Abstract
Objective
Cotrimoxazole prevents opportunistic infections including falciparum malaria in HIV-infected individuals but there are concerns of cross-resistance to other antifolate drugs such as sulphadoxine-pyrimethamine (SP). In this study, we investigated the prevalence of antifolate-resistance mutations in Plasmodium falciparum that are associated with SP resistance in HIV-infected individuals on antiretroviral treatment randomized to discontinue (STOP-CTX), or continue (CTX) cotrimoxazole in Western Kenya.
Design
Samples were obtained from an unblinded, non-inferiority randomized controlled trial where participants were recruited on a rolling basis for the first six months of the study, then followed-up for 12 months with samples collected at enrollment, quarterly, and during sick visits.
Method
Plasmodium DNA was extracted from blood specimens. Initial screening to determine the presence of Plasmodium spp. was performed by quantitative reverse transcriptase real-time PCR, followed by genotyping for the presence of SP-resistance associated mutations by Sanger sequencing.
Results
The prevalence of mutant haplotypes associated with SP-resistant parasites in pfdhfr (51I/59R/108N) and pfdhps (437G/540E) genes were significantly higher (P = 0.0006 and P = 0.027, respectively) in STOP-CTX compared to CTX arm. The prevalence of quintuple haplotype (51I/59R/108N/437G/540E) was 51.8% in STOP-CTX vs. 6.3% (P = 0.0007) in CTX arm. There was a steady increase in mutant haplotypes in both genes in STOP-CTX arm overtime through the study period, reaching statistical significance (P < 0.0001).
Conclusion
The frequencies of mutations in pfdhfr and pfdhps genes were higher in STOP-CTX arm compared to CTX arm, suggesting cotrimoxazole effectively controls and selects against SP-resistant parasites.
Trial registration
ClinicalTrials.gov NCT01425073
Author summary
Cotrimoxazole, an antifolate, is a fixed-dose trimethoprim-sulfamethoxazole used to prevent opportunistic infections including malaria in HIV-infected individuals. There are concerns that widespread use of cotrimoxazole for prophylaxis may result in selection of P. falciparum parasites with cross-resistance to other antifolate drugs such as sulphadoxine-pyrimethamine (SP), which is used as intermittent preventive treatment of malaria in pregnancy (IPTp) and in infants (IPTi) in Africa. This sub-study used samples from a clinical trial in which HIV-infected individuals on antiretroviral treatment were randomized to discontinue (STOP-CTX) or continue (CTX) cotrimoxazole prophylaxis for 12 months. The sub-study was designed to assess whether taking cotrimoxazole increased the risk of selecting for parasites with SP-resistant mutations in HIV-infected individuals. Samples were genotyped by sequencing to assess the prevalence of mutations associated with SP-resistance. We found there was no risk of selecting for parasites with SP-resistance mutations while on cotrimoxazole. In fact, the opposite was true; cotrimoxazole controlled parasites carrying SP-resistance mutations as evident by the gradual increase in the prevalence of parasites with mutant alleles in the STOP-CTX arm and not in the CTX-arm. We concluded that cotrimoxazole remains effective in controlling malaria infection despite of the high prevalence of SP-resistant parasites, and its use does not select for SP mutations.
Introduction
Despite the changes in the epidemiology and improvement in the control of HIV-infection and malaria, both remain important infectious diseases and global health priorities. Through immunosuppression, HIV infection affects the acquisition and persistence of immune response to malaria, causing substantial increase in the malaria prevalence and malaria-related morbidity and mortality [1]. Antiretroviral therapy (ART) and cotrimoxazole, a fixed-dose trimethoprim-sulfamethoxazole (an antifolate) widely used to prevent opportunistic infections in HIV-infected individuals, including falciparum malaria significantly reduces mortality and morbidity in HIV-infected individual. In countries with adequate health infrastructure, the World Health Organization (WHO) recommends daily cotrimoxazole prophylaxis for HIV-infected individuals with low CD4 cell count levels (< 350 cells/mm3), whereas in countries with high prevalence of HIV and limited health infrastructure, cotrimoxazole prophylaxis is recommended for all HIV-infected individuals regardless of the CD4 cell count levels [2]. However, there are concerns that widespread use of cotrimoxazole prophylaxis may result in selection of Plasmodium falciparum parasites with cross-resistance to closely related antifolate antimalarials such as sulphadoxine-pyrimethamine (SP) [1]. Although artemisinin based combination therapy is the mainstay for treatment of uncomplicated malaria in most malaria endemic countries, SP is widely used as intermittent preventive treatment of malaria in pregnancy (IPTp) and in infants (IPTi) in sub-Saharan Africa (SSA) [3–5].
Some of the important mutant alleles that confer P. falciparum parasite resistance to SP are in P. falciparum dihydrofolate reductase (pfdhfr) gene at codons 51, 59 and 108, and P. falciparum dihydropteroate synthase (pfdhps) gene at codons 437 and 540. Recent studies have shown high prevalence of these mutant alleles and haplotypes in Western Kenya, including mutant allele at codon 164 in the pfdhfr gene which is associated with high-grade resistance to SP [6–9]. Despite the high prevalence of SP-resistant mutations in parasite population in Western Kenya, there is limited clinical evidence associating these mutations with compromised efficacy of cotrimoxazole prophylaxis and IPTp/i [10]. Recent studies have indicated there is fixation of some of the key SP-resistant mutations in the parasite population despite discontinuation of SP as the first-line treatment for more than a decade [6,7,9].
Based on the malaria risk map and the eco-epidemiology of malaria, Kenya is stratified into four malaria ecological regions [11], with the lake region in Western Kenya having the highest, stable transmission of malaria with an estimated prevalence of 27% based on microscopy [12,13] and 37% based on PCR [14]. The HIV-1 prevalence in Kenya is estimated at 5.9%, with Homa Bay County, one of the eight counties in the lake region of Western Kenya having the highest prevalence estimated at 26% [15]. With such high HIV and malaria prevalence, the selective pressure due to cotrimoxazole prophylaxis and the risk of developing antifolate resistance in P. falciparum warrants further investigation. From February 2012 to August 2013, we conducted a randomized controlled trial (RCT) among adults on ART with evidence of immune recovery to determine whether discontinuation of cotrimoxazole was non-inferior to continuation of cotrimoxazole prophylaxis in decreasing morbidity in Homa Bay County [16]. Study participants were recruited in the first six months of the study on a rolling basis and randomized to discontinue or continue cotrimoxazole, then followed-up for 12 months with the primary endpoint a composite of malaria, pneumonia, diarrhea and non-trauma mortality events. Samples were collected at enrollment, quarterly, and at sick visits which the participants were encouraged to visit the clinic to see study providers for any illness. Malaria was defined as a fever, measured or self-reported, and either a positive rapid diagnostic test or thick smear showing the presence of parasites. Patients who were diagnosed with malaria were treated following the Kenyan Ministry of Health national guidelines. In the RCT study, we found increased incidence of malaria (13.0 in discontinuation of cotrimoxazole arm [STOP-CTX] vs. 0.4 in continuation of cotrimoxazole arm [CTX] per 100 person-years) [16]. In a follow-up study which we characterized the risk associated with stopping CTX therapy by determining parasite density, multiplicity of infecting parasites, and rates of new cases of parasitemia by PCR, malaria incidence was 42.0 in STOP-CTX vs. 9.9 in CTX per 100 person-years [17]. In this study, we determined and compared the prevalence of P. falciparum parasites with mutations associated with SP-resistance in HIV-infected individuals in the two study population arms, STOP-CTX and CTX.
Materials and methods
Ethical considerations
The study protocol was approved by the ethical review committee of the Kenya Medical Research Institute and the institutional review boards of the University of Washington and the Walter Reed Army Institute of Research. All participants gave informed consent. Consent was written if literate and fingerprint if illiterate, with the signature of an independent witness. For the clinical study, Vestergaard Frandsen donated insecticide-treated bednets and water filters. Alere donated cartridges for the Pima machines used for CD4 count measurements.
Study site and sample collection
Samples used in this study were collected between February 2012 and September 2013 in an unblinded, two-arm randomized non-inferiority clinical trial (clinical trials registration NCT01425073). The details of the study and sample collection have been described elsewhere [16]. Briefly, a total of 500 participants ≥18 years old, HIV seropositive, and taking first-line ART and cotrimoxazole with evidence of immune recovery (ART for ≥18 months and CD4 count > 350 cells/mm3) were enrolled in the study, and randomized to discontinue with cotrimoxazole prophylaxis (STOP-CTX; 250 individuals) or continue (CTX; 250 individuals). The study took place in Homa Bay County, Western Kenya, a malaria holoendemic lake endemic region where transmission is intense through-out the year with high annual entomological inoculation rates [12]. Generally, a bimodal pattern of rainfall is observed with the long rainy season from March to June and the short rainy season from November to December, but the periods vary each year with malaria prevalence peaking 1–2 months after the rainy season. Annual rainfall ranges from 700 mm to 1,200 mm with mean temperature of 25°C, with relatively high humidity [14]. This study lasted 18 months, enrollment taking place during the first six months (01 February 2012 to 27 August 2012) with participants randomized to STOP-CTX or CTX. This strategy ensured that participants were enrolled and followed over different malaria seasons.
Blood samples were collected from the participants during the scheduled visits at months 0, 3, 6, 9 and 12 (M0, M3, M6, M9 and M12 respectively), and sick visits. Participants were encouraged to come to the clinic to see study providers for any illness as a sick visit. At each sick visit, a standardized questionnaire was provided to assess participants’ symptoms and a clinician performed a physical exam. Additionally, available and clinically relevant basic diagnostic tests were performed (e.g. malaria smear, chest radiograph, stool ova and parasite exam) to assist with diagnosis as per routine clinic practice. Additionally, pertinent microbiological samples were taken in order to better evaluate cause of illness. If further evaluation was necessary, patients were referred for hospitalization at the nearest facility. Clinical and laboratory records from any hospitalization during participation were reviewed. Participants with malaria were treated following Kenyan national guidelines.
Genotypic analysis
In the RCT, dried blood spots samples were collected from the participants at enrollment, every 3 months and during sick visits (whether or not they were diagnosed with malaria) for the duration of the study which was 12 months. DNA was extracted from the FTA filter papers using the QIAamp DNA mini kit (Qiagen, Valencia, CA). The detection of P. falciparum positive samples was performed by quantitative reverse transcriptase real-time PCR (qRT-PCR) as previously described [17,18]. The presence of mutations in dihydrofolate reductase (pfdhfr: codons 16, 50, 51, 59, 108, and 164) and dihydropteroate synthase (pfdhps: codons 436, 437, 540, 581, and 613) genes which are associated with antifolate resistance in P. falciparum samples were assessed by Sanger sequencing as previously described [6]. Briefly, after successfully amplifying the target regions, the PCR amplicons were purified using Exosap-it (Affymetrix, Santa Clara, CA) per the manufacturer’s protocol. Sequencing of the target regions was done on the ABI 3500 xL genetic analyzer using version 3.1 of the big dye terminator method (Applied Biosystems, Foster City, CA). Bioinformatics analysis of the sequence data was done on the CLC Main Work Bench v6 software (Qiagen, Redwood City, California, USA). All sequences were compared against the pfdhfr (Accession Number; XM_001351443) or pfdhps (Accession Number; XM_001349382) 3D7 reference sequence published at the NCBI database.
Statistical analysis
The different Plasmodium species and genotype polymorphisms within pfdhfr and pfdhps genes of P. falciparum were analyzed as proportions showing frequency rates. The differences in frequencies were determined by the Chi-square test. All statistical analyses were performed at the 5% significance level. Graph pad Prism 4.0 software (Graph pad Software, San Diego, California, USA) was used for the analyses.
Results
Prevalence of falciparum and non-falciparum malaria
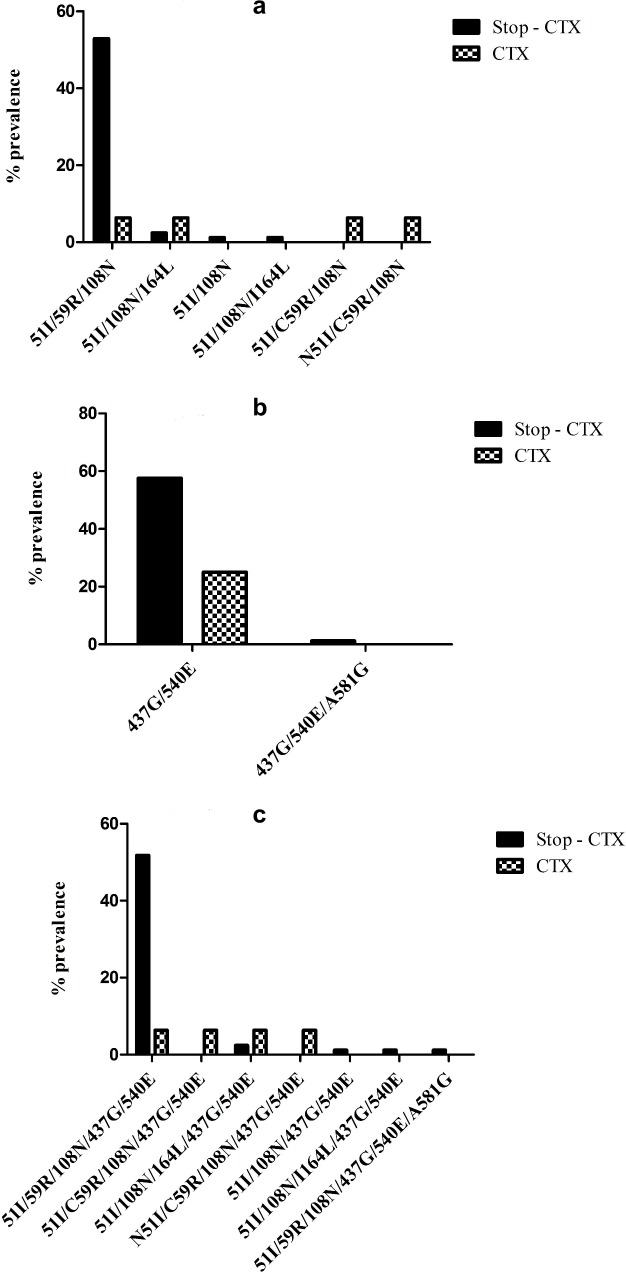
A total of 2,625 samples were initially screened for presence of malaria parasites by qRT-PCR [17,18]. Of these, 183 samples were positive for Plasmodium genus, 131 (71.6%) were P. falciparum, and 101 (55.2%) were successfully genotyped in pfdhfr (at codons 16, 50, 51, 59, 108, and 164) and pfdhps (at codons 436, 437, 540, 581, and 613) genes; 30 samples had non-falciparum parasites. The difference in the number of samples that were successfully genotyped by sequencing (n = 101) and those that were Plasmodium spp. positive as detected by qRT-PCR (n = 183) is due to the difference in sensitivities of the amplification assays used. For the detection of Plasmodium genus used for the initial screening for the presence of the parasite, Ottichilo et al. (2016) used previously described qRT-PCR assay (probe based assay) which amplifies total nucleic acids (RNA and DNA) of the 18S rRNA genes, increasing sensitivity several fold [17,18]. For genotypic analysis, nested PCRs that target DNA only [6] were used in the sequencing reactions. Table 1 shows the prevalence of the mutant alleles in pfdhfr and pfdhps genes. The prevalence was based on the total number of samples that were P. falciparum positive in each of the arms (total n = 101; STOP-CTX = 85 and CTX = 16). Single nucleotide polymorphisms (SNPs) were designated as wild, mutant or mixed alleles [6,7]. None of the parasite samples contained mutations in pfdhfr codons 16 and 50 or in pfdhps codons 436 and 613. Three samples, two in STOP-CTX arm and one in the CTX arm had the pfdhfr 164L mutation which confers high grade resistance to antifolate [19,20]. The two samples in the STOP-CTX arm were collected in M3 and M12 whereas the one sample in the CTX arm was collected at enrollment. Mutations in the STOP-CTX arm were present at a higher frequency compared to CTX arm in pfdhfr codons 51 (65.9% [n = 56/85] vs. 25.0% [n = 4/16]; P = 0.0043), 59 (60% [n = 51/85] vs. 12.5% [n = 2/16]; P = 0.0007) and 108 (65.9% [n = 56/85] vs. 31.3% [n = 5/16]; P = 0.0126). In the pfdhps gene, mutations were only present in codons 437 and 540 with frequencies higher in the STOP-CTX arm compared to the CTX arm (68.2% [n = 58/85] vs. 37.5% [n = 6/16] and 70.6% [n = 60/85] vs. 37.5% [n = 6/16] respectively). Fig 1 shows the prevalence of the different mutation haplotypes in pfdhfr and/or pfdhps genes. The prevalence of the triple mutant haplotype (pfdhfr 51I/59R/108N) was 52.9% (n = 45/85) in the STOP-CTX arm versus 6.3% (n = 1/16; P = 0.0006) in the CTX arm, and the pfdhps double mutant (437G/540E) was 57.6% (n = 49/85) in the STOP-CTX arm versus 25.0% (n = 4/16; P = 0.027) in the CTX arm. The prevalence of quintuple haplotype (51I/59R/108N/437G/540E) was 51.8% (n = 44/85) in the STOP-CTX arm versus 6.3% (n = 1/16; P = 0.0007) in the CTX arm.
Table 1. Prevalence of mutations in the pfdhfr and pfdhps genes.
| N51I | C59R | S108N | I164L | ||||||
| DHFR | STOP-CTX (N = 85) | CTX (N = 16) |
STOP-CTX (N = 85) | CTX (N = 16) |
STOP-CTX (N = 85) | CTX (N = 16) |
STOP-CTX (N = 85) | CTX (N = 16) |
|
| MUTANT | 65.9% (56) |
25% (4) |
60% (51) |
12.5% (2) |
65.9% (56) |
31.3% (5) |
2.4% (2) |
6.25% (1) |
|
| MIXED | 0 | 6.25% (1) |
0 | 12.5% (2) |
0 | 0 | 1.2% (1) |
0 | |
| A437G | K540E | A581G | A613S/T | ||||||
| DHPS | STOP-CTX (N = 85) | CTX (N = 16) | STOP-CTX (N = 85) | CTX (N = 16) | STOP-CTX (N = 85) | CTX (N = 16) |
STOP-CTX (N = 85) | CTX (N = 16) |
|
| MUTANT | 68.2% (58) |
37.5% (6) |
70.6% (60) |
37.5% (6) |
0 | 0 | 0 | 0 | |
| MIXED | 0 | 0 | 0 | 0 | 1.2% (1) |
0 | 0 | 0 | |
Note: Mutation distribution per codon was calculated as a percentage of the total number of P. falciparum positive samples in each arm as indicated (N). Mutations were tallied autonomously per codon as an overall prevalence.
Fig 1. Prevalence of haplotype mutations in pfdhfr and pfdhps genes in subjects continuing with cotrimoxazole prophylaxis therapy (CTX) and those who stopped CTX therapy (STOP CTX).
The prevalence was based on the total number of P. falciparum positive samples in each arm. The statistical difference in parasite prevalence between the two arms was determined. A) Haplotype mutations (51I, 59R, 108N and 164L) in pfdhfr gene; B) haplotype mutations (437G, 540E and 581G) in pfdhps gene and; C) haplotype mutations present in both genes. There were statistical significant differences between the STOP CTX and CTX arms in the pfdhfr gene haplotype 51I/59R/ 108N (P = 0.0006), in pfdhps gene haplotype 437G/540E (P = 0.027) and in both genes haplotype 51I/59R/108N/437G/540E (P = 0.0007).
Temporal trends of haplotype mutations in the pfdhfr and pfdhps genes
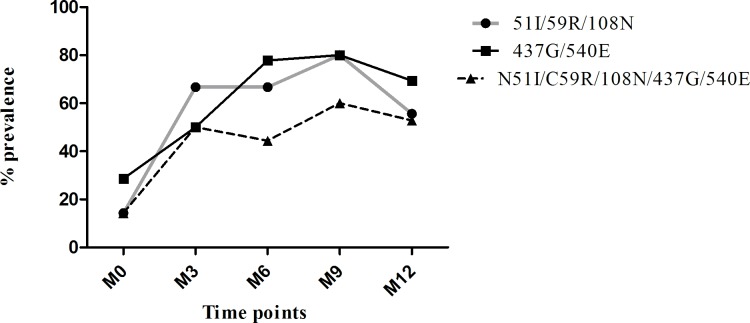
To determine change in the prevalence of the mutations over the study period for each study arm, we analyzed samples carrying mutations at each time-point, starting M0 –M12, and the sick visits. However, the sample sizes were small in the CTX arm. In the STOP-CTX arm, the percent prevalence of point mutations in both genes increased over time with marked increase occurring in M9 followed by a slight drop in M12 (Table 2). The difference in prevalence of mutations was less pronounced in the sick visits between the two arms. Fig 2 shows the prevalence of mutations at the different time-points of the triple mutant haplotype (51I/59R/108N) in the pfdhfr gene, the double mutant haplotype (437G/540E) in pfdhps gene and the quintuple haplotype (51I/59R/108N/437G/540E) in the STOP-CTX arm. All the changes (increases) over time reached statistical significance (P = 0.0069, 95% CI = 19.99–67.45; P = 0.008, 95% CI = 32.90–61.02; and P = 0.0044, 95% CI 18.35–52.09, respectively).
Table 2. Temporal change of mutations in the pfdhfr and pfdhps genes.
| M0 | M3 | M6 | M9 | M12 | SICK | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STOP (N = 7) | CTX (N = 4) | STOP (N = 6) | CTX (N = 1) | STOP (N = 9) | CTX (N = 1) | STOP (N = 15) | CTX (N = 3) | STOP (N = 36) | CTX (N = 3 | STOP (N = 12) | CTX (N = 4) | ||
|
DHFR |
N51I | 28.6% (2) | 25.0% (1) | 66.7% (4) | 0.0 | 66.7% (6) | 0.0 | 93.3% (14) | 33.3% (1) | 63.9% (23) | 0.0 | 58.3% (7) | 50.0% (2) |
| C59R | 14.3% (1) | 0.0 | 66.7% (4) | 0.0 | 66.7% (6) | 0.0 | 80.0% (12) | 33.3% (1) | 58.3% (21) | 0.0 | 58.3% (7) | 25.0% (1) | |
| S108N | 0.0 | 25% (1) | 66.7% (4) | 0.0 | 66.7% (6) | 0.0 | 93.3% (14) | 0.0 | 63.9% (23) | 0.0 | 58.3% (7) | 0.0 | |
| I164L | 0.0 | 25% (1) | 0.0 | 0.0 | 0.0 | 0.0 | 6.7% (1) | 0.0 | 2.8% (1) | 0.0 | 0.0 | 0.0 | |
|
DHPS |
A437G | 28.6% (2) | 50.0% (2) | 50.0% (3) | 0.0 | 77.8% (7) | 0.0 | 80.0% (12) | 33.3% (1) | 66.7% (24) | 0.0 | 83.3% (10) | 75.0% (3) |
| K540E | 28.6% (2) | 50.0% (2) | 50.0% (3) | 0.0 | 88.9% (8) | 0.0 | 80.0% (12) | 33.3% (1) | 69.4% (25) | 0.0 | 83.3% (10) | 75.0% (3) | |
| A581G | 0.0 | 0.0 | 0.0 | 0.0 | 77.8% (7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| DHFR HAPLOTYPES | 51I/59R/108N | 14.3% (1) | 0.0 | 66.7% (4) | 0.0 | 66.7% (6) | 0.0 | 80.0% (12) | 33.3% (1) | 55.6% (20) | 0.0 | 58.3% (7) | 25.0% (1) |
| DHPS HAPLOTYPES | 437G/540E | 28.6% (2) | 50.0% (2) | 50.0% (3) | 0.0 | 77.8% (7) | 0.0 | 80.0% (12) | 33.3% (1) | 69.4% (25) | 0.0 | 83.3% (10) | 75.0% (3) |
| DHFR/DHPS HAPLOTYPES | 51I/59R/108N/437G/540E | 14.3% (1) | 0.0 | 50.0% (3) | 0.0 | 44.4% (4) | 0.0 | 60.0% (9) | 0.0 | 52.8% (19) | 0.0 | 58.3% (7) | 25.0% (1) |
Data shows the prevalence of mutations at each time point, calculated as a percentage of the total number of P. falciparum positive samples at each time-point for each arm as indicated (N).
Fig 2. Temporal trends of haplotype mutations in the pfdhfr and pfdhps genes in the STOP-CTX arm.
The prevalence is based on the total number of samples collected at each time point. Temporal trends shown for haplotype mutations in pfdhfr gene (51I/59R/108N), pfdhps gene (437G/540E) and both genes combined (51I/59R/108N/437G/540E).
Discussion
The clinical benefits of using cotrimoxazole prophylaxis in controlling opportunistic infections including falciparum malaria in HIV-infected individuals are clear [21]. The widespread use of cotrimoxazole prophylaxis for individuals with HIV infection in malaria endemic countries has been a concern because of the risk of developing cross resistance to other antifolate drugs [22–24]. However, concern for cross resistance was based on in vitro data [3,25,26], and has not been substantiated by field clinical data (reviewed by [27]). Despite the high prevalence of SP-resistant mutations, cotrimoxazole continues to provide important benefits in reducing morbidity and mortality particularly in the setting of HIV infection [28–30], and does not lead to increased resistance [22,31–33]. Current field data clearly supports the continued use of cotrimoxazole as a prophylactic drug in HIV-infected populations [21,27]. Additional studies to investigate the use of cotrimoxazole as an alternative to SP in IPTp/i, malaria treatment and prophylaxis, and as a combined anti-malarial therapy with artemisinin are warranted.
In this study, we demonstrated cotrimoxazole prophylaxis did not results in increased risk of developing resistance, corroborating previous studies [22,28–32,34]. Interestingly, we found the prevalence of SP-resistant alleles increased steadily over the study period in the STOP-CTX arm for the first 9 months. Further, although the sample size was small, the prevalence of SP-resistant alleles in the CTX arm did not change over the study period. Taken together, cotrimoxazole prophylaxis lowered the overall incidence of SP-resistant parasites, consistent with previous studies [22,33]. Key SP-resistant mutations have become fixed in parasite populations despite discontinuation of SP as the first-line treatment for more than a decade [6,7,9], indicating these mutations might be providing benefit to the parasite population without fitness cost. It is possible that cotrimoxazole selects against parasites carrying SP-resistant alleles in the population, and removal of cotrimoxazole pressure allows the SP-resistant parasite population, which seems to be more fit than SP-susceptible population to dominate. As studies are underway to investigate expanded role of cotrimoxazole in developing countries [21], the use of cotrimoxazole in the prevention of malaria HIV-infected and HIV uninfected populations, especially as a tool for malaria elimination and as travelers’ prophylactic drug, needs to be further investigated.
Mutation in pfdhfr 164L codon is associated with high grade resistance to SP [35–38]. While some studies have shown evidence that cotrimoxazole prophylaxis might be associated with presence of pfdhfr 164L codon [31,39], other studies do not support this observation [40]. In Kenya, only low prevalence of pfdhfr 164L has been reported [6,7,36,41]. In our study, three parasite isolates had pfdhfr 164L, one in CTX arm collected at M0 and two in STOP-CTX collected in M9 and M12, indicating the presence of this mutation is unlikely due to cotrimoxazole drug pressure. Although cotrimoxazole has been speculated to contribute to antifolate selective pressure [40], additional studies are required to support this observation.
This study had several limitations. First, the study was unblinded clinical trial without a placebo or concurrent control group of HIV-uninfected individuals [16]. Second, the number of infections in the CTX arm was small, limiting statistical analysis or might have resulted in bias. This made it especially difficult in interpreting the data when evaluating prevalence at each time-point. Also, the use of highly sensitive qRT-PCR assay for the initial screening followed by multiple sequencing reactions, which uses multiple PCR steps that are not as sensitive was a limitation.
In conclusion, this study demonstrated cotrimoxazole does not select for SP-resistance P. falciparum parasites but instead, lowers the overall incidence of SP-resistant parasites. Since cotrimoxazole is available in malaria-HIV co-endemic regions with infrastructure in place and is effective against SP-resistant parasites, additional studies are required to validate these findings and to further explore the possibility of expanding the use of this drug for IPTp/i, prophylaxis for non-HIV population and travelers.
Supporting information
Consort figure from Polyak et al., (Ref: 16) showing study retention. A total of 490 participants (98%) were retained to the end of scheduled follow-up. Participants randomized to the CTX continuation arm self-reported that they took CTX every day in the past week at 90.5% of follow-up visits.
(PDF)
Acknowledgments
The authors would like to acknowledge the participants and the Homa Bay District Hospital staff as well as the research staff, Kenya Medical Research Institute and United States Medical Research Directorate—Africa and the US Military HIV Research Program for their support.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70-25.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Supported by the Merle A. Sande Award in International Infectious Diseases, Infectious Disease Society of America (IDSA)(http://www.idsociety.org/Index.aspx) and the Armed Forces Health Surveillance Branch (AFHSB), Global Emerging Infections Surveillance (GEIS) Section, ProMIS ID P0240_13_KY, 2013 (http://www.afhsc.mil/Home/Divisions/GEIS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Flateau C, Le Loup G, Pialoux G (2011) Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis 11: 541–556. 10.1016/S1473-3099(11)70031-7 [DOI] [PubMed] [Google Scholar]
- 2.WHO (2016) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva. [PubMed]
- 3.Peters PJ, Thigpen MC, Parise ME, Newman RD (2007) Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf 30: 481–501. 10.2165/00002018-200730060-00003 [DOI] [PubMed] [Google Scholar]
- 4.Garner P, Gulmezoglu AM (2006) Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev: CD000169 10.1002/14651858.CD000169.pub2 [DOI] [PubMed] [Google Scholar]
- 5.WHO (2009) Report of the Technical Consultations on Intermittent Preventive Treatment in Infants (IPTI), Technical Expert Group on Preventive Chemotherapy. Geneva.
- 6.Juma DW, Omondi AA, Ingasia L, Opot B, Cheruiyot A, et al. (2014) Trends in drug resistance codons in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in Kenyan parasites from 2008 to 2012. Malar J 13: 250 10.1186/1475-2875-13-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iriemenam NC, Shah M, Gatei W, van Eijk AM, Ayisi J, et al. (2012) Temporal trends of sulphadoxine-pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malar J 11: 134 10.1186/1475-2875-11-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, et al. (2009) High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One 4: e4569 10.1371/journal.pone.0004569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spalding MD, Eyase FL, Akala HM, Bedno SA, Prigge ST, et al. (2010) Increased prevalence of the pfdhfr/phdhps quintuple mutant and rapid emergence of pfdhps resistance mutations at codons 581 and 613 in Kisumu, Kenya. Malar J 9: 338 10.1186/1475-2875-9-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ter Kuile FO, van Eijk AM, Filler SJ (2007) Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 297: 2603–2616. 10.1001/jama.297.23.2603 [DOI] [PubMed] [Google Scholar]
- 11.Noor AM, Gething PW, Alegana VA, Patil AP, Hay SI, et al. (2009) The risks of malaria infection in Kenya in 2009. BMC Infect Dis 9: 180 10.1186/1471-2334-9-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Malaria Control Programme (NMCP) KNBoSK, and ICF International (2016) Kenya Malaria Indicator Survey 2015. Nairobi, Kenya and Rockville, Maryland, USA.
- 13.Ministry of Health, Kenya. (2015) Kenya Malaria Indicator Survey (2015 KMIS).
- 14.Idris ZM, Chan CW, Kongere J, Gitaka J, Logedi J, et al. (2016) High and Heterogeneous Prevalence of Asymptomatic and Sub-microscopic Malaria Infections on Islands in Lake Victoria, Kenya. Sci Rep 6: 36958 10.1038/srep36958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National AIDS Control Council, MOH. (2016) Kenya HIV County Profiles. National AIDS Control Council National AIDs and STI Control Program.
- 16.Polyak CS, Yuhas K, Singa B, Khaemba M, Walson J, et al. (2016) Cotrimoxazole Prophylaxis Discontinuation among Antiretroviral-Treated HIV-1-Infected Adults in Kenya: A Randomized Non-inferiority Trial. PLoS Med 13: e1001934 10.1371/journal.pmed.1001934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottichilo RK, Polyak CS, Guyah B, Singa B, Nyataya J, et al. (2017) Malaria Parasitemia and Parasite Density in Antiretroviral-Treated HIV-Infected Adults Following Discontinuation of Cotrimoxazole Prophylaxis. J Infect Dis 215: 88–94. 10.1093/infdis/jiw495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, et al. (2011) Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol 49: 2946–2953. 10.1128/JCM.00276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasconcelos KF, Plowe CV, Fontes CJ, Kyle D, Wirth DF, et al. (2000) Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase of isolates from the Amazon region of Brazil. Mem Inst Oswaldo Cruz 95: 721–728. [DOI] [PubMed] [Google Scholar]
- 20.Basco LK, Eldin de Pecoulas P, Wilson CM, Le Bras J, Mazabraud A (1995) Point mutations in the dihydrofolate reductase-thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol Biochem Parasitol 69: 135–138. [DOI] [PubMed] [Google Scholar]
- 21.Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ (2015) The expanding role of co-trimoxazole in developing countries. Lancet Infect Dis 15: 327–339. 10.1016/S1473-3099(14)71011-4 [DOI] [PubMed] [Google Scholar]
- 22.Hamel MJ, Greene C, Chiller T, Ouma P, Polyak C, et al. (2008) Does cotrimoxazole prophylaxis for the prevention of HIV-associated opportunistic infections select for resistant pathogens in Kenyan adults? Am J Trop Med Hyg 79: 320–330. [PubMed] [Google Scholar]
- 23.Sicuri E, Fernandes S, Macete E, Gonzalez R, Mombo-Ngoma G, et al. (2015) Economic evaluation of an alternative drug to sulfadoxine-pyrimethamine as intermittent preventive treatment of malaria in pregnancy. PLoS One 10: e0125072 10.1371/journal.pone.0125072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis NL, Barnett EJ, Miller WC, Dow A, Chasela CS, et al. (2015) Impact of daily cotrimoxazole on clinical malaria and asymptomatic parasitemias in HIV-exposed, uninfected infants. Clin Infect Dis 61: 368–374. 10.1093/cid/civ309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer JK, Milhous WK, Cortese JF, Kublin JG, Plowe CV (2001) Plasmodium falciparum cross-resistance between trimethoprim and pyrimethamine. Lancet 358: 1066–1067. 10.1016/S0140-6736(01)06201-8 [DOI] [PubMed] [Google Scholar]
- 26.Triglia T, Menting JG, Wilson C, Cowman AF (1997) Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A 94: 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manyando C, Njunju EM, D'Alessandro U, Van Geertruyden JP (2013) Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One 8: e56916 10.1371/journal.pone.0056916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SE, Brandeau ML, Bendavid E (2017) Cost-effectiveness of malaria preventive treatment for HIV-infected pregnant women in sub-Saharan Africa. Malar J 16: 403 10.1186/s12936-017-2047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suthar AB, Granich R, Mermin J, Van Rie A (2012) Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Organ 90: 128C–138C. 10.2471/BLT.11.093260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimwade K, Gilks C (2001) Cotrimoxazole prophylaxis in adults infected with HIV in low-income countries. Curr Opin Infect Dis 14: 507–512. [DOI] [PubMed] [Google Scholar]
- 31.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, et al. (2007) Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS 21: 2059–2066. 10.1097/QAD.0b013e3282ef6da1 [DOI] [PubMed] [Google Scholar]
- 32.Thera MA, Sehdev PS, Coulibaly D, Traore K, Garba MN, et al. (2005) Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis 192: 1823–1829. 10.1086/498249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malamba S, Mermin J, Reingold A, Lule J, Downing R, et al. (2006) Effect of cotrimoxazole prophylaxis taken by HIV-infected persons on the selection of sulfadoxine-pyritmethamine resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg 75: 375–380. [PubMed] [Google Scholar]
- 34.Malamba SS, Mermin J, Reingold A, Lule JR, Downing R, et al. (2006) Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HIV)-infected persons on the selection of sulfadoxine-pyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg 75: 375–380. [PubMed] [Google Scholar]
- 35.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, et al. (2001) Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol 17: 582–588. [DOI] [PubMed] [Google Scholar]
- 36.McCollum AM, Poe AC, Hamel M, Huber C, Zhou Z, et al. (2006) Antifolate resistance in Plasmodium falciparum: multiple origins and identification of novel dhfr alleles. J Infect Dis 194: 189–197. 10.1086/504687 [DOI] [PubMed] [Google Scholar]
- 37.Andriantsoanirina V, Durand R, Pradines B, Baret E, Bouchier C, et al. (2011) In vitro susceptibility to pyrimethamine of DHFR I164L single mutant Plasmodium falciparum. Malar J 10: 283 10.1186/1475-2875-10-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun V, Rempis E, Schnack A, Decker S, Rubaihayo J, et al. (2015) Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J 14: 372 10.1186/s12936-015-0909-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasasira A, Kamya M, Ochong E, Vora N, Achan J, et al. (2010) Effect of trimethoprim-sulfamethoxazole on the risk of malaria in HIV-infected Ugandan children living in an area of widespread antifolate resistance. Malaria Journal 9: 117 10.1186/1475-2875-9-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbogo GW, Nankoberanyi S, Tukwasibwe S, Baliraine FN, Nsobya SL, et al. (2014) Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 91: 54–61. 10.4269/ajtmh.13-0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oesterholt MJ, Alifrangis M, Sutherland CJ, Omar SA, Sawa P, et al. (2009) Submicroscopic gametocytes and the transmission of antifolate-resistant Plasmodium falciparum in Western Kenya. PLoS One 4: e4364 10.1371/journal.pone.0004364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort figure from Polyak et al., (Ref: 16) showing study retention. A total of 490 participants (98%) were retained to the end of scheduled follow-up. Participants randomized to the CTX continuation arm self-reported that they took CTX every day in the past week at 90.5% of follow-up visits.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.