Abstract
Objective:
Both estrogen and exercise may have cognition enhancing benefits; however, young oligo-amenorrheic athletes (OA) with estrogen deficiency have not been evaluated for cognitive deficits. Our objective was to determine whether 6 months of estrogen replacement will impact cognitive domains in OA. We hypothesized that estrogen replacement would improve verbal memory (VM) and executive control in OA.
Methods:
We performed cognitive assessments at baseline and after 6 months in 48 OA (14–25 years) randomized to estrogen (EST+) (oral 30 mcg ethinyl estradiol (n=16) or transdermal 100 mcg 17-beta-estradiol patch (n=13)), or no estrogen (EST-) (n=19) in an ongoing clinical trial. Neurocognitive testing included California Verbal Learning Test II (CVLT-II) (for VM), and Delis-Kaplan Executive Function System Color-Word Interference Test (D-KEFS-CWIT) (executive control).
Results:
On average, subjects (age: 19.9±3.1 years, BMI: 20.6±2.3 kg/m2) participated in 10.3±5.9 hours/week of weight-bearing activities of lower limbs. The EST+ group performed better for CVLT-II VM scores for immediate recall over 6 months of therapy compared to EST- (p<0.05) even after controlling for baseline scores and age. Changes in D-KEFS-CWIT over 6 months did not differ between the groups. However, the EST+ group had greater improvements in inhibition-switching completion time over 6 months compared with the EST- group after controlling for baseline scores and age (p=0.01).
Conclusion:
OA show improvements in VM and executive control following 6 months of estrogen replacement. These findings in athletes, who are in their prime of neurocognitive development, underscore the need for future studies exploring cognition in OA.
Keywords: Memory, executive control, athletes, estrogen, estrogen replacement
Introduction:
In addition to the known effects of estrogen in regulating reproductive function and bone metabolism, studies suggest that estrogen plays a role in enhancing cognitive performance1–3. Women with Turner syndrome and postmenopausal women, who are characterized by estrogen-deficiency, have cognitive deficits including altered memory and executive control, suggesting a role for estrogen in enhancing cognitive skills4–8. Similarly, use of aromatase inhibitors in breast cancer treatment to block estrogen production has been linked to impaired verbal memory (VM) and reduced information processing speed9, 10.
Consistent with these human studies, research in rodents supports a protective role of estrogen on cognition1, 11, 12 and provides mechanistic insights into this effect13. Brain-derived neurotrophic factor (BDNF), a nerve growth factor produced predominantly in the hippocampus, which represents the prime neural correlate of learning and memory14. The promoter of the BDNF gene harbors an estrogen response element15, suggesting that BDNF may mediate estrogen effects on cognitive function. Additionally, exercise increases BDNF expression, but this effect is evident in ovariectomized rats only after estrogen replacement13. Another postulated mechanism for estrogen is via catecholamine and cholinergic pathways in the prefrontal cortex (PFC)16. This region is important for mediating executive control, the processes that top-down regulate information processing to ensure goal achievement17. Despite evidence implicating estrogen deficiency in cognitive deficits in humans, studies evaluating cognitive effects of estrogen replacement have yielded equivocal results18–21.
While exercise has a positive impact on cognition22, excessive exercise can lead to low energy availability and inhibition of the hypothalamic-pituitary-gonadal axis, leading to estrogen deficiency and menstrual dysfunction (which occurs in up to 44% of female athletes23). Estrogen deficiency is a major component of the female athlete triad, which is characterized by low energy availability, menstrual dysfunction, and low bone density24, 25. Although the impact of estrogen deficiency on bone has been widely studied in oligo-amenorrheic athletes (OA), its effect on cognitive domains remains to be investigated. Further, female athletes comprise a distinctive population in that the negative impact of estrogen deficiency on cognitive performance may be counteracted by the beneficial effects of physical activity22. Estrogen replacement in OA athletes may serve as a physiological probe to investigate the cognitive domains that may be altered in these athletes who have estrogen deficiency. Of importance, the PFC matures through the second decade of life resulting in ongoing development of executive control during this period26. Thus, factors affecting cognitive development during the critical teenage/young adult years of neurobehavioral development merit careful investigation.
Our primary objective was to determine whether estrogen replacement for six months could improve cognitive measures of VM and executive control in adolescent and young adult OA (irrespective of route of administration or form). Our secondary objective was to determine whether a more physiologic form of estrogen administration, transdermal estrogen, would be more effective than oral estrogen in improving these cognitive outcomes. We hypothesized that OA randomized to estrogen replacement for six months would perform better on tasks evaluating VM and executive control compared to those randomized to no estrogen. Further, we hypothesized that transdermal estrogen will perform better than oral estrogen for these cognitive measures.
Methods and Material:
Study Subjects:
The study was approved by the Institutional Review Board of Partners HealthCare System and conducted between 2011 and 2015. Informed consent was obtained from subjects ≥18 years and parents of subjects <18, and informed assent from subjects <18. We performed baseline and six-month cognitive assessments in 48 OA aged 14–25 years randomized to estrogen or no estrogen in an ongoing clinical trial (NCT00946192). Subjects were informed during the consenting process that questionnaires would be administered over the course of the study, but we did not specify that we would examine the impact of estrogen on cognitive outcomes. All participants had a body mass index (BMI) between the 10th-90th percentiles. Athletes were required to engage in (i) ≥4 hours/week of weight-bearing exercise of their legs or (ii) ≥20 miles of running/week for ≥6 months in the preceding year. Our inclusion criteria for oligo-amenorrhea were rigorous and included (i) ≥3 missed menstrual periods in the preceding six months, and cycle length of >6 weeks for any menses that occurred during this 6-month period or (ii) menstrual onset at >15 years if subjects were pre-menarchal27. Subjects with other conditions that can cause amenorrhea such as polycystic ovary syndrome, thyroid dysfunction (unless euthyroid on medications for ≥3 months), primary ovarian insufficiency (indicated by an elevated follicle-stimulating hormone) or hyperprolactinemia, and subjects with known contraindications to estrogen were excluded. All athletes with oligo-amenorrhea were assessed for active eating disorders by the study psychologist, and if a diagnosis of active anorexia nervosa was made they were excluded from the study. Menstrual recovery, defined as at least three consecutive cycles in the past six months, was assessed for the no therapy group during the follow-up period. Assessing menstrual recovery consequent to lifestyle changes is not possible in groups receiving exogenous estrogen/progesterone, as cyclic menses would be expected on treatment.
Experimental Protocol:
Randomization of subjects to estrogen and progesterone vs. placebo cannot be adequately blinded as subjects receiving estrogen and progesterone will typically have cyclic menses on treatment. Therefore, a no-treatment arm was utilized instead of a placebo. Subjects determined eligible were randomized to one of three arms based on a predetermined algorithm: (i) 30 mcg ethinyl estradiol and 0.15 mg desogestrel taken orally daily for 21 days and a placebo pill for 7 days (EST+ORAL) (n=16), (ii) physiologic estrogen replacement as the transdermal estrogen patch (100 mcg 17-beta-estradiol patch applied twice weekly) with 200 mg of micronized progesterone (taken orally for the first 12 days of every month) (EST+PHYSIOL) (n=13), or (iii) no estrogen (EST-) (n=19). Participants were asked to maintain a medication calendar that was monitored by our research coordinators at follow-up visits. Participants were also asked to return the remaining medication and empty medication packs at subsequent study visits. Transdermal estradiol administration as 17-beta-estradiol in replacement doses is considered a physiologic form of estrogen administration because (i) in contrast to ethinyl estradiol (synthetic derivative of estrogen), 17-beta-estradiol is the endogenous form of estrogen, and (ii) unlike oral estrogen and similar to endogenous estrogen, transdermal estrogen does not undergo first pass hepatic metabolism, known to suppress Insulin-like growth factor 1 (IGF-I)28. For this study, we first report data for both estrogen-treated groups together (EST+) and then for EST+ORAL and EST+PHYSIOL groups separately. Neurocognitive tests were administered at baseline and 6 months following the respective intervention. We did not limit our assessments to times when subjects were on active estrogen only, given that animal data indicate that it is chronic (and not acute) estrogen administration that impacts cognitive parameters12.
Neurocognitive Tests:
a. Intelligence [Wechsler’s Scale of Intelligence (WASI)]29:
To test for general intellectual ability, we assessed fluid and crystallized intelligence using WASI subtests: (i) Matrix Reasoning: Subjects completed 35 gridded patterns by choosing the correct pattern out of five options, indicating fluid intelligence29. (ii) Vocabulary Test: Subjects defined a total of 42 items. Their verbal responses were scored for their match with the definition in the manual29, measuring crystallized intelligence.
b. Verbal memory [California Verbal Learning Test Edition 2 (CVLT-II)]30.
The CVLT-II assesses VM across several time delays. A 16-word list (List-A) was read to the subject five times. Subjects were instructed to recall this from memory after each read-through (immediate recall; Trials 1–5). Then, a different (interference) list of words (List-B) was read to the subjects and recalled by them (Trial-B). Following this, participants were asked to recall words of List-A (short-delay recall). Fifteen to 25 minutes later, long-delay recall took place, followed by cued recall, in which the four categories to which the words belonged were provided as cues. Finally, in a recognition test, participants indicated whether an item was on the original list or not. From these assessments, the following measures were calculated: (i) Semantic clustering, which measures the ability to learn by organizing target words into categorical groups; (ii) Serial clustering, which is the ability to recall the words in the order presented; (iii) Repetition errors are words repeatedly recalled; (iv) Intrusion error scores sum up non-list words; (v) Total recognition discriminability index is the ability to correctly identify words from List-A relative to total false positives, i.e., recalled words that were not on List-A. The test-retest reliability is high in the range of 0.80–0.84 when testing with the same standard form after one month, with minimal practice effects31.
c. Executive control [The Delis-Kaplan Executive Function System Color-Word Interference Test (D-KEFS-CWIT)]32:
Subjects were provided with a list of words representing color names (e.g., blue) or patches, displayed in varying ink colors. They completed four test conditions: (i) Color Naming: Participants named the color of 50 patches, providing an index of naming speed. (ii) Word Reading: Subjects read words representing color names (e.g., blue) displayed in black, providing a measure of reading speed. (iii) Response Inhibition: Participants named the color of the ink of words representing colors printed in dissonant ink colors (e.g., the word “blue” in red ink). Correctly naming the ink color requires inhibition of the response activation due to the meaning of the word, resulting in performance costs indicative of inhibitory ability. (iv) Inhibition Switching: Subjects named the color of the ink. However, if words were framed by a box, subjects were instructed to read the word instead. This condition adds mental-set shifting demands for successful task completion, interacting with inhibition in exerting executive control. From Conditions 1 and 2, a combined general processing speed measure was calculated (combined naming and word completion time). For Conditions 3 and 4, completion time and accuracy were calculated (inhibition and inhibition-switching completion time and errors, respectively). Eleven participants did not complete the inhibition-switching condition at the 6-month visit primarily due to time constraints, despite measures being taken to allocate sufficient time for study procedures (from subjects arriving late or technical issues with study procedures). For the D-KEFS CWLT, the test-retest reliability is high (range: 0.79–0.90) when tested with the same standard form after 9–74 days with minimal practice effects32.
For both tests, we employed the same version at baseline and six months following careful consideration of the test-retest literature on the measures. For all measures, raw scores were first calculated followed by T-scores using the respective manuals. Higher T scores corresponded with better performance.
Statistical Analysis:
JMP Pro12 was used for analysis. Student t test and ANOVA were used for comparisons of continuous variables (two- and three-group comparisons respectively) after excluding occasional outliers that otherwise skewed the distribution considerably. The Tukey Kramer test was used to account for multiple comparisons when comparing >2 groups. To determine associations of cognitive measures with possible covariates, we used Pearson correlations for all groups taken together (n=48). Furthermore, we performed multivariate analysis to control for confounding variables such as age (as cognitive scores increase with increasing age30) and baseline cognitive scores (to control for baseline functioning), when assessing the impact of estrogen administration on cognitive changes over six months. Finally, we performed exploratory analyses to determine whether there was any interaction of age with the treatment group or baseline scores. P values of ≤0.05 were considered significant.
Results:
Participant Characteristics:
EST+ and EST- groups did not differ for age, race, ethnicity, menarchal age, hours per week of physical activity, BMI, % ideal BMI, or weight change over the 6-month intervention period (Table 1). Six of the 19 OA from the EST- group resumed normal menses without any treatment during follow-up.
Table 1:
Participant Characteristics at Baseline across Treatment Groupsa
| EST-(n=19) | EST+(n=29) | P (t-test) | EST PHYSIOL (n=13) | EST ORAL (n=16) | P (ANOVA) | |
|---|---|---|---|---|---|---|
| Age at Screen (Years) | 19.0±0.6 | 20.4±0.6 | 0.12 | 21.04± 0.84 | 19.95±0.87 | 0.19 |
| Race (%) White | 90 | 86 | 0.23 | 77 | 94 | 0.05 |
| Ethnicity (%) Non-Hispanic | 100 | 97 | 0.41 | 100 | 94 | 0.36 |
| Age of Menarche (Years) | 13.4±0.4 | 13.7±0.5 | 0.66 | 14.5±0.6 | 13.1±0.6 | 0.16 |
| Total Activity (Hours/week) | 9.73±1.38 | 10.63±1.15 | 0.63 | 8.7±0.91 | 12.19±1.88 | 0.26 |
| BMI (kg/m2) | 20.7±0.5 | 20.5±0.4 | 0.83 | 20.70±0.60 | 20.35±0.64 | 0.90 |
| % Ideal BMI | 97.2±2.5 | 96.6±2.5 | 0.86 | 95.2±3.7 | 97.6±3.5 | 0.88 |
| Weight Change over 6 Months (kg) | 1.2±0.6 | 0.5±0.7 | 0.51 | −0.2±1.1 | 1.1±0.8 | 0.47 |
Table 1 gives the participant characteristics for the EST- and EST+ group and for EST PHYSIOL and EST ORAL groups. Values are presented as Mean±SEM, if not stated otherwise. Student t test and ANOVA were used to compare the means for EST+/EST- groups and EST PHYSIOL/ESTORAL/EST- groups respectively and P values are shown.
Abbreviations: BMI=Body mass index, EST-: No estrogen replacement, EST+: Estrogen replacement, EST ORAL: Oral estrogen groups and EST PHYSIOL: Transdermal estrogen
Neurocognitive Tests:
Baseline scores for WASI Matrix Reasoning and Vocabulary Test, CVLT-II, and D-KEFS-CWIT did not differ between groups (Table 2). When examining associations of these scores with the potential confounding variables of age and BMI, WASI vocabulary test scores (r=0.38, p=0.008) and CVLT-II total repetition scores (r=0.29, p=0.046) had significant correlations with age. There were no associations of WASI scores, CVLT-II, or D-KEFS-CWIT with BMI.
Table 2.
Baseline WASI, CVLT-II and D-KEFS Color Word Interference Test Scores by Treatment Groupsa
| EST-(n=19) | EST+(n=29) | P (t test) | EST PHYSIOL (n=13) | EST ORAL (n=16) | P (ANOVA) | |
|---|---|---|---|---|---|---|
| WASI | ||||||
| Matrix Reasoning | 54.47±1.70 | 56.76±1.13 | 0.25 | 57.77±1.46 | 55.94±1.68 | 0.40 |
| Vocabulary | 61.37±1.83 | 61.24±1.53 | 0.96 | 62.46± 2.03 | 60.25±2.24 | 0.77 |
| CVLT-II | ||||||
| Trial 1 | 49.47±2.62 | 47.93±2.13 | 0.65 | 45.77±2.52 | 49.69±3.27 | 0.60 |
| Trial 5 | 51.05±1.93 | 48.28±1.94 | 0.34 | 48.46±3.02 | 48.13±2.62 | 0.63 |
| Trials 1–5 Total | 55.58±1.86 | 52.69±1.97 | 0.32 | 53.92±2.95 | 51.69±2.70 | 0.51 |
| Trial B | 47.37±3.02 | 48.79±1.46 | 0.64 | 46.92±2.30 | 50.31±1.85 | 0.61 |
| Short-Delay Free Recall | 52.37±2.04 | 51.72±2.11 | 0.84 | 55.00±3.20 | 49.06±2.71 | 0.31 |
| Long-Delay Free Recall | 52.22±2.29 | 51.43±2.02 | 0.80 | 53.85±2.60 | 49.33±3.00 | 0.50 |
| Semantic Clustering | 57.63±3.23 | 53.28±2.64 | 0.30 | 58.07±4.75 | 49.38±2.58 | 0.15 |
| Serial Clustering | 49.47±3.13 | 50.52±2.35 | 0.79 | 44.62±2.56 | 55.31±3.31 | 0.08 |
| Total Repetitions | 49.47±2.91 | 49.83±2.02 | 0.92 | 48.85±3.36 | 50.63±2.54 | 0.92 |
| Total Intrusions | 49.74±0.89 | 49.14±1.03 | 0.68 | 49.23±1.59 | 49.06±1.39 | 0.92 |
| Total Recognition Discriminability | 52.63±1.64 | 51.72±1.72 | 0.72 | 51.92±2.86 | 51.56±2.18 | 0.93 |
| D-KEFS Color Word Interference Test | ||||||
| Combined Naming + Reading Time | 54.61±1.14 | 51.76±1.92 | 0.28 | 51.77±2.75 | 51.75±2.74 | 0.56 |
| Inhibition Completion Time | 56.94±1.37 | 52.86±2.10 | 0.16 | 53.69±2.86 | 52.19±3.10 | 0.35 |
| Inhibition Error Analysis | 54.22±1.83 | 52.52±1.19 | 0.42 | 49.85±1.91 | 54.69±1.30 | 0.12 |
| Inhibition/Switching Completion Time | 55.15±1.75 | 54.00±1.47 | 0.63 | 54.27±2.26 | 53.79±2.01 | 0.88 |
| Inhibition/Switching Error Analysis | 54.00±1.65 | 53.84±1.25 | 0.94 | 53.18±2.54 | 54.36±1.09 | 0.89 |
Table 2 provides the baseline scores for WASI, CVLT-II and D-KEFS Color Word Interference Test.
Values are presented as Mean±SEM. Student t test and ANOVA were used to compare the means for EST+/EST- groups and EST PHYSIOL/ESTORAL/EST- groups respectively and P values are shown. There were no significant differences between the EST+ and EST- groups and EST-, EST PHYSIOL and EST ORAL groups at baseline.
Abbreviations: CVLT-II: California Verbal Learning Test, Edition II, D-KEFS: Delis Kaplan Executive Function System, EST-: No estrogen replacement, EST+: Estrogen replacement, EST ORAL: Oral estrogen groups and EST PHYSIOL: Transdermal estrogen, WASI: Wechsler’s Scale of Intelligence.
Table 3 shows changes in WASI, CVLT-II, and D-KEFS-CWIT scores from baseline to six months in EST+ and EST- groups when these measures differed between groups. Changes in WASI Matrix Reasoning and Vocabulary Test scores did not differ across groups (Supplemental Table 1).
Table 3:
Changes in CVLT-II and D-KEFS Color Word Interference Test Scores over Six Months in Estrogen-Replaced vs. Non-replaced Groupsa,b
| EST+(n=29) | EST-(n=19) | Effect size (95% CI) | P | P* | EST PHYSIOL (n=13) | EST ORAL (n=16) | Effect size EST PHYSIOL vs. EST- (95% CI) | Effect size EST PHYSIOL vs. ORAL (95% CI) | Effect size EST Oral vs. EST-(95% CI) | P | P* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CVLT-II | ||||||||||||
| Immediate recall (Trial 1) | 13.79±2.55 | 3.95±2.77 | 9.85 (2.05,17.64) | 0.01 | 0.02 | 19.58±3.17 | 11.25±3.31 | 15.64* (4.70,26.57) | 8.33 (−2.99,19.65) | 7.30 (−2.76,17.36) | 0.005 | 0.04 |
| Immediate recall (Trial 5) | 3.62±2.13 | 5.26±2.15 | −1.64 (−8.00,4.72) | 0.61 | 0.24 | 6.25±2.55 | 3.13±3.02 | 0.99 (−8.18,10.15) | 3.13 (−6.37,12.62) | 2.14 (−6.30,10.57) | 0.70 | 0.47 |
| Immediate recall (Trial1–5) | 11.75±1.69 | 5.84±1.13 | 5.91 (1.35,10.47) | 0.01 | 0.10 | 14.00±2.38 | 10.06±2.34 | 8.16** (1.41,14.90) | 3.94 (−3.05,10.92) | 4.22 (−1.98,10.43) | 0.02 | 0.21 |
| Immediate recall (Trial B) | 3.97±1.69 | −1.05±1.93 | 5.02 (−0.24,10.27) | 0.06 | 0.01 | 5.38±2.23 | 2.81±2.50 | 6.44 (−1.31,14.19) | 2.57 (−5.47,10.61) | 3.87 (−3.44,11.17) | 0.13 | 0.04 |
| D-KEFS | ||||||||||||
| Inhibition Error Analysis | 2.19±0.79 | −0.11±1.96 | 2.30 (−1.48,6.09) | 0.23 | 0.22 | 4.92±1.65 | 0.23±0.51 | 5.03 (−0.55,10.62) | 4.69 (−1.33,10.71) | 0.34 (−5.25,5.93) | 0.08 | 0.47 |
| Inhibition/Switching Completion Time | 4.46±0.99 | 1.08±1.40 | 3.38 (−0.16,6.92) | 0.05 | 0.06 | 3.90±1.07 | 3.92±1.34 | 2.85 (−1.54,7.24) | 0.02 (−4.69,4.73) | 2.82 (−1.89,7.53) | 0.22 | 0.14 |
| Inhibition/Switching Error Analysis | 0.39±1.09 | −2.33 ±.1.79 | 2.72 (−1.32,6.77) | 0.18 | 0.07 | −0.70±1.50 | 1.23±1.56 | 1.63 (−4.27,7.54) | 1.93 (−3.87,7.73) | 3.56 (−1.95,9.08) | 0.30 | 0.19 |
Table 3 shows the changes in the CVLT-II and D-KEFS scores at 6 months by treatment groups. Student t test and ANOVA were used for initial comparisons (unadjusted p values reported).
Boldface indicates statistical significance.
Adjusted for baseline score.
p=0.003,
p=0.01 after Tukey Kramer’s adjustment for multiple comparisons.
Abbreviations: CVLT-II: California Verbal Learning Test, Edition II, D-KEFS: Delis Kaplan Executive Function System, EST-: No estrogen replacement, EST+: Estrogen replacement, EST ORAL: Oral estrogen groups and EST PHYSIOL: Transdermal estrogen
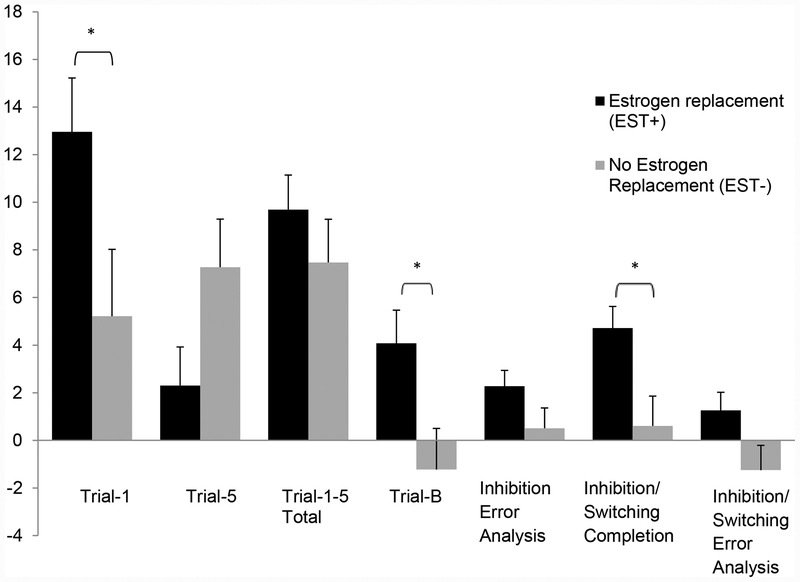
Improvement in immediate recall as assessed by CVLT-II Trial-1 and Trials-1–5, over six months of intervention, was greater in EST+ than EST- (p=0.01) (Table 3). After controlling for (i) baseline scores and (ii) baseline scores and age (Figure 1), the improvement in Trial-1 and Trial-B scores became more pronounced in the EST+ compared with EST- group (p<0.05). Serial clustering scores also improved in the EST+ group (p=0.01) compared with EST- group, but group differences did not persist after controlling for baselines scores and age. Groups did not differ for short- or long-delay recall, semantic clustering, total intrusions, repetition, and total discriminability scores.
Figure 1: Changes in cognitive scores in estrogen-replaced and no-therapy groups after adjusting for age and baseline scoresa.
a The figure shows the changes in CVLT-II and D-KEFS Color Word Interference test scores over six months adjusted for age and baseline value by treatment group. Immediate recall scores (Trial 1 and Trial B) and scores for cognitive flexibility assessed by Inhibition Switching Completion time were significantly improved in the EST+ compared with EST- groups. * p value <0.05
Abbreviations: CVLT-II: California Verbal Learning Test, Edition II, D-KEFS: Delis Kaplan Executive Function System, EST-: No estrogen replacement, EST+: Estrogen replacement
Changes in D-KEFS-CWIT measures over six months did not differ between EST+ and EST- groups (Table 3). However, after controlling for baseline measures, changes in inhibition-switching completion time (p=0.06) and inhibition-switching error analysis scores (p=0.07) trended towards significance. On inclusion of age and baseline scores in the multivariate model, EST+ had greater improvements in inhibition-switching completion time over six months than EST- (p=0.01) (Figure 1).
Finally, we compared effects of treatment in EST+ORAL versus EST+PHYSIOL versus EST- groups. Improvements in CVLT-II Trial-1 and Trial-1–5 scores were greater in EST+PHYSIOL compared to EST- groups (p<0.05) and the differences persisted after controlling for baseline scores +/− age for Trial 1 scores (p≤0.05). D-KEFS-CWIT changes over six months did not differ between groups for unadjusted scores and scores adjusted for baseline performance. After controlling for age and baseline scores, EST+PHYSIOL had greater improvements in inhibition-switching completion scores than EST- (p=0.04) with no difference between EST+ORAL and EST-.
For most variables, there was no significant interaction of age with treatment group (EST+ and EST-), randomization group (EST+PHYSIOL, EST+ORAL, EST-) or baseline scores. The only variable that improved with estrogen administration for which there was a significant interaction of age with randomization group was the change in Trial 1–5 scores for CVLT. However, after controlling for baseline scores, changes in this variable over time did not differ among the groups, and adding the interaction term to the model did not alter these results.
Discussion:
While studies have examined the effect of estrogen replacement on cognition in other estrogen-deficient states such as postmenopausal women and women with Turner’s syndrome, OA have not been examined for cognitive deficits with respect to their estrogen status. Our study is the first to assess the impact of estrogen replacement on cognitive measures in young female OA. Estrogen replacement is not the standard of care for athletes with functional hypothalamic amenorrhea33. In fact, estrogen replacement is not advised until athletes fail to respond to non-pharmacological therapy for at least one year or until they develop significant fractures with low bone density33. In our study we used estrogen as a physiological probe to investigate the cognitive domains that may be altered in athletes who have estrogen deficiency. We show that immediate free recall, a VM measure, improved significantly following estrogen replacement in OA compared to no treatment.
Female athletes in a state of relative energy deficit may develop hypothalamic amenorrhea from suppression of GnRH pulsatility subsequent to changes in hormones such as ghrelin, leptin, and cortisol34–36. The consequence of estrogen replacement on cognitive performance of OA (who have the mitigating influence of exercise activity) has never been studied. Additionally, a study in adult women with adolescent onset anorexia nervosa, another model of hypothalamic amenorrhea, has reported cognitive dysfunction, which improves with menstrual recovery or oral estrogen replacement, but not with weight recovery alone37. Similar to this study, we demonstrate that athletes with hypothalamic amenorrhea have improved immediate free recall after six months of estrogen replacement compared to no treatment.
The major setback to estrogen replacement came from the NIH-initiated Women’s Health Initiative study in postmenopausal women that showed no improvement in global cognitive decline with conjugated equine estrogen administration. Furthermore, in women >65 years, cognitive function worsened with estrogen replacement38, 39. In retrospect, the age of the participants and use of conjugated equine estrogen compared to natural estrogens such as 17-beta-estradiol used in more recent trials40 were thought to have impacted study results. With more promising results from recent studies40–42 estrogen has re-emerged as a potential positive modulator of cognitive function.
Our findings are consistent with the cognition-enhancing effects of estrogen demonstrated previously in animal and human models of estrogen deficiency. Ovariectomized rats treated with estradiol made more correct choices in the water maze test, suggesting that estrogen has a protective effect of working memory1, 11, 12. In our analysis, VM, assessed by the CVLT-II, improved for immediate-recall scores (Trial-1 and Trial-B) after six months of estrogen replacement, even after controlling for baseline scores. Girls with Turner syndrome (similar to our population for age and estrogen-deficiency) have decreased intelligence and deficits in executive function, non-verbal memory and attention; but verbal performance is preserved4, 6. It is unclear whether these deficits in Turner’s syndrome are consequent to the underlying genetic defect or estrogen-deficiency. However, estrogen replacement in these subjects results in improved motor speed of non-verbal processing2, 43. Of note, in our study, intelligence did not differ across treatment groups and cannot account for observed changes in memory and executive control following estrogen replacement.
In an older population, women aged 49–68 years who received 17-beta-estradiol treatment for a year demonstrated an improvement in VM40. Despite multiple positive studies3, 40–42 supporting the role of estrogen in augmenting VM, one study in 200 postmenopausal women randomized to testosterone, estrogen, or placebo found no effects of estrogen replacement on this cognitive measure44. However, the very short duration of estrogen replacement in this study (just 4 weeks) may have resulted in these negative findings. In fact, Luine et al showed in a rodent study that only chronic estrogen replacement (3d vs. 12d) leads to improved performance in the radial maze test with a higher number of correct choices, indicating that duration of treatment may impact the cognitive effects of estrogen12. In our study, we evaluated cognitive outcomes after six months (thus a longer duration) of estrogen replacement than in the study by Kocoska et al44. Of note, studies such as those by Asthana et al and Berent-Spillson et al have been able to demonstrate significant improvements in cognition in humans with estrogen replacement for a duration of 8 weeks and 3 months respectively3, 41. While our data are consistent with animal studies, it is still not clear why effects are evident only after chronic use. Future studies may be necessary to determine the shortest duration of estrogen therapy that will result in changes in cognitive tests.
Another finding that merits discussion is the improvement in executive control (assessed by the inhibition-switching completion time) following estrogen replacement. Although the EST+ group performed better for inhibition-switching completion times and inhibition-switching error, this was not statistically significant even after controlling for baseline performance. Nevertheless, when adjusted for age the inhibition-switching completion time was shortened in the EST+ group. The small number of subjects in the groups and missing entries for the inhibition-switching task might have contributed to this lack of significance. Although, it is well recognized that VM is the single most common domain altered with hormonal replacement20, our study opens an interesting area of executive control to be explored in future studies. Consistent with our results, ovariectomized monkeys treated with estrogen performed better on learning to shift to a new cognitive set in the Wisconsin Card Sort Task, a measure of the cognitive flexibility feature of executive control45. Furthermore, Colzato et al demonstrated that the inhibition of return effect, which reflects the ability to delay attention from returning to a previously attended location, hence a measure of cognitive flexibility, was high in the late follicular phase in women, when estradiol levels are higher compared with luteal and menstrual phases46.
Variables known to affect results of estrogen replacement in previous studies in post-menopausal women were the type of estrogen used, timing of initiation of estrogen replacement after menopause, and duration of estrogen replacement. Studies that used 17-beta-estradiol reported better results than those using conjugated equine estrogen20, 40. Further, the longer the duration of estrogen deficiency, the less was the response of neural tissues to estrogen replacement47–49. Our study provided estrogen replacement for a period of six months, a reasonable duration to demonstrate significant effects41, 50. We used oral ethinyl estradiol with progesterone, or the 17-beta-estradiol transdermal patch along with progesterone to reproduce a more physiologic form of estrogen replacement. For immediate recall and cognitive flexibility, subjects randomized to transdermal estradiol performed better than those who received no treatment after controlling for baseline scores and age. These associations merit further investigation in a larger group of subjects. Of note, a study conducted in 9 academic centers across the US on 693 postmenopausal women using transdermal estradiol (n=211) did not show significant changes for VM (using CVLT-II) and executive control (using a Stroop color interference test) when compared with oral conjugated estradiol or placebo21. Of studies that used transdermal estrogen replacement, Asthana et al. and Joffe et al. showed positive cognitive responses with estradiol, while Dunkin et al. showing neutral findings3, 18, 42.
Of note, the two modes of estrogen administration utilized different estrogen molecules. Although we attempted to minimize the difference by providing similar dose equivalents of estrogen (100 mcg of estradiol patch ~ 30 mcg of ethinyl estradiol), oral contraceptives typically have ethinyl estradiol, a synthetically derived estrogen, whereas, 17-beta estradiol used in the patch is the physiological form of estrogen. The 30 mcg of ethinyl estradiol used in our study in the oral estrogen arm is the most common form of estrogen used in oral contraceptives, and the study was designed to use an oral estrogen preparation that is commonly use in adolescents and young adults and is also commonly prescribed by practitioners to regulate menstrual cycles in athletes51. However, there could be differences stemming from the variation in the type of estrogen molecule as well as the route of administration. Further, for this study, we used a monophasic estrogen preparation (that does not mimic estrogen changes across a menstrual cycle), and future studies are necessary to determine whether the use of a biphasic or triphasic estrogen preparation that more closely mimics cyclic changes in estrogen levels across a menstrual cycle results in a different outcome.
Our sample size was based on other studies that have used neuro-cognitive testing in estrogen deficient states such as girls with Turner syndrome and postmenopausal women3, 4. Although our sample size is not large, it compares well with other studies such as that of Asthana et al, which reported a significant improvement in verbal memory in 12 postmenopausal women following estrogen replacement3. A limitation of the study is that we do not have data for the total duration of estrogen deficiency, because athletes may have periods of amenorrhea interspersed with periods of oligo-amenorrhea or eumenorrhea depending on the training season and kind of athletic activity. Also, it was difficult to accurately assess the degree of adherence to study medications given that ethinyl estradiol (in oral pills) cannot be assessed with standard assays. However, we did use tools such as a medication calendar and returned pill packs and backings of patches to determine adherence. Although subjects with active anorexia nervosa were excluded, subjects with a past history of anorexia nervosa or active bulimia were not excluded and we acknowledge this as a study limitation. Further, the impact on cognitive function of local estrogen produced in the brain versus estrogen produced in the ovaries remains unclear. Estradiol is known to cross the blood-brain barrier52, and changes in systemic estradiol levels (as observed in post-menopausal women and hypogonadal conditions such as Turner syndrome known to impact cognition) may alter local estradiol production in the brain through their effect on GnRH neurons53. Studies are needed to delineate the effects of peripheral versus locally produced estradiol on cognitive indices, and to also assess the impact of gonadal steroids on mood outcomes, as changes in mood can affect cognitive indices54.
Data regarding effects of estrogen on cognition and memory in humans have thus far been inconclusive and are lacking in adolescents and young adults with estrogen deficiency, except for a few studies in Turner’s syndrome. Our investigation supports the cognition-enhancing role of estrogens in another population that presents with estrogen deficiency early in life. In this study, we have used exogenous estrogen as a physiologic probe rather than as a treatment strategy. Importantly, our data provide the first clue that estrogen replacement may improve cognitive function in oligoamenorrheic athletes. However, further studies exploring the differences in cognitive indices following estrogen replacement in athletes, particularly focusing on verbal memory and executive control, are warranted.
Conclusion:
Female oligo-amenorrheic athletes show improvements in immediate-recall memory and cognitive flexibility with estrogen replacement supporting the role of gonadal steroids in regulation of higher cognitive functions. These youth are in the prime of their neural and behavioral development, and the impact of estrogen deficiency on cognition in this population needs further exploration.
Supplementary Material
Clinical Points.
The impact of estrogen replacement on cognition in athletes who lose their period due to excessive exercise and are estrogen deficient has not been previously assessed.
Estrogen replacement may have a role in improving memory and executive function in athletes who develop amenorrhea secondary to exercise.
Source of funding:
1. Adult Endocrinology Training grant T32 DK007028, NIH, Bethesda, MD, Dr. Baskaran: Primary Investigator (PI), corresponding author
2. National Institute of Health R01 HD060827, Dr. Misra:PI, Co-author
3. National Institute of Health K24 HD071843, Dr. Misra:PI, Co-author
Footnotes
Conflict of interest: Authors involved in the study report no conflict of interest to disclose.
Clinical Trial Registration: NCT00946192
References:
- 1.O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology 1996. January;21(1):51–65. [DOI] [PubMed] [Google Scholar]
- 2.Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB, Jr. Effects of estrogen on nonverbal processing speed and motor function in girls with Turner’s syndrome. The Journal of clinical endocrinology and metabolism 1998. September;83(9):3198–3204. [DOI] [PubMed] [Google Scholar]
- 3.Asthana S, Craft S, Baker LD, et al. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999. August;24(6):657–677. [DOI] [PubMed] [Google Scholar]
- 4.Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain 2006. May;129(Pt 5):1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaer ML, Geffner ME, Kaufman FR, Buckingham B, Hines M. Cognitive and behavioral characteristics of turner syndrome: exploring a role for ovarian hormones in female sexual differentiation. Horm Behav 2002. March;41(2):139–155. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q, Zhang Z, Xie S, et al. Cognitive impairment and gray/white matter volume abnormalities in pediatric patients with Turner syndrome presenting with various karyotypes. J Pediatr Endocrinol Metab 2013;26(11–12):1111–1121. [DOI] [PubMed] [Google Scholar]
- 7.Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res 1995. May-Jun;29(3):153–163. [DOI] [PubMed] [Google Scholar]
- 8.Sherwin BB. Sex hormones and psychological functioning in postmenopausal women. Exp Gerontol 1994. May-Aug;29(3–4):423–430. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology 2004. January;13(1):61–66. [DOI] [PubMed] [Google Scholar]
- 10.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology 2009. August;18(8):811–821. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 1994. May 2;644(2):305–312. [DOI] [PubMed] [Google Scholar]
- 12.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 1998. October;34(2):149–162. [DOI] [PubMed] [Google Scholar]
- 13.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 2001. December;14(12):1992–2002. [DOI] [PubMed] [Google Scholar]
- 14.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005. June 2;435(7042):673–676. [DOI] [PubMed] [Google Scholar]
- 15.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America 1995. November 21;92(24):11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Human brain mapping 2014. March;35(3):847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 18.Dunkin J, Rasgon N, Wagner-Steh K, David S, Altshuler L, Rapkin A. Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology 2005. April;30(3):284–296. [DOI] [PubMed] [Google Scholar]
- 19.Pefanco MA, Kenny AM, Kaplan RF, et al. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc 2007. March;55(3):426–431. [DOI] [PubMed] [Google Scholar]
- 20.Maki PM. Minireview: effects of different HT formulations on cognition. Endocrinology 2012. August;153(8):3564–3570. [DOI] [PubMed] [Google Scholar]
- 21.Gleason CE, Dowling NM, Wharton W, et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS Med 2015. June;12(6):e1001833; discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature reviews Neuroscience 2008. January;9(1):58–65. [DOI] [PubMed] [Google Scholar]
- 23.Loucks AB, Horvath SM. Athletic amenorrhea: a review. Medicine and science in sports and exercise 1985. February;17(1):56–72. [PubMed] [Google Scholar]
- 24.Joy E, De Souza MJ, Nattiv A, et al. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep 2014. Jul-Aug;13(4):219–232. [DOI] [PubMed] [Google Scholar]
- 25.Nazem TG, Ackerman KE The Female Athlete Triad. Sports Health 2012;4(4):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol 2000. October;54(1–3):241–257. [DOI] [PubMed] [Google Scholar]
- 27.Kramer J, Delis Dean, Mark Daniel. Sex differences in verbal learning. Journal of Clinical Psychology 1998;44(6):907–915. [Google Scholar]
- 28.Isotton AL, Wender MC, Casagrande A, Rollin G, Czepielewski MA. Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study. European journal of endocrinology/European Federation of Endocrine Societies 2012. February;166(2):207–213. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler PCD, ed. Wechsler abbreviated scale of intelligence San Antonio, Tex.: Psychological Corporation, ©2011; 2011. [Google Scholar]
- 30.Delis DC, Kramer JH, Kaplan E, & Ober BA. California Verbal Learning Test – Second edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 31.Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacology, biochemistry, and behavior 1992. November;43(3):855–861. [DOI] [PubMed] [Google Scholar]
- 32.Pearson. Delis-Kaplan Executive Function System™. In: DELIS DC, KAPLAN, Edith, KRAMER, Joel H, ed.; 2001. [Google Scholar]
- 33.De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. British journal of sports medicine 2014. February;48(4):289. [DOI] [PubMed] [Google Scholar]
- 34.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. The New England journal of medicine 2010. July 22;363(4):365–371. [DOI] [PubMed] [Google Scholar]
- 35.Ackerman KE, Slusarz K, Guereca G, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. American journal of physiology Endocrinology and metabolism 2012. April 1;302(7):E800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackerman KE, Patel KT, Guereca G, Pierce L, Herzog DB, Misra M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clinical endocrinology 2013. January;78(1):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chui HT, Christensen BK, Zipursky RB, et al. Cognitive function and brain structure in females with a history of adolescent-onset anorexia nervosa. Pediatrics 2008. August;122(2):e426–437. [DOI] [PubMed] [Google Scholar]
- 38.Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005. March;4(3):190–194. [DOI] [PubMed] [Google Scholar]
- 39.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama 2004. June 23;291(24):2959–2968. [DOI] [PubMed] [Google Scholar]
- 40.Wroolie TE, Kenna HA, Williams KE, et al. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17beta-estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry 2011. September;19(9):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berent-Spillson A, Briceno E, Pinsky A, et al. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology 2015. September;59:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 2006. May-Jun;13(3):411–422. [DOI] [PubMed] [Google Scholar]
- 43.Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB, Jr. Use of estrogen in young girls with Turner syndrome: effects on memory. Neurology 2000. January 11;54(1):164–170. [DOI] [PubMed] [Google Scholar]
- 44.Kocoska-Maras L, Zethraeus N, Radestad AF, et al. A randomized trial of the effect of testosterone and estrogen on verbal fluency, verbal memory, and spatial ability in healthy postmenopausal women. Fertil Steril 2011. January;95(1):152–157. [DOI] [PubMed] [Google Scholar]
- 45.Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci 2009. August 19;29(33):10362–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colzato LS, Pratt J, Hommel B. Estrogen modulates inhibition of return in healthy human females. Neuropsychologia 2012. January;50(1):98–103. [DOI] [PubMed] [Google Scholar]
- 47.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 2006. January;147(1):607–614. [DOI] [PubMed] [Google Scholar]
- 48.Daniel JM, Witty CF, Rodgers SP. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav 2015. August;74:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 2013. June;20(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992. October;17(5):485–495. [DOI] [PubMed] [Google Scholar]
- 51.Modahl C, Green L, Fein D, et al. Plasma oxytocin levels in autistic children. Biological psychiatry 1998. February 15;43(4):270–277. [DOI] [PubMed] [Google Scholar]
- 52.Walum H, Lichtenstein P, Neiderhiser JM, et al. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biological psychiatry 2012. March 1;71(5):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. The Journal of clinical endocrinology and metabolism 1987. January;64(1):27–31. [DOI] [PubMed] [Google Scholar]
- 54.Scantamburlo G, Hansenne M, Fuchs S, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007. May;32(4):407–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.