Abstract
Effects of baseline anxiety on the efficacy of venlafaxine extended release versus placebo were examined in a post hoc pooled subgroup analysis of 1573 patients enrolled in eight short-term studies of major depressive disorder. Anxiety subgroups were defined based on baseline 17-item Hamilton Rating Scale for Depression Item 10 score <3 (low) versus ≥3 (high). Change from baseline to final visit in Montgomery–Åsberg Depression Rating Scale total score and Montgomery–Åsberg Depression Rating Scale response and remission rates were analyzed. Change from baseline in Montgomery–Åsberg Depression Rating Scale total score and response and remission rates was significantly greater for venlafaxine extended release versus placebo in both low and high anxiety subgroups (all P < 0.0001). A statistically significant baseline anxiety by treatment interaction was observed for Montgomery–Åsberg Depression Rating Scale total score only (P = 0.0152). The adjusted mean change from baseline in Montgomery–Åsberg Depression Rating Scale total score was significantly greater in the high anxiety subgroup versus low anxiety subgroup for patients treated with venlafaxine extended release (−6.27 versus −3.89; P = 0.0440) but not placebo. These results support the efficacy of venlafaxine extended release for major depressive disorder treatment in patients with anxiety symptoms.
Keywords: anxiety, major depressive disorder, serotonin–norepinephrine reuptake inhibitor, treatment efficacy, venlafaxine extended release
Introduction
Symptoms of anxiety are common in patients with major depressive disorder (MDD) (Fava et al., 2008; Wiethoff et al., 2010). A recent review reported rates of co-occurring anxiety in depressed patients as high as 78% (Gaspersz et al., 2018). The prevalence of current or lifetime comorbid anxiety disorders is estimated at 51–57% in outpatients with depression (Fava et al., 2000; Zimmerman et al., 2002a). Anxious depression has been associated with a greater severity of illness, longer duration of illness, increased incidence of suicidal thoughts or behaviors, and more severe functional impairment compared with nonanxious depression (Altamura et al., 2004; Fava et al, 2006; Wiethoff et al, 2010), and MDD patients with comorbid anxiety at baseline are likely to have poorer clinical outcomes than those without anxious symptoms (Souery et al., 2007; Fava et al., 2008; Gaspersz et al., 2018).
Although antidepressant medications have generally similar efficacy within and between classes when used to treat depression (Gelenberg et al., 2010), and a range of antidepressant drugs have been shown to be effective in treating depression with anxiety (Tollefson et al., 1994; Bandelow et al., 2007; Nelson, 2010; Kornstein et al., 2014; Stein et al., 2013), several studies suggest that there may be efficacy differences among drugs in MDD patients with symptoms of anxiety. For example, in analyses of depressed patients with high levels of anxiety symptoms at baseline, patients treated with selective serotonin reuptake inhibitors (SSRIs) had greater response rates than in those treated with bupropion (Papakostas et al., 2008), and patients treated with the serotonin–norepinephrine reuptake inhibitor (SNRI) venlafaxine (extended release or immediate release) had significantly greater rates of remission compared with patients treated with fluoxetine (Davidson et al., 2002).
The antidepressant venlafaxine extended release has demonstrated efficacy for treating both MDD (Thase et al., 2017) and anxiety disorders in double-blind, placebo-controlled clinical trials (Rickels et al., 2000; Davidson et al., 1999; Allgulander et al., 2004b) and is approved for the treatment of generalized anxiety disorder (GAD), social anxiety disorder, and panic disorder in addition to MDD (Effexor package insert, 2017). Venlafaxine extended release also significantly improves symptoms of depression in MDD patients with comorbid anxiety (Silverstone and Ravindran, 1999; Silverstone and Salinas, 2001); however, results of anxiety subgroup analyses based on clinical trial data for other antidepressant drugs have suggested that antidepressant efficacy may be reduced in MDD patients with high baseline anxiety compared with patients with lower anxiety (Joliat et al., 2004; Fava et al., 2008; Wiethoff et al., 2010; Papakostas et al., 2012). This has not previously been examined with venlafaxine extended release in patients treated for MDD. The objective of this analysis was to determine the effect of baseline anxiety symptoms on improvement in depression symptoms in MDD patients treated with venlafaxine extended release or placebo. We therefore conducted a post hoc meta-analysis of data from eight clinical trials of venlafaxine extended release versus placebo for the treatment of MDD.
Methods
Data set
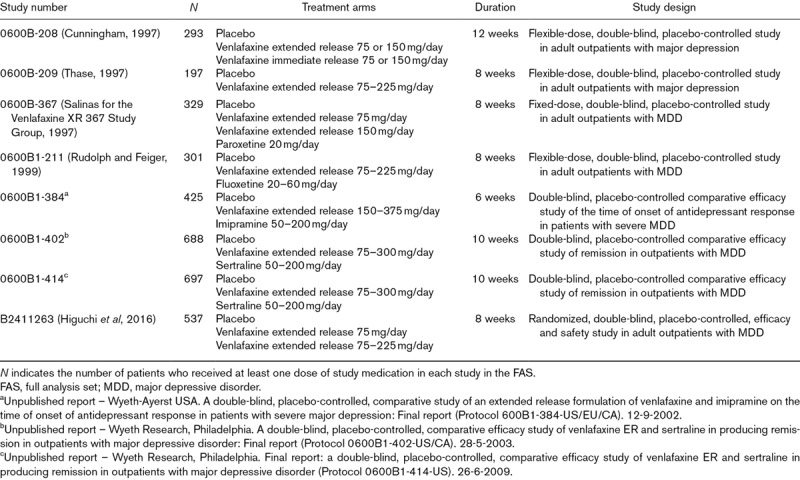
Patient-level data were pooled from eight short-term (6–12 weeks), randomized, placebo-controlled studies of venlafaxine extended release for the treatment of MDD. This data set includes all available short-term, Wyeth/Pfizer sponsored phase II, III, or IV, double-blind, placebo-controlled studies in adult patients with MDD that included at least one venlafaxine extended release treatment arm and that administered the Hamilton Rating Scale for Depression (HAM-D) at baseline and included a change from baseline in Montgomery–Åsberg Depression Rating Scale (MADRS) score as an efficacy outcome. Study characteristics are listed in Table 1. Venlafaxine extended release dose ranged from 75 to 375 mg/day. Six studies used flexible dosing, one used fixed dosing, and one included fixed- and flexible-dose arms. Five of the studies included an active control treatment arm and one included a venlafaxine immediate release arm; only data from venlafaxine extended release and placebo arms were included in the analysis.
Table 1.
Studies included in the pooled analysis
Protocols and any amendments received Institutional Review Board/Ethics Committee approval before the study began, and all patients were required to provide written informed consent prior to enrollment.
Patients
All eight studies included in the post hoc analysis enrolled adult outpatients (≥18 years; ≥20 years for one study) who met Diagnostic and Statistical Manual of Mental Disorders (DSM; Third Edition-Revised, Fourth Edition, or Fourth Edition-Text Revision) (American Psychiatric Association, 1987; American Psychiatric Association, 1994; American Psychiatric Association, 2000) criteria for major depression, severe depression, or MDD. Enrolled patients had symptoms of depression of at least 30 days duration and a HAM-D21 total score of 20 or greater [or MADRS total score ≥ 27 (1 study)] at screening and baseline. The HAM-D total score was calculated as the sum of items 1–17 (Hamilton, 1960), equivalent to the 17-item HAM-D (HAM-D17) total score. HAM-D items 18–21 were not scored or considered in the current analysis. Exclusion criteria were designed to select a sample of medically stable patients with a principal diagnosis of MDD (excluding bipolar and psychotic depression). Patients with recent treatment with the study drug, a history of drug or alcohol dependence within a year of study entry, or acute suicidality were also excluded.
Measures
The measure of efficacy in this analysis was change from baseline in MADRS total score at last visit (last observation carried forward). Other outcomes assessed were rate of response, defined as at least 50% reduction from baseline in MADRS total score, and rate of remission (MADRS total score ≤ 10) at the last visit.
Baseline anxiety symptoms were assessed using the HAM-D psychic anxiety item (item 10), scored from 0 (no difficulty) to 4 (fears expressed without questioning). Subgroups were defined using a HAM-D item 10 threshold score of 3 (low anxiety < 3; high anxiety ≥ 3), which has been used as a proxy for one of the criterion symptoms (‘Fear that something awful may happen’) for DSM (5th Edition; DSM-5) depressive disorder with anxious distress (McIntyre et al., 2016; American Psychiatric Association, 2013). Analyses were also conducted using two additional definitions for baseline anxiety subgroups; one based on the HAM-D item 11 (somatic anxiety) score, scores from 0 (absent) to 4 (incapacitating), and another based on the HAM-D anxiety/somatization factor score (sum of HAM-D items 10, 11, 12, 13, 15, and 17), which incorporates both psychic and somatic anxiety symptoms (Cleary and Guy, 1977). Somatic anxiety subgroups were defined using a baseline HAM-D item 11 threshold value of 3 (low anxiety < 3; high anxiety ≥ 3), as was used for HAM-D item 10. HAM-D anxiety/somatization score subgroups were defined using less than 7 (low anxiety) versus 7 or greater (high anxiety) as described previously (Tollefson et al., 1993).
Statistical analysis
Statistical analyses were based on the full analysis set (FAS), defined as all patients in the pooled studies who took at least 1 dose of the study drug during the double-blind treatment period. To examine the effect of baseline anxiety symptoms on venlafaxine extended release efficacy, change from baseline in MADRS total score was analyzed using an analysis of covariance model with terms for study, treatment group, baseline anxiety subgroup (low versus high), interaction of treatment group by baseline anxiety subgroup, and baseline MADRS total score. Binary end points (MADRS response and remission rates) were analyzed using logistic regression models with terms for study, treatment group, baseline anxiety subgroup, treatment group by baseline anxiety subgroup interaction, and baseline MADRS total score. All analyses based on HAM-D item 10 and 11 subgroups were repeated using a cutoff of 2. Only results for subgroups based on less than 3 and at least 3 were reported, as results using the different threshold values were similar.
Results
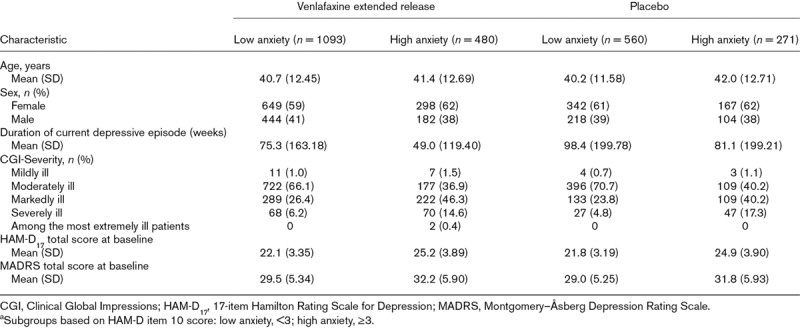
The FAS included 2405 patients; 1573 patients were treated with venlafaxine extended release and 832 were treated with placebo. The majority of patients were white (69.1%) and female (60.5%). At baseline, patients had a median age of 40.0 years (range: 18–85 years) and their severity of depression was generally moderate to severe (Table 2). Mean daily venlafaxine extended release dose was 143.5 mg/day (median: 143.9 mg/day; range: 37.5–471.7 mg/day).
Table 2.
Baseline demographics and clinical characteristics by treatment and baseline anxiety subgroup,a full analysis set
HAM-D item 10 subgroup analysis
The distribution of HAM-D item 10, psychic anxiety scores at baseline is shown in Fig. 1. A total of 751/2405 (31.2%) patients had a baseline HAM-D item 10 anxiety score greater than three and were included in the high baseline anxiety subgroup. Baseline mean MADRS total scores were 29.4 (venlafaxine extended release) and 28.9 (placebo) for patients in the low baseline anxiety subgroup and 32.0 (venlafaxine extended release) and 31.7 (placebo) for those with high baseline anxiety symptoms.
Fig. 1.
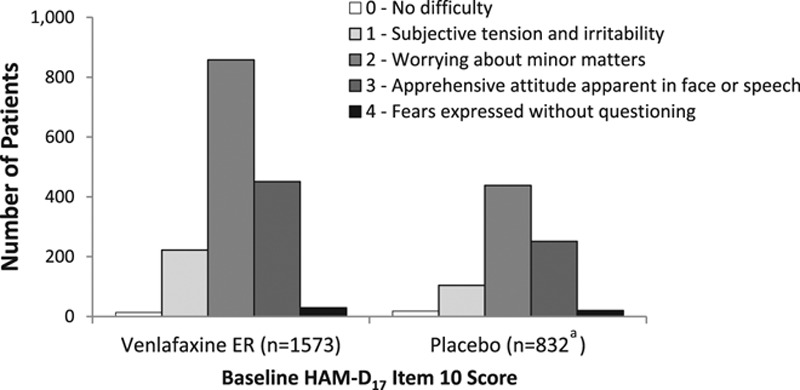
Distribution of HAM-D item 10 scores at baseline. aOne patient assigned to placebo had missing HAM-D item score at baseline. HAM-D17, 17-item Hamilton Rating Scale for Depression.
A statistically significant effect of interaction between baseline anxiety subgroup and treatment was observed (P = 0.0152). Venlafaxine extended release showed a significantly greater improvement from baseline in MADRS total score at final visit compared with placebo for both low and high baseline anxiety subgroups (both P < 0.0001; Fig. 2) based on HAM-D item 10 score. Among patients treated with venlafaxine extended release, those with high baseline anxiety (HAM-D item 10 scores ≥ 3) had greater improvement from baseline in MADRS total score at final visit (adjusted mean change [standard error], −16.98 [0.51]) compared with the low baseline anxiety subgroup (−15.76 [0.35]; P = 0.0440). However, no significant difference was found between baseline anxiety subgroups in improvement with placebo treatment (low, −11.87 [0.46]; high, −10.71 [0.65]).
Fig. 2.
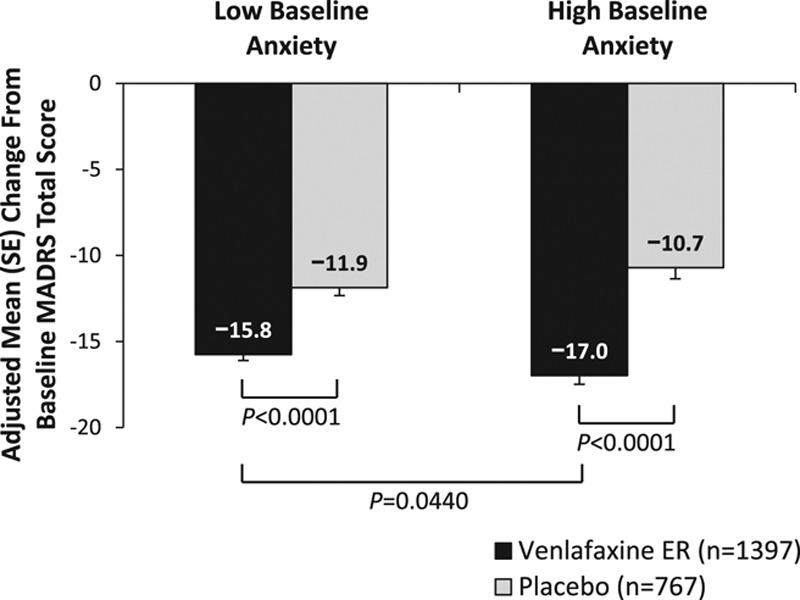
Change from baseline to final visit in MADRS total score, HAM-D item 10 anxiety subgroups. Low baseline anxiety, HAM-D item 10 score < 3; high baseline anxiety, HAM-D item 10 score ≥ 3. HAM-D17, 17-item Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; SE, standard error.
The test of interaction between treatment and baseline anxiety for MADRS response yielded a P value of 0.0616. Significantly, higher rates of MADRS response were observed at final visit for venlafaxine extended release versus placebo in both low and high baseline anxiety subgroups (low anxiety, 58.9% versus 40.5%; high anxiety, 64.2% versus 36.9%; both P < 0.0001; Fig. 3a). The adjusted odds ratio (OR) of MADRS response between venlafaxine extended release versus placebo was 2.02 (95% confidence interval [CI]: 1.61, 2.52) for the low anxiety subgroup and 2.94 (95% CI 2.11, 4.09) for the high anxiety subgroup. For patients receiving venlafaxine extended release, the probability of achieving MADRS response was significantly greater for patients with high baseline anxiety versus low baseline anxiety (OR: 1.31 [95% CI: 1.02, 1.68]; P = 0.0318). Among those receiving placebo, MADRS response rates, however, were not found to be significantly different for low versus high anxiety subgroups (OR: 0.90 [95% CI: 0.65, 1.24]).
Fig. 3.
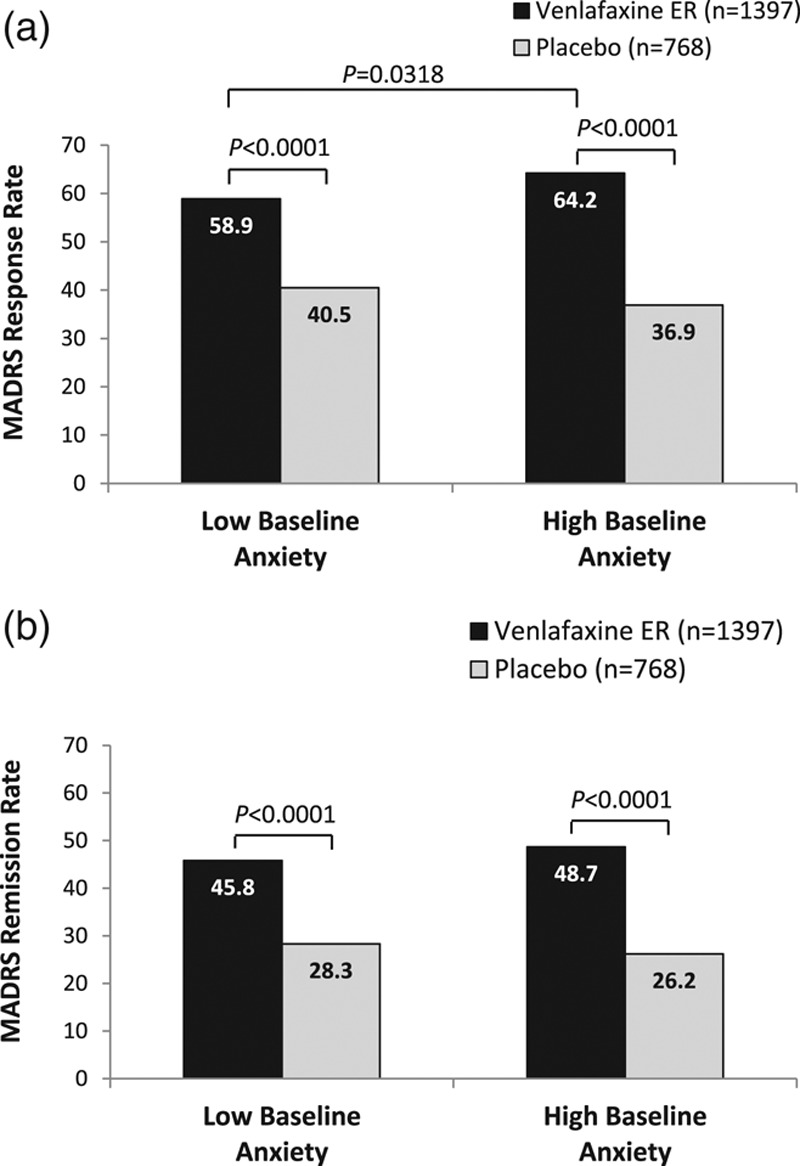
MADRS response (a) and remission (b) rates at final visit, HAM-D item 10 anxiety subgroups. Low baseline anxiety, HAM-D item 10 score < 3; high baseline anxiety, HAM-D item 10 score ≥ 3. (a) MADRS response. Response was defined as reduction from baseline of ≥50% in MADRS total score. (b) MADRS remission. Remission was defined as MADRS total score ≤ 10. ER, extended release; HAM-D17, 17-item Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale.
The effect of interaction between treatment and baseline anxiety was not statistically significant in the analysis of MADRS remission (P = 0.2865), allowing for an interpretation of the overall effects of treatment group and baseline anxiety subgroup. Overall, significantly higher proportions of patients treated with venlafaxine extended release versus placebo achieved MADRS remission at final visit (P < 0.0001; low baseline anxiety subgroup: 45.8 versus 28.3%, OR: 2.19 [95% CI: 1.72, 2.78]; high baseline anxiety subgroup: 48.7 versus 26.2%, OR: 2.76 [95% CI: 1.93, 3.93]; Fig. 3b). Rates of remission were numerically higher for patients with high versus low baseline anxiety treated with venlafaxine extended release, but the odds of achieving remission did not differ significantly between baseline anxiety groups treated with either venlafaxine extended release (OR: 1.27 [0.99, 1.63]) or placebo (OR: 1.01 [0.71, 1.44]).
HAM-D anxiety/somatization factor and HAM-D item 11 subgroup analyses
A greater proportion of patients (1554/2405 [64.6%]) were defined as having high baseline anxiety based on HAM-D anxiety/somatization factor score (≥7) compared with the HAM-D item 10 score definition. A total of 263/2405 (10.9%) patients were categorized as having high baseline somatic anxiety using a baseline HAM-D item 11 score cutoff of three or greater.
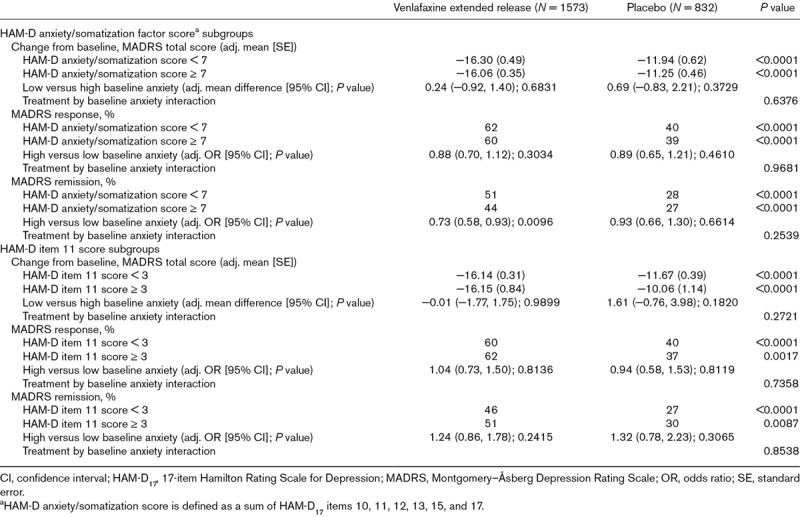
Venlafaxine extended release was associated with significantly greater improvement from baseline in MADRS total score and significantly higher rates of response and remission compared with placebo for low and high baseline anxiety subgroups based on either baseline HAM-D anxiety/somatization factor score or baseline HAM-D somatic anxiety (item 11) score (all P ≤ 0.0087; Table 3). When baseline anxiety subgroups were defined based on HAM-D anxiety/somatization factor score, the probability of achieving MADRS remission was significantly lower for patients with high baseline anxiety compared with those with low baseline anxiety among patients receiving venlafaxine extended release (OR: 0.73 [95% CI: 0.58, 0.93]; P = 0.0096), but not among patients in the placebo group. No other significant differences between low and high anxiety subgroups were observed using either definition. The tests for interactions between treatment and anxiety subgroup based on either definition (HAM-D anxiety/somatization factor score or HAM-D item 11) were not significant for any efficacy measure (Table 3).
Table 3.
Results based on alternate anxiety subgroup definitions, full analysis set
Discussion
In this pooled, post hoc analysis of data from eight short-term, placebo-controlled clinical trials, treatment with venlafaxine extended release (75–375 mg/day) significantly reduced symptoms of depression in MDD patients regardless of the level of anxiety at baseline. A significant treatment effect on MADRS total score was observed in both low and high anxiety subgroups based on psychic anxiety symptoms (HAM-D item 10 score < or ≥3) at baseline. Odds of achieving response or remission were significantly higher for patients treated with venlafaxine extended release versus placebo, for low and high psychic anxiety subgroups. Among patients treated with venlafaxine extended release – but not placebo – patients with high baseline anxiety based on HAM-D item 10 score had significantly greater improvement from baseline in MADRS total score at final visit. That finding was supported by the significantly greater probability of achieving MADRS response compared with the low baseline anxiety subgroup; the effect of baseline anxiety on remission rates in venlafaxine extended release-treated patients approached significance.
In both low and high somatic anxiety symptom subgroups, based on baseline HAM-D item 11 score, venlafaxine extended release treatment was associated with significantly higher rates of response and remission versus placebo. However, no effect of baseline somatic anxiety was observed. Differences between psychic and somatic symptoms of anxiety in response to treatment previously have been noted in the literature: several studies reported greater improvement in psychic symptoms than somatic anxiety symptoms with SSRI/SNRI treatment in GAD or MDD patients (Rocca et al., 1997; Allgulander et al., 2004a; Dahl et al., 2005; Davidson et al., 2008; Russell et al., 2007; Bandelow et al., 2007). It should be noted that those studies measured improvement in psychic versus somatic anxiety symptoms, but did not examine improvement in total depression score in patients with psychic versus somatic anxiety. The effectiveness of HAM-D approaches to identify patients with anxious depression has been recently examined. A kappa coefficient analysis demonstrated only a modest (but significant) concordance between HAM-D anxiety/somatization factor scores and DSM-5 anxious distress specifier criteria; HAM-D item 10 or 11 scores were not similarly assessed (Zimmerman et al., 2018). Observed differences in outcomes in analyses based on different anxiety subgroup definitions do not necessarily indicate that one definition is more accurate or applicable than others, however. Rather, the current results suggest that definitions for high baseline anxiety based on psychic vs. somatic symptoms capture different subsets of patients who may respond differentially to venlafaxine extended release treatment. Interestingly, in the current analysis of subgroups defined based on the HAM-D anxiety/somatization factor, which combines both psychic and somatic anxiety symptoms scores (Cleary and Guy, 1977), there was a statistically significant effect of baseline anxiety on likelihood of remission in venlafaxine extended release-treated patients, but no effect on improvement from baseline in MADRS total score or on MADRS response.
The significantly greater effect of venlafaxine extended release in patients with high versus low baseline psychic anxiety reported here is in contrast with results of several previously published analyses, in which high levels of anxiety in MDD patients were associated with poor response to medication (Joliat et al., 2004; Fava et al., 2008; Wiethoff et al., 2010; Papakostas et al., 2012). Each of those analyses defined baseline anxiety subgroups using the HAM-D anxiety/somatization factor score. In contrast, in an analysis that used psychic anxiety symptoms to define baseline anxiety subgroups, duloxetine treatment significantly improved symptoms of depression in the high anxiety group (baseline HAM-D item 10 score ≥ 2) but not in the low anxiety group (Russell et al., 2007). Subgroups based on somatic anxiety symptoms were not examined. Other analyses showed no difference in antidepressant efficacy outcome based on improvement in depression scale scores or remission rate in low versus high anxiety subgroups (Farabaugh et al., 2010; Nelson, 2010).
Clinical practice guidelines support the use of SSRIs or SNRIs as first-line antidepressant treatment of MDD with anxious symptoms (Schaffer et al., 2012). The Canadian Network for Mood and Anxiety Treatments guidelines recommend the use of an antidepressant with efficacy for GAD in MDD patients with anxious distress, including SSRIs and SNRIs (Kennedy et al., 2016). Several SSRIs/SNRIs in addition to venlafaxine extended release have demonstrated efficacy versus placebo for treating symptoms of depression in low and high baseline anxiety subgroups using the HAM-D anxiety/somatization factor score. Fluoxetine improved symptoms of depression, based on 21-item HAM-D total score, compared with placebo in both anxious and nonanxious depressed patients (Tollefson et al., 1994). Duloxetine had significantly greater efficacy for treating depression compared with placebo in both anxious and nonanxious patients, based on response, remission, and depression scale scores (Nelson, 2010). Desvenlafaxine treatment was associated with significantly greater improvement from baseline in HAM-D17 total score, and significantly higher rates of HAM-D response and remission compared with placebo in low and high anxiety groups (Kornstein et al., 2014), although it has never formally been studied or approved for any anxiety indication.
Studies suggest that the majority of patients diagnosed with MDD have symptoms of anxiety or a comorbid anxiety disorder (Fava et al., 2000; Zimmerman et al., 2002a; Fava et al., 2008; Wiethoff et al., 2010; Gaspersz et al., 2018). Clinicians are well aware of the need to address anxiety symptoms in depressed patients: severe anxiety in such patients may be associated with suicidality (Allgulander, 2000) and cardiovascular disease (Allgulander, 2016). Prospective studies of population samples and of patients with cardiovascular disease point to the emergence of anxiety driving other well-known risk factors such as sedentary lifestyle, tobacco smoking, and depression (Allgulander, 2016). Therefore, assessment for and effective treatment of anxiety in MDD is critical. The observed efficacy of venlafaxine extended release in MDD patients with either low or high levels of anxiety at baseline is not unexpected, as it is approved for the treatment of several anxiety disorders. Nonetheless, given the high comorbidity of major depression with anxiety symptoms, this is a reassuring result for clinicians treating patients with both depression and strong anxiety symptoms, and underscores that venlafaxine extended release is an effective therapeutic option for those patients. Furthermore, patients with GAD may present with depression in primary care settings where clinicians may be less likely to diagnose a primary anxiety disorder (Bandelow et al., 2013; Roberge et al., 2015). From a pragmatic point, the results of this analysis indicate that venlafaxine extended release can be valuable in patients with anxiety and depression treated in settings that cannot offer a qualified diagnostic procedure for the anxiety disorder. On the basis of the results of this meta-analysis, physicians can expect venlafaxine extended release to be effective in depressed patients in both primary and tertiary care settings with and without anxiety symptoms.
Although specific changes to treatment of MDD are not recommended for patients with anxious depression as a group (Schaffer et al., 2012), the APA clinical practice guideline suggests that some patients with prominent anxiety may need adjunctive treatment with an anxiolytic or sedative-hypnotic medication (e.g. buspirone, benzodiazepines, or selective γ-aminobutyric acid agonists) (Gelenberg et al., 2010). In the authors’ experience, it may be beneficial to coprescribe an anxiolytic while titrating an effective antidepressant dose, usually for 2–4 weeks. While patients with depression often have periods when they are well, symptoms of GAD are chronic and thus may require maintenance therapy (Bandelow et al., 2013; Roberge et al., 2015). Indeed, treatment guidelines recommend maintenance therapy in MDD patients with factors associated with anxiety, including comorbid disorders and risk of suicide (Gelenberg et al., 2010; Lam et al., 2016). Anxiety Disorders Association of Canada guidelines cite benefits of maintenance therapy with SSRIs/SNRIs, whereas long-term treatment with benzodiazepines is not recommended (Katzman et al., 2014).
Several limitations of the current analysis should be considered in the discussion of results. First, this was a post hoc analysis of data from clinical trials that were not designed to assess the effect of anxiety symptoms, and individual HAM-D items have not been validated as measures separate from their use in the scale. Few randomized controlled trials of venlafaxine extended release for MDD utilize in-depth assessments of anxiety such as the Hamilton Anxiety Rating Scale (Hamilton, 1959) (HAM-A); the HAM-A was administered in only one trial included in this analysis. Therefore, the HAM-D psychic anxiety item (item 10) was selected as an indicator of anxious symptoms for this analysis, with the somatic anxiety item (item 11) and the anxiety/somatization factor included for comparison. Definitions based on each of the three measures have been used in the published literature to characterize the severity of baseline anxiety symptoms (Nelson, 2010). An additional limitation of the analysis was that the eight pooled trials differed in their enrolled populations based on enrollment criteria and varied in aspects of their design, including the use of fixed versus flexible dosing, dosages assessed, and trial duration. Three of the studies in this analysis were flexible-dose trials that allowed venlafaxine extended release doses exceeding the recommended therapeutic dose range; however, the median daily venlafaxine extended release dose was 143.9 mg (mean: 143.5 mg). In each trial, generally healthy patients with few comorbidities were selected for enrollment; therefore, results may not generalize to a broader population of MDD patients (Zimmerman et al., 2002b). Finally, it is important to note that the efficacy end points used in this analysis included depression measures only. Improvement in anxiety symptoms was not determined, and therefore, these results do not address the efficacy of venlafaxine extended release for treating anxiety symptoms in MDD patients.
Conclusion
The results of this meta-analysis of eight short-term, double-blind, placebo-controlled studies support the efficacy of venlafaxine extended release for the treatment of MDD in those patients with either low or high severity of anxiety symptoms at baseline. Patients with high baseline anxiety based on HAM-D item 10 score had significantly greater improvement from baseline in MADRS total score at final visit and a significantly greater probability of achieving MADRS response compared with the low baseline anxiety subgroup. All three end points assessed (change from baseline in MADRS total score, MADRS response, and MADRS remission rates) remained statistically significant for venlafaxine extended release versus placebo when analyzed for the low or high anxiety subgroups.
Acknowledgements
Medical writing support was provided by Kathleen M. Dorries, PhD, of Peloton Advantage and was funded by Pfizer. Statistical analysis plan and supervision of programming was performed by Xuemei Wang from Syneos Health (funded by Pfizer). Gavin J. Lyndon, Rita Prieto, and Dalia B. Wajsbrot participated in analysis design, assembly of data, data analysis, and manuscript preparation. All authors participated in data interpretation, manuscript review and revisions, and final approval of manuscript.
Conflicts of interest
Gavin J. Lyndon is a full-time employee of Pfizer Ltd. and holds Pfizer stock and stock options. Rita Prieto is a full-time employee of Pfizer GEP SLU Spain and holds Pfizer stock and stock options. Dalia B. Wajsbrot is a full-time employee of Pfizer Inc and holds Pfizer stock and stock options. Christer Allgulander has no potential financial conflicts. In the last 36 months and in the near future, Borwin Bandelow will receive/has received speaker’s honorarium from Hexal, Novartis, Janssen, Lilly, Lundbeck, and Pfizer, will serve or has served on an advisory board for Mundipharma, and will receive or has received publication honorarium from Servier.
References
- Allgulander C. Hawton K, Van Heeringen K. Psychiatric aspects of suicidal behaviour: anxiety disorders. The International Handbook of Suicide and Attempted Suicide. 2000, Chichester, UK: John Wiley & Sons, Ltd [Google Scholar]
- Allgulander C. Anxiety as a risk factor in cardiovascular disease. Curr Opin Psychiatry. 2016;29:13–17. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Dahl AA, Austin C, Morris PL, Sogaard JA, Fayyad R, et al. Efficacy of sertraline in a 12-week trial for generalized anxiety disorder. Am J Psychiatry. 2004a;161:1642–1649. [DOI] [PubMed] [Google Scholar]
- Allgulander C, Mangano R, Zhang J, Dahl AA, Lepola U, Sjödin I, Emilien G: SAD 388 Study Group. Efficacy of venlafaxine ER in patients with social anxiety disorder: a double-blind, placebo-controlled, parallel-group comparison with paroxetine. Hum Psychopharmacol. 2004b;19:387–396. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Montresor C, Salvadori D, Mundo E. Does comorbid subthreshold anxiety affect clinical presentation and treatment response in depression? A preliminary 12-month naturalistic study. Int J Neuropsychopharmacol. 2004;7:481–487. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association : American Psychiatric Association. Diagnostic and statistical manual of mental disorders, third revised edition. 1987, Washington, DC:: American Psychiatric Press [Google Scholar]
- American Psychiatric Association : American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 1994, Washington, DC: American Psychiatric Publishing [Google Scholar]
- American Psychiatric Association : American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR). 2000, Washington, DC:: American Psychiatric Publishing [Google Scholar]
- American Psychiatric Association : American Psychiatric Association. Depressive disorders. Diagnostic and statistical manual of mental disorders. 2013, Washington, DC: American Psychiatric Association [Google Scholar]
- Bandelow B, Andersen HF, Dolberg OT. Escitalopram in the treatment of anxiety symptoms associated with depression. Depress Anxiety. 2007;24:53–61. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Boerner JR, Kasper S, Linden M, Wittchen HU, Moller HJ. The diagnosis and treatment of generalized anxiety disorder. Dtsch Arztebl Int. 2013;110:300–309; quiz 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P, Guy W. Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res. 1977;1:115–120. [Google Scholar]
- Cunningham LA. Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Venlafaxine XR 208 study group. Ann Clin Psychiatry. 1997;9:157–164. [DOI] [PubMed] [Google Scholar]
- Dahl AA, Ravindran A, Allgulander C, Kutcher SP, Austin C, Burt T. Sertraline in generalized anxiety disorder: efficacy in treating the psychic and somatic anxiety factors. Acta Psychiatr Scand. 2005;111:429–435. [DOI] [PubMed] [Google Scholar]
- Davidson J, Allgulander C, Pollack MH, Hartford J, Erickson JS, Russell JM, et al. Efficacy and tolerability of duloxetine in elderly patients with generalized anxiety disorder: a pooled analysis of four randomized, double-blind, placebo-controlled studies. Hum Psychopharmacol. 2008;23:519–526. [DOI] [PubMed] [Google Scholar]
- Davidson JR, DuPont RL, Hedges D, Haskins JT. Efficacy, safety, and tolerability of venlafaxine extended release and buspirone in outpatients with generalized anxiety disorder. J Clin Psychiatry. 1999;60:528–535. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Meoni P, Haudiquet V, Cantillon M, Hackett D. Achieving remission with venlafaxine and fluoxetine in major depression: its relationship to anxiety symptoms. Depress Anxiety. 2002;16:4–13. [DOI] [PubMed] [Google Scholar]
- 2017, Philadelphia, Pennsylvania:: Wyeth Pharmaceuticals Inc [Google Scholar]
- Farabaugh AH, Bitran S, Witte J, Alpert J, Chuzi S, Clain AJ, et al. Anxious depression and early changes in the HAMD-17 anxiety-somatization factor items and antidepressant treatment outcome. Int Clin Psychopharmacol. 2010;25:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, Rosenbaum JF. Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Carmin CN, Balasubramani GK, Wisniewski SR, et al. What clinical and symptom features and comorbid disorders characterize outpatients with anxious major depressive disorder: a replication and extension. Can J Psychiatry. 2006;51:823–835. [DOI] [PubMed] [Google Scholar]
- Gaspersz R, Nawijn L, Lamers F, Penninx BWJH. Patients with anxious depression: overview of prevalence, pathophysiology and impact on course and treatment outcome. Curr Opin Psychiatry. 2018;31:17–25. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, et al. Practice guideline for the treatment of patients with major depressive disorder [online]. 2010, Washington, DC: American Psychiatric Association, http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. [Accessed 24 March 2015]. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Kamijima K, Nakagome K, Itamura R, Asami Y, Kuribayashi K, Imaeda T. A randomized, double-blinded, placebo-controlled study to evaluate the efficacy and safety of venlafaxine extended release and a long-term extension study for patients with major depressive disorder in japan. Int Clin Psychopharmacol. 2016;31:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliat MJ, Schmidt ME, Fava M, Zhang S, Michelson D, Trapp NJ, Miner CM. Long-term treatment outcomes of depression with associated anxiety: efficacy of continuation treatment with fluoxetine. J Clin Psychiatry. 2004;65:373–378. [DOI] [PubMed] [Google Scholar]
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. : Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14Suppl 1S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. : CANMAT Depression Work Group. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Guico-Pabia CJ, Fayyad RS. The effect of desvenlafaxine 50 mg/day on a subpopulation of anxious/depressed patients: a pooled analysis of seven randomized, placebo-controlled studies. Hum Psychopharmacol. 2014;29:492–501. [DOI] [PubMed] [Google Scholar]
- Lam RW, McIntosh D, Wang J, Enns MW, Kolivakis T, Michalak EE, et al. : CANMAT Depression Work Group. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1. Disease burden and principles of care. Can J Psychiatry. 2016;61:510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Weiller E, Zhang P, Weiss C. Brexpiprazole as adjunctive treatment of major depressive disorder with anxious distress: results from a post-hoc analysis of two randomised controlled trials. J Affect Disord. 2016;201:116–123. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Anxiety does not predict response to duloxetine in major depression: results of a pooled analysis of individual patient data from 11 placebo-controlled trials. Depress Anxiety. 2010;27:12–18. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fan H, Tedeschini E. Severe and anxious depression: combining definitions of clinical sub-types to identify patients differentially responsive to selective serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2012;22:347–355. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Stahl SM, Krishen A, Seifert CA, Tucker VL, Goodale EP, Fava M. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry. 2008;69:1287–1292. [DOI] [PubMed] [Google Scholar]
- Rickels K, Pollack MH, Sheehan DV, Haskins JT. Efficacy of extended-release venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am J Psychiatry. 2000;157:968–974. [DOI] [PubMed] [Google Scholar]
- Roberge P, Normand-Lauzière F, Raymond I, Luc M, Tanguay-Bernard MM, Duhoux A, et al. Generalized anxiety disorder in primary care: mental health services use and treatment adequacy. BMC Fam Pract. 2015;16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca P, Fonzo V, Scotta M, Zanalda E, Ravizza L. Paroxetine efficacy in the treatment of generalized anxiety disorder. Acta Psychiatr Scand. 1997;95:444–450. [DOI] [PubMed] [Google Scholar]
- Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. J Affect Disord. 1999;56:171–181. [DOI] [PubMed] [Google Scholar]
- Russell J, Raskin J, Wiltse C, Walker D, Brawman-Mintzer O. Efficacy and tolerability of duloxetine treatment in elderly patients with major depressive disorder and concurrent anxiety symptoms. Psychiatry (Edgmont). 2007;4:33–45. [PMC free article] [PubMed] [Google Scholar]
- Salinas E: For the Venlafaxine XR 367 Study Group. Once-daily extended release (XR) venlafaxine versus paroxetine in outpatients with major depression [abstract 90-49]. Biol Psychiatry. 1997;42Suppl 1244S [Google Scholar]
- Schaffer A, McIntosh D, Goldstein BI, Rector NA, McIntyre RS, Beaulieu S, et al. : Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force. The CANMAT task force recommendations for the management of patients with mood disorders and comorbid anxiety disorders. Ann Clin Psychiatry. 2012;24:6–22. [PubMed] [Google Scholar]
- Silverstone PH, Ravindran A. Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. Venlafaxine XR 360 study group. J Clin Psychiatry. 1999;60:22–28. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Salinas E. Efficacy of venlafaxine extended release in patients with major depressive disorder and comorbid generalized anxiety disorder. J Clin Psychiatry. 2001;62:523–529. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, et al. : Group for the Study of Resistant Depression. Clinical factors associated with treatment resistance in major depressive disorder: results from a european multicenter study. J Clin Psychiatry. 2007;68:1062–1070. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Picarel-Blanchot F, Kennedy SH. Efficacy of the novel antidepressant agomelatine for anxiety symptoms in major depression. Hum Psychopharmacol. 2013;28:151–159. [DOI] [PubMed] [Google Scholar]
- Thase M, Asami Y, Wajsbrot D, Dorries K, Boucher M, Pappadopulos E. A meta-analysis of the efficacy of venlafaxine extended release 75-225 mg/day for the treatment of major depressive disorder. Curr Med Res Opin. 2017;33:317–326. [DOI] [PubMed] [Google Scholar]
- Thase ME. Efficacy and tolerability of once-daily venlafaxine extended release (XR) in outpatients with major depression. The venlafaxine XR 209 study group. J Clin Psychiatry. 1997;58:393–398. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Holman SL, Sayler ME, Potvin JH. Fluoxetine, placebo, and tricyclic antidepressants in major depression with and without anxious features. J Clin Psychiatry. 1994;55:50–59. [PubMed] [Google Scholar]
- Tollefson GD, Souetre E, Thomander L, Potvin JH. Comorbid anxious signs and symptoms in major depression: impact on functional work capacity and comparative treatment outcomes. Int Clin Psychopharmacol. 1993;8:281–293. [DOI] [PubMed] [Google Scholar]
- Wiethoff K, Bauer M, Baghai TC, Möller HJ, Fisher R, Hollinde D, et al. Prevalence and treatment outcome in anxious versus nonanxious depression: results from the german algorithm project. J Clin Psychiatry. 2010;71:1047–1054. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, McDermut W. Major depressive disorder and axis I diagnostic comorbidity. J Clin Psychiatry. 2002a;63:187–193. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Clark H, McGonigal P, Harris L, Guzman Holst C, Martin J. Relationship between the DSM-5 anxious distress specifier and the hamilton depression rating scale anxiety/somatization factor. J Nerv Ment Dis. 2018;206:152–154. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry. 2002b;159:469–473. [DOI] [PubMed] [Google Scholar]


