [PubMed] [Google Scholar]
- 3.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–5. Epub 1992/12/24. 10.1056/NEJM199212243272602 . [DOI] [PubMed] [Google Scholar]
- 4.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370–84. Epub 2012/01/19. 10.3390/nu3030370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNulty H, Strain JJ, Pentieva K, Ward M. C(1) metabolism and CVD outcomes in older adults. The Proceedings of the Nutrition Society. 2012;71(2):213–21. Epub 2011/12/14. 10.1017/S0029665111003387 . [DOI] [PubMed] [Google Scholar]
- 6.Kim YI. Does a high folate intake increase the risk of breast cancer? Nutr Rev. 2006;64(10 Pt 1):468–75. Epub 2006/10/27. 10.1111/j.1753-4887.2006.tb00178.x . [DOI] [PubMed] [Google Scholar]
- 7.Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302(19):2119–26. Epub 2009/11/19. 10.1001/jama.2009.1622 . [DOI] [PubMed] [Google Scholar]
- 8.Hartman TJ, Woodson K, Stolzenberg-Solomon R, Virtamo J, Selhub J, Barrett MJ, et al. Association of the B-vitamins pyridoxal 5'-phosphate (B(6)), B(12), and folate. Am J Epidemiol. 2001;153(7):688–94. 10.1093/aje/153.7.688 [DOI] [PubMed] [Google Scholar]
- 9.Johansson M, Relton C, Ueland PM, Vollset SE, Midttun O, Nygard O, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303(23):2377–85. Epub 2010/06/17. 10.1001/jama.2010.808 . [DOI] [PubMed] [Google Scholar]
- 10.Ozkan Y, Yardim-Akaydin S, Firat H, Caliskan-Can E, Ardic S, Simsek B. Usefulness of homocysteine as a cancer marker: total thiol compounds and folate levels in untreated lung cancer patients. Anticancer Res. 2007;27(2):1185–9. Epub 2007/05/01. . [PubMed] [Google Scholar]
- 11.Shen H, Spitz MR, Wang LE, Hong WK, Wei Q. Polymorphisms of methylene-tetrahydrofolate reductase and risk of lung cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 2001;10(4):397–401. Epub 2001/04/25. . [PubMed] [Google Scholar]
- 12.Truong T, Sauter W, McKay JD, Hosgood HD 3rd, Gallagher C, Amos CI, et al. International Lung Cancer Consortium: coordinated association study of 10 potential lung cancer susceptibility variants. Carcinogenesis. 2010;31(4):625–33. Epub 2010/01/29. 10.1093/carcin/bgq001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vineis P, Veglia F, Garte S, Malaveille C, Matullo G, Dunning A, et al. Genetic susceptibility according to three metabolic pathways in cancers of the lung and bladder and in myeloid leukemias in nonsmokers. Ann Oncol. 2007;18(7):1230–42. Epub 2007/05/15. 10.1093/annonc/mdm109 . [DOI] [PubMed] [Google Scholar]
- 14.Piskac-Collier AL, Monroy C, Lopez MS, Cortes A, Etzel CJ, Greisinger AJ, et al. Variants in folate pathway genes as modulators of genetic instability and lung cancer risk. Genes Chromosomes Cancer. 2011;50(1):1–12. Epub 2010/09/16. 10.1002/gcc.20826 . [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Matsuo K, Hiraki A, Saito T, Sato S, Yatabe Y, et al. Impact of one-carbon metabolism-related gene polymorphisms on risk of lung cancer in Japan: a case control study. Carcinogenesis. 2007;28(8):1718–25. Epub 2007/05/01. 10.1093/carcin/bgm104 . [DOI] [PubMed] [Google Scholar]
- 16.Hung RJ, Hashibe M, McKay J, Gaborieau V, Szeszenia-Dabrowska N, Zaridze D, et al. Folate-related genes and the risk of tobacco-related cancers in Central Europe. Carcinogenesis. 2007;28(6):1334–40. Epub 2007/03/29. 10.1093/carcin/bgm067 . [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Zhang Z, Li G, Pillow PC, Hernandez LM, Spitz MR, et al. Polymorphisms of methionine synthase and methionine synthase reductase and risk of lung cancer: a case-control analysis. Pharmacogenet Genomics. 2005;15(8):547–55. Epub 2005/07/12. 01213011-200508000-00003. . [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Jin G, Wang H, Wu W, Liu Y, Qian J, et al. Association of polymorphisms in one-carbon metabolizing genes and lung cancer risk: a case-control study in Chinese population. Lung Cancer. 2008;61(1):21–9. Epub 2008/01/29. 10.1016/j.lungcan.2007.12.001 . [DOI] [PubMed] [Google Scholar]
- 19.Shen M, Rothman N, Berndt SI, He X, Yeager M, Welch R, et al. Polymorphisms in folate metabolic genes and lung cancer risk in Xuan Wei, China. Lung Cancer. 2005;49(3):299–309. Epub 2005/06/01. 10.1016/j.lungcan.2005.04.002 . [DOI] [PubMed] [Google Scholar]
- 20.Arslan S, Karadayi S, Yildirim ME, Ozdemir O, Akkurt I. The association between methylene-tetrahydrofolate reductase gene polymorphism and lung cancer risk. Mol Biol Rep. 2011;38(2):991–6. Epub 2010/06/10. 10.1007/s11033-010-0194-z . [DOI] [PubMed] [Google Scholar]
- 21.Jeng YL, Wu MH, Huang HB, Lin WY, You SL, Chu TY, et al. The methylenetetrahydrofolate reductase 677C—>T polymorphism and lung cancer risk in a Chinese population. Anticancer Res. 2003;23(6D):5149–52. Epub 2004/02/26. [PubMed] [Google Scholar]
- 22.Siemianowicz K, Gminski J, Garczorz W, Slabiak N, Goss M, Machalski M, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms in patients with small cell and non-small cell lung cancer. Oncol Rep. 2003;10(5):1341–4. Epub 2003/07/29. 10.3892/or.10.5.1341 . [DOI] [PubMed] [Google Scholar]
- 23.Kiyohara C, Horiuchi T, Takayama K, Nakanishi Y. Methylenetetrahydrofolate reductase polymorphisms and interaction with smoking and alcohol consumption in lung cancer risk: a case-control study in a Japanese population. BMC Cancer. 2011;11:459 Epub 2011/10/26. 10.1186/1471-2407-11-459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Lu J, An J, Shi Q, Spitz MR, Wei Q. Polymorphisms of cytosolic serine hydroxymethyltransferase and risk of lung cancer: a case-control analysis. Lung Cancer. 2007;57(2):143–51. Epub 2007/04/11. 10.1016/j.lungcan.2007.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Q, Zhang Z, Neumann AS, Li G, Spitz MR, Wei Q. Case-control analysis of thymidylate synthase polymorphisms and risk of lung cancer. Carcinogenesis. 2005;26(3):649–56. Epub 2004/12/08. 10.1093/carcin/bgh351 . [DOI] [PubMed] [Google Scholar]
- 26.Swartz MD, Peterson CB, Lupo PJ, Wu X, Forman MR, Spitz MR, et al. Investigating multiple candidate genes and nutrients in the folate metabolism pathway to detect genetic and nutritional risk factors for lung cancer. PLoS One. 2013;8(1):e53475 Epub 2013/02/02. 10.1371/journal.pone.0053475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summers CM, Cucchiara AJ, Nackos E, Hammons AL, Mohr E, Whitehead AS, et al. Functional polymorphisms of folate-metabolizing enzymes in relation to homocysteine concentrations in systemic lupus erythematosus. J Rheumatol. 2008;35(11):2179–86. Epub 2008/09/12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skibola CF, Smith MT, Hubbard A, Shane B, Roberts AC, Law GR, et al. Polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and risk of adult acute lymphocytic leukemia. Blood. 2002;99(10):3786–91. Epub 2002/05/03. 10.1182/blood.V99.10.3786 . [DOI] [PubMed] [Google Scholar]
- 29.Summers CM, Mitchell LE, Stanislawska-Sachadyn A, Baido SF, Blair IA, Von Feldt JM, et al. Genetic and lifestyle variables associated with homocysteine concentrations and the distribution of folate derivatives in healthy premenopausal women. Birth Defects Res A Clin Mol Teratol. 2010;88(8):679–88. Epub 2010/06/15. 10.1002/bdra.20683 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skibola CF, Forrest MS, Coppede F, Agana L, Hubbard A, Smith MT, et al. Polymorphisms and haplotypes in folate-metabolizing genes and risk of non-Hodgkin lymphoma. Blood. 2004;104(7):2155–62. Epub 2004/06/17. 10.1182/blood-2004-02-0557 . [DOI] [PubMed] [Google Scholar]
- 31.Brown KS, Kluijtmans LA, Young IS, McNulty H, Mitchell LE, Yarnell JW, et al. The thymidylate synthase tandem repeat polymorphism is not associated with homocysteine concentrations in healthy young subjects. Hum Genet. 2004;114(2):182–5. Epub 2003/10/31. 10.1007/s00439-003-1039-9 . [DOI] [PubMed] [Google Scholar]
- 32.Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Genet A. 2004;124A(4):339–45. Epub 2004/01/22. 10.1002/ajmg.a.20505 . [DOI] [PubMed] [Google Scholar]
- 33.Barbaux S, Kluijtmans LA, Whitehead AS. Accurate and rapid "multiplex heteroduplexing" method for genotyping key enzymes involved in folate/homocysteine metabolism. Clin Chem. 2000;46(7):907–12. Epub 2000/07/15. . [PubMed] [Google Scholar]
- 34.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. Jama. 2008;300(17):2012–21. Epub 2008/11/06. 10.1001/jama.2008.555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. Epub 2006/03/15. 10.1056/NEJMoa060900 . [DOI] [PubMed] [Google Scholar]
- 36.Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nature reviews Cancer. 2016;16(11):694–707. Epub 2016/10/25. 10.1038/nrc.2016.82 . [DOI] [PubMed] [Google Scholar]
- 37.Dougherty SM, Mazhawidza W, Bohn AR, Robinson KA, Mattingly KA, Blankenship KA, et al. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocrine-related cancer. 2006;13(1):113–34. Epub 2006/04/08. 10.1677/erc.1.01118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(14):4417–26. Epub 2008/06/27. 10.1158/1078-0432.ccr-07-1950 . [DOI] [PubMed] [Google Scholar]
- 39.Stanislawska-Sachadyn A, Woodside JV, Brown KS, Young IS, Murray L, McNulty H, et al. Evidence for sex differences in the determinants of homocysteine concentrations. Mol Genet Metab. 2008;93(4):355–62. Epub 2008/01/09. 10.1016/j.ymgme.2007.11.004 . [DOI] [PubMed] [Google Scholar]
- 40.Stanislawska-Sachadyn A, Mitchell LE, Woodside JV, Buckley PT, Kealey C, Young IS, et al. The reduced folate carrier (SLC19A1) c.80G>A polymorphism is associated with red cell folate concentrations among women. Ann Hum Genet. 2009;73(Pt 5):484–91. Epub 2009/08/05. 10.1111/j.1469-1809.2009.00529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) folate concentration determined from serum samples within a day of blood collection vs. serum samples frozen for a few days; B) folate concentrations determined from serum samples frozen for 34–48 days vs. serum samples frozen for 10 months; C) homocysteine concentrations determined from serum samples frozen for 34–48 days vs. serum samples frozen for 10 months; D) RBC folate concentrations determined from whole blood vs. whole blood diluted 1:10 in 1% ascorbic acid, frozen for 2 years. The measures were corrected for the hematocrit values. Correlations were calculated using Pearson statistics.
(PDF)
(PDF)
(PDF)
(PDF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.