Abstract
Aim
This study compared the setting time, radiopacity, solubility and pH changes between Hisperidin cement, MTA-Angelus and Calcium hydroxide cements.
Methods
The study was conducted on 3 equal groups of samples of the evaluated capping materials including; Hisperidin (group I, N = 24), MTA-Angelus (group II, N = 24) and Dycal (group III, N = 24). According to the assessed property, these groups were further subdivided into three equal subgroups (8 samples each) including; subgroup A for assessment of the setting time, subgroup B for assessment of radiopacity and subgroup C for assessment of the solubility of the material and evaluation of pH. All recorded data were tabulated and statistically analyzed.
Results
The highest mean value of setting time was for the MTA-Angelus followed by Hesperidin and Calcium hydroxide with 72.83, 48.26 and 1.58 min, respectively. MTA-Angelus had the highest radiopacity value and followed by Calcium hydroxide then Hesperidin. Hesperidin showed the solubility in distilled water (≈45% mass loss) in relation to Calcium hydroxide (≈19% mass loss). On the other hand, MTA-Angelus showed 9% increase in weight. On contrast to MTA and Calcium hydroxide, Hesperidin showed decrease in pH value throughout the evaluation periods. Higher pH values in MTA-Angelus and Calcium hydroxide were reported in comparison with Hesperidin.
Conclusion
Despite its slight acidic nature, lower radiopacity and longer initial setting time, Hesperidin, as a natural product, is a promising pulp capping material. Further research on Hesperidin powder is recommended to improve its physicochemical properties and to assess its biological actions.
Keywords: Dental pulp, pH, Radiopacity, Setting time, Solubility
1. Introduction
Historically, various materials such as ivory, gold-beaters skin, Canada Balsam, oiled skin, quill, plaster of Paris, paper, oxychloride, oxyphosphate, asbestos, gutta percha, lactophosphate of lime and oxyxulphate of zinc cement have been used in pulp capping (Dominguez et al., 2003).
In the past, Eugenol and Zinc oxide had the best outcomes after several comparative researches on different capping materials. Then Calcium hydroxide was successfully applied due to its good biocompatibility (Dammaschke, 2008).
Therefore, Calcium hydroxide became the pulp capping material of choice for several decades. By the time, the studies have been continued on new materials as pulp capping agents therefore new materials such as Mineral Trioxide Aggregate (MTA), composite resin, Propolis, Portland cement and finally Bioaggregate were introduced to the markets (Almas and Mahmoud, 2000, Accorinte et al., 2008, Asgary et al., 2008, Borges et al., 2010, Barbosa Silva et al., 2000, Bavana et al., 2015, Bollu et al., 2016, El Ashry et al., 2016, Negm et al., 2017).
In earliest ancestors, natural materials have often used as a sole therapy for both injuries and diseases. Nowadays, the medicinal use of natural agents gains a great renewed interest for treatment of several diseases (Abu-Seida, 2015). During the last few years, several dental studies had been carried out on Propolis, a resinous material collected by honey bees from different plants (Barbosa Silva et al., 2000, Saleh et al., 2016). Propolis had been applied as an antimicrobial and anti- inflammatory material for many decades. One of the main components of Propolis is flavonoids that have several therapeutic actions including; reduction of free radicals release, inhibition of bacterial and fungal growth, and regulation of the immune response. These therapeutic actions suggested that flavonoids have natural antimicrobial, anti-inflammatory and immuno-regulatoy proprieties. Hesperidin is one of the most important flavonoids (Burdock, 1998).
For any pulp capping material, the most important physical and chemical characteristics are setting time, radio-opacity, solubility and pH value (Camilleri, 2010, Borges et al., 2011, Negm et al., 2016).
The authors hypothesized that Hesperidin – as a flavonoid – could be a beneficial natural new pulp capping material. Therefore, this part of the study evaluated the physicochemical properties including; setting time, radiopacity, solubility and pH value of Hesperidin in comparison with MTA-Angelus and Calcium hydroxide.
2. Materials and methods
2.1. Materials
The materials used in the present study are listed in Table 1.
Table 1.
Tested materials and their composition according to the manufacturers.
| Materials | Composition | Manufacturer |
|---|---|---|
| MTA-Angelus | Silicon dioxide or silica (SiO2), potassium oxide (K2O), aluminum oxide (Al2O3), sodium oxide (Na2O), iron oxide (Fe2O3), sulfur trioxide (SO3), calcium oxide (CaO), bismuth trioxide (Bi2O3), magnesium oxide (MgO), potassium sulphate (KSO4), sodium sulphate (NaSO4), crystalline silica. | QED Ltd., Bakewell Road, Orton Southgate; Peterborough, Brazil |
| Hesperidin | Pure Hesperidin powder | SEDICO Pharmaceutical Company, 6th October City, Egypt |
| Dycal |
Base paste: Disalicylate ester of 1, 3 butylene glycol, calcium phosphate, calcium tungstate, zinc oxide, iron oxide. Catalyst paste: Calcium hydroxide, ethyl toluenesulfonamide, zinc state, titanium dioxide, zinc oxide, iron oxide. |
Dentsply Caulk Milford, DE, USA |
2.2. Samples
Seventy-two samples were divided into three main groups (24 samples each) according to the evaluated material. These groups included; group I (Hisperidin), group II (MTA Angelus) and group III (Dycal). These groups were further subdivided into three equal subgroups (8 samples each) according to the tested property; subgroup A: for evaluation of the setting time, subgroup B: for evaluation of the radiopacity and subgroup C: for evaluation of the solubility of the material and determining the pH.
2.3. Methods of evaluation
2.3.1. Determination of the setting time of the tested materials
Hesperidin was mixed at water to powder ratio of 1:3 (Camilleri, 2010). MTA-Angelus and Dycal were mixed according to the manufacturer's instructions. The mixture was placed in a ring mold with an internal diameter of 10 mm ± 0.1 mm and a thickness of 2 mm ± 0.1 (Fig. 1a). For each material, 8 specimens were fabricated. At 150 ± 10 s from the commencement of mixing (Borges et al., 2010), a Gilmore needle (100 ± 0.5 g with a flat end diameter of 2 mm ± 0.1 mm) was vertically lowered onto the horizontal surface of the tested material and allowed there for 5 s (Fig. 1b). This procedure was repeated at 30 s intervals until the needle failed to make a complete circular indentation in the material (Kataia, 2011). The indenter was wiped clean between indentations. After assessment of the initial setting time, a 456 ± 0.5 g Gilmore needle with a flat end diameter of 1.0 ± 0.1 mm was used to evaluate the final setting time (Fig. 1c). The needle was inserted at 60 s regular intervals until complete circular indentations could not be observed on the surface of the evaluated capping material (Borges et al., 2010). The setting time was calculated as the time passed from the beginning of mixing to the time of absence of indentation on the surface of tested material.
Fig. 1.
(a) Ring mold used for measurement of the setting time. (b) Gillmore needle for determination of initial setting time. (c) Gillmore needle for determination of final setting time.
2.3.2. Radiopacity evaluation using digital radiographic system
Four acrylic plates (2.2 cm × 4.5 cm × 1 mm) with 6 holes of 1 mm in depth and 5 mm in diameter were placed between two glass plates covered with cellophane sheets. Each tested material was placed immediately in eight holes. Any excess material was removed once set then the plates were stored at room temperature during the setting time of all tested materials. The radiographic evaluation was conducted after the final setting. To standardize and locate the tested materials during radiopacity evaluation, the tested materials were placed on the acrylic plates in the same position (Borges et al., 2011).
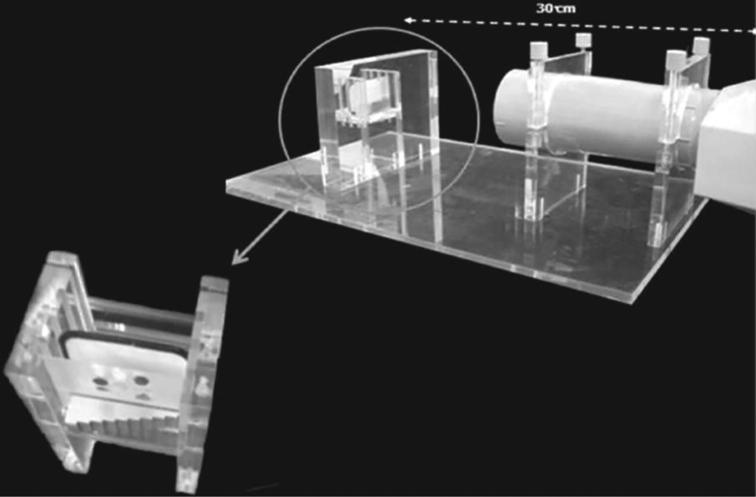
At the time of radiographic exposure, each acrylic plate with the contained material was positioned alongside with another acrylic plate (1.3 cm × 4.5 cm × 1 mm) containing a graduated aluminum step-wedge with a thickness varied from 1 to 10 mm in uniform steps of 1 mm each (American National Standard Institute (ANSI/American Dental Association, 2000). This set of acrylic plates had a similar size of a digital radiography phosphor plate of the Digora system (Sordex Orion Corporation, Helsinki, Finland). For digital radiography, the set of acrylic plates was placed in front of this phosphoric plate with placing the samples in the middle of the phosphor plate and next to the aluminum step-wedge. Radiographic unit (MINRAY®, Soredex, Tuusula, Finland) was applied for radiography of the samples and the aluminum step-wedge. The exposure time was set at 0.04 s and the radiographic setting factors were 65 kVp, 7 mA and 30 cm focal distance (ANSI/ADA, 2000).
The same radiographic setting factors were used for exposure of the phosphor plate and the plate was scanned using a suitable tool from the Digora system. The same phosphor plate was used for all exposure to avoid the possible differences between plates. An acrylic device was prepared for positioning of the phosphor plate and assuring standard focal distance (Fig. 2). The imaged plates of the tested materials were scanned just after the exposure using the Digora plate scanner according to the manufacturer's standard instructions for obtaining gold standard images (Martins et al., 2006). Then the phosphor plate was cleaned and re-used. The radiographs were viewed using Digora for Windows 1.51 software. The grey-scale values of the materials and the aluminum step-wedge corresponded to 30 × 30 pixels, identified by the coordinates dX and dY. For each analyzed area of interest, the initial coordinates were observed and the average measures of grey levels were described by the function 'density mean'. The regions with air bubbles inside the evaluated material were avoided. This procedure was repeated three times and the average was calculated for each specimen.
Fig. 2.
Acrylic device for positioning the phosphor plate at standardized object-to-focus distance.
2.3.3. Evaluation of the solubility of tested materials
The tested materials were placed in split Teflon ring molds with 20 mm internal diameter and 1.5 ± 0.5 mm thickness (Fig. 3a) and were placed on thin polyethylene sheets backed by glass plates (Kataia, 2011). Each mold was filled to a slight excess with the tested material. Eight discs were fabricated and evaluated for each material. Before setting, an impermeable nylon thread was inserted through the cement for hanging of the samples and immersion in solution throughout the experimental time. Another glass plate faced with polyethylene sheet was placed on top of the filled rings. After three times of the setting time, the samples of tested materials were removed from the molds and any loose material particles were removed from the surface using a soft brush (Ricci Vivan et al., 2010). The samples were weighed in an analytical balance with 0.001 g precision, then the net weight of each sample was recorded (M0). The samples suspended by the nylon threads were immersed in a wide-mouthed glass bottles containing 50 ± 1 mL distilled water with keeping the samples not touching the bottles, walls (Fig. 3b). The bottles were kept hermitically closed for 28 days at constant temperature of 37 ± 1 °C. Samples were then removed from the bottles, gently washed with distilled water, dried with filter paper and desiccated for 1 h at 37 °C (Torabinejad et al., 1995). Each disc was reweighed to the nearest micro-gram for the second time (M1). The material's solubility was measured as the percentage of lost mass compared to the initial mass according to Carvalho et al. (2003) as follows:
Fig. 3.
(a) Ring molds used for determination of solubility. (b) Disc of a capping material hung in distilled water to measure solubility.
The solubility of each material was calculated as the mean percent mass loss of the eight samples.
2.3.4. Determination of the pH values of tested materials
The recovered solutions of solubility test were used for measurement of pH changes using pH meter (Orion PerpHect Log R meter, Orion Research Inc., USA). Measurement of pH was carried out at 7, 14, 21 and 28 days from mixing according to Camilleri (2010). During the evaluation periods, each disc was re-placed in the same glass bottle without any change in the water. The pH was measured eight times for each tested material.
2.4. Statistical analysis
All data were collected and statistically analyzed. Values of the physical properties were presented as mean and standard deviation (SD) values. Data were explored for normality using Kolmogorov-Smirnov test of normality. The results of Kolmogorov-Smirnov test showed that most of data were normally distributed (parametric data), so one way analysis of variance ANOVA test was applied for comparison between groups and different pH within the same group, followed by Tukey’s post hoc test when the difference was significant. The significance level was set at P ≤ .05. Statistical analysis was performed using SPSS 16.0 (Statistical Package for Scientific Studies, SPSS, Inc., Chicago, IL, USA) for Windows.
3. Results
3.1. Setting times
The data are tabulated and statistically analyzed in Table 2.
Table 2.
Initial and final setting times (in minutes) of Hesperidin, MTA and Dycal.
| Capping materials | Initial setting time (min.) | Final setting time (min.) | |
|---|---|---|---|
| Hesperidin | Mean ± SD | 15.64a ± 0.38 | 48.26b ± 3.47 |
| Min | 15.10 | 45.95 | |
| Max | 16.13 | 56.68 | |
| MTA | Mean ± SD | 13.33b ± 2.18 | 72.83a ± 17.29 |
| Min | 11.03 | 57.65 | |
| Max | 17.62 | 112.83 | |
| Dycal | Mean ± SD | 0.85c ± 0.15 | 1.58c ± 0.26 |
| Min | 0.50 | 1.38 | |
| Max | 0.98 | 2.20 | |
| F value | 308.99 | 101.08 | |
| P value | <0.0001* | <0.0001* | |
Significance level P < .05, *Significant. Tukey's post hoc test: means with different superscript letters (within the same setting time) are significantly different.
Regarding the initial setting time, the greatest mean value was recorded in Hesperidin (15.64 ± 0.38 min), followed by MTA (13.33 ± 2.18 min), with the least value recorded in Dycal (0.85 ± 015 min).
Regarding the final setting time, the greatest mean value was recorded in MTA (72.83 ± 17.29 min), followed by Hesperidin (48.26 ± 3.47 min), with the least value recorded in Dycal (1.58 ± 0.26 min).
3.2. Radiopacity
The data are tabulated and statistically analyzed in Table 3. The greatest mean value was recorded in MTA (179.85 ± 7.78), followed by Dycal (135.56 ± 9.78) overcoming the value of three steps of aluminum step-wedge which was the minimum radio-opacity recommended by the ANSI/ADA specification number 57. The least value was recorded in Hesperidin (72.63 ± 7.38) and it was less than three steps of aluminum step wedge.
Table 3.
Radiopacity of Hesperidin, MTA and Dycal.
| Values | Hesperidin | MTA | Dycal |
|---|---|---|---|
| Mean ± SD | 72.63a ± 7.38 | 179.85b ± 7.78 | 135.56c ± 9.78 |
| Min | 64.20 | 171.07 | 122.27 |
| Max | 81.86 | 188.89 | 146.92 |
| F value | 319.71 | ||
| P value | <0.0001* | ||
Significance level P < .05. *Significant. Tukey's post hoc test: means with different superscript letters are significantly different.
3.3. Solubility (% of mass change)
The data are tabulated and statistically analyzed in Table 4. The greatest mean percent decrease was recorded in Hesperidin (−44.76 ± 11.53 g), followed by Dycal (−18.87 ± 5.17 g), while MTA recorded a percent increase of 9.41 ± 1.36 g. One way analysis of variance (ANOVA) test of the initial and final setting times, radiopacity and solubility revealed a significant difference between different groups (P < .0001). Tukey’s post hoc test revealed a significant difference between each two groups.
Table 4.
Solubility (percentage of mass change) of Hesperidin, MTA and Dycal.
| Values | Hesperidin | MTA | Dycal |
|---|---|---|---|
| Mean ± SD | −44.76c ± 11.53 | 9.41a ± 1.36 | −18.87b ± 5.17 |
| Min | 34.98 | 7.89 | 7.58 |
| Max | 62.86 | 12.11 | 28.11 |
| F value | 216.11 | ||
| P value | <0.0001* | ||
Significance level P < .05. *Significant, − = weight loss. Tukey's post hoc test: means with different superscript letters are significantly different.
3.4. The pH values
3.4.1. Within the same group
The data are collected, tabulated and statistically analyzed in Table 5.
Table 5.
pH values of Hesperidin, MTA and Dycal within the same group.
| Materials | Values | pH at 7 days | pH at 14 days | pH at 21 days | pH at 28 days | F value | P value |
|---|---|---|---|---|---|---|---|
| Hesperidin | Mean ± SD | 5.71a ± 0.37 | 5.58a,b ± 0.07 | 5.43a,b ± 0.10 | 5.37b ± 0.17 | 4.23 | 0.0138* |
| Min | 5.26 | 5.40 | 5.25 | 5.23 | |||
| Max | 6.18 | 5.63 | 5.60 | 5.76 | |||
| MTA | Mean ± SD | 11.48b ± 0.11 | 11.56b ± 0.15 | 11.82a ± 0.21 | 11.12c ± 0.14 | 26.6 | <0.0001* |
| Min | 11.32 | 11.48 | 11.72 | 11.04 | |||
| Max | 11.58 | 11.94 | 12.34 | 11.46 | |||
| Dycal | Mean ± SD | 11.40a,b ± 0.06 | 11.49a ± 0.08 | 11.53a ± 0.11 | 11.33b ± 0.15 | 5.94 | 0.0028* |
| Min | 11.32 | 11.37 | 11.40 | 11.18 | |||
| Max | 11.48 | 11.59 | 11.69 | 11.54 |
Significance level P < .05, *Significant. Tukey's post hoc test: means sharing the same superscript letter (within the same group) are not significantly different.
In Hesperidin group, the pH values were 5.71 ± 0.37, 5.58 ± 0.07, 5.4 ± 0.10 and 5.37 ± 0.17 at 7, 14, 21 and 28 days, respectively. The mean value decreased with increasing the evaluation period. One way analysis of variance revealed that the difference was statistically significant (P = 0.0138). Tukey’s post hoc test revealed a significant difference between pH value at 7 and 28 days.
In MTA group at 7, 14, 21 and 28 days, the mean value of pH was 11.48 ± 0.11, 11.56 ± 0.15, 11.82 ± 0.21 and 11.12 ± 0.14 respectively. The mean value increased with increasing pH evaluation period from 7 days to 21 days, then decreased at 28 days. One way analysis of variance revealed a statistically significant difference (P < .0001). Tukey’s post hoc test revealed a non-significant difference between pH value at 7 and 14 days.
In Dycal group, the mean value of pH at 7 days was 11.40 ± 0.06, it increased to 11.49 ± 0.08 and 11.53 ± 0.11 at 14 and 21 days respectively. At 28 days, the mean value decreased to 11.33 ± 0.15. One way analysis of variance revealed that the difference was statistically significant (P = .0028). Tukey’s post hoc test revealed a significant difference between pH value at 28 days and each of 14 and 21 days.
3.4.2. Comparison between groups at different observation periods
The data are collected in Table 6. At each pH evaluation period, significantly higher values were recorded in MTA and Dycal groups in comparison with Hesperidin group. A Statistically significant difference was recorded between groups at the same evaluation period (P < .0001).
Table 6.
Comparison of pH values of Hesperidin, MTA and Dycal between all groups at different evaluation periods.
| Materials | Values | pH at 7 days | pH at 14 days | pH at 21 days | pH at 28 days |
|---|---|---|---|---|---|
| Hesperidin | Mean ± SD | 5.71b ± 0.37 | 5.58b ± 0.07 | 5.43b ± 0.10 | 5.37b ± 0.17 |
| MTA | Mean ± SD | 11.48a ± 0.11 | 11.56a ± 0.15 | 11.82a ± 0.21 | 11.12a ± 0.14 |
| Dycal | Mean ± SD | 11.40a ± 0.06 | 11.49a ± 0.08 | 11.53a ± 0.11 | 11.33a ± 0.15 |
| F value | 1726.43 | 7987.04 | 4679.69 | 3833.95 | |
| P value | <0.0001* | <0.0001* | <0.0001* | <0.0001* | |
Significance level P < .05, *Significant. Tukey's post hoc test: means sharing the same superscript letter (within the same pH period) are not significantly different.
4. Discussion
Several studies have been carried out to discover new pulp capping materials for preservation of vitality and function of the dental pulp. The present study investigated the possible application of Hesperidin as a new natural pulp capping material. To achieve this target, the study was divided into two parts, the first part dealt with the physical and chemical properties of Hesperidin and the second part dealt with its biological properties as a direct pulp capping material in dogs, teeth. Despite its slight acidic nature, longer initial setting time and lower radiopacity, Hesperidin is a promising pulp capping material and further studies are recommended to improve its physicochemical properties.
In the present study, Hesperidin was compared with both MTA and Calcium hydroxide, commonly used pulp capping materials. Calcium hydroxide was most common direct pulp capping material in 1940s and mid 1950s because it was thought to be biologically acceptable, has antibacterial property, enhances dentin bridge formation and keeps pulp vitality (Dominguez et al., 2003, Asgary et al., 2006). However, there are several disadvantages of Calcium hydroxide such as its degeneration over time, poor sealing ability and tunnel defects and necrosis in dentinal bridges formed under Calcium hydroxide leading to bacterial micro-leakage and impeding dentinal bridge formation (El-Ashry et al., 2013).
The success rate of cases directly capped with Calcium hydroxide in ten-year follow-up studies was 30–85% (Jitaru et al., 2016). Therefore, MTA was applied in 1995 and introduced to the market in 1998 to overcome the aforementioned disadvantages of Calcium hydroxide (Torabinejad et al., 1995). MTA is a non-resorbable biocompatible filling material composed of fine hydrophilic particles that set in the presence of moisture. Due to MTA has excellent sealing ability, high ability of induction hard tissue formation and antibacterial effects, its biological properties are excellent. Direct pulp capping using MTA had a higher success rate (80.5%) than Calcium hydroxide (59%) at 24–123 months period (Mente et al., 2014). However, MTA also has few disadvantages such as a delayed setting time; poor handling characteristics and high expensiveness which are limiting its use (Asgary et al., 2007). Therefore the studies on new pulp capping materials – of lesser cost- are continued.
Propolis (bee glue) has a beneficial role in many dental diseases because it has a regenerative effect on dental pulp through prevention of microbial infection, inflammation and pulp necrosis. Moreover, it stimulates the stem cells to form an efficient tubular dentin (Kumar, 2014). These advantages of Propolis are mainly related to its content of flavonoids and derivatives of hydroxycinnamic acids (Sabir et al., 2005). Therefore, the hypothesis of the present study was that Hesperidin as a flavonoid could be a beneficial natural material for pulp capping.
Regarding the setting time evaluation, the highest mean value of initial setting time was recorded for Hesperidin group followed by MTA and Dycal. For the final setting time, the highest mean value was for the MTA followed by Hesperidin and Dycal. The longer setting time of MTA after mixing could be attributed to its content of gypsum and tricalcium aluminoferrate which are important factors of setting time (Roberts et al., 2008). Although Calcium sulphate had been removed in MTA-Angelus to decrease the setting time (Salehimehr et al., 2014), it still requires a longer setting time than Hesperidin and Dycal. These results are in agreement with Salehimehr et al. (2014).
Ideal capping materials should have a sufficient radiopacity for differentiation from dental tissues. Radiopacity is evaluated by an aluminium step-wedge according to ANSI/ADA specification #57 and an endodontic sealing material should present radiopacity correspondent to at least 3 mm Aluminium steps (Tagger and Katz, 2004, Tanomaru-Filho et al., 2007). Recently, digital methods have been used for evaluation of the grey-tones values measured in pixels by using specific software. X-ray digital periodontal phosphor plate enhanced with Digora software was applied for obtaining an immediate image on the screen eliminating the need of conventional radiographic film and its chemical processing thus saving time (McDonnell and Price, 1993) and decreasing the interference factors of the radiographic quality (Syriopoulos et al., 2000). Another disadvantage of the digital software are the detailed analysis of the digital image and evaluation of it by a numerical value (Nummikoski et al., 1992). The acrylic device with holders keeps the same object-to-focus distance. Transparent radiolucent plates with 5 mm wells were applied in sample preparation, according to ANSI/ADA specification # 57, thus decreasing volume of the used sample and allowing several samples to be placed in the central part of the X-ray phosphor plate. To balance out the effect of localized irregularities not seen by the naked eye, three different regions were assessed as mentioned before (Caravalho et al., 2007).
Among the tested materials, MTA-Angelus had higher radiopacity values than Dycal. This is in agreement with (McComb, 1983, Borges et al., 2012). On the contrary, Hesperidin had not sufficient radiopacity to be distinguished on a digital radiograph. This is due to the radiopacifying materials present in both MTA-Angelus (Bismuth trioxide) and Dycal (Titanium dioxide), while pure Hesperidin powder lacks any radiopacifier. Therefore, future studies dealing with addition of radiopacifier to Hesperidin are recommended.
Solubility or % of mass change was also measured according to ANSI/ADA specification # 57. Dycal showed a distinct dissolution in distilled water that was necessary for Calcium hydroxide to be released for the development of therapeutic actions as reported before (McComb, 1983). Hesperidin showed a higher solubility of the pure powder in distilled water after 28 days due to the presence of hydroxyl rings that combine with those of water. Although solubility of a material is important for the development of its therapeutic actions, it should not be high but the ideal solubility is not documented. In contrast, MTA showed increase in weight because it has a hydrophilic property resulted from capillary action of the pores present in the set cement. These pores result from the incorporation of air bubbles during mixing. Similar findings were recorded by Borges et al. (2012). On the contrary, Ceci et al. (2015) recorded a slight solubility of MTA. This difference in solubility might be due to the differences in the method and time used of evaluation where this loss in weight might be due to solubility of the radiopacifier and ions leached in the solution; however in this study, solubility was evaluated at 7 days interval and by weighing the original mass in the mold.
High pH and released Calcium and Phosphorous ions are essential in the hard tissue healing. Furthermore; high pH assists in antibacterial action which is also a critical factor for pulp healing and new hard tissue formation (McHugh et al., 2004). Close similarity of the media to the oral tissue fluid may be a source of Calcium and Phosphate nucleation; hence distilled water was selected as a storage media for measurement of pH of the tested materials. The pH greater than 9 can inactivate the bacterial cell wall. Calcium hydroxide ionization results in high pH, thus MTA had a higher pH than Dycal due to its contents of Tricalcium silicate (C3S) and Dicalcium silicate (C2S) where the hydration reaction of C3S results in more Calcium hydroxide than C2S. Our results indicated that both MTA and Dycal produced an alkaline environment of pH less than 12 but greater than 9. These results are nearly similar to the previous findings of Salehimehr et al. (2014) who reported a higher pH of MTA above 12. This might be due to the difference in the method used of evaluation where Salehimehr et al. (2014) measured the pH directly from the set cement mass using a temperature compensated electrode rather than immersing the samples in distilled water as in our study. Measurement of pH at long time represents the ability of the cements to increase pH thus increasing hydroxyapatite formation with good hard tissue deposition. Therefore, pH was measured in this study during 4 periods up to 28 days.
In all periods of evaluation, pH remained high indicating the continual reactions of MTA and Dycal and continual setting of MTA concurring with results obtained by Ghazivini et al. (2009). On the contrary, Hesperidin had acidic pH values during all evaluation periods. This might be due to the fact that Hesperidin was obtained from citrus fruits that are slightly acidic in nature. Therefore, further studies to improve the properties of Hesperidin are recommended.
5. Conclusions
Despite its slight acidic nature, longer initial setting time and lower radiopacity, Hesperidin, as a natural product, is a promising pulp capping material. Further studies on Hesperidin powder are recommended to improve its physicochemical properties and to evaluate its biological properties.
Conflict of interest
All authors have no competing interests to declare.
Ethical statement
All international and institutional ethical measures were followed in this study. All author approved the writing and submission of this article and none of them has any conflict of interests. This article did not published elsewhere before in any form.
Acknowledgments
Acknowledgements
None.
Funding
This research did not receive any grant from funding agencies in the commercial, public, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ebtesam O. Abo El-Mal, Email: ebtesam.oaboelmal@must.edu.eg.
Ashraf M. Abu-Seida, Email: ashrafseida@cu.edu.eg.
References
- Abu-Seida A.M. Effect of propolis on experimental cutaneous wound healing in dogs. Vet. Med. Int. 2015:4. doi: 10.1155/2015/672643. Article ID 672643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorinte M.D., Holland R., Reis A., Bortoluzzi M.C., Murata S.S., Dezan E. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp capping agents in human teeth. J. Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Almas K., Mahmoud A. A comparative study of propolis and saline application on human dentin: an SEM study. J. Dent. Res. 2000;79:1281–1285. [PubMed] [Google Scholar]
- American National Standard Institute/ American Dental Association ANSI/ADA . ANSI/ADA; Chicago: 2000. Specification # 57: Endodontic Sealing Material. [Google Scholar]
- Asgary S., Eghbal M.J., Parirokh M., Ghanavati F., Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg. Oral Med. Oral Path. Oral Radiol. Endod. 2008;106:609–614. doi: 10.1016/j.tripleo.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Asgary S., Karami F.A., Taheri S. Evaluation of antimicrobial effect of MTA, calcium hydroxide and CEM cement. Iran. Endod. J. 2007;2:105–109. [PMC free article] [PubMed] [Google Scholar]
- Asgary S., Parirokh M., Eghbal M.J., Ghoddusi J., Eskandarizadeh A. SEM evaluation of neodentinal bridging after direct pulp protection with mineral trioxide aggregate. Aust. Endod. J. 2006;32:26–30. doi: 10.1111/j.1747-4477.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Barbosa Silva E., Peruchi C.M., Ramalho L.T. Histological study of the propolis effects in rats connective tissue. J. Dent. Res. 2000;79:1084–1088. [Google Scholar]
- Bavana S., Fazlyab M., Heshmat H., Mojtahedzadeh F., Motahhary P. Histological evaluation of single and double-visit pulp capping with different materials on sound human premolars: a randomized controlled clinical trial. Iran. Endod. J. 2015;102:82–88. [PMC free article] [PubMed] [Google Scholar]
- Bollu I.P., Velagula D., Bolla N., Kumar K.R., Hari A., Thumu J. Histological evaluation of mineral trioxide aggregate and enamel-matrix derivative combination in direct pulp capping: an in vivo study. J. Conserv. Dent. 2016;19:536–540. doi: 10.4103/0972-0707.194031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A.H., Pedro F.L., Miranda C.E., Semanoff-Segundo A., Pécora J.D., CruzFilho A.M. Comparative study of the physico-chemical properties of MTA-Based and Portland cements. Acta Odont. Latin. 2010;23:175–181. [PubMed] [Google Scholar]
- Borges A.H., Pedro F.L., Semanoff A., Miranda C.E., Pécora J.D., Cruz Filho A.M. Radio-opacity evaluation of Portland and MTA- based cements by digital radiographic system. J. Appl. Oral. Sci. 2011;19:228–232. doi: 10.1590/S1678-77572011000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges R.P., Sousa-Neto M.D., Versiana M.A., Rached-Júnior F.A., De-Deus J., Miranda C.E.S. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int. Endod. J. 2012;45:419–428. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- Burdock G.A. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem. Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- Camilleri J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int. Endod. J. 2010;43:231–240. doi: 10.1111/j.1365-2591.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- Caravalho J.R., Correr A.B., Sinhoreti M.A., Consani S., Sousa M.D. Radio-opacity of root filling materials using digital radiography. Int. Endod. J. 2007;40:514–520. doi: 10.1111/j.1365-2591.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- Carvalho J.R., Guimarães L.F., Sobrino L.C., Pécora J.D., Sousa-Neto M.D. Evaluation of solubility, disintegration and dimensional alteration of a Glass Ionomer root canal sealer. Braz. Dent. J. 2003;14:114–118. doi: 10.1590/s0103-64402003000200008. [DOI] [PubMed] [Google Scholar]
- Ceci M., Beltrami R., Chiesa M., Colombo M., Poggio C. Biological and chemical-physical properties of root-end filling materials: a comparative study. J. Conserv. Dent. 2015;18:94–99. doi: 10.4103/0972-0707.153058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammaschke T. The history of direct pulp capping. J. Hist. Dent. 2008;56:9–23. [PubMed] [Google Scholar]
- Dominguez M.S., Witherspoon D.E., Gutmann J.L., Opperman L.A. Histological and scanning electron microscopy assessment of various vital pulp therapy materials. J. Endod. 2003;29:324–333. doi: 10.1097/00004770-200305000-00003. [DOI] [PubMed] [Google Scholar]
- El-Ashry S., Abu-Seida A.M., Al-Boghdady H., El-Batouty K., Abdel-Fattah M. The effect of different formulations of calcium hydroxide on healing of intentionally induced periapical lesions in dogs. Pak. Vet. J. 2013;33:48–52. [Google Scholar]
- El Ashry S.H., Abu-Seida A.M., Emara R.A. The influence of addition of osteogenic supplements to mineral trioxide aggregate on the gene expression level of odontoblastic markers following pulp capping in dogs. Vet. Arhiv. 2016;86:685–697. [Google Scholar]
- Ghazivini S.A., Tabrizi M.A., Kobafard F., Baghban A.K., Asgary S. Ion release and pH of a new endodontic cement, MTA and Portland cement. Iran. Endod. J. 2009;4:74–78. [PMC free article] [PubMed] [Google Scholar]
- Jitaru S., Hodisan I., Timis L., Lucian A., Bud M. The use of bioceramics in endodontics – literature review. Clujul. Med. 2016;89:470–473. doi: 10.15386/cjmed-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataia E.M.A. Endodontics, Faculty of Oral and Dental Medicine – Cairo University; 2011. Physical Properties and Adaptation of Synthesized Calcium Phosphate Cements: Comparative Study with New and Standard Root Canal Cements. Ph.D. Thesis. [Google Scholar]
- Kumar V.L.S. Propolis in dentistry and oral cancer management. North Am. J. Med. Sci. 2014;6:11–20. doi: 10.4103/1947-2714.134369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M.G., Whaites E.J., Ambrosano G.M., Haiter Nelo F. What happens if you delay scanning Digora phosphor storage plates (PSPs) for up to 4 hrs? Dentomax. Radiol. 2006;35:143–146. doi: 10.1259/dmfr/29710762. [DOI] [PubMed] [Google Scholar]
- McComb D. Comparison of physical properties of commercial calcium hydroxide lining cements. J Am. Dent. Assoc. 1983;107:610–613. doi: 10.14219/jada.archive.1983.0311. [DOI] [PubMed] [Google Scholar]
- McDonnell D., Price C. An evaluation of the Sens-A-Ray digital imaging system. Dentomax. Radiol. 1993;22:121–126. doi: 10.1259/dmfr.22.3.8299829. [DOI] [PubMed] [Google Scholar]
- McHugh C.P., Zhang P., Michalek S., Eleazer P.D. pH required to kill enterococcus faecalis in vitro. J Endod. 2004;30:218–219. doi: 10.1097/00004770-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Mente J., Hufnagel S., Leo M., Michel A., Gehig H., Panagidis D. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014;40:1746–1751. doi: 10.1016/j.joen.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Negm A., Hassanien E., Abu-Seida A.M., Nagy M. Physical evaluation of a new pulp capping material developed from Portland cement. J. Clin. Exper. Dent. 2016;8:e278–283. doi: 10.4317/jced.52748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm A.M., Hassanien E.E., Abu-Seida A.M., Nagy M.M. Biological evaluation of a new pulp capping material developed from Portland cement. Exp. Toxicol. Pathol. 2017;69:115–122. doi: 10.1016/j.etp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Nummikoski P.V., Martinez T.S., Matteson S.R., McDavid W.D., Dove S.B. Digital subtraction radiography in artificial recurrent caries detection. Dentomax. Radiol. 1992;29:677–684. doi: 10.1259/dmfr.21.2.1397457. [DOI] [PubMed] [Google Scholar]
- Ricci Vivan R., Ordinola-Zapata R., Zeferino M.A., Bramante C.M., Bernardineli N., Garcia R.B. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg. Oral Med. Oral Path. Oral Radiol. Endod. 2010;110:250–256. doi: 10.1016/j.tripleo.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Roberts H.W., Toth J.M., Berzin W., Charlton D.G. Mineral trioxide aggregate use in endodontic treatment: a review of literature. Dent. Mat. 2008;24:149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Sabir A., Tabbu C.R., Agustiono P., Sosroseno W. Histological analysis of rat dental pulp tissue capped with propolis. J. Oral Sci. 2005;47:135–138. doi: 10.2334/josnusd.47.135. [DOI] [PubMed] [Google Scholar]
- Saleh R., Nagi S.M., Khallaf M.E., Abd El-Alim S.H., Zaazou M.H., Abu-Seida A.M. In-Vivo assessment of dentin bridge formation after using MTA and experimental propolis paste as direct pulp capping material. Res. J. Pharmaceut. Biol. Chem. Sci. 2016;7:1244–1250. [Google Scholar]
- Salehimehr G., Nabahar S., Hosseini-Zijoud S.M., Yari S. Comparison of physical and chemical properties of Angelus MTA and new endodontic restorative material. J. App. Pharmaceut. Sci. 2014;4:105–109. [Google Scholar]
- Syriopoulos K., Sanderink G.C., Velders X.L., van der Stelt P.F. Radiographic detection of approximal caries: a comparison of dental films and digital imaging systems. Dentomax. Rdiol. 2000;29:312–318. doi: 10.1038/sj/dmfr/4600553. [DOI] [PubMed] [Google Scholar]
- Tagger M., Katz A. A standard for radiopacity of root-end filling materials is urgently need. Int. Endod. J. 2004;37:260–264. doi: 10.1111/j.0143-2885.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- Tanomaru-Filho M., Jorge E.G., Guerreiro Tanumaru J.M., Gonçalves M. Radiopacity evaluation of new root canal filling materials by digitalization of images. J. Endod. 2007;33:249–251. doi: 10.1016/j.joen.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Torabinejad M., Hong C.U., McDonald F., Pitt Ford T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995;21:349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]