Abstract
Introduction
Periodontitis is a chronic disease in humans induced by several pathogens including Porphyromonas gingivalis (P. gingivalis). Although mouse models of human periodontitis have been developed for study using an oral gavage of P. gingivalis, existing models take over a month to develop in order to ensure adequate periodontal destruction. The aim of the present study is to determine if using an injection of a cocktail of type II collagen antibodies along with an oral gavage of P. gingivalis in mice induces adequate periodontal destruction in a shorter time so as to potentially serve as a more useful mouse model of periodontitis.
Methods
Twenty-eight DBA1/BO male mice were placed in four groups: Group A (antibody injection plus gavage), Group B (gavage only), Group C (antibody injection only), and Group D (neither antibody injection nor gavage, control). Between six and eight weeks old, all mice underwent antibiotic administration, and at eight weeks old, were given antibody injection (Groups A and C) and oral P. gingivalis gavage (Groups A and B). Fifteen days after gavage Groups A and B received gavage, all mice were euthanized. Histomorphometric, morphometric, and cell counting analyses were conducted using analysis of variance (ANOVA) and Kruskal Wallis analysis followed by pairwise t-tests using Bonferroni correction.
Results
For histomorphometric analysis, mean distance from the cemento-enamel junction to the alveolar bone crest (CEJ-ABC) and the mean epithelial downgrowth (ED) in μm was statistically significantly highest for Group A (CEJ-ABC 1.49.81 vs. Group B 101.46, Group C 78.74, and Group D 66.23, p < 0.0083; ED 66.76 vs. Group B 25.92, Group C 9.21, and Group D 9.10, p < 0.0083). Morphometric analysis also showed that Group A had a significantly higher mean CEJ-ABC in μm compared to all other groups (265.50 vs. Group B 195.77, Group C 150.33, and Group D 133.93, p < 0.0083). A similar pattern was seen in cell counting, in which Group A had a significantly lower mean count of fibroblasts per 45 × 50 μm field (8.02 vs. Group B 9.56, Group C 12.09, and Group D 11.02, p < 0.0083), and a significantly higher mean count polymorphonuclear leukocytes per 45 × 50 μm (4.59 vs. Group B 1.74, Group C 0.83, and Group D 0.68, p < 0.0083).
Conclusion
The results of this study provide proof-of-concept for a mouse model that can be quickly developed for human periodontitis using a type II collagen antibody cocktail injection coupled with oral gavage of P. gingivalis in DBA1/BO male mice. Future studies should verify the results of this proof-of-concept, compare this new model to existing models, and evaluate the extent of this model’s usefulness.
Keywords: SstT protein, Porphyromonas gingivalis, Periodontitis, Models, Animal, Image cytometry
1. Introduction
Periodontitis is a chronic disease in humans (Tonetti et al., 2017), with more recent studies demonstrating that over 47% of the United States (US) adult population suffers from the disease (Eke et al., 2012). Porphyromonas gingivalis (P. gingivalis) is a well-documented pathogen in chronic periodontitis; hence, animal models of human periodontitis induced through P. gingivalis have been developed that have demonstrated similarities to a human disease at a level adequate for research (Genco et al., 1998, Graves et al., 2012). In general, these models allow the study of the level of virulence of a variety of oral pathogens (including P. gingivalis), the natural history of periodontal tissue destruction, and the efficacy of periodontal treatment (Baker et al., 1994, Genco et al., 1998, Kesavalu et al., 2007, Schou et al., 1993).
These animal models, however, have differences. Polak et al. (2009) used a mouse model of periodontitis by infecting Balb/C mice with either P. gingivalis, Fusobacterium nucleatum (F. nucleatum), or both. By contrast, Kesavalu et al. (2007) infected Sprague-Dawley rats with either monoinfections of P. gingivalis, Treponema denticola (T. denticola), or Tannerella forsythia (T. forsythia), or with all three pathogens. Assuma et al. (1998) used a primate model and induced periodontitis by tying P. gingivalis-soaked silk ligatures around the posterior mandibular teeth, copying a procedure that had been done in the past. A different group led by Jain (2003) also used silk ligatures and P. gingivalis to induce periodontitis in their model, but they used age- and sex-matched New Zealand White rabbits (Jain et al., 2003).
One of the challenges in developing animal models for human periodontitis is that the time between infection and bone loss secondary to periodontal disease can be over a month. In the model by Polak and colleagues mentioned earlier, to create the oral infection, the Balb/C mice were super-infected three times at two-day intervals, and maxillary jaws were not harvested until 42 days afterward (Polak et al., 2009). For the rat model described earlier, rats were infected on five consecutive days in four or six alternate weeks (depending upon the group), and stable oral infections were to be maintained over a twelve- to sixteen-week period (Kesavalu et al., 2007). For the primate model, animals were infected three times per week for six weeks following suture placement (Assuma et al., 1998). In the rabbit model, the animals took seven weeks to infect, and were euthanized after fourteen weeks (Jain et al., 2003).
The long period required for infectivity reduces the usefulness of these animal models of human periodontitis. Developing a mouse model for human periodontitis that reduces this time period would be preferable. An innovative idea for this was originally inspired by a landmark paper by Levon M. Khachagian (2006) that described the development of a mouse model of rheumatoid arthritis through using a process to create collagen anti-body induced arthritis (CAIA) in the mouse. His model highlights the fact that the type II collagen protein structure has epitopes in region CB11 that could be targeted by monoclonal antibodies to cause destruction of the protein (Khachigian, 2006). These antibodies are D1, F10, A2, and D8, and Khachagian successfully induced arthritis in mice by creating an antibody cocktail (2–4 mg total) and administered it intraperitoneally or intravenously to the mouse on day 0 of his experiment (Khachigian, 2006).
As part of improving upon Khachagian’s rheumatoid arthritis mouse model, one research group experimented with using Khachagian’s approach, but also adding in P. gingivalis. As this study is part of a line of research behind a business innovation, detailed data cannot be shared. However, the group observed that not only was this mouse model of rheumatoid arthritis superior to the original one proposed by Khachagian, the jaws of the mice revealed significant periodontal destruction.
That finding led this researcher to propose experimenting using Khachagian’s approach plus P. gingivalis to develop a mouse model of human periodontitis that does not require the extended length of time as existing animal models. The aim of the present study is to determine if using an injection of a cocktail of type II collagen antibodies D1, F10, A2, and D8 along with an oral gavage of P. gingivalis in mice induces adequate periodontal destruction in a shorter time so as to potentially serve as a more useful mouse model of periodontitis. The hypothesis is that injecting type II collagen antibodies into mice who undergo an oral gavage of P. gingivalis will induce significant evidence of experimental periodontitis in less than one month.
2. Materials and methods
In this study, 28 mice were divided into four groups representing four different conditions (see Table 1).
Table 1.
Study design.
| Group | Number of mice | Type II collagen antibody injection | Daily P. gingivalis gavage |
|---|---|---|---|
| A | 7 | 7 mg Day 1, and 4 mg Day 5 | 1 through 15 days |
| B | 7 | None | 1 through 15 days |
| C | 7 | 7 mg Day 1, and 4 mg Day 5 | None |
| D | 7 | None | None |
| Total | 28 | ||
Group A represents the proposed novel method of developing a mouse model for human periodontitis. Group A mice underwent both injections of type II collagen antibody and daily P. gingivalis gavage. Group B mice only underwent the daily P. gingivalis gavage; this group represents the traditional method of inducing periodontitis in mice (Polak et al., 2009). Group C mice only underwent the type II collagen antibody injections, and was included to provide comparison with Group A to see the independent effect of the injections. Group D mice underwent neither procedure and serve as a control group.
Mice were assigned to these procedures at 8 weeks old. Prior to this, between 6 weeks and 8 weeks old, all mice underwent pre-treatment with antibiotics consistent with prior protocols (Kesavalu et al., 2007). To assess the success of inducing periodontitis, after mice were euthanized, histomorphometry, morphometry, and cell counting were conducted on jaws from each group and compared.
2.1. Mouse conditions
Pathogen-free DBA1/BO male mice (Taconic Farm, Rensselaer, NY) were used in this study (Huck et al., 2018). Pathogen-free female mice with ovariectomy were not used to be consistent with prior protocols (Huck et al., 2018). Mice were fed sterile food and distilled water ad libitum. Mice were euthanized by CO2 inhalation at scheduled time points 15 days after gavage inoculation. Tissue from euthanized mice was harvested and compared using histomorphometry, morphometry, and inflammatory cell counting. Prior to study conduct, this study was reviewed and approved by the Animal Care and Use Committee (IACUC) at the Boston University Medical Center (BUMC) as part of BUMC’s routine research and compliance process (protocol #13957).
2.2. Antibiotic administration
At the age of 6 weeks, mice were given sulfamethoxazole at 0.87 mg/ml and trimethoprim at 0.17 mg/ml (Hi-Tech Pharmacal Co. Inc., Amityville, NY, USA) in milli-Q water ad libitum for ten days, followed by three days without antibiotics.
2.3. Type II collagen antibody injection
Mice in Groups A and C were injected intraperitoneally with ArthritoMab (CIA-MAB-2C, MD Bioproducts) on day 1 (7 mg/mouse) and day 5 (4 mg/mouse). ArithritoMab is a cocktail of the type II collagen antibodies D1, F10, A2, and D8.
2.4. P. Gingivalis gavage
The W83 P. gingivalis strain used in this study (BAA-308; ATCC, Manassas, VA, USA). This pathogen was cultured and maintained in Schaedler anaerobe broth (Oxoid Ltd., Basingstoke, Hampshire, England), then supplemented with hemin (5 μg/ml, Sigma-Aldrich, St. Louis, MO), menadione (1 μg/ml, Sigma-Aldrich, St. Louis, MO), and sodium bicarbonate (420 μg/ml, Sigma-Aldrich, St. Louis, MO) in an anaerobic chamber with 85% N2, 10% H2, and 5% CO2 at 37 °C. Bacteria at early logarithmic-phase growth were used for inoculation procedures. An average 5 × 108 colony-forming units of W83 (BAA-308; ATCC, Manassas, VA, USA) in 100 μl phosphate-buffered saline (PBS) with 2% carboxymethylcellulose (Sigma-Aldrich, St Louis, MO, USA).
Mice in Groups A, B, and C were anesthetized with Isophrane and inoculated with an average 5 × 108 colony-forming units of P. gingivalis strain W83 (BAA-308; ATCC, Manassas, VA, USA) in 100 μl PBS with 2% carboxymethylcellulose (Sigma-Aldrich, St Louis, MO, USA). They were administered by oral gavage for 15 days (1 inoculation/day).
2.5. Tissue specimen preparation for histomorphometric analysis
After euthanizing the mice and harvesting their jaws, alveolar bone and intact surrounding tissue from each animal was dissected and fixed with 4% freshly prepared paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in PBS (pH 7.2) for 24 h at 4 °C. Following fixation, specimens were consecutively washed with 5%, 10%, and 15% glycerol (American Bioanalytical, Natick, MA) in PBS, each for 15 min at 4 °C, and decalcified in an ethylenediaminetetraacetic acid tetrasodium salt dihydrate (EDTA) (Sigma-Aldrich, St. Louis, MO) for 14 days at 4 °C.
Once per day during this period, the specimens were gently stirred and the solutions refreshed. Samples were then immersed in 30% sucrose (Sigma-Aldrich, St. Louis, MO) in PBS until embedding. The tissue block was embedded with a HISTO PREP® compound (Fisher Scientific, Hanover Park, IL) for cryostat sectioning. Serial mesiodistal sections (5 μm) parallel to the long axis of the teeth were made. The correctly oriented sections were stained with hematoxylin and eosin (H & E) for histomorphometric analysis.
H & E sections were fixed by immersing the slides in acetone (ACROS Organics, Morris Plains, NJ) for 5 min, then air dried for 5 min at room temperature. Slides were rinsed three times every 5 min in PBS to remove the tissue-freezing matrix, and were then immersed in hematoxylin solution (Fisher Scientific, Hanover Park, IL) for 3 min. Slides were then washed with tap water for 3 min, fast-dipped in acid ethanol (Sigma-Aldrich, St. Louis, MO) 8 to 12 times, washed with tap water two times for 1 min, and then cleansed with deionized water for 2 min. Slides were then placed in an eosin solution (ACROS Organics, Morris Plains, NJ) for 30 s and dehydrated for 3 min in each solution of alcohol series: 95%, 95%, 95%, 100%, 100%, and 100%. Finally, slides were bathed in Xylene (Fisher Scientific, Hanover Park, IL) three times for 5 min and cover slipped with Permount (Fisher Scientific, Hanover Park, IL).
2.6. Histomorphometric analysis
One section was taken from each animal. This section captured the interdental areas between the first and second molars of the right maxillary quadrants. H & E-stained sections from images captured at 100× magnification using an image analysis system (Image-Pro Plus Version 5.0, Media Cybernetics, Silver Spring, MD) were evaluated. Measurements included an extension of the apical migration of epithelium (epithelial downgrowth, or ED) and a measurement of bone resorption. ED was defined by measuring the distance from the cemento-enamel junction (CEJ) to the apical extent of the junctional epithelium in μm. Bone resorption was measured as the distance between the CEJ and the alveolar bone crest (ABC) in μm, and this measurement will be called CEJ-ABC. Sections from different specimens were measured in a random sequence, and one measurement was taken per mouse. Data were recorded in an Excel spreadsheet and analyzed in R (R Core Team, 2015).
2.7. Preparation for morphometric analysis
After euthanasia, mice skulls were mechanically defleshed after 15 min of treatment in boiling water, washed with PBS, and then exposed overnight in 3% hydrogen peroxide. They then were washed with PBS three times for five minutes, immersed in bleach for one minute, washed again with PBS for three times for five minutes, and then dried in an incubator at 37 °C for 1 h. The skulls were then stained at room temperature with 1% methylene blue (Sigma-Aldrich, St. Louis, MO) for one minute, after which, they were dried in an incubator for 30 min at 37 °C.
2.8. Morphometric analysis
As another measurement of bone resorption, CEJ-ABC was measured in μm as described previously (Klausen et al., 1989). Measurements were made under a dissecting microscope fitted with a video image marker measurement system (Image-Pro Plus version 6.0) standardized to give measurements in μm. Three sites (mesio-buccal, mid-buccal, and disto-buccal) on the three molar teeth on each mouse were measured for CEJ-ABC.
2.9. Counting of cells
A six fields sized 45 × 50 μm from each interdental area (from CEJ to the root apex of the second molar) were analyzed for cell counting. Analysis was performed only for sections where the root canal systems of the adjacent teeth were visible and properly oriented. In each area of interest, the total number of cells (fibroblasts, mononuclear leukocytes [ML], and polymorphonuclear neutrophils [PMN]) were counted manually from images captured at 400× magnification on H & E-stained sections. The fibroblasts, ML, and PMN were identified by their characteristic morphologies. The data were then reported as the number of each type of cell per field.
2.10. Statistical analysis
To quantify whether or not using an injection of a type II collagen antibody cocktail coupled with an oral gavage of P. gingivalis in mice induced adequate periodontal destruction in a shorter time in this study, evidence of periodontal destruction was quantified in three ways: Through histomorphometric analysis, through morphometric analysis, and through the analysis of the presence of inflammatory cells. With respect to histomorphometric analysis, mean differences in CEJ-ABC and ED were compared between all four groups using an analysis of variance (ANOVA) for each, where the independent variable was group membership and the dependent variable was either CEJ-ABC or ED. Results were verified by using a non-parametric Kruskal Wallis test as well, as because of the low number of experimental units in each group, it was hard to tell outcome distributions.
Alpha was set at 0.05. If the F-test on the ANOVA was found to be statistically significant, pairwise t-tests were conducted using a Bonferroni correction. Because there were six pairwise tests per ANOVA, the adjusted alpha = 0.0083. When possible, these were verified by also running non-parametric pairwise Mann–Whitney U tests, although these suffered from being non-calculable at times because of ties.
For the morphometric analysis, first, the measurement of CEJ-ABC on all sites of all teeth of each mouse were averaged to provide one measurement estimate per mouse. Next, the same ANOVA and Kruskal Wallis analysis was conducted on the outcome of average CEJ-ABC as measured from morphometric analysis and calculated above. If the F-test p-value was statistically significant, pairwise t-tests were used with the same Bonferroni correction, and verified through running pairwise Mann-Whitney U tests.
To analyze cell counts, first, the frequencies of fibroblasts, ML, and PMN for each of the six fields were mouse were averaged to provide one measurement per mouse. Next, the same approach was used for each measurement, considering it a dependent variable in an ANOVA and Kruskal Wallis with the independent variable as group membership. If the F-test p-value on any of these analyses was statistically significant, pairwise t-tests were used with the same Bonferroni correction. Mann-Whitney U tests were also run for verification.
3. Results
This section presents the results of all three analyses.
3.1. Histomorphometric and morphometric results
Table 2 presents the histomorphometric and morphometric results.
Table 2.
Histomorphometric and morphometric results.
| Histomorphometric analysis |
Morphometric analysis | ||
|---|---|---|---|
| Group | Cemento-enamel junction to alveolar bone crest (μm) mean, SD | Epithelial downgrowth (μm) mean, SD | Cemento-enamel junction to alveolar bone crest (μm) mean, SD |
| Group A | 159.81a, 39.00 | 66.76a, 19.78 | 265.50a, 36.59 |
| Group B | 101.46b, 17.02 | 25.92b, 6.80 | 195.77b, 29.29 |
| Group C | 78.74b, 17.12 | 9.21b, 2.28 | 150.33b,c, 34.89 |
| Group D | 66.23b, 30.63 | 9.10b, 2.69 | 133.93c, 35.36 |
Note: Letters indicate significance results of pairwise t-tests after Bonferroni correction. SD = standard deviation.
The ANOVA and Kruskal Wallis analyses for CEJ-ABC and ED from the histomorphometric analysis and for the CEJ-ABC from the morphometric analysis all resulted in statistical significance at a very high level (p < 0.0001 for all analyses). Therefore, pairwise t-tests were conducted, with the results labeled with letters in Table 2. In terms of the histomophometric analysis, Group A, representing the new experimental mouse model, demonstrated statistically significantly higher levels of mean CEJ-ABC and ED compared to the other three groups. With respect to the morphometric analysis, although the other groups failed to show statistically significant differences from each other, the mean CEJ-ABC in Group A was statistically significantly higher than all other groups. Fig. 1 provides a visualization of the information in Table 2.
Fig. 1.
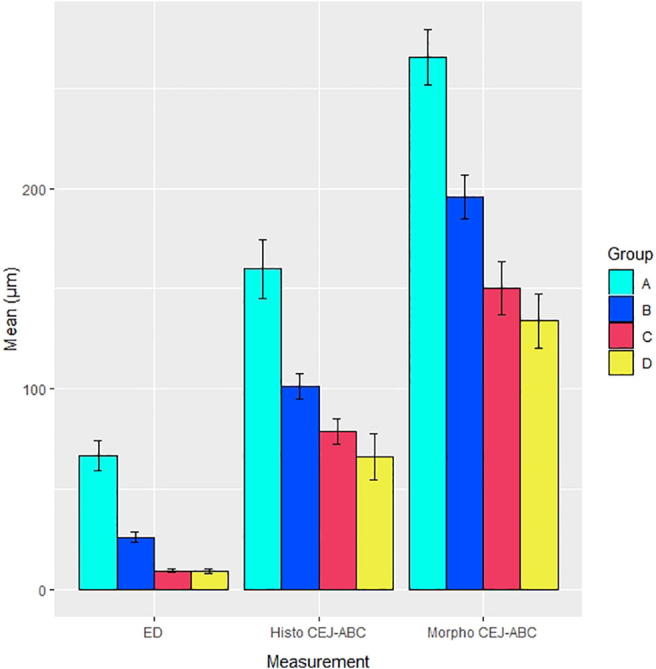
Results of histomorphometric and morphometric analysis. ED = epithelial downgrowth, Histo CEJ-ABC = measurement of cemento-enamel junction to alveolar bone crest in histomorphometric analysis, and Morpho CEJ-ABC = measurement of cemento-enamel junction to alveolar bone crest in morphometric analysis. Means are graphed with error bars ± one standard error of the mean. In all analyses, both analysis of variance and Kruskal Wallis analysis were highly statistically significant (p < 0.0001). Also, in all pairwise t-test analysis with Bonferroni correction, Group A, representing the proposed new mouse model, had means statistically significantly higher than all other groups.
3.2. Cell count results
Table 3 presents the results from inflammatory cell counting.
Table 3.
Cell count results.
| Group | Average number of fibroblasts per 45 × 50 μm mean, SD |
Average number of mononuclear leukocytes per 45 × 50 μm mean, SD |
Average number of polymorphonuclear leukocytes per 45 × 50 μm mean, SD |
|---|---|---|---|
| Group A | 8.02a, 1.41 | 2.12a, 0.70 | 4.59a, 1.63 |
| Group B | 9.56b, 1.71 | 1.19ab, 0.80 | 1.74b, 0.86 |
| Group C | 12.09c, 0.88 | 0.39b, 0.19 | 0.83b, 0.47 |
| Group D | 11.02c, 0.58 | 0.43b, 0.19 | 0.68b, 0.26 |
Note: Letters indicate significance results of pairwise t-tests after Bonferroni correction. SD = standard deviation.
The ANOVA and Kruskal Wallis analyses average number of fibroblasts, ML, and PMN from the cell counting all resulted in statistical significance at a very high level (p < 0.0001 for all analyses). Therefore, pairwise t-tests were conducted, with the results labeled with letters in Table 3. Consistent with periodontal disease, Group A demonstrated statistically significantly lower levels of mean numbers of fibroblasts compared to the other three groups, and statistically significantly higher levels of PMN compared to the other three groups. In terms of ML, the mean for Group A was the highest, but it was not statistically significantly higher than the next highest mean which was Group B, the group that received gavage of P. gingivalis but no type II collagen antibodies. However, Group A was statistically significantly higher in terms of average ML compared to Groups C and D. Fig. 2 provides a visualization of the information in Table 3.
Fig. 2.

Results of histomorphometric and morphometric analysis. ML = mononuclear leukocytes and PMN = polymorphonuclear neutrophils. Means are graphed with error bars ± one standard error of the mean. In all analyses, both analysis of variance and Kruskal Wallis analysis were highly statistically significant (p < 0.0001). In the pairwise t-test analysis of average fibroblasts per group (with Bonferroni correction), Group A, representing the proposed new mouse model, had means statistically significantly lower than all other groups. In the pairwise t-test analysis of PMN, Group A had means statistically significantly higher than all other groups. For mononuclear leukocytes, Group A was not statistically significantly higher than Group B, but was statistically significantly higher than the other two groups, C and D.
4. Discussion
The results of this study suggest that using an injection of a cocktail of type II collagen antibodies along with oral gavage of P. gingivalis in DBA1/BO male mice results in a mouse model of human periodontitis that displays adequate periodontal destruction in approximately two weeks’ time. Histomorphometric and morphometric analysis confirmed that accelerated bone resorption took place during that time, and cell counting confirmed that inflammatory cells were proliferating. Compared to mice without the gavage, the cocktail, or both, the new proposed mouse model outperformed on all metrics.
The success of this mouse model with respect to cell counts is consistent with existing literature. A previous study using a periodontitis mouse model of female NOD2−/− and male ApoE−/− mice reported increased mean inflammatory cell counts and decreased fibroblast counts after induction of periodontitis (Yuan et al., 2013, Alshammari et al., 2017). Being able to more quickly produce an animal model of human periodontitis using the methods described here will increase the opportunity for accelerated protocols in laboratory science to investigate potential clinical applications.
Some other mouse models have not used a monoinfection with P. gingivalis as was used in this model, such as the study mentioned previously that created a mixed infection in rats using P. gingivalis, T. denticola, T. forsythia and F. nucleatum (Kesavalu et al., 2007). Although there was significant bone loss induced in this model, using mixed infection vs. mono-infection will increase risk of complications due to increased period of periodontitis induction and may also result in increased mouse death. Inducing a mixed infection typically requires repeated anesthesia or increases in bacterial concentration, and this can be both cost- and time-intensive as well as result in a low yield. In this study, the combination effect of the P. gingivalis gavage model primed with type II collagen antibody injections will accelerated and aggravated bone loss while minimizing risk of these type of complications.
This study is not without limitations. First, fewer studies have been done using DBA1/BO male mice, making the results of this study hard to compare with previous studies. Next, because no competing mouse model was developed in this study, the mouse model tested should only be considered a proof-of-concept, and not a fully competitive model to existing ones. It is possible that had the experiment continued, the natural history of periodontitis in this new mouse model may have been different than with existing ones that are well-documented in the literature. Finally, another limitation was that the measurements and counts conducted in this study were all done in two dimensions. A three-dimensional analysis could improve the accuracy of the results.
5. Conclusions
In conclusion, this study demonstrated a proof-of-concept for a mouse model for human periodontitis using a type II collagen antibody cocktail injection coupled with oral gavage of P. gingivalis in DBA1/BO male mice that can be created in less than two weeks. Further studies need to be conducted to verify the results of this proof-of-concept, compare this new model to other models, and document the extent of this model’s usefulness.
Acknowledgements
I would like to thank Dr. Salomon Amar at New York Medical College for helpful discussion and assistance with experiments. I also would like to thank Monika Wahi for her effort on this manuscript. This work was supported by NIH grants RO1HL076801 and RO1DE014079 to SA.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alshammari A., Patel J., Al-Hashemi J., Cai B., Panek J., Huck O., Amar S. Kava-241 reduced periodontal destruction in a collagen antibody primed Porphyromonas gingivalis model of periodontitis. J. Clin. Periodontol. 2017;44:1123–1132. doi: 10.1111/jcpe.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assuma R., Oates T., Cochran D., Amar S., Graves D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Baker P.J., Evans R.T., Roopenian D.C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral. Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Eke P.I., Dye B.A., Wei L., Thornton-Evans G.O., Genco R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;10:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Genco C.A., Van Dyke T., Amar S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998;6:444–449. doi: 10.1016/s0966-842x(98)01363-8. [DOI] [PubMed] [Google Scholar]
- Graves D.T., Kang J., Andriankaja O., Wada K., Rossa C. Animal Models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 2012;15:117–132. doi: 10.1159/000329675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck O., You J., Han X., Cai B., Panek J., Amar S. Reduction of articular and systemic inflammation by Kava-241 in a Porphyromonas gingivalis-induced arthritis murine model. Infect. Immun. 2018;86 doi: 10.1128/IAI.00356-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Batista E.L., Serhan C., Stahl G.L., Van Dyke T.E. Role for periodontitis in the progression of lipid deposition in an animal model. Infect. Immun. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavalu L., Sathishkumar S., Bakthavatchalu V., Matthews C., Dawson D., Steffen M., Ebersole J.L. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian L.M. Collagen antibody-induced arthritis. Nat. Protoc. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- Klausen B., Evans R.T., Sfintescu C. Two complementary methods of assessing periodontal bone level in rats. Scandinavian J. Dental Res. 1989;97:494–499. doi: 10.1111/j.1600-0722.1989.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Polak D., Wilensky A., Shapira L., Halabi A., Goldstein D., Weiss E.I., Houri-Haddad Y. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J. Clin. Periodontol. 2009;36:406–410. doi: 10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A language and environment for statistical computing. [Google Scholar]
- Schou S., Holmstrup P., Kornman K.S. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J. Periodontol. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- Yuan H., Zelkha S., Burkatovskaya M., Gupte R., Leeman S.E., Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc. Natl. Acad. Sci. USA. 2013;110:E5059–E5068. doi: 10.1073/pnas.1320862110. [DOI] [PMC free article] [PubMed] [Google Scholar]


