Anantharam and Kreutzberger describe the reductionist approaches that have begun to unveil the mechanistic basis of secretion.
Abstract
Ca2+-dependent secretion is a process by which important signaling molecules that are produced within a cell—including proteins and neurotransmitters—are expelled to the extracellular environment. The cellular mechanism that underlies secretion is referred to as exocytosis. Many years of work have revealed that exocytosis in neurons and neuroendocrine cells is tightly coupled to Ca2+ and orchestrated by a series of protein–protein/protein–lipid interactions. Here, we highlight landmark discoveries that have informed our current understanding of the process. We focus principally on reductionist studies performed using powerful model secretory systems and cell-free reconstitution assays. In recent years, molecular cloning and genetics have implicated the involvement of a sizeable number of proteins in exocytosis. We expect reductionist approaches will be central to attempts to resolve their roles. The Journal of General Physiology will continue to be an outlet for much of this work, befitting its tradition of publishing strongly mechanistic, basic research.
Introduction
Life in multicellular organisms depends on the proper execution of exocytosis. In the context of cell-to-cell communication, the process serves to signal the status of one cell to another, or to modulate the functional status of neighboring or more distant cells. At higher levels of organization, within mammalian endocrine tissue, for example, it enables coordinated physiological responses that are essential for organismal homeostasis. Over the past several decades, many of the molecular regulators of exocytosis have been systematically identified. A great deal is now known about the biochemistry of the core fusion machine and the structure of its constituents. We have arrived at this point in our understanding of exocytosis through the combined efforts of a number of investigators using diverse experimental preparations. It would be an impossible task to detail that immense body of work in the pages of this brief article and do justice to the individual accomplishments. Instead, we will focus on the value of landmark reductionist studies using model secretory systems, including the sea urchin egg, the frog neuromuscular junction, and the adrenal chromaffin cell. These studies have been critical to elucidating the signature features of exocytosis, especially its steep Ca2+ dependence and requirement for ATP. Moreover, they have enabled roles for specific actors in fusion to be conceptualized without knowing anything about their identity. We will end this article by discussing the current phase of reconstitution assays, which has required cloning and identification of the actual proteins.
A second goal of this article is to highlight articles of importance related to secretion that have been published in JGP. Although the themes of exocytosis and membrane fusion have not been an explicit focus of the journal, a number of influential studies have been published within its pages over the years. In fact, one of the very first quantitative measurements of exocytosis appears in a 1935 edition of the journal. In that article, K.S. Cole calculated changes in the membrane surface area of a suspension of sea urchin eggs after fertilization (Cole, 1935). We now know this to result from the fusion of cortical granules with the plasma membrane during the creation of the fertilization envelope. Consistent with the mission of the journal, there has always been a place for fundamental studies “elucidating biological, chemical, or physical mechanisms of broad physiological significance.” In this article, we will highlight some of those studies within the framework of what has been and is being published elsewhere. We issue the requisite disclaimer that aspects of this article are necessarily narrow in their focus. By no means does it constitute the final word on this topic, nor does it seek to recount all of the varied contributions that bring us to the present time.
The first descriptions of the basic morphological features of exocytosis
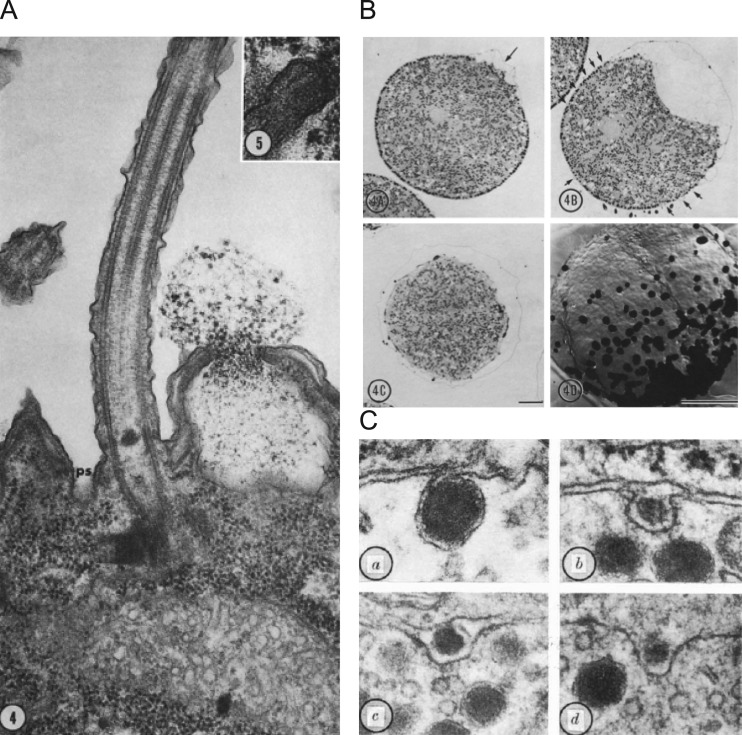
We begin this article in the 1950s when George Palade, publishing extensively in the Journal of Biophysical and Biochemical Cytology (soon to be renamed the Journal of Cell Biology) reported in vivid detail the hidden, inner life of a secretory cell within the exocrine pancreas. A series of landmark biochemical and morphological studies followed, illuminating the structure and function of subcellular organelles we now recognize as ribosomes, the ER, and transport organelles (Palade, 1956a,b; Palade and Siekevitz, 1956; Siekevitz and Palade, 1958a,b,c). Armed with autoradiography and EM as an alternative to cell fractionation, Palade and his colleagues, Lucien Caro and Jim Jamieson, elaborated the export pathway of a digestive enzyme, from ER exit to discharge from the cell (Caro and Palade, 1964; Jamieson and Palade, 1966, 1967a,b). Over the course of these studies, Palade remarked that for enzyme secretion to occur, there must be “…continuity established between the granule compartment and extracellular medium (lumen), concomitantly with continuity of the granule membrane with the plasmalemma all around the orifice through which the granule content reaches the lumen” (Palade, 1959, 1975). Because of Palade’s elegant work, terms that had recently been coined and were entering the common scientific parlance, such as “membrane fusion” and “exocytosis,” began to take on morphological meaning. The structural rearrangements that accompany fusion were elaborated upon by others, notably Bill Douglas and colleagues at the posterior pituitary (Nagasawa et al., 1970) and John Heuser and Thomas Reese at the frog neuromuscular junction (Heuser and Reese, 1973, 1981; Heuser et al., 1979). By this time, it was understood that exocytosis in neurons and neuroendocrine cells required Ca2+ (Del Castillo and Katz, 1954a; Douglas and Rubin, 1961b; Katz and Miledi, 1965). Other studies published in the 1970s established that the process shares a common form in a variety of organisms and settings, including, for example, mucocyst discharge in Tetrahymena and deposition of the fertilization envelope in sea urchin eggs (Chandler and Heuser, 1979; Fig. 1).
Figure 1.
Morphological changes accompanying secretion are identified by EM. (A) Mucocyst discharge in the ciliated protist Tetrahymena pyriformis. A longitudinal section showing a cilium, its accompanying parasomal sac (ps), and a discharging mucocyst. Magnification × 72,000. Taken from Satir et al. (1973), A is reprinted with the permission of the Journal of Cell Biology. (B) A wave of sea urchin cortical granule exocytosis in light micrographs and in freeze-fracture replicas. Top left: Initiation of exocytosis at arrow with raising of fertilization membrane. Magnification × 800. Top right: Numerous small elevations at arrows are evident as wave of fusion propagates. Bottom left: Exocytosis is complete as indicated by a fully elevated membrane and absence of granules. Bottom right: “Face-on” view of exocytotic “wave” with darkened spots indicating locations of fusion events. Magnification × 2,000. Bar, 10 µm for all images. Taken from Chandler and Heuser (1979), B is reprinted with the permission of the Journal of Cell Biology. (C) Images of a secretory event in the rat posterior pituitary. a: A granule fused with the cell membrane and possibly communicating with the extracellular space via a narrow opening. b: A fusion-associated “omega figure” in which the opening to the extracellular space is more evident. c and d: The opening has now expanded to the point where the granule contents may be discharged completely to the extracellular space. Magnification × 96,000. Taken from Nagasawa et al. (1970), C is reprinted with the permission of Nature.
Early attempts to reconstitute fusion between liposomes
It was widely appreciated that exocytosis was a phenomenon of immense biological import in the 1970s. Because laboratory techniques had not yet matured to the point where the process could be conveniently studied in living cells, investigators turned to reductionist approaches in an attempt to address mechanistic questions. Thus, a series of studies was published in that decade attempting to reconstitute membrane fusion using artificial lipid vesicles (Papahadjopoulos et al., 1974; Papahadjopoulos and Poste, 1975; Holz and Stratford, 1979). The hope was that meaningful biological insights would emerge by recapitulating the process from first principles.
Two basic conditions had to be met for artificial vesicle–vesicle fusion to have any bearing on our understanding of the cellular process: there had to be coalescing of membrane phospholipids along with mixing of their respective aqueous contents. A common problem was that there was no standard assay in place with the sensitivity to confirm that these events had actually occurred. The various approaches that were employed typically used Ca2+ in the high millimolar range because it was not yet established that a Ca2+-binding protein was needed for fusion (Holz and Stratford, 1979; Wilschut and Papahadjopoulos, 1979). More broadly, it was not clear to what extent proteins participated in the fusion reaction. As a result, some of the earliest efforts to understand fusion labored to define the physico-chemical basis for phenomena that had only a tenuous link to cellular exocytosis. For example, Ca2+ alone, at millimolar levels, has complicated effects on phospholipid membranes (Papahadjopoulos et al., 1978; Portis et al., 1979; Wilschut and Papahadjopoulos, 1979), inducing vesicle aggregation and sometimes fusion (Holz and Stratford, 1979).
Reconstituting fusion between a vesicle and planar bilayer
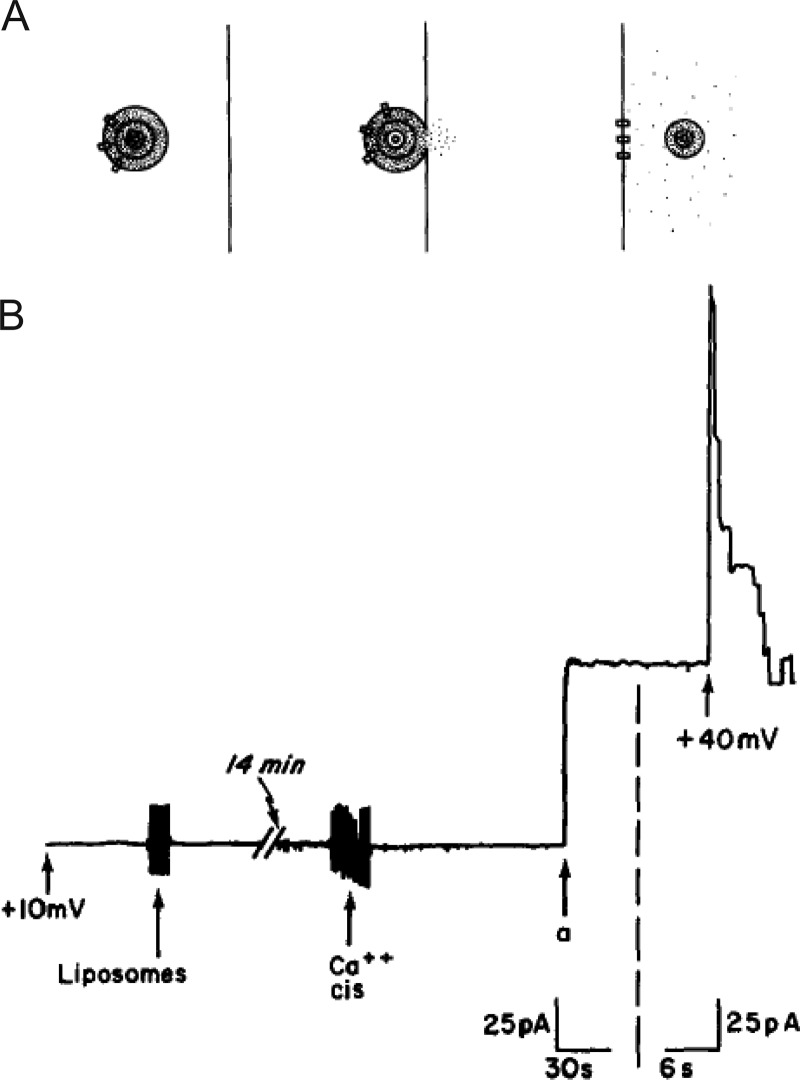
The very first studies providing convincing evidence that artificial vesicles could fuse with an artificial planar membrane appeared in back-to-back articles in JGP in 1980 by Alan Finkelstein, Fred Cohen, and Josh Zimmerberg (Cohen et al., 1980; Zimmerberg et al., 1980a). There are remarkable aspects of these studies that merit a more detailed retelling.
To establish an assay system appropriate for monitoring fusion, the authors adapted a method established some years before from a thin 60-Å bilayer barrier between two aqueous compartments (Mueller et al., 1962). The compartments were denoted as “cis” and “trans” based on which side the vesicles were added. The vesicles themselves were multilammelar and housed a fluorescent soluble dye within all lipid compartments. Based on this design, the criterion used to score fusion was the appearance of fluorescein marker on the side of the planar membrane opposite (trans) to the side on which vesicles were added (Zimmerberg et al., 1980a; Fig. 2 A). The use of the soluble marker distinguished this study from others assaying membrane continuity, which were confounded by the possible nonfusion exchange of markers including diffusion of membrane intercalating dyes from one compartment to the other. In this case, the evidence for fusion was unambiguous as the aqueous lumenal marker could not otherwise traverse the hydrophobic core of the membrane. Moreover, the very fact that transfer occurred and that the fluorescent “packaged” material could be detected on the opposite side of the barrier satisfied the key requirements for an assay system with potential usefulness for investigations of exocytotic mechanisms.
Figure 2.
Events associated with fusion in the planar bilayer assay. (A) Progression of events associated with fusion from left to right. A multilamellar vesicle is situated on the cis side of a planar bilayer. It contains ion-permeable channels (rectangles) and a fluorescent dye (dots) trapped in its aqueous compartments. With fusion of the vesicle with the membrane, the fluorescent dots begin to escape to the trans side of the membrane. The ion-permeable channels are now in the planar membrane. The key to detection of fluorescence on the trans side is the presence of a vesicle which has lost only its outer lamella during fusion but retains fluorescence dye within aqueous compartments. Taken from Zimmerberg et al. (1980a). (B) When VDAC-containing liposomes are added to the cis compartment, no conductance increases are initially detectable. After addition of 30 mM CaCl2, a single jump is observed (a). When voltage is switched to 40 mV, several channels are seen turning off, demonstrating that the current jump resulted from the insertion of several channels. Taken from Cohen et al. (1980).
Despite its elegance, the method was not yet ready for routine use. High background signal, membrane breakage, and malfunctions of a fluorescence-activated cell sorter all conspired to make investigations cumbersome and inconvenient. Indeed, the discussion offers the trenchant observation that the “small number [of successful experiments] reflects not a lack of diligence and zeal on the part of the authors, but rather their finite capacity for heroics” (Zimmerberg et al., 1980a). Thus, in a companion article, the authors describe how an additional membrane reporter could be used to complement the dye-based reporter described above, or to be used in its stead (Cohen et al., 1980). The alternate reporter, in this case, was a membrane-associated protein—an ion channel. The channel represented a voltage-dependent conductance (VDAC) with current-voltage characteristics distinct from the nonspecific conductance changes that might otherwise be measured in membranes. When vesicles containing the protein were added to one side of the membrane, current jumps appeared that were consistent with the addition of many ion channels upon vesicle fusion (Fig. 2 B).
Because VDAC was an integral membrane protein, neither diffusion nor contact-initiated transfer were likely routes of transfer (Cohen et al., 1980). Moreover, the number of channels inserted into the planar bilayer was correlated with the number of VDAC molecules in multilammelar vesicles and the size distribution of those vesicles. Again, the fact that transfer could be readily detected provided encouragement that the assay could lead to better understanding of exocytotic mechanisms. The counter argument was that a synthetic assay, consisting of a limited number of phospholipids and proteins, bore only a superficial resemblance to the more complex process at work within cells. Nonetheless, observations made in this simple assay spurred a number of important investigations to follow. For example, the authors noted that in order for fusion to occur with any degree of reliability, two requirements had to be satisfied: (1) multivalent cations had to be present in the cis compartment (note that concentrations of Ca2+ ≥ 30 mM, Ba2+ ≥ 20 mM, and Mg2+ ≥ 80 mM were all effective in inducing fusion; Cohen et al., 1980), and (2) an osmotic pressure difference had to exist across the plasma membrane (cis side higher). If only the second condition was satisfied, very modest levels of fusion were observed (Zimmerberg et al., 1980a). If only the first condition was satisfied, no fusion was detectable.
By this time, it was established fact that Ca2+ was indispensable for exocytosis in many forms of mammalian exocytosis (Douglas and Rubin, 1961b; Katz and Miledi, 1965). However, the underlying mechanism by which Ca2+ triggered fusion and vesicle content release remained unclear. A number of ideas had been posited, including Ca2+-dependent bridging of bilayers, electrostatic screening of negative charges between bilayers, and local dehydration of charged groups, without a definitive verdict (Papahadjopoulos et al., 1978; Portis et al., 1979; Zimmerberg et al., 1980a). The notion that an osmotic gradient might be important for membrane fusion arose out of work performed some years earlier by Chris Miller, who at the time was a postdoctoral fellow in Ephraim Racker’s laboratory. In his assay, as in others, the fusion of unilamellar liposomes was shown to be dependent on the presence of acidic phospholipids as a major membrane constituent in addition to high millimolar Ca2+. However, after the growth of liposomes (by fusion) to large diameters in excess of 0.1 µm, further growth, and fusion, cease. In fact, the only way to restore the ability of larger liposomes to fuse was through the imposition of a positive transmembrane osmotic gradient (Miller et al., 1976).
The conditions that enabled liposome-to-liposome fusion seemed on their face to have little resemblance to what occurs within cells. It was already known that micromolar, not millimolar, amounts of Ca2+ were required to trigger fusion at biological membranes (Baker and Knight, 1978; Baker and Whitaker, 1978). The fact that other divalent cations, especially Mg2+, had similar effects to Ca2+ in vitro with respect to induction of lipid phase separations and promoting liposome aggregation, complicated efforts to attribute fusion strictly to the interaction of Ca2+ and phospholipids (Portis et al., 1979). Moreover, large osmotic pressure differences between the cytosol and extracellular space were not typical for excitable cells in the brain or periphery. With respect to Ca2+, Finkelstein and colleagues reasoned that in both synthetic and “real” systems, Ca2+ may function to bring membranes into close apposition in a way that promoted subsequent fusion. All that was missing here were high, divalent ion affinity binding sites probably provided by some as of yet unidentified protein (Zimmerberg et al., 1980a). Moreover, osmotic differences may serve a more localized function in cells, where possibly before fusion an increase in ionic permeability drives swelling and rupture by mobilizing inactive vesicular contents.
In an attempt to address the issue of nonphysiological levels of Ca2+ being required to trigger fusion, a “Ca2+-binding protein,” partially purified from calf brain, was dispersed into the planar bilayer (Zimmerberg et al., 1980b). Remarkably, the system now exhibited Ca2+ sensitivity in the micromolar range that better fit expectations based on cellular data. Later studies by Zimmerberg, Holz, and others would reveal that osmotic swelling was not directly relevant to the biology of the fusion reaction (Holz, 1986; Zimmerberg et al., 1987). Taken together, the work outlined above did suggest that there were two experimentally separable steps in vesicle fusion with planar bilayer membranes: (1) vesicular “attachment” to the bilayer during a prefusion state, and (2) destabilization of lipids at their points of contact. For the second to occur with the strong Ca2+ dependence most familiar to cell biologists, some Ca2+-binding protein was necessary. That such conditions could be identified highlights the value of reductionist approaches, through which the many elements of a complicated biological process could be distilled to core principles.
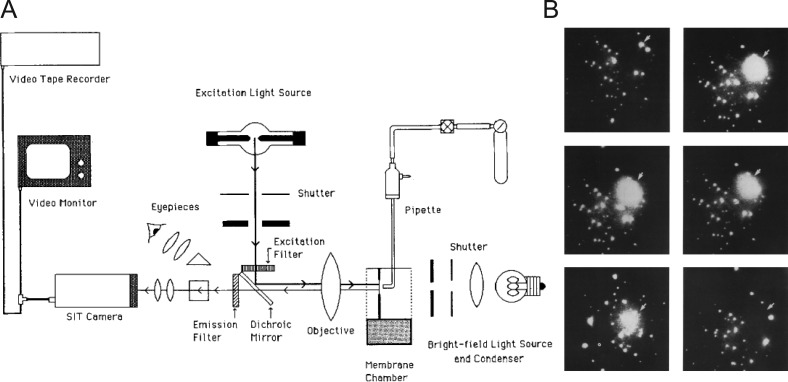
The planar bilayer system would remain an important element in the experimentalist’s toolkit, finding use in revealing biophysical characteristics of ion channels (Cohen and Niles, 1993) and later, soluble N-ethylmaleimide sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE)–mediated fusion (Woodbury and Rognlien, 2000; Bao et al., 2018). We should note that one limitation of the assay as originally described was that it could not describe, in real time, the efficient transfer of contents from one compartment into the other. Transfer of contents across membranes is necessarily a defining feature of vesicular exocytosis. Cohen developed optical techniques to overcome that limitation by loading vesicles with calcein and using a planar bilayer configuration that could accommodate a fluorescence microscope with video-rate imaging capability (Niles and Cohen, 1987; Fig. 3 A). The vesicles themselves were large (2–10 µm) and thus easily resolvable. These modifications would allow Cohen and colleagues to directly monitor the release of dye resulting from vesicle fusion and rupture as a bright flash of light (Fig. 3 B). There was another clever element in the design of the assay. The side of the planar membrane to which dye was released contained cobalt, which had the effect of quenching the calcein fluorescence. Therefore, release of calcein into cobalt-containing solution resulted in flashes of a short duration, whereas release into a cobalt-free solution (e.g., during vesicular rupture, not fusion) resulted in flashes of a long duration. This allowed proper fusion to be definitively distinguished from aberrant events. We note that this study provided the first example of how one might monitor individual fusion events, as they happened, with fluorescence microscopy.
Figure 3.
Fluorescence-based imaging of single-vesicle fusion. (A) Diagram of the fluorescence microscope arrangement and video equipment. The light sources, filters, dichroic mirror, objective, condenser, and eyepieces were components of an upright microscope laid on its backbrace. (B) Individual movie frames from the video record are temporally arranged from left to right and top to bottom. The first panel indicates the time immediately before vesicle rupture and release of calcein (subsequent frames); the intact vesicle is indicated by an arrowhead. The final frame indicates the original location of the vesicle, which is no longer evident. Taken from Niles and Cohen (1987).
Exploring the Ca2+ dependence of secretion in adrenal chromaffin cells
To directly address questions relevant to the regulation of cellular exocytosis, one needed access to biological systems that were amenable to manipulation. Bear in mind that, at this time, there was not a strong appreciation for how genetics might inform studies on exocytosis. Indeed, it was only in the late 1970s that Randy Schekman and Peter Novick began to demonstrate how the yeast system could be harnessed to dissect the process of secretion (Novick and Schekman, 1979). Thus, the clearest way forward was to biochemically modify the protein and ionic composition of the cell interior so that critical control points for exocytosis could be identified. A system that would prove to be immeasurably valuable in these efforts was the adrenal chromaffin cell.
It had been known since the 1950s that secreted constituents of the adrenal chromaffin cell were stored within organelles and released via exocytosis (De Robertis and Vaz Ferreira, 1957). The experiments of Douglas and colleagues (Douglas and Rubin, 1961a,b) established the idea that Ca2+ acted intracellularly to drive fusion, just as at the neuromuscular junction (Del Castillo and Katz, 1954a). But how much Ca2+ was necessary to initiate fusion in any quantitative sense was not yet known. An answer to this question appeared to be within reach after Reed and Lardy (1972) reported on the ability of a newly identified carboxylic acid antibiotic, A23187, to transport Ca2+ across phase boundaries. They proposed that its properties might be useful in investigations of “various biological systems.” Shortly thereafter, others demonstrated that the application of A23187 to perfused cat adrenals prompted catecholamine secretion even in the absence of depolarization (Cochrane et al., 1975; Garcia et al., 1975). Johnson and Scarpa (1976) subsequently showed that chromaffin granules had a low intrinsic permeability to Ca2+, and that the addition of A23187 to purified granules did not cause them to release catecholamines. These findings supported the idea that release of catecholamines occurred as a result of fusion and not a generalized leakage into the cytosol followed by release by other means.
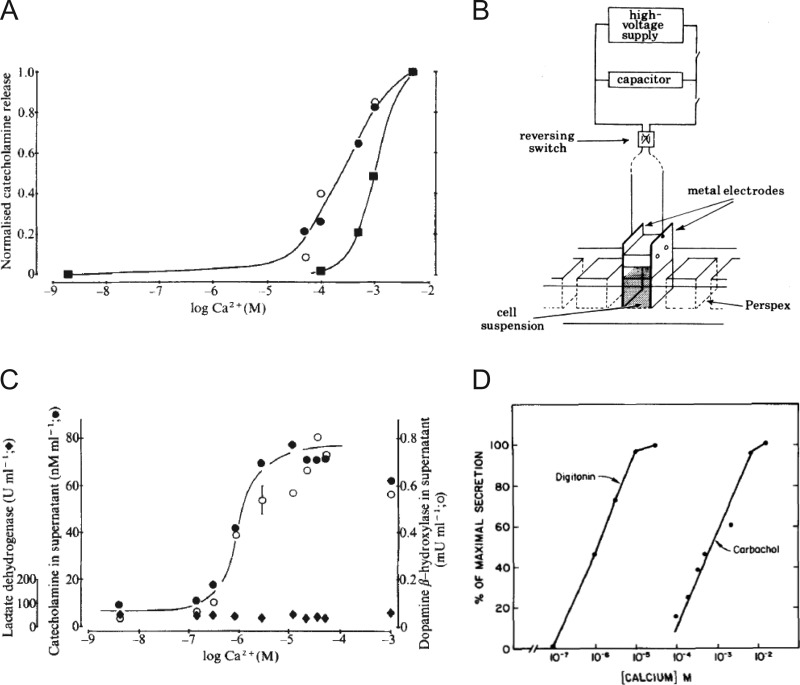
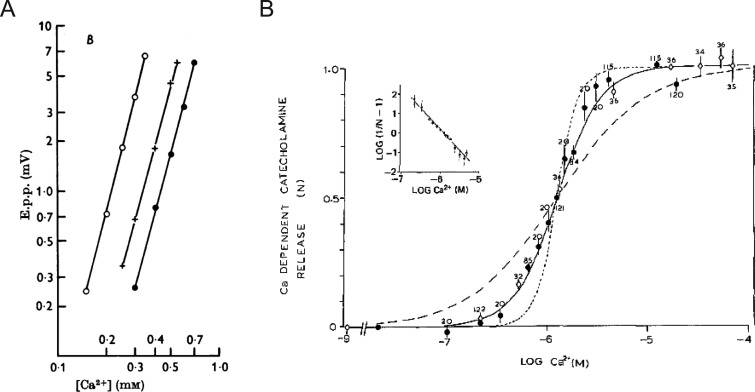
As useful as the ionophores were in validating the absolute Ca2+ dependence of exocytosis, the final level of intracellular Ca2+ that was achieved as a result of ionophore-mediated transport, and therefore the level necessary to evoke secretion, was still not obvious. Thus, work continued on alternate methods that might afford a greater degree of control over the intracellular milieu and allow a more precise determination of Ca2+ requirements. This effort culminated in a seminal study by Peter Baker and Derek Knight, who made the observation that permeabilized cells could actually be stimulated to secrete in a Ca2+-dependent manner (Baker and Knight, 1978; Fig. 4). They recognized that exposing suspensions of bovine chromaffin cells to a series of high-voltage discharges generated holes in the plasma membrane by causing localized dielectric breakdown (Fig. 4 B). They further demonstrated that the field strength could be modulated so that the plasma membrane could be breached without perturbing the structural integrity of the granule membrane (Rink and Knight, 1988). When cells were exposed to high-voltage discharges in media containing Ca2+, catecholamines were released. In fact, a major result of the early experiments on chromaffin cells was the demonstration that although the plasma membrane was freely permeable to solutes of up to 1 kD, less than 1% of the total catecholamine leaked out of the cell (Rink and Knight, 1988). This result argued strongly for a vesicular mechanism of secretion rather than secretion coming out of a cytosolic pool. Electropermeabilization also allowed direct estimates of the amount of Ca2+ necessary for provoking catecholamine discharge, with half-maximal discharge occurring in the range of 1 µM Ca2+ (Baker and Knight, 1978; Fig. 4, A–C). In intact cells, a much greater concentration of extracellular Ca2+ was necessary to observe a comparable level of release (Fig. 4 A). The extent of catecholamine release from electropermeabilized chromaffin cells increased as a sigmoidal function of buffered Ca2+ concentration (Knight and Baker, 1982). The relationship between secretion and Ca2+ was fit by a Hill function with a coefficient of 2, suggesting that the binding of 2 Ca2+ ions at an intracellular site was required to trigger fusion (Fig. 8 B).
Figure 4.
Ca2+-dependent release of intracellular constituents from intact and permeabilized bovine adrenal chromaffin cells. (A) Ca2+-dependent release from intact bovine chromaffin cells in response to 500 µM of the cholinergic agonist carbamylcholine (closed circles), to 500 µM of veratridine (closed squares), and to a potassium challenge (open circle). In all cases, secretion is half-maximal at Ca2+ concentrations between 0.1 and 1 mM and is associated with release of the granule protein dopamine β-hydroxylase but not the cytosolic protein lactate dehydrogenase. A and C are taken from Baker and Knight (1978) and are reprinted with permission from Nature. (B) The experimental setup used to expose a suspension of cells to a brief electric field. Taken from Baker and Knight (1981), B is reprinted with permission from Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. (C) Ca2+-dependent release of catecholamines (closed circles), dopamine β-hydroxylase (open circles), and lactate dehydrogenase (closed diamond) in response to electrical discharges. (D) Ca2+-dependent release of [3H]norepinephrine into the medium of digitonin permeabilized chromaffin cells or intact cells exposed to the nicotinic agonist carbochol. Taken from Dunn and Holz (1983), D is reprinted with permission from the Journal of Biological Chemistry.
Figure 8.
The dependence of secretion on Ca2+. (A) The relationship between Ca2+ concentration and the amplitude of the end-plate potential (e.p.p.) displayed as a log–log plot. Fits at different Mg2+ concentrations are shown (open circle, 0.5 mM; plus, 2 mM; filled circle, 4 mM), all with an approximate slope of 4. This indicates that a fourth power relationship exists between the e.p.p. and Ca2+. Note, Mg2+ does not change the slope, but merely shifts the line along the x axis. Taken from Dodge and Rahamimoff (1967), A is reprinted with permission from the Journal of Physiology. (B) Catecholamine released from leaky cells as a result of raising Ca2+ concentration from 10−9 M (open diamonds) or 2 × 10−8 M (filled circle). The release (N) is expressed relative to the amount released at 10−5 M Ca2+. The numbers represent the number of observations, and error bars represent SEM. The solid line is a theoretical curve based on two Ca2+ ions involved in the secretory process; the dotted curve is based on 4; the dashed curve is based on 1. The inset is a Hill plot. The regression line has a slope of 2.2. Taken from Knight and Baker (1982), B is reprinted with permission from the Journal of Membrane Biology.
Inspired by this important advance, other techniques to bypass the plasma membrane barrier appeared in the literature. Howell and Gomperts used streptolysin O as a membrane permeabilizing agent to demonstrate that rat mast cells secrete histamine in response to buffered Ca2+ in the micromolar range (Howell and Gomperts, 1987). Possibly the most successful of these efforts were those of Ron Holz and his collaborators, who reported that chromaffin cells in culture could be permeabilized with the detergent digitonin (Dunn and Holz, 1983). In the presence of this detergent, openings rapidly appeared in the membrane that were large enough to allow low-molecular-weight ions (e.g., Ca2+ and ATP) to rapidly equilibrate between the extracellular and intracellular spaces. Despite the fact that permeabilization allowed some cytosolic proteins to escape, chromaffin granules remained intact, did not leak catecholamines, and were able to maintain a proton gradient (Holz and Senter, 1985; Sarafian et al., 1987; Ali et al., 1989). Experiments in digitonin-permeabilized cells provided further evidence of the highly Ca2+-sensitive nature of the secretory response (Dunn and Holz, 1983; Wilson and Kirshner, 1983). Secretion, monitored by determining the amount of catecholamine released, increased in the presence of micromolar Ca2+ and continued for many minutes (Fig. 4 D). Overall, the approach demonstrated the feasibility of increasing plasma membrane permeability to molecules as large as 134 kD while maintaining the essential Ca2+-dependent reactions for exocytosis (Dunn and Holz, 1983).
The role of ATP in exocytosis
It was recognized early on that secretion from digitonin-permeabilized cells decayed with time, even in the continuous presence of Ca2+ (Holz and Senter, 1985; Sarafian et al., 1987; Ali et al., 1989). Some of the lost response could be recovered by including MgATP in the medium—a result also noted by Baker and Knight (1978) some years before in electropermeabilized cells. It was further demonstrated that when cells were initially permeabilized in the presence of MgATP, subsequent Ca2+-triggered norepinephrine release was greater than when MgATP was absent. This suggested that ATP was important for supporting essential biochemical reactions that precede Ca2+-dependent secretion in intact cells. What was still not known at the time was why ATP was necessary and what protein or lipid target it acted upon to maintain the normal secretory capacity of the cell. In later experiments, Eberhard et al. (1990) would demonstrate that the removal of ATP caused a rapid decrease in the levels of membrane polyphosphoinositides. The addition of a bacterial phospholipase C specific for inositol phospholipids to permeabilized chromaffin cells inhibited Ca2+-dependent secretion in a manner that was comparable to the removal of ATP. Furthermore, preincubation of permeabilized cells with neomycin in the absence of ATP maintained both the polyphosphoinositides (by binding to the lipids and protecting them from lipid phosphatases) and secretion (Eberhard et al., 1990). Thus, it became apparent that inositol phospholipids, despite being only a minor constituent of cell membranes, were critical for maintaining the normal secretory response of a chromaffin cell. The next step was to identify the endogenous factors responsible for ATP-dependent priming. This was soon achieved by Tom Martin and Jesse Hay using another powerful secretory model cell—the PC12 cell.
Rat pheochromocytoma cells (PC12 cells) were initially developed and characterized by Lloyd Greene and Arthur Tischler in 1976 (Greene and Tischler, 1976). They became a widely used secretory system once it was demonstrated that they secrete norepinephrine and also acetylcholine in response to membrane depolarization (Greene and Rein, 1977). In 1989, Tom Martin published a method for “cracking” cells for studies of regulated exocytosis (Martin and Walent, 1989). It was first applied to a GH3 pituitary secretory cell line and, a little later, to the PC12 cell (Martin and Walent, 1989; Walent et al., 1992). In both cases, the cells were permeabilized by passing them once through a steel ball homogenizer, thereby creating a large “scar” on the surface membrane. By subsequently rinsing the cracked cell with physiological media, soluble cytoplasmic factors could be completely depleted while still maintaining the essential intracellular structure necessary for regulated exocytosis to occur (Hay and Martin, 1992). This allowed the authors to reconstitute fusion in semi-intact cells with cytosolic proteins purified from rat brain homogenates. Two of the factors were subsequently identified as a phosphatidylinositol (PI) transfer protein (Hay and Martin, 1993) and PI(4)P-5 kinase (Hay et al., 1995).
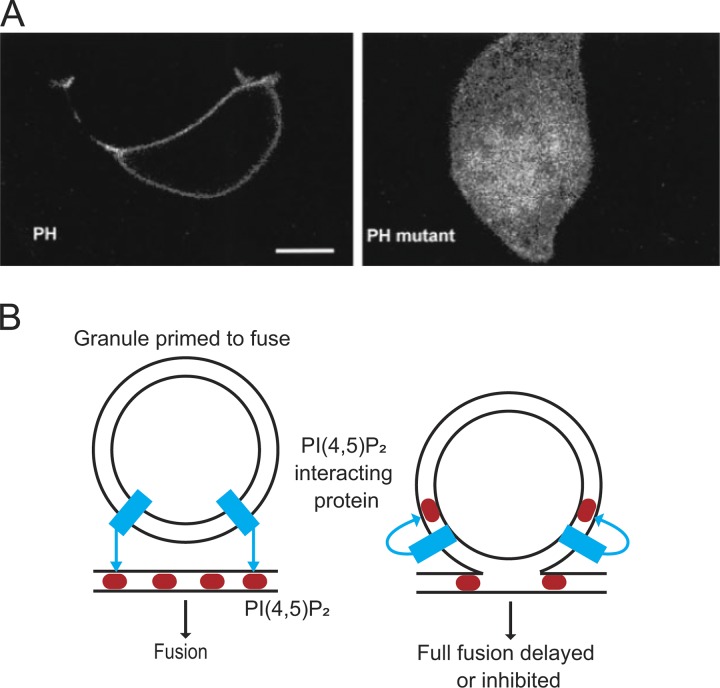
The studies referenced above indicated that the requirement for ATP in secretion reflected, at least in part, its role in the sequential phosphorylation of phosphatidylinositol in a pathway consisting of a phosphatidylinositol (PI) transfer protein, a PI-4 kinase and a PI(4)P-5 kinase. It is now clear that the presence of PI 4,5-bisphosphate (PI(4,5)P2) is necessary for Ca2+-dependent secretion. Over the years, a number of proteins with important roles in exocytosis, including synaptotagmin (Bai et al., 2004), Munc13 (Shin et al., 2010), and CAPS (Grishanin et al., 2002), have been shown to depend on PI(4,5)P2 for their function. Imaging studies performed using a pleckstrin homology (PH)-GFP probe that binds to membrane PI(4,5)P2 (Várnai and Balla, 1998; Holz et al., 2000) have helped establish the now accepted view that the relevant interactions occur at the plasma membrane (Fig. 5 A). Note, however, that there is recent evidence that some granules apposing the plasma membrane obtain PI(4,5)P2 without fusion (Zhao et al., 2016). A recent Viewpoint in JGP discusses this finding and the implications for exocytosis (Abbineni et al., 2018; Fig. 5 B).
Figure 5.
The role of inositol phospholipids in fusion. (A) PH-GFP identifies primarily PI(4,5)P2 in the plasma membrane (note fluorescence in cell periphery; left panel). When a critical basic residue for the interaction is mutated, GFP does not label the plasma membrane. Taken from Holz et al. (2000), A is reprinted with permission from the Journal of Biological Chemistry. (B) The localization of PI(4,5)P2 (red) in the membrane may promote protein–lipid interactions (blue) for formation of fusion pores. Expression of PI(4,5)P2 on the granule membrane may thwart those interactions. B is based on Abbineni et al. (2018).
We mention here that ATP has at least two other important uses in regulated exocytosis aside from a role in phosphoinositide synthesis as described above. First, ATP supports the function of N-ethylmaleimide Sensitive Factor (NSF) which, together with the adaptor Soluble NSF Attachment Protein (SNAP), disassembles post-fusion, cis SNARE complexes (Block et al., 1988; Malhotra et al., 1988; Banerjee et al., 1996). This ATP-dependent action of NSF is important for readying the fusion apparatus for subsequent rounds of exocytosis. Although NSF was at one time suggested to catalyze the fusion of transport vesicles with membranes, this idea became untenable when it was shown in permeabilized PC12 and chromaffin cells that ATP was not required for fusion per se, but for a step that preceded fusion (Holz et al., 1989; Hay and Martin, 1992). Another role for ATP is in phosphorylation of protein substrates important for exocytosis. This topic is reviewed elsewhere in detail (Klenchin and Martin, 2000; Laidlaw et al., 2017; Kadkova et al., 2018).
Release mechanisms exhibit heterogeneous Ca2+ sensitivities
An intrinsic feature of the secretory response, conserved across diverse evolutionary phyla, is that at very low concentrations of Ca2+ approximating resting cytosolic levels, very little, if any, exocytosis is observed. At many micromolar Ca2+, the response becomes “maximal” or saturates. Paul Blank, Josh Zimmerberg, and colleagues examined cortical granule fusion in sea urchin eggs to examine the basis for submaximal responses in Ca2+-triggered exocytosis (Blank et al., 1998a,b, 2001). These studies, published in JGP, provided critical insights into the basis for the heterogeneous secretory response to Ca2+, since observed in other systems.
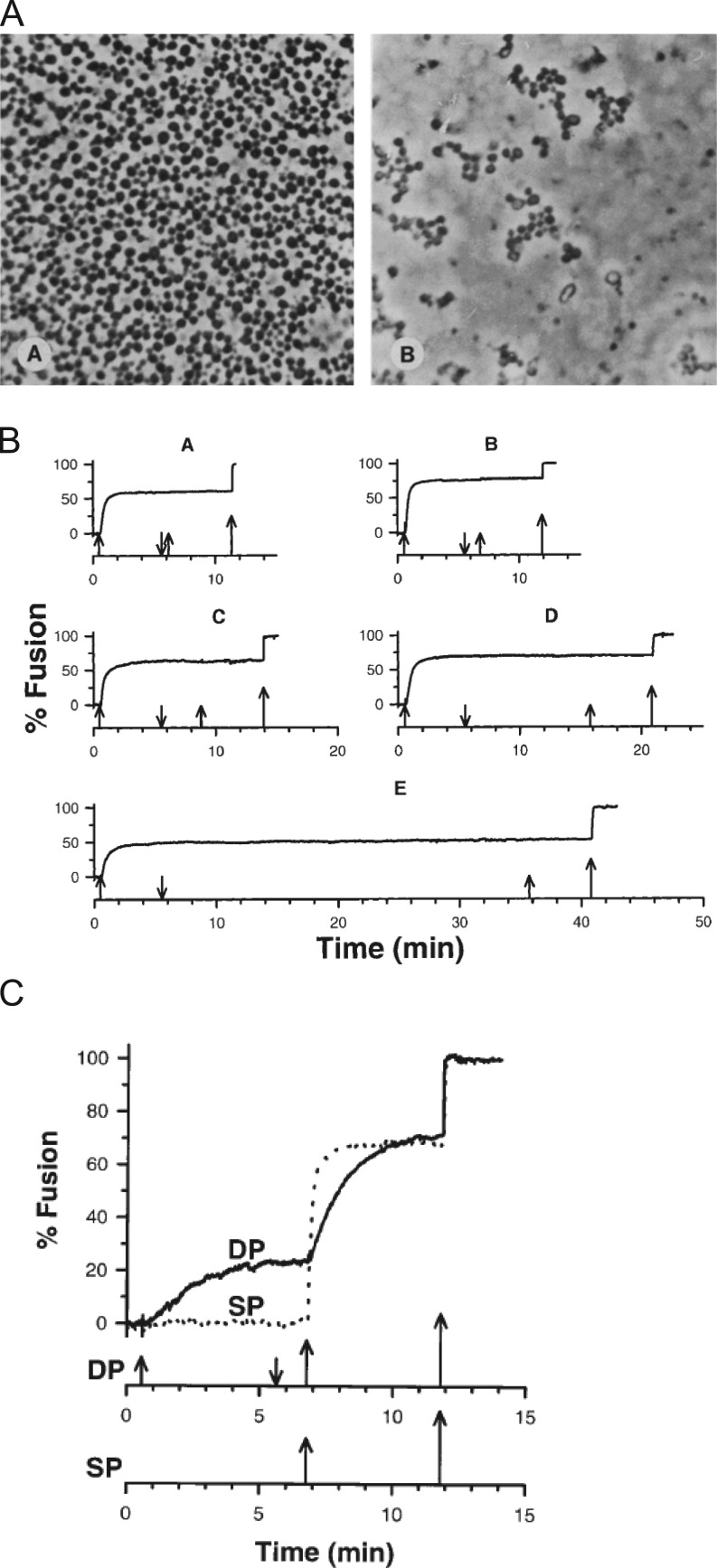
In sea urchin eggs, cortical granules line the inner leaflet of the plasma membrane. Upon fertilization, there is a rise in intracellular Ca2+ that triggers their fusion and leads to the discharge of proteins that form the fertilization envelope, which acts as a barrier to polyspermy. The eggs, immobilized on a solid support, can be effectively “unroofed” by applying a shear force, leaving behind a sheet of plasma membrane with docked granules (Vacquier, 1975; Baker and Whitaker, 1978). The preparation can then be washed free of cytosolic components, and fusion can subsequently be triggered by the addition of only micromolar Ca2+. In this system, there is only one round of fusion, and all cortical granules are primed and release ready—thus, the Ca2+-triggered stages of exocytosis can be effectively separated from upstream docking and priming reactions (for review, see Abbineni et al., 2013). When these isolated planar cortices are perfused with a solution containing more than 0.5 µM Ca2+, exocytosis ensues. Because of the large 1-µm diameter of each granule, fusion can be readily visualized even using a standard light microscopy (Zimmerberg et al., 1987; Fig. 6 A).
Figure 6.
Heterogeneity of Ca2+ responses of cortical granules in the planar cortex assay. (A) Cortices treated with 0.1 µM Ca2+ (left panel) or 17 µM Ca2+ (right panel). Taken from Zimmerberg et al. (1985), A is reprinted with permission from the Journal of Cell Biology. (B) Fusion of cortical granules depends on the final concentration of Ca2+ used and not the time between Ca2+ challenges. (C) Fusion of cortical granules does not depend on the number of Ca2+ steps used to reach the final concentration. For B and C, the upward and downward arrows signify Ca2+ addition or removal. The cortices in B were stimulated with 24 µM Ca2+ and then washed for 0.5, 1, 3, 10, or 30 min in 0 Ca2+ before being stimulated again. The largest arrow signifies >300 µM Ca2+. The Ca2+ concentrations in C are 14, 24, and >300 µM for the double challenge curve (DP) and 24 and >300 µM for the single challenge curve (SP). B and C are taken from Blank et al. (1998a).
Using the sea urchin egg, Blank et al. (1998a) examined two hypotheses that could explain the graded response to Ca2+: (1) within a homogenous population of granules, submaximal doses of Ca2+ cause an inactivation of fusion such that only a subset of granules undergo fusion at any given dose; or (2) there are differences in the Ca2+ sensitivity of individual granules, and the ability of granules to fuse is thus dependent on the magnitude of the Ca2+ stimulus. The study found that granules that do not fuse in response to the first Ca2+ stimulus could be triggered to fuse by using a second, higher dose of Ca2+ (Fig. 6, B and C). Repeated or extended exposure to a submaximal dose of Ca2+ did not cause an inactivation of fusion. Thus, there appeared to be populations of cortical granules with highly variable Ca2+ sensitivities. In a subsequent study, the authors went on to suggest that “Poisson-distributed fusion complexes” with distinct Ca2+ affinities are sufficient to explain the strong Ca2+ dependence of exocytosis in sea urchin eggs as well as mammalian secretory cells (Blank et al., 2001). This model differs from others that are based on a linear reaction scheme where Ca2+ influences the rate of exocytosis via Ca2+-dependent rate constants (Heinemann et al., 1993, 1994).
Identifying the sites of Ca2+ interaction within secretory cells
It is worth emphasizing that, in the 1980s, although there was abundant evidence that Ca2+ was required to trigger exocytosis, it was still not known whether the intracellular locus of Ca2+ action was on the secretory granule/vesicle or the plasma membrane. A breakthrough came in 1992, when Holz and colleagues demonstrated that chromaffin cell secretory granules injected into frog oocytes undergo regulated exocytosis and require a different range of Ca2+ compared with the endogenous cortical granules present in oocytes (Scheuner et al., 1992; Scheuner and Holz, 1994). At about the same time, it was shown that granules isolated from sea urchin eggs had the ability to fuse together as demonstrated by transfer of a fluorescent lipophilic dye and a combination of aqueous contents (Vogel and Zimmerberg, 1992). These studies provided evidence that the molecule necessary for Ca2+ sensing is likely to be present on the granule membrane.
The studies referenced above were remarkably prescient. In fact, we now know that secretory granules are heterogeneous with respect to Ca2+ sensing as a result of the nonuniform sorting of the protein synaptotagmin (Syt). These proteins are members of a large family (numbering some 17 isoforms) that couple Ca2+ binding to vesicle fusion in a wide variety of secretory tissue (for review, see MacDougall et al., 2018). A property of these isoforms that sets one apart from the other is diversity in divalent ion affinity, such as Sr2+ and Ba2+ (Bhalla et al., 2005; Zhang et al., 2011). The impact of Syt isoform diversity on the secretory response has recently been examined in chromaffin cells. Chromaffin cells endogenously express Syt-1 and Syt-7 on dense core granules (Rao et al., 2017). Two crucial findings demonstrate that Syt isoforms may be important components underlying heterogeneous responses to depolarizing stimuli in this secretory system: (1) Syt-1 and Syt-7 are sorted to different populations of granules (Rao et al., 2014), and (2) Syt-7-bearing granules trigger fusion in response to smaller increases in cytosolic Ca2+ relative to Syt-1–bearing granules (Rao et al., 2017). As such, it is now evident that the protein composition of granules is heterogeneous within individual cells, and may thereby lead to diversity in the regulated secretory response. The physiological consequences of heterogeneity remain to be determined and are the focus of ongoing investigations. Nevertheless, that heterogeneity exists and is conserved among organisms separated by hundreds of millions of years of evolution suggests it to be a fundamental feature of secretory systems.
We note that qualitatively similar phenomena (i.e., where multiple Ca2+-sensing sites operate within cells to control exocytosis) had been proposed to exist at the frog neuromuscular junction some years earlier (Zengel and Magleby, 1977, 1980). In that system, it was known that repetitive depolarizing impulses (i.e., conditioning impulses) modify the characteristics of neurotransmitter release in a manner that depends on the duration or frequency of stimulation (Del Castillo and Katz, 1954b; Liley, 1956; Hubbard, 1963). The amount of neurotransmitter released is approximately proportional to the end plate potential, measured using intracellular or surface microelectrode techniques. Therefore, depending on the time constant of the decay of the endplate potential to baseline, one could classify the effect on presynaptic release as “facilitation” (Mallart and Martin, 1967; Magleby, 1973), “augmentation” (Magleby and Zengel, 1976; Erulkar and Rahamimoff, 1978), or “potentiation” (Rosenthal, 1969; Magleby and Zengel, 1975).
In the late 1970s, Karl Magleby and Janet Zengel showed that these phenomena could be differentiated not only on the basis of their respective time constants of decay, but also on their sensitivity to extracellular divalent ions, including Sr2+ and Ba2+ (Zengel and Magleby, 1977). For example, when extracellular Sr2+ replaces Ca2+, the magnitude and time constant of decay of the second component of facilitation increased, but there was little effect on augmentation. Ba2+ increased the magnitude of augmentation but had no effect on facilitation. Based on these results, Magleby and colleagues proposed that “Ba2+ and Sr2+ could modify release by acting differentially on various parts of the transmitter releasing system…” and that the “underlying factors” on which they act may operate relatively independently (Zengel and Magleby, 1980; Zengel et al., 1980).
It is tempting to posit a molecular basis for these phenomena. Syt isoforms, for example, are known to mediate forms of presynaptic plasticity such as facilitation. In fact, the second time constant of decay of facilitation (i.e., the component that is sensitive to Sr2+) is also the component that is abrogated in Syt-7 KO synapses (Jackman et al., 2016; Jackman and Regehr, 2017). Thus, specialized Ca2+ sensors, distinguished by their divalent ion affinity, might function within a synapse to modify the efficacy of release in a manner that is analogous to neuroendocrine cell secretion.
Moving toward studies of secretion in intact cells
By the early 1990s, studies in permeabilized neuroendocrine cells had firmly validated the supposition that exocytosis was steeply Ca2+-dependent and provided a means to quantify the relationship between Ca2+ and catecholamine release. A weakness of the experiments, as they were performed at the time, was that the released catecholamines had to be manually collected (i.e., via a pipette) before analysis and quantification, which limited the speed with which the progression of exocytosis could be monitored. Moreover, these experiments were designed to measure exocytosis in bulk, typically from many hundreds of thousands of cells at once. Variations in responses of individual cells or granules were simply not resolvable. In recent years, a number of new assays have been developed that offer improved temporal resolution and, importantly, allow direct observation of individual exocytotic events in single cells. A lengthy exposition on these contemporary imaging and electro-physiological/-chemical assays is beyond the scope of this article. But for the sake of completeness, it is worthwhile to mention some of the key observations enabled by these more recently developed technologies.
It had been known since the 1930s that changes in cellular membrane area could be measured as a change in membrane capacitance (Cole, 1935). Once the patch-clamp technique was developed (Neher and Sakmann, 1976; Neher et al., 1978), it was clear that discrete increases in the area of the surface membrane of a cell, associated with fusion of individual secretory granules, could be monitored at high resolution. Assuming a specific capacitance of 1 μF/cm2 for biological membranes, and with knowledge of secretory granule size (i.e., from electron micrographs), one could even predict the number of granules that must have fused to elicit a particular stimulus-dependent capacitance increase. Erwin Neher and Alain Marty performed the first capacitance measurements during exocytosis in the bovine adrenal chromaffin cell (Neher and Marty, 1982). Separately, Julio Fernandez, Bastien Gomperts, and Josh Zimmerberg subsequently applied the technique to degranulation in mast cells (Fernandez et al., 1984; Zimmerberg et al., 1987).
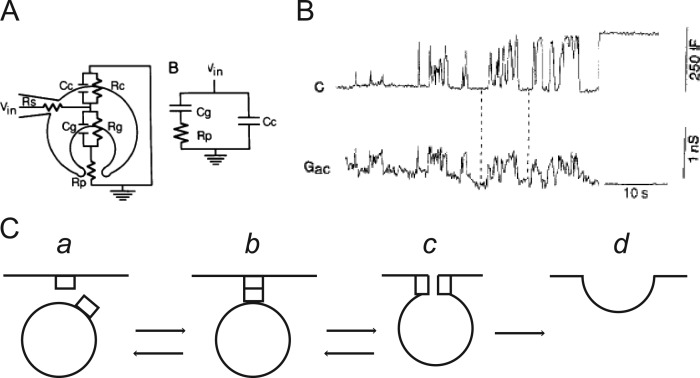
A remarkable observation made by Fernandez et al. (1984) was that changes in membrane capacitance were not always stable. Sometimes, the capacitance would flicker, suggestive of a “reversible aqueous pore between the secretory vesicle and the plasma membrane.” This idea would be more completely articulated by Zimmerberg and colleagues, who proposed that “…such flickers result from the formation of an exocytotic pore, the lumen of the membrane neck that forms upon fusion connecting the interior of the granule with the extracellular space” (Zimmerberg et al., 1987). Because the dilation of the pore itself is variable, the release of granule contents would be “nonquantal.” Zimmerberg and colleagues went on to suggest how pore resistance, and ultimately, pore diameter, could be calculated based on a simplified equivalent circuit model (Zimmerberg et al., 1987; Fig. 7 A). Time resolved measurements of fusion pore conductance based on the admittance of the patch-clamped cells were subsequently published by Wolf Almers’s group (Breckenridge and Almers, 1987; Spruce et al., 1990; Fig. 7, B and C). These methods were later combined with amperometric measurements of catecholamine release to demonstrate that pore expansion was not immediate, but subject to regulation by Ca2+ (Wightman et al., 1991; Chow et al., 1992; Albillos et al., 1997).
Figure 7.
Patch-clamp electrophysiology enables real-time detection of granule fusion in mast cells. (A) A sketch of the exocytotic fusion pore that has resulted from fusion. Superimposed on the cartoon is the equivalent circuit. Rc and Cc, resistance and capacitance of the cell membrane; Rg and Cg, resistance and capacitance of the granule membrane; Rp, resistance of the fusion pore; and Rs, series resistance of the patch electrode. Taken from Zimmerberg et al. (1987), A is reprinted with permission from the Proceedings of the National Academy of Sciences of the United States of America. (B) Capacitance, C, and conductance, Gac, of a mast cell granule as it undergoes exocytosis. Taken from Spruce et al. (1990), B is reprinted with permission from Neuron. (C) Hypothetical sequence of events during exocytosis. The “flicker” between conducting and nonconducting states in the trace in B may arise from transitions between b and c in the model. Based on Breckenridge and Almers (1987), C is reprinted with permission from the Proceedings of the National Academy of Sciences of the United States of America.
The secretory response exhibits a steep Ca2+ dependence
Classical electrophysiological studies of synaptic transmission revealed that the relationship between secretion and Ca2+ was highly nonlinear (Jenkinson, 1957). Dodge and Rahamimoff (1967) subsequently described this correspondence in quantitative terms at the NMJ. There, the authors noted that a log–log plot of end plate potential amplitudes (i.e., release) against extracellular Ca2+ concentrations yielded a line with a slope of ∼4 (Dodge and Rahamimoff, 1967; Fig. 8 A). They proposed, based on the numerical value of the slope, that multiple Ca2+ ions (i.e., four) acted cooperatively at an intracellular locus with four “independent binding sites” to provoke release. Results in other preparations, including the Calyx of Held, chromaffin cell, bipolar ribbon synapse, and crayfish motor neuron, are in broad agreement with the idea that there is a steep power dependence of exocytosis on Ca2+ concentration (Knight and Baker, 1982; Heidelberger et al., 1994; Heinemann et al., 1994; Lou et al., 2005; Yang et al., 2005). This means that even a small change in Ca2+ sensitivity has a large effect on the release rate (Yang et al., 2005).
A review of the literature indicates that the Ca2+ sensitivity of secretion is not uniform, but variable across secretory cell types (Augustine and Neher, 1992; Thomas et al., 1993; Landò and Zucker, 1994; Jarsky et al., 2010; Matveev et al., 2011). As a general rule, there is a steeper dependence of exocytosis on Ca2+ in neurons than there is in neuroendocrine cells. An effort to quantify this relationship in chromaffin cells was first made by Knight and Baker (1982). Based on a fit of the Hill function to Ca2+ activation curve, they proposed that exocytosis involved at least two Ca2+ binding steps (Knight and Baker, 1982; Fig. 8 B). A decade later, Bittner and Holz (1992) identified a similar relationship between Ca2+ and secretion in digitonin-permeabilized chromaffin cells. With the advent of Ca2+ flash photolysis, patch-clamp electrophysiology, and the greatly improved time resolution these techniques afforded, it was estimated that a third- or fourth-order cooperative relationship existed between the rate of fusion and Ca2+ (Neher, 2006). Neher and colleagues calculated that exocytosis operated at a maximum rate of 103 fusion events per second when intracellular Ca2+ concentrations were raised to above 100 µM (Heinemann et al., 1994; Voets, 2000; Sørensen, 2004; Neher, 2006). The interpretation of cooperativity obtained from the log–log slope or Hill function fit of Ca2+ activation curves is complicated by the fact that secretion is the end result of multiple Ca2+-dependent process (e.g., Ca2+ influx through channels and diffusion from sites of entry, buffering by endogenous Ca2+ buffers, binding to proteins triggering exocytosis, etc.; Neher and Augustine, 1992; Klingauf and Neher, 1997; Schneggenburger and Neher, 2005; Matveev et al., 2011). Nevertheless, cooperativity is an important element of models that aim to recapitulate the sensitivity and time-course of the secretory response by taking into account multiple Ca2+-binding steps and estimates of Ca2+ concentrations at fusion sites (Heinemann et al., 1993, 1994; Chow et al., 1994; Heidelberger et al., 1994; Schneggenburger and Neher, 2000; Voets, 2000; Sørensen et al., 2003; Neher, 2015).
A high-order dependence of exocytosis on Ca2+ concentration is considered to be a central feature of synchronous neurotransmitter release (Augustine et al., 1985, 1987; Goda and Stevens, 1994). Therefore, one presumes that a shallower dependence of exocytosis on Ca2+ in chromaffin cells (i.e., compared with neurons) reflects the fact that secretion is less “synchronized” to Ca2+ influx, perhaps as a result of different physiological pressures for endocrine versus synaptic signaling (Smith, 1999; Yang et al., 2005). In terms of their morphological arrangement, it is therefore possible that channels and sites of exocytosis are not as tightly coupled in chromaffin cells and other neuroendocrine cell types (Thomas et al., 1993; Chow et al., 1994; Barg et al., 2001; Zhu et al., 2002; Wu et al., 2009). Secretion, set up in this way, will be tuned to respond to spatially averaged rather than spatially restricted Ca2+ levels where there is a steep fall-off from sites of entry (i.e., channels; Schneggenburger and Neher, 2005). The net outcome is a secretory response with a lower Ca2+ threshold so that granules even at some distance from channels will undergo efficient exocytosis (Klingauf and Neher, 1997). Thus, even among systems that share a steep Ca2+ dependence, release mechanisms have the potential to differ in ways that satisfy their distinct physiological purposes.
Reconstituting fusion in the molecular age
The lessons learned over many years of work with cell-based and cell-free systems have been immensely valuable to current efforts to reconstitute exocytosis. Throughout the course of this article, we have attempted to highlight insights pertinent to ongoing studies, including the exquisite Ca2+ dependence of exocytosis and its requirement for specific lipids. With the ability to resolve individual fusion events, we have also become aware that exocytosis is not monolithic but capable of a striking degree of regulated variation. These observations guide current efforts at reconstitution that aim to recapitulate the essential features of the cellular process, especially its Ca2+ dependence, in a completely synthetic system (Malsam et al., 2012; Ma et al., 2013; Lai et al., 2017). In fact, the literature is replete with efforts that have come a long way toward achieving this goal.
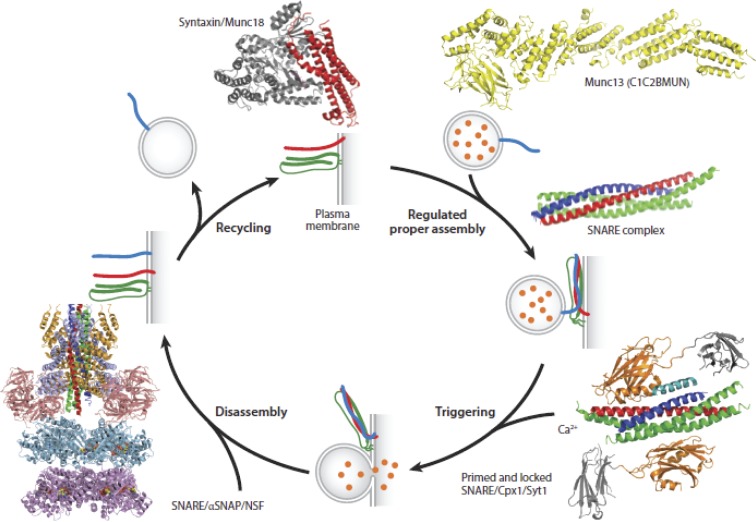
It is best left to others to describe the inspired efforts that culminated in the identification of the core fusion machinery for exocytosis, which is necessary for the inclusion of relevant proteins for the reconstitution of exocytosis (for an excellent perspective on this topic, see Südhof, 2014). This work benefited from important genetic studies in yeast (Novick et al., 1980, 1981; Barlowe and Schekman, 1993), the discovery of the SNARE proteins as the highly specific targets for proteolysis by the clostridial neurotoxins (Blasi et al., 1993a,b; Binz et al., 1994; Yamasaki et al., 1994), and successful reconstitution of membrane traffic between intracellular compartments (Balch et al., 1984a,b; Braell et al., 1984). We do now know that SNARE proteins catalyze membrane fusion (Söllner et al., 1993a,b; Weber et al., 1998), and Syt couples this reaction to Ca2+ (Brose et al., 1992; Elferink et al., 1993; Geppert et al., 1994). Munc13 and Munc18 interact and regulate SNARE protein behavior (Hata et al., 1993; Brose et al., 1995; Betz et al., 1997). Complexin binds to SNAREs and regulates spontaneous and evoked release (McMahon et al., 1995; Reim et al., 2001). The phosphoinositol, PI(4,5)P2, plays an important role in coupling Ca2+ and fusion (Tucker et al., 2003; Bai et al., 2004). A model illustrating the structures of these various proteins and their actions during the course of exocytosis is shown in Fig. 9.
Figure 9.
Structures of synaptic proteins and their complexes, which participate in Ca2+-triggered exocytosis. Shown are structures of the neuronal SNARE complex, the SNARE/Cpx1/Syt1 complex, the SNARE/αSNAP/NSF complex, the Munc18/syntaxin complex, and the C1C2MUN fragment of Munc13. Cpx1, complexin-1; Munc, mammalian uncoordinated. Taken from Brunger et al. (2018), Fig. 9 is reprinted with permission from the Annual Review of Biophysics.
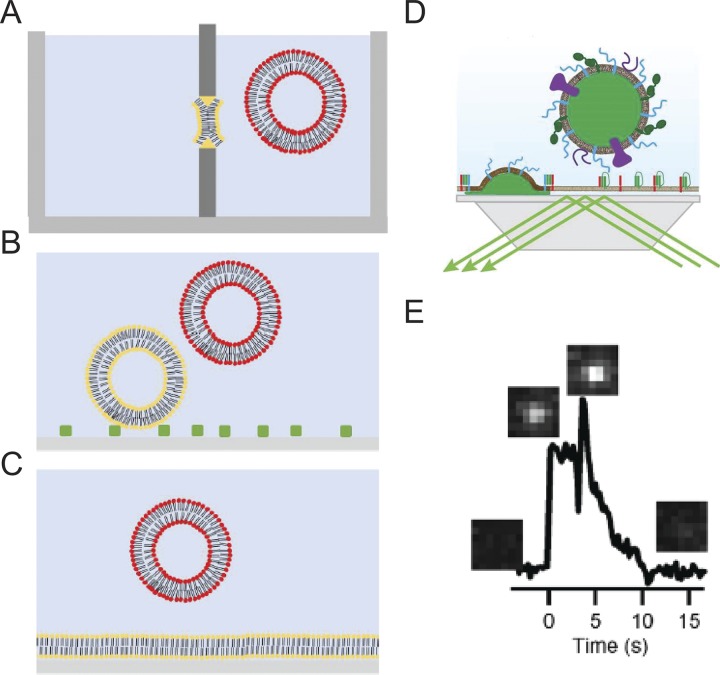
Reconstitution methods have begun to utilize single-particle imaging to resolve different steps in exocytosis and determine the roles of various key proteins. Currently, two “types” of assay systems are most frequently used to reconstitute SNARE-mediated vesicle fusion: (1) liposome fusion to planar supported bilayers (Domanska et al., 2009; Karatekin et al., 2010; Karatekin and Rothman, 2012), and (2) tethered vesicle-to-vesicle fusion (Yoon et al., 2006; Lee et al., 2010; Kyoung et al., 2011; Fig. 10, A–C). The incorporation of Syt into planar supported bilayer fusion assays accelerates fusion in the presence of Ca2+, but high amounts of fusion still exist in its absence (Kiessling et al., 2013; Park et al., 2015). The vesicle–vesicle fusion assay has achieved full Ca2+ dependence in the presence of the SNARE proteins, Syt, complexin, Munc13, and Munc18; moreover, fusion events exhibit characteristics comparable to those observed in electrophysiology measurements when mutants of Syt and complexin are employed (Zhou et al., 2015; Gong et al., 2016). Despite the advances demonstrated by this assay system, fusion is not yet as robust as in the cell setting (Lai et al., 2017).
Figure 10.
Assays for reconstitution of membrane fusion. (A) Fusion between a planar membrane (yellow) and liposomes. (B) Vesicle–vesicle fusion where target vesicles (yellow) are tethered to the surface and fusion with secretory mimicking vesicles (red) is imaged. (C) Fusion between target planar supported bilayer (yellow) and secretory mimicking vesicle (red). (D) Cartoon of a “hybrid” granule docking and fusion assay performed using a prism-based TIRF microscope system. (E) A single fusion event of an Neuropeptide (NPY)-Ruby-labeled granule with a planar-supported bilayer containing t-SNAREs. Images corresponding to the appearance of the granule and release of NPY in the evanescent field are placed above an intensity versus time graph. Taken from Kreutzberger et al. (2017), E is reprinted with the permission of Science Advances.
A reconstituted fusion assay that shows particular promise, in terms of its robust Ca2+ dependence, incorporates secretory vesicles/granules purified from endogenous sources and a synthetic plasma membrane substrate. Scheuner et al. (1992) first demonstrated that such an assay could actually work by triggering Ca2+-dependent fusion of bovine chromaffin granules with frog oocyte membranes. Many years later, Reinhard Jahn's group demonstrated that synaptic vesicles or bovine chromaffin granules will fuse with proteoliposomes containing the plasma membrane SNARE proteins (Holt et al., 2008; Park et al., 2012, 2015). Given the appropriate buffer and lipid conditions, fusion was increased by Ca2+ (Park et al., 2012, 2015). More recently, Kiessling et al. (2013) showed that purified synaptic vesicles from rat brain could fuse with acceptor SNARE-containing planar bilayers at least as fast as synthetic vesicles with a fusion efficiency that is clearly enhanced by Ca2+.
An obvious benefit of incorporating actual secretory organelles in reconstitution assays is that one can presume some or many of the important ingredients for exocytosis are already in place. Recently, Lukas Tamm’s group has taken this approach one step further by reconstituting fusion with granules purified from PC12 cells and plasma membrane SNARE–containing supported bilayers (Kreutzberger et al., 2017; Fig. 10, D and E). The strength of the assay is that it recapitulates key characteristics of the regulated pathway in cells, including its micromolar Ca2+ dependence and requirement for PI(4,5)P2. These elements had been difficult to recapitulate by other means. Because the assay employed a cell-derived organelle, simple genetic approaches could be used to alter expression of specific membrane constituents in advance of granule isolation. This allowed the investigators to show, by selective addition and deletion, that fast and efficient fusion responses to Ca2+ required a MUN-domain protein, such as CAPS or Munc13.
As a strategy, a hybrid reconstitution assay using one part “real” material holds tremendous promise for studies of exocytotic mechanisms. Earlier investigations had adopted similar schemes to good effect, using them to demonstrate that a Ca2+-binding protein was necessary to endow micromolar Ca2+ dependence to fusion (Zimmerberg et al., 1980b) and that Ca2+ dependence is likely conferred by a factor on the secretory granule (Scheuner et al., 1992; Vogel and Zimmerberg, 1992; Scheuner and Holz, 1994). The catalog of proteins with a presumed involvement in exocytosis is large and expanding (Fig. 9). The goal for future years is to define where and when they act as fusion and content release progress.
Conclusions and outlook
We have condensed a vast amount of scientific work into the pages of this Milestones article. It is bookended by descriptions of past and current efforts to reconstitute the fusion process. The article relates how our understanding of Ca2+-triggered exocytosis has grown over the course of just a few decades. Tentative predictions of fusion intermediates (e.g., “omega” structures; see Fig. 1) based on electron micrographs have given way to high-resolution, electrophysiological measurements of a dynamic fusion pore. Because of patch-clamp recording, it has become possible to describe the organization of the secretory response in quantitative terms. We now recognize that synaptic vesicles and dense core granules are organized into kinetically separable “pools” whose fusion characteristics are defined by their responses to intracellular Ca2+ (Heinemann et al., 1993, 1994; Chow et al., 1994). Fluorescence-based imaging of exocytosis—most notably, TIRF microscopy—have offered a number of insights of at least equivalent significance (Axelrod, 1981; Steyer et al., 1997). They have clarified the relationship between pore expansion and secretion (Taraska et al., 2003; Perrais et al., 2004; Bohannon et al., 2017) and provided visual evidence for heterogeneity in the fusion characteristics of individual secretory granules (Zhang et al., 2011; Rao et al., 2017), suggested first by Blank et al. (1998a,b, 2001).
The sophistication of ongoing studies using reconstitution has only been possible because of the cloning and identification of the “core” fusion machines. But what we have hoped to highlight is that many of the important insights into exocytosis did not require a knowledge of molecular mechanisms. A current phase of studies on membrane fusion, launched by the advent of cryo-EM and crystallography (notably by the groups of Axel Brunger and Reinhard Jahn), have provided us with an unprecedented view of the ultrastructure of the exocytotic apparatus (Fig. 9; Brunger et al., 2018). However, an argument could well be made that our knowledge of protein structure has outpaced our understanding of what the proteins actually do. When one considers the sheer number of actors with roles or presumed roles in the secretory pathway, this is especially problematic. The studies discussed in this article demonstrate the value of clear analytical thinking allied to the selection of apt experimental preparations. One predicts that there will always be a role for reductionist approaches, especially reconstitution, in unraveling the function of the multitude of molecules implicated in Ca2+-dependent secretion.
Acknowledgments
This article is dedicated to my mentor, Dr. Ronald W. Holz, whose unrelenting enthusiasm, optimism, and encouragement have been instrumental in shaping the course of my scientific career (A. Anantharam). We wish to thank Drs. Andrea S.B. Anantharam, J. David Castle, Prabhodh Abbineni, and Kevin Bohannon, as well as members of the Anantharam laboratory (especially Amanda Gibson, Noah Schenk, and Dr. Mounir Bendahmane), for comments and suggestions. The authors also thank Dr. Volker Kiessling for providing the cartoon in Figure 10 D.
Grant support was provided by the National Institutes of Health (grant GM111997 to A. Anantharam; A.J.B. Kreutzberger is supported by grant GM072694 to Dr. Lukas Tamm).
The authors declare no competing financial interests.
Author contributions: A.J.B. Kreutzberger wrote the section "Reconstituting fusion in the molecular age" and assisted in editing other sections. A. Anantharam wrote and edited all sections of the paper.
Olaf S. Andersen served as editor.
References
- Abbineni P.S., Hibbert J.E., and Coorssen J.R.. 2013. Critical role of cortical vesicles in dissecting regulated exocytosis: overview of insights into fundamental molecular mechanisms. Biol. Bull. 224:200–217. 10.1086/BBLv224n3p200 [DOI] [PubMed] [Google Scholar]
- Abbineni P.S., Axelrod D., and Holz R.W.. 2018. Visualization of expanding fusion pores in secretory cells. J. Gen. Physiol. 150:1640–1646. 10.1085/jgp.201812186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A., Dernick G., Horstmann H., Almers W., Alvarez de Toledo G., and Lindau M.. 1997. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 389:509–512. 10.1038/39081 [DOI] [PubMed] [Google Scholar]
- Ali S.M., Geisow M.J., and Burgoyne R.D.. 1989. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 340:313–315. 10.1038/340313a0 [DOI] [PubMed] [Google Scholar]
- Augustine G.J., and Neher E.. 1992. Calcium requirements for secretion in bovine chromaffin cells. J. Physiol. 450:247–271. 10.1113/jphysiol.1992.sp019126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G.J., Charlton M.P., and Smith S.J.. 1985. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J. Physiol. 367:163–181. 10.1113/jphysiol.1985.sp015819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G.J., Charlton M.P., and Smith S.J.. 1987. Calcium action in synaptic transmitter release. Annu. Rev. Neurosci. 10:633–693. 10.1146/annurev.ne.10.030187.003221 [DOI] [PubMed] [Google Scholar]
- Axelrod D. 1981. Cell-substrate contacts illuminated by total internal reflection fluorescence. J. Cell Biol. 89:141–145. 10.1083/jcb.89.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Tucker W.C., and Chapman E.R.. 2004. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11:36–44. 10.1038/nsmb709 [DOI] [PubMed] [Google Scholar]
- Baker P.F., and Knight D.E.. 1978. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 276:620–622. 10.1038/276620a0 [DOI] [PubMed] [Google Scholar]
- Baker P.F., and Knight D.E.. 1981. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 296:83–103. 10.1098/rstb.1981.0174 [DOI] [PubMed] [Google Scholar]
- Baker P.F., and Whitaker M.J.. 1978. Influence of ATP and calcium on the cortical reaction in sea urchin eggs. Nature. 276:513–515. 10.1038/276513a0 [DOI] [PubMed] [Google Scholar]
- Balch W.E., Dunphy W.G., Braell W.A., and Rothman J.E.. 1984a Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 39:405–416. 10.1016/0092-8674(84)90019-9 [DOI] [PubMed] [Google Scholar]
- Balch W.E., Glick B.S., and Rothman J.E.. 1984b Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 39:525–536. 10.1016/0092-8674(84)90459-8 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Barry V.A., DasGupta B.R., and Martin T.F.J.. 1996. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J. Biol. Chem. 271:20223–20226. 10.1074/jbc.271.34.20223 [DOI] [PubMed] [Google Scholar]
- Bao H., Das D., Courtney N.A., Jiang Y., Briguglio J.S., Lou X., Roston D., Cui Q., Chanda B., and Chapman E.R.. 2018. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature. 554:260–263. 10.1038/nature25481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S., Ma X., Eliasson L., Galvanovskis J., Göpel S.O., Obermüller S., Platzer J., Renström E., Trus M., Atlas D., et al. . 2001. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys. J. 81:3308–3323. 10.1016/S0006-3495(01)75964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C., and Schekman R.. 1993. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 365:347–349. 10.1038/365347a0 [DOI] [PubMed] [Google Scholar]
- Betz A., Okamoto M., Benseler F., and Brose N.. 1997. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J. Biol. Chem. 272:2520–2526. 10.1074/jbc.272.4.2520 [DOI] [PubMed] [Google Scholar]
- Bhalla A., Tucker W.C., and Chapman E.R.. 2005. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell. 16:4755–4764. 10.1091/mbc.e05-04-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz T., Blasi J., Yamasaki S., Baumeister A., Link E., Südhof T.C., Jahn R., and Niemann H.. 1994. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 269:1617–1620. [PubMed] [Google Scholar]
- Bittner M.A., and Holz R.W.. 1992. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J. Biol. Chem. 267:16219–16225. [PubMed] [Google Scholar]
- Blank P.S., Cho M.S., Vogel S.S., Kaplan D., Kang A., Malley J., and Zimmerberg J.. 1998a Submaximal responses in calcium-triggered exocytosis are explained by differences in the calcium sensitivity of individual secretory vesicles. J. Gen. Physiol. 112:559–567. 10.1085/jgp.112.5.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank P.S., Vogel S.S., Cho M.S., Kaplan D., Bhuva D., Malley J., and Zimmerberg J.. 1998b The calcium sensitivity of individual secretory vesicles is invariant with the rate of calcium delivery. J. Gen. Physiol. 112:569–576. 10.1085/jgp.112.5.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank P.S., Vogel S.S., Malley J.D., and Zimmerberg J.. 2001. A kinetic analysis of calcium-triggered exocytosis. J. Gen. Physiol. 118:145–156. 10.1085/jgp.118.2.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi J., Chapman E.R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T.C., Niemann H., and Jahn R.. 1993a Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 365:160–163. 10.1038/365160a0 [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E.R., Yamasaki S., Binz T., Niemann H., and Jahn R.. 1993b Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 12:4821–4828. 10.1002/j.1460-2075.1993.tb06171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M.R., Glick B.S., Wilcox C.A., Wieland F.T., and Rothman J.E.. 1988. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl. Acad. Sci. USA. 85:7852–7856. 10.1073/pnas.85.21.7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon K.P., Bittner M.A., Lawrence D.A., Axelrod D., and Holz R.W.. 2017. Slow fusion pore expansion creates a unique reaction chamber for co-packaged cargo. J. Gen. Physiol. 149:921–934. 10.1085/jgp.201711842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W.A., Balch W.E., Dobbertin D.C., and Rothman J.E.. 1984. The glycoprotein that is transported between successive compartments of the Golgi in a cell-free system resides in stacks of cisternae. Cell. 39:511–524. 10.1016/0092-8674(84)90458-6 [DOI] [PubMed] [Google Scholar]
- Breckenridge L.J., and Almers W.. 1987. Final steps in exocytosis observed in a cell with giant secretory granules. Proc. Natl. Acad. Sci. USA. 84:1945–1949. 10.1073/pnas.84.7.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N., Petrenko A.G., Südhof T.C., and Jahn R.. 1992. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 256:1021–1025. 10.1126/science.1589771 [DOI] [PubMed] [Google Scholar]
- Brose N., Hofmann K., Hata Y., and Südhof T.C.. 1995. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 270:25273–25280. 10.1074/jbc.270.42.25273 [DOI] [PubMed] [Google Scholar]
- Brunger A.T., Choi U.B., Lai Y., Leitz J., and Zhou Q.. 2018. Molecular Mechanisms of Fast Neurotransmitter Release. Annu. Rev. Biophys. 47:469–497. 10.1146/annurev-biophys-070816-034117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L.G., and Palade G.E.. 1964. Protein Synthesis, Storage, and Discharge in the Pancreatic Exocrine Cell. An Autoradiographic Study. J. Cell Biol. 20:473–495. 10.1083/jcb.20.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D.E., and Heuser J.. 1979. Membrane fusion during secretion: cortical granule exocytosis in sex urchin eggs as studied by quick-freezing and freeze-fracture. J. Cell Biol. 83:91–108. 10.1083/jcb.83.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R.H., von Rüden L., and Neher E.. 1992. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 356:60–63. 10.1038/356060a0 [DOI] [PubMed] [Google Scholar]
- Chow R.H., Klingauf J., and Neher E.. 1994. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc. Natl. Acad. Sci. USA. 91:12765–12769. 10.1073/pnas.91.26.12765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D.E., Douglas W.W., Mouri T., and Nakazato Y.. 1975. Calcium and stimulus-secretion coupling in the adrenal medulla: contrasting stimulating effects of the ionophores X-537A and A23187 on catecholamine output. J. Physiol. 252:363–378. 10.1113/jphysiol.1975.sp011148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F.S., and Niles W.D.. 1993. Reconstituting channels into planar membranes: a conceptual framework and methods for fusing vesicles to planar bilayer phospholipid membranes. Methods Enzymol. 220:50–68. 10.1016/0076-6879(93)20073-C [DOI] [PubMed] [Google Scholar]
- Cohen F.S., Zimmerberg J., and Finkelstein A.. 1980. Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J. Gen. Physiol. 75:251–270. 10.1085/jgp.75.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K.S. 1935. Electric Impedance of Hipponoe Eggs. J. Gen. Physiol. 18:877–887. 10.1085/jgp.18.6.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J., and Katz B.. 1954a Quantal components of the end-plate potential. J. Physiol. 124:560–573. 10.1113/jphysiol.1954.sp005129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J., and Katz B.. 1954b Statistical factors involved in neuromuscular facilitation and depression. J. Physiol. 124:574–585. 10.1113/jphysiol.1954.sp005130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E., and Vaz Ferreira A.. 1957. A multivesicular cathecol-containing body of the adrenal medulla of the rabbit. Exp. Cell Res. 12:575–581. 10.1016/0014-4827(57)90173-8 [DOI] [PubMed] [Google Scholar]
- Dodge F.A. Jr., and Rahamimoff R.. 1967. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. 193:419–432. 10.1113/jphysiol.1967.sp008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska M.K., Kiessling V., Stein A., Fasshauer D., and Tamm L.K.. 2009. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem. 284:32158–32166. 10.1074/jbc.M109.047381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W.W., and Rubin R.P.. 1961a Mechanism of nicotinic action at the adrenal medulla: calcium as a link in stimulus-secretion coupling. Nature. 192:1087–1089. 10.1038/1921087b0 [DOI] [PubMed] [Google Scholar]
- Douglas W.W., and Rubin R.P.. 1961b The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J. Physiol. 159:40–57. 10.1113/jphysiol.1961.sp006791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.A., and Holz R.W.. 1983. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J. Biol. Chem. 258:4989–4993. [PubMed] [Google Scholar]
- Eberhard D.A., Cooper C.L., Low M.G., and Holz R.W.. 1990. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268:15–25. 10.1042/bj2680015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L.A., Peterson M.R., and Scheller R.H.. 1993. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 72:153–159. 10.1016/0092-8674(93)90059-Y [DOI] [PubMed] [Google Scholar]
- Erulkar S.D., and Rahamimoff R.. 1978. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J. Physiol. 278:501–511. 10.1113/jphysiol.1978.sp012320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J.M., Neher E., and Gomperts B.D.. 1984. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 312:453–455. 10.1038/312453a0 [DOI] [PubMed] [Google Scholar]
- Garcia A.G., Kirpekar S.M., and Prat J.C.. 1975. A calcium ionophore stimulating the secretion of catecholamines from the cat adrenal. J. Physiol. 244:253–262. 10.1113/jphysiol.1975.sp010795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R.E., Li C., Rosahl T.W., Stevens C.F., and Südhof T.C.. 1994. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 79:717–727. 10.1016/0092-8674(94)90556-8 [DOI] [PubMed] [Google Scholar]
- Goda Y., and Stevens C.F.. 1994. Two components of transmitter release at a central synapse. Proc. Natl. Acad. Sci. USA. 91:12942–12946. 10.1073/pnas.91.26.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Lai Y., Li X., Wang M., Leitz J., Hu Y., Zhang Y., Choi U.B., Cipriano D., Pfuetzner R.A., et al. . 2016. C-terminal domain of mammalian complexin-1 localizes to highly curved membranes. Proc. Natl. Acad. Sci. USA. 113:E7590–E7599. 10.1073/pnas.1609917113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L.A., and Rein G.. 1977. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 268:349–351. 10.1038/268349a0 [DOI] [PubMed] [Google Scholar]
- Greene L.A., and Tischler A.S.. 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA. 73:2424–2428. 10.1073/pnas.73.7.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishanin R.N., Klenchin V.A., Loyet K.M., Kowalchyk J.A., Ann K., and Martin T.F.. 2002. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J. Biol. Chem. 277:22025–22034. 10.1074/jbc.M201614200 [DOI] [PubMed] [Google Scholar]
- Hata Y., Slaughter C.A., and Südhof T.C.. 1993. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 366:347–351. 10.1038/366347a0 [DOI] [PubMed] [Google Scholar]
- Hay J.C., and Martin T.F.J.. 1992. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J. Cell Biol. 119:139–151. 10.1083/jcb.119.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J.C., and Martin T.F.J.. 1993. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 366:572–575. 10.1038/366572a0 [DOI] [PubMed] [Google Scholar]
- Hay J.C., Fisette P.L., Jenkins G.H., Fukami K., Takenawa T., Anderson R.A., and Martin T.F.J.. 1995. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 374:173–177. 10.1038/374173a0 [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., and Matthews G.. 1994. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 371:513–515. 10.1038/371513a0 [DOI] [PubMed] [Google Scholar]
- Heinemann C., von Rüden L., Chow R.H., and Neher E.. 1993. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 424:105–112. 10.1007/BF00374600 [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R.H., Neher E., and Zucker R.S.. 1994. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys. J. 67:2546–2557. 10.1016/S0006-3495(94)80744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J.E., and Reese T.S.. 1973. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57:315–344. 10.1083/jcb.57.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J.E., and Reese T.S.. 1981. Structural changes after transmitter release at the frog neuromuscular junction. J. Cell Biol. 88:564–580. 10.1083/jcb.88.3.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J.E., Reese T.S., Dennis M.J., Jan Y., Jan L., and Evans L.. 1979. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J. Cell Biol. 81:275–300. 10.1083/jcb.81.2.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M., Riedel D., Stein A., Schuette C., and Jahn R.. 2008. Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+. Curr. Biol. 18:715–722. 10.1016/j.cub.2008.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz R.W. 1986. The role of osmotic forces in exocytosis from adrenal chromaffin cells. Annu. Rev. Physiol. 48:175–189. 10.1146/annurev.ph.48.030186.001135 [DOI] [PubMed] [Google Scholar]
- Holz R.W., and Senter R.A.. 1985. Plasma membrane and chromaffin granule characteristics in digitonin-treated chromaffin cells. J. Neurochem. 45:1548–1557. 10.1111/j.1471-4159.1985.tb07226.x [DOI] [PubMed] [Google Scholar]
- Holz R.W., and Stratford C.A.. 1979. Effects of Divalent Ions on Vesicle-Vesicle Fusion Studied by a New Luminescence Assay for Fusion. J. Membr. Biol. 46:331–358. 10.1007/BF01868754 [DOI] [Google Scholar]
- Holz R.W., Bittner M.A., Peppers S.C., Senter R.A., and Eberhard D.A.. 1989. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J. Biol. Chem. 264:5412–5419. [PubMed] [Google Scholar]
- Holz R.W., Hlubek M.D., Sorensen S.D., Fisher S.K., Balla T., Ozaki S., Prestwich G.D., Stuenkel E.L., and Bittner M.A.. 2000. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem. 275:17878–17885. 10.1074/jbc.M000925200 [DOI] [PubMed] [Google Scholar]
- Howell T.W., and Gomperts B.D.. 1987. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim. Biophys. Acta. 927:177–183. 10.1016/0167-4889(87)90132-7 [DOI] [PubMed] [Google Scholar]
- Hubbard J.I. 1963. Repetitive Stimulation at the Mammalian Neuromuscular Junction, and the Mobilization of Transmitter. J. Physiol. 169:641–662. 10.1113/jphysiol.1963.sp007286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S.L., and Regehr W.G.. 2017. The Mechanisms and Functions of Synaptic Facilitation. Neuron. 94:447–464. 10.1016/j.neuron.2017.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S.L., Turecek J., Belinsky J.E., and Regehr W.G.. 2016. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature. 529:88–91. 10.1038/nature16507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J.D., and Palade G.E.. 1966. Role of the Golgi complex in the intracellular transport of secretory proteins. Proc. Natl. Acad. Sci. USA. 55:424–431. 10.1073/pnas.55.2.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J.D., and Palade G.E.. 1967a Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J. Cell Biol. 34:577–596. 10.1083/jcb.34.2.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J.D., and Palade G.E.. 1967b Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J. Cell Biol. 34:597–615. 10.1083/jcb.34.2.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T., Tian M., and Singer J.H.. 2010. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J. Neurosci. 30:11885–11895. 10.1523/JNEUROSCI.1415-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D.H. 1957. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J. Physiol. 138:434–444. 10.1113/jphysiol.1957.sp005860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.G., and Scarpa A.. 1976. Ion permeability of isolated chromaffin granules. J. Gen. Physiol. 68:601–631. 10.1085/jgp.68.6.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadkova A., Radecke J., and Sorensen J.B.. 2018. The SNAP-25 Protein Family. Neuroscience. 10.1016/j.neuroscience.2018.09.020 [DOI] [PubMed] [Google Scholar]
- Karatekin E., and Rothman J.E.. 2012. Fusion of single proteoliposomes with planar, cushioned bilayers in microfluidic flow cells. Nat. Protoc. 7:903–920. 10.1038/nprot.2012.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin E., Di Giovanni J., Iborra C., Coleman J., O’Shaughnessy B., Seagar M., and Rothman J.E.. 2010. A fast, single-vesicle fusion assay mimics physiological SNARE requirements. Proc. Natl. Acad. Sci. USA. 107:3517–3521. 10.1073/pnas.0914723107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., and Miledi R.. 1965. The Effect of Calcium on Acetylcholine Release from Motor Nerve Terminals. Proc. R. Soc. Lond. B Biol. Sci. 161:496–503. 10.1098/rspb.1965.0017 [DOI] [PubMed] [Google Scholar]
- Kiessling V., Ahmed S., Domanska M.K., Holt M.G., Jahn R., and Tamm L.K.. 2013. Fast Snare Mediated Synaptic Vesicle Fusion in Supported Membranes. Biophys. J. 104:88a–88a. 10.1016/j.bpj.2012.11.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin V.A., and Martin T.F.. 2000. Priming in exocytosis: attaining fusion-competence after vesicle docking. Biochimie. 82:399–407. 10.1016/S0300-9084(00)00208-X [DOI] [PubMed] [Google Scholar]
- Klingauf J., and Neher E.. 1997. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys. J. 72:674–690. 10.1016/S0006-3495(97)78704-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D.E., and Baker P.F.. 1982. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J. Membr. Biol. 68:107–140. 10.1007/BF01872259 [DOI] [PubMed] [Google Scholar]
- Kreutzberger A.J.B., Kiessling V., Liang B., Seelheim P., Jakhanwal S., Jahn R., Castle J.D., and Tamm L.K.. 2017. Reconstitution of calcium-mediated exocytosis of dense-core vesicles. Sci. Adv. 3:e1603208 10.1126/sciadv.1603208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung M., Srivastava A., Zhang Y., Diao J., Vrljic M., Grob P., Nogales E., Chu S., and Brunger A.T.. 2011. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA. 108:E304–E313. 10.1073/pnas.1107900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Choi U.B., Leitz J., Rhee H.J., Lee C., Altas B., Zhao M., Pfuetzner R.A., Wang A.L., Brose N., et al. . 2017. Molecular Mechanisms of Synaptic Vesicle Priming by Munc13 and Munc18. Neuron. 95:591–607. 10.1016/j.neuron.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw K.M.E., Livingstone R., Al-Tobi M., Bryant N.J., and Gould G.W.. 2017. SNARE phosphorylation: a control mechanism for insulin-stimulated glucose transport and other regulated exocytic events. Biochem. Soc. Trans. 45:1271–1277. 10.1042/BST20170202 [DOI] [PubMed] [Google Scholar]
- Landò L., and Zucker R.S.. 1994. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J. Neurophysiol. 72:825–830. 10.1152/jn.1994.72.2.825 [DOI] [PubMed] [Google Scholar]
- Lee H.K., Yang Y., Su Z., Hyeon C., Lee T.S., Lee H.W., Kweon D.H., Shin Y.K., and Yoon T.Y.. 2010. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 328:760–763. 10.1126/science.1187722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley A.W. 1956. The quantal components of the mammalian end-plate potential. J. Physiol. 133:571–587. 10.1113/jphysiol.1956.sp005610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X., Scheuss V., and Schneggenburger R.. 2005. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 435:497–501. 10.1038/nature03568 [DOI] [PubMed] [Google Scholar]
- Ma C., Su L., Seven A.B., Xu Y., and Rizo J.. 2013. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 339:421–425. 10.1126/science.1230473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall D.D., Lin Z., Chon N.L., Jackman S.L., Lin H., Knight J.D., and Anantharam A.. 2018. The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis. J. Gen. Physiol. 150:783–807. 10.1085/jgp.201711944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K.L. 1973. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J. Physiol. 234:327–352. 10.1113/jphysiol.1973.sp010348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K.L., and Zengel J.E.. 1975. A dual effect of repetitive stimulation on post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J. Physiol. 245:163–182. 10.1113/jphysiol.1975.sp010839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K.L., and Zengel J.E.. 1976. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J. Physiol. 257:449–470. 10.1113/jphysiol.1976.sp011378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B.S., Block M.R., and Rothman J.E.. 1988. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 54:221–227. 10.1016/0092-8674(88)90554-5 [DOI] [PubMed] [Google Scholar]
- Mallart A., and Martin A.R.. 1967. Two components of facilitation at the neuromuscular junction of the frog. J. Physiol. 191:19P–20P. [PMC free article] [PubMed] [Google Scholar]
- Malsam J., Parisotto D., Bharat T.A., Scheutzow A., Krause J.M., Briggs J.A., and Söllner T.H.. 2012. Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release. EMBO J. 31:3270–3281. 10.1038/emboj.2012.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.F.J., and Walent J.H.. 1989. A new method for cell permeabilization reveals a cytosolic protein requirement for Ca2+ -activated secretion in GH3 pituitary cells. J. Biol. Chem. 264:10299–10308. [PubMed] [Google Scholar]
- Matveev V., Bertram R., and Sherman A.. 2011. Calcium cooperativity of exocytosis as a measure of Ca2+ channel domain overlap. Brain Res. 1398:126–138. 10.1016/j.brainres.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Missler M., Li C., and Südhof T.C.. 1995. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 83:111–119. 10.1016/0092-8674(95)90239-2 [DOI] [PubMed] [Google Scholar]
- Miller C., Arvan P., Telford J.N., and Racker E.. 1976. Ca++-induced fusion of proteoliposomes: dependence on transmembrane osmotic gradient. J. Membr. Biol. 30:271–282. 10.1007/BF01869672 [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D.O., Tien H.T., and Wescott W.C.. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 194:979–980. 10.1038/194979a0 [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W.W., and Schulz R.A.. 1970. Ultrastructural evidence of secretion by exocytosis and of “synaptic vesicle” formation in posterior pituitary glands. Nature. 227:407–409. 10.1038/227407a0 [DOI] [PubMed] [Google Scholar]
- Neher E. 2006. A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse. Pflugers Arch. 453:261–268. 10.1007/s00424-006-0143-9 [DOI] [PubMed] [Google Scholar]
- Neher E. 2015. Merits and Limitations of Vesicle Pool Models in View of Heterogeneous Populations of Synaptic Vesicles. Neuron. 87:1131–1142. 10.1016/j.neuron.2015.08.038 [DOI] [PubMed] [Google Scholar]
- Neher E., and Augustine G.J.. 1992. Calcium gradients and buffers in bovine chromaffin cells. J. Physiol. 450:273–301. 10.1113/jphysiol.1992.sp019127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., and Marty A.. 1982. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 79:6712–6716. 10.1073/pnas.79.21.6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., and Sakmann B.. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 260:799–802. 10.1038/260799a0 [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., and Steinbach J.H.. 1978. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 375:219–228. 10.1007/BF00584247 [DOI] [PubMed] [Google Scholar]
- Niles W.D., and Cohen F.S.. 1987. Video fluorescence microscopy studies of phospholipid vesicle fusion with a planar phospholipid membrane. Nature of membrane-membrane interactions and detection of release of contents. J. Gen. Physiol. 90:703–735. 10.1085/jgp.90.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., and Schekman R.. 1979. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 76:1858–1862. 10.1073/pnas.76.4.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., and Schekman R.. 1980. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 21:205–215. 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., and Schekman R.. 1981. Order of events in the yeast secretory pathway. Cell. 25:461–469. 10.1016/0092-8674(81)90064-7 [DOI] [PubMed] [Google Scholar]
- Palade G.E. 1956a The endoplasmic reticulum. J. Biophys. Biochem. Cytol. 2(4, suppl):85–98. 10.1083/jcb.2.4.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G.E. 1956b Intracisternal granules in the exocrine cells of the pancreas. J. Biophys. Biochem. Cytol. 2:417–422. 10.1083/jcb.2.4.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G.E. 1959. Functional changes in the structure of cell components. In Subcellular Particles. Hayashi T., editor. Ronald press, New York. 64–83. [Google Scholar]
- Palade G. 1975. Intracellular aspects of the process of protein synthesis. Science. 189:347–358. 10.1126/science.1096303 [DOI] [PubMed] [Google Scholar]
- Palade G.E., and Siekevitz P.. 1956. Pancreatic microsomes; an integrated morphological and biochemical study. J. Biophys. Biochem. Cytol. 2:671–690. 10.1083/jcb.2.6.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., and Poste G.. 1975. Calcium-induced phase separation and fusion in phospholipid membranes. Biophys. J. 15:945–948. 10.1016/S0006-3495(75)85872-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B.E., and Vail W.J.. 1974. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim. Biophys. Acta. 352:10–28. 10.1016/0005-2736(74)90175-8 [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Portis A., and Pangborn W.. 1978. Calcium-induced lipid phase transitions and membrane fusion. Ann. N. Y. Acad. Sci. 308(1 Liposomes and):50–66. 10.1111/j.1749-6632.1978.tb22013.x [DOI] [PubMed] [Google Scholar]
- Park Y., Hernandez J.M., van den Bogaart G., Ahmed S., Holt M., Riedel D., and Jahn R.. 2012. Controlling synaptotagmin activity by electrostatic screening. Nat. Struct. Mol. Biol. 19:991–997. 10.1038/nsmb.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Seo J.B., Fraind A., Pérez-Lara A., Yavuz H., Han K., Jung S.R., Kattan I., Walla P.J., Choi M., et al. . 2015. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat. Struct. Mol. Biol. 22:815–823. 10.1038/nsmb.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D., Kleppe I.C., Taraska J.W., and Almers W.. 2004. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J. Physiol. 560:413–428. 10.1113/jphysiol.2004.064410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., and Papahadjopoulos D.. 1979. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 18:780–790. 10.1021/bi00572a007 [DOI] [PubMed] [Google Scholar]
- Rao T.C., Passmore D.R., Peleman A.R., Das M., Chapman E.R., and Anantharam A.. 2014. Distinct fusion properties of synaptotagmin-1 and synaptotagmin-7 bearing dense core granules. Mol. Biol. Cell. 25:2416–2427. 10.1091/mbc.e14-02-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T.C., Santana Rodriguez Z., Bradberry M.M., Ranski A.H., Dahl P.J., Schmidtke M.W., Jenkins P.M., Axelrod D., Chapman E.R., Giovannucci D.R., and Anantharam A.. 2017. Synaptotagmin isoforms confer distinct activation kinetics and dynamics to chromaffin cell granules. J. Gen. Physiol. 149:763–780. 10.1085/jgp.201711757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P.W., and Lardy H.A.. 1972. A23187: a divalent cation ionophore. J. Biol. Chem. 247:6970–6977. [PubMed] [Google Scholar]
- Reim K., Mansour M., Varoqueaux F., McMahon H.T., Südhof T.C., Brose N., and Rosenmund C.. 2001. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 104:71–81. 10.1016/S0092-8674(01)00192-1 [DOI] [PubMed] [Google Scholar]
- Rink T.J., and Knight D.E.. 1988. Stimulus-secretion coupling: a perspective highlighting the contributions of Peter Baker. J. Exp. Biol. 139:1–30. [PubMed] [Google Scholar]
- Rosenthal J. 1969. Post-tetanic potentiation at the neuromuscular junction of the frog. J. Physiol. 203:121–133. 10.1113/jphysiol.1969.sp008854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., and Bader M.F.. 1987. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J. Biol. Chem. 262:16671–16676. [PubMed] [Google Scholar]
- Satir B., Schooley C., and Satir P.. 1973. Membrane fusion in a model system. Mucocyst secretion in Tetrahymena. J. Cell Biol. 56:153–176. 10.1083/jcb.56.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., and Holz R.W.. 1994. Evidence that the ability to respond to a calcium stimulus in exocytosis is determined by the secretory granule membrane: comparison of exocytosis of injected bovine chromaffin granule membranes and endogenous cortical granules in Xenopus laevis oocytes. Cell. Mol. Neurobiol. 14:245–257. 10.1007/BF02088323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Logsdon C.D., and Holz R.W.. 1992. Bovine chromaffin granule membranes undergo Ca(2+)-regulated exocytosis in frog oocytes. J. Cell Biol. 116:359–365. 10.1083/jcb.116.2.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R., and Neher E.. 2000. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 406:889–893. 10.1038/35022702 [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., and Neher E.. 2005. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 15:266–274. 10.1016/j.conb.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Shin O.H., Lu J., Rhee J.S., Tomchick D.R., Pang Z.P., Wojcik S.M., Camacho-Perez M., Brose N., Machius M., Rizo J., et al. . 2010. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat. Struct. Mol. Biol. 17:280–288. 10.1038/nsmb.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P., and Palade G.E.. 1958a A cyto-chemical study on the pancreas of the guinea pig. III. In vivo incorporation of leucine-1-C14 into the proteins of cell fractions. J. Biophys. Biochem. Cytol. 4:557–566. 10.1083/jcb.4.5.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P., and Palade G.E.. 1958b A cytochemical study on the pancreas of the guinea pig. I. Isolation and enzymatic activities of cell fractions. J. Biophys. Biochem. Cytol. 4:203–218. 10.1083/jcb.4.2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz P., and Palade G.E.. 1958c A cytochemical study on the pancreas of the guinea pig. II. Functional variations in the enzymatic activity of microsomes. J. Biophys. Biochem. Cytol. 4:309–318. 10.1083/jcb.4.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. 1999. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. J. Neurosci. 19:589–598. 10.1523/JNEUROSCI.19-02-00589.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Bennett M.K., Whiteheart S.W., Scheller R.H., and Rothman J.E.. 1993a A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 75:409–418. 10.1016/0092-8674(93)90376-2 [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., and Rothman J.E.. 1993b SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. 10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- Sørensen J.B. 2004. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 448:347–362. 10.1007/s00424-004-1247-8 [DOI] [PubMed] [Google Scholar]
- Sørensen J.B., Fernández-Chacón R., Südhof T.C., and Neher E.. 2003. Examining synaptotagmin 1 function in dense core vesicle exocytosis under direct control of Ca2+. J. Gen. Physiol. 122:265–276. 10.1085/jgp.200308855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A.E., Breckenridge L.J., Lee A.K., and Almers W.. 1990. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 4:643–654. 10.1016/0896-6273(90)90192-I [DOI] [PubMed] [Google Scholar]
- Steyer J.A., Horstmann H., and Almers W.. 1997. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 388:474–478. 10.1038/41329 [DOI] [PubMed] [Google Scholar]
- Südhof T.C. 2014. The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl. 53:12696–12717. 10.1002/anie.201406359 [DOI] [PubMed] [Google Scholar]
- Taraska J.W., Perrais D., Ohara-Imaizumi M., Nagamatsu S., and Almers W.. 2003. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc. Natl. Acad. Sci. USA. 100:2070–2075. 10.1073/pnas.0337526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Wong J.G., Lee A.K., and Almers W.. 1993. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 11:93–104. 10.1016/0896-6273(93)90274-U [DOI] [PubMed] [Google Scholar]
- Tucker W.C., Edwardson J.M., Bai J., Kim H.J., Martin T.F., and Chapman E.R.. 2003. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 162:199–209. 10.1083/jcb.200302060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V.D. 1975. The isolation of intact cortical granules from sea urchin eggs: calcium lons trigger granule discharge. Dev. Biol. 43:62–74. 10.1016/0012-1606(75)90131-1 [DOI] [PubMed] [Google Scholar]
- Várnai P., and Balla T.. 1998. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143:501–510. 10.1083/jcb.143.2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T. 2000. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 28:537–545. 10.1016/S0896-6273(00)00131-8 [DOI] [PubMed] [Google Scholar]
- Vogel S.S., and Zimmerberg J.. 1992. Proteins on exocytic vesicles mediate calcium-triggered fusion. Proc. Natl. Acad. Sci. USA. 89:4749–4753. 10.1073/pnas.89.10.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walent J.H., Porter B.W., and Martin T.F.J.. 1992. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 70:765–775. 10.1016/0092-8674(92)90310-9 [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Söllner T.H., and Rothman J.E.. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. 10.1016/S0092-8674(00)81404-X [DOI] [PubMed] [Google Scholar]
- Wightman R.M., Jankowski J.A., Kennedy R.T., Kawagoe K.T., Schroeder T.J., Leszczyszyn D.J., Near J.A., Diliberto E.J. Jr., and Viveros O.H.. 1991. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA. 88:10754–10758. 10.1073/pnas.88.23.10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., and Papahadjopoulos D.. 1979. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 281:690–692. 10.1038/281690a0 [DOI] [PubMed] [Google Scholar]
- Wilson S.P., and Kirshner N.. 1983. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J. Biol. Chem. 258:4994–5000. [PubMed] [Google Scholar]
- Woodbury D.J., and Rognlien K.. 2000. The t-SNARE syntaxin is sufficient for spontaneous fusion of synaptic vesicles to planar membranes. Cell Biol. Int. 24:809–818. 10.1006/cbir.2000.0631 [DOI] [PubMed] [Google Scholar]
- Wu M.M., Llobet A., and Lagnado L.. 2009. Loose coupling between calcium channels and sites of exocytosis in chromaffin cells. J. Physiol. 587:5377–5391. 10.1113/jphysiol.2009.176065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Baumeister A., Binz T., Blasi J., Link E., Cornille F., Roques B., Fykse E.M., Südhof T.C., Jahn R., and Niemann H.. 1994. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem. 269:12764–12772. [PubMed] [Google Scholar]
- Yang H., Liu H., Hu Z., Zhu H., and Xu T.. 2005. PKC-induced sensitization of Ca2+-dependent exocytosis is mediated by reducing the Ca2+ cooperativity in pituitary gonadotropes. J. Gen. Physiol. 125:327–334. 10.1085/jgp.200409230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T.Y., Okumus B., Zhang F., Shin Y.K., and Ha T.. 2006. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA. 103:19731–19736. 10.1073/pnas.0606032103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J.E., and Magleby K.L.. 1977. Transmitter release during repetitive stimulation: selective changes produced by Sr2+ and Ba2+. Science. 197:67–69. 10.1126/science.17160 [DOI] [PubMed] [Google Scholar]
- Zengel J.E., and Magleby K.L.. 1980. Differential effects of Ba2+, Sr2+, and Ca2+ on stimulation-induced changes in transmitter release at the frog neuromuscular junction. J. Gen. Physiol. 76:175–211. 10.1085/jgp.76.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J.E., Magleby K.L., Horn J.P., McAfee D.A., and Yarowsky P.J.. 1980. Facilitation, augmentation, and potentiation of synaptic transmission at the superior cervical ganglion of the rabbit. J. Gen. Physiol. 76:213–231. 10.1085/jgp.76.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu Y., Wang Z., Dunning F.M., Rehfuss J., Ramanan D., Chapman E.R., and Jackson M.B.. 2011. Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Mol. Biol. Cell. 22:2324–2336. 10.1091/mbc.e11-02-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.D., Hamid E., Shin W., Wen P.J., Krystofiak E.S., Villarreal S.A., Chiang H.C., Kachar B., and Wu L.G.. 2016. Hemi-fused structure mediates and controls fusion and fission in live cells. Nature. 534:548–552. 10.1038/nature18598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Lai Y., Bacaj T., Zhao M., Lyubimov A.Y., Uervirojnangkoorn M., Zeldin O.B., Brewster A.S., Sauter N.K., Cohen A.E., et al. . 2015. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 525:62–67. 10.1038/nature14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Hille B., and Xu T.. 2002. Sensitization of regulated exocytosis by protein kinase C. Proc. Natl. Acad. Sci. USA. 99:17055–17059. 10.1073/pnas.232588899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Cohen F.S., and Finkelstein A.. 1980a Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. I. Discharge of vesicular contents across the planar membrane. J. Gen. Physiol. 75:241–250. 10.1085/jgp.75.3.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Cohen F.S., and Finkelstein A.. 1980b Micromolar Ca2+ stimulates fusion of lipid vesicles with planar bilayers containing a calcium-binding protein. Science. 210:906–908. 10.1126/science.7434004 [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Sardet C., and Epel D.. 1985. Exocytosis of sea urchin egg cortical vesicles in vitro is retarded by hyperosmotic sucrose: kinetics of fusion monitored by quantitative light-scattering microscopy. J. Cell Biol. 101:2398–2410. 10.1083/jcb.101.6.2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F.S., and Brodwick M.. 1987. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc. Natl. Acad. Sci. USA. 84:1585–1589. 10.1073/pnas.84.6.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]