Abstract
Eusocial insects live in societies in which distinct family members serve specific roles in maintaining the colony and advancing the reproductive ability of a few select individuals. Given the genetic similarity of all colony members, the diversity of morphologies and behaviors is surprising. Social communication relies on pheromones and olfaction, as shown by mutants of orco, the universal odorant receptor coreceptor, and through electrophysiological analysis of neuronal responses to pheromones. Additionally, neurohormonal factors and epigenetic regulators play a key role in caste-specific behavior, such as foraging and caste switching. These studies start to allow an understanding of the molecular mechanisms underlying social behavior and provide a technological foundation for future studies of eusocial insects. In this review, we highlight recent findings in eusocial insects that advance our understanding of genetic and epigenetic regulations of social behavior and provide perspectives on future studies using cutting-edge technologies.
Keywords: social behavior, neuroplasticity, caste development, odorant receptor, critical periods, epigenetics
1. INTRODUCTION
Many animals live in cooperative societies that confer specific advantages in increasing group fitness. While these animal societies maintain a traditional reliance on individual reproductive success, eusocial insects live in colonies, where the fitness of the colony greatly outweighs individual fitness. Individuals in a colony are divided into castes, which exhibit unique and complex caste-specific behaviors. The vast majority of individuals in a colony is reproductively inactive and serves one or a few fertile female queen(s). Although all workers in a colony are closely related siblings, they have widely different behaviors that are adaptive and responsive to changing circumstances.
Colonies can be viewed as superorganisms (49). Each caste fulfills a specific role that contributes to the functioning of the whole colony but cannot survive on its own. The queen is the “ovary” of the colony, as the only (or one of very few) reproductively active member(s) of the colony. Nurses are reproductive accessories, feeding and caring for the queen, larvae, and pupae. Foragers are the “muscle” and fulfill multiple functions, including defending the colony and gathering food. Stable colonies have a strictly maintained social distribution of roles. However, individuals in several eusocial species have the remarkable ability to switch between castes, and in exceptional cases, this caste transition can extend the life span of the colony past the death of the queen or other members of the colony. When various castes act in a coordinated manner, the result is a wildly successful species, which is showcased by the often-quoted fact that the biomass of all ants on Earth is equivalent to that of humans (48).
Multicellular organisms with disrupted intercellular communication suffer severe consequences owing to a lack of coordination between cells and organs (12). Similarly, intracolony communication is paramount for coordination of actions and resource sharing. Ants have two main routes of communication: chemosensory and tactile. Chemosensory communication is the most essential, as it dictates caste status and colony affiliation (138). As such, it has long been assumed that olfaction plays an important role in determining social deference and dominance (72, 118). Accordingly, given the reliance of communication on chemical signals, the number of odorant receptors (ORs) in ants is dramatically expanded compared with that of solitary insects such as fruit flies and mosquitoes, indicating the considerable importance of the olfactory system in eusocial insects (147, 148). Recently, functional analysis of the universal odorant receptor coreceptor (Orco) was performed in ants to test the role of their chemosensory system, providing major clues into the mechanisms by which environmental signals influence social behavior via olfaction (130, 143).
Since 2010, several research and review papers have highlighted the rich diversity of behaviors in eusocial species and revealed the underlying molecular mechanisms controlling social behaviors. We provide an expanded understanding of subsequent recent successes in genetic manipulation, pharmacological inhibition, and RNA interference in eusocial insects and discuss what this reveals about the cellular and molecular regulatory mechanisms controlling behavior and behavioral transitions. Additionally, we discuss the implications of achieving genetic manipulation in eusocial insects, a stated goal of previous papers (144). We argue that, owing to the rich repertoire of individual behaviors, social interactions, and strong neurobehavioral plasticity during development and in adults, as well as the current genetic and epigenetic tools available, eusocial insects provide a fascinating model that allows researchers to study the interactions of molecular, neural, and synaptic controls of behavior.
2. COMPLEX SOCIALITY IN EUSOCIAL INSECTS
Eusociality is the highest level of social organization in an animal colony and is broadly defined by its division of labor including distinct reproductive individuals (22). Eusociality is postulated to have arisen in hymenopterans more than 100 million years ago (37, 95), which has allowed for a rich diversity of intra- and intercolony dynamics to develop. In this section, we discuss several unique caste systems, whose determination can be either rigid or flexible, and the range of social interactions (which are antecedent to colony fitness) between members of a single colony and between different colonies.
Rigid caste determination is irreversible and happens during prepupal development (8, 48, 136). This is the case in the carpenter ant Camponotus floridanus, in which colony reproduction depends exclusively on a single queen (called a monogynous structure) determined irreversibly during early development. Additionally, worker subcastes in C. floridanus, termed majors and minors owing to their differing sizes, are determined irreversibly in late larval development (Figure 1a). Leaf-cutter ants are another example of a rigid caste species, as worker size, which is optimized for specified caste-associated duties, is established during larval development (139). When castes have distinctive alternate morphologies, as in the case of drastic size differences, the species is said to be polyphenic (87). Worker subcaste polyphenism is a characteristic associated with rigid caste societies; however, polyphenisms between queens and workers exist in both rigid and flexible caste systems. Rigid determination allows for multiple physically optimized castes or subcastes, but adults in these species are limited by an inability to adapt immediately to situational demands.
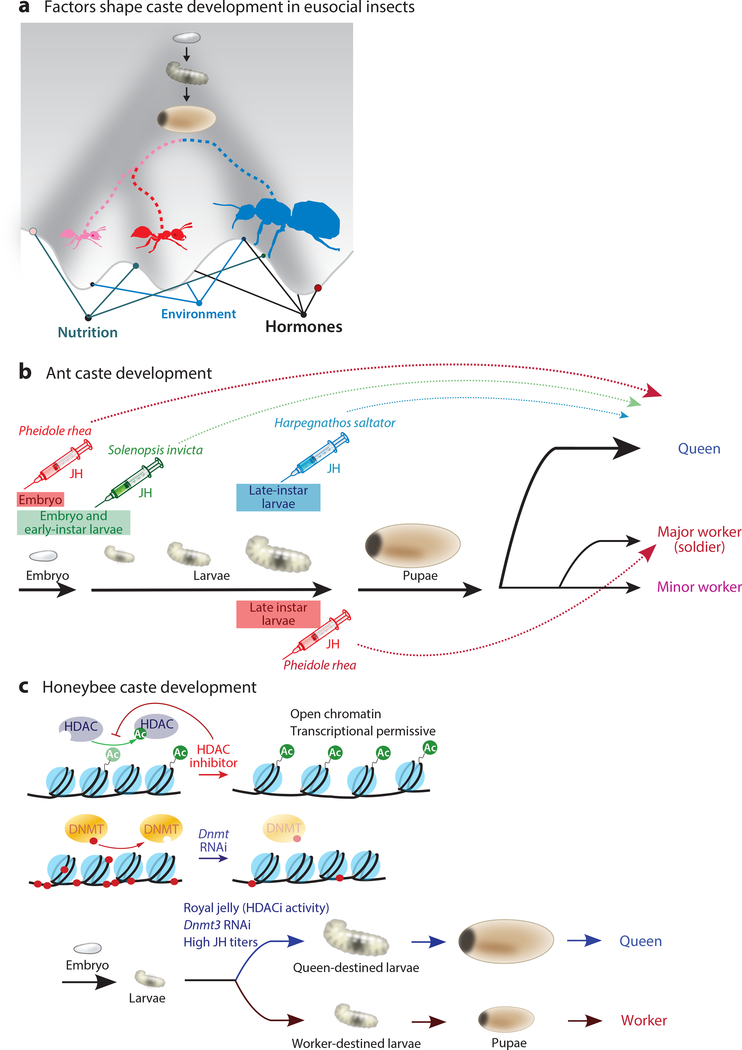
Figure 1. Major regulators during critical periods in caste determination.
(a) Factors including nutrition, environment, and hormones determine caste fates in eusocial insects. Embryos are pluripotent for caste determination but are highly responsive to external cues. Combinations of external cues shape development trajectories for each caste as represented by the Waddington’s epigenetic landscape (134). This allows single, fertilized embryos to give rise to different female castes including queens (blue) and major or minor workers (red and pink, respectively). The caste is determined at early developmental stages and is maintained throughout the lifetime. (b) JH acts at different species-dependent prepupal stages to trigger caste-specific development. In Harpegnathos, JH induces queen development at the late larval stages (94). In Solenopsis, JH induces queen development at the embryonic and early-instar larval stages (94, 99a, 132). In Pheidole, JH triggers both queen and major development at embryonic and late-instar larval stages, respectively (88, 97, 136, 137). (c) Queen development in the honeybee Apis mellifera is dependent on larval nutrition with royal jelly containing HDACi (58, 121). HDACi results in reduced removal of an acetyl group on histone tails, which may lead to an open chromatin conformation and altered transcriptional levels. Knockdown of Dnmt3 by RNAi in young larvae mimics the developmental effect of royal jelly (63). Abbreviations: Ac, acetyl; Dnmt, DNA methyltransferase; HDACi, histone deacetylase inhibitor; JH, juvenile hormone; RNAi, RNA interference.
Harpegnathos saltator is considered an extremely flexible caste system; these ants can transition from a worker to a pseudoqueen (gamergate) and revert back to a worker (C. Opachaloemphan, unpublished data) (144). This adaptation allows Harpegnathos colonies to survive queen death (provided colony members still remain), unlike the rigid caste species discussed above. Although initial establishment of worker versus queen castes occurs during development, transition and reversion processes can occur in adulthood and are accompanied by physiological and behavioral changes, such as increased ovary size and reduced optic lobe volume in the gamergate and loss of foraging behavior in the workers (42). Varying degrees of caste flexibility are evident, as some species display adult plasticity only between certain castes (Figure 2). Although adult honeybee workers (Apis mellifera) cannot become queens, they do display age polyethism, behavioral change that is characteristic of eusocial insects: In a stable colony, a young honeybee worker is tasked with nursing the young and cleaning inside the hive, while an older worker (>3 weeks) forages for food. This division of labor establishes a caste ratio, a ratio of the number of workers within each subcaste (50, 100). In colony instability, caste ratios may shift to suboptimal levels (e.g., not enough foragers or nurses). When this occurs in honeybees, older foragers can transition into nurses to compensate and vice versa, and this transition is accompanied by a shift in the DNA methylation profiles of brain tissue (47).
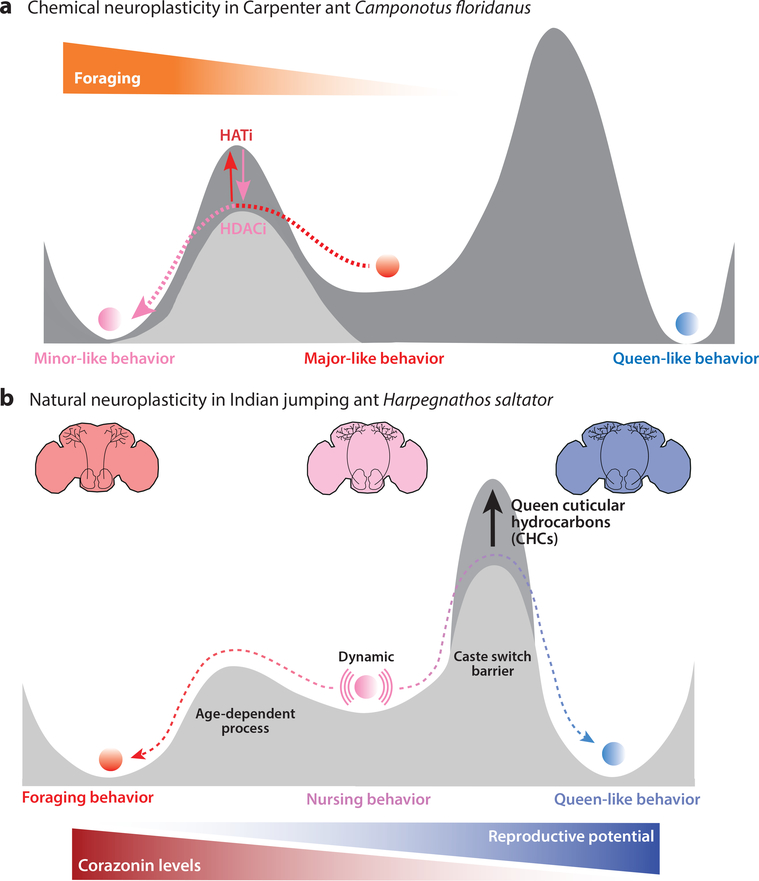
Figure 2. Neurobehavioral plasticity in eusocial insects.
(a) The carpenter ant Camponotus floridanus has two worker subcastes: minor and major. Even though Camponotus has a rigid caste system, caste-specific behaviors in young workers can be altered by chemical treatments: Injecting HDACi in the brain can induce minor-specific foraging behavior in majors. Coinjecting HATi antagonizes the behavioral effect (115). (b) In Harpegnathos saltator, natural environmental cues can change caste-specific behaviors: Queen CHCs repress the reproductive capabilities of Harpegnathos workers and prevent a caste switch. In the absence of a queen, workers develop into gamergates (blue), which exhibit queen-like behavior and physiology. Nursing workers (pink) naturally become foraging workers (red) as they age, in a transition related to increasing neuropeptide corazonin levels in the brain (40). Abbreviations: CHCs, cuticular hydrocarbons; HATi, histone acetyltransferase inhibitor; HDACi, histone deacetylase inhibitor.
Rigid division of labor in a massive colony necessitates extensive social interactions to ensure collaboration. Interactions can be simple, as in the case of tandem running (repeated abdominal and hind-leg tapping used to lead nestmates to a new destination) (34), or complex, as in the case of leaf-cutter ants, who are able to form sustainable agricultural farming societies. Leaf-cutter workers collaboratively forage for, clean, reserve, and culture leaves to grow fungi (83). Another interesting example of highly organized cooperation is in Eciton hamatum, an army ant, which has learned to create an ant bridge in the face of an obstacle blocking its path. This allows the ants to travel and transport food efficiently in unfamiliar situations (99). These behaviors showcase the rich diversity of social interactions within a colony. Furthermore, colonies can also interact with one another in a multitude of ways.
Intercolony social dynamics of ants are not well studied, although an astounding array of intercolony interactive behaviors has been reported. Polyergus lucidus, or slave-making ants, practice intercolony parasitism; these ants survive by invading the nests of other ant species, mimicking the chemical profile of the queen (effectively stealing her identity), and using this newfound social position to manipulate the enslaved ants into serving the counterfeit queen (19). When Camponotus ants, which have a precise nestmate recognition system, encounter another colony, they engage in immediate warfare (14, 82, 91). In contrast, Harpegnathos colonies often accept a nonnestmate worker from a different colony (9).
Colonies have a diverse set of complex hierarchical structures that fit the specific needs of the environment. Diversity in structures is paired with diversity in caste plasticity, routes of communication, complex coordinated behavior, and intercolony interactions. The vast range of social dynamics regulating colony coordination fosters an interesting comparison of caste-specific behaviors and distinct neurodevelopment from individuals with closely related genetic traits.
3. DEVELOPMENT OF SOCIAL BRAINS IN EUSOCIAL INSECTS
3.1. Developmental Trajectories
As described above, an ant colony acts as a superorganism: To maintain its homeostasis, members of the somatic lineage (wingless workers) stay in the colony and perform tasks such as nursing, foraging, and defending, while members of the germline lineage (newborn virgin queens and males, which bear wings) normally leave the nest to mate and found new colonies or die (in the case of males). Differential behaviors between castes are important for both colony survival and expansion of the species.
Earlier studies identified temporal critical periods (see Section 3.3) when individuals have heightened sensitivity to exogenous hormonal treatment: The response window for queen versus worker differentiation occurs in late embryos or early-instar larvae, whereas subcaste differentiation normally occurs in late-instar larvae (136). How is development in separate trajectories differentially regulated, thereby giving rise to distinct neural anatomies and behaviors? What hormones, signaling pathways, transcription factors (TFs), and chromatin factors are involved? Earlier studies on wing development in queens versus workers shed light on the differential mechanisms underlying tissue fate (1). The era for tackling the genetic and epigenetic mechanisms underlying neuronal differentiation and neural circuit formation has now arrived.
3.2. Peripheral Neuron Development
Eusocial insects rely primarily on chemosensory communication to organize social cooperation. Chemosensory receptor neurons, which underwent positive selection during social evolution, play a crucial role in animal communication (43, 147). Here we compare the development of vertebrate and invertebrate olfactory systems and discuss the importance of olfactory plasticity in establishing social communication.
3.2.1. Odorant receptors.
Chemosensory receptors include ORs, gustatory receptors (GRs), and ionotropic receptors (IRs) (56). Whereas both vertebrate and insect ORs comprise 7-transmembrane domains, vertebrate ORs are G-protein-coupled receptors, and insect ORs form heteromultimers with Orco, an obligate common coreceptor (6, 61). Together, ORs and Orco form odorant-gated ion channels. Interestingly, there are only 60 Orgenes in Drosophila. By contrast, the number of Or genes increases in hymenopteran insects, with the largest numbers (300–500) in ants, thus strongly suggesting that the olfactory system plays an important role in social communication (148). Notably, a major Or gene subfamily that encodes 9-exon ORs is dramatically expanded in hymenopteran insects, with the most dramatic expansion in ants. Indeed, 9-exon ORs play an important, but not exclusive, role in sensing cuticular hydrocarbon (CHC) pheromones, as evidenced by functional analyses using the Drosophila system discussed in Section 4.1. Both vertebrates and invertebrates follow a general one neuron–one receptor rule: Each odorant receptor neuron (ORN) expresses only a single type of OR, with axons of ORNs that express the same OR converging to the same glomerulus located in the olfactory bulbs in vertebrates or the antennal lobes (ALs) in invertebrates, thereby forming highly organized glomerular maps of the olfactory world (6, 61).
3.2.2. Odorant receptor neuron development and axon targeting.
Insect ORNs are located mainly in the antennae and maxillary pulp. Dendrites of ORNs are held in hair-like structures called sensilla. Different types of sensilla (e.g., basiconic, trichoid, and coeloconic) hold various combinations of ORN types and numbers. A Drosophila antenna contains a total of 410 sensilla, with each sensillum containing 1–4 ORNs; therefore, a Drosophila antenna normally has ∼1,000 ORNs (65). These numbers are dramatically expanded in ants: A Camponotus worker contains a total of ∼7,500 sensilla, and basiconic sensilla can have up to 130 ORNs (86). Thus, the total number of ORNs in Camponotus might reach 60,000!
Insect ORNs project axons into glomeruli and form synapses with projection neurons that project further into the mushroom bodies (MBs) and the lateral horns. Consistent with the expansion of ORs and the increased number of ORNs, the number of glomeruli is also dramatically expanded from 60 in Drosophila to 250–500 in female ants (35, 130, 143, 149).
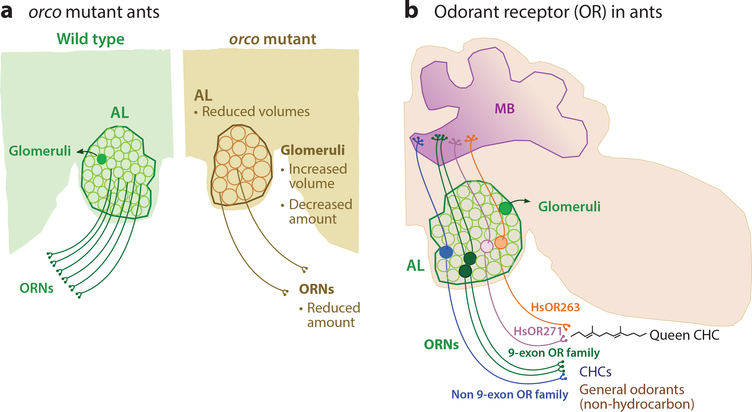
In mice, genetic inactivation of ORNs (e.g., via kir2.1) leads to the loss of these neurons (145), although whether they die or convert to a different type of neuron is not clear. In Drosophila, loss of Orco that also inactivates ORNs does not affect the neuroanatomy of ORNs and glomeruli during development (66). However, it leads to the degeneration of axons and morphological changes in glomeruli after eclosion (16). In contrast, orco mutations in two ant species, H. saltator and Ooceraea biroi, dramatically reduce the number of ORNs and glomeruli. Strikingly, only ∼20% of glomeruli remain in mutant workers (130, 143) (Figure 3a) in these two ant species that separated 120 million years ago, suggesting that the function of Orco in regulating neural development is conserved in ants and perhaps in all hymenopteran eusocial insects whose Or genes were expanded in evolution. Although dramatically reduced, the number of glomeruli remaining in Harpegnathos mutant workers is 62, more than the number of ORN subtypes that express one of the other chemosensory receptors, 17 GRs and 23 IRs (148). This finding suggests that a small number of OR-expressing ORNs are Orco independent and survive. Reliance on Orco for ORN development is not limited to female Harpegnathos: Wild-type male AL has only 79 glomeruli (fewer than one-third compared with females) and displays a striking reduction in orco mutants—only 31 glomeruli remain in mutant male ants (143).
Figure 3. Anatomy and function of the olfactory system in eusocial insects.
ALs in insects consist of numerous compacted neuropil structures called glomeruli. Each glomerulus receives odorant signals from a set of ORNs that contains the same OR, while projection neurons relay the signals to the MBs and lateral horns. (a) orco mutant ants (right) have fewer ORNs than the wild type (left). The volume of mutant ALs is reduced, with fewer but enlarged glomeruli (143). (b) Ants have an amplified family of Or genes that sense a broad spectrum of odorants. In particular, the 9-exon OR family is significantly expanded in eusocial insects (147, 148). Two members of this class, HsOR263 and HsOR271, mediate response to the Harpegnathos queen CHC pheromone. The 9-exon and non-9-exon ORs display a broad overlap of sensitivity to general odorants and CHCs (93, 119). Abbreviations: ALs, antennal lobes; CHC, cuticular hydrocarbon; MBs, mushroom bodies; Or, odorant receptor gene; orco, odorant receptor coreceptor gene; ORNs, odorant receptor neurons.
Developmental defects in Harpegnathos occur at the pupal stage, suggesting a critical period after ORNs are differentiated (143). Further investigation is required to elucidate the temporal window of this critical period. Importantly, it is not yet clear whether the activity required for the survival of ORNs is spontaneous (independent of odorant sensory input) or induced by odorants (as ant pupae are likely exposed to nest odorants). If the latter is true, the selection of neurons may depend on the early social environment, as only the neurons responsive to nest odorants survive, which may explain the discrimination between nestmates and nonnestmates observed in ant species such as C. floridanus (14, 82, 91).
3.2.3. Regulation of odorant receptor expression and odorant receptor neuron development.
A group of TFs controls the development of olfactory neurons in both vertebrates and invertebrates (3, 98). In mice, Or genes are expressed in a stochastic manner through a process that remains to be understood (81). Two conserved cis-regulatory sites are required in every Orpromoter: a homeodomain site bound by the TFs Lhx2 and Emx2 and an O/E (Olf1/early B cell factor)-like site. Before a given ORN expresses a specific Or,Or loci are marked with heterochromatic histone modifications: H3K9 and H4K20 trimethylations (77, 81). Only the selected Or genes are derepressed by the transcriptional regulators, including the TFs Lhx2 and Emx2. This leads to expression of a single Or gene, and presence of the corresponding OR protein suppresses expression of all other Or genes via a negative-feedback loop that involves the unfolded protein response pathway and other factors, such as the histone demethylase LSD1, that ultimately reestablish global gene silencing via formation of densely compacted foci (24). Reversing formation of these foci by overexpression of the lamin B receptor results in coexpression of many ORs in a single ORN (17).
In Drosophila, ORs are expressed in neurons derived from a sensory organ precursor (SOP) that develops into a sensillum. Differentiation of an SOP is regulated by the combined expression of patterning factors, including Lozenge (Lz), Amos, and Atonal, which control the fate of sensilla. Atonal lineage develops into coeloconic sensilla, many of which contain IRs and not ORs. Amos specifies basiconic sensilla when coexpressing high levels of Lz but leads to the development of trichoid sensilla when coexpressing low levels of Lz. Within sensilla, further differentiation of an SOP into neurons depends on the activity of Notch signaling. In the first round of division, the initial SOP divides into two daughter cells: PIIa and PIIb. PIIb is Notch-off, and PIIa is Notch-on. Two more rounds of division follow, generating the sensillum as well as ORNs derived from PIIb and glia derived from PIIa (3). Because each round relies on the same Notch-on/off signaling, it is generally assumed that the chromatin state is crucial in selecting the final OR expressed. Indeed, the transcriptional corepressors Hamlet (a homolog of Prdm16) and C-terminal-binding protein specifically increase H3K27 trimethylation and decrease H3K4 trimethylation on Notch target loci, thereby erasing their inherited Notch-on status and enabling subsequent responses to Notch signaling (27). At the later stage of ORN maturation, seven TFs (Acj6, zf30c, Sim, Xbp1, Fer1, E93, and Onecut) regulate the selection of an Or gene (3, 54, 55). In Drosophila, only two more rounds of division from the PII precursor cells generate one to four neuron(s) in each sensillum. However, given the dramatic expansion in ants, at least seven to eight more rounds must be required to generate the necessary number of ORNs, which, with an increased number of SOPs, might explain why there are ∼60-fold more ORNs in an ant antenna than in Drosophila. How TFs and chromatin modifications achieve this requires further investigation.
3.2.4. Regulation of axon targeting of odorant receptor neurons.
Proper axon targeting requires expression of different adhesion molecules. In adult mice, ORNs undergo continuous turnover (5). How do young postnatal ORNs target the correct glomeruli? There appear to be two stages of ORN axon targeting: one for ORNs born within a temporal window before postnatal day 7 (critical period) and the other for ORNs born later (75, 131). In the critical period, ORNs do not follow the track of existing ORNs but instead use their own targeting adhesion molecules—such as Robo, neuropilins, semaphorins, Kirrels, and ephrins (104)—to find their correct glomerulus targets. How each class of ORNs targets a specific glomerulus remains unknown. In contrast, later-born ORNs follow the track of existing ORNs that express the same OR via homotypic interactions, which may also use guidance molecules such as Kirrels. As such, the olfactory/glomerular map may be established during the critical period in mice.
Similar to early ORN axon targeting in mice, a wide range of adhesion molecules—such as Robo, Dscam, N-cadherin, Semaphorin-1a, and Teneurins (3)—regulate the projection of ORN axons to specific glomeruli in Drosophila. However, whereas the presence of an OR controls adhesion molecule expression and axon targeting via G-protein signaling in mice, ORs have no influence on axon targeting in Drosophila.
As discussed above, mutations in orco lead to loss of ORNs and glomeruli in ants. However, Orco-independent ORNs survive in mutant ants. It is not yet clear whether and how ORs or ORN activity regulates adhesion molecule expression and axon targeting in Orco-independent versus Orco-dependent ORNs (which disappear in orco mutant ants). Intriguing questions include the following: (a) Do Orco-dependent (unlike Orco-independent) ORNs rely on Orco or proper OR activity to express adhesion molecules, thereby targeting correct glomeruli, which is essential for their survival? (b) As the volume of glomeruli increases in orco mutant ants (143) (Figure 3a), how do survivor neurons, i.e., Orco-independent ORNs, project their axons to these larger glomeruli? These questions merit further investigation.
3.3. Development of Central Nervous System
In the Drosophila central brain, approximately 30,000 neurons, including peripheral and central neurons, are generated from a pool of 100 neuroblasts. Each neuroblast expresses a specific set of TFs and divides asymmetrically to produce a distinct group of neurons (i.e., a lineage) (106). During embryonic stages, neuroblasts generate a primary set of neurons and glia, which serves as a scaffold for later development. At larval stages, neuroblasts proliferate and produce differentiated neurons, generating secondary lineages. During this second phase of neuronal development, axons project along the primary scaffold, generating a progressively more specialized neural network. After metamorphosis, the newly emerged adult brain contains mature neural circuits (122). Identifying the developmental pathway and specific circuitry in eusocial insects is required to provide fundamental insights on the neuroanatomical and neurophysiological basis of caste-associated behaviors.
3.3.1. Epigenetic regulation of caste development and behavior in eusocial insects.
Since most individuals in eusocial insects are highly related genetically, caste determination is controlled mainly by environmental cues rather than genetics. This more evolutionarily viable alternative to genetic determination provides the opportunity for adaptation: For example, caste ratios, a ratio of worker subcastes, can be adjusted to increase colony survival in the face of new demands (100, 101, 140). Differential trajectories governing caste determination are established by gene expression patterns induced by multiple interacting factors, including temperature and larval nutrition (10, 58, 127).
Specialized feeding controls queen versus worker fate in the honeybee A. mellifera, such that early feeding of larvae with royal jelly nutritionally determines the queen (58). Royal jelly contains up to 5% histone deacetylase inhibitor (HDACi) and perhaps has an effect on the early larval chromatin state (121). Interestingly, Sir2, an HDAC, is also nutritionally responsive in honeybees (92); higher Sir2 levels in Caenorhabditis elegansand mice are correlated with extended life span, which is also a feature of honeybee queens (105, 129). Whereas the exact downstream targets of royal jelly HDACi are not yet known, histone modifications are essential mediators of important caste determinations in C. floridanus. Camponotusshows distinct patterns of histone H3K27 acetylation between castes (116). The resulting differential gene expression in Camponotus occurs in genes related to neural development and olfactory learning (115, 116).
Besides histone modifications, DNA methylation, which is associated with honeybee queen larval development (63), appears to be another important caste determinant and has been extensively investigated in honeybees. Differing DNA methylation profiles of the brains of workers and future queens can be observed as early as 96 h posthatch. Some differentially methylated genes affect expression of metabolically associated pathways (33). However, most DNA methylation is enriched in gene bodies of actively transcribed genes and correlates with moderately and highly expressed genes, such as housekeeping genes (8, 142). In the honeybee, this regulatory methylation pattern was functionally analyzed by knocking down DNA methyltransferase 3 (Dnmt3), a gene encoding an enzyme that catalyzes DNA methylation (63). Dnmt3-knockdown bees display reduced genome-wide DNA methylation levels and altered RNA splicing patterns, suggesting the functional role of DNA methylation in honeybee queen caste determination (63, 69) (Figure 1c).
The role of DNA methylation in caste determination is further supported by studies in other eusocial insects such as the termite Zootermopsis nevadensis, in which queen and worker larvae have significantly different methylation patterns (39). However, additional confirmation of the association between caste determination and DNA methylation in eusocial insects is needed, as the limited number of replicates in the DNA methylome studies was recently called into question (71). Nevertheless, DNA methylation and histone modifications both show key roles in caste development and social behavior in eusocial insects (63, 115, 116).
3.3.2. Juvenile hormone and critical periods.
Juvenile hormone (JH) is one of the main insect hormones broadly regulating insect development and reproduction (44, 88). It plays a major conserved role in early caste determination; functionally, JH regulates queen development in ants (1, 88, 94), wasps (4, 7), stingless bees (22a, 45), bumble bees (88, 113a), and honeybees (25a, 88) (Figure 1b,c). JH is synthesized from the corpora allatum, an endocrine gland in the brain (32), and positively correlates with insulin signaling in honeybees (21), thus suggesting a connection with the specialized feeding of queen-destined larvae.
Interestingly, JH is associated with differentiation of the soldier caste in the termite Hodotermopsis sjostedti (20, 78). The application of a JH analog (methoprene) before molting induces upregulation of several genes in the central nervous system (CNS) of soldiers (52). These genes may contribute to the enlargement of the mandibular motor neurons that is observed in workers. Upregulation occurs only during the transitional period and does not continue throughout adulthood, indicating that high JH levels coincide with the enlargement period (51). JH, however, is effective only during certain critical periods.
Long assumed to exist, critical periods in development represent intervals when an organism is particularly sensitive to environmental stimuli (46). Enhanced sensitivity to particular regulatory hormones defines critical periods in caste development (88, 136). Injection of JH is an invaluable tool for identifying the time frame of these sensitive periods in different species (Figure 1b). Harpegnathos larvae are responsive to the use of JH to induce queen development when the hormone is introduced in the third- and fourth-instar larvae, but not before (94). In contrast, the fire ant Solenopsis invicta is responsive only in the embryonic and early-instar larval phases (94, 99a). Even more interesting is that ants with dimorphic (polyphenic) subcastes appear to have more than one critical period; early treatment with a JH analog in Pheidole ants triggers queen development, whereas late treatment (during the last instar phase) triggers the development of major workers (88, 97, 136, 137). While the branching point for polyphenic development occurs at the larval stages, certain drug treatments of dimorphic adults may alter behavior, but not morphology, at crucial sensitive periods. In C. floridanus, treatments that affect epigenetic marks induce behavioral changes only in young adult workers (discussed in Section 4.2.2).
Identifying windows of heightened sensitivity is important in understanding exactly when upstream caste-determining effectors are acting. Once a temporal window is established, a spatial map of gene- and chromatin-level activities can be elucidated, giving valuable information on the often-irreversible caste-determining steps that regulate behavior.
4. FUNCTION OF THE SOCIAL BRAIN IN EUSOCIAL INSECTS
The advanced repertoire of cooperative social behaviors that eusocial insects display necessitates a sophisticated peripheral nervous system as well as CNS to achieve collective fitness. In this section, we summarize the current understanding of how peripheral and central neurons in eusocial insects respond to and process social cues and how these signals regulate social behavior, social status, and reproduction. Importantly, we highlight progress in the investigation of epigenetic regulation of neural functions and social behavior.
4.1. Function of Peripheral Neurons
As described above, pheromones and general odorants trigger behavioral responses (68) through chemosensory receptors in insects. There are up to 130 ORNs in each ant sensillum, which makes electrophysiological analysis of chemoresponses challenging. Nevertheless, single-sensillum recording on Harpegnathos indicates that reproductive gamergates have an overall reduced response to a wide range of long-chain CHCs compared to workers (38), perhaps explaining their lack of repression by the queen pheromone.
Furthermore, Or genes underwent dramatic expansion during the evolution of hymenopteran eusocial insects. Or genes belonging to the 9-exon family experienced the most dramatic expansion and account for one-third of all Orgenes in ants but are not represented in Drosophila (148), although other families are also expanded, indicating the importance of olfactory sensing in social communication. Interestingly, 9-exon ORNs are enriched in basiconic sensilla and specifically target T6 glomeruli in the AL (80, 149), leading to the hypothesis that the 9-exon OR family particularly mediates CHC sensing. To test this hypothesis, two groups (93, 119) used transgenic Drosophilathat express a single ant OR in an otherwise empty ORN and analyzed the responses of Harpegnathos 9-exon and non-9-exon ORs to social CHC pheromones versus general odors. They identified two H. saltator ORs (HsOR263, HsOR271) that mediate the response to the queen pheromone (Figure 3b). Importantly, they found that both 9-exon and non-9-exon ORs respond to CHCs and general odors, suggesting that the spatial segregation of ORNs and glomeruli does not translate to functional specificity. Therefore, amplification of several OR subfamilies increases the number of ORs for CHC detection, likely allowing ants to distinguish subtle differences of CHC profiles among individuals inside or between social groups (23). Indeed, C. floridanus ants can discriminate between closely related hydrocarbons, allowing them to cooperate with nestmates that display the same pheromones but attack nonnestmates with slightly different pheromone cocktails (111).
Available orco mutant ants (see Section 3.2.2) enable behavioral analysis of their defective social communication (130, 143). The dramatically reduced number of ORNs in orco mutant ants disrupts their responses to general odorants (Figure 3a). In addition, orco mutant ants appear to be unable to sense brood, sex, and trail pheromones, among others. As a consequence, they display a lack of social interactions, abnormal social behaviors, and reduced fitness. Because mutant ants have intact GRs, IRs, and other chemosensory receptors, expanded ORs likely play a critical role in regulating social communication and social behavior. Interestingly, although HsOR263 and HsOR271 are strongly responsive to the queen pheromone, loss of this OR function in orco mutant ants is not sufficient to allow them to become gamergates in the presence of a queen (143). This is likely because becoming a gamergate requires positive signals by other pheromones, rather than simply the lack of repression by queen pheromones. Alternatively, chemosensory receptors beyond the OR family might mediate queen pheromone perception.
4.2. Function of Central Neurons
Eusocial insects can display dramatic adult plasticity in regulating caste transition (see Section 2): For example, nurses can switch to foragers in honeybees, and workers to gamergates in Harpegnathos. How genetic and epigenetic processes regulate the functionality and plasticity of the mature brain has become a major field of inquiry for researchers of eusocial insects. Mechanisms of regulation, including DNA methylation, histone modifications, and noncoding RNAs, are responsible for regulating and, importantly, maintaining gene expression patterns. These changes can result in massive shifts in neuronal organization and behavioral phenotypes (8, 47, 74, 115, 116). The growing number of studies in animals and humans emphasizes the importance of this field, revealing that epigenetic alterations in the CNS result in neurological disorders (57, 135). For example, loss of function of methyl-CpG-binding protein 2 (MECP2) leads to symptoms characteristic of Rett syndrome, a neurological disorder characterized by loss of language acquisition and intellectual impairment (2).
4.2.1. Central nervous system.
Animal behaviors are inherently responsive to external stimuli. Stimuli are first sensed and transmitted to the CNS, where they are processed and integrated, and the regulatory response is enacted. Eusocial insects have a unique sensitivity to a wide variety of stimuli, such as infrared light (112), polarized ultraviolet light (36, 133), magnetism (107), and a vast range of odors, all of which contribute to the behavior of colony members (68). Given the essential role that the CNS plays in behavioral regulation, it is no surprise that differing brain morphologies exist between individuals with vastly distinct social roles. Surprisingly, however, this morphological shift can occur in an adult insect within its lifetime and is sometimes reversible.
To understand morphological brain changes in eusocial insects, deep knowledge of the Drosophila nervous system is important, as it shares common organization and function (28). The ALs and MBs are highly developed brain regions in both species and are similarly responsible for olfaction and learning/processing. Accordingly, well-established Drosophilaneurodevelopmental tools and insights can serve as useful models in studying hymenopteran brain development. The MBs in invertebrates, which may be analogous to the hippocampi in vertebrates (126), coordinate responses to external stimuli. They have been implicated in behavioral responses and the consolidation and retrieval of memory (79).
Both ALs and MBs are highly altered in individuals with very different social behaviors. The noneusocial but well-studied desert locust provides evidence of the fluidity of these brain regions in varying social settings. The desert locust has two different life phases: solitary and gregarious. Population density catalyzes the transition between phases: An isolated condition yields a solitary-phase insect, and a highly populated condition yields a gregarious swarm-forming locust. The gregarious locust engages in migratory behavior, but the solitary locust does not; this distinction is correlated with dramatic differences in their brain morphology. Compared with solitary locusts, gregarious locusts have a considerably larger overall brain, larger absolute volume in MBs, and reduced AL proportions to midbrain (89). Broader diet preferences and more extensive foraging strategies may explain the larger MBs in gregarious locusts, whereas higher sensitivity to food odorants and more limited food preferences may explain the relatively larger ALs in solitary locusts (25, 89).
Sociality has a pronounced effect on MB development, as evidenced in Megalopta genalis, the sweat bee whose queen can switch from being solitary to social (110). The social queen has more developed MBs than its solitary counterpart (120). This is likely due to the reliance of social communication on olfactory processing. Even within a lifetime, behavior has a pronounced effect on brain morphology: In the honeybee, organization of the MBs of a newly emerged queen is very plastic and responsive to early experiences, such as mating and leaving the hive (29). In worker castes of A. mellifera, C. floridanus, and Cataglyphis bicolor (a desert ant), foraging induces a direct increase in MB neuropil size (28a, 41, 64, 141). Caste-specific behaviors can require particular neural functions, as in the genus Pheidole, where dimorphic worker subcastes have dramatically different brain organizations and relative volumes, particularly in the MBs (84).
Given the predominance of age polyethism in eusocial insects, age and experience likely play a role in the morphological shifts of brain mass. As expected, experience-based memory formation in eusocial species can alter neuronal reorganization and increase the density of synaptic complexes in the MBs (30, 108, 124). For example, in honeybees, the density of microglomeruli (a synaptic complex formed by axonal ends of projection neurons and dendritic branches of Kenyon cells) in the MBs increases while the volume remains constant as the insect ages (31). This is observed in all regions of the MBs, which include both visual and olfactory processing areas. Specifically, MB density increases as foraging behavior increases, likely owing to a heightened need for visual processing, spatial orientation, and memory, all of which are necessary for successful foraging (26, 53, 64, 124, 125).
Eusocial insects have a stark division of labor between castes that defines each caste’s specialized function in the colony. Importantly, this behavioral exclusivity is independent of the original genomic content of the organism, leading to mysterious questions of how epigenetics shapes neuroplasticity and enables complex social interactions. Given naturally occurring morphological and behavioral plasticity, environmentally induced neuroplasticity and neurodevelopmental trajectories can be readily studied and manipulated in eusocial insects.
4.2.2. Functional analysis of behavior in eusocial insects.
The carpenter ant C. floridanus has no naturally occurring behavioral switch between its two distinct worker subcastes, minors and majors. Both types of workers have highly specific behaviors and morphologies that are irreversible. Nevertheless, a recent study (115) showed that the specific foraging behavior of a subcaste can be reprogramed pharmaceutically in early adulthood by changing histone acetylation levels in the brain and altering the expression levels of genes involved in specifying neuronal activity. Young major brains were injected with an epigenetic drug that disrupts the removal of histone acetylation (HDACi), resulting in major workers displaying minor worker–specific behaviors (Figure 2a). These results were confirmed through knockdown of the HDAC Rpd3 in majors. Conversely, young major brains coinjected with histone acetyltransferase inhibitors display reduced foraging activity, suggesting the dependence of minor and major worker behaviors on histone acetylation (115). Interestingly, this study also identified an epigenetic window of sensitivity (critical period) in the young brain, which shows a heightened early responsiveness to histone-modifying inhibitors that decreases sharply with age (115).
A similar decrease in plasticity is speculated to occur in Harpegnathos; compared with old workers (foragers), young workers (nurses) have a heightened ability to become gamergates. The neuropeptide corazonin stimulates foraging behavior (40) (Figure 2b). Likewise, in other eusocial insects including ants, termites, and honeybees, nonreproductive foraging workers have high expression levels of corazonin, and nonforaging individuals have low expression (59, 90, 128). Corazonin is thus considered a putative key neural regulator of complex social behavior. Further analysis (40) confirmed its functional role: Injection of synthetic corazonin peptide into Harpegnathos brains induced hunting behavior and suppressed egg-laying ability. Additionally, knockdown of corazonin receptor gene by RNAi conferred the opposite behavioral modifications (40). The study displays a stark example of a behavioral switch in eusocial insects that is regulated by a simple gene encoding a neuropeptide.
Behavioral changes may occur as a result of cascades leading to general pathway inhibition or upregulation. Transcriptomic analysis in honeybees has revealed a molecular basis for aggressive worker-associated behaviors: Decreased mitochondrial activity in the brain correlates with increased aggressive behaviors (15). In accordance, pharmaceutical-based inhibition of oxidative phosphorylation significantly promotes aggression in honeybees (70). In the mature adult stage, females in stable eusocial insect societies have well-established caste affinities: What set of genes (genetic tool kit) regulates their behaviors? Functional analyses discussed in this section provide a basis for understanding the genetic and epigenetic mechanisms controlling caste-specific behaviors, such as aggression in honeybees and foraging in ants.
5. DEVELOPMENT OF GENOMIC, GENETIC, AND EPIGENETIC TOOLS IN EUSOCIAL INSECTS
Defining characteristics of model organisms include ease of genetic manipulation and availability of experimental technologies. To establish eusocial insects as model organisms, the development and use of a wide array of technologies are paramount. In this section, we provide an overview of recent technological advances developed in and applicable to eusocial insects.
5.1. Traditional Transcriptomics
In recent years, comparative genomic approaches have been widely integrated into eusocial insect studies to identify the genes responsible for organizing societies, analyze the evolution of sociality from solitariness, and address the common molecular mechanisms underlying social communication and behaviors between humans and animals (114). The main goal has been to grasp how the genomes of eusocial insects program their complex social behaviors yet allow the insects to retain plasticity and adaptability to environmental cues. A growing number of genomes in eusocial insects including bees, ants, wasps, and termites have been sequenced to date; however, the relatively low quality of many draft genomes in nonmodel organisms impedes sophisticated genome-wide analyses. Two ant genomes (C. floridanus and H. saltator) have recently been improved through the generation of long genome reads, which provide long, contiguous, and accurate assemblies as well as comprehensive protein coding and long noncoding RNA annotations (113). High-quality genome assemblies have allowed scientists to apply bioinformatic approaches to eusocial insects, thus opening the door to more sophisticated studies and functional analyses.
Nowadays, most studies attempting to identify a key gene controlling caste development, behavior, or aging rely heavily on prerequisite transcriptomic data. The genes identified are often based on whole-insect or whole-tissue transcriptomes that compare bulk gene expression profiles among castes. Owing to the heterogeneity of these tissue samples, gene expression measurements provide average information from multiple cell populations, resulting in imprecise outcomes.
In the past few years, rapid advances in high-throughput technology have enabled highly sensitive approaches toward quantifying gene profiles from single cells. Drop-seq and 10x Genomics, approaches for collecting messenger RNA (mRNA) transcripts simultaneously from multiple cells and barcoding each cell individually in a distinctive droplet, have become critical for studying transcriptomes in complex tissues (62, 76, 146). These valuable tools use transcriptomic data to identify key genes in specific cells of highly complex and heterogeneous tissues such as the brain. However, these single-cell transcriptomic approaches do not provide spatial information pertaining to neural circuitry.
5.2. Spatial Transcriptomics and Chromatin-State Analysis
Once a key gene has been identified, its spatiotemporal expression patterns must be confirmed. This can be achieved by performing serial hybridizations or multiplexing barcoding probes (73). Single-molecule fluorescent in situ hybridization (FISH) can produce patterns that are quantitative and sensitive enough to count individual mRNA molecules, thus potentially mapping the spatial distribution of expression in tissue and pinpointing functional circuitry. An alternative approach to FISH is in situ sequencing, which uses a spotted-barcoded microarray to capture mRNA from tissue and sequence the spatially barcoded transcripts (123). This alternative has the advantage of not requiring prerequisite sequence knowledge, but its resolution is limited by the spot size.
Recent rapid advancements in experimental tools are not limited to gene expression measurement. Tools to detect chromatin state through chromatin accessibility (ATAC-seq) (13), chromatin structure (Hi-C) (85), and histone modifications (ChIP-seq) (11, 103, 117) are becoming sensitive enough to detect single-cell inputs. Given that DNA methylation and histone modifications have been implicated in regulating caste differentiation, these low-input genomic tools are useful for determining the spatial patterns of chromatin modifications.
5.3. Genetic Manipulation
Genomic methods can likely specify an area where epigenetic modifications responsible for caste determination occur, but using them to track cells through development would be difficult, time consuming, and expensive. Clonal lineage analysis is a powerful tool for studying neuronal lineage development in Drosophila (106). This method requires the introduction of a label into early cells, which can be accomplished using established CRISPR technology in ants. This technique can both precisely label cells and manipulate gene expression levels, allowing for visual tracking of neurodevelopment. As such, it would be possible to answer several pressing questions about developmental trajectories and the propagation of epigenetic signals.
Development of genetic tools in eusocial insects is considered essential in neuroepigenetics (144). Recently, mutant and transgenic honeybees have been derived using CRISPR and a piggyBac transposon, respectively (60, 109), thus providing proof of principle for genetic manipulation and germline transformation of eusocial insects. Also recently and for the first time, two groups (130, 143) performed functional analyses using two species of orco mutant ants generated by CRISPR technology. They demonstrated the essential function of ORs in regulating social communication, behavior, and neural development (130, 143).
We envision that the next steps of genetic analysis in eusocial insects will harness the immense array of genetic technologies available, such as Gal4-UAS, FLP-FRT, Cre-LoxP systems, and GCaMP. Development of these transgenic tools will require extensive collaboration among entomologists to unravel the genetic and epigenetic mechanisms underlying complex eusociality.
6. PERSPECTIVES
With the combination of cutting-edge technologies in genomics, genetics, and epigenetics, the study of eusocial insects has made tremendous progress in recent years. Eusocial insects provide an ideal platform to study the phenotypic effects of epigenetic changes, as different caste members, despite sharing a very similar genome, differ widely in morphology and complex caste-specific behaviors. Additionally, their relatively simple brain structure and rich repertoire of tractable social behaviors as well as the availability of tools for genetic manipulation make neuroepigenetic investigation in these organisms attractive. Important questions in the field include how epigenetic modifications are translated into functional consequences and how these changes regulate neuroplasticity.
Emerging methods in single-cell and spatial transcriptomics help visually connect a cell-type-specific gene expression profile with neural architecture (67). Use of these methods will allow an understanding of the fundamental steps of neuronal development and pinpoint specific neural circuits responsible for neuroplasticity in eusocial insects. Applying these technologies to eusocial insects could help identify caste-associated patterns of neural networks, for example, in the MBs. Identification of the functional neural circuitry in caste differentiation would be highly valuable for understanding the cellular basis of the extensive neuroplasticity in eusocial insects and, perhaps, other organisms.
ACKNOWLEDGMENTS
The authors thank Jakub Mlejnek (New York University) for comments on the manuscript. This work was supported by a Howard Hughes Medical Institute Collaborative Innovation Award (HCIA), #2009005, to D.R., the National Institutes of Health (NIH) grant EY13010 to C.D., and an NIH Ruth L. Kirschstein National Research Service Award (NRSA) postdoctoral fellowship, F32AG044971, to H.Y.
LITERATURE CITED
- 1.Abouheif E, Wray GA. 2002. Evolution of the gene network underlying wing polyphenism in ants. Science 297:249–52 [DOI] [PubMed] [Google Scholar]
- 2.Amir RE, Van den Veyver IB,Wan M, Tran CQ, Francke U, Zoghbi HY. 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet 23:185–88 [DOI] [PubMed] [Google Scholar]
- 3.Barish S, Volkan PC. 2015. Mechanisms of olfactory receptor neuron specification in Drosophila. Wiley Interdiscip. Rev. Dev. Biol 4:609–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth RH, Lester LJ, Sroka P, Kessler T, Hearn R. 1975. Juvenile hormone promotes dominance behavior and ovarian development in social wasps (Polistes annularis). Experientia 31:691–92 [DOI] [PubMed] [Google Scholar]
- 5.Beites CL, Kawauchi S, Crocker CE, Calof AL. 2005. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp. Cell Res 306:309–16 [DOI] [PubMed] [Google Scholar]
- 6.Benton R, Sachse S, Michnick SW, Vosshall LB. 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLOS Biol. 4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohm MK. 1972. Effects of environment and juvenile hormone on ovaries of the wasp, Polistes metricus. J. Insect Physiol 18:1875–83 [Google Scholar]
- 8.Bonasio R, Li Q, Lian J, Mutti NS, Jin L, et al. 2012. Genome-wide and caste-specific DNA methylomes of the ants Camponotus floridanus and Harpegnathos saltator. Curr. Biol 22:1755–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonasio R, Zhang G, Ye C, Mutti NS, Fang X, et al. 2010. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329:1068–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brian MV. 1973. Temperature choice and its relevance to brood survival and caste determination in the ant Myrmica rubra L. Physiol. Zool 46:245–52 [Google Scholar]
- 11.Brind’Amour J, Liu S, Hudson M, Chen C, Karimi MM, Lorincz MC. 2015. An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat. Commun 6:6033. [DOI] [PubMed] [Google Scholar]
- 12.Bryant DM, Mostov KE. 2008. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol 9:887–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, et al. 2015. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523:486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlin NF, Holldobler B. 1983. Nestmate and kin recognition in interspecific mixed colonies of ants. Science 222:1027–29 [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekaran S, Rittschof CC, Djukovic D, Gu H, Raftery D, et al. 2015. Aggression is associated with aerobic glycolysis in the honey bee brain. Genes Brain Behav. 14:158–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. 2009. Neuronal activity and Wnt signaling act through Gsk3-βto regulate axonal integrity in matureDrosophila olfactory sensory neurons. Development 136:1273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clowney EJ, LeGros MA, Mosley CP, Clowney FG, Markenskoff-Papadimitriou EC, et al. 2012. Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell 151:724–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnaani J, Robinson GE, Bloch G, Borst D, Hefetz A. 2000. The effect of queen-worker conflict on caste determination in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol 47:346–52 [Google Scholar]
- 19.Cool-Kwait E, Topoff H. 1984. Raid organization and behavioral development in the slave-making ant Polyergus lucidus Mayr. Insectes Soc. 31:361–74 [Google Scholar]
- 20.Cornette R, Gotoh H, Koshikawa S, Miura T. 2008. Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae). J. Insect Physiol 54:922–30 [DOI] [PubMed] [Google Scholar]
- 21.Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, et al. 2007. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. PNAS 104:7128–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespi BJ, Yanega D. 1995. The definition of eusociality. Behav. Ecol. 6:109–15 [Google Scholar]; 22a. de Oliveira Campos LA, Velthuis-Kluppell FM, Velthuis HHW. 1975. Juvenile hormone and caste determination in a stingless bee. Naturwissenschaften 62:98–99 [Google Scholar]
- 23.d’Ettorre P, Deisig N, Sandoz JC. 2017. Decoding ants’ olfactory system sheds light on the evolution of social communication. PNAS 114:8911–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalton RP, Lomvardas S. 2015. Chemosensory receptor specificity and regulation. Annu. Rev. Neurosci 38:331–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Despland E, Simpson SJ. 2005. Food choices of solitarious and gregarious locusts reflect cryptic and aposematic antipredator strategies. Anim. Behav 69:471–79 [Google Scholar]; 25a. Dietz A, Hermann HR, Blum MS. 1979. The role of exogenous JH I, JH III and anti-JH (precocene II) on queen induction of 4.5-day-old worker honey bee larvae. J. Insect Physiol 25:503–12 [Google Scholar]
- 26.Durst C, Eichmuller S, Mensel R. 1994. Development and experience lead to increased volume of ¨ subcompartments of the honeybee mushroom body. Behav. Neural Biol 62:259–63 [DOI] [PubMed] [Google Scholar]
- 27.Endo K, Karim MR, Taniguchi H, Krejci A, Kinameri E, et al. 2011. Chromatin modification of Notch targets in olfactory receptor neuron diversification. Nat. Neurosci 15:224–33 [DOI] [PubMed] [Google Scholar]
- 28.Fahrbach SE. 2006. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 51:209–32 [DOI] [PubMed] [Google Scholar]; 28a. Fahrbach SE, Dobrin S. 2009. The how and why of structural plasticity in the adult honeybee brain In Cognitive Ecology II, ed. Dukas R, Ratcliffe JM, pp. 27–46. Chicago: Univ. Chicago Press [Google Scholar]
- 29.Fahrbach SE, Giray T, Robinson GE. 1995. Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol. Learn. Mem 63:181–91 [DOI] [PubMed] [Google Scholar]
- 30.Falibene A, Roces F, Rossler W. 2015. Long-term avoidance memory formation is associated with a transient increase in mushroom body synaptic complexes in leaf-cutting ants. Front. Behav. Neurosci 9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farris SM, Robinson GE, Fahrbach SE. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci 21:6395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feyereisen R, Tobe SS. 1981. A rapid partition assay for routine analysis of juvenile hormone release by insect corpora allata. Anal. Biochem 111:372–75 [DOI] [PubMed] [Google Scholar]
- 33.Foret S, Kucharski R, Pellegrini M, Feng S, Jacobsen SE, et al. 2012. DNA methylation dynamics, metabolic fluxes, gene splicing, and alternative phenotypes in honey bees. PNAS 109:4968–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin EL, Franks NR. 2012. Individual and social learning in tandem-running recruitment by ants. Anim. Behav 84:361–68 [Google Scholar]
- 35.Friedman DA, Gordon DM, Luo L. 2017. The MutAnts are here. Cell 170:601–2 [DOI] [PubMed] [Google Scholar]
- 36.von Frisch K 1949. Die Polarisation des Himmelslichtes als orientierender Faktor bei den Tanzen der ¨ Bienen. Experientia 5:142–48 [DOI] [PubMed] [Google Scholar]
- 37.Gadau J, Helmkampf M, Nygaard S, Roux J, Simola DF, et al. 2012. The genomic impact of 100 million years of social evolution in seven ant species. Trends Genet. 28:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaninia M, Haight K, Berger SL, Reinberg D, Zwiebel LJ, et al. 2017. Chemosensory sensitivity reflects reproductive status in the ant Harpegnathos saltator. Sci. Rep 7:3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glastad KM, Gokhale K, Liebig J, Goodisman MA. 2016. The caste- and sex-specific DNA methylome of the termite Zootermopsis nevadensis. Sci. Rep 6:37110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gospocic J, Shields EJ, Glastad KM, Lin Y, Penick CA, et al. 2017. The neuropeptide corazonin controls social behavior and caste identity in ants. Cell 170:748–59.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gronenberg W, Heeren S, Holldobler B. 1996. Age-dependent and task-related morphological changes ¨ in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol 199:2011–19 [DOI] [PubMed] [Google Scholar]
- 42.Gronenberg W, Liebig J. 1999. Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften 86:343–45 [Google Scholar]
- 43.Hansson BS, Stensmyr MC. 2011. Evolution of insect olfaction. Neuron 72:698–711 [DOI] [PubMed] [Google Scholar]
- 44.Hartfelder K, Emlen DJ. 2012. Endocrine control of insect polyphenism In Insect Endocrinology, ed. Gilbert LI, pp. 464–522. New York: Academic [Google Scholar]
- 45.Hartfelder K, Makert GR, Judice CC, Pereira GAG, Santana WC, et al. 2006. Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie 37:144–63 [Google Scholar]
- 46.Hensch TK. 2005. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci 6:877–88 [DOI] [PubMed] [Google Scholar]
- 47.Herb BR, Wolschin F, Hansen KD, Aryee MJ, Langmead B, et al. 2012. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat. Neurosci 15:1371–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holldobler B, Wilson EO. 1990. ¨ The Ants. Cambridge, MA: Belknap [Google Scholar]
- 49.Holldobler B, Wilson EO. 2008. ¨ The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. New York: W.W. Norton [Google Scholar]
- 50.Huang Z-Y, Robinson GE. 1996. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol 39:147–58 [Google Scholar]
- 51.Ishikawa Y, Aonuma H, Miura T. 2008. Soldier-specific modification of the mandibular motor neurons in termites. PLOS ONE 3:e2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa Y, Okada Y, Ishikawa A, Miyakawa H, Koshikawa S, Miura T. 2010. Gene expression changes during caste-specific neuronal development in the damp-wood termite Hodotermopsis sjostedti. BMC Genom. 11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismail N, Robinson GE, Fahrbach SE. 2006. Stimulation of muscarinic receptors mimics experiencedependent plasticity in the honey bee brain. PNAS 103:207–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafari S, Alenius M. 2015Cis-regulatory mechanisms for robust olfactory sensory neuron class-restricted odorant receptor gene expression in Drosophila. PLOS Genet. 11:e1005051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafari S, Alkhori L, Schleiffer A, Brochtrup A, Hummel T, Alenius M. 2012. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLOS Biol. 10:e1001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joseph RM, Carlson JR. 2015. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 31:683–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jowaed A, Schmitt I, Kaut O, Wullner U. 2010. Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J. Neurosci 30:6355–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamakura M 2011. Royalactin induces queen differentiation in honeybees. Nature 473:478–83 [DOI] [PubMed] [Google Scholar]
- 59.Khamis AM, Hamilton AR, Medvedeva YA, Alam T, Alam I, et al. 2015. Insights into the transcriptional architecture of behavioral plasticity in the honey bee Apis mellifera. Sci. Rep 5:11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohno H, Suenami S, Takeuchi H, Sasaki T, Kubo T. 2016. Production of knockout mutants by CRISPR/Cas9 in the European honeybee, Apis mellifera L. Zool. Sci 33:505–12 [DOI] [PubMed] [Google Scholar]
- 61.Komiyama T, Luo L. 2006. Development of wiring specificity in the olfactory system. Curr. Opin. Neurobiol 16:67–73 [DOI] [PubMed] [Google Scholar]
- 62.Konstantinides N, Kapuralin K, Fadil C, Barboza L, Satija R, Desplan C. 2018. Phenotypic convergence: distinct transcription factors regulate common terminal features. Cell 174:622–35.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kucharski R, Maleszka J, Foret S, Maleszka R. 2008. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319:1827–30 [DOI] [PubMed] [Google Scholar]
- 64.Kuhn-Buhlmann S,Wehner R. 2006. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants, Cataglyphis bicolor. J. Neurobiol 66:511–21 [DOI] [PubMed] [Google Scholar]
- 65.Laissue PP, Vosshall LB. 2008. The olfactory sensory map in Drosophila. Adv. Exp. Med. Biol 628:102–14 [DOI] [PubMed] [Google Scholar]
- 66.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43:703–14 [DOI] [PubMed] [Google Scholar]
- 67.Lein E, Borm LE, Linnarsson S. 2017. The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing. Science 358:64–69 [DOI] [PubMed] [Google Scholar]
- 68.Leonhardt SD, Menzel F, Nehring V, Schmitt T. 2016. Ecology and evolution of communication in social insects. Cell 164:1277–87 [DOI] [PubMed] [Google Scholar]
- 69.Li-Byarlay H, Li Y, Stroud H, Feng S, Newman TC, et al. 2013. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. PNAS 110:12750–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li-Byarlay H, Rittschof CC, Massey JH, Pittendrigh BR, Robinson GE. 2014. Socially responsive effects of brain oxidative metabolism on aggression. PNAS 111:12533–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Libbrecht R, Oxley PR, Keller L, Kronauer DJ. 2016. Robust DNA methylation in the clonal raider ant brain. Curr. Biol 26:391–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liebig J, Peeters C, Oldham NJ, Markstadter C, Holldobler B. 2000. Are variations in cuticular hy- ¨ drocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? PNAS 97:4124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L. 2014. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods 11:360–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. 2010. The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLOS Biol 8:e1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma L, Wu Y, Qiu Q, Scheerer H, Moran A, Yu CR. 2014. A developmental switch of axon targeting in the continuously regenerating mouse olfactory system. Science 344:194–97 [DOI] [PubMed] [Google Scholar]
- 76.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, et al. 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161:1202–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magklara A, Yen A, Colquitt BM, Clowney EJ, Allen W, et al. 2011. An epigenetic signature for monoallelic olfactory receptor expression. Cell 145:555–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masuoka Y, Yaguchi H, Suzuki R, Maekawa K. 2015. Knockdown of the juvenile hormone receptor gene inhibits soldier-specific morphogenesis in the damp-wood termite Zootermopsis nevadensis (Isoptera: Archotermopsidae). Insect Biochem. Mol. Biol 64:25–31 [DOI] [PubMed] [Google Scholar]
- 79.McGuire SE, Le PT, Davis RL. 2001. The role of Drosophila mushroom body signaling in olfactory memory. Science 293:1330–33 [DOI] [PubMed] [Google Scholar]
- 80.McKenzie SK, Fetter-Pruneda I, Ruta V, Kronauer DJ. 2016. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. PNAS 113:14091–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monahan K, Lomvardas S. 2015. Monoallelic expression of olfactory receptors. Annu. Rev. Cell Dev. Biol 31:721–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morel L, Blum MS. 1988. Nestmate recognition in Camponotus floridanus callow worker ants: Are sisters or nestmates recognized? Anim. Behav 36:718–25 [Google Scholar]
- 83.Mueller UG. 1998. The evolution of agriculture in ants. Science 281:2034–38 [DOI] [PubMed] [Google Scholar]
- 84.Muscedere ML, Traniello JF. 2012. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste- and age-related patterns of worker brain organization. PLOS ONE 7:e31618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagano T, Lubling Y, Stevens TJ, Schoenfelder S, Yaffe E, et al. 2013. Single-cell Hi-C reveals cellto-cell variability in chromosome structure. Nature 502:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakanishi A, Nishino H, Watanabe H, Yokohari F, Nishikawa M. 2009. Sex-specific antennal sensory system in the ant Camponotus japonicus: structure and distribution of sensilla on the flagellum. Cell Tissue Res. 338:79–97 [DOI] [PubMed] [Google Scholar]
- 87.Nijhout HF. 1999. Control mechanisms of polyphenic development in insects. BioScience 49:181–92 [Google Scholar]
- 88.Nijhout HF,Wheeler DE. 1982. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol 57:109–33 [Google Scholar]
- 89.Ott SR, Rogers SM. 2010. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. Biol. Sci 277:3087–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oxley PR, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, et al. 2014. The genome of the clonal raider ant Cerapachys biroi. Curr. Biol 24:451–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, et al. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309:311–14 [DOI] [PubMed] [Google Scholar]
- 92.Paoli PP, Wakeling LA, Wright GA, Ford D. 2014. The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. Age 36:9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pask GM, Slone JD, Millar JG, Das P, Moreira JA, et al. 2017. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun 8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Penick CA, Prager SS, Liebig J. 2012. Juvenile hormone induces queen development in late-stage larvae of the ant Harpegnathos saltator. J. Insect Physiol 58:1643–49 [DOI] [PubMed] [Google Scholar]
- 95.Peters RS, Krogmann L, Mayer C, Donath A, Gunkel S, et al. 2017. Evolutionary history of the Hymenoptera. Curr. Biol 27:1013–18 [DOI] [PubMed] [Google Scholar]
- 96.Rachinsky A, Strambi C, Strambi A, Hartfelder K. 1990. Caste and metamorphosis: hemolymph titers of juvenile hormone and ecdysteroids in last instar honeybee larvae. Gen. Comp. Endocrinol 79:31–38 [DOI] [PubMed] [Google Scholar]
- 97.Rajakumar R, San Mauro D, Dijkstra MB, Huang MH, Wheeler DE, et al. 2012. Ancestral developmental potential facilitates parallel evolution in ants. Science 335:79–82 [DOI] [PubMed] [Google Scholar]
- 98.Ravi N, Sanchez-Guardado L, Lois C, Kelsch W. 2017. Determination of the connectivity of newborn neurons in mammalian olfactory circuits. Cell. Mol. Life Sci 74:849–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reid CR, Lutz MJ, Powell S, Kao AB, Couzin ID, Garnier S. 2015. Army ants dynamically adjust living bridges in response to a cost-benefit trade-off. PNAS 112:15113–18 [DOI] [PMC free article] [PubMed] [Google Scholar]; 99a. Robeau RM, Vinson SB. 1976. Effects of juvenile hormone analogues on caste differentiation in the imported fire ant, Solenopsis invicta. J. Georgia Entomol. Soc 11:198–202 [Google Scholar]
- 100.Robinson GE. 1992. Regulation of division of labor in insect societies. Annu. Rev. Entomol 37:637–65 [DOI] [PubMed] [Google Scholar]
- 101.Robinson GE, Page RE, Strambi C, Strambi A. 1992. Colony integration in honey bees: mechanisms of behavioral reversion. Ethology 90:336–48 [Google Scholar]
- 102.Deleted in proof [Google Scholar]
- 103.Rotem A, Ram O, Shoresh N, Sperling RA, Goren A, et al. 2015. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nat. Biotechnol 33:1165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakano H 2010. Neural map formation in the mouse olfactory system. Neuron 67:530–42 [DOI] [PubMed] [Google Scholar]
- 105.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, et al. 2013. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18:416–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmid A, Chiba A, Doe CQ. 1999. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development 126:4653–89 [DOI] [PubMed] [Google Scholar]
- 107.Schmitt DE, Esch HE. 1993. Magnetic orientation of honeybees in the laboratory. Naturwissenschaften 80:41–43 [Google Scholar]
- 108.Scholl C, Wang Y, Krischke M, Mueller MJ, Amdam GV, Rossler W. 2014. Light exposure leads to reorganization of microglomeruli in the mushroom bodies and influences juvenile hormone levels in the honeybee. Dev. Neurobiol. 74:1141–53 [DOI] [PubMed] [Google Scholar]
- 109.Schulte C, Theilenberg E, Muller-Borg M, Gempe T, Beye M. 2014. Highly efficient integration and ¨ expression of piggyBac-derived cassettes in the honeybee (Apis mellifera). PNAS 111:9003–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwarz MP, Richards MH, Danforth BN. 2007. Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu. Rev. Entomol 52:127–50 [DOI] [PubMed] [Google Scholar]
- 111.Sharma KR, Enzmann BL, Schmidt Y, Moore D, Jones GR, et al. 2015. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 12:1261–71 [DOI] [PubMed] [Google Scholar]
- 112.Shi NN, Tsai CC, Camino F, Bernard GD, Yu N, Wehner R. 2015. Keeping cool: enhanced optical reflection and radiative heat dissipation in Saharan silver ants. Science 349:298–301 [DOI] [PubMed] [Google Scholar]
- 113.Shields EJ, Sheng L, Weiner AK, Garcia BA, Bonasio R. 2018. High-quality genome assemblies reveal long non-coding RNAs expressed in ant brains. Cell Rep. 23:3078–90 [DOI] [PMC free article] [PubMed] [Google Scholar]; 113a. Shpigler H, Amsalem E, Huang ZY, Cohen M, Siegel AJ, et al. 2014. Gonadotropic and physiological functions of juvenile hormone in bumblebee (Bombus terrestris) workers. PLOS ONE 9:e100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shpigler HY, Saul MC, Corona F, Block L, Ahmed AC, et al. 2017. Deep evolutionary conservation of autism-related genes. PNAS 114:9653–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simola DF, Graham RJ, Brady CM, Enzmann BL, Desplan C, et al. 2016. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science 351:aac6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simola DF, Ye C, Mutti NS, Dolezal K, Bonasio R, et al. 2013. A chromatin link to caste identity in the carpenter ant Camponotus floridanus. Genome Res. 23:486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skene PJ, Henikoff S. 2017. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6:e21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sledge MF, Boscaro F, Turillazzi S. 2001. Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol 49:401–9 [Google Scholar]
- 119.Slone JD, Pask GM, Ferguson ST, Millar JG, Berger SL, et al. 2017. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. PNAS 114:8586–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith AR, Seid MA, Jimenez LC, Wcislo WT. 2010. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. Biol. Sci 277:2157–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spannhoff A, Kim YK, Raynal NJ, Gharibyan V, Su MB, et al. 2011. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 12:238–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spindler SR, Hartenstein V. 2010. The Drosophila neural lineages: a model system to study brain development and circuitry. Dev. Genes Evol 220:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sta˚hl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, et al. 2016. Visualization and analysis of ´ gene expression in tissue sections by spatial transcriptomics. Science 353:78–82 [DOI] [PubMed] [Google Scholar]
- 124.Stieb SM, Hellwig A, Wehner R, Rossler W. 2012. Visual experience affects both behavioral and neuronal aspects in the individual life history of the desert ant Cataglyphis fortis. Dev. Neurobiol 72:729–42 [DOI] [PubMed] [Google Scholar]
- 125.Stieb SM, Muenz TS, Wehner R, Rossler W. 2010. Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Dev. Neurobiol 70:408–23 [DOI] [PubMed] [Google Scholar]
- 126.Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. 1998. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn. Mem 5:11–37 [PMC free article] [PubMed] [Google Scholar]
- 127.Tarver MR, Florane CB, Zhang D, Grimm C, Lax AR. 2012. Methoprene and temperature effects on caste differentiation and protein composition in the Formosan subterranean termite, Coptotermes formosanus. J. Insect Sci 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Terrapon N, Li C, Robertson HM, Ji L, Meng X, et al. 2014. Molecular traces of alternative social organization in a termite genome. Nat. Commun 5:3636. [DOI] [PubMed] [Google Scholar]
- 129.Tissenbaum HA, Guarente L. 2001. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–30 [DOI] [PubMed] [Google Scholar]
- 130.Trible W, Olivos-Cisneros L, McKenzie SK, Saragosti J, Chang NC, et al. 2017. orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170:727–35.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsai L, Barnea G. 2014. A critical period defined by axon-targeting mechanisms in the murine olfactory bulb. Science 344:197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vinson SB, Robeau R. 1974. Insect growth regulator effects on colonies of the imported fire ant. J. Econ. Entomol 67:584–87 [DOI] [PubMed] [Google Scholar]
- 133.Vowles DM. 1950. Sensitivity of ants to polarized light. Nature 165:282–83 [DOI] [PubMed] [Google Scholar]
- 134.Waddington CH. 1957. The Strategy of the Genes. London: George Allen-Unwin [Google Scholar]
- 135.Wang SC, Oelze B, Schumacher A. 2008. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLOS ONE 3:e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wheeler DE. 1986. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am. Nat 128:13–34 [Google Scholar]
- 137.Wheeler DE, Nijhout HF. 1981. Soldier determination in ants: new role for juvenile hormone. Science 213:361–63 [DOI] [PubMed] [Google Scholar]
- 138.Wilson EO. 1965. Chemical communication in the social insects. Science 149:1064–71 [DOI] [PubMed] [Google Scholar]
- 139.Wilson EO. 1983. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). III. Ergonomic resiliency in foraging by A. cephalotes. Behav. Ecol. Sociobiol 14:47–54 [Google Scholar]
- 140.Wilson EO. 1984. The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera, Formicidae). Behav. Ecol. Sociobiol 16:89–98 [Google Scholar]
- 141.Withers GS, Fahrbach SE, Robinson GE. 1993. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364:238–40 [DOI] [PubMed] [Google Scholar]
- 142.Yan H, Bonasio R, Simola DF, Liebig J, Berger SL, Reinberg D. 2015. DNA methylation in social insects: how epigenetics can control behavior and longevity. Annu. Rev. Entomol 60:435–52 [DOI] [PubMed] [Google Scholar]
- 143.Yan H, Opachaloemphan C, Mancini G, Yang H, Gallitto M, et al. 2017. An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170:736–47.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan H, Simola DF, Bonasio R, Liebig J, Berger SL, Reinberg D. 2014. Eusocial insects as emerging models for behavioural epigenetics. Nat. Rev. Genet 15:677–88 [DOI] [PubMed] [Google Scholar]
- 145.Yu CR, Power J, Barnea G, O’Donnell S, Brown HEV, et al. 2004. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron 42:553–66 [DOI] [PubMed] [Google Scholar]
- 146.Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, et al. 2017. Massively parallel digital transcriptional profiling of single cells. Nat. Commun 8:14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou X, Rokas A, Berger SL, Liebig J, Ray A, Zwiebel LJ. 2015. Chemoreceptor evolution in Hymenoptera and its implications for the evolution of eusociality. Genome Biol. Evol 7:2407–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou X, Slone JD, Rokas A, Berger SL, Liebig J, et al. 2012. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLOS Genet. 8:e1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zube C, Rossler W. 2008. Caste- and sex-specific adaptations within the olfactory pathway in the brain of the ant Camponotus floridanus. Arthropod Struct. Dev 37:469–79 [DOI] [PubMed] [Google Scholar]