Abstract
Although epidemiologic studies of telomere length have become increasingly common, few population-based, multi-ethnic studies include data on telomere shortening, which may be a better predictor of morbidity and mortality than a single measure of telomere length. This study used stored blood samples from 1,169 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) to examine age, sex, race/ethnicity, marital status, income, and education as predictors of change in telomere length over a 10-year period in linear mixed effects models. Mean age at baseline was 61 years, and the sample was 54% female, 27% white, 30% African-American, and 43% Hispanic. At baseline, 58% of the sample was married; 32% had household income below $25,000 per year, 35% had income between $25,000 and $49,999 per year, and 34% had income above $50,000 per year; 41% had a high school education or less, 30% had some college, and 29% had a college degree or more. Relative telomere length (T/S ratio) was measured by the quantitative polymerase chain reaction method. In general, ten-year telomere attrition was greater for groups that had longer telomere length at baseline, including younger people, whites, and women. After adjusting for baseline telomere length, race/ethnic differences in telomere attrition were attenuated, and age and sex differences were reversed, such that older people and men showed greater telomere shortening. There were no significant differences in telomere attrition by marital status, income, or education. There is not yet a consensus in the field regarding whether to adjust for baseline telomere length in models examining predictors of telomere attrition. To ensure comparability across studies, researchers should report results both with and without adjustment for baseline telomere length.
Keywords: telomere attrition, age, sex, race/ethnicity, epidemiology, methods
Telomere attrition is a hallmark indicator of biological aging (Kennedy et al., 2014; Lopez-Otin et al., 2013), and leukocyte telomere length has been proposed as a biomarker of aging (Aubert and Lansdorp, 2008). Telomeres cap the ends of chromosomes and promote chromosomal stability (Blackburn et al., 2015). Due to the end replication problem, telomeres naturally shorten with mitosis (Blackburn, 2005). Oxidative damage and DNA replication stress also contribute to telomere loss (Blackburn et al., 2015; von Zglinicki, 2002). When telomeres become critically shortened, cellular senescence is triggered, and cells lose their ability to divide (Blackburn, 2000; Blasco, 2005). This end stage of cellular senescence, reached through replicative senescence and cell stress pathways, is implicated in the pathophysiology of biological aging and is thought to contribute to the development of the aging phenotype and age-related diseases (Campisi, 2005; Kennedy et al., 2014). A number of studies have shown that leukocyte telomere length is associated with morbidity (e.g., Haycock et al., 2014; Willeit et al., 2014) and mortality (e.g., Rode et al., 2015) independent of chronological age. Although relatively few studies have examined telomere attrition as a risk factor for health-related outcomes, there is some evidence that the rate of telomere shortening may be a better predictor of morbidity (Masi et al., 2014) and mortality (Duggan et al., 2014; Epel et al., 2009; Goglin et al., 2016) than a single measure of telomere length (for exceptions, see Toupance et al., 2017; Weischer et al., 2014). Thus, more work is needed to identify factors that increase the rate of telomere attrition.
The purpose of the current study was to use stored blood samples from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based cardiovascular cohort study, to examine sociodemographic predictors (age, sex, race/ethnicity, marital status, income, and education) of within-person change in telomere length over a 10-year period. This work builds on previous cross-sectional studies of telomere length in MESA, which found that younger people, women, and whites had longer telomere length than older people, men, and African-Americans and Hispanics (Diez Roux et al., 2009) and that income and education were not associated with telomere length (Carroll et al., 2013). Given the relative paucity of longitudinal studies in large, multi-ethnic US samples (for exceptions, see the Coronary Artery Risk Development in Young Adults Study, the Bogalusa Heart Study, the Atherosclerosis Risk in Communities Study, and the Baltimore Longitudinal Study of Aging), this and future studies using MESA data have the potential to fill an important gap in the telomere epidemiology literature.
DATA AND METHODS
Data
MESA is a population-based longitudinal study designed to identify risk factors for the progression of subclinical cardiovascular disease (CVD) (Bild et al., 2002). Between July 2000 and August 2002, 6,814 women and men aged 45-84 without clinically apparent CVD were recruited from six regions in the US. To date, participants have completed a baseline examination and four additional follow-up exams. Telomeres were assessed on a random subsample of 1,295 white, African-American, and Hispanic MESA participants from the New York, Los Angeles, and Baltimore sites who agreed to participate in an ancillary study examining the effects of stress on cardiovascular outcomes. Although data on telomere length at Exam 1 was already available for over half of our sample as part of a previous cross-sectional study (Diez Roux et al., 2009), we repeated the Exam 1 telomere assay for these participants in the same lab and at the same time as the Exam 5 telomere assay to ensure comparability of results. Stored blood samples from Exam 1 (July 2000-August 2002) and Exam 5 (April 2010-December 2011) were used to assess telomere length. The analytic sample excluded 126 respondents with missing data on one or more study variables (final n=1,169). At baseline, mean body mass index for the analytic sample was 29.09 (SD=5.42), mean systolic blood pressure was 124.38 (SD=20.35), mean diastolic blood pressure was 71.93 (SD=10.09), and 38.1% of the sample reported use of anti-hypertensive medications. Mean follow-up time between Exams 1 and 5 was 9.46 years (SD=0.46).
Measures
Leukocyte telomere length.
DNA (Exam 1 and Exam 5) was isolated at the Collaborative Studies Clinical Laboratory at University of Minnesota Medical Center, Fairview (Minneapolis, MN) from packed EDTA and citrate cells that were frozen at −70C. The DNA extraction and purification method used sodium dodecylsulfate cell lysis followed by a salt precipitation method for protein removal using commercial Puregene® reagents (Gentra System, Inc., Minneapolis, MN 55447). A mean yield of 39.1 μg DNA/mL packed cell was obtained, and DNA was of high quality (mean purity A260/280=1.77) and high molecular weight as determined by gel electrophoresis.
Telomere length assays were performed in the Blackburn Lab at the University of California, San Francisco, using the quantitative polymerase chain reaction (PCR) method to measure telomere length relative to the single-copy gene (human beta-globulin) (T/S ratio), as described in detail elsewhere (Cawthon, 2002; Lin et al., 2009). DNA samples were coded and the lab was blinded to all other measurements in the study. Each sample was assayed 3 times on 3 different days. The samples were assayed on duplicate wells, resulting in 6 data points. Sample plates were assayed in groups of three plates, and no two plates were grouped together more than once. Each assay plate contained 96 control wells, which included 8 standard curves using 3-fold serial dilution of 8 control DNA samples from various cancer cell lines for normalizing batch to batch variations. Any assay run with 8 or more invalid control wells was considered a failed run and was excluded from further analysis (100% of runs passed this criterion). The mean of the T/S values was calculated, and the largest or the smallest T/S value in the set (whichever deviated most from the mean) was marked as a potential outlier. Then the mean of the T/S value was calculated without the potential outlier. If the absolute value of the log of the ratio between the recalculated mean (excluding the potential outlier) to the value of the potential outlier was greater than 0.4, then the value was marked as an outlier (99.8% of all samples contained no outliers). The average inter-assay coefficient of variation was 2.9%.
Sociodemographic characteristics.
Age (in years), sex (1=male, 0=female), race (dummy variables for African-American and Hispanic, with white as the reference category), marital status (1=married, 0=not married), annual household income (in $10,000s), and education (dummy variables for high school or less and some college, with college degree or more as the reference category) were self-reported at the baseline exam.
Analysis Plan
First, we conducted descriptive analyses to examine the average telomere length at each exam and the average within-person change in telomere length between Exams 1 and 5 for the full sample and by age, sex, race/ethnicity, marital status, income, and education. Next, we used a linear mixed effects model with time-varying covariates that were centered to person-level means (hereafter referred to as the hybrid model) (Allison, 2005) to estimate associations between person-level factors and the rate of within-person change in telomere length between Exams 1 and 5, controlling for study site. In this analysis, time since baseline was the only time-varying covariate. The hybrid model accounts for correlations between telomere samples at baseline and the follow-up exam for the same person via the inclusion of an individual-level random intercept. Robust standard errors were used to account for possible misspecification of the covariance structure among repeated observations. The linear mixed effects model (hybrid model) is shown in the appendix.
In a second step, we added an interaction term between Exam 1 telomere length and time since baseline to the model described above in order to obtain baseline-adjusted estimates of change. Previous studies have found a strong correlation between baseline telomere length and telomere attrition (Aviv et al., 2009; Nordfjall et al., 2009; Revesz et al., 2016; Revesz et al., 2015; Weischer et al., 2014). While this correlation could be a statistical artifact resulting from measurement error in the telomere length assay (i.e., regression to the mean), biological evidence from non-human species suggests that individuals lose more base pairs from their longer telomeres than from their shorter telomeres (Bauch et al., 2014; Salomons et al., 2009); and prior research in humans has shown that telomere attrition increases with increasing baseline telomere length even after correcting for regression to the mean (Verhulst et al., 2013). In accordance with the recommendation that studies examining change in telomere length adjust for baseline telomere length in order to increase statistical power by reducing residual variation (Verhulst et al., 2013), it has become increasingly common for studies of telomere attrition to control for baseline telomere length (Biegler et al., 2012; Huzen et al., 2014; van Ockenburg et al., 2015). It should be noted, however, that the research question being addressed in a baseline-adjusted model (i.e., Holding baseline telomere length constant, what is the association between a predictor and change in telomere length?) differs from that being addressed in an unadjusted model (i.e., What is the association between a predictor and change in telomere length?).
RESULTS
Means for the Exam 1 and Exam 5 measures of telomere length (T/S ratio), as well as 10-year telomere attrition, are shown in Table 1. In the full sample, mean telomere length at Exam 1 was 0.92 (SD=0.20); mean telomere length at Exam 5 was 0.71 (SD=0.14); and mean telomere attrition between exams was 0.22 (SD=0.19) units. Younger respondents had longer telomere length than older respondents at both exams, but 10-year telomere attrition was not significantly different by age. Similarly, women had longer telomere length than men at Exams 1 and 5, but telomere attrition was not significantly different. Next, whites had longer telomere length than African-Americans and Hispanics at Exam 1, but mean telomere length did not differ by race/ethnicity at Exam 5 because whites experienced greater telomere attrition than African-Americans and Hispanics. Married respondents had longer telomere length than unmarried respondents at both exams, but telomere attrition was not significantly different. Respondents with greater household income had longer telomere length at Exam 5, but income was not associated with baseline telomere length or 10-year telomere attrition. Finally, education was not associated with telomere length at either exam or with telomere attrition. In the full sample, 83.4% of respondents experienced at least a 5% decrease in telomere length between Exams 1 and 5; 6.6% experienced at least a 5% increase in telomere length; and the remaining 10% experienced less than a 5% change in telomere length. The distributions of the telomere data at Exams 1 and 5 are shown in Figure S1.
Table 1.
Mean Telomere Length (T/S Ratio) and 10-year Telomere Attrition for the Full Sample and by Sociodemographic Characteristics
| Exam 1 | Exam 5 | 10-year attrition | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | p-value | Mean (SD) | p-value | Mean (SD) | p-value | |
| Full sample | 1,169 | 0.92 (0.20) | 0.71 (0.14) | −0.22 (0.19) | |||
| By Exam 1 age | |||||||
| 45-54 | 362 | 0.98 (0.21) | <0.0001 | 0.76 (0.15) | <0.0001 | −0.23 (0.20) | 0.18 |
| 55-64 | 369 | 0.93 (0.19) | 0.71 (0.13) | −0.23 (0.19) | |||
| 65-74 | 331 | 0.86 (0.19) | 0.67 (0.14) | −0.20 (0.19) | |||
| 75-84 | 107 | 0.81 (0.18) | 0.63 (0.10) | −0.20 (0.17) | |||
| By sex | |||||||
| Female | 628 | 0.94 (0.20) | <0.0001 | 0.72 (0.15) | 0.0007 | −0.23 (0.18) | 0.12 |
| Male | 541 | 0.89 (0.20) | 0.69 (0.14) | −0.21 (0.20) | |||
| By race/ethnicity | |||||||
| White | 315 | 0.94 (0.21) | 0.03 | 0.71 (0.14) | 0.14 | −0.24 (0.20) | 0.0009 |
| African-American | 345 | 0.89 (0.20) | 0.72 (0.15) | −0.19 (0.19) | |||
| Hispanic | 509 | 0.92 (0.21) | 0.70 (0.14) | −0.23 (0.19) | |||
| By Exam 1 marital status | |||||||
| Married | 680 | 0.93 (0.20) | 0.0003 | 0.72 (0.15) | 0.01 | −0.23 (0.20) | 0.07 |
| Not married | 489 | 0.89 (0.20) | 0.69 (0.14) | −0.21 (0.19) | |||
| By Exam 1 household income | |||||||
| $0-$24,999 | 370 | 0.90 (0.21) | 0.28 | 0.69 (0.13) | 0.004 | −0.23 (0.19) | 0.59 |
| $25,000-$49,999 | 404 | 0.92 (0.21) | 0.71 (0.14) | −0.22 (0.20) | |||
| $50,000+ | 395 | 0.93 (0.20) | 0.72 (0.15) | −0.21 (0.19) | |||
| By educational attainment | |||||||
| High school or less | 478 | 0.92 (0.21) | 0.41 | 0.70 (0.15) | 0.27 | −0.23 (0.19) | 0.14 |
| Some college | 355 | 0.93 (0.20) | 0.71 (0.14) | −0.22 (0.20) | |||
| College or more | 336 | 0.90 (0.19) | 0.71 (0.14) | −0.20 (0.18) | |||
Notes: 10-year attrition = 10*(Exam 5 TL – Exam 1 TL)/(Exam 5 visit date – Exam 1 visit date). P-value based on t-test for variables with two categories and one-way ANOVA for variables with more than two categories. SD=standard deviation.
Results of the multivariable hybrid models are presented in Table 2. The estimate for follow-up time between exams was −0.22 (SE=0.01; p<0.0001), which indicates that the average within person change in telomere length over the 10-year study period was a reduction of 0.22 units. As shown in Model 1 of Table 2, younger people, women, and whites experienced greater telomere attrition than older people, men, and African-Americans and Hispanics. For every 10-years of higher age at Exam 1, 10-year telomere attrition was 0.01 units lower (est.=0.01; SE=0.01; p=0.02). Telomere attrition was 0.02 units lower for men compared to women (est.=0.02; SE=0.01, p=0.03). Compared to whites, 10-year telomere attrition was 0.05 units lower for African-Americans (est.=0.05; SE=0.02; p=0.0001) and 0.04 units lower for Hispanics (est.=0.04; SE=0.02; p=0.04). Telomere attrition was not significantly different for African-Americans compared to Hispanics (results not shown). Finally, there were no significant differences in telomere attrition by marital status, income, or education. With the exception of marital status, these results suggest that telomere attrition was greater for groups that had longer baseline telomere length.
Table 2.
Estimated Change in 10-year Telomere Attrition by Sociodemographic Characteristics from the Linear Mixed Effects (Hybrid) Model with a Random Intercept and Robust Standard Errors (n=1,169)
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Interpretation | Est. | SE | p-value | Est. | SE | p-value |
| Intercept | Average TL over two exams | 0.81 | 0.00 | <0.0001 | 0.81 | 0.00 | <0.0001 |
| Time | Average 10-year telomere attrition | −0.22 | 0.01 | <0.0001 | −0.22 | 0.00 | <0.0001 |
| Time × Exam 1 Age | Difference in 10-year telomere attrition per 10-year higher Exam 1 age | 0.01 | 0.01 | 0.02 | −0.03 | 0.00 | <0.0001 |
| Time × Male | Difference in 10-year telomere attrition for males compared to females | 0.02 | 0.01 | 0.03 | −0.02 | 0.01 | 0.02 |
| Time × African-American | Difference in 10-year telomere attrition for African-Americans compared to whites | 0.05 | 0.02 | 0.003 | 0.02 | 0.01 | 0.15 |
| Time × Hispanic | Difference in 10-year telomere attrition for Hispanics compared to whites | 0.04 | 0.02 | 0.04 | −0.01 | 0.01 | 0.32 |
| Time × Married at Exam 1 | Difference in 10-year telomere attrition for married respondents compared to unmarried respondents | −0.01 | 0.01 | 0.52 | 0.01 | 0.01 | 0.10 |
| Time × Exam 1 Household Income | Difference in 10-year telomere attrition per $10,000 higher Exam 1 household income | 0.00 | 0.00 | 0.73 | 0.00 | 0.00 | 0.68 |
| Time × High School | Difference in 10-year telomere attrition for respondents with high school or less compared to college or more | −0.02 | 0.02 | 0.20 | 0.00 | 0.01 | 0.66 |
| Time × Some College | Difference in 10-year telomere attrition for respondents with some college compared to college or more | −0.02 | 0.02 | 0.19 | −0.01 | 0.01 | 0.48 |
| Time × Baseline TL | Difference in 10-year telomere attrition per 1-unit increase in baseline TL | --- | --- | --- | −0.70 | 0.02 | <0.0001 |
Notes: Follow-up time was centered to the individual’s average follow-up time and is presented on a 10-year scale. Demographic variables and Exam 1 telomere length were centered to the population mean. A negative coefficient for an interaction with time indicates greater 10-year telomere attrition. All models control for study site. Model 1 does not adjust for baseline telomere length, while Model 2 includes baseline telomere length as a covariate. Est.=estimate; SE=standard error; TL=telomere length (T/S ratio).
As shown in Model 2 of Table 2, we found that longer baseline telomere length was associated with greater 10-year telomere attrition. For every one unit increase in baseline telomere length, telomere attrition was 0.70 units greater (est.=−0.70; SE=0.02; p<0.0001). Because we were unable to correct for regression to the mean in the hybrid models, the estimate for baseline telomere length is likely inflated and should be interpreted with caution (see Verhulst et al., 2013). Next, we found that older people and men experienced greater telomere attrition than younger people and women in the baseline-adjusted model. Notably, the results for age and sex in Model 2 are in the opposite direction of those in Model 1. For every 10-years of higher age at Exam 1, 10-year telomere attrition was 0.03 units greater (est.=−0.03; SE=0.00; p<0.0001). Telomere attrition was 0.02 units greater for men compared to women (est=−0.02; SE=0.01, p=0.02). Race/ethnic differences in telomere attrition were no longer significant after adjusting for baseline telomere length, and there were no significant differences in telomere attrition by marital status, income, or education in baseline-adjusted models.
DISCUSSION
While research on telomere length has become increasingly common in the epidemiologic literature, most previous studies have examined cross-sectional data. However, several recent studies suggest that the rate of telomere attrition may be a better predictor of long-term health outcomes than a single measure of telomere length (Duggan et al., 2014; Epel et al., 2009; Goglin et al., 2016; Masi et al., 2014). Thus, we obtained repeat measures of telomere length for over 1,000 participants in MESA in order to facilitate research on the antecedents and health-related consequences of telomere shortening in a large, multi-ethnic, population-based sample. The purpose of the current study was to examine age, sex, race/ethnicity, marital status, income, and education as predictors of within-person change in telomere length over a 10-year period.
In a multivariable model not adjusted for baseline telomere length, we found that telomere attrition was greater for younger people, women, and whites compared to older people, men, and African-Americans and Hispanics. Consistent with other prior longitudinal studies (see, for example, Aviv et al., 2009; Nordfjall et al., 2009; Revesz et al., 2016; Revesz et al., 2015; Weischer et al., 2014), we found that baseline telomere length was a strong predictor of telomere attrition. After adjusting for baseline, race/ethnic differences in telomere attrition were no longer significant. Furthermore, when baseline telomere length was held constant, older people and men were found to experience greater attrition than younger people and women.
Currently, there is not a consensus in the literature regarding whether to adjust for baseline telomere length in models examining predictors of telomere attrition. If baseline is a true confounder (i.e., it is associated with the exposure and is a predictor of attrition but is not a mediator), then adjustment for baseline may be warranted, although it is known that measurement error can introduce important bias in baseline-adjusted models (Glymour et al., 2005; Yanez et al., 1998). However, if baseline telomere length is hypothesized to be in the causal pathway from the exposure to telomere attrition, then regression coefficients from baseline-adjusted models will provide estimates of the direct effect, rather than the total effect, of the exposure on change in telomere length. In order to provide an unbiased estimate of the direct effect, it is necessary to adjust for potential confounders of the mediator-outcome association, which in this case includes any potential confounders of the association between baseline telomere length and telomere attrition. Failure to adjust for confounders of the mediator-outcome association could lead to overestimation of the indirect effect and underestimation of the direct effect (see http://davidakenny.net/cm/mediate.htm). To the extent that age, sex, and race/ethnicity are either causally related to telomere length themselves or are proxies for factors, such as cell division history, sex steroid hormone concentrations, or psychosocial stress exposure, that are causally related to telomere length, then baseline-adjusted models that fail to adjust for confounders of the baseline-attrition association may produce downwardly biased estimates of the direct effects of these sociodemographic characteristics on telomere attrition.
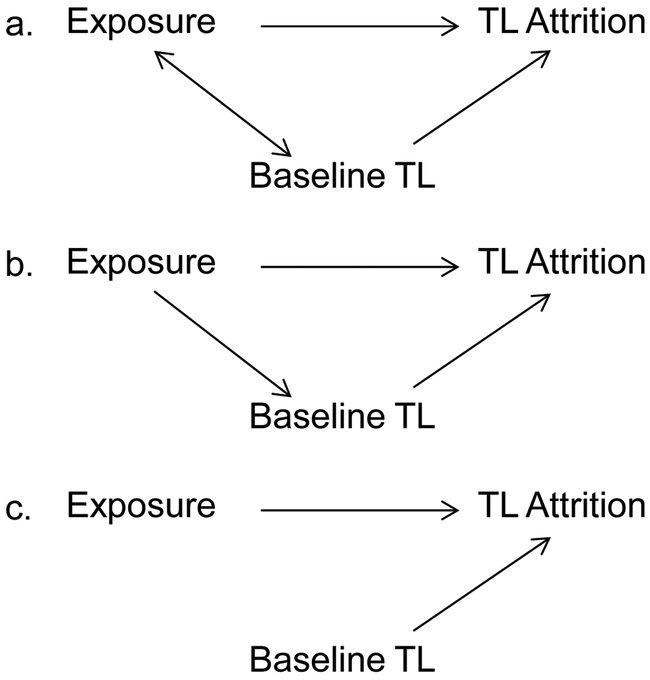
While there is compelling biological evidence that the rate of telomere attrition depends on baseline telomere length (Aviv et al., 2009; Verhulst et al., 2013), researchers must carefully consider the implications of adjusting for baseline telomere length when interpreting the results of baseline-adjusted models, particularly when there is measurement error (Glymour et al., 2005), which is known to exist for measures of relative telomere length, or when baseline telomere length is an intermediate. The choice of whether or not to adjust for baseline should be based on the specific research question under study, and researchers should clearly state whether they believe that baseline telomere length is a confounder, a mediator, or neither (Schisterman et al., 2009) (see Figure 1). For example, if we conceptualize baseline telomere length as a confounder of the association between race/ethnicity and telomere attrition, then the baseline-adjusted model provides a better estimate of the causal effect of race/ethnicity (or factors closely associated with race/ethnicity, such as discrimination) on telomere attrition, assuming no residual or unmeasured confounding. In this case, attenuation of the coefficient for race/ethnicity after adjusting for baseline is interpreted as evidence of confounding. (An added complexity in this case is regression to the mean, which, as previously discussed, will may result in an artifactual association of longer baseline telomere length with faster attrition). However, if we conceptualize baseline telomere length as a mediator of the association between race/ethnicity and telomere attrition, then the baseline-adjusted model could result in an underestimate of the causal effect of race/ethnicity (or factors associated with race/ethnicity) on telomere attrition. This is because baseline levels may capture prior effects of race/ethnicity on attrition, so by adjusting for it we are potentially adjusting away a major component of the causal effect we are trying to estimate. On one hand we need to control for any confounding effects of baseline telomere length (or artifactual associations resulting from regression to the mean), but on the other hand, controlling for baseline may adjust away effects of prior history on attrition, making it difficult to isolate a causal effect over the relatively short 10-year period we have available to study. If researchers choose to present baseline-adjusted models, we recommend that they also present unadjusted models in order to ensure comparability of results across studies. As demonstrated here, adjustment for baseline telomere length can lead to changes in the directionality of associations, producing very different conclusions regarding which groups experience greater telomere attrition.
Figure 1.
Graphical representation of baseline telomere length as a confounder (a), a mediator (b), or neither (c) in the analysis of telomere attrition
Notes: TL=telomere length.
Strengths and Limitations
A strength of this study was the use of a large, racially and ethnically diverse, population-based sample, which enhances generalizability. While some previous longitudinal studies have included African-Americans (Aviv et al., 2009; Rewak et al., 2014; Sanders et al., 2015), most prior research on telomere attrition has focused on samples of European descent (Bendix et al., 2014; Dalgard et al., 2015; Ehrlenbach et al., 2009; Shalev et al., 2014; van Ockenburg et al., 2015). To our knowledge, this was the first study to examine change in telomere length among Hispanics.
Other strengths of this study include a low average inter-assay coefficient of variation (2.9%) for the telomere length assay and the relatively long period of time – approximately 10 years – between the first and second telomere measurements. Some research suggests that change in telomere length observed in studies conducted over shorter time periods may reflect measurement error rather than true change (Chen et al., 2011; Steenstrup et al., 2013). While we cannot rule out the possibility that measurement error accounts for the finding that 6.6% of our sample experienced at least a 5% increase in telomere length, a recent simulation study suggests that scenarios in which true lengthening occurs provides a better fit for the empirical data than scenarios in which no lengthening occurs (Bateson and Nettle, 2017), and prior experimental studies suggest that non-technical factors, such as weight loss (Carulli et al., 2016; Garcia-Calzon et al., 2014), may contribute to telomere lengthening.
Finally, a key strength of this study was the use of multivariable hybrid models, which estimated the effect of aging (via the measure of follow-up time) using only within-person variability in telomere length across exams. Furthermore, the models accounted for any observed and unobserved time-invariant confounders, such as genetic factors, which may be important determinants of both telomere length and telomere attrition. Despite these advantages, we were unable to correct for regression to the mean using Verhulst’s (2013) formula in the hybrid models, which means that the estimate for baseline telomere length is likely inflated. For studies in which the primary goal is to estimate the effect of baseline telomere length on telomere attrition, the hybrid model may not be appropriate.
This study also had some limitations. Because we only had two measures of telomere length, we were unable to examine non-linearity in trajectories of change. Ongoing data collection efforts may provide additional biological specimens for MESA study participants, making it possible to obtain more data on telomere length in the future. Previous research has shown that relative telomere rankings, as measured by deciles, are generally fixed in mid- to late-life (Benetos et al., 2013). This is likely due to the fact that telomere dynamics are largely determined by telomere length at birth and the rate of attrition during childhood and adolescence (Benetos et al., 2013). Thus, the age of the MESA cohort (45-84 at baseline) is a limitation of this study, and more work is needed to identify the determinants of telomere length and telomere attrition in early life.
Next, given that all MESA participants were in mid- to late-life and free from CVD at baseline, a second limitation is that our sample represented an unusually healthy group – particularly the older men and older racial/ethnic minorities. Unobserved factors, which could be related to telomere length and telomere attrition, may have conferred a survival advantage to those who were included in the study. Future studies could avoid this form of selection bias by focusing on younger, randomly selected samples. The inclusion of unusually healthy older African-Americans in MESA may explain why the results of this study differ from other multi-ethnic studies, which have found that African-Americans tend to have longer telomere length than whites (Hunt et al., 2008; Needham et al., 2013), as well as greater telomere attrition (Chen et al., 2011; Rewak et al., 2014).
A third limitation is that the measure of relative telomere length used in this study is an average of telomere length across all leukocyte cell types, including neutrophils, eosinophils, basophils, lymphocytes, and monocytes. Previous research has shown that, within the same individual, telomere length in different cell types varies (Lin et al., 2010). Thus, changes in telomere length observed in this study may be due to changes in the composition of leukocyte cell subpopulations. Future longitudinal studies should either measure telomere length in a single cell type, such as monocytes, or control for changes in white blood cell composition (which we were unable to do in the current study because these measures were not available for the MESA Exam 1 data).
Finally, a number of sample handling procedures, such as variability in blood preservative and time between collection and freezer storage, could have introduced error in estimates of telomere length. Future research that examines these factors is needed to determine the relative contribution of each on estimates of telomere length.
Conclusions
In a population-based sample of adults aged 45-84 at baseline, groups that started out with longer telomere length, including younger people, women, and whites, experienced greater telomere attrition over a 10-year period than those who started out with shorter telomere length. After adjusting for baseline telomere length, race/ethnic differences in telomere attrition were no longer significant, and age and sex differences were reversed. These results highlight the significance of adjusting for baseline telomere length when drawing conclusions about predictors of telomere attrition. Although controlling for baseline telomere length has become increasingly common in the telomere epidemiology literature, researchers should carefully consider the pros and cons of baseline-adjustment, particularly when telomere length is measured with error (as indicated by a high CV) or when baseline telomere length is thought to be an intermediate between an exposure and telomere attrition. To ensure comparability across studies and to enhance consistency in the field of telomere epidemiology, we strongly recommend that researchers report results both with and without adjustment for baseline telomere length since results may be very different, as shown here. Future studies in MESA should examine telomere dynamics in relation to health-related outcomes, including cardiovascular events and mortality. This type of work could contribute to the emerging literature on the potential clinical utility of monitoring the rate of telomere attrition over time.
Supplementary Material
HIGHLIGHTS.
This study examined age, sex, race/ethnicity, marital status, income, and education as predictors of 10-year telomere attrition in a multi-ethnic population-based cardiovascular cohort
With the exception of marital status, ten-year telomere attrition was greater for groups that had longer telomere length at baseline, including younger people, whites, and women
After adjusting for baseline telomere length, race/ethnic differences in telomere attrition were no longer significant, and age and sex differences were reversed
Researchers should carefully consider the pros and cons of baseline-adjustment in studies examining predictors of telomere attrition
ACKNOWLEDGEMENTS
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The MESA Stress Study was supported by R01HL076831 and R01HL101161 (PI: Ana Diez Roux). Work was also partially supported by funding from the UCLA Older Americans Independence Center, NIH/NIA Grant P30AG028748, and the USC/UCLA Biodemography Center through a P30 grant from the NIA (P30AG017265).
Appendix. The linear mixed effects model (hybrid model)
A key advantage of the hybrid model is that it allows estimation of associations of within-person changes in predictors with within-person changes in the outcome, tightly controlling for observed and unobserved time-invariant characteristics. Further, the hybrid model is able to examine the effect of baseline factors (e.g., age, sex, and race/ethnicity) on the rate of within-person change in telomere length between Exams 1 and 5 through the inclusion of interaction terms between time since baseline and time-invariant sociodemographic variables. The hybrid model estimates the difference in measures more efficiently than the simple change score method, particularly when the variances of the two repeated measures differ (Diggle et al., 2002) because the hybrid model separately models the variance of each repeated measure, and the difference between exams is weighted to account for the reliability of each measure based on their respective variances. The model is shown below:
yij: Telomere length for individual i at Exam j.
: Time (years) since baseline exam (Exam 1) for individual i at Exam j. is centered to individual i’s average follow-up time: where Timeij is the follow-up years and is the average follow-up years for individual i.
β0: Model intercept representing the population mean for telomere length over two exams.
bi0: Individual-level random intercept representing individual specific deviation from the population mean on telomere length over two exams.
β1: Average annual change in telomere length in the population.
β2: Baseline age effect on annual change in telomere length.
β3: Difference in telomere length annual change between men and women.
β4: Difference in telomere length annual change between African American and white.
β5: Difference in telomere length annual change between Hispanic and white.
ϵij: Unexplained random error term.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- Allison PD, 2005. Fixed effects regression methods for longitudinal data using SAS. SAS Institute, Cary, NC. [Google Scholar]
- Aubert G, Lansdorp PM, 2008. Telomeres and aging. Physiological reviews 88, 557–579. [DOI] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, 2009. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. American journal of epidemiology 169, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M, Nettle D, 2017. The telomere lengthening conundrum - it could be biology. Aging Cell 16, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauch C, Becker PH, Verhulst S, 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol Ecol 23, 300–310. [DOI] [PubMed] [Google Scholar]
- Bendix L, Thinggaard M, Fenger M, Kolvraa S, Avlund K, Linneberg A, Osler M, 2014. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol A Biol Sci Med Sci 69, 231–239. [DOI] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A, 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL, 2012. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer prevention research; (Philadelphia, Pa: ) 5, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP, 2002. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology 156, 871–881. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, 2000. Telomere states and cell fates. Nature 408, 53–56. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. Febs Lett 579, 859–862. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J, 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Blasco MA, 2005. Telomeres and human disease: ageing, cancer and beyond. Nature reviews. Genetics 6, 611–622. [DOI] [PubMed] [Google Scholar]
- Campisi J, 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Diez-Roux AV, Adler NE, Seeman TE, 2013. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA). Brain, behavior, and immunity 28, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli L, Anzivino C, Baldelli E, Zenobii MF, Rocchi MB, Bertolotti M, 2016. Telomere length elongation after weight loss intervention in obese adults. Molecular genetics and metabolism 118, 138–142. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, 2002. Telomere measurement by quantitative PCR. Nucleic Acids Res 30, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A, 2011. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci 66, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgard C, Benetos A, Verhulst S, Labat C, Kark JD, Christensen K, Kimura M, Kyvik KO, Aviv A, 2015. Leukocyte telomere length dynamics in women and men: menopause vs age effects. International journal of epidemiology 44, 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T, 2009. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 8, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P, Liang K, Zeger S, 2002. Analysis of Longitudinal Data. Oxford Press, New York. [Google Scholar]
- Duggan C, Risques R, Alfano C, Prunkard D, Imayama I, Holte S, Baumgartner K, Baumgartner R, Bernstein L, Ballard-Barbash R, Rabinovitch P, McTiernan A, 2014. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J Natl Cancer Inst 106, dju035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A, 2009. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. International journal of epidemiology 38, 1725–1734. [DOI] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE, 2009. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging-Us 1, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calzon S, Moleres A, Marcos A, Campoy C, Moreno LA, Azcona-Sanjulian MC, Martinez-Gonzalez MA, Martinez JA, Zalba G, Marti A, Group ES, 2014. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PloS one 9, e89828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM, 2005. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American journal of epidemiology 162, 267–278. [DOI] [PubMed] [Google Scholar]
- Goglin SE, Farzaneh-Far R, Epel ES, Lin J, Blackburn EH, Whooley MA, 2016. Change in Leukocyte Telomere Length Predicts Mortality in Patients with Stable Coronary Heart Disease from the Heart and Soul Study. PloS one 11, e0160748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P, 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. Bmj 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Chen W, Gardner J, Kimura M, Srinivasan S, Eckfeldt J, Berenson G, Aviv A, 2008. Leukocyte telomeres are longer in African Americans than in whites: The NHLBI Family Heart Study and the Bogalusa Heart Study. Aging Cell Postprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzen J, Wong LS, van Veldhuisen DJ, Samani NJ, Zwinderman AH, Codd V, Cawthon RM, Benus GF, van der Horst IC, Navis G, Bakker SJ, Gansevoort RT, de Jong PE, Hillege HL, van Gilst WH, de Boer RA, van der Harst P, 2014. Telomere length loss due to smoking and metabolic traits. J Intern Med 275, 155–163. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, 2014. Geroscience: linking aging to chronic disease. Cell 159, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E, 2009. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods 352, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E, 2010. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. Journal of immunological methods 352, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi S, D'Aiuto F, Martin-Ruiz C, Kahn T, Wong A, Ghosh AK, Whincup P, Kuh D, Hughes A, von Zglinicki T, Hardy R, Deanfield JE, scientific, N., data collection, t., 2014. Rate of telomere shortening and cardiovascular damage: a longitudinal study in the 1946 British Birth Cohort. European heart journal 35, 3296–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, Epel ES, 2013. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Social science & medicine (1982) 85, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G, 2009. The individual blood cell telomere attrition rate is telomere length dependent. PLoS genetics 5, e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz D, Milaneschi Y, Terpstra EM, Penninx BW, 2016. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology 67, 153–162. [DOI] [PubMed] [Google Scholar]
- Revesz D, Milaneschi Y, Verhoeven JE, Lin J, Penninx BW, 2015. Longitudinal Associations Between Metabolic Syndrome Components and Telomere Shortening. The Journal of clinical endocrinology and metabolism 100, 3050–3059. [DOI] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, Non AL, Kubzansky LD, 2014. Race-related health disparities and biological aging: does rate of telomere shortening differ across blacks and whites? Biological psychology 99, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, Bojesen SE, 2015. Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst 107, djv074. [DOI] [PubMed] [Google Scholar]
- Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MH, Verhulst S, 2009. Telomere shortening and survival in free-living corvids. Proceedings. Biological sciences 276, 3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AE, Divaris K, Naorungroj S, Heiss G, Risques RA, 2015. Telomere length attrition and chronic periodontitis: an ARIC Study nested case-control study. Journal of clinical periodontology 42, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW, 2009. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass.) 20, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A, 2014. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Molecular psychiatry 19, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A, 2013. The telomere lengthening conundrum--artifact or biology? Nucleic acids research 41, e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toupance S, Labat C, Temmar M, Rossignol P, Kimura M, Aviv A, Benetos A, 2017. Short Telomeres, but Not Telomere Attrition Rates, Are Associated With Carotid Atherosclerosis. Hypertension 70, 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ockenburg SL, Bos EH, de Jonge P, van der Harst P, Gans RO, Rosmalen JG, 2015. Stressful life events and leukocyte telomere attrition in adulthood: a prospective population-based cohort study. Psychological medicine 45, 2975–2984. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD, 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for 'regression to the mean'. European journal of epidemiology 28, 859–866. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, 2002. Oxidative stress shortens telomeres. Trends in biochemical sciences 27, 339–344. [DOI] [PubMed] [Google Scholar]
- Weischer M, Bojesen SE, Nordestgaard BG, 2014. Telomere shortening unrelated to smoking, body weight, physical activity, and alcohol intake: 4,576 general population individuals with repeat measurements 10 years apart. PLoS genetics 10, e1004191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Raschenberger J, Heydon EE, Tsimikas S, Haun M, Mayr A, Weger S, Witztum JL, Butterworth AS, Willeit J, Kronenberg F, Kiechl S, 2014. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PloS one 9, e112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez ND 3rd, Kronmal RA, Shemanski LR, 1998. The effects of measurement error in response variables and tests of association of explanatory variables in change models. Stat Med 17, 2597–2606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.