Abstract
Objective
To examine the literature and summarize studies that describe the potential ocular hazards that are posed by different systems of light curing units mainly used in the dental clinics, to ensure the safety of the operator, patient and the auxiliary staff in the dental clinic.
Methods
This systematic review was reported and conducted according to the PRISMA guidelines. The online databases PubMed and Google Scholar were used for data search. MeSH terms were used for PubMed search. Randomized controlled clinical trials, original studies and in-vitro studies conducted up to 2018 in English language were included in the review. Eight articles were included in the study after application of eligibility criteria, all of which were in accordance to the review protocol.
Results
The total wavelength dose received can cause Ocular damage which suggest that light intensity is correlated to the duration required to cause a certain level of damage, and we can substitute the long light exposure by using of a lower intensity light.
Conclusion
This review concludes that blue light poses maximum risk to cause retinal degeneration based on the evaluated studies. Most of the studies recommend the use of protective eyewear in order to limit exposure of the patient, operator and assistant to the LCUs. It is not advisable to stare directly into the light source and the recommended safe exposure times and distances for patient, operator and assistant must be strictly adhered to in the dental practice.
Keywords: Blue light, Dental curing light, Ocular hazards, Radiation
1. Introduction
Dentistry has made giant leaps in the last few decades with regards to material science. With the development of modern materials and techniques in restoration of cavities, veneering, orthodontic bonding procedures and pits fissure sealing which depend on converting monomers to polymers, there has been simultaneous development in curing lights (Sofan et al., 2017). The ability to photo-polymerize resins within a few seconds in the mouth made a huge revolutionized dentistry. Light has a dual wave-particle nature and its particle nature is more important while describing its effects when absorbed by a photopolymer. The part of the electromagnetic spectrum that effect with the eye ranges from ultraviolet (100–400 nm wavelengths) to infrared (760–10,000 or more nm wavelengths) and visible light lies in the center of this spectrum (400–760 nm). Visible light can be further referred to as blue (short wavelength), green (medium wavelength) and red (long wavelength) according to the maximum absorption spectrum of the human eye cells (Santini, 2012).
Initially, the dental light curing unit (LCU) delivered ultraviolet (UV) light for photopolymerization (Mahn et al., 2013, Conte et al., 2017) later the LCU transitioned into blue light emitting units because of the health concerns with the use of UV light. Currently, three main types of LCUs are used in the dental setting – halogen, plasma arc and light emitting diodes (LEDs). Most of these units emit intense blue light within the 400–500 nm wavelength but some of them also produce the UV(A) range (315–400 nm) (David et al., 2016, Tenkate et al., 2017). These lights are being continuously developed and improved to become more powerful and produce higher intensities (mW/cm2) and this has led to several advantages for both the dentist and the patient which includes shorter working times, saving time, less chance of bracket movement during orthodontic applications, reduced risk of saliva contamination and less discomfort for the patient due to short chair side time (Pelissier et al., 2011, Carine, 2009). However, these also have potential disadvantages.
In the dental clinic, the operator is usually exposed to active use of LCUs for several hours a day. Part of this radiation is scattered to the neighboring structures, some absorbed by the target organ and some of it is reflected which all depends upon the angle of the light beam, distance from the light source to the object and the spectrum of the emitted light (Rachel, 2009) estimated the maximum acceptable exposure times according to the exposure limit guidelines set by the American Conference of Government and Industrial Hygienists (Deveau et al., 2015) and the International Commission on non-Ionizing Radiation Protection (Bruzell et al., 2004). They assumed a thirty percentage reflection of the curing light at a distance of thirty cm and concluded that the total blue light exposure and UV component exposure of halogen lamps for the eye should not exceed 1 min/day and direct accidental exposure should not exceed 1 s (Deveau et al., 2015). In the 80 s, studies had been conducted to assess the ocular hazards of quartz-tungsten halogen (QTH) LCUs and it was found that due to their low emission intensities of around 300 mW/cm2, they had little potential to cause ocular damage (Rueggebergf et al., 2017, Satromi et al., 1987, Al-Samadani et al., 2013). However, the plasma arc and LED curing units today have a very different spectra emission and emit irradiances of up to 3000 mW/cm2 or more (Omidi et al., 2018).
The objective of this systematic review is to examine the literature and summarize the studies that describe the effects and potential hazards on the eye that are posed by different systems of curing light units commonly used in the dental practice today to ensure the safety of the operator, patient and the auxiliary staff in the dental clinic.
2. Methodology
This systematic review was reported and conducted according to the PRISMA guidelines.
2.1. Focused question
“To study how different types of curing lights in the dental office affect the eyes and what ocular hazards are posed by each”.
2.2. Search strategy
The systematic review was conducted to study how dental curing light affects the eyes. A protocol-based search strategy was employed to mine available literature and extract relevant studies in accordance with the focused question. The search was conducted in November 2017 and updated in January 2018. Two online databases - Google Scholar and PubMed - were used to conduct the search.
The MeSH 2017 browser from the online portal of the National Library of Health was used to generate the Medical Subject Headings (MeSH) equivalents of the proposed terms to conduct the PubMed search. The above terms were converted to “curing light, dental” and “light”.
A combination of these terms was used for the PubMed search without applying any filters to retrieve maximum results.
2.3. Study selection
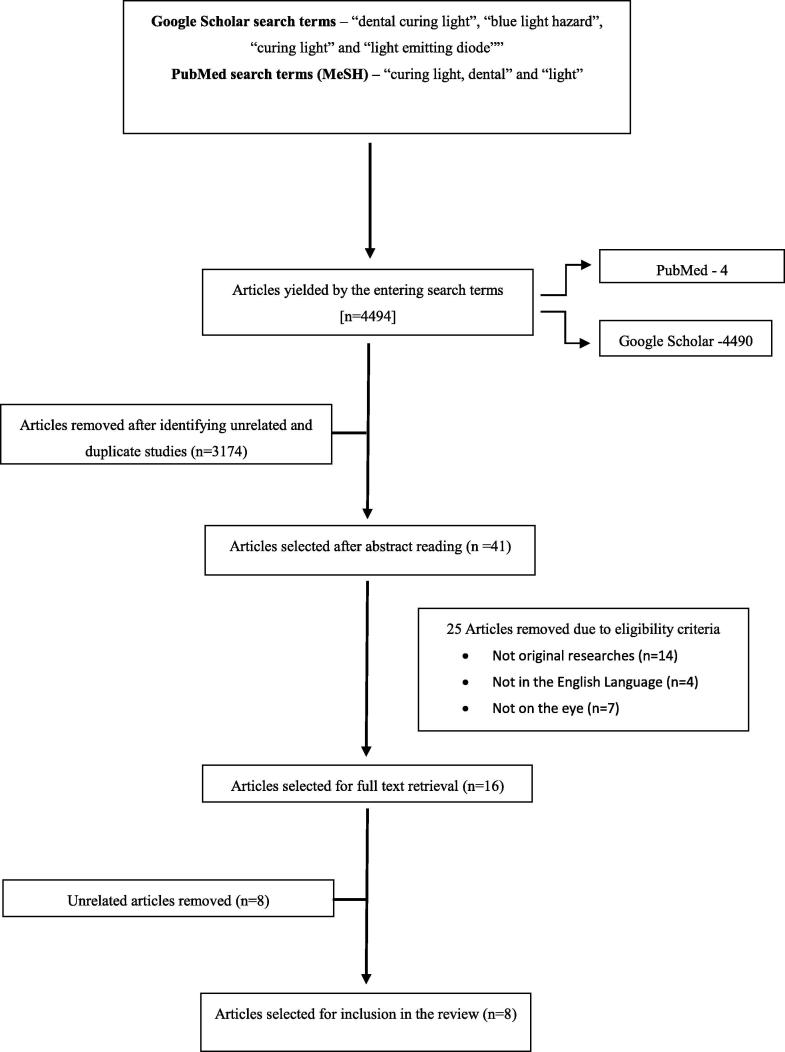
Studies in this review were selected based on a strategy as depicted in (Fig. 1). The two electronic databases generated a total of 4494 titles out of which, 4490 titles were generated from Google Scholar while the remaining four were generated from PubMed. These titles were then screened to remove unrelated and duplicate studies which left 1320 studies to be assessed. These were further scrutinized by abstract reading and 41 articles were selected which contained reviews, articles in other languages and articles which measured parameters of LCUs other than their ocular hazards. 14 of these were eliminated because they were not original researches. Further, four articles were eliminated because they were in languages other than English and seven of them measured the effects of LCUs on restorative resins and curing times. Eight articles were removed because they were unrelated. Finally, the reviewers were left with eight articles that were in accordance to the review protocol according to the reviewed articles our concerns were mainly to Type of dental curing lights, Distance from dental curing lights to the objects and Type of Hazard.
Fig. 1.
Flowchart outlining the search strategy for the review.
The following inclusion and exclusion criteria were deemed acceptable by all the reviewers:
2.4. Inclusion criteria
-
(1)
Randomized controlled trials, original research and in-vitro studies.
-
(2)
Studies published till 2018.
-
(3)
Studies done on the effects of dental curing light on eyes.
2.5. Exclusion criteria
-
(1)
Articles describing the effects of dental curing light on parts other than eyes.
-
(2)
Articles not in the English language.
-
(3)
Studies done on other forms of lights used in the dental clinic other than curing lights.
Titles and abstracts that were generated after entering the search terms in the two online databases were then screened independently by two reviewers by applying the eligibility criteria. Full text was accessed of those articles that qualified the eligibility criteria. Free full text articles were directly downloaded from the said databases while the restricted access articles were downloaded using the institutional access account of at King Abdul Aziz University.
3. Results
This present review aimed to include original research, randomized controlled trials and in-vitro experimental studies. Key data pertaining to the included studies is summarized in (Table 1). All articles in this review (Jiangmei et al., 1999, McCusker et al., 2013, Labrie et al., 2011, Rassaei et al., 2013, Price et al., 2016, Chang et al., 2016, Lee et al., 2016) are in-vitro experimental except one (Eriksen et al., 1987) which is an in-vivo experimental study. Three studies (Jiangmei et al., 1999, Chang et al., 2016, Lee et al., 2016) were conducted on live rodents (rats and mice) while one study (Rassaei et al., 2013) employed bovine superfused retina. One study (Price et al., 2016) used human teeth model while another study (Labrie et al., 2011) used extracted human maxillary teeth. Another study (McCusker et al., 2013) used three different types of orthodontic brackets (ceramic, stainless steel and composites) while one study (Eriksen et al., 1987) did not specify the type of study sample used in their research.
Six of the eight studies (Jiangmei et al., 1999, McCusker et al., 2013, Labrie et al., 2011, Rassaei et al., 2013, Price et al., 2016, Chang et al., 2016, Lee et al., 2016) used LED LCUs of varying wavelengths while three studies (McCusker et al., 2013, Labrie et al., 2011, Price et al., 2016) used plasma arc LCUs and two studies (McCusker et al., 2013, Labrie et al., 2011) used halogen LCUs. The lights used in all the studies ranged from 380 nm wavelength to more than 600 nm wavelength and intensities ranging from 29.2 mW/cm2 to 2000 mW/cm2 while three studies (Eriksen et al., 1987, Price et al., 2016, Chang et al., 2016) did not specify the intensity of radiations used. Three studies (Eriksen et al., 1987, Labrie et al., 2011, Rassaei et al., 2013) used weighted irradiances and safe exposure times to measure retinal damage while three studies (Jiangmei et al., 1999, Chang et al., 2016, Lee et al., 2016) measured cell byproducts released during apoptosis to ascertain retinal degeneration. One article (Chang et al., 2016) studied the effects of LCU on the eye while using five types of dental loupes while one study (Price et al., 2016) used changes in a and b waves on an electroretinogram as indicator of retinal damage. Three studies (Jiangmei et al., 1999, Chang et al., 2016, Lee et al., 2016) used TUNEL staining while four studies (Eriksen et al., 1987, McCusker et al., 2013, Labrie et al., 2011, Price et al., 2016) used a spectroradiometer. One study (Rassaei et al., 2013) used the electroretinogram to access retinal damage. Ocular effect obtained from those studies is summarized in (Table 1).
4. Discussion
To the best of our knowledge, this is the first comprehensive review that aims to summarize the effects of dental light curing units on the eyes after an in-depth qualitative analysis of eight articles that were generated in the search conducted based on the review protocol. A total of 35 LCUs were used in the selected articles which consisted of 17 LEDs, 3 plasma arc, 3 halogen and 12 unspecified LCUs. The studies included a various types of study samples. This included twenty-four female C57BL/6 mice, 45 male mice and 20 female Sprague-Dawley rats. The study samples in this review also included one human teeth model, four extracted human maxillary teeth, one bovine superfused retina and 8 orthodontic brackets. All samples were exposed to a different sources of dental lights cure with similar results as ocular damage. The most commonly used lights in the studies were blue LEDs. Weighted irradiances and cell apoptosis byproducts were the most commonly used parameters used to measure retinal degeneration. Even though the recent development in different types of curing lights in dentistry, mainly three types of LCUs are used in the dental setting –plasma arc, halogen and light emitting diodes (LEDs). Over exposure to blue light cure without protective measurements can induce apoptosis to the cornea, increased ocular inflammation and dryness of the eye.
The short term risks associated with dental Lights cure is particularly low if safety measures are used. The same result for reflected light from orthodontic brackets during bonding procedure.
4.1. Key findings for ocular hazards posed by dental curing lights
The retina is the innermost portion of the human eye and the area that contains the photoreceptor cells; rods and cones which initiate the visual process by converting images of the physical world generated by the dyotropic media of the eye into neural signals (Roh et al., 1994). The photobiologic effects produced in the eye are a function of the manner in which light penetrates a tissue. The ocular media of the normal human eye transmits at least one percentage of the radiation within the range of 400–1400 nm and this is known as “retinal hazard region” (Catherine et al., 2013). Light is focused by the eye in order to produce images and this focusing process increases the power density of light by concentrating it on the retina. As a consequence, light with a radiant intensity insufficient to cause skin damage may cause ocular injury when focused into the retina.
The damage caused to eye can be photomechanical, photothermal or photochemical. Absorption of harmful wavelength and adjustment of the pupil size (from less than one mm to eight mm) are two natural protective mechanisms of the human eye (Hunter et al., 2012). Wavelengths under 400 nm are absorbed by the lens of the eye and cannot reach the retina, but more blue spectrum radiation can reach the retina in the young eye than in the aged eye (Roh et al., 1994), because in the young eye, ocular transmittance is high, reaching close to 90% at 450 nm. The majority of the studies in this review used LCUs emitting blue light. Wu et al. studied the blue light induced apoptosis in rat retina (Jiangmei et al., 1999). Electron microscopy revealed pathological changes immediately and within 24 h after 3 and 6 h of exposure to light. These changes included progressive condensation and margination of the chromatin, shrinkage or convolution and fragmentation of the nucleus, condensation of the cytoplasm, and formation of apoptotic bodies along with rapid removal of dying cells from damaged areas in the absence of inflammatory response. They concluded that apoptosis is seen early after the retina is damaged by blue light. Blue light exposure irreversibly inhibits cytochrome oxiuase, causing inner segment photoreceptor damage (Soares et al., 2017). Rassaei et al. also studied the effects of blue light on the superfused bovine retina (Rassaei et al. 2013). They concluded that the light intensity which is applied by the UV-Z-lamp is potent enough to cause severe damage of the visual signal transduction in the service personnel, if the appropriate filter device were to be omitted. Using the light filter, which is well-adapted to the emission spectrum of the UV-Z-lamp, may reduce the remaining irradiation to tolerable intensities and avoid photochemical damage of the retina. Lee et al. studied the influence of light emitting diode -derived blue light over exposure on mouse ocular surface. Their study revealed that over exposure to blue light with short wavelengths can induce oxidative damage and apoptosis to the cornea, which may manifest as increased ocular surface inflammation and resultant dry eye. Similarly, Chang et al. studied increased expression of osteopontin in retinal degeneration induced by blue light emitting diode exposure in mice. They found that increases in OPN expression were selectively observed in the central retina, the primary site of photo receptor apoptosis.
Ocular damage depends on the total wavelength dose received which mean that light intensity is correlated to the duration required to cause a certain level of damage, and a longer light exposure can substitute for the use of a lower intensity. This has led researchers to establish safe exposure times for various lights used in the dental practice. Labrie et al. evaluated the ocular hazards from four types of curing lights and found that the plasma arc unit delivered both the greatest total radiant power and the greatest weighted blue-light irradiance, because most of its spectral emission lies within the blue-light range. The peak irradiance values of the low-power and high-power LED units were close to the wavelength at which maximum blue light hazard occurs (440 nm). They concluded that higher-powered LCUs showed the potential to cause ocular damage mediated by blue light at shorter distances, with the potential damage occurring after cumulative viewing of about six seconds at a distance of Thirty cm (over an Eight-hour workday). However, the study by McCusker et al. reported a different conclusion. They studied the effects of three different types of LCUs on the eyes when light reflected off from three different types of ceramic brackets. They concluded that the short-term risks associated with dental LCUs is low particularly if protective measures are used. The same is right for reflected light from orthodontic brackets during bonding.
5. Conclusion
Ocular hazards posed by the curing light units used in the dental practice are well established. This review concludes that blue light poses maximum risk to cause retinal degeneration based on the evaluated studies. Most of the studies recommend the use of protective eyewear in order to limit exposure of the patient, operator and assistant to the LCUs. It is not advisable to stare directly into the light source and the recommended safe exposure times and distances for patient, operator and assistant must be strictly adhered to in the dental practice.
Conflict of interest
The authors of this research have no conflict of interest to declare.
Ethical statement
By submitting this manuscript to the Saudi Dental Journal, all authors explicitly confirms that the manuscript meets the highest ethical standards including proper statistical investigations and thorough ethical reviews by the data owning organisations.
Footnotes
Peer review under responsibility of King Saud University.
Appendix A.
Table 1.
Key data pertaining to the included studies.
| Authors/study design | Type/number of samples | Type of light | Intensity of radiation (mW/cm2) | Parameters evaluated | Type of assay/test/equipment |
|---|---|---|---|---|---|
| Lee et al. (in-vitro, experimental) | Twenty-Four female C57BL/6 Mice divided into four groups – red, green, blue and untouched (UT) |
Red (630 ± 8) Green (525 ± 2) Blue (410 ± 10) |
48.8 59.5 29.2 |
Tear volume, Tear film breakup time (TBUT), Interferon (INF)-γ, Interleukin (IL)-1β, IL-6, Tumor necrosis factor (TNF)-α, Malondialdehyde (MDA), CD4 + CCR5 + T cells | Corneal Fluorescein staining, multiplex immunobead assay, enzyme linked immunosorbent Assay (ELISA), Flow cytometry, 2′7′-diachloroflouroscein diacetate (DCF-DA) assay, terminal de-oxynucleotidyl transferase-mediated dUTP-nick end labeling (TUNEL) staining |
| Chang et al. (in-vitro, experimental) | 45 male mice 36 – exposed 9 – controls |
Dim red light (>600 nm) Blue LED light (410 ± 10) |
Not specified | Osteopontin (OPN) | TUNEL assay, Western blotting, Immunohistochemistry, immunogold electron microscopy |
| Price et al. (in-vitro experimental) | Human teeth model | Sapphire Plus Plasma Arc LCU (Den-Mat, Lompoc CA) | Not specified | Effect of dental loupes – five types used Three loupes of 3.5× magnification (Design for Vision, Carl Zeiss, Quality Aspirator) and two 2.5× magnification (Design for Vision, Quality Aspirator) |
6-in integrating sphere (Labsphere, North Sutton, NH) connected to a fiber optic spectrometer (USB 4000, Ocean Optics, Dunedin, FL) |
| Rassaei et al. (in-vitro experimental) | Bovine superfused retina | Blue LED LCU (Delma Medical Instrument, Guangzhou, Guangdong, China) 420–480 nm |
>1000 (1200 lx for isolated retina) |
a and b waves used as indicators of retinal damage | Electroretinogram (ERG) |
| Labrie et al. (in-vitro experimental) | Extracted human maxillary teeth |
PAC: Sapphire (Den-Mat Santa Maria, CA) LED:
QTH: Optilux 501 (Kerr Corporation Orange, CA) |
826 ± 2 325 ± 1 740 ± 2 630 ± 5 |
Weighted blue light and effective ultraviolet (UV) irradiances received by the eye | A laboratory-grade light detector (3.9-mm diameter CC3-UV probe, Ocean Optics, Dunedin, FL) attached by a 1-mm fibre optic cable to a fibre optic spectroradiometer (USB 4000, Ocean Optics), with a spectral range of 300–890 nm, fully covering the spectral emission from the LCU |
| McCusker et al. (in-vitro experimental) | 8 different orthodontic brackets Ceramics Clarity, Clarity SL (3 M Unitek), Encore (Ortho Technology) Stainless Steel Victory (3 M Unitek), Microarch, Mini Ovation (GAC International), TOC Bracket (TOC) Composite Tiger (TOC) |
11 LCUs 1 Plasma Arc Apollo 95E(460–490 nm) 2 Halogens Cromalux (400–500 nm) CU80 (380–510 nm) 8 LEDs DEMIOrtho (420–465 nm) Elipar Freelight2 (430–480 nm) Fusion (385–430 nm) Mini LED (420–480 nm) Mini LED2 (420–480 nm) Ortholux (430–480 nm) Smartlite (450–490 nm) Starlite (440–480 nm) |
1600 650–800 600 1100-1330 1000 1500 1250 2000 1600 950 100 |
Weighted irradiance and safe exposure times | Integrated spectroradiometer (DMc150-MDE, Bentham Instruments Ltd. UK) |
| Jiangmei et al. (in-vitro experimental) | Female Sprague-Dawley rats | Blue light (400–480 nm) | 0.64 | Cell death following blue light exposure | TUNEL, gel electrophoresis |
| Eriksen et al. (in-vivo experimental) | Not specified | 12 dental LCUs | Not specified | Total irradiance(E), Effective UV irradiance (Eeff)), UV--A irradiance (EUVA), blue light radiance (Lb), Thermal hazard radiance (LR) and the Luminance (LV) | Spectroradiometer |
References
- Al-Samadani K. Light intensity decay in quartz-tungsten-halogen polymerization units. J. Int. Oral Health. 2013;5:23–30. [PMC free article] [PubMed] [Google Scholar]
- Bruzell R. Health hazards associated with curing light in the dental clinic. Clin Oral Investig. 2004;8:113–117. doi: 10.1007/s00784-003-0248-x. [DOI] [PubMed] [Google Scholar]
- Carine D. Effect of curing time on the bond strength of a bracket-bonding system cured with a light-emitting diode or plasma arc light. Eur. J. Orthod. 2009;2011(33):55–59. doi: 10.1093/ejo/cjq027. [DOI] [PubMed] [Google Scholar]
- Catherine S. The possible ocular hazards of LED dental illumination applications. J. Tenn Dent Assoc. 2013;93:25–29. [PubMed] [Google Scholar]
- Chang S. Increased expression of osteopontin in retinal degeneration induced by blue light-emitting diode exposure in mice. Front. Mol. Neurosci. 2016;9:58. doi: 10.3389/fnmol.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte G. Curing effectiveness of single-peak and multi-peak led light curing units on tpo-containing resin composites with different chromatic characteristics. Oral Implantol. 2017;10:140–150. doi: 10.11138/orl/2017.10.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. Ultraviolet safety assessments of insect light traps. J. Occup. Environ. Hygiene. 2016;13:413–424. doi: 10.1080/15459624.2015.1125489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau M. The global landscape of occupational exposure limits-implementation of harmonization principles to guide limit selection. J. Occup. Environ. Hygiene. 2015;12:127–144. doi: 10.1080/15459624.2015.1060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen P. Optical hazard evaluation of dental curing lights. Commun. Dent. Oral Epidemiol. 1987;15:197–201. doi: 10.1111/j.1600-0528.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Hunter J. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin Eye Res. 2012;31:28–42. doi: 10.1016/j.preteyeres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiangmei Wu. Blue light induced apoptosis in rat retina. Eye. 1999;13:577–583. doi: 10.1038/eye.1999.142. [DOI] [PubMed] [Google Scholar]
- Labrie D. Evaluation of ocular hazards from 4 types of curing lights. J. Can. Dent. Assoc. 2011;77:b116. [PubMed] [Google Scholar]
- Lee H. Influence of light emitting diode-derived blue light overexposure on mouse ocular surface. J. Pone. 2016;11:8. doi: 10.1371/journal.pone.0161041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn E. Clinical criteria for the successful curing of composite materials. Periodoncia Implantol. Rehabil. 2013;6:148–153. [Google Scholar]
- McCusker N. Light curing in orthodontics; should we be concerned? Dent. Mater. 2013;29:85–90. doi: 10.1016/j.dental.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Omidi B. Intensity output and effectiveness of light curing units in dental offices. J. Clin. Exp. Dent. 2018;10:555–560. doi: 10.4317/jced.54756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier B. Three generations of LED lights and clinical implications for optimizing their use. Dent Update. 2011;38:660–664. doi: 10.12968/denu.2011.38.10.660. [DOI] [PubMed] [Google Scholar]
- Price R. The dental curing light: A potential health risk. J. Occup. Environ. Hygiene. 2016;13:639–646. doi: 10.1080/15459624.2016.1165822. [DOI] [PubMed] [Google Scholar]
- Rachel S. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematous. Autoimmun Rev. 2009;8:320–324. doi: 10.1016/j.autrev.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassaei M. Effect of high-intensity irradiation from dental photopolymerization on the isolated and superfused vertebrate retina. Graefes Arch. Clin. Exp. Ophthalmol. 2013;251:751–762. doi: 10.1007/s00417-012-2235-x. [DOI] [PubMed] [Google Scholar]
- Roh S. Light damage to the eye. J. Fla Med. Assoc. 1994;81:248–251. [PubMed] [Google Scholar]
- Rueggebergf Light curing in dentistry and clinical implications. Braz. Oral Res. 2017;31:61. doi: 10.1590/1807-3107BOR-2017.vol31.0061. [DOI] [PubMed] [Google Scholar]
- Santini A. Degree of conversion and microhardness of TPO-containing resin-based composites cured by polywave and monowave LED units. J. Dent. 2012;40:577–584. doi: 10.1016/j.jdent.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Satromi K. Potential retinal hazards of visible-light photopolymerization units. J. Dent. Res. 1987;66:731–736. doi: 10.1177/00220345870660030501. [DOI] [PubMed] [Google Scholar]
- Soares C. Evaluation of eye protection filters used with broad-spectrum and conventional LED curing lights. Braz. Dental J. 2017;28:9–15. doi: 10.1590/0103-6440201701380. [DOI] [PubMed] [Google Scholar]
- Sofan E., Sofan A., Palaia G., Tenore G., Romeo U., Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann. Stomatol. 2017;8:1–17. doi: 10.11138/ads/2017.8.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenkate T.D. Ocular ultraviolet radiation exposure of welders. Scand. J. Work Environ. Health. 2017;43:287–288. doi: 10.5271/sjweh.3630. [DOI] [PubMed] [Google Scholar]