Abstract
Understanding regulators of folliculogenesis remains limited in the domestic dog (Canis familiaris), which challenges our ability to develop in vitro follicle culture systems for canid genome rescue efforts. Here, we investigated the influence of activin on dog follicle development and survival, oocyte quality, and FSH receptor expression in culture. Preantral (150 – ≤230 µm diameter), early antral (231 – ≤330 µm), and antral (>330 – 550 µm) stage follicles were encapsulated in a fibrin-alginate hydrogel with 0, 100, or 200 ng/ml rhActivin plus 0, 0.1, 1, or 10 µg/ml FSH for 12 or 21 d of in vitro culture. All follicle groups increased in diameter (P < 0.05) with activin acting synergistically with FSH to improve (P < 0.05) growth and antral cavity expansion (to >630 µm) in early antral and antral cohorts. This complementary effect was not linked to changes in FSHR mRNA expression (P > 0.05). Although not influencing (P > 0.05) follicle survival or transzonal projection (TZP) density in shorter term 12 d culture, activin in the presence of 1 ng/ml FSH maintained TZP density from the 12 to 21 d interval. Activin also increased oocyte diameter and improved nuclear integrity compared to un-supplemented controls. These results indicate that activin acts synergistically with FSH to promote growth and antral cavity expansion of the dog follicle in vitro, information useful to formulating an effective culture microenvironment for this species.
Keywords: Activin, domestic dog, FSH, ovarian follicle, trans-zonal projection
1. Introduction
The International Union for Conservation of Nature has designated five of the 35 extant, wild canid species as endangered or critically endangered [1]. As the closest, domesticated relative, the dog is an excellent model for understanding the intricacies of canid reproductive biology, including developing knowledge and tools to foster species survival and improved conservation management. The unique biology of canids poses challenges to controlling reproductive function, including technologies common in humans as well as some laboratory and livestock species. Firstly, it is known from longitudinal measures of ovarian steroid hormone activity [2–4] and ultrasonography [5] that the ovaries of the dog and other canids generally are quiescent, lacking mature follicular development and yielding sporadic estrus only once or twice annually [6]. An explicit reasoning for this protracted, suppressed ovarian activity is not well understood. Secondly, when ovulation does occur in the dog, oocytes are immature and resume meiosis to achieve metaphase II (MII) only after intraoviductal residence for 48 to 72 hours [7, 8]. Because of this reproductive complexity, the dog offers unique opportunities for understanding regulation of canid follicle and oocyte maturation and viability.
Domestic dog oocytes from antral follicles >2 mm in diameter are more likely to resume meiosis and achieve Metaphase II in vitro (as high as 79.5% in our laboratory [9]) than those recovered from <0.05 mm counterparts (16.9% [9]), although in general achievement of MII dog oocytes in vitro ranges from 20–45% [10–12]. Thus, our objective is to develop an improved in vitro culture system that consistently produces antral stage follicles and, thereby, maturation-competent oocytes. Previously we explored an alginate culture system developed originally for the mouse [13, 14] to maintain the three-dimensional (3D) architecture of isolated dog follicles. Although preantral (compared with early antral) follicles produced the more robust percent growth in vitro, we also observed that follicles: 1) rarely grew beyond ~500 µm diameter (compared with 5–7 mm preovulatory follicle diameter [15]); 2) were unable to maintain an antral cavity; and 3) contained oocytes that became increasingly pale over a 20 d culture period, suggesting loss of intracellular lipid (the dog is known for its dark colored, lipid-filled oocytes [16, 17]). These morphological anomalies may have resulted from poor communication between the oocyte and cumulus cells via cumulus cell trans-zonal projections (TZPs), or extensions of the plasma membrane through the zona pellucida which form junctions with the oocyte. This communication is known to direct antral cavity development [18–20], and modulate oocyte intracellular lipids [21, 22]. In sum, there is a need to explore factors which may act to promote TZP function and antrum formation for canine in vitro folliculogenesis.
Recent work in in vitro follicle culture has demonstrated a role of the protein hormone activin in promoting antrum cavitation in rat, cow, and human follicles [23–25]. Activin also has been suggested to improve cumulus-oocyte communications in cultured bovine follicles by maintaining TZPs [26] to, in turn, promote antrum formation and oocyte viability. Activin is a glycoprotein member of the transforming growth factor β (TGFβ) superfamily, produced by granulosa cells of the ovarian follicle [27–32], and is believed to act both as an endocrine factor signaling pituitary FSH release, and as a paracrine/autocrine factor in the ovary [33]. Supplementing culture medium with activin has been shown to promote murine follicle growth [34] and survival [35] as well as oocyte maturation in humans and zebrafish [30, 36]. Previous investigations into activin mechanisms have focused mainly on its role in increasing follicle sensitivity to FSH by increasing transcription of FSH receptor mRNA [37, 38]. The purpose of the present study was to understand the role of activin as a regulator of antral cavity development and cumulus cell-oocyte communication in the domestic dog.
Here, we took advantage of the dynamic Fibrin-Alginate Interpenetrating Network (FA-IPN) [39], a 3D hydrogel system which confers structural support through its alginate constituent and flexibility via its fibrin component that can be degraded by the follicle, thereby allowing further growth, including antrum expansion. FA-IPN has increased rates of successful antrum formation and oocyte maturation in murine follicle culture [40]. Our specific objectives were to determine the influence of activin, with or without FSH, on follicle growth, survival, antral cavity expansion, FSH receptor expression, TZP density, and oocyte growth and viability. We hypothesized that activin supplementation (1) acts synergistically with FSH via up-regulation of FSH receptors to promote follicle growth, (2) supports maintenance of TZP density, and (3) promotes antrum development and oocyte viability in in vitro culture.
2. Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and media from Irvine Scientific (Irvine, CA), unless otherwise stated.
2.1. Follicle Isolation and Encapsulation
Ovaries from 42 dogs (age 5 mo - 9 yr, breed information in Supplemental Table 1) were obtained during routine ovariohysterectomies conducted at local veterinary clinics. Each freshly-excised reproductive tract (from cervix to bilateral ovaries) were stored on ice in Leibovitz’s L15 medium containing 84.2 µM penicillin G sodium, 41.2 µM streptomycin sulfate, and 50 mM ascorbic acid for transport to the laboratory. Within 3 hours of excision, each ovary was visually examined to estimate reproductive stage of the donor. Virtually all females were in anestrus or diestrus based on ovarian morphology, specifically absence of visible antral follicles and/or presence of corpora lutea, respectively (Anderson & Simpson 1973).
Ovarian tissue then was manipulated under a stereomicroscope using a scalpel blade and 25 g needle to mechanically-isolate follicles. Follicles were isolated from the ovarian tissue within 10 hours of excision. Only those having at least two granulosa cell layers with a homogeneously dark, circular oocyte and intact basement membrane [41] were included in the study. Isolated follicles meeting these criteria were individually-encapsulated in a bead with 22.5 mg/ml fibrinogen (bovine, Sigma) and 0.38% (w/v) alginate (FMC BioPolymers, Philadelphia, PA) according to methods described by [39]. Briefly, follicles in fibrinogen-alginate (5 µl) were pipetted into 50 U/ml thrombin in Tris BuPH with 40 mM CaCl2. Alginate crosslinks in the presence of a divalent cation, such as calcium [42], and fibrinogen polymerizes into fibrin when thrombin activates factor XIIIa [39]. Beads then were washed in α-Minimum Essential Medium containing 3 mg/ml (~45 µM) BSA, 4.2 µg/ml (0.72 µM) insulin, 3.8 µg/ml (47.5 nM) transferrin, and 5 ng/ml (63.3 nM) selenium, hereafter called ‘Growth Medium’ [41].
2.2. Follicle Culture and Growth
Each encapsulated follicle was transferred to its own individual well of a flat-bottomed 96-well culture plate in 100 µl Growth Medium supplemented with a specific concentration of recombinant human activin (Sigma) and/or FSH (Folltropin V, BioNiche Animal Health, Athens, GA). Follicles were assigned randomly to one of 12 treatments of 0, 100 (3.8 nM), or 200 ng/ml (7.7 nM) activin and 0, 0.1, 1, or 10 µg/ml (0, 0.2, 1.7, or 17.5 IU/L) FSH (3 × 4 factorial design).
As mechanical isolation can result in a small layer of ovarian stromal cells covering the isolated follicles [43], two size evaluations were taken for each follicle on the day of culture onset (Day 0) – one for determination of follicle stage (only measuring diameter within the basal lamina) and another of full the structure (diameter of follicle plus surrounding stromal cell layer) (Fig. S1). This latter size measurement was used to assess follicle growth over the culture period. Determination of stages have been defined in our previous work [44]: preantral follicle (100 – ≤230 µm diameter); early antral (231 – ≤330 µm); and antral (331 – 550 µm). For both sizing approaches, the widest apparent diameter was measured followed by determining perpendicular width. A mean for these two values was calculated and reported as diameter [45]. We used the same technique on Day 0 to determine oocyte diameter (excluding the zona pellucida). All follicle and initial oocyte diameters were assessed using an inverted microscope (Leitz DM-IL, Research Instrument Limited, Falmouth, Cornwall, UK) with a heated stage, 10x objective, and optical micrometer.
Follicles were cultured at 38.5°C in a humidified atmosphere of 5% CO2 with existing medium exchanged with fresh every 72 hours. Diameter of each follicle was also measured on Days 3, 6, 9, 12, 15, and 21 following culture onset. Percentage change in diameter from Day 0 of incubation was calculated as follicle growth. Incubation of follicles was terminated at one of two time points (Day 12 or 21) and cumulus-oocyte complexes (COCs) collected to determine oocyte diameter (for subsequent growth analysis) and to evaluate TZP integrity and nuclear status (see below).
2.3. Follicle Survival
Follicles that survived were defined as those sustaining a normal follicular appearance. Those that failed to survive had decreased in diameter over at least two consecutive observation time points, and/or contained a degenerate or extruded oocyte (emerging from the follicle’s basement membrane), or displayed fragmented granulosa cells [46]. Follicles with these degenerating traits apparent before Day 3 were considered to have had an undetectable defect prior to, or as result of, the isolation/encapsulation process. A total of 955 follicles were included in the study (136 representing the preantral stage; 480 early antral; 339 antral; Supplemental Table 2).
2.4. Antral Cavity Expansion
For the antrum assessment, we focused on antral cavity expansion, rather than initiation of cavitation, for two reasons. First, ~85% of our isolated follicles already had begun the cavitation process (i.e., met early antral or antral criteria; [47]. Second, we had determined in a previous study [45] that dog follicles incubated in 0.5% hydrogel developed antral cavities that subsequently collapsed. Therefore, our aim here was to both maintain antrum integrity and expand this cavity’s size. We recorded follicles as being ‘expanded antral’ when the diameter exceeded 630 µm and the fluid-filled cavity was maintained throughout the culture interval. For this assessment, we fixed 205 follicles on Day 21 (10 – 16 per treatment) in Bouin’s solution followed by embedding in paraffin blocks. For each block, 6 µm thick sections were cut, mounted on slides, and stained with haemotoxylin and eosin for subsequent microscopic evaluations of presence of theca cells and an antral cavity.
2.5. Oocyte Trans-Zonal Projections (TZPs), Growth, and Intact Nuclear Status
At the end of culture on Day 12 or 21, two 25 g needles were used to tease each follicle from its encompassing FA-IPN, followed by careful tearing of the basement membrane to release the COCs. Each COC was fixed in 4% paraformaldehyde for 12 hours and then stored in wash buffer (0.2% azide, 2% normal goat serum, 1% bovine serum albumin, 0.1 M glycine, and 0.1% Triton X-100 [48] at 4ºC for subsequent analyses. COCs were grouped based on hormone treatment for subsequent assessment of oocyte size, TZP density, and nuclear status. Actin staining of fixed COCs (n = 242) was achieved using Alexa Fluor 488 Rhodamine Phalloidin [48] (Invitrogen at 1:100 dilution in wash buffer) for at least 30 min (room temperature) with simultaneous nuclear staining using 1 µg/ml (2.2 µM) Hoechst 33342 (in a 90% glycerol, 10% PBS solution). No more than four oocytes per treatment group were mounted on a slide (with GVA Aqueous Mounting Solution; Genemed Biotechnologies, San Francisco, CA). Imaging was at 100x with an LSM 510 laser scanning confocal microscope (Carl Zeiss, Germany) and a 488 nm krypton/argon laser and ultraviolet light. Z-stack images were taken of each COC. The z-stack slice imaging the oocyte at its widest diameter was used for TZP assessment. The circumference of the oocyte was measured and TZPs were pseudo-colored using ImageJ software (NIH, Bethesda, MD). TZPs were counted and density calculated per 10 µm oocyte circumference. Oocyte diameter (in µm excluding the zona pellucida) was determined by taking the mean of the widest diameter across the vitelline membrane and then a perpendicular width to the initial assessment. Presence of an intact nucleus (as opposed to fragmented chromatin, or oocytes no visible nucleus) was recorded for each COC.
2.6. Granulosa Cell FSHR Expression
Additional short-term (3 d) cultures were carried out to evaluate the influence of activin supplementation on dog FSH receptor expression. This evaluation required collecting the ovaries from another 14 donors (age, 6 months – 2.5 years) and then the recovery of 639 additional follicles representing each of the three target stages; Supplemental Table 3). After isolation, these follicles were cultured in FA-IPN (as above), including in 0, 100, or 200 ng/ml activin and 0 or 10 µg/ml FSH (3 × 2 design). On the third day after culture onset, granulosa cells were collected in RNAlater and stored at −20°C on the basis of the three follicle stages by six hormone treatments (4 – 5 cultures per pool; 18 total groups). After later thawing, total RNA was isolated using an Agilent Absolutely RNA Nanoprep kit with on-column DNase digestion (manufacturer’s instructions). Synthesis of cDNA was performed with a Transcriptor High Fidelity cDNA Synthesis Kit (Roche) with 30 ng total RNA. A reverse-transcription polymerase chain reaction (RT-PCR) for FSH receptor was performed with FastStart Essential DNA Green Master (Roche) using β-actin as an endogenous control (primer details, Supplemental Table 4) on a LightCycler 96 (Roche).
2.7. Statistical Analyses
Follicle growth data were log-transformed to achieve normality followed by model selection using an Akaike information criterion (AIC) [49] in RStudio (Version 1.0.143) with the following factors: activin concentration; FSH concentration; follicle stage at isolation; and all related interactions, as well as age of follicle donor. Culture replicate (i.e. cultured follicles from an individual donor dog for all but nine experiments, in which follicles isolated from two dogs were pooled: See Supplemental Table 1) was included as a random variable in the model. All factors and interactions were included in the final model that relied on a standard least squares model and post-hoc Tukey-Kramer HSD test for significance via JMP 9.0 software (SAS, Cary, NC).
Follicle survival was evaluated using a proportional hazards model with donor dog age, follicle stage at isolation, activin concentration, FSH concentration, and the interactions of the latter three as model effects. Donor dog age was treated as a categorical variable on the basis of <8 mo of age (prepubertal, n = 4 culture replicates, Supplemental Table 1), 8 mo to <2 y (peripubertal, n = 15 replicates), 2 to 4 y (adult, n = 5 replicates), and >8 y (aged, n = 2 replicates). Due to the random nature of collections from local, commercial spay clinics, precise animal age was not always available; furthermore, there were no donors 5 to 8 y of age. Follicles that survived through the 12 or 21 d culture end points were censored. We used a Chi square likelihood ratio test to examine a specific type of follicle mortality associated with oocyte extrusion; our earlier data have suggested that this subcategory of follicle loss may be associated with a breakdown in oocyte and surrounding cumulus cell connectivity [45].
Proportions of follicles forming an expanded antrum were compared among groups using a Chi square likelihood ratio test. TZP density, proportion of oocytes with intact nuclei, and oocyte diameter differences among treatment groups and time points (Days 0, 12, and 21) were evaluated using a nonparametric Wilcoxon test. RT-PCR data were analyzed via the Pfaffl method, accounting for primer efficiency [50]. Results are expressed as the mean ± SEM, and significant differences set at P < 0.05.
3. Results
3.1. Follicle Growth
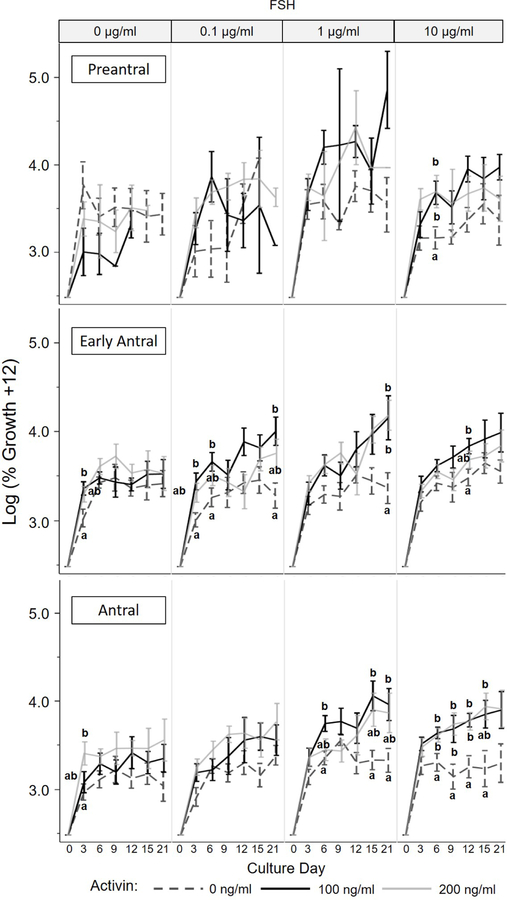
Ovarian follicles, regardless of stage or treatment group, experienced growth over the 21 d culture. Overall, this growth tended to be rapid in the first 3–6 days of culture, and level off to a more steady, sustained growth in the latter half of the incubation period. Our statistical model demonstrated that follicle stage at isolation, activin concentration, FSH concentration, and interaction of follicle stage x activin, activin x FSH, and follicle stage x activin x FSH influenced (P < 0.05) subsequent follicular growth in vitro. Early antral follicles tended to increase in size over 21 day culture the most, on average 40.7 ± 3.4% compared to 37.5 ± 7.9 and 32.4 ± 2.9 for preantral and antral counterparts, respectively. Providing activin during incubation (in the absence of FSH) increased (P < 0.05) follicle size compared to the controls as early as Day 3 (Fig. 1). For example, in the absence of FSH and activin, early antral follicles grew only 11.8 ± 3.6% by Day 3 compared to the same stage follicles exposed to 100 ng/ml activin and 0 µg/ml FSH, which increased 22.7 ± 3.3% in diameter.
Figure 1.
Growth (log transformation of percent diameter increase compared to Day 0 defined as onset of culture) ± SEM of preantral, early antral, and antral stage dog ovarian follicles cultured in vitro with various activin (0, 100, or 200 ng/ml) and FSH (0, 0.1, 1, or 10 μg/ml) concentrations. Values with differing letters within the same follicle stage, FSH treatment, and culture day represent differences (P < 0.05) among activin concentration groups.
Figure 1 illustrates the interaction between follicle stage and hormonal supplementations. Preantral follicles experienced no benefit (P > 0.05) of supplemental activin. Conversely, presence of 100 or 200 ng/ml activin enhanced (P < 0.05) follicle growth for the early antral and antral cohorts, but only in the presence of FSH. For example, early antral follicles exposed to 0.1 µg/ml or 1 µg/ml FSH plus 100 ng/ml activin increased (P < 0.05) in size 52.6 ± 6.7% and 63.8 + 14.1%, respectively, by Day 21 compared to only 17.9 ± 6.5% and 23.2 ± 14.8% in these FSH dosages alone. There was no influence (P > 0.05) of increased activin concentration (100 versus 200 ng/ml) on in vitro growth, regardless of follicle stage.
There was an effect of donor dog age on the ability of ovarian follicles to grow in vitro. Specifically, follicles isolated from adult / prime breeding age individuals (2 – 4 y old) increased in size the most (P < 0.05) compared to the same stage follicles recovered from prepubertal (<8 mo) or peripubertal (8 mo - <2 y) bitches (Fig. S2A).
3.2. Follicle Survival
The parametric survival model demonstrated that the only significant factors were FSH dosage and donor age. Original follicle stage (Fig. S3A), activin supplementation (Fig. 2A) or their interactions had no influence (P < 0.05) on follicular survival. Follicles had improved (P < 0.05) survival when cultured with 0 or 10 µg/ml FSH compared to the lowest rate after treatment with 0.1 µg/ml (Fig. S3B). Follicles recovered from <2 y old donors also underperformed with higher mortality (P < 0.05) compared to all older groups (Fig. S2B).
Figure 2:
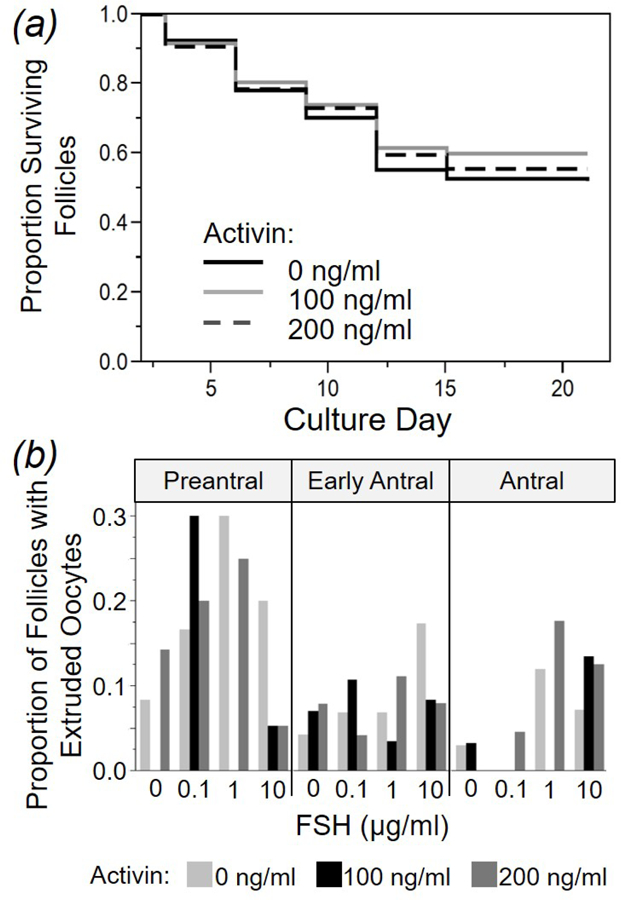
Proportions of dog ovarian follicles (A) surviving in 0, 100, or 200 ng/ml activing (combining all follicle stages and FSH treatment groups) over a 21 d culture, and (B) experiencing oocyte extrusion based on original stage at isolation and hormone concentration in vitro (Activin, 0, 100, or 200 ng/ml; FSH, 0, 0.1, 1, or 10 µg/ml).
When evaluating follicle loss specifically due to oocyte extrusion, there was an impact (P < 0.05) of follicle stage and FSH presence. The greatest frequency (13%) of this anomaly occurred in preantral follicles exposed to exogenous FSH (Fig. 2B). Although oocyte extrusion was not attenuated by activin presence, age of donor bitch increased the likelihood (P < 0.05) of this phenomenon occurring (circa 53% and 57% in bitches < 8 mo and 8 mo - 1.5 yr of age compared to 75% and 86% for donors 2 – 4 yr and > 8 y of age, respectively).
3.3. Antral Cavity Expansion
Antral cavity expansion (Fig. 3A) occurred in early antral and antral stage follicles cultured in the presence of both activin and FSH (Fig. 3B). Only a single follicle categorized as ‘preantral’ at the onset of culture developed a large antral cavity (in presence of 100 ng/ml activin and 10 µg/ml FSH). No follicle experienced expanded cavitation in the absence of activin or FSH. The combined supplementation of 100 or 200 ng/ml of activin and 0.1, 1, or 10 µg/ml FSH produced 36 total follicles with cavity expansion (3.4% of total cultured), with most of this cavitation (88.9%) occurring Days 6 to 15 from incubation onset (i.e., midculture). Histology at 21 d of incubation revealed an absence of cells with theca-like morphology compared to freshly-isolated, sized-matched control follicles (Fig. 3C). Presumptive small antral cavities also were observed in ~40% of follicles; these cavities stained pink in the presence of eosin (Fig. 3C), a trait not observed in cavities of fresh controls. Donor dog age had no influence (P > 0.05) on antral cavity expansion.
Figure 3:
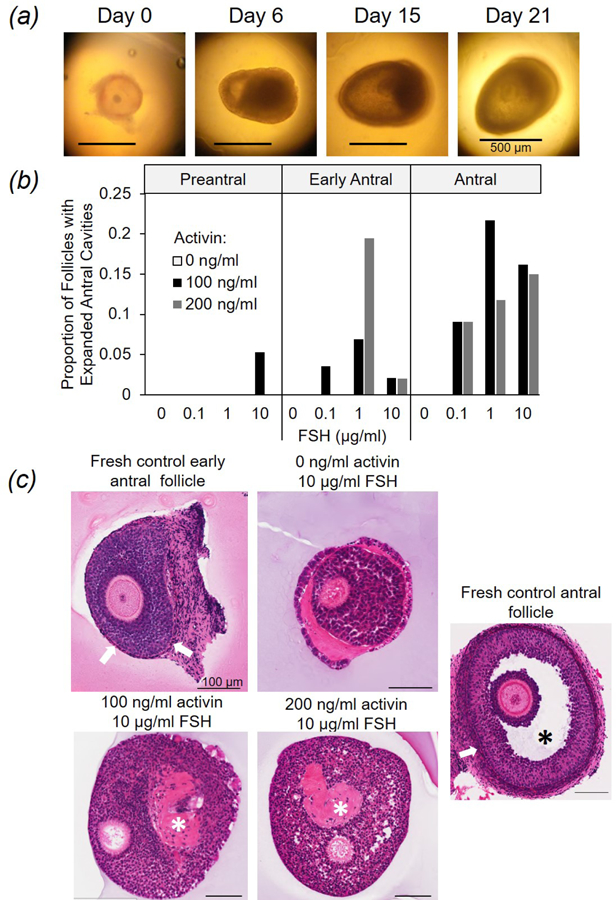
Antral cavity formation in cultured dog ovarian follicles. (A) Images of a single, early antral follicle exposed to 100 ng/ml activin and 1 µg/ml FSH developing an antral cavity over 21 d (Day 0 = culture onset). (B) Proportion of follicles developing an expanded antral cavity based on original stage at isolation and hormone concentration (activin, 0, 100, or 200 ng/ml; FSH, 0, 0.1, 1, or 10 µg/ml FSH). (C) Hematoxylin and eosin staining of freshly-isolated early antral and antral follicles (controls) and counterparts cultured 21 d (activin, 0, 100, or 200 ng/ml and FSH, 10 µg/ml). White arrows indicate theca cell layers, and asterisks (*) mark antral cavities in fresh (black) versus cultured (white) follicles.
3.4. Oocyte Trans-Zonal Projections (TZPs), Growth, and Intact Chromatin
Confocal microscopic images of TZPs in freshly-isolated oocytes and cultured follicles on Days 12 and 21 after FSH and activin exposure (and respective oocytes on Day 21) are depicted in Figure 4A–C). Regardless of treatment, vesiculation was notable along the cortical edge of oocytes at the junction with TZPs in 66% and 58% of Day 12 and 21 oocytes with TZPs, respectively (Fig. 4B,C). Average TZP density for COCs from freshly-isolated preantral versus antral follicles was 2.4 ± 0.4 and 3.5 ± 0.1 TZPs/10 µm oocyte perimeter. Both these values were higher (P < 0.05) than for COCs from cultured follicles (average across all treatments, 0.51 ± 0.10 TZPs/10 µm). Neither hormone influenced (P > 0.05) incidence of vesiculation. TZP density was reduced (P < 0.05) in follicles exposed to the highest (200 ng/ml) activin concentration over the 12 d incubation (Fig. 4E). For example, oocytes from follicles cultured for this interval in 0 or 100 ng/ml activin and 1 µg/ml FSH averaged 0.72 ± 0.20 and 0.54 ± 0.27 TZPs/10 µm, respectively. By contrast, those cultured in the same FSH concentration, but with 200 ng/ml activin averaged only 0.37 ± 0.25 TZPs/10 µm (P < 0.05). Follicles cultured in 200 ng/ml activin consistently experienced reduced TZP density on Day 12 compared to 0 or 100 ng/ml activin counterparts (regardless of FSH dosage). However, at the Day 21 evaluation and in the presence of 1 µg/ml FSH, it was clear that activin was required to maintain TZP density. At this time, the average number of TZPs/10 µm was less (P < 0.05) in absence of activin (0.09 ± 0.5) compared to 100 ng/ml (0.89 ± 0.40,) or 200 ng/ml (0.46 ± 0.31) activin, respectively. These latter values were similar to the highest average TZP density measured in oocytes at the abbreviated (12 d) incubation time point.
Figure 4:
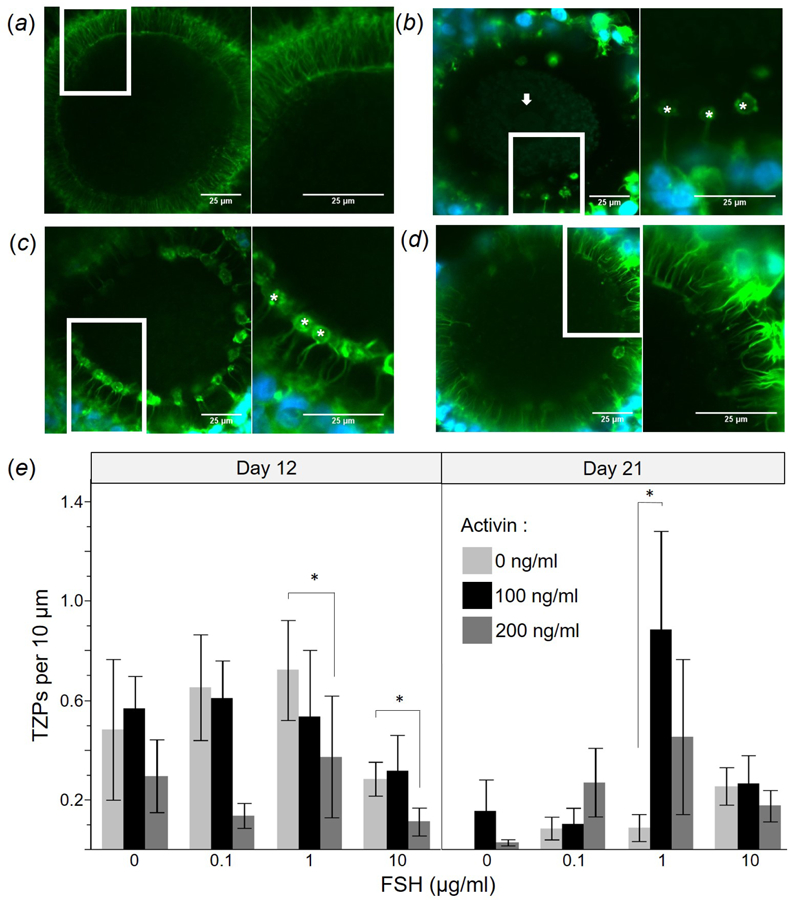
Confocal microscopy images depicting trans-zonal projections (TZPs) in oocytes of dog ovarian follicles that were (A) freshly-isolated (control) or cultured for 21 d in 1 µg/ml FSH and (B) 0, (C) 100, or (D) 200 ng/ml activin. Inserts within panels indicate magnified (2x) areas depicted in each right panel; asterisk (*) indicates vesicularisation, and arrow denotes nucleus. (E) TZP density (mean ± SEM) after 12 and 21 d culture (activin, 0, 100, or 200 ng/ml; FSH, 0, 0.1, 1, or 10 µg/ml). Asterisks (*) indicate differing (P < 0.05) values among activin concentrations within a given FSH dosage and culture day.
For oocytes from surviving follicles, growth occurred from Day 0 to 12 followed by a plateau through to Day 21 (Fig. 5A). Supplementation with 100 ng/ml activin increased (P < 0.05) oocyte diameter (87.5 ± 2.9 µm) at Day 21 compared to non-supplemented controls (77.2 ± 3.1 µm) (Fig 5A). FSH concentration had no influence (P > 0.05) on oocyte size at Day 12 or 21. Presence of activin at 100 or 200 ng/ml, compared to activin absence, produced a higher proportion of oocytes with intact chromatin, with significance reached on Day 12 for 0.1 µg/ml FSH and Day 21 for 10 µg/ml FSH (Fig. 5B). FSH concentration had no effect (P > 0.05) on intact nuclear status of recovered oocytes on Days 12 or 21 (Fig. 5B).
Figure 5:
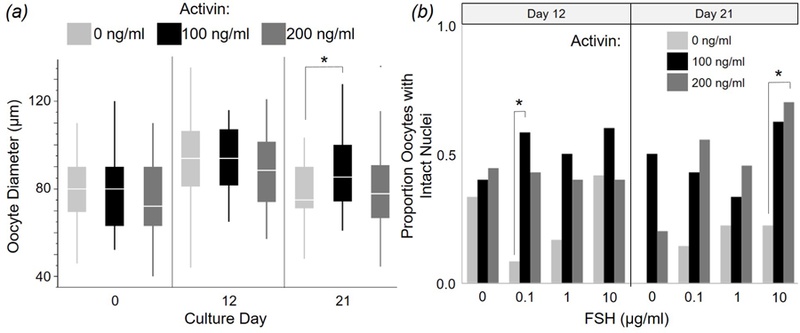
(A) Oocyte growth based on diameter changes during culture of dog follicles for 12 or 21 d while exposed to 0, 100, or 200 ng/ml activin (combining all FSH treatment groups). (B) Proportion of chromatin-intact oocytes from donor follicles supplemented with activin (0, 100, or 200 ng/ml), and FSH (0, 0.1, 1, or 10 µg/ml) in vitro. All values are means ± SEM. Asterisks (*) indicate differing (P < 0.05) values among activin concentrations within a time point.
3.5. Granulosa Cell FSHR Expression
There was no statistical inference (P > 0.05) to be made from an activin impact on FSHR expression, largely because of variation among replicates (Fig. 6). Of all the comparisons, activin may have been stimulative only in the preantral cohort in the absence of FSH compared to the fresh, non-cultured control. FSH and activin supplementation alone or in combination had no influence (P > 0.05) on level of the FSHR transcript in incubated early antral and antral follicles.
Figure 6:
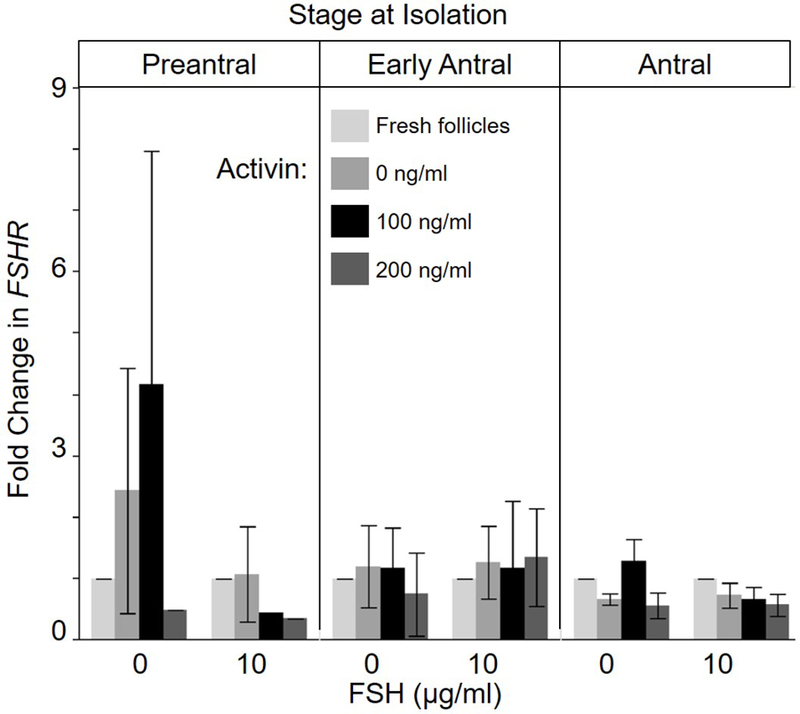
Fold change (mean ± SEM) in FSHR expression on Day 3 of cultured dog ovarian follicles (Day 0 = incubation onset) using B actin as reference gene. Four to five independent cultures were pooled by treatment group (0, 100, or 200 ng/ml activin, and 0 or 10 µg/ml FSH) and initial follicle stage (preantral, early antral, or antral). No differences (P > 0.05) were found.
4. Discussion
Until now, all studies examining the role of activin have focused on traditional livestock, laboratory rodent, and non-human primate models. Here we were interested in exploring the influence of this hormone and its mechanisms of action in the reproductively-complex dog that only displays ovarian follicular activity only 1–2 times yearly. Previous investigations have revealed that FSH can stimulate growth of cultured dog follicles [41], but additional LH supplementation fails to promote antral cavity formation [45]. Sequentially increasing FSH concentrations [51], supplementing with both FSH and high concentrations of insulin [52] or growth hormone [53] in vitro results in antrum development. Yet, in each of these previous studies, follicle growth and antral cavity expansion have been limited to <500 µm in diameter (only ~25% of the size needed to produce an oocyte capable of achieving MII [9]). The goal of our present study was to explore, for the first time, the potential role of activin on follicle/oocyte growth and survival, including in regulating two crucial functions, antral cavity expansion and cumulus cell-oocyte communication.
From the adaptation of a 3D culture system (already known useful in other models) [39, 54], we made four significant discoveries useful to the dog while extending the informational base on activin’s role in mammals at large. First, the influence of activin was specific to follicle stage, with virtually all effects exerted at the level of the early antral or antral cohorts. The preantral stage likely lacks an active mechanism or receptor to respond to activin stimulation. Second, activin’s role required presence of FSH to achieve a synergistic effect on follicle growth and antral cavitation in both the early antral and antral stage follicle. In the absence of FSH, there was no influence of activin on follicle growth or cavity expansion. Third, although having a negligible effect on follicle survival in vitro, activin supplementation promoted cumulus cell-oocyte communication by partially sustaining TZP density in long-term culture. Finally, although having no growth promotion influence on the resident oocyte, it was clear that activin facilitated preservation of egg nuclear integrity and normality. There was no evidence that these impacts of activin were mediated via changes in FSHR mRNA expression.
Although we identified multiple roles for activin in the dog system, it was somewhat unexpected that activin had no discernible effect on the preantral follicle, especially as activin A is known to promote growth of these same stage follicles in the cow [55]. This hormone clearly was not the primary driver for initiating antrum development in the dog. Cavity initiation in the preantral follicle most likely demands theca cells, an obligation lacking in our current microenvironment (per our histological findings). The pink eosin staining in antral cavities, indicative of an internal richness in intrafollicular proteins, may also be due to the lack of theca cells that facilitates fluid incursions into the follicle via aquaporins [56]. One possible resolution for the dog system, currently under exploration, is evaluating other promoters of theca cell development, for example, kit-ligand that is known to help fully stimulate antrum cavitation in early stage bovine follicles [57].
Once at the early antral stage, the dog follicle had developed a sensitivity to the presence of combined activin and FSH. This synergism resulted in increased growth and antral cavity expansion, which was similar to what has been observed in the cow, human, and rat [23–25]. Interestingly, supplementing primary rat follicles with activin seems to serve a priming function for a more robust response to gonadotrophin when the activin is withdrawn [58]. In this same species. activin also appears to promote FSH receptor mRNA expression in granulosa cells [37, 38, 59], a mechanism that we did not observe in the dog. As activin also has been postulated to block the stimulatory effects of FSH on early stage preantral follicles in the cow [55] and rat [58], it would be useful to examine the influence of this hormone only on this stage dog follicle before FSH exposure.
Incubation with a specific combination of activin and FSH (1 µg/ml) supported the maintenance of TZP density from culture days 12 through 21. The benefit of activin to TZP density was reduced by the highest activin supplementation (200 ng/ml) during the first 12 d of culture, possibly due to accelerated granulosa cell proliferation and follicle expansion that impeded new projections and/or normal connectivity to oocytes. This same phenomenon was also likely occurring during oocyte extrusion. Premature oocyte discharge has been described previously in the domestic dog [51] and has occurred in follicular cultures of the cow [60] goat [61], and human [62]. As none of our control follicles (0 FSH) experienced extrusion, it is likely that our preantral cohort was inadequately primed for the level of granulosa cell proliferation produced in the presence of FSH. However, our ability to recover TZP density in the presence of both activin and FSH indicated the exciting potential of achieving longer-term, successful follicular cultures. The phenomenon of vesiculations at the junction of the oocyte cortex and TZP (observed here in 57% of dog follicles) has also been described in an ultrastructural evaluation of in vitro and in vivo matured dog oocytes [63, 64], as well as in the human COC [65]. In these studies, deeply embedded TZPs with a ‘bulbous ending’, perhaps resulting from an in-folding of the oolemma, were noted, although frequency of this observation went unreported. Ultrastructural evaluations in other species have demonstrated that TZPs end in foot-processes adjoining the oocyte’s plasma membrane [66]. Therefore, these vesiculations may have originated from a normal feature, but one where the TZP simply grew atypically large. Although our findings revealed no definitive cause for this structural peculiarity, we would suggest that such formations may serve as useful markers for effectiveness of in vitro maturation of both the follicle and its resident oocyte. Further, as TZP density in cultured follicles, regardless of treatment, were reduced compared to fresh controls, it is evident that activin is not the sole factor promoting this interaction. Future studies should explore the function of growth differentiation factor 9 in simulating TZP formation, as demonstrated in the mouse [67], in dog follicles.
We determined that activin played a beneficial role in ensuring both oocyte form and integrity. It is known from previous in vivo studies that the dog oocyte reaches a maximal size of ~113 µm (range, 86 to 132; [8], typically near the early antral follicular stage [44]. It was encouraging that our described microenvironment was capable of producing oocytes of this desired diameter from incubated follicles by 12 d post-culture onset. However, there was as much as 26% reduction in oocyte size during the second phase of culture (through 21 d) in the presence of 100 ng/ml activin and 10 µg/ml FSH. A more detailed analysis revealed that follicles exposed to 200 ng/ml activin in the presence of 0, 0.1, or 10 µg/ml FSH maintained oocyte diameter throughout the 3 wk incubation (growing 0.4, 6.1, and 5.8%, respectively). Resident oocytes from follicles supplemented with 100 ng/ml activin and 1 µg/ml FSH (the group also producing the highest TZP densities at Day 21) increased in size a modest 2% over the latter half of culture. This information plus finding that activin supplemented follicles produced oocytes with more intact nuclei at both mid- and late incubation clearly demonstrated that this hormone promoted oocyte growth, survival, and integrity.
Lastly, although not a major focus of the present study, the influence of ovarian donor age on follicle viability in vitro was important. We identified clear advantages for developing these microenvironments using ovarian materials recovered from adult bitches. Prepubertal donors presented more challenges to achieving successful follicle growth and survival in culture but a lower prevalence of premature oocyte extrusions, probably at least in part due to naivete and lack of earlier gonadotropin priming. Therefore, our observations on the donor-age dependent performance of follicles should influence the source of ovaries for future studies.
In summary, we identified a probable role for activin in domestic dog folliculogenesis, promoting growth of early antral and antral stage follicles, largely through antrum cavity expansion and in cooperation with FSH. This improved ability to reach later developmental stages in vitro appears due to activin maintaining TZP density and, therefore, oocyte viability for a 21 d culture interval. Using the permissible FA-IPN incubation system, the follicle growth cap of 500 µm diameter achieved in our recent previous investigation [45] was surpassed 1.8-fold (to 800 to 900 µm), largely by the current protocol expanding the antral cavity. As a large antral follicle is critical to producing a meiotically-competent oocyte, our findings here edge us closer to the ultimate goal of developing an in vitro folliculogenesis system for the domestic dog.
Supplementary Material
Highlights.
Domestic dog ovarian follicles have a stage-specific response to activin supplementation, with early antral and antral but not preantral stage follicles responding to activin in culture.
Activin and FSH have a synergistic effect on growth and antral cavitation in both early antral and antral stage domestic dog follicles that is not mediated via changes in FSHR mRNA expression.
Activin facilitated improved preservation of dog oocyte nuclear integrity and trans-zonal projection density, but not follicle survival, during in vitro culture.
Acknowledgements
The authors thank: Dr. Ariella Shikanov (University of Michigan) and the laboratories of Drs. Lonnie Shea and Teresa Woodruff (Northwestern University) for instruction in fibrinogen preparation; veterinary clinics in Front Royal, Stephen’s City, and Harrisonburg, VA for donating domestic dog ovaries; Dr. Megan Brown (SCBI) for assisting with model selection statistical analyses; and Dr. Lara Mouttham and two anonymous contributors for thoughtful reviews of the manuscript.
Funding
This research was supported by a Smithsonian Institution Doctoral Fellowship (to J.N.), the Smithsonian Scholarly Studies Program, and by a grant from the National Institutes of Health KO1RR020564 (to N.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- [1].IUCN. IUCN Red list of threatened species 2017. p. < www.iucnredlist.org >. [DOI] [PMC free article] [PubMed]
- [2].Wildt DE, Panko WB, Chakraborty PK, Seager SWJ. Relationship of serum estrone, estradiol-17β and progesterone to LH, sexual behavior and time of ovulation in the bitch. Biol Reprod 1979;20:648–58. [DOI] [PubMed] [Google Scholar]
- [3].Olson PN, Bowen RA, Behrendt MD, Olson JD, Nett TM. Concentrations of reproductive hormones in canine serum throughout late anestrus, proestrus and estrus. Biol Reprod 1982;27:1196–206. [DOI] [PubMed] [Google Scholar]
- [4].Concannon PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci 2011;124:200–10. [DOI] [PubMed] [Google Scholar]
- [5].England GCW, Russo M, Freeman SL. Follicular dynamics, ovulation and conception rates in bitches. Reprod Domest Anim 2009;44:53–8. [DOI] [PubMed] [Google Scholar]
- [6].Concannon PW, McCann JP, Temple M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J Reprod Fertil Suppl 1989;39:3–25. [PubMed] [Google Scholar]
- [7].Chastant-Maillard S, Viaris de Lesegno C, Chebrout M, Thoumire S, Meylheuc T, Fontbonne A, et al. The canine oocyte: uncommon features of in vivo and in vitro maturation. Reprod Fertil Dev 2011;23:391–402. [DOI] [PubMed] [Google Scholar]
- [8].Reynaud K, Fontbonne A, Marseloo N, Thoumire S, Chebrout M, de Lesegno CV, et al. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005;130:193–201. [DOI] [PubMed] [Google Scholar]
- [9].Songsasen N, Wildt DE. Size of the donor follicle, but not stage of reproductive cycle or seasonality, influences meiotic competency of selected domestic dog oocytes. Mol Reprod Dev 2005;72:113–9. [DOI] [PubMed] [Google Scholar]
- [10].Luvoni GC, Chigioni S, Allievi E, Macis D. Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology 2005;63:41–59. [DOI] [PubMed] [Google Scholar]
- [11].No J, Zhao M, Lee S, Ock SA, Nam Y, Hur T-Y. Enhanced in vitro maturation of canine oocytes by oviduct epithelial cell co-culture. Theriogenology 2018;105:66–74. [DOI] [PubMed] [Google Scholar]
- [12].Salama A, Fathi M, Badr MR, Moawad AR. Carnitine supplementation during in vitro maturation improves developmental competence of canine oocytes. Reprod Fertil Dev 2018;30:203-. [Google Scholar]
- [13].Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006;27:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006;12:2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reynaud K, Fontbonne A, Marseloo N, de Lesegno CV, Saint-Dizier M, Chastant-Maillard S. In vivo canine oocyte maturation, fertilization and early embryogenesis: A review. Theriogenology 2006;66:1685–93. [DOI] [PubMed] [Google Scholar]
- [16].Durrant BS, Pratt NC, Russ KD, Bolamba D. Isolation and characterization of canine advanced preantral and early antral follicles. Theriogenology 1998;49:917–32. [DOI] [PubMed] [Google Scholar]
- [17].Guraya SS. A histochemical analysis of lipid yolk deposition in the oocytes of cat and dog. J Exp Zool 1965;160:123–35. [DOI] [PubMed] [Google Scholar]
- [18].Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001;233:258–70. [DOI] [PubMed] [Google Scholar]
- [19].Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 2007;305:300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eppig J Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001;122:829–38. [DOI] [PubMed] [Google Scholar]
- [21].del Collado M, da Silveira JC, Sangalli JR, Andrade GM, Sousa LRdS, Silva LA, et al. Fatty acid binding protein 3 and transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes. Scientific Reports 2017;7:2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Auclair S, Uzbekov R, Elis S, Sanchez L, Kireev I, Lardic L, et al. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am J Physiol Endocrinol Metab 2013;304:E599–E613. [DOI] [PubMed] [Google Scholar]
- [23].Zhao J, Taverne MAM, van der Weijden GC, Bevers MM, van den Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod 2001;65:967–77. [DOI] [PubMed] [Google Scholar]
- [24].Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008;23:1151–8. [DOI] [PubMed] [Google Scholar]
- [25].McLaughlin M, Bromfield J, Albertini D, Telfer E. Activin promotes follicular integrity and oogenesis in cultured pre-antral bovine follicles. Mol Hum Reprod 2010;16:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction 2010;139:971–8. [DOI] [PubMed] [Google Scholar]
- [27].Silva JRV, van den Hurk R, van Tol HTA, Roelen BAJ, Figueiredo JR. Gene expression and protein localisation for activin-A, follistatin and activin receptors in goat ovaries. J Endocrinol 2004;183:405–15. [DOI] [PubMed] [Google Scholar]
- [28].Van den Hurk R, Van de Pavert SA. Localization of an activin/activin receptor system in the porcine ovary. Mol Reprod Dev 2001;60:463–71. [DOI] [PubMed] [Google Scholar]
- [29].Schwall RH, Mason AJ, Wilcox JN, Bassett SG, Zeleznik AJ. Localization of inhibin/activin subunit mRNAs within the primate ovary. Mol Endocrinol 1990;4:75–9. [DOI] [PubMed] [Google Scholar]
- [30].Da Silva SJM, Bayne RAL, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ gell survival and proliferation before primordial follicle formation. Dev Biol 2004;266:334–45. [DOI] [PubMed] [Google Scholar]
- [31].Rabinovici J, Spencer SJ, Doldi N, Goldsmith PC, Schwall R, Jaffe RB. Activin-A as an intraovarian modulator: actions, localization, and regulation of the intact dimer in human ovarian cells. J Clin Invest 1992;89:1528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marino G, Zanghì A. Activins and inhibins: expression and role in normal and pathological canine reproductive organs: a review. Anatomia, Histologia, Embryologia 2013;42:1–8. [DOI] [PubMed] [Google Scholar]
- [33].Ethier J, Findlay J. Roles of activin and its signal transduction mechanisms in reproductive tissues. Reproduction 2001;121:667–75. [DOI] [PubMed] [Google Scholar]
- [34].Oktay K, Karlikaya G, Akman O, Ojakian GK, Oktay M. Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod 2000;63:457–61. [DOI] [PubMed] [Google Scholar]
- [35].Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 1996;137:1447–56. [DOI] [PubMed] [Google Scholar]
- [36].Pang Y, Ge W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol Reprod 1999;61:987–92. [DOI] [PubMed] [Google Scholar]
- [37].Xiao S, Robertson DM, Findlay JK. Effects of activin and follicle-stimulating hormone (FSH)-suppressing protein/follistatin on FSH receptors and differentiation of cultured rat granulosa cells. Endocrinology 1992;131:1009–16. [DOI] [PubMed] [Google Scholar]
- [38].Nakamura M, Minegishi T, Hasegawa Y, Nakamura K, Igarashi S, Ito I, et al. Effect of an activin A on follicle-stimulating hormone (FSH) receptor messenger ribonucleic acid levels and FSH receptor expressions in cultured rat granulosa cells. Endocrinology 1993;133:538–44. [DOI] [PubMed] [Google Scholar]
- [39].Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 2009;30:5476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril 2010;93:2633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles as influenced by the physical and hormonal microenvironment. Reproduction 2011;142:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007;28:4439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RML. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997;68:682–8. [DOI] [PubMed] [Google Scholar]
- [44].Songsasen N, Fickes A, Pukazhenthi BS, Wildt DE. Follicular morphology, oocyte diameter and localisation of fibroblast growth factors in the domestic dog ovary. Reprod Domest Anim 2009;44:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nagashima J, Wildt DE, Travis AJ, Songsasen N. Follicular size and stage and gonadotropin concentration affect alginate-encapsulated in vitro growth and survival of pre- and early antral dog follicles. Reprod Fertil Dev 2015. [DOI] [PubMed]
- [46].Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009;81:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Songsasen N, Fickes A, Pukazhenthi BS, Wildt DE. Follicular morphology, oocyte diameter and localisation of fibroblast growth factors in the domestic dog ovary. Reprod Domest Anim 2009;44:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod 2007;76:949–57. [DOI] [PubMed] [Google Scholar]
- [49].Sakamoto Y, Ishiguro M, Kitagawa G. Akaike information criterion statistics Dordrecht, The Netherlands: D Reidel; 1986. [Google Scholar]
- [50].Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 2001;29:e45-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Serafim MKB, Araújo VR, Silva GM, Duarte ABG, Almeida AP, Chaves RN, et al. Canine preantral follicles cultured with various concentrations of follicle-stimulating hormone (FSH). Theriogenology 2010;74:749–55. [DOI] [PubMed] [Google Scholar]
- [52].Serafim MK, Silva GM, Duarte AB, Araújo V, Silva T, Lima A, et al. High insulin concentrations promote the in vitro growth and viability of canine preantral follicles. Reprod Fertil Dev 2013;25:927–34. [DOI] [PubMed] [Google Scholar]
- [53].Serafim MKB, Duarte ABG, Silva GM, Souza CEA, Magalhães-Padilha DM, Moura AAA, et al. Impact of growth hormone (GH) and follicle stimulating hormone (FSH) on in vitro canine preantral follicle development and estradiol production. Growth Horm IGF Res 2015;25:85–9. [DOI] [PubMed] [Google Scholar]
- [54].Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three- dimensional culture. Hum Reprod 2013;28:2187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Silva AWB, Bezerra FTG, Costa JJN, Rossi RODS, Passos MJ, Vasconcelos GL, et al. Differential effects of activin-A and FSH on growth, viability and messenger RNA expression in cultured bovine preantral follicles. Livestock Science 2014;160:199–207. [Google Scholar]
- [56].McConnell NA, Yunus RS, Gross SA, Bost KL, Clemens MG, Hughes FM. Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology 2002;143:2905–12. [DOI] [PubMed] [Google Scholar]
- [57].Parrott JA, Skinner MK. Direct actions of lit-ligand on theca cell growth and differentiation during follicle development. Endocrinology 1997;138:3819–27. [DOI] [PubMed] [Google Scholar]
- [58].Mizunuma H, Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, et al. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology 1999;140:37–42. [DOI] [PubMed] [Google Scholar]
- [59].Cossigny DA, Findlay JK, Drummond AE. The effects of FSH and activin A on follicle development in vitro. Reproduction 2012;143:221–9. [DOI] [PubMed] [Google Scholar]
- [60].Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000;62:1322–8. [DOI] [PubMed] [Google Scholar]
- [61].Saraiva MVA, Celestino JJH, Araújo VR, Chaves RN, Almeida AP, Lima-Verde IB, et al. Expression of follicle-stimulating hormone receptor (FSHR) in goat ovarian follicles and the impact of sequential culture medium on in vitro development of caprine preantral follicles. Zygote 2011;19:205–14. [DOI] [PubMed] [Google Scholar]
- [62].Hovatta O, Wright C, Krausz T, Hardy K, Winston RML. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod 1999;14:2519–24. [DOI] [PubMed] [Google Scholar]
- [63].Viaris de Lesegno C, Reynaud K, Pechoux C, Chebrout M, Chastant-Maillard S. Ultrastructural evaluation of in vitro-matured canine oocytes. Reprod Fertil Dev 2008;20:626–39. [DOI] [PubMed] [Google Scholar]
- [64].de Lesegno CV, Reynaud K, Pechoux C, Thoumire S, Chastant-Maillard S. Ultrastructure of canine oocytes during in vivo maturation. Mol Reprod Dev: Incorporating Gamete Research 2008;75:115–25. [DOI] [PubMed] [Google Scholar]
- [65].Motta PM, MAKABE S, NAGURO T, CORRER S. Oocyte follicle cells association during development of human ovarian follicle. A study by high resolution scanning and transmission electron microscopy. Arch Histol Cytol 1994;57:369–94. [DOI] [PubMed] [Google Scholar]
- [66].Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008;14:159–77. [DOI] [PubMed] [Google Scholar]
- [67].El-Hayek S, Yang Q, Abbassi L, FitzHarris G, Clarke HJ. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol 2018;28:1124–31.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.