Supplemental Digital Content is available in the text.
This and Related “Classic” Articles Appear on Prsjournal.com for Journal Club Discussions.
Abstract
Background:
Anatomical variations in perforator arrangement may impair the surgeon’s ability to effectively avoid rectus muscle transection without compromising flap perfusion in the deep inferior epigastric artery perforator (DIEP) flap.
Methods:
A single surgeon’s experience was reviewed with consecutive patients undergoing bilateral abdominal perforator flap breast reconstruction over 6 years, incorporating flap standardization, pedicle disassembly, and algorithmic vascular rerouting when necessary. Unilateral reconstructions were excluded to allow for uniform comparison of operative times and donor-site outcomes. Three hundred sixty-four flaps in 182 patients were analyzed. Operative details and conversion rates from DIEP to abdominal perforator exchange (“APEX”) arms of the algorithm were collected. Patients with standardized DIEP flaps served as the controlling comparison group, and outcomes were compared to those who underwent abdominal perforator exchange conversion.
Results:
The abdominal perforator exchange conversion rate from planned DIEP flap surgery was 41.5 percent. Mean additional operative time to use abdominal perforator exchange pedicle disassembly was 34 minutes per flap. Early postsurgical complications were of low incidence and similar among the groups. One abdominal perforator exchange flap failed, and there were no DIEP flap failures. One abdominal bulge occurred in the DIEP flap group. There were no abdominal hernias in either group. Fat necrosis rates (abdominal perforator exchange flap, 2.4 percent; DIEP flap, 3.4 percent) were significantly lower than that historically reported for both transverse rectus abdominis musculocutaneous and DIEP flaps.
Conclusions:
This study revealed no added risk when using pedicle disassembly to spare muscle/nerve structure during abdominal perforator flap harvest. Abdominal bulge/hernia was nearly completely eliminated. Fat necrosis rates were extremely low, suggesting benefit to pedicle disassembly and vascular routing exchange when required.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, III.
Autologous breast reconstruction has undergone progressive evolution since introduction of the transverse rectus abdominis musculocutaneous (TRAM) flap in 1979.1 Major shifts have been a reflection of improved technique and understanding of anatomy. Within this timeline, development of the deep inferior epigastric artery perforator (DIEP) flap represented a significant step forward. Its foundational premise was based on the concept of structural preservation of the rectus abdominis in contradistinction to its predecessor, the TRAM flap. Thus, the advent and popularization of abdominal perforator flap breast reconstruction, as surgeons adopted the technique to deliver natural-tissue breast reconstruction with the implied surety of protecting the abdominal wall from sacrificial damage. There has subsequently been much comparison of the DIEP to the TRAM flap in an effort to define the benefits relative to the added technical complexity.
Despite these analyses, significant questions remain regarding technique for the DIEP flap. Anatomical variations in perforator arrangement may hinder the operator’s ability to effectively avoid muscle and motor nerve transection without impairing flap perfusion. In addition, to date, no standard has been adopted to define the point where rectus fiber transection in the DIEP flap harvest equates to a muscle-sparing TRAM flap equivalent. This remains a confounding factor for patient disclosure, counseling, and outcomes reporting. The wide variability of muscle wall preservation and opinions on flap perfusion requirements among different surgeons has resultantly clouded uniform reporting.2–5 Not all DIEP flaps are the same, and to make fair comparisons, one must know how the flaps were harvested with objective reference standards. Our study aims to address and overcome these obstacles. We introduce an option for meeting the challenge of merging quality perfusion with full rectus preservation. Multiple, misaligned abdominal perforators are included in the dissection through “exchange” of their native routing patterns with pedicle disassembly and vascular rerouting to preserve interposed muscle/nerve structure. This report, and associated introduction of the abdominal perforator exchange (“APEX”) flap, is an effort to improve flap and donor-site outcomes for women undergoing abdominally based autologous breast reconstruction.
PATIENTS AND METHODS
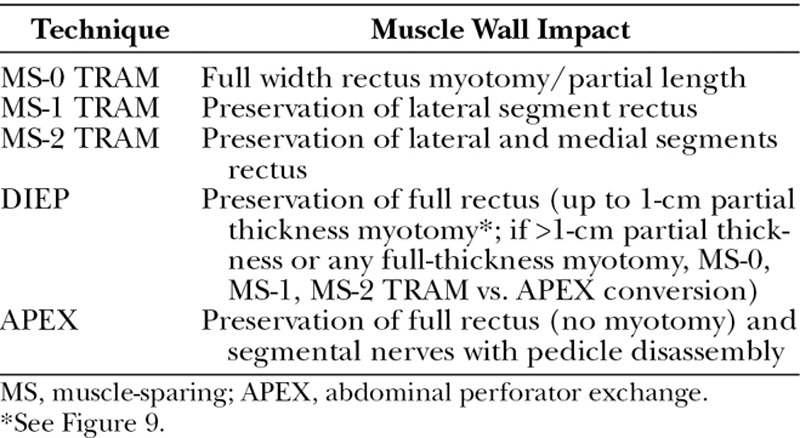
A retrospective review of a single surgeon’s (F.J.D.) experience was undertaken on consecutive patients undergoing bilateral abdominal perforator flap breast reconstruction over 6 years. Unilateral breast reconstructions were excluded to allow for uniform comparison of operative times and donor-site outcomes among the differing flap groups. A total of 364 flaps in 182 patients were performed between 2011 and 2017. Patient histories were collected from office charts. Intraoperative decisions for flap conversion and supercharging were based on our algorithm (Fig. 1), and the flap selected was classified according to our nomenclature system (Table 1). Operative details, including surgical times, perforators selected, rectus structure impact, and conversion rates within the arms of our algorithm, were recorded. Early postsurgical outcomes/complications were collected from hospital charts.
Fig. 1.
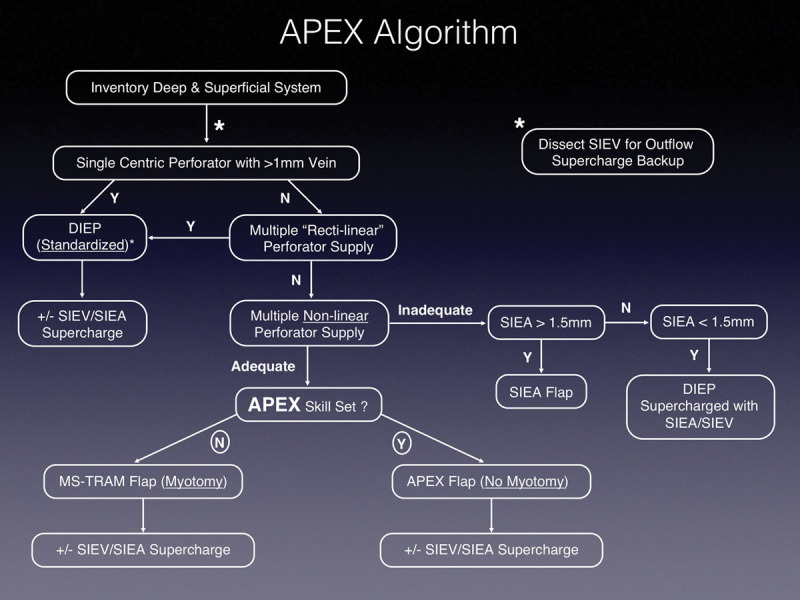
Abdominal perforator exchange (APEX) algorithm. SIEV, superficial inferior epigastric vein; SIEA, superficial inferior epigastric artery; N, no; Y, yes; MS, muscle-sparing.
Table 1.
Standardized Flap Classification System
Patients lost to follow-up before 3 months were excluded from the analysis. Fat necrosis and abdominal bulge/hernia occurrences were identified and recorded by the primary author (F.J.D.) during follow-up clinical examination. Fat necrosis was defined as a palpable nodule greater than or equal to 1 cm according to the Rao grading system.6
Technique
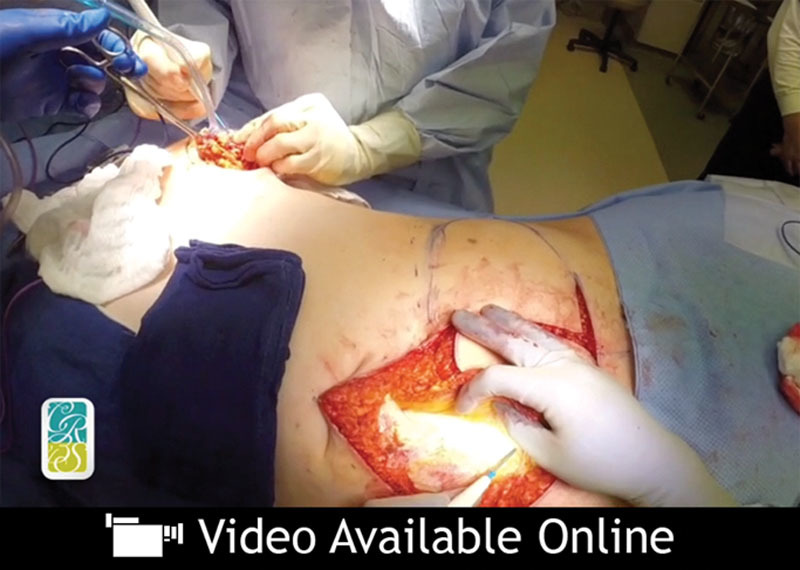
Incisional planning is carried out with attention toward dropping the pattern low enough to leave the scar line at a level fitting for the desired aesthetic. The upper limb is typically drawn at the level of the umbilicus or just below it (Fig. 2).
Fig. 2.
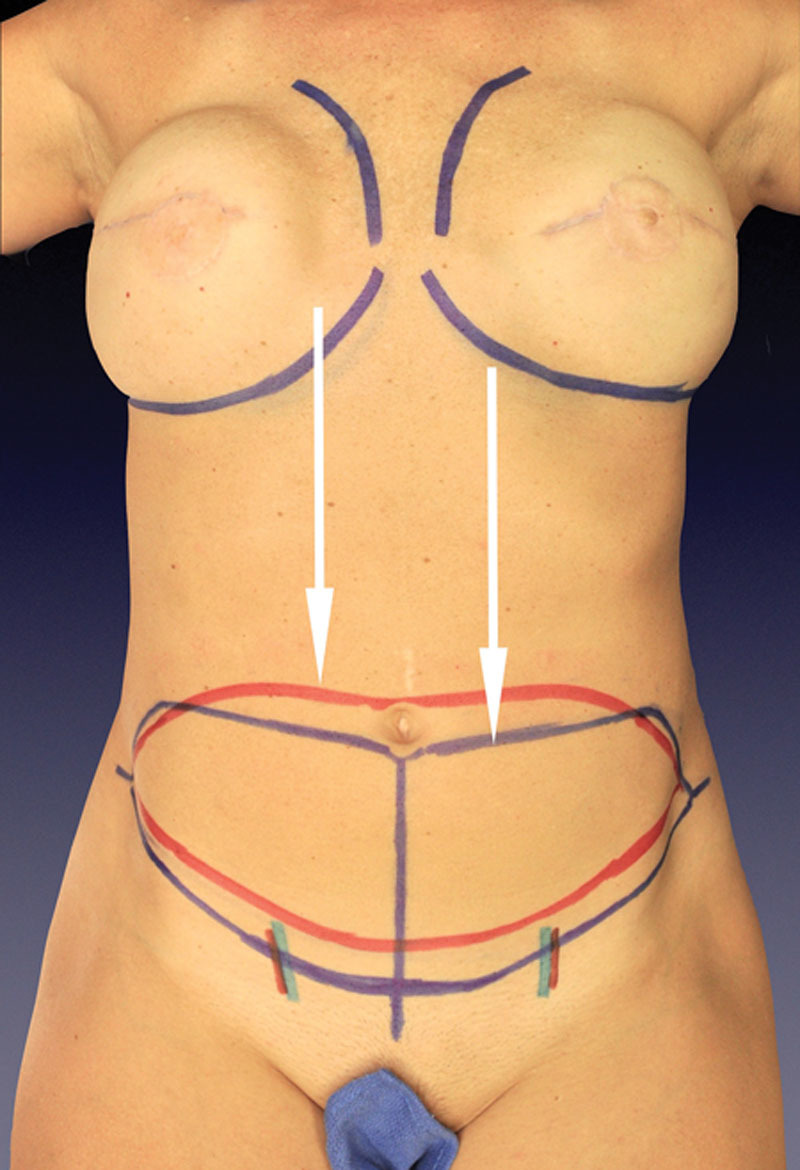
Abdominal incision pattern lowered (blue) with abdominal perforator exchange option for inclusion of multiple medial and lateral perforators lower on the abdominal wall without interim muscle transection. An improved aesthetic versus patterns (red) that leave a high-riding scar line is delivered.
Although preoperative computed tomographic angiography is used to direct attention to larger perforators and reveal iatrogenic vascular injury in nonvirgin abdomens, each perforator is inspected visually for its arterial and venous composition and associated location in the flap base (Fig. 3). (See Video, Supplemental Digital Content 1, which demonstrates the abdominal perforator exchange conversion technique, available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D341.) The algorithm is then followed to direct which vessels merit inclusion and which flap technique is used. (See Video, Supplemental Digital Content 2, which demonstrates the abdominal perforator exchange algorithm review, available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D342.) Criteria for supercharging are subjective and based on flap performance observations of the surgeon during harvest. Irrespective of these considerations, the superficial inferior epigastric vein is always dissected to length, when present, to serve as a venous outflow lifeboat.7,8
Fig. 3.
Perforators surgically “inventoried” to direct the algorithm decision pathway. (Left) Peripheral perforator superior edge of flap base with substantial venous component. (Right) Lower perforator of similar gross overall size made up of large sensory nerve and arterial flow element but underdeveloped venous outflow component.
Video 1.
Supplemental Digital Content 1, which demonstrates the abdominal perforator exchange conversion technique, is available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D341.
Video 2.
Supplemental Digital Content 2, which demonstrates the abdominal perforator exchange algorithm review, is available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D342.
If two or more perforators are selected, no concern is had for whether they appear to be in different “rows.” The fascial incision is angled from one perforator’s emergence point to the next to produce a continuous single access line. At this point, the decision is made to convert to an abdominal perforator exchange flap or continue with a DIEP flap harvest (Table 1). When selected perforators fall into a single muscular cleavage line, a DIEP dissection pattern follows without myotomy (Fig. 4). When the selected perforators do not fall in the same intramuscular fiber line, consideration of abdominal perforator exchange conversion ensues (Fig. 5). Transection of any full-thickness muscle was found to have invariably required a greater than 1-cm impact, and therefore any full-thickness myotomy requirements led to abdominal perforator exchange conversion or muscle-sparing TRAM flap classification.
Fig. 4.
Multiple perforators aligned in single rectus fiber line (“rectilinear”).
Fig. 5.
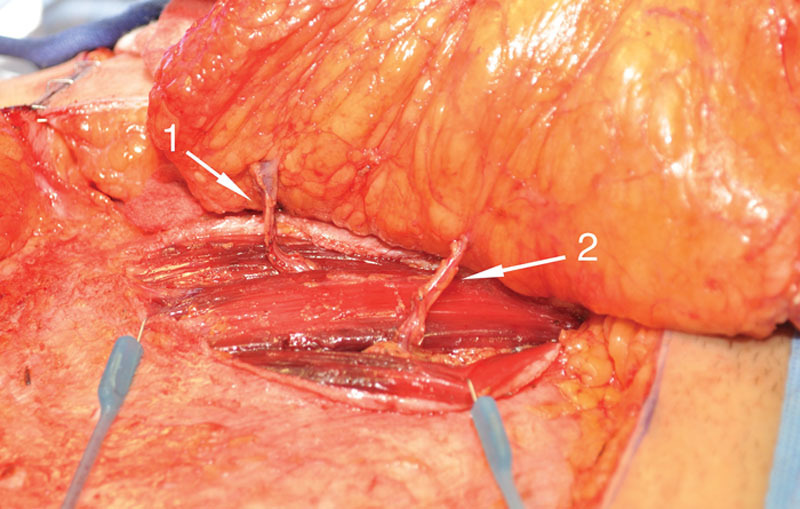
Perforators emerging from different muscular fiber cleavage lines. (1) Superior perforator with well-developed venous component but eccentric location. (2) Centric perforator with underdeveloped venous component.
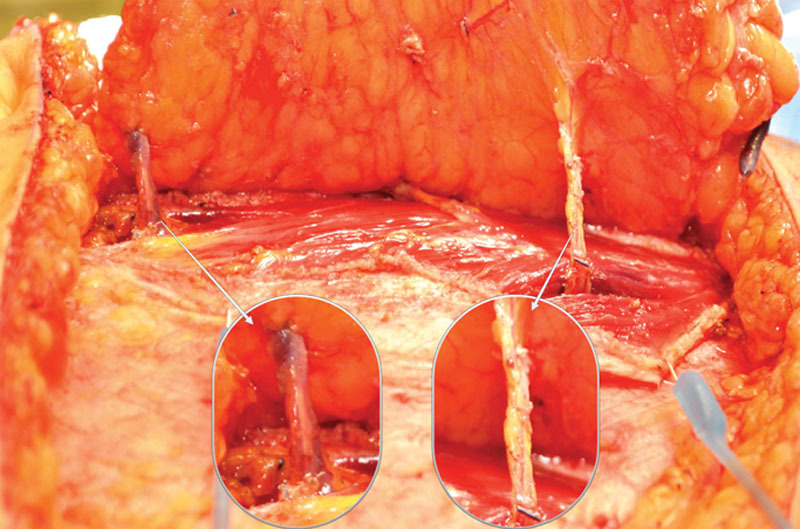
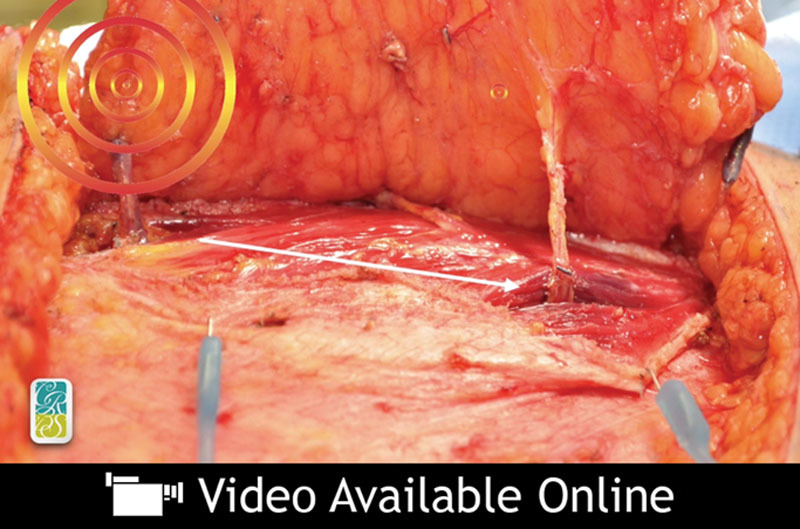
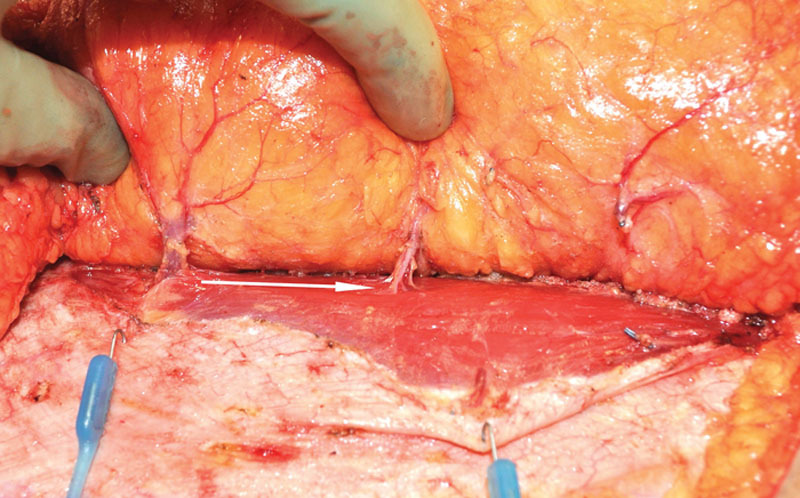
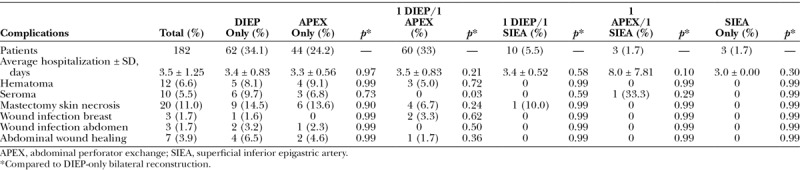
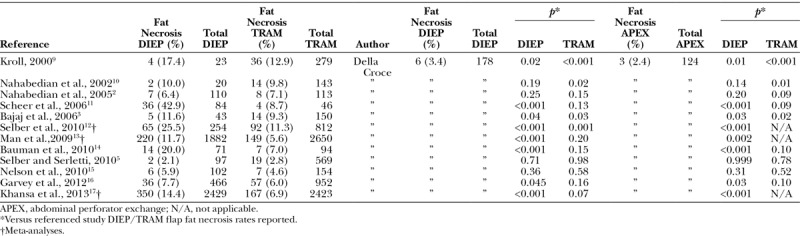
When the abdominal perforator exchange arm of the algorithm is followed, the primary pedicle is disassembled beneath the interposed muscle bundle (Fig. 6) and each perforator is slipped from its respective muscular dissection plane, preserving intervening muscle and associated motor nerves (Figs. 7 and 8). A photograph of the harvest site is taken before the fascia is closed as an objective record of dissection quality. Continuity is restored at the disassembly point on a sterile side table using 1.5-mm venous couplers and hand-sewn arterial anastomosis. Anastomosis to the recipient internal mammary system follows. Implantable Doppler monitoring was used on the primary arterial anastomoses only in this series; however, monitoring may be adjusted to operator preference.
Fig. 6.
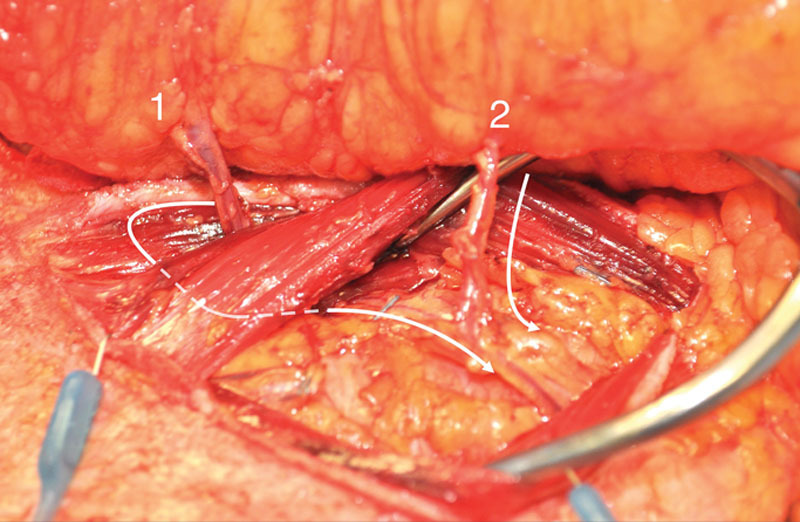
Perforators 1 and 2 followed to the common deep inferior epigastric vascular source beneath an interposed 4-cm-wide muscular bundle.
Fig. 7.
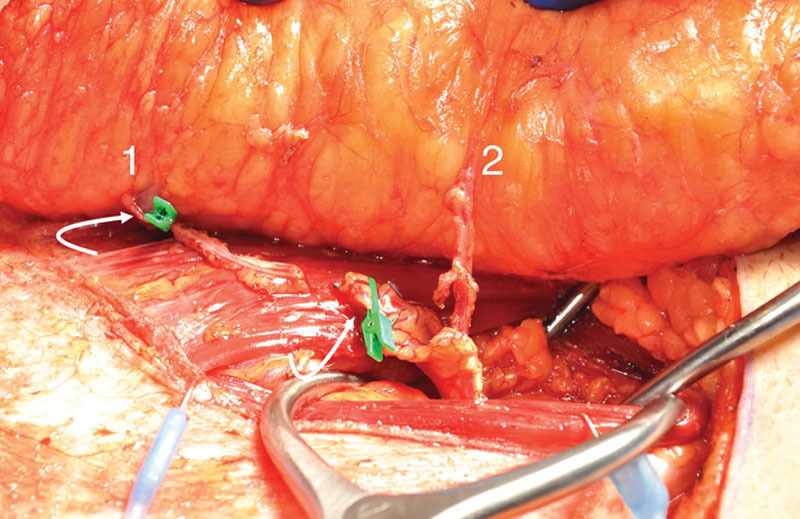
Deep inferior epigastric pedicle disassembled between desired perforators and slipped from respective muscular fiber planes, leaving interposed muscle and segmental motor nerves intact.
Fig. 8.
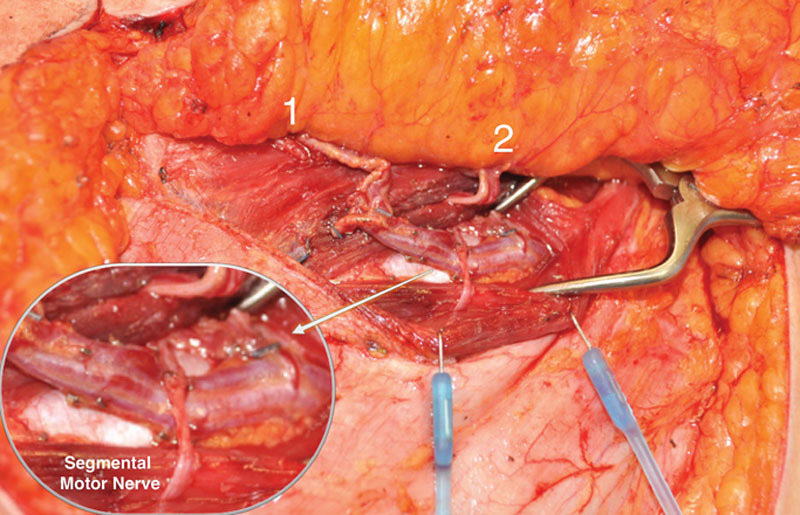
Segmental nerve preservation demonstrated, as facilitated by abdominal perforator exchange technique. The primary pedicle may be divided beneath nerve and muscle fibers to allow for preservation of structural components.
Statistical Analysis
Statistical analysis was performed on continuous variables with the Wilcoxon rank sum and Welch two-tailed t test, depending on data distribution within tested groups. Dichotomous outcome variables were compared using chi-square or Fisher’s exact test, depending on the frequency of targeted data. Results with a value of p < 0.05 were regarded as significant.
RESULTS
Patient characteristics and comorbidities were similar among the treatment groups, with a notable exception of a lower body mass index in the abdominal perforator exchange–only subset (p = 0.01) and the one DIEP/one abdominal perforator exchange (p < 0.001) subset (Table 2). One hundred ninety-four flaps (53 percent) were DIEP flaps, whereas 151 flaps (42 percent) were converted to the abdominal perforator exchange technique (Table 3). The remaining 5 percent (19 flaps) were superficial inferior epigastric artery flaps. An average of 2.09 ± 0.45 perforators were included in the abdominal perforator exchange flaps and 1.82 ± 0.44 perforators were included in the DIEP flaps (p < 0.001). Twelve percent of our flaps were based on a single perforator. Eleven DIEP flaps (5.6 percent) were supercharged with the superficial inferior epigastric artery and 18 (9.1 percent) with a superficial inferior epigastric vein; 2.7 percent of abdominal perforator exchange flaps were supercharged with the superficial inferior epigastric artery and 5.3 percent were supercharged with a superficial inferior epigastric vein.
Table 2.
Patient Demographics
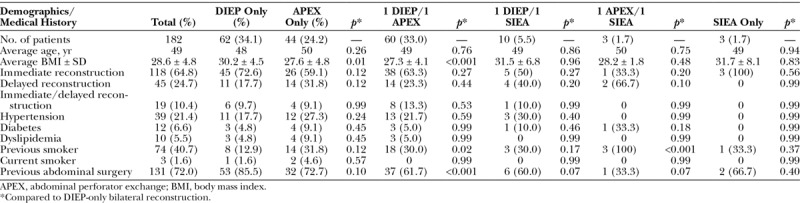
Table 3.
Algorithmic Flap Selection Product and Associated Performance
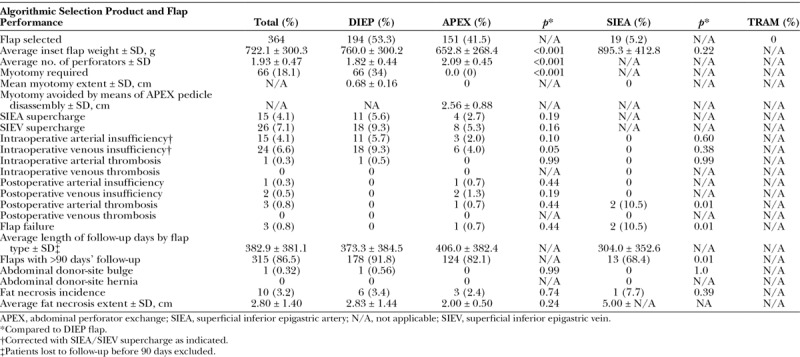
Of those patients meeting criteria for myotomy (34 percent), the average width of transected muscle was 0.68 ± 0.16 cm, and all were partial thickness (Fig. 9). Sixty-six percent of DIEP flaps were harvested with no myotomy. The average width of muscle spared, as a result of abdominal perforator exchange conversion, was 2.56 ± 0.88 cm, and all were harvested without rectus fiber transection (Fig. 10). No patients were converted to TRAM or muscle-sparing TRAM flaps.
Fig. 9.
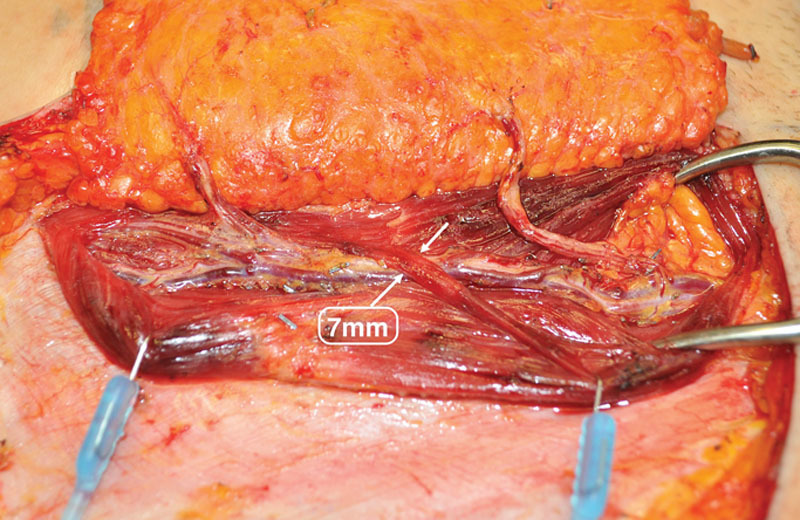
Perforator course through muscle fiber structure with 7-mm tangential, partial-thickness fiber crossing. Myotomy up to 1-cm partial thickness maintains categorization as a DIEP flap under institutional standard and associated classification system (Table 1).
Fig. 10.
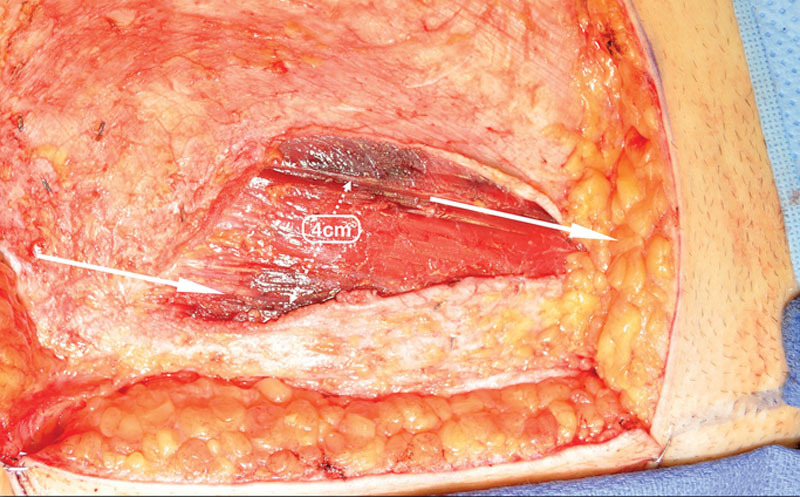
Dissection field demonstrating full preservation of 4-cm-wide interposed muscle bundle. Photographic documentation of harvest-site quality routinely performed before fascial closure.
The added operative time for an abdominal perforator exchange flap versus a DIEP flap was 34 minutes on average (Table 4). Hospitalization averaged 3.5 ± 0.83 days and did not differ significantly among the various patient subsets. There were three flap failures (0.8 percent of 364 flaps performed): two occurred in the immediate postoperative period and one presented at the time of the second stage with diffuse fat necrosis. There were no DIEP flap failures. Excluding superficial inferior epigastric artery flaps, the overall DIEP/abdominal perforator exchange flap failure rate was 0.27 percent. There were 12 hematomas requiring operative evacuation; the majority of these were immediate reconstructions with bleeding from the mastectomy wound bed. There were no other significant early flap complications (Table 5).
Table 4.
Total Surgical Time for DIEP versus Abdominal Perforator Exchange Flaps
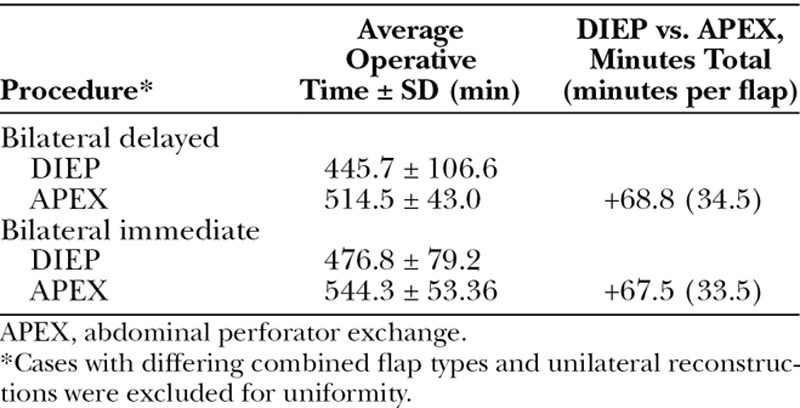
Table 5.
Postsurgical Course among Flap Combinations
Overall, long-term follow-up averaged 383 days. One patient presented at the second stage with a clinically detectable, unilateral abdominal bulge in the DIEP flap group. There were no bulges in the abdominal perforator exchange or superficial inferior epigastric artery flap groups, and no patients developed abdominal hernias during the study period. Fat necrosis did not differ significantly among the flap types produced from the algorithmic decision pathway. Minor clinically detectable areas between 1 and 3 cm were found in 3.4 percent of standardized DIEP flaps and 2.4 percent of abdominal perforator exchange flaps (Tables 3 and 6).2,3,5,6,9–17
Table 6.
Fat Necrosis Rate Comparison
DISCUSSION
Anatomy
Varied descriptions of the vascular anatomy of the DIEP flap have been a source of bewilderment with respect to standardization of technique. The anatomical description of the transverse and oblique routes perforators take as they course through the rectus described by Rozen et al. was cited as support of the argument that fibers must be routinely divided when harvesting a DIEP flap.18 Others have reported similarly, with commentary on the unpredictable routes perforators take as they pass through rectus structure.14,19,20 These findings are in stark contrast to reports by Munhoz et al. and others that describe orderly, consistent perforator rows that drop directly through aligned muscular cleavage planes to the inferior epigastric system.21–25 Our observations are that there is no consistent medial or lateral row arrangement of the perforators in the infraumbilical abdominal wall (Fig. 11). Vessels, which may appear aligned in “rows” at the fascial level, frequently take different tracks around muscle fibers as they descend toward the primary pedicle (Fig. 12). Such is the source of the “DIEP dilemma.” When multiple perforators are necessary to vigorously perfuse a flap, the operator often finds that interposed bundles of rectus muscle separate them. This dilemma demands that either the muscle be cut to bring perforators together or that the misaligned perforator is sacrificed to maintain muscle structure.5
Fig. 11.
Deep inferior epigastric perforators emerge from variable points in the rectus fiber structure. Developmental patterns are not productive of classically described perforator “rows” in the clinical setting.
Fig. 12.
Courses individual vessels take through rectus structure are confounding when perforators track tangentially from fascial exit points to the primary source. Rectus fiber structure is noted to be woven with crisscrossing, overlapping bundles.
Abdominal Morbidity
To date, no standard has been adopted regarding muscle fiber transection during a DIEP flap harvest. Several authors have written about this issue.2–5,20,26,27 This reality contradicts the popular perception of the basic advantage of the DIEP flap over the TRAM flap.
Many published studies support the DIEP flap as a means of reducing overall morbidity in the abdominal donor site compared with the TRAM flap, but all have lacked consistency and clarity with respect to muscle fiber transection in the DIEP arm of their reports.12,13,28–31 Despite this lack of a standard, multiple studies have shown that the DIEP flap produces at least half the risk of hernia/bulge compared with the muscle-sparing TRAM flap.5,13,28,31–33 The consensus indicates that abdominal wall function is correlated to muscle/nerve/fascia preservation and that patients undergoing the DIEP flap enjoy less overall donor-site morbidity, with quicker recoveries and better overall preservation of abdominal wall strength compared with TRAM flap recipients.34–38 Shubinets et al. found that the amount of rectus muscle sacrificed correlated with the likelihood of undergoing subsequent surgical hernia repair, and that the added costs to the health care system ranged from $39,704 to $48,378.39
Innervation
A number of scholarly articles have touched on the potential impact of rectus motor nerve transection as a contribution to abdominal bulges and weakness.4,10,40–42 When dissection is confined to a sagittal split along the muscle fiber line, the segmental motor nerves crossing the pedicle may be easily identified. If the nerves cross between two perforators that are to be included with an abdominal perforator exchange disassembly, the associated neural structures may be preserved, along with any interposed muscle (Fig. 8).
Fat Necrosis
Fat necrosis is intimately tied to perfusion quality, and provision of perfusion quality is intimately tied to abdominal morbidity. Fat necrosis can mimic recurrent breast cancer both clinically and radiographically.6,17 It can be painful, and added cost for clinical encounters, imaging, and surgical intervention may be considerable.
The quandary experienced with misaligned perforators has been the vexing decision of whether to cut across muscle to join them or ligate the misaligned vessel and risk fat necrosis and/or venous congestion. A number of authors have studied whether lateral or medial perforators are superior and whether a single-perforator dissection is preferred.43–46 Baumann et al. found that single-perforator flaps developed fat necrosis more often than multiperforator flaps, as did Garvey et al.14,16,33 A recent report by Kamali et al. suggests that medial perforators alone are prone to produce fat necrosis nearly three times as often as hemiabdominal flaps that include a lateral row perforator.42 Mohan et al. reported fat necrosis rates of 9.8 percent in single-perforator flaps and 4.9 percent in multiperforator flaps.47 Grover et al. found a threefold higher rate of fat necrosis in single-perforator DIEP flaps compared with multiperforator flaps.48 A single perforator in the base of a DIEP flap constitutes a maximal reduction in blood flow relative to its natural state (thus, the many reports of lesser fat necrosis in TRAM flaps versus DIEP flaps).11–13,17,33 In some cases, a large, centrally located perforator with a high-quality venous component is adequate to fully perfuse a flap, but as Blondeel pointed out, centricity is important.49
In essence, the abdominal perforator exchange conversion delivers the benefit of TRAM flap perfusion quality without the tradeoff of structural sacrifice. Complementing this approach with superficial inferior epigastric artery/superficial inferior epigastric vein supercharging, when necessary, delivers full control of perfusion quality. When compared with the great majority of previous reports, our algorithm for DIEP flap selection and abdominal perforator exchange conversion resulted in a statistically significant lowering of the rate of fat necrosis compared with both DIEP and TRAM flaps in those series (Table 6).2,3,5,9,11–17,33,50 This suggests meaningful benefit when flap perfusion is tailored to the anatomy on an individual basis.
Various authors have described decision pathways to determine when a DIEP flap should be abandoned in favor of a TRAM flap.3,4,8,10,13,15,46,51,52 We are in agreement with authors who admit that conversion from a DIEP flap to a TRAM flap is warranted when the only alternative is a poorly perfused flap. The abdominal perforator exchange flap represents an alternative to TRAM flap conversion, and we use it exclusively when the DIEP flap is not the best choice for maximization of perfusion and structural preservation. Our algorithm requires that operator skill be sufficiently advanced to accommodate pedicle disassembly and include supercharging when deemed of benefit (Fig. 1).
The added time and complexity to perform the abdominal perforator exchange flap may, theoretically, be more than offset by the avoidance of ongoing donor-site problems and fat necrosis in the newly reconstructed breast. Disassembling the pedicle did not increase complications compared to flaps without pedicle transection within our patient cohort (Tables 3 and 5). Because pedicle division is undertaken only through the large vessels of the primary trunk between selected perforators, this anastomosis should have no more likelihood of a problem than the anastomoses to the recipient vasculature. The fact that none of our abdominal perforator exchange flaps suffered substantial fat necrosis, as would be expected with interim pedicle anastomosis failure, supports this assertion.
Notably, among the three flap failures, two were superficial inferior epigastric artery flaps and both were of arterial origin (10.53 percent of superficial inferior epigastric artery flaps performed) (Table 3). This reflects the experience of other authors’ commentary on arterial mismatch and associated failure rates with the superficial inferior epigastric artery flap.53–56 The superficial inferior epigastric artery is resultantly a tertiary arm within our algorithm.
Aesthetics
Part of the appeal of the DIEP flap is the byproduct expectation of an improved abdominal aesthetic. However, this does not always translate within the clinical outcome.57–59 Incisional patterns that push to include the higher paraumbilical perforators are primarily to blame. Because these perforators often represent some of the larger vessels in the flap base, the want to include them is intrinsic. This drives the surgeon to raise the incisional pattern to capture them.48 The resultant high-riding scar line is aesthetically displeasing and does not represent the “tummy tuck” that was anticipated (Fig. 13). Abdominal perforator exchange perforator inclusion delivers the liberty to move away from high perforators and associated incisional designs and reliably perfusing flaps with a greater number of lower/multiple perforators (Fig. 14). Our finding of a tendency toward a lower body mass index in our abdominal perforator exchange patients likely resulted, in part, from the dropping of the upper incision height to keep scar lines low in these patients. This pattern shift moved the paraumbilical vessels to the far periphery of these flaps or excluded them entirely, adding greater perfusion requirement from the less well-developed vasculature lower on the abdominal wall.
Fig. 13.
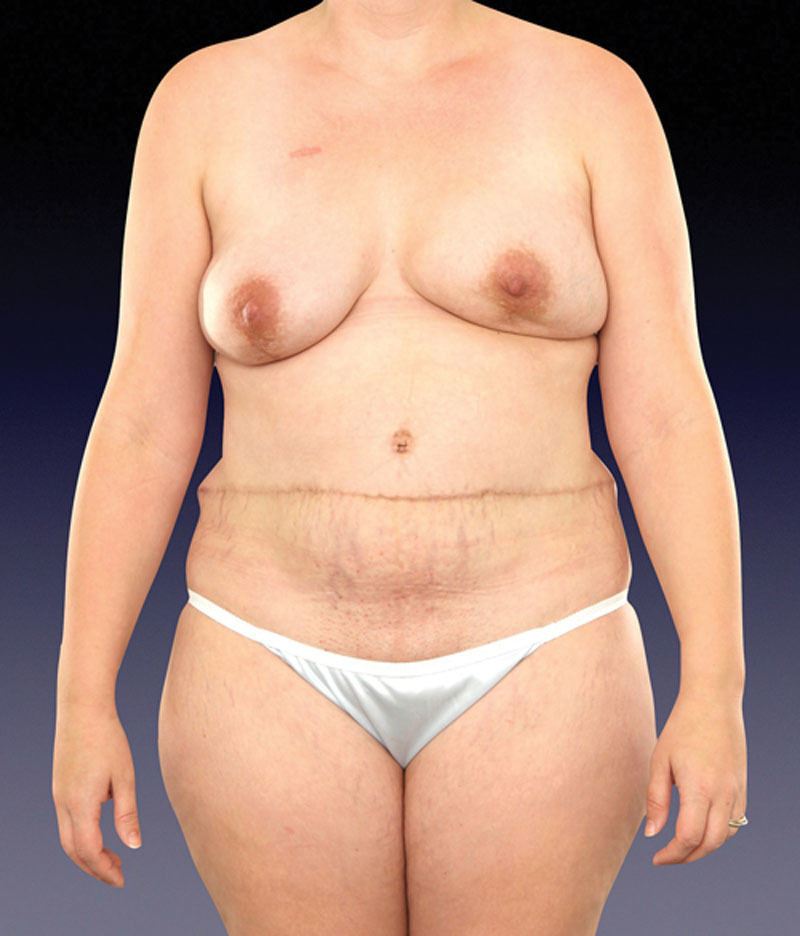
Unfavorable donor aesthetic with high-riding abdominal scar. It is improved with a low-incisional-pattern design as supported by abdominal perforator exchange conversion and inclusion of multiple, lower abdominal wall perforators without muscle sacrifice.
Fig. 14.

Before and after abdominal perforator exchange flap with strong abdominal wall and no clinically detectable breast fat necrosis. Donor-site aesthetic is complemented by a low scar line.
Standard
The standard described herein addresses issues that have clouded the definition of the DIEP flap and impacted uniform reporting for decades.5,27 Our institutional definition is simple and offers a standard against which future reporting may be based. We expand on well-adopted system described by Nahabedian et al.10 and add specific parameters to clarify what qualifies as a DIEP flap and when abdominal perforator exchange or TRAM flap conversion is the technique product (Table 1). A DIEP flap is defined as a flap that is harvested with full preservation of rectus structure without myotomy or, if myotomy is required to include more than one perforator, it is held to an absolute minimal amount (≤1 cm and partial thickness) (Fig. 9). Beyond that, the flap is classified as a muscle-sparing TRAM flap. This institutional standard is biased toward minimization of morbidity and avoiding introduction of complexity for the sake of complexity. We thereby afford standardization of the DIEP flap within the framework of its premise in concert with conceptual and technical reason. Transection of partial-thickness rectus muscle bundles of greater than 1 cm or any full-thickness transection to join more than one perforator to the primary trunk is either classified as a muscle-sparing TRAM flap or is the threshold for which abdominal perforator exchange conversion is encountered. (See Video, Supplemental Digital Content 3, which demonstrates the abdominal flap nomenclature and classification system review, available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D343.)
Video 3.
Supplemental Digital Content 3, which demonstrates the abdominal flap nomenclature and classification system review, is available in the “Related Videos” section of the full-text article on PRSJournal.com or, for Ovid users, at http://links.lww.com/PRS/D343.
Objective measurement, documentation, and reporting of myotomy and motor nerve transection should be included in reports that compare abdominal donor-site morbidity going forward. Only then can we reasonably compare outcomes among procedures that spare structure versus those that do not. This report is not without limitations, and the reader must consider the retrospective nature of outcomes reported along with associated potential for observer bias.
CONCLUSIONS
The abdominal perforator exchange (APEX) algorithm allows the operator to reliably harvest flaps with robust perfusion while preserving structure to the maximum extent possible. Pedicle disassembly delivers the freedom to engineer the vascularity required irrespective of the anatomy encountered. The abdominal perforator exchange conversion option allows the surgeon to exchange the vascular anatomy encountered for the anatomy preferred; to exchange myotomy for intact rectus structure; to exchange neurolysis for motor nerve continuity; to exchange fat necrosis for soft breast flaps; and to exchange high-riding scar lines for lower ones. This advancement delivers a technique to maximize donor-site integrity and improve flap performance. We further describe a new classification system for the standardization of abdominal flap nomenclature, which may provide an opportunity to improve uniform reporting going forward.
Supplementary Material
Footnotes
Presented at the Fourth Annual London Breast Meeting, in London, United Kingdom, September 8, 2017; and the American Society for Reconstructive Microsurgery Annual Scientific Meeting, in Phoenix, Arizona, January 15, 2017.
Disclosure: Authors have no financial interest in any of the products or devices drugs mentioned in this article. No funding was received for this article.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s website (www.PRSJournal.com).
REFERENCES
- 1.Holmström H. The free abdominoplasty flap and its use in breast reconstruction: An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13:423–427. [DOI] [PubMed] [Google Scholar]
- 2.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: Is there a difference? Plast Reconstr Surg. 2005;115:436–444. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:737–746; discussion 747750. [DOI] [PubMed] [Google Scholar]
- 4.Chang DW. Discussion: Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: Is there a difference? Plast Reconstr Surg. 2005;115:445–446. [DOI] [PubMed] [Google Scholar]
- 5.Selber JC, Serletti JM. The deep inferior epigastric perforator flap: Myth and reality. Plast Reconstr Surg. 2010;125:50–58. [DOI] [PubMed] [Google Scholar]
- 6.Rao A, Saadeh PB. Defining fat necrosis in plastic surgery. Plast Reconstr Surg. 2014;134:1202–1212. [DOI] [PubMed] [Google Scholar]
- 7.Sbitany H, Mirzabeigi MN, Kovach SJ, Wu LC, Serletti JM. Strategies for recognizing and managing intraoperative venous congestion in abdominally based autologous breast reconstruction. Plast Reconstr Surg. 2012;129:809–815. [DOI] [PubMed] [Google Scholar]
- 8.Galanis C, Nguyen P, Koh J, Roostaeian J, Festekjian J, Crisera C. Microvascular lifeboats: A stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg. 2014;134:20–27. [DOI] [PubMed] [Google Scholar]
- 9.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. [DOI] [PubMed] [Google Scholar]
- 10.Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475; discussion 476477. [DOI] [PubMed] [Google Scholar]
- 11.Scheer AS, Novak CB, Neligan PC, Lipa JE. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg. 2006;56:355–358. [DOI] [PubMed] [Google Scholar]
- 12.Sailon AM, Schachar JS, Levine JP. Free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps for breast reconstruction: A systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg. 2009;62:560–563. [DOI] [PubMed] [Google Scholar]
- 13.Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: A meta-analysis and critical review. Plast Reconstr Surg. 2009;124:752–764. [DOI] [PubMed] [Google Scholar]
- 14.Baumann DP, Lin HY, Chevray PM. Perforator number predicts fat necrosis in a prospective analysis of breast reconstruction with free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2010;125:1335–1341. [DOI] [PubMed] [Google Scholar]
- 15.Nelson JA, Guo Y, Sonnad SS, et al. A Comparison between DIEP and muscle-sparing free TRAM flaps in breast reconstruction: A single surgeon’s recent experience. Plast Reconstr Surg. 2010;126:1428–1435. [DOI] [PubMed] [Google Scholar]
- 16.Garvey PB, DelBello SM, Liu J, Kronowitz SJ, Butler CE. DIEP and MS FTRAM flaps based on both branches of the deep inferior epigastric artery result in fewer perfusion-related complications than single DIEA branch flaps: A study of 1127 patients. Plast Reconstr Surg. 2012;130(Suppl):12.22774230 [Google Scholar]
- 17.Khansa I, Momoh AO, Patel PP, Nguyen JT, Miller MJ, Lee BT. Fat necrosis in autologous abdomen-based breast reconstruction: A systematic review. Plast Reconstr Surg. 2013;131:443–452. [DOI] [PubMed] [Google Scholar]
- 18.Rozen WM, Ashton MW, Pan WR, Taylor GI. Raising perforator flaps for breast reconstruction: The intramuscular anatomy of the deep inferior epigastric artery. Plast Reconstr Surg. 2007;120:1443–1449. [DOI] [PubMed] [Google Scholar]
- 19.El-Mrakby HH, Milner RH. The vascular anatomy of the lower anterior abdominal wall: A microdissection study on the deep inferior epigastric vessels and the perforator branches. Plast Reconstr Surg. 2002;109:539–543; discussion 544547. [DOI] [PubMed] [Google Scholar]
- 20.Vandevoort M, Vranckx JJ, Fabre G. Perforator topography of the deep inferior epigastric perforator flap in 100 cases of breast reconstruction. Plast Reconstr Surg. 2002;109:1912–1918. [DOI] [PubMed] [Google Scholar]
- 21.Munhoz AM, Ishida LH, Sturtz GP, et al. Importance of lateral row perforator vessels in deep inferior epigastric perforator flap harvesting. Plast Reconstr Surg. 2004;113:517–524. [DOI] [PubMed] [Google Scholar]
- 22.Itoh Y, Arai K. The deep inferior epigastric artery free skin flap: Anatomic study and clinical application. Plast Reconstr Surg. 1993;91:853–863; discussion 864. [PubMed] [Google Scholar]
- 23.Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg. 1984;73:1–16. [DOI] [PubMed] [Google Scholar]
- 24.Konerding MA, Gaumann A, Shumsky A, Schlenger K, Hockel M. The vascular anatomy of the inner anterior abdominal wall with special reference to the transversus and rectus abdominis musculoperitoneal (TRAMP) composite flap for vaginal reconstruction. Plast Reconstr Surg. 1997;99:705–710; discussion 711712. [DOI] [PubMed] [Google Scholar]
- 25.Stokes RB, Whetzel TP, Sommerhaug E, Saunders CJ. Arterial vascular anatomy of the umbilicus. Plast Reconstr Surg. 1998;102:761–764. [DOI] [PubMed] [Google Scholar]
- 26.Lee BT, Chen C, Nguyen MD, Lin SJ, Tobias AM. A new classification system for muscle and nerve preservation in DIEP flap breast reconstruction. Microsurgery 2010;30:85–90. [DOI] [PubMed] [Google Scholar]
- 27.Weissler JM, Albino FP, Carney MJ, Wu LC. Revisiting the abdominal donor site: Introducing a novel nomenclature for autologous breast reconstruction. Plast Reconstr Surg. 2017;140:1110–1118. [DOI] [PubMed] [Google Scholar]
- 28.Egeberg A, Rasmussen MK, Sørensen JA. Comparing the donor-site morbidity using DIEP, SIEA or MS-TRAM flaps for breast reconstructive surgery: A meta-analysis. J Plast Reconstr Aesthet Surg. 2012;65:1474–1480. [DOI] [PubMed] [Google Scholar]
- 29.Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg. 1997;50:322–330. [DOI] [PubMed] [Google Scholar]
- 30.Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg. 2013;132:1383–1391. [DOI] [PubMed] [Google Scholar]
- 31.Mennie JC, Mohanna PN, O’Donoghue JM, Rainsbury R, Cromwell DA. Donor-site hernia repair in abdominal flap breast reconstruction: A population-based cohort study of 7929 patients. Plast Reconstr Surg. 2015;136:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: A comparison of outcomes. Plast Reconstr Surg. 2006;117:1711–1719; discussion 17201721. [DOI] [PubMed] [Google Scholar]
- 33.Garvey PB, DelBello SM, Liu J, Kronowitz SJ, Butler CE. Balancing flap perfusion & donor site morbidity: An evidence-based approach to optimizing outcomes for free flap breast reconstruction. Plast Reconstr Surg. 2012;130(Suppl):76. [Google Scholar]
- 34.Selber JC, Nelson J, Fosnot J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part I. Unilateral reconstruction. Plast Reconstr Surg. 2010;126:1142–1153. [DOI] [PubMed] [Google Scholar]
- 35.Selber JC, Fosnot J, Nelson J, et al. A prospective study comparing the functional impact of SIEA, DIEP, and muscle-sparing free TRAM flaps on the abdominal wall: Part II. Bilateral reconstruction. Plast Reconstr Surg. 2010;126:1438–1453. [DOI] [PubMed] [Google Scholar]
- 36.Kroll SS, Schusterman MA, Reece GP, Miller MJ, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616–619. [DOI] [PubMed] [Google Scholar]
- 37.Mizgala CL, Hartrampf CR, Jr, Bennett GK. Abdominal function after pedicled TRAM flap surgery. Clin Plast Surg. 1994;21:255–272. [PubMed] [Google Scholar]
- 38.Kroll SS, Sharma S, Koutz C, et al. Postoperative morphine requirements of free TRAM and DIEP flaps. Plast Reconstr Surg. 2001;107:338–341. [DOI] [PubMed] [Google Scholar]
- 39.Shubinets V, Fox JP, Sarik JR, Kovach SJ, Fischer JP. Surgically treated hernia following abdominally based autologous breast reconstruction: Prevalence, outcomes, and expenditures. Plast Reconstr Surg. 2016;137:749–757. [DOI] [PubMed] [Google Scholar]
- 40.Rozen WM, Ashton MW, Murray AC, Taylor GI. Avoiding denervation of rectus abdominis in DIEP flap harvest: The importance of medial row perforators. Plast Reconstr Surg. 2008;122:710–716. [DOI] [PubMed] [Google Scholar]
- 41.Blondeel PN. Discussion: Contour abnormalities of the abdomen after transverse rectus abdominis muscle flap breast reconstruction: A multifactorial analysis. Plast Reconstr Surg. 2002;109:88–90. [DOI] [PubMed] [Google Scholar]
- 42.Kamali P, Lee M, Becherer BE, et al. Medial row perforators are associated with higher rates of fat necrosis in bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2017;140:19–24. [DOI] [PubMed] [Google Scholar]
- 43.Bailey SH, Saint-Cyr M, Wong C, et al. The single dominant medial row perforator DIEP flap in breast reconstruction: Three-dimensional perforasome and clinical results. Plast Reconstr Surg. 2010;126:739–751. [DOI] [PubMed] [Google Scholar]
- 44.Schaverien M, Saint-Cyr M, Arbique G, Brown SA. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg. 2008;121:1909–1919. [DOI] [PubMed] [Google Scholar]
- 45.Rahmanian-Schwarz A, Rothenberger J, Hirt B, Luz O, Schaller HE. A combined anatomical and clinical study for quantitative analysis of the microcirculation in the classic perfusion zones of the deep inferior epigastric artery perforator flap. Plast Reconstr Surg. 2011;127:505–513. [DOI] [PubMed] [Google Scholar]
- 46.Rozen WM, Ashton MW, Le Roux CM, Pan WR, Corlett RJ. The perforator angiosome: A new concept in the design of deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 2010;30:1–7. [DOI] [PubMed] [Google Scholar]
- 47.Mohan AT, Zhu L, Wang Z, Vijayasekaran A, Saint-Cyr M. Techniques and perforator selection in single, dominant DIEP flap breast reconstruction: Algorithmic approach to maximize efficiency and safety. Plast Reconstr Surg. 2016;138:790e–803e. [DOI] [PubMed] [Google Scholar]
- 48.Grover R, Nelson JA, Fischer JP, Kovach SJ, Serletti JM, Wu LC. The impact of perforator number on deep inferior epigastric perforator flap breast reconstruction. Arch Plast Surg. 2014;41:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blondeel PN. Discussion: Perfusion-related complications are similar for DIEP and muscle-sparing free TRAM flaps harvested on medial or lateral deep inferior epigastric artery branch perforators for breast reconstruction. Plast Reconstr Surg. 2011;128:590e–592e. [DOI] [PubMed] [Google Scholar]
- 50.Garvey PB, Salavati S, Feng L, Butler CE. Perfusion-related complications are similar for DIEP and muscle-sparing free TRAM flaps harvested on medial or lateral deep inferior epigastric artery branch perforators for breast reconstruction. Plast Reconstr Surg. 2011;128:581e–589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindsey JT. Integrating the DIEP and muscle-sparing (MS-2) free TRAM techniques optimizes surgical outcomes: Presentation of an algorithm for microsurgical breast reconstruction based on perforator anatomy. Plast Reconstr Surg. 2007;119:18–27. [DOI] [PubMed] [Google Scholar]
- 52.Nahabedian MY. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:747–750. [DOI] [PubMed] [Google Scholar]
- 53.Rozen WM, Chubb D, Grinsell D, Ashton MW. The variability of the superficial inferior epigastric artery (SIEA) and its angiosome: A clinical anatomical study. Microsurgery 2010;30:386–391. [DOI] [PubMed] [Google Scholar]
- 54.Park JE, Shenaq DS, Silva AK, Mhlaba JM, Song DH. Breast reconstruction with SIEA flaps: A single-institution experience with 145 free flaps. Plast Reconstr Surg. 2016;137:1682–1689. [DOI] [PubMed] [Google Scholar]
- 55.Selber JC, Samra F, Bristol M, et al. A head-to-head comparison between the muscle-sparing free TRAM and the SIEA flaps: Is the rate of flap loss worth the gain in abdominal wall function? Plast Reconstr Surg. 2008;122:348–355. [DOI] [PubMed] [Google Scholar]
- 56.Coroneos CJ, Heller AM, Voineskos SH, Avram R. SIEA versus DIEP arterial complications: A cohort study. Plast Reconstr Surg. 2015;135:802e–807e. [DOI] [PubMed] [Google Scholar]
- 57.Eom JS, Kim DY, Kim EK, Lee TJ. The low DIEP flap: An enhancement to the abdominal donor site. Plast Reconstr Surg. 2016;137:7e–13e. [DOI] [PubMed] [Google Scholar]
- 58.Akita S. Differences between low DIEP flap and low-rise scar DIEP flap for breast reconstruction. Plast Reconstr Surg. 2016;138:365e–366e. [DOI] [PubMed] [Google Scholar]
- 59.Eom JS. Reply: Low-rise scar deep inferior epigastric artery perforator flap for breast reconstruction. Plast Reconstr Surg. 2016;138:366e–367e. [DOI] [PubMed] [Google Scholar]


