Abstract
Bone metastases are common in men with metastatic castrate-resistant prostate cancer (mCRPC), occurring in 30% of patients within 2 years of castrate resistance and in >90% of patients over the disease course. There are 6 US Food and Drug Administration-approved therapies for mCRPC with demonstrated survival benefit. Of these, only radium-223 (Ra-223) specifically targets bone metastases, delays development of skeletal-related events, and improves survival. This review discusses key data from the ALSYMPCA trial, which contributed to the approval of Ra-223. Data from other trials are highlighted to provide further insight into which patients might benefit from Ra-223. Special patient populations are described, as well as other considerations for the administration of Ra-223. Finally, ongoing trials of Ra-223 combined with other therapies for mCRPC are discussed. These include combining Ra-223 with sipuleucel-T or immunooncology agents, to enhance immune responses, and trials in mildly symptomatic or asymptomatic patients. To date, the optimal timing, sequence, and combinations of Ra-223 with other agents are yet to be determined. The goals of this review are to provide insight into practical aspects of patient selection for Ra-223 treatment and to discuss key therapeutic strategies using the 6 approved mCRPC agents in patients with bone metastases. Results from ongoing trials should help guide the practitioner in using Ra-223 in patients with mCRPC.
Key Words: prostate cancer, castrate-resistant prostate cancer, bone metastases, radium-223, alpharadin
Bone metastases develop in 30% of patients with castrate-resistant prostate cancer (CRPC) within 2 years of castrate resistance and in >90% of patients over the disease course through interactions between bone-derived and cancer-derived factors.1–3 Bone metastases cause pain, fractures, and spinal cord compression. Their presence is a prognostic marker.4 Optimal treatments for bone metastases is critical for metastatic CRPC (mCRPC) management, given their high prevalence and clinical impact.
There are 6 US Food and Drug Administration (FDA)-approved therapies for mCRPC with demonstrated overall survival (OS) benefit (abiraterone, enzalutamide, docetaxel, cabazitaxel, radium-223 [Ra-223], sipuleucel-T; Table 1).9 Supportive treatments include denosumab, zoledronic acid, and external-beam radiation therapy (EBRT). EBRT plus the β-emitter samarium-153 (Sm-153) has been shown to be beneficial relative to Sm-153 alone; the toxicity of the combination was similar to Sm-153 monotherapy.10 Strontium-89 (Sr-89), another β-emitter, has demonstrated efficacy to treat the bone metastases associated with CRPC, due to its ability to accumulate in bone metastases.11,12 Denosumab and zoledronic acid delay development of skeletal-related events (SREs) and are FDA-approved in mCRPC, but neither prolongs survival.13–15
TABLE 1.
Phase 3 Trials for Currently Approved CRPC Therapy Other Than Ra-223
Ra-223 is an α-emitting radionuclide approved for treatment of men with mCRPC having symptomatic bone metastases and no known visceral metastases. Ra-223 is a calcium-mimetic preferentially taken up into areas of high bone turnover, such as those surrounding bone metastases. Ra-223 induces local apoptosis of tumor cells through nonrepairable double-stranded DNA breaks.16 Ra-223 inhibits bone metastases through effects on bone-tumor microenvironment.16,17 The α particles of Ra-223 have a very short range; nearly 99% of daughter nuclides remain in bone, limiting damage to surrounding normal tissue.18,19 In a preclinical model, >30% of injected Ra-223 activity/gram was in bone, 10.5% in spleen, 5.7% in intestines, and 2.3% in kidneys.20 In trials, Ra-223 treatment improved OS,21 prolonged time to SREs,22,23 decreased serum alkaline phosphatase (ALP), and improved quality of life. To date, optimal timing, sequence, and combinations of Ra-223 with other agents for mCRPC are undetermined. Cross-resistance may be an issue for agents targeting the androgen axis, but sequencing with Ra-223 may prove useful.24 This review provides insight into practical aspects of patient selection for Ra-223 and discusses key therapeutic strategies using approved agents in patients with bone metastases.
PERCEPTION OF PAIN IN mCRPC
Bone pain, the driving symptom for Ra-223 use, may be inconsistent among patients with mCRPC. The prevalence, intensity, and frequency of analgesic use among patients with prostate cancer showed great variability.25 Comparing patients predocetaxel and postdocetaxel treatment, pain prevalence, and severity were higher predocetaxel. In addition, analgesics were underused. Moreover, bone metastases may be reported or perceived variably, for example, as pain, weakness, or difficulty climbing stairs. Some physicians reported pain as present or absent, rather than detailing intensity, type, or duration.26
Results from a 2017 survey showed that patients with bone metastases often reported bone pain in terms of difficulty performing daily activities.27 Patients may also develop anorexia, asthenia, or cachexia related to bone metastases.26 Physicians often underreported patients’ pain intensity, when compared with patients’ assessment of pain severity.25 The latter finding would imply that patient-reported outcomes should be preferred for assessing pain, particularly those associated with bone metastases.
Quality of life may be adversely affected by pain. For example, in a study of 248 patients with mCRPC, SREs from bone metastases caused decreases in health-related quality of life (QoL) across all outcome measures.28 Preventing SREs and pain progression together may help improve patient QoL during treatment for mCRPC.29
INSIGHTS FROM ALSYMPCA
The phase 3 ALSYMPCA trial (NCT00699751) showed that Ra-223 prolonged survival (14.9 vs. 11.3 mo; P<0.0001) and time to first SRE (median, 15.6 vs. 9.8 mo; 95% CI 0.52-0.83) versus placebo. OS was calculated based on the intention-to-treat (ITT) population having completed the 6 cycles of Ra-223 administration.21 Baseline biochemical values were similar, with the exception of prostate-specific antigen (PSA) levels; these were higher in the previous docetaxel use group than in the docetaxel-naive group.21 Other baseline characteristics were balanced between groups and reflected a patient population with advanced mCRPC.
The majority in ALSYMPCA who received Ra-223 had >6 bone metastases; of these, 41% of patients had >20 bone metastases and 43% had 6 to 20 bone metastases; only 16% of patients had <6 bone metastases.21 Most patients in the Ra-223 arm had Eastern Cooperative Oncology Group (ECOG) performance status 0 (27%) or 1 (60%). Over half received prior docetaxel (57% per group). Median hemoglobin and PSA in the Ra-223 group were 12.2 g/dL and 146 µg/L, respectively. Rates of grade 3-4 hematologic toxicity were low (anemia, 13%; thrombocytopenia, 6%; neutropenia, 3%) and not increased in the Ra-223 group, compared with placebo. Nonhematologic toxicity included nausea (40%, previous docetaxel; 30%, docetaxel-naive), vomiting (24%, previous docetaxel; 11% docetaxel-naive), and diarrhea (25% previous docetaxel; 26% docetaxel-naive) due to fecal elimination of the agent.21,30 Long-term safety data confirmed that Ra-223 was well tolerated, with low incidence of myelosuppression and no new safety signals.31 On the basis of these data, the US Nuclear Regulatory Commission licensed Ra-223 in 2013 as an investigational drug for use in mCRPC.32,33 Shortly thereafter, Ra-223 was granted FDA approval for use in patients with mCRPC having symptomatic bone metastases and no known visceral metastases.34
The FDA label for Ra-223 does not require progression on prior therapies; a prespecified subgroup analysis from ALSYMPCA supports a role for Ra-223 in patients without prior docetaxel use: OS (previous docetaxel use: HR 0.70; P=0.002; docetaxel-naive use: HR 0.69; P=0.01); fewer grade 3-4 adverse events (AEs; 62% vs. 54%, respectively); and lower rates of grade 3-4 thrombocytopenia (9% vs. 3%, respectively).21 These data demonstrate that Ra-223 is safe and effective before or after docetaxel.
ALSYMPCA established a clear benefit of Ra-223 before or after chemotherapy. Symptomatic bone disease was defined in ALSYMPCA as either regular use of analgesic drugs for bone pain or treatment with EBRT within the previous 12 weeks.21 Evidence from an international early access program (EAP) supports Ra-223 treatment earlier in the disease course.35 This prospective, single-arm phase 3b study of Ra-223 enrolled 696 patients with mCRPC. In contrast to ALSYMPCA, both symptomatic (77%) and asymptomatic (defined as no pain or opioid use at baseline; 19%) patients were included in the EAP.35,36 Asymptomatic patients had more favorable baseline characteristics and received fewer prior treatments than symptomatic patients. Seventy-one percent of asymptomatic patients received all 6 doses of Ra-223, compared with 55% of symptomatic patients. In addition, asymptomatic patients from the US EAP tolerated Ra-223 better than did symptomatic patients, with lower rates of any grade AE (61% vs. 79%), grade 3-4 AEs (29% vs. 40%), and serious AEs (22% vs. 40%). Median OS was 17 versus 14.9 months in ALSYMPCA.37 More studies are needed to determine if the higher treatment course completion rate and better tolerability with Ra-223 earlier in the disease course translates to improved outcomes.
Ra-223 earlier in the disease course may facilitate retreatment with Ra-223. This was evaluated in a recent trial (NCT01934790), in which patients completed 6 cycles of Ra-223 before retreatment with ≤6 additional cycles. As of this writing, median time to radiologic progression or median OS has not been reached; the median time to first symptomatic skeletal event (SSE) was 16.7 months, and the median SSE-survival was 12.8 months.38,39
Ra-223 requires no separate precautions or dose adjustments for special populations, for example, the elderly.40 Results of ALSYMPCA showed no differences between age groups <75 and ≥75 years, the latter being a subgroup thought too frail to receive chemotherapy (ECOG performance status ≥2).41 As Ra-223 is not metabolized by the liver nor excreted by bile, any hepatic impairment is unlikely to affect elimination; excretion in urine is minimal. Because Ra-223 is eliminated through the gastrointestinal tract, patients with Crohn’s disease or ulcerative colitis should take precautions.40
INSIGHTS FROM OTHER PHASE 3 TRIALS
We evaluated patient eligibility criteria and patient populations of those treated with other mCRPC agents in phase 3 trials to determine how those compared with men enrolled in ALSYMPCA. The studies (Table 1) enrolled >7000 men with CRPC, most of whom had bone or bone and lymph node disease.5–8,42–45 Analysis of these showed that most presented with bone metastases and later developed visceral disease. Furthermore, at detection most occurrences of visceral disease were symptomatic.46 Visceral metastases were associated with bone or lymph node metastases, and visceral disease plus bone metastases resulted in poorer outcomes.46 These findings may argue for earlier use of Ra-223 in mCRPC.
Early unblinded evidence from the ERA-223 trial (NCT02043678, randomized double-blinded, placebo-controlled, multicenter phase 3 trial investigating efficacy and safety of Ra-223 plus abiraterone and prednisone/prednisolone in asymptomatic or mildly symptomatic chemotherapy-naive patients with bone-predominant mCRPC) indicated that such combinations in patients with asymptomatic mCRPC may not give best outcomes, and further studies may be warranted.
TREATMENT OF BONE AND VISCERAL METASTASES IN mCRPC
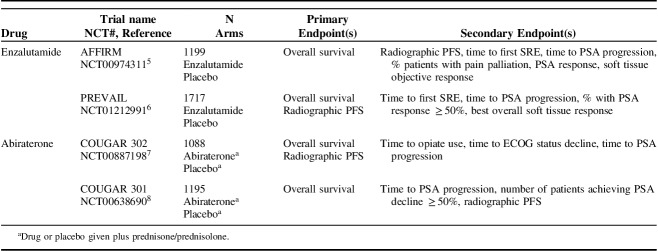
To address how bone metastases affect survival, a meta-analysis of 9 phase 3 clinical trials in nearly 9000 men was performed.4 Most (72%) had bone metastases, with or without lymph node metastases; fewer had visceral disease (21%) or lymph node–only metastases (6%). Results showed that having lung and liver metastases predicted shorter OS, compared with having bone metastases and nonvisceral disease (Fig. 1A).4
FIGURE 1.
A, Median overall survival (OS) by site of metastases and by trial start date across the different trials. CALGB, Cancer and Leukemia Group B; ENTHUSE, Endothelin A Use; LN, lymph node. From Halabi et al4 with permission. B, Frequency of bone-related pain in relation to treatment line and to time from acquisition of metastatic castration-resistance status until death. Visceral and bone metastases frequencies are from Pezaro et al.46 Pain prevalence is based on Autio et al.25 Ra-223 use scenarios referred to: (1) as first or second treatment lines; (2) as third treatment line; or (3) after >3 treatment lines. Adapted from Rodriguez-Vida et al26 with permission.
Although the number of bone metastases may remain relatively flat over time, the number of visceral metastases rises until death (Fig. 1B). Possible Ra-223 scenarios include part of first-line or second-line treatment before docetaxel, as third-line following docetaxel, or after ≥3 lines of treatment with docetaxel. These observations imply that Ra-223 may have a role when visceral disease is present.26 The St Gallen Advanced Prostate Cancer Consensus Conference observed that only 12% of patients enrolled in phase 3 trials were recommended to receive Ra-223 as first-line treatment; their panel therefore did not recommend Ra-223 as first-line treatment. However, a majority of the panel (65%) thought it was appropriate to study some men with asymptomatic CRPC and bone metastases.47 The current practice is to give Ra-223 when it may be less likely to benefit patients, after bone metastases have become symptomatic. In one study of patients with epidural disease, 93% of patients had prior CRPC therapy, and 75% had >20 metastases, compared with roughly 40% of patients in ALSYMPCA; the patient population in the former study therefore had a high number of cord compressions, and 37% of patients failed to complete all 6 cycles of therapy.48 This study highlights the need to determine optimal sequencing for Ra-223, especially in heavily pretreated patients.
RA-223 IN COMBINATION WITH OTHER AGENTS
Several ongoing trials investigate safety and efficacy of Ra-223 with other agents (Table 2). Results of a phase 3, expanded-access, single-arm trial (NCT01618370; Table 2) showed that Ra-223 could be given safely with abiraterone or enzalutamide.36 However, the ERA-223 study was recently unblinded by the Independent Data Monitoring Committee; preliminary analysis by an independent ad-hoc committee showed increased incidence of fractures (24% vs. 7%) and deaths (27% vs. 20%) among patients who received Ra-223 plus abiraterone (n=401), compared with patients who received placebo plus abiraterone (n=405).
TABLE 2.
Clinical Trials for Ra-223 to Help Sequencing Decisions
Two trials examine adding Ra-223 to abiraterone or enzalutamide early in the disease course, based on results from the EAP showing higher rates of treatment completion and better tolerability in asymptomatic patients.35 Combining androgen-deprivation therapy (ADT) with Ra-223 could target 2 key aspects of mCRPC: the ability of cancer cells to stimulate androgen signaling (thus stimulating cancer regrowth), and the ability of cancer to grow where bone remodeling has been disrupted. Two studies investigating these questions include NCT02225704, to assess safety and tolerability of first-line Ra-223 plus enzalutamide in patients with progressive mCRPC, and NCT03002220, evaluating the safety of Ra-223 after abiraterone or enzalutamide in patients with asymptomatic progression of mCRPC, as well as the association between AR-V7 mutation status and Ra-223 (Table 2). These results and those of investigator-initiated research into the same combinations will evaluate whether Ra-223 plus abiraterone or enzalutamide improve outcomes and will provide insight into Ra-223 early in treatment. Prior to final analyses, Ra-223 should be not be used with next-generation antihormonal therapy, except in a clinical trial.
Preclinical evidence suggests that Ra-223 may enhance certain antitumor immune responses, such as antigen-specific cytotoxic T cell-mediated lysis.50 A study investigating Ra-223 plus sipuleucel-T focuses on asymptomatic or minimally symptomatic mCRPC (NCT02463799; Table 2). Sipuleucel-T is an autologous cellular immunotherapy to stimulate immune responses against prostate cancer, and is FDA-approved for treatment of asymptomatic or minimally symptomatic mCRPC. Evidence suggests that immune responses may be enhanced by radiation; this study examines whether Ra-223 added to sipuleucel-T increases the anticancer immune response and antitumor effect against mCRPC.51
Two trials examine Ra-223 plus immunotherapy or immunooncology treatment. Ra-223 is believed to induce apoptosis among metastasized CRPC cells, releasing CRPC-specific proteins, which serve as antigens to educate the patient’s T cells. Agents such as pembrolizumab and atezolizumab can increase the number and activity of antigen-specific T cells, thus creating a T-cell subset that recognizes and attacks cancer. Thus, combining Ra-223 with immune checkpoint inhibitors may result in better control of mCRPC than does Ra-223 alone. Two trials (NCT03093428 and NCT02814669; Table 2) will evaluate Ra-223 plus pembrolizumab or atezolizumab, versus Ra-223.
Niraparib is a PARP inhibitor approved to treat ovarian cancer.52 A phase 1 study (NCT03076203; Table 2) will evaluate safety and determine the recommended phase 2 dose of niraparib when added to Ra-223. Olaparib, another PARP inhibitor, will be evaluated in a phase 1/2 study examining combination with Ra-223 (NCT03317392) to determine the best dose of olaparib plus Ra-223 and to track AEs.
PRACTICAL CONSIDERATIONS FOR RA-223 ADMINISTRATION
Patients should be maintained on androgen-suppression treatment while on treatment with Ra-223.53 The dose regimen of Ra-223 is 55 kBq per kg body weight, given at 4-week intervals for 6 injections, based on ALSYMPCA41 and the most recent National Institute of Standards standardization for Ra-223 dosimetry.34,54
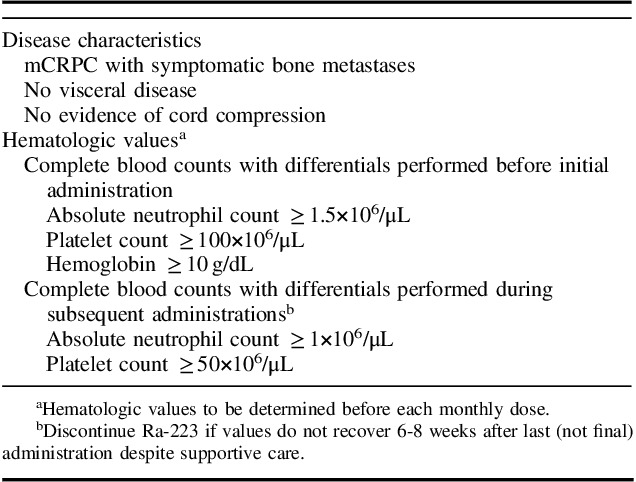
In addition to distribution of metastases, prior therapy history, potential future treatments, and symptomatology, clinicians must also weigh patient factors, such as performance status, hematologic parameters, and comorbidities, when deciding whether to administer Ra-223. Hematologic evaluation should be performed at baseline and prior to every dose of Ra-223. It is recommended that before the first dose is administered, absolute neutrophil count (ANC) should be ≥1.5×106/μL, the platelet count ≥100×106/μL, and hemoglobin ≥10 g/dL. Before subsequent administrations, ANC should be ≥1×106/μL and the platelet count ≥50×106/μL (Table 3). In a single-dose phase 1 study of Ra-223, neutrophil and platelet count nadirs occurred 2 to 3 weeks after Ra-223 administration at doses up to 1 to 5 times the recommended dose, and most patients recovered approximately 6 to 8 weeks after administration. If there is no recovery within 6 to 8 weeks after the last (not final) administration of Ra-223, treatment should be discontinued.
TABLE 3.
Specifications for Ra-223 Administration
Patients with compromised bone marrow reserves, including patients with prior chemotherapy use or who had extensive prior EBRT, should have more frequent blood count monitoring, owing to increased risk of myelosuppression. In addition to hematologic monitoring, patients should watch food and liquid intake to maintain good hydration and should report signs of bleeding, infection, dehydration, hypovolemia, urinary retention, or renal failure/insufficiency.55 No studies to date evaluate Ra-223 in patients on an antiplatelet and anticoagulant regimen. Such therapies are not considered contraindications to Ra-223; however, close hematologic monitoring in these patients is recommended owing to increased risk of bleeding if thrombocytopenia develops. One grade 5 event because of thrombocytopenia that may have been treatment related was reported in ALSYMPCA.41
The important role of family must be discussed, as they will be exposed to radiation if Ra-223 is used. The radiation dose administered by Ra-223 is much lower than that for most nuclear medicine procedures and, therefore, poses less hazard to family and caregivers. The estimated dose to a family member is <5 mSv, and <1 mSv for those with casual interaction.56 Therefore, family members of patients receiving Ra-223 do not have significant excess exposure to radiation, and patients with mCRPC should not be excluded from receiving treatment when living with family.
Owing to the nature of α particles, extensive shielding is unnecessary. Latex gloves suffice to protect caregivers’ hands. The most significant exposure may happen if spills occur during preparation or administration; one study estimated maximal exposure at 72 mSv; the authors concluded exposure likely would be an order of magnitude lower.33 Precautions should be taken so that no Ra-223 enters an open wound. In general, patients are treated as outpatients, without recommended special precautions for those they might interact with.40,56 Patients should be instructed to follow good hygiene practices to minimize others’ radiation exposure.55 Precautions are minimal enough that patients living in group-home situations should receive Ra-223.
BIOMARKERS AND NOVEL IMAGING TECHNIQUES
Currently, there is no generally accepted method to identify patients with mCRPC who would benefit from Ra-223 or to assess its efficacy, other than through symptoms. Although PSA, ALP, and lactate dehydrogenase (LDH) values are established prognostic biomarkers in mCRPC,57 they are not predictive of response to Ra-223.
PSA levels in particular may be of limited value. Studies reported varying changes in serum PSA levels following Ra-223. Early-phase studies did not find correlations between PSA and Ra-223.58 In ALSYMPCA, only 16% of patients receiving Ra-223 showed ≥30% decrease in PSA by week 12, compared with 6% receiving placebo.41 A single-institution retrospective study demonstrated decreased PSA levels in 22% of patients who received Ra-223 monotherapy versus 35% of patients who received Ra-223 plus other treatment.59 No trials of enzalutamide have shown decreased PSA levels as an outcome marker.60
ALP and LDH levels have been investigated as possible surrogate markers for Ra-223 efficacy. LDH and ALP are established prognostic biomarkers in mCRPC. In patients with mCRPC, both preantiandrogen and postantiandrogen therapy, rising ALP was the strongest of 3 quantified markers (PSA, LDH, and ALP) for outcome.57 In contrast to PSA, biomarker analysis from ALSYMPCA showed significant decreases in ALP levels as early as 4 weeks after Ra-223 commenced and significant decline in ALP levels, by up to 80%, at 12 weeks after treatment initiation.61 The decreases in tissue ALP and in LDH (mean decline 51%) correlated with longer OS in ALSYMPCA but did not meet statistical surrogacy requirements and therefore cannot be considered predictive of response to Ra-223. Nevertheless, they may be useful to monitor during treatment.61 Given the multiple treatment options available for patients with mCRPC, biomarkers are needed to identify which patients are most likely to respond to and benefit from Ra-223.
Trials evaluating initiation of an agent with a different mechanism of action (MOA) at the time of PSA progression on a first hormone may reveal additional insights. In one prospective trial of patients treated with enzalutamide (n=31) or abiraterone (n=31), patients expressing the androgen receptor splice variant AR7 had lower PSA response rates and shorter PSA progression-free survival (PFS), clinical PFS, and OS than did patients not expressing AR7.62 These imply that sequential hormonal agents may not be optimal for some patients and that using agents with differing MOAs may be effective.
Higher-sensitivity imaging techniques may reveal presymptomatic bone metastases. One technique uses prostate-specific membrane antigen (PMSA) as a target molecule, combined with probes for positron emission tomography (PET) scanning. PMSA-based imaging has been reported to improve detection of metastatic disease compared with computed tomography (CT) or magnetic resonance imaging (MRI) in patients with prostate cancer; it has rendered additional cross-sectional imaging unnecessary.63 18F-FDG PET/CT has not been recommended by the National Comprehensive Cancer Network64 guidelines for prostate cancer, although its utility in detecting locally recurrent or metastatic disease, or effect on outcome, has not been fully examined. Tumor burden indices determined by 18F-PET/CT at baseline were highly correlative and significant predictors of OS following treatment with Ra-223.65 A 2015 study showed that 18F-NaF/18F-FDG PET/CT is superior to whole-body MRI plus 99mTc-MDP scintigraphy for evaluation of skeletal disease extent. Moreover, 18F-NaF/18F-FDG PET/CT plus whole-body MRI detected extraskeletal disease that may change management of mCRPC.66
SUMMARY AND FUTURE DIRECTIONS
Ra-223 demonstrated safety and efficacy and is FDA-approved for patients with mCRPC having symptomatic bone metastases and no visceral metastases, regardless of prior treatment. Importantly, bone metastases have deleterious effects on patients beyond causing pain.
Optimal sequence of Ra-223 with other approved therapies for mCRPC is unknown. Evidence shows benefit and tolerability of Ra-223 in patients with prior docetaxel use, and in chemotherapy-naive patients. Data also support Ra-223 in asymptomatic patients; ongoing studies evaluate whether survival benefit observed with Ra-223 in symptomatic mCRPC is preserved in asymptomatic patients. Finally, additional studies investigate combinations of Ra-223 with other agents, including PARP and checkpoint inhibitors. Because the toxicity profile of Ra-223 does not overlap with other approved drugs for mCRPC, combination treatment may be feasible. Ra-223 should not be used with next-generation novel antihormonal therapy, except in a clinical trial.
Predictive biomarkers are needed to identify patients most likely to respond to Ra-223. The development and use of these biomarkers, plus emerging data around timing, sequencing, and combinations with Ra-223, will hopefully lead to improved patient selection and optimal outcomes.
ACKNOWLEDGMENTS
Beverly E. Barton, PhD, and Leonard Lionnet, PhD, CMPP, of ScioScientific, LLC, assisted with writing and editing this manuscript. The authors received no compensation from Bayer HealthCare Pharmaceuticals Inc. for their participation. No funded research is reported herein.
Footnotes
R.B.D.: has grants from Astellas and GenomeDx and serves on advisory boards for Bayer and GenomeDx. D.G.: consults for Acceleron Pharma, Astellas/Medivation, AstraZeneca, Bayer, Bristol-Myers Squibb, Exelixis, Genentech, Innocrin (Viamet), Janssen Pharmaceuticals, Myovant Sciences (until 3/2017), OncLive, Pfizer, and Sanofi-Aventis. He receives research support from Acerta, AstraZeneca, Bayer, Bristol-Myers Squibb, Dendreon/Valeant, Exelixis, Innocrin (Viamet), Janssen Pharmaceuticals, Millenium, Novartis, Pfizer, and Sanofi-Aventis. He has received an honorarium from Axess Oncology and speaking fees from Bayer, Exelixis, and Sanofi-Aventis. He served on the Capio Biosciences Inc Scientific Advisory Board until 4/1/2018. M.M.’s institution has received funds from Agensys, Bayer, Clovis, Janssen Pharmaceuticals, and Seattle Genetics. She has received travel funds from Clovis and Agensys. C.P.: is a paid investigator for clinical studies by Bayer and is part of Bayer’s speakers bureau.
REFERENCES
- 1.Kingsley LA, Fournier PGJ, Chirgwin JM, et al. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–2617. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundy GR. Metastasis: metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. [DOI] [PubMed] [Google Scholar]
- 4.Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad F, de Bono J, Shore N, et al. Efficacy outcomes by baseline prostate-specific antigen quartile in the AFFIRM trial. Eur Urol. 2015;67:223–230. [DOI] [PubMed] [Google Scholar]
- 6.Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16:509–521. [DOI] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis CJ, Basch E, Molina A, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–1217. [DOI] [PubMed] [Google Scholar]
- 9.El-Amm J, Aragon-Ching JB. The changing landscape in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Med Oncol. 2013;5:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baczyk M, Milecki P, Pisarek M, et al. A prospective randomized trial: a comparison of the analgesic effect and toxicity of 153Sm radioisotope treatment in monotherapy and combined therapy including local external beam radiotherapy (EBRT) among metastatic castrate resistance prostate cancer (mCRPC) patients with painful bone metastases. Neoplasma. 2013;60:328–333. [DOI] [PubMed] [Google Scholar]
- 11.Furubayashi N, Negishi T, Ura S, et al. Palliative effects and adverse events of strontium-89 for prostate cancer patients with bone metastasis. Mol Clin Oncol. 2015;3:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake GM, Zivanovic MA, Blaquiere RM, et al. Strontium-89 therapy: measurement of absorbed dose to skeletal metastases. J Nucl Med. 1988;29:549–557. [PubMed] [Google Scholar]
- 13.Hegemann M, Bedke J, Stenzl A, et al. Denosumab treatment in the management of patients with advanced prostate cancer: clinical evidence and experience. Ther Adv Urol. 2017;9:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R. Treatment of metastatic bone disease and the emerging role of Radium-223. Sem Nucl Med. 2016;46:99–104. [DOI] [PubMed] [Google Scholar]
- 17.Aprile C, Persico MG, Lodola L, et al. Radium-223 and metastatic castration-resistant prostate cancer: all that glitters is not gold. World J Radiol. 2016;8:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henriksen G, Fisher DR, Roeske JC, et al. Targeting of osseous sites with α-emitting 223Ra: comparison with the β-emitter 89Sr in mice. J Nucl Med. 2003;44:252–259. [PubMed] [Google Scholar]
- 19.Maffioli L, Florimonte L, Costa DC, et al. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q J Nucl Med Mol Imaging. 2015;59:420–438. [PubMed] [Google Scholar]
- 20.Abou DS, Ulmert D, Doucet M, et al. Whole-body and microenvironmental localization of radium-223 in naïve and mouse models of prostate cancer metastasis. J Natl Cancer Inst. 2016;108:djv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397–1406. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson S, Franzén L, Parker C, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20–26. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. [DOI] [PubMed] [Google Scholar]
- 24.Sartor O, Gillessen S. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian J Androl. 2014;16:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autio KA, Bennett AV, Jia X, et al. Prevalence of pain and analgesic use in men with metastatic prostate cancer using a patient-reported outcome measure. J Oncol Pract. 2013;9:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Vida A, Torregrosa MD, Pinto Á, et al. Selection and monitoring of patients with metastatic castration-resistant prostate cancer for treatment with radium-223. Clin Transl Oncol. 2018;20:679–686. [DOI] [PubMed]
- 27.International Prostate Cancer Coalition. Global Prostate Symptoms Survey; 2017. Available at: https://www.menwhospeakup.com/index.php. Accessed November 10, 2017.
- 28.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16:579–584. [DOI] [PubMed] [Google Scholar]
- 29.von Moos R, Costa L, Ripamonti CI, et al. Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer. 2017;71(suppl C):80–94. [DOI] [PubMed] [Google Scholar]
- 30.Sartor O, Coleman RE, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–746. [DOI] [PubMed] [Google Scholar]
- 31.Parker CC, Coleman RE, Sartor O, et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur Urol. 2018;73:427–435. [DOI] [PubMed] [Google Scholar]
- 32.United States Nuclear Regulatory Agency Licensing Decision of Radium-223 Dichloride. Washington, DC: Government Printing Office; 2013. Available at: https://www.nrc.gov/reading-rm/doc-collections/nuregs/brochures/br0117/13-01.pdf. [Google Scholar]
- 33.Stabin MG, Siegel JA. Radiation dose and hazard assessment of potential contamination events during use of 223Ra dichloride in radionuclide therapy. Health Phys. 2015;109:212–217. [DOI] [PubMed] [Google Scholar]
- 34.Bayer HealthCare Pharmaceuticals, Inc. Xofigo Full Prescribing Information Wayne, NJ; 2017. [Google Scholar]
- 35.Saad F, Carles J, Gillessen S, et al. Radium-223 in an international early access program (EAP): effects of concomitant medication on overall survival in metastatic castration-resistant prostate cancer (mCRCP) patients. J Clin Oncol. 2015;33(suppl 15):5034. [Google Scholar]
- 36.Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–1316. [DOI] [PubMed] [Google Scholar]
- 37.Vogelzang NJ, Fernandez DC, Morris MJ, et al. Radium-223 dichloride (Ra-223) in U.S. expanded access program (EAP). J Clin Oncol. 2015;33(suppl 7):247. [Google Scholar]
- 38.National Cancer Institute. Re-treatment safety of Radium-223 dichloride in castration-resistant prostate cancer with bone metastases; NCT01934790. 2017; A re-treatment safety study of Radium-223 dichloride in subjects with castration-resistant prostate cancer with bone metastases who received an initial course of six doses of Radium-223 dichloride 250 kBq/kg every four weeks. Available at: https://clinicaltrials.gov/ct2/show/study/NCT01934790?sect=Xj0156. Accessed November 10, 2017.
- 39.Sartor AO, Heinrich D, Mariados N, et al. Radium-223 retreatment in an international, open-label, phase 1/2 study in patients with castration-resistant prostate cancer and bone metastases: 2-year follow-up. J Clin Oncol. 2018;36(suppl 6):178. [Google Scholar]
- 40.Lien LME, Tvedt B, Heinrich D. Treatment of castration-resistant prostate cancer and bone metastases with radium-223 dichloride. Int J Urol Nurs. 2015;9:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. [DOI] [PubMed] [Google Scholar]
- 42.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 43.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. [DOI] [PubMed] [Google Scholar]
- 44.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. [DOI] [PubMed] [Google Scholar]
- 45.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 46.Pezaro C, Omlin A, Lorente D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26:1589–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spratt DE, Osborne JR, Zumsteg ZS, et al. Radium-223 outcomes after multiple lines of metastatic castration-resistant prostate cancer therapy in clinical practice: implication of pre-treatment spinal epidural disease. Prostate Cancer Prostatic Dis. 2016;19:271–276. [DOI] [PubMed] [Google Scholar]
- 49.Olmos D, Brewer D, Clark J, et al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol. 2012;13:1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malamas AS, Gameiro SR, Knudson KM, et al. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937–86947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res. 2017;23:6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. [DOI] [PubMed] [Google Scholar]
- 53.Dan TD, Doyle L, Raval AJ, et al. Dosing, administration, and safety of radium-223: how I do it. Can J Urol. 2016;23:8301–8305. [PubMed] [Google Scholar]
- 54.Zimmerman BE, Bergeron DE, Cessna JT, et al. Revision of the NIST standard for 223Ra: new measurements and review of 2008 data. J Res Nat Inst Stand Technol. 2015;120:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Den R, Doyle LA, Knudsen KE. Practical guide to the use of radium 223 dichloride. Can J Urol. 2014;21(suppl 1):70–76. [PubMed] [Google Scholar]
- 56.Dauer LT, Williamson MJ, Humm J, et al. Radiation safety considerations for the use of (223)RaCl(2) de in men with castration-resistant prostate cancer. Health Phys. 2014;106:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mikah P, Krabbe L-M, Eminaga O, et al. Dynamic changes of alkaline phosphatase are strongly associated with PSA-decline and predict best clinical benefit earlier than PSA-changes under therapy with abiraterone acetate in bone metastatic castration resistant prostate cancer. BMC Cancer. 2016;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson S, Strang P, Aksnes AK, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–686. [DOI] [PubMed] [Google Scholar]
- 59.Dan TD, Eldredge-Hindy HB, Hoffman-Censits J, et al. Hematologic toxicity of concurrent administration of Radium-223 and next generation anti-androgen therapies. Am J Clin Oncol. 2017;40:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armstrong AJ, Saad F, Phung D, et al. Clinical outcomes and survival surrogacy studies of prostate-specific antigen declines following enzalutamide in men with metastatic castration-resistant prostate cancer previously treated with docetaxel. Cancer. 2017;123:2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maurer T, Eiber M, Schwaiger M, et al. Current use of PSMA–PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. [DOI] [PubMed] [Google Scholar]
- 64.Prostate Cancer. NCCN. 2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed May 1, 2017.
- 65.Etchebehere EC, Araujo JC, Fox PS, et al. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–1184. [DOI] [PubMed] [Google Scholar]
- 66.Minamimoto R, Loening A, Jamali M, et al. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J Nucl Med. 2015;56:1862–1868. [DOI] [PubMed] [Google Scholar]